Abstract
Purpose
Radiation Therapy Oncology Group (RTOG) 9508 showed a survival advantage for patients with 1 but not 2 or 3 brain metastasis (BM) treated with whole-brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS) versus WBRT alone. An improved prognostic index, the graded prognostic assessment (GPA) has been developed. Our hypothesis was that if the data from RTOG 9508 were poststratified by the GPA, the conclusions may vary.
Methods and Materials
In this analysis, 252 of the 331 patients were evaluable by GPA. Of those, 211 had lung cancer. Breast cancer patients were excluded because the components of the breast GPA are not in the RTOG database. Multiple Cox regression was used to compare survival between treatment groups, adjusting for GPA. Treatment comparisons within subgroups were performed with the log-rank test. A free online tool (brainmetgpa.com) simplified GPA use.
Results
The fundamental conclusions of the primary analysis were confirmed in that there was no survival benefit overall for patients with 1 to 3 metastases; however, there was a benefit for the subset of patients with GPA 3.5 to 4.0 (median survival time [MST] for WBRT + SRS vs WBRT alone was 21.0 versus 10.3 months, P = .05) regardless of the number of metastases. Among patients with GPA 3.5 to 4.0 treated with WBRT and SRS, the MST for patients with 1 versus 2 to 3 metastases was 21 and 14.1 months, respectively.
Conclusions
This secondary analysis of predominantly lung cancer patients, consistent with the original analysis, shows no survival advantage for the group overall when treated with WBRT and SRS; however, in patients with high GPA (3.5-4), there is a survival advantage regardless of whether they have 1, 2, or 3 BM. This benefit did not extend to patients with lower GPA. Prospective validation of this survival benefit for patients with multiple BM and high GPA when treated with WBRT and SRS is warranted.
Introduction
Brain metastases are a common problem. In 2013 in the United States, an estimated 1.66 million cases of new cancer cases were diagnosed and more than 580,000 cancer deaths occurred (1). In an estimated 15% to 30% (250,000-500,000) of these new cancer patients, brain metastases (BM) will develop during the course of their illness (2-4). For perspective, secondary brain tumors (metastases) are more than 10 times as common as all primary brain tumors combined (23,000) (1).
The American Society for Radiation Oncology recently published an evidence-based guideline for the management of newly diagnosed brain metastases (4). The conclusions were consistent with guidelines published by the American Association of Neurological Surgeons/Congress of Neuro-surgeons (AANS/CNS) (5-9). These efforts reviewed more than 2000 publications and found 36 randomized controlled trials offering level 1 evidence. One of those trials is Radiation Therapy Oncology Group (RTOG) protocol 9508, which was a phase 3 randomized trial of whole brain radiation therapy (WBRT) versus WBRT and stereotactic radiosurgery (SRS) (10). That study showed a survival advantage for patients with 1 brain metastasis treated with WBRT and SRS versus WBRT alone but no such advantage for the study overall (1-3 metastases). The median survival times (MST) for patients with single BM treated with WBRT and SRS versus WBRT alone were 6.5 and 4.9 months, respectively (P=.04), whereas the MST for the study overall (1-3 BM) were 6.5 and 5.7 months, respectively (P=.14). It is noteworthy that many patients who were randomized to receive SRS did not actually receive it (15% among the solitary BM patients and 24% among the patients with 2-3 BM; 19% overall). This suggests that if more of the patients who were randomized to receive SRS had actually received SRS, a survival advantage might have been detected in the study overall. RTOG 9508 was stratified by the number of BM (1 vs 2 or 3) and a prognostic index, the recursive partitioning analysis (RPA) class (I versus II; class III was excluded). The RPA class definitions are as follows: class I: age under 65, controlled primary tumor, Karnofsky performance score (KPS) >60, no extracranial metastases; class III: KPS under 70; class II: all others (11). An improved and diagnosis-specific prognostic index, the graded prognostic assessment (GPA) has been developed (12-15) and independently validated (16-25). A user-friendly GPA worksheet (Table 1) and a free online tool at brainmetgpa.com have simplified use of the GPA.
Table 1. GPA worksheet to estimate survival from brain metastases by diagnosis.
| Diagnosis | Prognostic factor | GPA Scoring Criteria | Patient Score | ||||
|---|---|---|---|---|---|---|---|
| Non-small cell and small cell lung cancer | 0 | 0.5 | 1.0 | ||||
| Age | >60 | 50-60 | <50 | ___ | |||
| KPS | <70 | 70-80 | 90-100 | ___ | |||
| ECM | Present | - | Absent | ___ | |||
| No. of BM | >3 | 2-3 | 1 | ___ | |||
| Sum total = | _____ | ||||||
| MST (mo) by GPA: 0-1.0 = 3.0, 1.5-2.0 = 5.5, 2.5-3.0 =9.4, 3.5-4.0 =14.8 | |||||||
| Melanoma | 0 | 1.0 | 2.0 | ||||
| KPS | <70 | 70-80 | 90-100 | ___ | |||
| No. of BM | >3 | 2-3 | 1 | ___ | |||
| Sum total = | ______ | ||||||
| MST (mo) by GPA: 0-1.0 = 3.4, 1.5-2.0 = 4.7, 2.5-3.0 =8.8, 3.5-4.0 =13.2 | |||||||
| Breast cancer | 0 | 0.5 | 1.0 | 1.5 | 2.0 | ||
| KPS | <50 | 60 | 70-80 | 90-100 | n/a | ___ | |
| Subtype | basal | n/a | LumA | HER2 | LumB | ___ | |
| Age | >60 | <60 | n/a | n/a | n/a | ___ | |
| Sum total = | _____ | ||||||
| Subtype | Basal = triple negative (ER/PR/HER2-neg), LumA = luminal A (ER/PR-pos, HER2-neg) LumB = luminal B (triple positive, ER/PR/HER2-pos) HER2 = HER2-pos, ER/PR-neg |
||||||
| MST (mo) by GPA: 0-1.0 = 3.4, 1.5-2.0 = 7.7, 2.5-3.0 =15.1, 3.5-4.0 =25.3 | |||||||
| Renal cell carcinoma | 0 | 1.0 | 2.0 | ||||
| KPS | <70 | 70-80 | 90-100 | ___ | |||
| No. of BM | >3 | 2-3 | 1 | ___ | |||
| Sum total = | _____ | ||||||
| MST (mo) by GPA: 0-1.0 = 3.3, 1.5-2.0 = 7.3, 2.5-3.0 =11.3, 3.5-4.0 =14.8_____ | |||||||
| GI cancers | 0 | 1 | 2 | 3 | 4 | ||
| KPS | <70 | 70 | 80 | 90 | 100 | ___ | |
| MST (mo) by GPA: 0-1.0 = 3.1, 2.0 = 4.4, 3.0 = 6.9, 4.0= 13.5 | |||||||
Abbreviations: BM = brain metastases; ECM = extracranial metastases; ER = estrogen receptor; GI = gastrointestinal; GPA = graded prognostic assessment; HER2 = human epidermal growth factor receptor 2; KPS = Karnofsky performance score; MST = median survival time; PR = progesterone receptor.
The purpose of this study was to determine whether, if the data from RTOG 9508 were poststratified by the GPA instead of the RPA, the conclusions would vary. More specifically, would there be a survival advantage for patients with 2 or 3 BM when poststratified by the GPA?
Methods and Materials
Table 2 shows the patient characteristics for patients in the primary analysis and this secondary analysis. In the original analysis, 331 patients were randomized. Patients had a median age of 60 years (range, 19-90 years), 1-3 brain metastases, and no previous cranial radiation (10). In this analysis, 252 of those patients were evaluable by GPA (those with lung, gastrointestinal, and renal cancers and melanoma, for which a diagnosis-specific GPA index is available), and 79 were excluded from repeated analysis because data needed to calculate the breast GPA (estrogen and progesterone receptor status and HER2 status) were not in the RTOG database (34 patients) or they had other types of cancers (45 patients). Multiple Cox regression was used to compare overall survival (OS) between the treatment groups, adjusting for GPA. Subgroups defined by GPA class and number of metastases were also compared for treatment effects, using the log-rank test. All analyses were based on the intent to treat.
Table 2. Patient characteristics.
| Primary analysis n=331 | Secondary analysis n=252 | Patients excluded from secondary analysis n=79 | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Characteristic | WBRT + SRS n (%) | WBRT n (%) | WBRT + SRS n (%) | WBRT n (%) | WBRT + SRS n (%) | WBRT n (%) |
| n | 164 | 167 | 126 | 126 | 38 | 41 |
| GPA | ||||||
| 0.0-1.0 | 7 (6) | 3 (2) | ||||
| 1.5-2.0 | 25 (20) | 31 (25) | ||||
| 2.5-3.0 | 69 (55) | 70 (56) | ||||
| 3.5-4.0 (best) | 25 (20) | 22 (17) | ||||
| RPA | ||||||
| Class 1 (best) | 46 (28) | 45 (27) | 34 (27) | 35 (28) | 12 (32) | 10 (24) |
| Class 2 | 118 (72) | 122 (73) | 92 (73) | 91 (72) | 26 (68) | 31 (75) |
| Primary tumor site | ||||||
| GI | 9 (5) | 6 (4) | 9 (7) | 6 (5) | ||
| Lung | 105 (64) | 106 (63) | 105 (83) | 106 (84) | ||
| Melanoma | 7 (4) | 9 (5) | 7 (6) | 9 (7) | ||
| Renal | 5 (3) | 5 (3) | 5 (4) | 5 (4) | ||
| Breast | 15 (9) | 19 (11) | 0 (0) | 0 (0) | 15 (39) | 19 (46) |
| Other | 23 (14) | 22 (13) | 0 (0) | 0 (0) | 23 (61) | 22 (54) |
| Age, y | ||||||
| <65 | 109 (66) | 101 (60) | 81 (64) | 72 (57) | 27 (71) | 29 (71) |
| ≥65 | 55 (34) | 66 (40) | 45 (36) | 54 (43) | 11 (29) | 12 (29) |
| Extracranial metastases | ||||||
| Absent | 99 (60) | 100 (60) | 82 (65) | 81 (64) | 17 (45) | 19 (46) |
| Present | 65 (40) | 66 (40) | 44 (35) | 45 (36) | 21 (55) | 21 (51) |
| No. of BM | ||||||
| 1 | 92 (56) | 94 (56) | 70 (56) | 73 (58) | 22 (58) | 21 (51) |
| 2 | 39 (24) | 46 (28) | 30 (24) | 34 (27) | 9 (24) | 12 (29) |
| 3 | 33 (20) | 27 (16) | 26 (21) | 19 (14) | 7 (18) | 8 (20) |
| KPS | ||||||
| 70-80 | 71 (43) | 62 (37) | 57 (45) | 44 (35) | 14 (37) | 18 (44) |
| 90-100 | 93 (57) | 105 (63) | 69 (55) | 82 (65) | 24 (63) | 23 (56) |
Abbreviations: BM = brain metastases; CI = confidence interval; GI = gastrointestinal; GPA = graded prognostic assessment; KPS = Karnofsky performance score; RPA = recursive partitioning analysis; SRS = stereotactic radiosurgery; WBRT = whole-brain radiation therapy.
Results
The patient population in RTOG 9508 was predominantly lung cancer (64% and 84% of the primary analysis and this secondary analysis, respectively). Table 3 shows survival by treatment group, number of metastases, and GPA. There was no survival difference between treatments when analyzing the group overall (hazard ratio for WBRT versus WBRT+SRS: 1.0; 95% CI: 0.8-1.4; P=.78); however, patients with GPA 3.5 to 4.0 had better OS when treated with WBRT+SRS (MST 21.0 months; 2-year OS 43%) than with WBRT alone (MST 10.3 months; 2-year OS 21%; P=.05). Figure 1 shows the Kaplan-Meier survival curves for the WBRT+SRS and WBRT alone arms, for the overall group (upper panel) and for the GPA 3.5-4.0 group only (lower panel). Inasmuch as the number of brain metastases is a component of GPA, most (35/47) patients with GPA 3.5 to 4.0 had a single metastasis; however, data in this limited sample of high-GPA patients showed better survival for WBRT+SRS regardless of the number of metastases. The subgroup analyses (Table 3, middle and lower panels) showed that in patients with GPA 3.5 to 4.0 and 1 metastasis, the MST for patients in the WBRT+SRS arm versus the WBRT arm was 21.0 and 11.4 months, respectively, corresponding to a 9.6-month survival benefit for the WBRT+SRS arm. Similarly, among those with GPA 3.5 to 4.0 and 2 or 3 metastases, the MST for the 2 arms was 14.1 and 8.9 months, respectively, corresponding to a 5.2-month survival benefit for the WBRT+SRS arm. However, in patients with GPA <3.5, the 2 treatment arms showed similar MST regardless of the number of metastases. None of the subgroup analyses reached statistical significance, perhaps because of the limited sample size within each subgroup (see sample sizes in Table 3). All surviving patients (16 patients) were followed up for a minimum of 1 year and a median of 5 years.
Table 3. Median survival time (MST) in months, by treatment, GPA, and number of metastases.
| WBRT | WBRT + SRS | ||||
|---|---|---|---|---|---|
|
|
|
||||
| n | MST (95% CI) | n | MST (95% CI) | P value | |
| Overall | 126 | 5.8 (4.7-6.8) | 126 | 5.7 (4.6-8.6) | .21 |
| GPA <3.5 | 104 | 5.4 (3.3-6.3) | 101 | 5.0 (3.2-6.5) | .97 |
| GPA 3.5-4.0 | 22 | 10.3 (5.3-19.4) | 25 | 21.0 (10.5-36.0 | .05 |
| 1 metastasis | 73 | 5.0 (3.0-6.2) | 70 | 6.6 (4.6-11.2) | .07 |
| GPA <3.5 | 56 | 3.3 (2.2-5.3) | 52 | 5.4 (3.4-6.6) | .16 |
| GPA 3.5-4.0 | 17 | 11.4 (4.7-23.1) | 18 | 21.0 (10.0-36.0) | .21 |
| 2-3 metastases | 52 | 6.7 (5.7-9.1) | 55 | 5.3 (2.7-9.0) | .92 |
| GPA <3.5 | 47 | 6.5 (5.4-9.1) | 48 | 4.0 (2.5-7.9) | .26 |
| GPA 3.5-4.0 | 5 | 8.9 (2.4-14.6) | 7 | 14.1 (1.7-36.0) | .10 |
Abbreviations: CI = confidence interval; GPA = graded prognostic assessment; SRS = stereotactic radiosurgery; WBRT = whole-brain radiation therapy.
Figure 1.
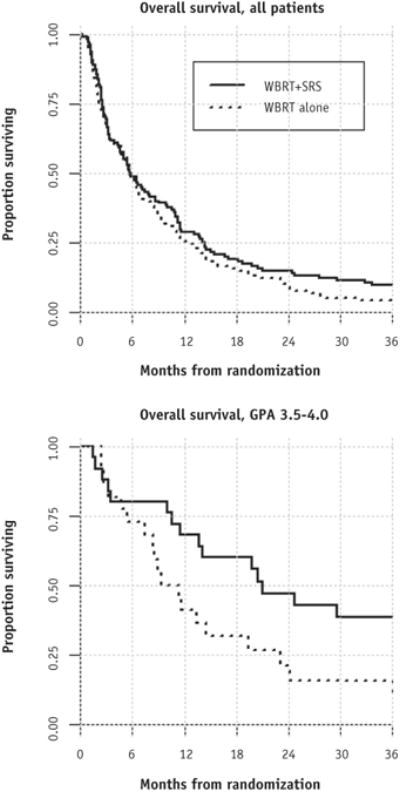
Kaplan-Meier curves for overall survival, by intent to treat, for all patients (above) and for patients with graded prognostic assessment (GPA) 3.5-4.0 (below). SRS = stereotactic radiosurgery; WBRT = whole-brain radiation therapy.
A sensitivity analysis of the 79 patients excluded from secondary analysis (Table 1; breast and other cancers) showed no difference in overall survival compared with the included group (MST 6.0 vs 5.8 months; P=.92). Survival in subgroup classes (treatment, RPA class, and number of metastases) was also very similar between the 2 cohorts.
Discussion
It is important to note that both the primary analysis and this secondary analysis primarily reflect patients with lung cancer. As in the primary analysis, there was no survival advantage for patients when treated with WBRT and SRS compared with WBRT alone for the group overall (patients with 1-3 BM); however, there was an advantage for the patients with a good prognosis (GPA 3.5-4.0) regardless of whether they had 1, 2, or 3 BM. Specifically, subset analyses in both the primary and secondary analyses showed a survival benefit for patients with 1, 2, or 3 brain metastases treated with WBRT and SRS in the best prognostic groups (GPA 3.5-4.0 or RPA class I). This benefit did not extend to patients with lower GPA or RPA and 2 or 3 metastases. Prospective validation of this survival benefit for patients with multiple BM and high GPA when treated with WBRT and SRS is warranted.
The GPA identifies a more select group of patients than does the RPA, as shown by the difference in survival for the best prognostic group by each index (MST for GPA 3.5-4 and RPA class I were 21 and 11.6 months, respectively). Accordingly, the GPA should be used to stratify future randomized clinical trials, estimate survival, and individualize management for patients with brain metastases. A free online tool, available at brainmetgpa.com, has simplified GPA use and is now widely used.
As with all post-hoc analyses, this study has several limitations; a particularly notable limitation is the observation in the initial report of this trial that patients with up to 3 brain metastases had a survival benefit on post-hoc analysis if they had non-small cell lung cancer. This would therefore have excluded the breast cancer patients. In our current analysis, all breast cancer patients were excluded because the data for grouping them into GPA categories do not exist in the RTOG database. It is possible that the SRS survival benefit seen in the current analysis in the high-GPA subgroup, irrespective of the number of brain metastases, could be absent in breast cancer patients; however, we note the strong similarity in survival between included and excluded patients as detailed in the aforementioned sensitivity analysis.
The enduring debate regarding the role of WBRT continues to evolve. These data suggest, despite a trend toward SRS alone, that WBRT and SRS may continue to play a role, especially in light of emerging data suggesting that the neurocognitive effects of WBRT can be mitigated. The Radiation Therapy Oncology Group has completed 2 trials (RTOG 0614 and 0933) aimed at reducing the toxicity of WBRT. RTOG 0614 was a randomized trial of WBRT versus WBRT and memantine in patients with brain metastases and showed better cognitive function over time in the memantine group. Specifically, memantine delayed the time to cognitive decline and reduced the rate of decline in memory, executive function, and processing speed (26). RTOG 0933 was a phase 2 trial of WBRT with hippo-campal avoidance (HA-WBRT). Preliminary analysis demonstrated memory preservation that was significantly better with HA-WBRT than in historical controls treated with WBRT (27-29). A trial investigating memantine and HA-WBRT is in development.
Prospective validation of this survival benefit for patients with GPA 3.5 to 4.0 and 1 to 3 brain metastases when treated with WBRT and SRS is warranted.
Summary.
This is a secondary analysis of Radiation Therapy Oncology Group 9508 poststratified by the graded prognostic assessment (GPA). There was no survival benefit overall for patients with 1 to 3 metastases; however, there was a benefit for the subset of patients with GPA 3.5 to 4.0 (median survival time for whole-brain radiation therapy [WBRT] + stereotactic radiosurgery vs WBRT alone was 21.0 vs 10.3 months, P=.05) regardless of the number of metastases.
Acknowledgments
Supported in part by NIH P30 CA77598 using the Biostatistics and Bioinformatics shared resource of the Masonic Cancer Center, University of Minnesota, and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number 8UL1TR000114-02. RTOG 9508 was conducted by the Radiation Therapy Oncology Group (RTOG) and was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). This article's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Presented at the 55th Annual Meeting of the American Society for Radiation Oncology (ASTRO) September 24, 2013, Atlanta, GA, and at the Best of ASTRO Inaugural Meeting, November 8, 2013, San Diego, CA.
Conflict of interest: Dr Mehta is a consultant with Abbott, BMS, Elekta, Merck, Novocure, Novelos, Phillips, Roche; has stock options with Accuray and Pharmacyclics; has a research grant from Novocure; and is on the Board of Directors of Pharmacyclics. The other authors report no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 3.Posner JB. Neurologic Complications of Cancer. Philadelphia: FA Davis; 1995. [Google Scholar]
- 4.Mehta MP, Tsao MN, Whelan TJ, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2005;63:37–46. doi: 10.1016/j.ijrobp.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:17–32. doi: 10.1007/s11060-009-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:71–83. doi: 10.1007/s11060-009-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta MP, Paleogos NA, Mikkelsen T, et al. The role of chemotherapy in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:71–83. doi: 10.1007/s11060-009-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rykin TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:103–114. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar LE, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 12.Sperduto PW, Berkey B, Gaspar LE, Mehta MP, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-insitutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the Graded Prognostic Assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperduto PW, Kased N, Roberge D, et al. Summary Report on the Graded Prognostic Assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Likacheva A, Pinnix CC, Parikh N, et al. Validation of recursive partitioning analysis and diagnosis-specific graded prognostic assessment in patients treated initially with radiosurgery alone. J Neurosurg. 2012;117(Suppl):38–44. doi: 10.3171/2012.3.GKS1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo S, Reddy CA, Chao ST, et al. Impact of non-small cell lung cancer histology on survival predicted from the graded prognostic assessment for patients with brain metastases. Lung Cancer. 2012;77:389–393. doi: 10.1016/j.lungcan.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Viana GA, da Silva LG, Stefano EJ. Prognostic indexes for brain metastases: Which is the most powerful? Int J Radiat Oncol Biol Phys. 2012;83:e325–e330. doi: 10.1016/j.ijrobp.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 19.Nieder C, Andratschke NH, Geinitz H, et al. Diagnosis-specific graded prognostic assessment score is valid in patients with brain metastases treated in routine clinical practice in two European countries. Med Sci Monit. 2012;(18):CR450–CR455. doi: 10.12659/MSM.883213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieder C, Geinitz H, Molls M. Validation of the graded prognostic assessment index for surgically treated patients with brain metastases. Anticancer Res. 2008;28:3015–3017. [PubMed] [Google Scholar]
- 21.Nieder C, Bremnes RN, Andratschke NH. Prognostic scores in patients with brain metastases from non-small cell lung caner. J Thor Oncol. 2009;11:1337–1341. doi: 10.1097/JTO.0b013e3181b6b6f4. [DOI] [PubMed] [Google Scholar]
- 22.Nieder C, Marienhagen K, Geinitz H, et al. Validation of the graded prognostic assessment index for patients with brain metastases. Acta Oncol. 2009;48:457–459. doi: 10.1080/02841860802342390. [DOI] [PubMed] [Google Scholar]
- 23.Nieder C, Mehta M. Prognostic indices for brain metastases: Usefulness and challenges. Radiat Oncol. 2009;4:10. doi: 10.1186/1748-717X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villa S, Weber DC, Moretones C, et al. Validation of the new graded prognostic assessment scale for brain metastases: A multicenter prospective study. Radiat Oncol. 2011;6:23. doi: 10.1186/1748-717X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoni D, Clavier JB, Pop M, et al. Institutional, retrospective analysis of 777 patients with brain metastases: Treatment outcomes and diagnosis-specific prognostic factors. Int J Radiat Oncol Biol Phys. 2013;86:630–637. doi: 10.1016/j.ijrobp.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy (WBRT): First report of RTOG 0614, a placebo-controlled, double-blind, randomized controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gondi V, Tome WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97:370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: Safety profile for RTOG 0933. Radiother Oncol. 2010;95:327–331. doi: 10.1016/j.radonc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gondi V, Mehta M, Pugh S, et al. Memory preservation with conformal avoidance of the hippocampus during whole-brain radiotherapy for patients with brain metastasis: Preliminary results of RTOG 0933. Int J Radiat Oncol Biol Phys. 2013;87:1186. late breaking abstract 1. [Google Scholar]


