Abstract
Background
While the oral cavity harbors more than 680 bacterial species, the interaction and association of selected bacterial species play a role in periodontal diseases. Bacterial species including Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, a consortium previously designated as the “red complex” is now being expanded to include other new emerging pathogens that are significantly associated with periodontal disease.
Highlight
In addition to novel mechanisms for oxidative resistance of individual species, community dynamics may lead to an overall strategy for survival in the inflammatory environment of the periodontal pocket. Complex systems controlled by response regulators protect against oxidative and nitrosative stress.
Conclusion
The combination of these multifaceted strategies would provide a comprehensive defense and support system against the repetitive host immune response to promote microbial persistence and disease.
Keywords: Porphyromonas gingivalis, oxidative stress, red complex, oral microbiome, Filifactor alocis
1. Background
Periodontal diseases are polymicrobial inflammatory-associated infectious diseases that can lead to the destruction of periodontal ligaments and adjacent supportive alveolar bone. The oral cavity harbors more than 680 bacterial species [1–3], and some of these microorganisms have been shown to be responsible for the initiation and progression of periodontal diseases [4,5]. Multiple methods including DNA-DNA hybridization, microarrays and next generation sequencing have shown that certain bacterial complexes associate with each other and that some were more likely to potentiate disease [6]. Bacterial species including Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, a consortium previously designated as the red complex, has been shown to have the highest association with the severity of periodontal disease [7]. Additionally, Fusobacterium nucleatum, Prevotella species, Eikenella corrodens, Parvimonas micra (formerly Peptostreptococcus micros), and Campylobacter rectus have an increased abundance in deep periodontal pockets and are also implicated as periodontopathogens [4,5,7,8]. Recent microbiome studies of healthy and periodontal disease patients in conjunction with microbial pathogenesis evaluation, have demonstrated that emerging new pathogens such as Filofacter alocis may play an increasingly significant role in periodontal disease [9–12]. In this review, “red complex” terminology is redefined as a representation of the original characterized periodontal pathogens and the new emerging bacteria. These bacteria are not usually found alone, but in combination, in the periodontal pocket, suggesting that some bacteria may cause destruction of the periodontal tissue in a cooperative manner [13,14]. Furthermore, coaggregation, nutrient effects, and modulation of virulence factors by periodontopathogens or by interspecies interactions between periodontopathogenic and nonpathogenic organisms have been reported to contribute to oral microbial pathogenesis (Figure 1) [15].
Figure 1. General strategies displayed by interacting oral biofilm microbial species to promote survival.
Diagram shows survival strategies emerging from the interaction and cohabitation of oral biofilm species. The purple arrow indicates that species from the intermediate colonizer group uses acidic pH neutralization to promote the establishment of late colonizers. The orange arrows show that species from the intermediate and late colonizer groups employ attachment to late colonizer motile species to acquire motility for deeper colonization. The red arrows demonstrate that species from early, intermediate and late colonizers use the strategies of common physiological support, toxin-antitoxin systems and modulation of gene expression to promote community-living, while combining their stress and virulence resistance mechanisms to survive host repetitive immune attacks.
The primary factor that affects survival of organisms in the oral cavity is oxidative stress. Oxidative stress can be defined as an excess of pro-oxidants, such as reactive oxygen species (ROS), in the cell. Reactive oxygen species, such as the superoxide radical (O2*), hydroxyl radical (HO·), hydrogen peroxide (H2O2) and oxidant nitric oxide (NO), pose a significant threat to cellular integrity [16]. The damage produced by intracellular ROS induces mutagenesis resulting in the generation of a wide spectrum of oxidative DNA lesions [17] many of which are toxic and/or mutagenic [18]. Thus, mutation prevention or avoidance is of utmost priority.
Additionally, exposure of periodontal pathogens to air can give rise to the metabolic conversion of atmospheric oxygen to ROS inside bacterial cells [17] and ROS are also produced by macrophages and neutrophils during the immune inflammatory response mediated by a process called the oxidative burst [19]. Thus, it is crucial that these organisms utilize an arsenal of mechanisms to either prevent or fix oxidative damage resulting from ROS in order to survive in a hostile environment.
This paper focuses on the pattern of microbial synergy exhibited by members of the expanded red complex and other bacterial species, which enables them to survive cooperatively and individually in the oxidatively stressed environment of the periodontal pocket.
2. Sensory Response
Numerous studies have shown that the formation of biofilms is controlled by cell-to-cell signaling mechanisms and that gene regulation during biofilm growth is due to the accumulation of signal molecules [20]. These signal molecules encapsulate what is known as the Quorum sensing (QS) mechanism, which is defined as cell-density dependent bacterial intercellular communication [20,21]. In general, bacteria behave as single cellular organisms at low cell densities; but may shift their behavior to a ‘multicellular’ type as their population density reaches a threshold level during the formation of a biofilm [22]. As the cells sense the change in population density, they are able to communicate through small signaling molecules. This results in bacteria within the biofilm being able to express genes for different phenotypes, in particular, those that function in virulence [20,22]. QS also influences gene expression which can affect outcomes in invasion, defense, spread, and resistance to stress conditions in bacterial pathogens [23].
QS may be used in bacteria for intraspecies or interspecies communication, a feat that is achieved through two types of QS systems, each mediated by distinct classes of autoinducers; N-acylated-l-homoserine lactones (AHLs) and autoinducer AI-2, respectively [24]. AI-2 is thought to be a non-species-specific autoinducer that mediates intra- and interspecies communication among Gram-negative and Gram-positive bacteria [25]. The AI-2 and its synthase LuxS have been shown to correlate with pathogenicity in a variety of organisms [26,27]. For our purposes, the AI-2 system is of particular importance, since it is proposed to be a universal language for interspecies communication, and may provide insights into how periodontal pathogens are able to combat oxidative stress within the periodontal pocket.
The enzyme LuxS is responsible for AI-2 biosynthesis. It is the product of the gene luxS, and is widely conserved throughout the bacterial kingdom [24]. LuxS synthesizes 4,5-dihydroxy-2,3-pentanedione (DPD), which undergoes spontaneous rearrangements to form a variety of DPD derivatives, known as the AI-2 pool [28]. It is important to note that the chemical nature of the active signaling molecule from aforementioned AI-2 pool varies between species, as does the nature of the AI-2 receptor for these signals [24].
It was previously shown that P. gingivalis possesses a gene that encodes a peptide that has 29% identity with LuxS of Vibrio harveyi. An insertional luxS mutation failed to induce luciferase activity in V. harveyi while wild type P. gingivalis ATCC 33277 induced luciferase expression [21]. Based on these findings, it has been proposed that P. gingivalis uses a LuxS protein in its AI-2 signaling system [21,29]. In P. gingivalis, QS has been implicated in the control of genes responsible for the acquisition of hemin [21] as well as promoting survival during host-induced stresses such as temperature, H2O2, and pH [30]. QS in the other red complex bacteria, including T. forsythia and T. denticola, has not been extensively studied, however previous data suggests the presence of an AI-2 signaling system in both these organisms [31]. F. nucleatum AI-2 was shown to induce biofilm growth, coaggregation between species, and expression of adhesion molecules of the red complex periodontopathogens [31]. This is significant because, as an intermediate colonizer, F. nucleatum is thought to be involved in facilitating the survival of other anaerobic bacteria within the periodontal biofilm [32,33], a feat that may be accomplished through AI-2 quorum sensing. Additionally, the induced virulence of each of the red complex species by F. nucleatum AI-2, was shown to be inhibited by quorum sensing inhibitors (QSIs), suggesting that AI-2 plays an essential role in the interspecies interactions between the periodontopathogens [31].
It has been previously shown that luxS is involved in stress gene responses in P. gingivalis as there was an induction of oxidative stress related genes in a luxS mutant [30]. Though the results were unexpected, the data showed a clear correlation between AI-2 and oxidative stress resistance in the organism. In addition, the induction of biofilm growth in red complex bacteria in response to AI-2 is another indication of a role for QS in oxidative stress resistance among the organisms. It is possible that this synergistic pathogenicity occurs as a byproduct of AI-2 signaling systems in the red complex [14] and that these signaling systems may potentiate these species individual and collective response to oxidative stress conditions in the oral cavity.
3. Oral Biofilms
Bacteria can attach to oral surfaces and/or each other by coadhesion and coaggregation, multiply, and form biofilms [34,35]. This microbial ecosystem undergoes a succession of different species, which is related to development of caries as well as periodontal disease [1,2].
In periodontal disease, there is a shift in the microbial population within the biofilm, which may be caused by changes in diet, poor hygiene [36,37], as well as increasing age of an individual [38]. It has been proposed that succession is also determined by coaggregation of bacteria [2] which occurs as a result of complex interactions between different bacteria and between bacteria and the surrounding environment [39]. The chronic inflammatory host response to this microbial shift, in addition to products released by bacteria, results in destruction of tissues that support and anchor the teeth.
The main initial colonizers in biofilm formation are streptococci and actinomyces where the former binds to receptors on the salivary pellicle, composed of various host molecules that coat the tooth enamel, while the latter binds to host proteins rich in proline and statherin (phosphate-containing protein). These two microbes provide attachment sites for other bacteria by the coadhesion process. Other initial colonizers include Capnocytophaga spp., Eikenella spp., Haemophilus spp., Prevotella spp., Propionibacterium spp., and Veillonella spp. [1]. One bacterial species in particular, Fusobacterium nucleatum, is found in high numbers in both healthy and diseased tissues and it coaggregates with many bacterial species, including early and late colonizers [2,39]. F. nucleatum is thus an important component of the biofilm formation process as studies suggest that it may be needed for colonization of late colonizers such as P. gingivalis, T. denticola, and T. forsythia [2].
P. gingivalis, T. denticola, and T. forsythia are found in higher numbers and are often isolated together from subgingival plaque taken from patients suffering from periodontitis [14]. Other bacteria that are found in elevated numbers in periodontal lesions include F. alocis, Eubacterium nodatum, Parvimonas micra, Prevotella intermedia, and F. nucleatum. P. gingivalis is considered a keystone species as it expresses virulence factors which disrupt host defense mechanisms that benefits itself as well as the periodontal biofilm as a whole [40,41]. The virulence factors secreted by P. gingivalis include gingipains and lipopolysaccharides (LPS). Data suggests that the gingipains (Arg-specific cysteine proteinases) of P. gingivalis can enhance local inflammation which may fuel further changes to the biofilm and stabilize the transition to a disease-inducing microbiota [42]. Metatranscriptomic analysis of oral microbial gene expression has shown that when P. gingivalis was introduced into a healthy multispecies biofilm the patterns of community gene expression was altered [43]. However, it is important to note that a new model for pathogenesis is emerging in which the data now suggests that periodontal disease is initiated and sustained by dysbiosis of the oral microbial community that’s initiated by keystone pathogens [10,41].
Studies indicate that synergism exists between various members of the red complex as P. gingivalis, T. denticola, T. forsythia, and F. alocis can be found together in periodontal lesions [14,39,41]. For example, an interaction of P. gingivalis with F. alocis can enhance its potential virulence [11,12,44] and P. gingivalis and T. denticola mutually enhance each other’s growth by producing isobutyric acid and succinic acid respectively [14]. Furthermore, a component present in sonicated cell extracts of T. forsythia can stimulate the growth of P. gingivalis in nutrition-decreased medium in a dose-dependent manner [14]. The data suggests that the growth-promoting factor from T-forsythia extracts was degraded by the gingipains of P. gingivalis [14,45]. Also, the growth of T. forsythia is enhanced when grown together with P. gingivalis [14]. In addition to nutritional synergism, there also exists cooperation in terms of coaggregation. Using visual assay and phase-contrast microscope, studies done by Onagawa et al showed that P. gingivalis coaggregates with T. denticola [46].
Two-dimensional electrophoresis and a ligand overlay assay demonstrated that P. gingivalis fimbriae and T. denticola dentilisin are responsible for the coaggregation observed between these two microbes [47]. More recent research shows that the hemagglutinin domain (Hgp44), RgpA, and Kgp of P. gingivalis also play an important role in coaggregation with T. denticola [48]. In fact, two triple mutants, one in RgpA, Kgp, and hemagglutinin A and another mutant in RgpA, RgpB and Kgp both had decreased coaggregation with T. denticola. In addition, there was a significant decrease in coaggregation between P. gingivalis with T. denticola when anti-Hgp44 antibodies were added. Interestingly, other studies using RT-PCR and western blot analysis demonstrate that incubation of P. gingivalis with T. denticola increases expression of RgpA, Kgp, and HagA (hemagglutinin A) [49].
Finally, there is a strong synergy during in-vitro biofilm formation between P. gingivalis and T. denticola [50]. In fact, coincubation of P. gingivalis with T. denticola resulted in formation of a mixed biofilm in significantly less time than by P. gingivalis alone. This was due to enhanced adhesive abilities of P. gingivalis [49]. In addition, using microtiter plate assays and confocal laser scanning microscopy, there was an increase in mass of the two microbes as well as an increase in adherence of the biofilm to the substratum when the cells were grown together versus when each was grown individually.
4. Oxidative Stress Response
As previously mentioned, F. nucleatum is found within mature oral biofilms where it coaggregates with both late and early colonizers and acts as an intermediary between the early and late stages of oral biofilm formation [51]. Futhermore, F. nucleatum coaggregates with non-coaggregating obligate anaerobe-oxygen tolerant species such as P. gingivalis and Actinomyces naeslundii. Data suggests that F. nucleatum plays a protective role against ROS, atmospheric O2 and H2O2 within the oral biofilm through the involvement of proteins of the alkyl hydroperoxide reductase/thioredoxin reductase system, modification of glycolytic enzymes and modulation of the intracellular concentrations of molecular chaperone proteins and related proteins [51].
P. gingivalis displays a particularly strong synergistic relationship with F. nucleatum which was supported by the fact that P. gingivalis was shown to survive increased O2 levels when grown in co-culture with F. nucleatum compared to being grown in mono-culture [51]. This protective effect exhibited by F. nucleatum could also benefit other late anaerobic colonizers in the oral biofilm as it was recently shown that biofilm formation could regulate the survival and invasiveness of this organism in an aerobic environment. Similarly, survival and virulence of T. denticola is dependent on the ability of this organism to form biofilms, grow in this milieu, interact with other species in the biofilm and escape from the biofilm, depending on environmental conditions [52]. The formation and maintenance of biofilms are intimately linked to the production of an extracellular matrix [53]. Extra polymeric substances (EPS) may constitute complex and compact structures within which ROS could find difficulty to penetrate and reach internal layers, thus hampering their efficacy.
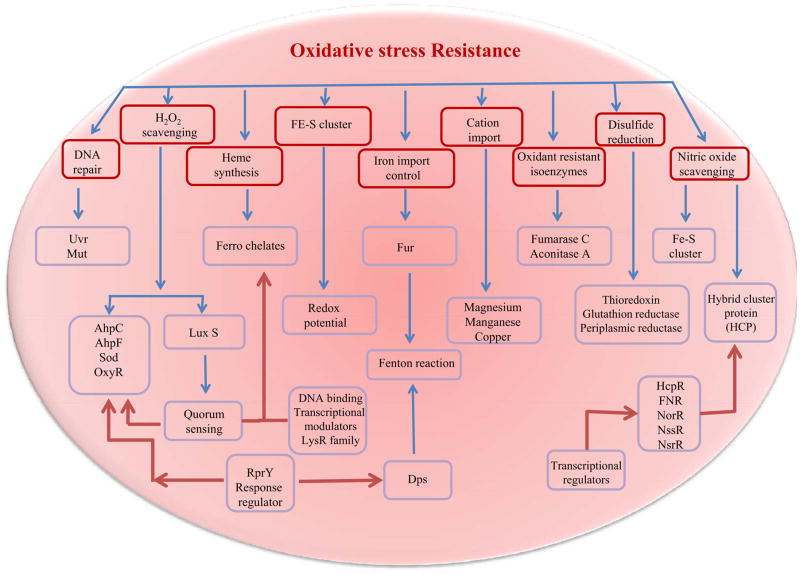
Different genes have been shown to be involved in oxidative stress response and may afford protection for bacteria against the activity of oxidizing agents (Figure 2). Data has indicated that in T. forsythia there is marked increase in the levels of several stress response proteins in the biofilm grown cells, namely Dps (a non-specific DNA-binding protein), AhpC (alkylhydroperoxide reductase subunit C) and Hsp20 [54]. Of these, both Dps and AhpC have been previously shown to be induced under conditions of osmotic and oxidative stress, and form part of a defense mechanism to these types of stress in several bacterial species, including the related periodontal pathogen P. gingivalis [55,56]. Another transcriptional response to oxidative stress employs the use of the RprY response regulator which can activate and/or repress transcription of their target genes and also regulate various oxidative stress proteins. This regulator has been identified in P. gingivalis [reviewed in [57]], B. fragilis [58] and T. forsythia [54]. The RprY protein of P. gingivalis positively regulates both AhpC and Dps at the transcriptional level [59] and in T. forsythia this protein was significantly downregulated which might explain a possible upregulation of AhpC and Dps in this organism. The niche occupied by red complex bacteria presents a constantly changing set of environmental challenges and in T. forsythia, the upregulation of Dps and AhpC suggests that biofilm cells are more resistant to oxidative stress. This was confirmed in cell viability assays after exposure to elevated H2O2 levels [54]. Furthermore, in P. gingivalis it was observed that AhpC plays a role in peroxide resistance but did not contribute significantly to virulence [56].
Figure 2. General biochemical mechanisms of oxidative stress resistance in anaerobic oral pathogens.
Mechanisms of oxidative stress resistance in bacteria are shown in hatched boxes (Red). The effector gene/gene product through which these mechanisms function are shown in blue boxes. Blue arrow indicate the primary pathways of oxidative stress resistance. Red arrows indicate the signaling pathways which modulate primary pathways during oxidative stress resistance. Further to the above mentioned common mechanisms, some organisms in the polymicrobial niche could also possess a well developed but yet explored mechanisms that could play a role through community dynamics in maintaining a conducive anaerobic environment.
OxyR is a redox-sensitive protein in the LysR family of DNA-binding transcription modulators, and regulates the expression of genes important in defense against oxidative stress in Gram-negative organisms [60]. The genome of T. forsythia has putative genes encoding an OxyR homologue and enzymes such as SodF (superoxide dismutase), Dps, rubrerythrin and AhpC are all involved in antioxidant responses. The data suggests that T. forsythia OxyR is at its maximum level of activation during anaerobic growth as there were no changes in expression of antioxidant genes before and after H2O2 treatment [33]. The expression of these antioxidant response genes has been shown to be OxyR-dependent in P. gingivalis [61,62] and similar results were seen in T. forsythia [33]. While OxyR is mainly responsible for tolerance to peroxide in aerobic E. coli and the facultative anaerobe B. fragilis, it appears to play an essential role against peroxide as well as oxygen (air) stress in obligate anaerobes such as P. gingivalis [63] and T. forsythia [33]. To date, except for the role of OxyR in P. gingivalis and T. forsythia, this protein has not been well studied in Gram-negative obligate anaerobes.
The anaerobic spriochete T. denticola, can metabolize oxygen to a certain extent [64] and genome analysis suggested the presence of an alkyl hydroperoxide reductase peroxiredoxin, a desulfoferrodoxin neelaredoxin, and NADPH oxidase (Nox) for tolerating oxygen stress [65]. A recent study showed that T. denticola possesses oxidoreductase activity [64] and removes oxygen from aerobic environments to generate anaerobic microenvironments [66]. In response to oxygen stress, other anaerobic oral bacteria, including many oral Streptococcus species, make use of various NADH-oxidases, NADH-peroxidases, superoxide dismutases, glutathione reductases, and pyruvate oxidases to metabolize oxygen into less harmful derivatives [67]. Based on microarray analysis data [64], T. denticola appears to employ different mechanisms for detoxification upon extended oxygen exposure since its NADH-oxidase and glutathione peroxidase homologs were not induced. Instead, a number of redoxins and proteins involved in iron homeostasis were differentially regulated. Several of the open reading frames (ORFs) suggested to be involved in the oxidative stress response [65], were significantly induced under these experimental conditions, while others could not be confirmed. Most strikingly, transcript levels for the peroxiredoxin were significantly higher than untreated controls. In the periodontal pathogen P. gingivalis, a peroxiredoxin, a thioredoxin, and a ferritin are under the control of OxyR [63,68]. Since the T. denticola genome does not contain an OxyR homologue, differential regulation of these genes could be controlled by one of the several transcriptional regulators that are specifically induced under oxygen stress conditions or by a yet unidentified transcriptional regulator.
5. Nitrosative Stress
Nitric oxide (NO) has been shown to be important in the pathogenesis and progression of periodontal disease due to its significant oxidative properties. Because of the high reactivity of NO with other molecules, NO-derivatives are often referred to as reactive nitrogen species (RNS) [69,70]. The biological chemistry of NO and RNS is complex because of the range of potential reactions that can happen with surrounding molecules [71]. These interactions have the potential to cause cellular responses ranging from minor changes in cell signaling to lethal oxidative insult [72].
Under normal conditions, minimal NO levels are found in human saliva. However, NO is produced in large amounts in immune and epithelial cells by an inducible nitric oxide synthase (iNOS) upon activation by an inflammatory stimulus [69,73,74]. When T. denticola was treated with lipopolysaccharide (LPS) and other surface material from P. gingivalis in murine macrophages, iNOS expression was induced which resulted in NO and lipooligosaccharide (LOS) being produced [75,76]. Gingival fibroblasts also released NO, inflammatory cytokines and matrix metalloproteinase 3 (MMP-3) when stimulated by T. denticola LOS [76].
Since excess intracellular NO is toxic, a crucial part of NO or nitrosative stress resistance in anaerobes such as P. gingivalis, T. denticola and T. forsythia, is their ability to keep intracellular NO/RNS at non-toxic or minimal levels. Pathogenic bacteria showed that they can resist the antimicrobial activities of RNS through various mechanisms which include hemoglobins that detoxify NO, NO reductases, peroxynitrite reductase and various repair mechanisms such as those that reverse S-nitrosothiol generation [69,71]. Additionally, bacteria’s capacity to repair damages caused by nitrosative stress is also crucial for their resistance potential. For instance, bacteria that are unsuccessful at repairing DNA lesions were shown to be more susceptible to RNS and phagocytes, and were less virulent [69]. These resistance strategies often extend to oxidative stress resistance because the targets of ROS and RNS overlap (Figure 2) [69]. In P. gingivalis, the gene expression for Dps was significantly upregulated under NO stress suggesting a role in NO stress resistance [77]. This protein displayed protective ferroxidase activity as well as DNA binding properties under oxidative stress, which could also be relevant to nitrosative stress. Dps did not seem to repair DNA itself, but interacted with DNA and DNA binding proteins to allow the repair of damaged DNA [78].
The NO detoxification pathway seems to involve a non-enzymatic interconversion of NO to hydroxylamine, a toxic nitrogen oxide, and the reduction of the latter to ammonia through hydroxylamine reduction to ammonia via the action of the hybrid cluster protein (HCP), a 4Fe-4S cluster binding oxidoreductase enzyme [77,79]. HCP, which was found to be essential for NO stress resistance in P. gingivalis, was induced by nitrite and NO, and was regulated by a FNR-like regulator called HcpR [77,80]. Interestingly, HCP genes were also predicted in Bacteroides species and T. denticola, along with HcpR-encoding genes, suggesting their possible involvement in nitrosative stress resistance [79]. Additionally, the important role of HCP in NO, hydroxylamine and H2O2 stress reduction and detoxification was demonstrated in other strictly or facultatively anaerobic bacteria [77].
Nitrosative stress resistance also involves transcriptional regulators, which can either act as secondary (e.g. FNR), or primary sensors (e.g. NorR, NsrR, NssR) that first detect NO and then regulate the expression of genes that encode for enzymes with NO as a substrate [71]. A study of the effects of NO stress on P. gingivalis revealed that the expression of a few transcription regulators predicted to belong to the TetR and LuxR families was significantly modulated [77]. These types of regulators are reported to usually be involved in stress resistance, sensing, multidrug resistance, biofilm formation, control of metabolic pathways, and virulence [77]. Further studies on the role of these transcriptors are ongoing.
Additional nitrosative stress resistance bacterial strategies use numerous types of proteins. First, the expression of genes generally induced by stress or implicated in mechanisms of adaptation to atypical conditions e.g., uspA, htpG, ustA and dps was significantly upregulated under NO stress, suggesting a role in NO stress resistance in P. gingivalis [77]. UspA, a universal stress protein, and UstA have been recognized to participate in oxidative stress resistance, although their exact mechanism(s) of action are not totally understood at present [57]. They might play a similar role in nitrosative stress. Second, the expression of genes encoding for proteins involved with protein fate, degradation, and stabilization, e.g. GroES and PG0778, was also upregulated under NO stress in P. gingivalis [77]. GroES, the 10kDA chaperonin heat shock protein is usually implicated in protein repair under conditions of environmental stress [77], and could play a similar role under NO stress. PG0778 encodes an O-sialoglycoprotease that was shown to be important in gingipain biogenesis and virulence modulation through the release of sialic acid, a ROS scavenger with protective properties against oxidative stress [77]. Research is ongoing to fully understand the effects of nitrosative stress in red complex bacteria.
6. Filofactor alocis in Oxidative Stress Resistance
Recently, studies have demonstrated it is likely that emerging new pathogens may play an increasingly significant role in periodontal disease than was previously thought possible [10,81–84].
One such organism that has generated significant interest is F. alocis. While this organism has been identified in endodontic infections [85–87] and localized aggressive periodontitis patients [10], its presence is now observed at significantly higher levels in adult periodontitis or refractory periodontitis patients [5,88–90]. Also, its colonization properties [90] and potential virulence attributes described by our lab [12,44] and others [91] could further support the proposal that F. alocis should be included as a diagnostic indicator of periodontal disease [88–90,92].
Our previous studies have shown that F. alocis was relatively resistant to oxidative stress and its growth was stimulated under those conditions [44]. These facts suggest that F. alocis is more resistant to oxidative stress than P. gingivalis. While the precise mechanism of oxidative stress resistance in F. alocis is yet to be explored, the presence of sialidase activity in F. alocis could suggest an important virulence attribute as recently demonstrated in P. gingivalis [93] and other periodontal pathogens [93,93,94]. While a number of periodontal bacteria are known to exploit host sialylated glycoproteins as a nutrient source, sialidase activity can also play an important role in their pathogenicity [44,95,96]. The released sialic acid can act as a ROS scavenger to reduce the oxidative stress in the inflammatory environment of the periodontal pocket [97]. Hence this could be a possible mechanism of F. alocis oxidative stress resistance.
F. alocis was shown to have pathogen synergy with P. gingivalis. Such co-infection has shown upregulation of many proteins involved in oxidative stress resistance. Proteins such as superoxide reductase, iron-sulfur cluster protein, iron permease, ruberythrin, the ferrous hydrogenase family protein, and the thioredoxin family protein were among them [12]. One of the unique genome organization of F. alocis include its possession of 3-methyladenine DNA glycosylase (HMPREF0389_1529), that is involved in oxidative and nitrosative stress resistance in other pathogenic bacteria [98]. The genome of F. alocis has also shown to possess a well-developed iron sulfur cluster proteins and ferrous iron transport system which are unique to the organism compared to other red complex bacteria. Additionally, F. alocis showed a well-developed protein sorting and transport system [12].
Infection-induced periodontitis is accompanied by an inflammatory response [99]. In the inflammatory response, neutrophils and macrophages are the most abundant cell types encountered at the site of infection [100] and are responsible for killing and clearance mediated through phagocytosis [100,101]. F. alocis infection in the mouse chamber model results in significant neutrophil recruitment. There is also evidence that F. alocis can induce dysfunction of select neutrophil responses. For example, Wang et al [102] suggest that F. alocis was able to induce degranulation of secretory vesicle, gelatinase, and specific granules, but failed to induce azurophil granule exocytosis. Further, both non-opsonized and opsonized F. alocis failed to activate the respiratory burst response but did not suppress the oxidase activity in response to other stimuli. Our previous studies have shown that neutrophil activating protein A, that may be involved in neutrophil modulation, was in high abundance in the secretome and/or membrane-associated in F. alocis [12]. Taken together, these results suggest that F. alocis may have unique characteristics that can modulate neutrophil function and which could have an impact on the disease process. Also, F. alocis could resist the oxidative burst adversities of the immune cells during their function.
7. A Corporate Survival Strategy
Overall there seems to be a pattern of microbial synergy that exists between the late colonizers of the periodontal pocket. Interbacterial signaling that occurs during the development of multispecies cohabitation, affect gene and protein expression, and survival dynamics of each species involved (Figure 1) [103].
Not all bacteria found in the oral cavity are motile or good colonizers. Colonization requires motility and adhesion among other attributes. Some bacteria have some attributes but lack others, which ultimately compromises their ability to colonize effectively. Interactions between periodontal strains were shown to result in bacterial associations with different characteristics that capitalize on their strengths and increase their chance of survival. For example, P. gingivalis and T. forsythia, both non-motile bacteria, can bind to T. denticola, a motile spirochete, in a ‘piggyback’ manner to gain mobility, augment their colonization activities, and establish themselves deeper in the periodontal pocket where anaerobic conditions are greatly improved (Figure 1) [104].
Interactions between oral biofilm bacteria have also demonstrated beneficial effects on biofilm formation and persistence. One example would be the toxin-antitoxin (TA) systems (Figure 1). These systems encode a ‘toxin’ protein that either promotes growth arrest or cell death, and an antitoxin that hinders toxin activity. (p)ppGpp is usually produced under stress conditions such as amino acid starvation and cell growth at the stationary phase, and cause the generation of polyphosphate which in turn activate enzymes responsible for the degradation of antitoxins, thereby allowing the release of cell growth inhibitory toxins and/or cell death. A study on multispecies biofilm showed that P. gingivalis reduced the expression of SpoT, a GTP pyrophosphokinase involved in the production of (p)ppGpp, when in communication with Streptococcus gordonii and Fusobacterium nucleatum, thus promoting cell growth and survival [103]. Also, when growing as a biofilm, T. denticola was reported to increase the production of functional TA homologs, a putative prophage, a family of putative transposases, and bacteriophage particles, to increase its genetic mobility [103]. This would increase its readiness to respond to signals from adjacent bacteria as well as its potential to adapt to changing environmental conditions.
Oral bacteria interactions between early and intermediate colonizers promote the establishment and longevity of late colonizers (Figure 1). The pH of the periodontal pocket was shown to increase with pocket depth and the severity of the inflammatory host response to periodontitis [103]. Early colonizers such as non-mutans streptococci use carbohydrates as nutrients, which results in fermentation and acidic conditions [105,106]. The intermediate colonizer F. nucleatum was shown to neutralize the acidic environment, which allowed the subsequent attachment and survival of late colonizers that do not grow well under acidic pH stress, such as P. gingivalis. To do so, F. nucleatum upregulated the expression of 22 soluble cytoplasmic proteins, some being linked to increased energy production via the 2-oxoglutarate and Embden-Meyerhof pathways [103].
Interactions between cohabitating species provide common physiological support (Figure 1). Firstly, studies have demonstrated that such interactions have led to the noticeable downregulation of DNA repair protein expression and upregulation in protein synthesis. Higher protein synthesis involved proteins for several vitamin synthesis pathways in all cohabitating species. Thus, it would become apparent that interacting cohabitations would render the environment less stressful, and that the bacteria were able to promote physiological support for each other instead of individual growth [103]. Secondly, bacterial strains with metabolic restrictions, can benefit from cohabiting with other strains of different metabolic processing, as nutrients of interest that would not be readily available if they were surviving on their own, will now be accessible to them because of this microbial synergy. Data confirms that the growth of T. forsythia is enhanced on blood agar when co-cultivated with P. gingivalis and F. nucleatum [14]. Additionally, the digestion of T. forsythia growth promoting factors by gingipains from P. gingivalis promoted the latter’s growth. P. gingivalis and T. denticola association also enabled synergistic growth in response to a combination of their respective growth-stimulating factors isobutyric acid and succinic acid [14]. Furthermore, an elevated production of uracil permease in cohabitating species was observed to facilitate the intake of nucleosides and nucleobases as nutrients by P. gingivalis, and S. gordonii was shown to support Veillonella alcalesens and P. gingivalis through the generation of lactate [103]. Similarly, the production of succinate by other oral bacteria benefited P. gingivalis ATP production [43]. Moreover, P. gingivalis Arg- and Lys-gingipain proteases digested hemoglobin to release heme [103], which would thus facilitate iron acquisition for its own and interacting bacteria benefits. Finally, the choice of catabolizing amino acids to form butyrate and propionate was suggested to be a calculated strategy against host cells while supplying the nutritional needs of cohabitating oral species [43]. Therefore, bacterial interspecies interactions seem to condition their biological setup for common nutritional benefit and anti-host defense.
It is likely that the interactions between oral bacteria strains would lead to higher outputs of virulence (Figure 1). Firstly, studies have shown that the P. gingivalis and T. forsythia mixed infection results in higher abscess formation, compared to monoinfections [14]. Secondly, data suggests that P. gingivalis gingipains is an important virulence factor that helps to create an environment that not only promotes the survival of P. gingivalis but may also aid in the survival of other organisms associated with it [14,104]. Gingipains can act as complement C5 convertases, leading to C5a receptor activation and signaling with Toll-like receptors 2, which eventually leads to aggrevated local inflammation. Thus, gingipains would indirectly promote bleeding, thereby providing heme for the nutritional benefit of the periodontal microbiota [43].
Metatranscriptomic analysis of the oral bacterial community gene expression also showed that adding P. gingivalis to a multispecies bacterial group caused changes in the pattern of community gene expression affecting proteins related to growth and division, chaperones, ABC-transport systems and several transcription factors [43]. Secondly, T. denticola was shown to interact and bind to P. gingivalis fimbriae via dentilisin, a chymotrypsin-like proteinase. The resulting association of both bacteria would provide T. denticola with the opportunity to coaggregate and possibly form biofilms thus providing the bacteria with a protective strategy against oxidative insults [14,104]. Thirdly, bacteria interactions leading to oral biofilm formation seem to contribute to the dispersion of antibiotic resistance properties found in participating species. As a matter of fact, Prevotella intermedia, one of the ‘orange complex’ bacteria species lining the gingival crevice, was reported to be crucial in the initiation and development of periodontal disease through the stimulation of host inflammatory reponses and its innate resistance to several antibiotics such as penicillins, cephalosphorins and tetracyclins. P. intermedia’s involvement with the oral biofilm provided interacting bacteria species with additional protection from antibiotic assault thus increased biofilm antibiotic resistance [103].
Oxidative and nitrosative stress resistance are strengthened by the interaction and cohabitation of oral bacteria species. F. nucleatum was found to be a shield for anaerobic late colonizers such as P. gingivalis from the hydrogen peroxide secreted by early streptococcal colonizers (Figure 1) [51]. This interaction would be beneficial to the establishment of the late oral periodontal species. Additionally, F. nucleatum and red complex bacteria consolidate resistance to oxidative stress by overproducing proteins of similar functions. Under oxidative stress, F. nucleatum and red complex species augmented the expression of several stress response, chaperones, regulators, and antioxidant proteins to possibly decrease the amount of intracellular ROS and counteract the destructive effects of ROS [33,51,54–59,63–65,68,103]. A similar protective strategy was also noticed with nitrosative stress since the biological insults caused by ROS and RNS overlap. Furthermore, interactions between F. alocis and P. gingivalis showed upregulation of similar antioxidant proteins and sialidase-mediated ROS scavenging mechanisms for oxidative stress resistance [12,97]. Moreover, expansion of the red complex to include other microbes could reveal community-dependent mechanisms for survival. For example, it is likely that organisms that are present in higher numbers could act as the ‘oxidative sink’ for the community. F. alocis is relatively more resistant to oxidative stress compared to P. gingivalis. Furthermore, its growth is stimulated under these conditions. Given its high density in the periodontal pocket, it is tempting to speculate that this could be a protective strategy for community survival. This community dynamics could likely involve novel antioxidant mechanisms in the more abundant members, for example F. alocis. This awaits further confirmation in the lab.
The interactions and cohabitations between the bacterial species belonging to the oral biofilm, especially the red complex, have resulted in the development of efficient stress resistance mechanisms involving antioxidative (e.g. hemin, hemoglobins, oxidoreductases), repair mechanisms (e.g. protein and DNA repair proteins), physical barriers (e.g. biofilm formation), antibiotic resistance dispersion, sialidase activity, and transcription regulation (e.g. oxidant-sensing transcription regulators) strategies to overcome oxidative and nitrosative stress attacks from host cells (Figure 2). Furthermore, these interactions have led to changes in genetic and protein expression profiles promoting the physiological support of the bacterial community. The combination of these multifaceted strategies would provide a comprehensive defense and support system against the repetitive host immune attacks and promote disease persistence in the periodontal pocket (Figures 1 and 2).
8. Conclusion
Proteomics studies have proven very insightful in the revelation of biological bacterial events that are relevant to multispecies interactions [103]. Systems approaches, which have been successful in modeling and simulating gut microbiome-host intestinal transcriptome interactions, are gaining momentum in their application to acquire further understanding of oral microbiome interactions [43]. Moreover, the complex interactions between the diverse oral microbial community and host, as well as the potential mechanism(s) in which the oral microbiome impact disease is starting to emerge [43]. Further application of this approach to the study of periodontal disease would have a tremendous impact on the development of novel therapeutics in the near future.
Acknowledgments
Grants
This work was supported by Loma Linda University and Public Health Services Grants DE13664 DE019730, DE019730 04S1, DE022508, DE022724 from NIDCR (to H.M.F).
We would like to thank our colleagues in the laboratory and from the Division of Microbiology and Molecular Genetics at Loma Linda University.
Footnotes
Conflicts of interest
The author declares no competing financial interests.
Ethical approval
Ethical approval or consent is not required.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolenbrander PE, Palmer RJ, Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–80. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- 3.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–7. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 5.Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80:1421–32. doi: 10.1902/jop.2009.090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teles FR, Teles RP, Siegelin Y, Paster B, Haffajee AD, Socransky SS. RNA-oligonucleotide quantification technique (ROQT) for the enumeration of uncultivated bacterial species in subgingival biofilms. Mol Oral Microbiol. 2011;26:127–39. doi: 10.1111/j.2041-1014.2010.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 8.Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–37. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- 9.Shaddox LM, Huang H, Lin T, Hou W, Harrison PL, Aukhil I, et al. Microbiological characterization in children with aggressive periodontitis. J Dent Res. 2012;91:927–33. doi: 10.1177/0022034512456039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine DH, Markowitz K, Fairlie K, Tischio-Bereski D, Ferrendiz J, Furgang D, et al. A Consortium of Aggregatibacter actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis Is Present in Sites Prior to Bone Loss in a Longitudinal Study of Localized Aggressive Periodontitis. J Clin Microbiol. 2013;51:2850–61. doi: 10.1128/JCM.00729-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Wright CJ, Dingming H, Uriarte SM, Lamont RJ. Oral community interactions of Filifactor alocis in vitro. PLoS One. 2013;8:e76271. doi: 10.1371/journal.pone.0076271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aruni AW, Roy F, Sandberg L, Fletcher HM. Proteome variation among Filifactor alocis strains. Proteomics. 2012;12:3343–64. doi: 10.1002/pmic.201200211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol 2000. 1997;14:12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki N, Yoneda M, Hirofuji T. Mixed red-complex bacterial infection in periodontitis. Int J Dent. 2013;2013:587279. doi: 10.1155/2013/587279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev. 2007;71:653–70. doi: 10.1128/MMBR.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strand KR, Sun C, Li T, Jenney FE, Jr, Schut GJ, Adams MW. Oxidative stress protection and the repair response to hydrogen peroxide in the hyperthermophilic archaeon Pyrococcus furiosus and in related species. Arch Microbiol. 2010;192:447–59. doi: 10.1007/s00203-010-0570-z. [DOI] [PubMed] [Google Scholar]
- 17.Cadet J, Douki T, Gasparutto D, Ravanat JL. Oxidative damage to DNA: formation, measurement and biochemical features. Mutat Res. 2003;531:5–23. doi: 10.1016/j.mrfmmm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Salmon TB, Evert BA, Song B, Doetsch PW. Biological consequences of oxidative stress-induced DNA damage in Saccharomyces cerevisiae. Nucleic Acids Res. 2004;32:3712–23. doi: 10.1093/nar/gkh696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freitas M, Lima JL, Fernandes E. Optical probes for detection and quantification of neutrophils’ oxidative burst. A review Anal Chim Acta. 2009;649:8–23. doi: 10.1016/j.aca.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 20.Lazar V. Quorum sensing in biofilms--how to destroy the bacterial citadels or their cohesion/power? Anaerobe. 2011;17:280–5. doi: 10.1016/j.anaerobe.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Chung WO, Park Y, Lamont RJ, McNab R, Barbieri B, Demuth DR. Signaling system in Porphyromonas gingivalis based on a LuxS protein. J Bacteriol. 2001;183:3903–9. doi: 10.1128/JB.183.13.3903-3909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–45. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 23.LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev. 2013;77:73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galloway WR, Hodgkinson JT, Bowden SD, Welch M, Spring DR. Quorum sensing in Gram-negative bacteria: small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem Rev. 2011;111:28–67. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- 25.Thiel V, Vilchez R, Sztajer H, Wagner-Dobler I, Schulz S. Identification, quantification, and determination of the absolute configuration of the bacterial quorum-sensing signal autoinducer-2 by gas chromatography-mass spectrometry. Chembiochem. 2009;10:479–85. doi: 10.1002/cbic.200800606. [DOI] [PubMed] [Google Scholar]
- 26.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005;3:383–96. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed NA, Petersen FC, Scheie AA. AI-2/LuxS is involved in increased biofilm formation by Streptococcus intermedius in the presence of antibiotics. Antimicrob Agents Chemother. 2009;53:4258–63. doi: 10.1128/AAC.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell-cell communication. Nature. 2005;437:750–3. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69:3431–4. doi: 10.1128/IAI.69.5.3431-3434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan L, Hillman JD, Progulske-Fox A. Microarray analysis of quorum-sensing-regulated genes in Porphyromonas gingivalis. Infect Immun. 2005;73:4146–54. doi: 10.1128/IAI.73.7.4146-4154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang YJ, Choi YJ, Lee SH, Jun HK, Choi BK. Autoinducer 2 of Fusobacterium nucleatum as a target molecule to inhibit biofilm formation of periodontopathogens. Arch Oral Biol. 2013;58:17–27. doi: 10.1016/j.archoralbio.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Pollanen MT, Paino A, Ihalin R. Environmental stimuli shape biofilm formation and the virulence of periodontal pathogens. Int J Mol Sci. 2013;14:17221–37. doi: 10.3390/ijms140817221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honma K, Mishima E, Inagaki S, Sharma A. The OxyR homologue in Tannerella forsythia regulates expression of oxidative stress responses and biofilm formation. Microbiology. 2009;155:1912–22. doi: 10.1099/mic.0.027920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–84. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 35.Lappin-Scott HM, Bass C. Biofilm formation: attachment, growth, and detachment of microbes from surfaces. Am J Infect Control. 2001;29:250–1. doi: 10.1067/mic.2001.115674. [DOI] [PubMed] [Google Scholar]
- 36.Al-Ahmad A, Roth D, Wolkewitz M, Wiedmann-Al-Ahmad M, Follo M, Ratka-Kruger P, et al. Change in diet and oral hygiene over an 8-week period: effects on oral health and oral biofilm. Clin Oral Investig. 2010;14:391–6. doi: 10.1007/s00784-009-0318-9. [DOI] [PubMed] [Google Scholar]
- 37.Avila M, Ojcius DM, Yilmaz O. The oral microbiota: living with a permanent guest. DNA Cell Biol. 2009;28:405–11. doi: 10.1089/dna.2009.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jakubovics NS. Talk of the town: interspecies communication in oral biofilms. Mol Oral Microbiol. 2010;25:4–14. doi: 10.1111/j.2041-1014.2009.00563.x. [DOI] [PubMed] [Google Scholar]
- 40.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–90. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 41.Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–19. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajishengallis G, Krauss JL, Liang S, McIntosh ML, Lambris JD. Pathogenic microbes and community service through manipulation of innate immunity. Adv Exp Med Biol. 2012;946:69–85. doi: 10.1007/978-1-4614-0106-3_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimitrov DV, Hoeng J. Systems approaches to computational modeling of the oral microbiome. Front Physiol. 2013;4:172. doi: 10.3389/fphys.2013.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aruni AW, Roy F, Fletcher HM. Filifactor alocis has virulence attributes that can enhance its persistence under oxidative stress conditions and mediate invasion of epithelial cells by Porphyromonas gingivalis. Infect Immun. 2011;79:3872–86. doi: 10.1128/IAI.05631-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoneda M, Yoshikane T, Motooka N, Yamada K, Hisama K, Naito T, et al. Stimulation of growth of Porphyromonas gingivalis by cell extracts from Tannerella forsythia. J Periodontal Res. 2005;40:105–9. doi: 10.1111/j.1600-0765.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- 46.Onagawa M, Ishihara K, Okuda K. Coaggregation between Porphyromonas gingivalis and Treponema denticola. Bull Tokyo Dent Coll. 1994;35:171–81. [PubMed] [Google Scholar]
- 47.Hashimoto M, Ogawa S, Asai Y, Takai Y, Ogawa T. Binding of Porphyromonas gingivalis fimbriae to Treponema denticola dentilisin. FEMS Microbiol Lett. 2003;226:267–71. doi: 10.1016/S0378-1097(03)00615-3. [DOI] [PubMed] [Google Scholar]
- 48.Ito R, Ishihara K, Shoji M, Nakayama K, Okuda K. Hemagglutinin/Adhesin domains of Porphyromonas gingivalis play key roles in coaggregation with Treponema denticola. FEMS Immunol Med Microbiol. 2010;60:251–60. doi: 10.1111/j.1574-695X.2010.00737.x. [DOI] [PubMed] [Google Scholar]
- 49.Meuric V, Martin B, Guyodo H, Rouillon A, Tamanai-Shacoori Z, Barloy-Hubler F, et al. Treponema denticola improves adhesive capacities of Porphyromonas gingivalis. Mol Oral Microbiol. 2013;28:40–53. doi: 10.1111/omi.12004. [DOI] [PubMed] [Google Scholar]
- 50.Yamada M, Ikegami A, Kuramitsu HK. Synergistic biofilm formation by Treponema denticola and Porphyromonas gingivalis. FEMS Microbiol Lett. 2005;250:271–7. doi: 10.1016/j.femsle.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 51.Steeves CH, Potrykus J, Barnett DA, Bearne SL. Oxidative stress response in the opportunistic oral pathogen Fusobacterium nucleatum. Proteomics. 2011;11:2027–37. doi: 10.1002/pmic.201000631. [DOI] [PubMed] [Google Scholar]
- 52.Dashper SG, Seers CA, Tan KH, Reynolds EC. Virulence factors of the oral spirochete Treponema denticola. J Dent Res. 2011;90:691–703. doi: 10.1177/0022034510385242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Branda SS, Vik S, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–6. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Pham TK, Roy S, Noirel J, Douglas I, Wright PC, Stafford GP. A quantitative proteomic analysis of biofilm adaptation by the periodontal pathogen Tannerella forsythia. Proteomics. 2010;10:3130–41. doi: 10.1002/pmic.200900448. [DOI] [PubMed] [Google Scholar]
- 55.Ueshima J, Shoji M, Ratnayake DB, Abe K, Yoshida S, Yamamoto K, et al. Purification, gene cloning, gene expression, and mutants of Dps from the obligate anaerobe Porphyromonas gingivalis. Infect Immun. 2003;71:1170–8. doi: 10.1128/IAI.71.3.1170-1178.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson NA, Liu Y, Fletcher HM. Alkyl hydroperoxide peroxidase subunit C (ahpC) protects against organic peroxides but does not affect the virulence of Porphyromonas gingivalis W83. Oral Microbiol Immunol. 2004;19:233–9. doi: 10.1111/j.1399-302X.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 57.Henry LG, McKenzie RM, Robles A, Fletcher HM. Oxidative stress resistance in Porphyromonas gingivalis. Future Microbiol. 2012;7:497–512. doi: 10.2217/fmb.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasmussen BA, Kovacs E. Cloning and identification of a two-component signal-transducing regulatory system from Bacteroides fragilis. Mol Microbiol. 1993;7:765–76. doi: 10.1111/j.1365-2958.1993.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 59.Duran-Pinedo AE, Nishikawa K, Duncan MJ. The RprY response regulator of Porphyromonas gingivalis. Mol Microbiol. 2007;64:1061–74. doi: 10.1111/j.1365-2958.2007.05717.x. [DOI] [PubMed] [Google Scholar]
- 60.Zheng M, Storz G. Redox sensing by prokaryotic transcription factors. Biochem Pharmacol. 2000;59:1–6. doi: 10.1016/s0006-2952(99)00289-0. [DOI] [PubMed] [Google Scholar]
- 61.Diaz PI, Zilm PS, Wasinger V, Corthals GL, Rogers AH. Studies on NADH oxidase and alkyl hydroperoxide reductase produced by Porphyromonas gingivalis. Oral Microbiol Immunol. 2004;19:137–43. doi: 10.1111/j.0902-0055.2004.00120.x. [DOI] [PubMed] [Google Scholar]
- 62.Ohara N, Kikuchi Y, Shoji M, Naito M, Nakayama K. Superoxide dismutase-encoding gene of the obligate anaerobe Porphyromonas gingivalis is regulated by the redox-sensing transcription activator OxyR. Microbiology. 2006;152:955–66. doi: 10.1099/mic.0.28537-0. [DOI] [PubMed] [Google Scholar]
- 63.Diaz PI, Slakeski N, Reynolds EC, Morona R, Rogers AH, Kolenbrander PE. Role of oxyR in the oral anaerobe Porphyromonas gingivalis. J Bacteriol. 2006;188:2454–62. doi: 10.1128/JB.188.7.2454-2462.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McHardy I, Keegan C, Sim JH, Shi W, Lux R. Transcriptional profiles of Treponema denticola in response to environmental conditions. PLoS One. 2010;5:e13655. doi: 10.1371/journal.pone.0013655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, Dodson RJ, et al. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci U S A. 2004;101:5646–51. doi: 10.1073/pnas.0307639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai Y, Chu L. Novel mechanism for conditional aerobic growth of the anaerobic bacterium Treponema denticola. Appl Environ Microbiol. 2008;74:73–9. doi: 10.1128/AEM.01972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hojo K, Nagaoka S, Ohshima T, Maeda N. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88:982–90. doi: 10.1177/0022034509346811. [DOI] [PubMed] [Google Scholar]
- 68.Meuric V, Gracieux P, Tamanai-Shacoori Z, Perez-Chaparro J, Bonnaure-Mallet M. Expression patterns of genes induced by oxidative stress in Porphyromonas gingivalis. Oral Microbiol Immunol. 2008;23:308–14. doi: 10.1111/j.1399-302X.2007.00429.x. [DOI] [PubMed] [Google Scholar]
- 69.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–32. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 70.Hughes MN. Chemistry of nitric oxide and related species. Methods Enzymol. 2008;436:3–19. doi: 10.1016/S0076-6879(08)36001-7. [DOI] [PubMed] [Google Scholar]
- 71.Bowman LA, McLean S, Poole RK, Fukuto JM. The diversity of microbial responses to nitric oxide and agents of nitrosative stress close cousins but not identical twins. Adv Microb Physiol. 2011;59:135–219. doi: 10.1016/B978-0-12-387661-4.00006-9. [DOI] [PubMed] [Google Scholar]
- 72.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mydel P, Takahashi Y, Yumoto H, Sztukowska M, Kubica M, Gibson FC, III, et al. Roles of the host oxidative immune response and bacterial antioxidant rubrerythrin during Porphyromonas gingivalis infection. PLoS Pathog. 2006;2:e76. doi: 10.1371/journal.ppat.0020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ugar-Cankal D, Ozmeric N. A multifaceted molecule, nitric oxide in oral and periodontal diseases. Clin Chim Acta. 2006;366:90–100. doi: 10.1016/j.cca.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 75.de Sa Siqueira MA, Fischer RG, da Silva Figueredo CM, Brunini TM, Mendes-Ribeiro AC. Nitric oxide and oral diseases: can we talk about it? Cardiovasc Hematol Agents Med Chem. 2010;8:104–12. doi: 10.2174/187152510791170942. [DOI] [PubMed] [Google Scholar]
- 76.Tanabe S, Bodet C, Grenier D. Treponema denticola lipooligosaccharide activates gingival fibroblasts and upregulates inflammatory mediator production. J Cell Physiol. 2008;216:727–31. doi: 10.1002/jcp.21447. [DOI] [PubMed] [Google Scholar]
- 77.Boutrin MC, Wang C, Aruni W, Li X, Fletcher HM. Nitric oxide stress resistance in Porphyromonas gingivalis is mediated by a putative hydroxylamine reductase. J Bacteriol. 2012;194:1582–92. doi: 10.1128/JB.06457-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiancone E, Ceci P. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding. Biochim Biophys Acta. 2010;1800:798–805. doi: 10.1016/j.bbagen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 79.Rodionov DA, Dubchak IL, Arkin AP, Alm EJ, Gelfand MS. Dissimilatory metabolism of nitrogen oxides in bacteria: comparative reconstruction of transcriptional networks. PLoS Comput Biol. 2005;1:e55. doi: 10.1371/journal.pcbi.0010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis JP, Yanamandra SS, Anaya-Bergman C. HcpR of Porphyromonas gingivalis is required for growth under nitrosative stress and survival within host cells. Infect Immun. 2012;80:3319–31. doi: 10.1128/IAI.00561-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahn J, Yang L, Paster BJ, Ganly I, Morris L, Pei Z, et al. Oral microbiome profiles: 16S rRNA pyrosequencing and microarray assay comparison. PLoS One. 2011;6:e22788. doi: 10.1371/journal.pone.0022788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–85. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–25. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomes BP, Pinheiro ET, Jacinto RC, Zaia AA, Ferraz CC, Souza-Filho FJ. Microbial analysis of canals of root-filled teeth with periapical lesions using polymerase chain reaction. J Endod. 2008;34:537–40. doi: 10.1016/j.joen.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 86.Sakamoto M, Rocas IN, Siqueira JF, Jr, Benno Y. Molecular analysis of bacteria in asymptomatic and symptomatic endodontic infections. Oral Microbiol Immunol. 2006;21:112–22. doi: 10.1111/j.1399-302X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 87.Siqueira JF, Jr, Rocas IN, Alves FR, Silva MG. Bacteria in the apical root canal of teeth with primary apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:721–6. doi: 10.1016/j.tripleo.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 88.Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–73. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–55. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schlafer S, Riep B, Griffen AL, Petrich A, Hubner J, Berning M, et al. Filifactor alocis--involvement in periodontal biofilms. BMC Microbiol. 2010;10:66. doi: 10.1186/1471-2180-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moffatt CE, Whitmore SE, Griffen AL, Leys EJ, Lamont RJ. Filifactor alocis interactions with gingival epithelial cells. Mol Oral Microbiol. 2011;26:365–73. doi: 10.1111/j.2041-1014.2011.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dahlen G, Leonhardt A. A new checkerboard panel for testing bacterial markers in periodontal disease. Oral Microbiol Immunol. 2006;21:6–11. doi: 10.1111/j.1399-302X.2005.00243.x. [DOI] [PubMed] [Google Scholar]
- 93.Stinson MW, Safulko K, Levine MJ. Adherence of Porphyromonas (Bacteroides) gingivalis to Streptococcus sanguis in vitro. Infect Immun. 1991;59:102–8. doi: 10.1128/iai.59.1.102-108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roy S, Douglas CW, Stafford GP. A novel sialic acid utilization and uptake system in the periodontal pathogen Tannerella forsythia. J Bacteriol. 2010;192:2285–93. doi: 10.1128/JB.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aruni W, Vanterpool E, Osbourne D, Roy F, Muthiah A, Dou Y, et al. Sialidase and sialoglycoproteases can modulate virulence in Porphyromonas gingivalis. Infect Immun. 2011;79:2779–91. doi: 10.1128/IAI.00106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brigham C, Caughlan R, Gallegos R, Dallas MB, Godoy VG, Malamy MH. Sialic acid (N-acetyl neuraminic acid) utilization by Bacteroides fragilis requires a novel N-acetyl mannosamine epimerase. J Bacteriol. 2009;191:3629–38. doi: 10.1128/JB.00811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iijima R, Takahashi H, Namme R, Ikegami S, Yamazaki M. Novel biological function of sialic acid (N-acetylneuraminic acid) as a hydrogen peroxide scavenger. FEBS Lett. 2004;561:163–6. doi: 10.1016/S0014-5793(04)00164-4. [DOI] [PubMed] [Google Scholar]
- 98.Slade D, Lindner AB, Paul G, Radman M. Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans. Cell. 2009;136:1044–55. doi: 10.1016/j.cell.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 99.Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333:1–9. doi: 10.1111/j.1574-6968.2012.02579.x. [DOI] [PubMed] [Google Scholar]
- 100.Bascones-Martinez A, Munoz-Corcuera M, Noronha S, Mota P, Bascones-Ilundain C, Campo-Trapero J. Host defence mechanisms against bacterial aggression in periodontal disease: Basic mechanisms. Med Oral Patol Oral Cir Bucal. 2009;14:e680–e685. doi: 10.4317/medoral.14.e680. [DOI] [PubMed] [Google Scholar]
- 101.Gemmell E, Bird PS, Bowman JJ, Xu L, Polak B, Walsh LJ, et al. Immunohistological study of lesions induced by Porphyromonas gingivalis in a murine model. Oral Microbiol Immunol. 1997;12:288–97. doi: 10.1111/j.1399-302x.1997.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q, Jotwani R, Le J, Krauss JL, Potempa J, Coventry SC, et al. Filifactor alocis infection and inflammatory responses in the mouse subcutaneous chamber model. Infect Immun. 2013 doi: 10.1128/IAI.01434-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuboniwa M, Tribble GD, Hendrickson EL, Amano A, Lamont RJ, Hackett M. Insights into the virulence of oral biofilms: discoveries from proteomics. Expert Rev Proteomics. 2012;9:311–23. doi: 10.1586/epr.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bodet C, Chandad F, Grenier D. Pathogenic potential of Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia, the red bacterial complex associated with periodontitis. Pathol Biol (Paris) 2007;55:154–62. doi: 10.1016/j.patbio.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 105.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi N, Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 2008;42:409–18. doi: 10.1159/000159604. [DOI] [PubMed] [Google Scholar]