Abstract
Background
Our recent study has shown that acute treatment with ethanol increases oxidative stress and cytotoxicity through cytochrome P450 2E1 (CYP2E1)-mediated pathway in U937 monocytic cells. U937 cells are derived from blood monocytes and are considered as the model system for HIV-related study. Since the prevalence of alcohol use in HIV-infected population is high, and HIV+ patients are on antiretroviral therapy (ART) soon after they are diagnosed, it is important to study the interactions between ethanol and ART in monocytes.
Methods
This study examined the chronic effects of ethanol and ART (darunavir/ritonavir), alone and in combination, on expression/levels of cytochrome P450 enzymes (CYPs), antioxidant enzymes (AOEs), reactive oxygen species (ROS), and cytotoxicity in U937 cells. The mRNA and protein levels were measured using quantitative RTPCR and Western blot, respectively. ROS and cytotoxicity were measured using flow cytometry and XTT assay, respectively.
Results
While chronic ART treatment increased CYP2E1 protein expression by 2-fold, ethanol and ethanol+ART increased CYP2E1 by ~5-fold. In contrast, ART and ethanol treatments decreased CYP3A4 protein expression by 38±17% and 74±15%, respectively, and the combination additively decreased CYP3A4 level by 90±8%. Expressions of superoxide dismutase (SOD1) and peroxiredoxin (PRDX6) were decreased by both ethanol and ART, however, the expressions of SOD2 and catalase were unaltered. These results suggested increased ethanol metabolism, increased ART accumulation, and decreased defense against ROS. Therefore, we determined the effects of ethanol and ART on ROS and cytotoxicity. While ART showed a slight increase, ethanol and ethanol+ART displayed significant increase in ROS and cytotoxicity. Moreover, the combination showed additive effects on ROS and cytotoxicity.
Conclusions
These results suggest that chronic ethanol, in the absence and presence of ART, increases ROS and cytotoxicity in monocytes, perhaps via CYPs and AOEs mediated pathways. This study has clinical implications in HIV+ alcohol users who are on ART.
Keywords: Ethanol, darunavir/ritonavir, cytochrome P450s, antioxidant enzymes, oxidative stress
Introduction
Ethanol is primarily metabolized by liver enzymes including alcohol dehydrogenase, cytochrome P450 (CYP) 2E1, and catalase (Cederbaum, 2012). Importantly, ethanol metabolism by CYP2E1 is associated with increased production of reactive oxygen species (ROS) resulting in enhanced oxidative stress and tissue injury, especially in the liver (Lieber, 1997, Comporti et al., 2010, Yun et al., 2014). Moreover, chronic exposure to ethanol is known to induce the levels of CYP2E1 expression, which further modulates the ethanol-mediated deleterious effects in various tissues (Cederbaum, 2012, Lieber, 1997). Thus, CYP2E1 plays the major role in ethanol metabolism in chronic alcohol users and causes toxicity in liver and other tissues/cells including blood cells (Sharma et al., 2012). Considering the elevated oxidative stress conditions following HIV infection (Kimura et al., 1993) and reports that oxidative stress potentiates HIV pathogenesis (Schreck et al., 1991), we rationalize a critical role of CYP2E1 in oxidative stress-mediated enhanced HIV replication in HIV+ alcohol users
Darunavir and ritonavir-boosted darunavir (darunavir/ritonavir; DRV/RTV) are commonly prescribed first line drugs from the antiretroviral class of protease inhibitors (PIs) that comprise the ART regimen (Guidelines for the Use of Antiretroviral Agents, 2014). DRV and RTV are mainly metabolized by CYP3A4 in the liver and this, in turn, modulate the bioavailability of these drugs in target cells, namely T-lymphocytes and monocytes (Back et al., 2008, Kumar et al., 1996). In addition, as a potent inhibitor of CYP3A4, and inducers of other CYP isoforms, RTV carries a strong potential for drug-drug interactions (DDI) (Josephson, 2010). In addition to DDI, we have shown a three-way physical interactions of ethanol and PIs, including DRV and RTV, with CYP3A4 suggesting a broader scope for DDI with these PIs (Kumar and Kumar, 2011). In general, CYP-mediated DDI, comprising of substance of abuse, ART, and dietary supplements, can potentially lead to toxicity in hepatic and extrahepatic cells (Kumar et al., 2015, Pal and Mitra, 2006).
The importance and role of myeloid cells, both monocytes and monocyte-derived macrophages, in propagation and maintenance of HIV infection has been reviewed extensively (Cassol et al., 2006, Campbell et al., 2014, Williams et al., 2014). Monocytes/macrophages (M/M) are important targets of HIV infection and comprise a major viral reservoir during HIV infection (Gendelman et al., 1989). Following their infection with HIV, M/M are known to mediate the transmigration of HIV infection across the blood-brain barrier (Williams et al., 2014). Several studies have identified specific changes facilitating the spread of HIV infection to brain by M/M (Buckner et al., 2011, Guha et al., 2015). A direct evidence supporting the involvement of these cells in HIV propagation and maintenance has been demonstrated previously (Collins et al., 2015, Harman et al., 2015, Kramski et al., 2012). Moreover, a correlation between HIV infection and change/loss of myeloid lineage cell function has been established in recent articles (Harman et al., 2015, Wonderlich et al., 2015). Overall, these evidences strongly support the detailed examination of changes in myeloid lineage cells to assess and predict the impact of drug abuse on HIV pathogenesis. Therefore, M/M lineage of cells, and cell lines derived from these M/M such as U937, are considered important systems to perform HIV-related study.
In the past five years we have utilized U937 cell lines and primary M/M to study the acute effects of alcohol and tobacco constituents. Recently, we have demonstrated relatively high expressions of CYP2E1 and CYP3A4 in U937 monocytic cell lines (Jin et al., 2011) and primary M/M (Ande et al., 2015a). Importantly, we have also demonstrated significant induction of these CYP enzymes by acute exposure to ethanol and increase in oxidative stress in U937 cells (Jin et al., 2013, Jin et al., 2011). Furthermore, we have clearly demonstrated the role of CYP2E1 in acute ethanol treatment-mediated oxidative stress in other cell types including astrocytic cells (Jin et al., 2013). In this study, we have examined the effects of chronic exposure to ethanol and/or ART on the regulation of CYPs and AOEs, as well as, the induction of ROS and cytotoxicity in U937 cells. We have also examined whether chronic exposure of ethanol and ART exhibit an additive or synergistic effect.
Materials and Methods
Materials
The U937 monocytic cell line was obtained from ATCC (Manassas, VA). Roswell Park Memorial Institute (RPMI) 1640 media, L-glutamine, non-essential amino acid solution, and sodium bicarbonate solution were purchased from Corning Inc. (Tewksbury, MA). Premium heat-inactivated fetal bovine serum was purchased from Atlanta biologicals (Atlanta, GA). The Allprep DNA/RNA/protein and RNeasy mini kits were obtained from Qiagen, (Valencia, CA). Gene expression reagents and primer probes (catalase, Hs00156308_m1; CYP2E1, Hs00559367_m1; CYP3A4, Hs00430021_m1; Superoxide dismutase 1 (SOD1), Hs00533490_m1; SOD2, Hs00167309_m1; Peroxiredoxin 6 (PRDX6), Hs00705355_s1; Actin, Hs9999903_m1) were obtained from Life Technologies (Grand Island, NY). All Western blot reagents were obtained from Bio-Rad laboratories, Inc. (Hercules, CA). Primary antibodies for detection of CYP2E1, CYP3A4, SOD1, SOD2, and catalase were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX). The primary antibody for PRDX6 was obtained from LifeSpan BioSciences Inc. (Seattle, WA). Primary antibody for β-actin and the XTT cell viability kit were purchased from Cell Signaling Technology (Danvers, MA). The IRDye® secondary antibodies and Odyssey® blocking buffer for Western blot analysis were obtained from LI-COR Biosciences (Lincoln, NE). Pierce BCA protein assay kit, Halt protease inhibitor cocktail and 2′,7′-dichlorodihydrofluoroscein diacetate (DCFDA) were purchased from Life Technologies (Grand Island, NY). Ghost dye for measuring cell viability was purchased from Tonbo Biosciences (San Diego, CA).
Cell culture and treatments
U937 cells were grown in RPMI media, supplemented with fetal bovine serum, L-glutamine, non-essential amino acid solution, sodium bicarbonate, and Gentamycin, at 37° C in a humidified incubator maintained at 5% CO2. To evaluate the effects of DRV+RTV and/or ethanol treatments, four treatment paradigms were set up for this study (Fig. 1) : a) Control: methanol-vehicle treated cells; b) ART: DRV and RTV treated cells; c) Ethanol: Ethanol treated; d) Ethanol+ART: DRV+RTV and ethanol treated cells. One milliliters of media with U937 cells was plated at 0.2*106 million cells/ml density in a 6-well plate and treated every 12 hours either with a combination of DRV (4 μM) and RTV (1 μM) or equivalent volume of methanol-vehicle control in presence and absence of ethanol (50 mM) for 4 days. The doses of ethanol and DRV/RTV for studying their chronic effects were selected based on existing studies, and were at upper physiological levels (Bagby et al., 2006, Yilmaz et al., 2009). The dosing for RTV, at one-fourth concentration of the co-administered PI, is based on the marketed RTV formulation, Kaletra® (Qazi et al., 2002). In the subsequent sections ART term was used to represent DRV+RTV. With every dosing, one-half milliliters of media was added to the cells to avoid toxicity. In addition, to compensate for any loss of ethanol treatment due to evaporation, the ethanol-treated cells were cultured in an incubator humidified with 50 mM ethanol during the entire course of treatment. On fifth day, 12 h after the last treatment, cells were collected and re-plated at 0.2*106 million cells/ml and treated for four more days. Cell were again collected and re-plated on Day 9 at 0.2*106 million cells/ml and subjected to another four days of treatment. Final cell pellets were collected on Day 13 of the study. Total RNA was collected following 4-day and 8-day treatments, and was extracted using the RNeasy mini kit. Collected cell pellets after 12-day treatment of U937 cells were subjected to extraction of total RNA, DNA, and protein using the Allprep DNA/RNA/protein mini kit using the protocols described by the manufacturer.
Figure 1.
Treatment paradigm for studying the chronic effects of ART, ethanol, and ethanol+ART in U937 cells. Cells were collected and re-plated at day 5 and day 9, prior to final harvest at day 13. The cells were dosed every 12-h with the addition of fresh 0.5 ml media.
RTPCR
Quantitative RTPCR was performed as described in our previous studies (Ande et al., 2015a). Briefly, 120 ng of RNA was reverse transcribed to cDNA and then amplified in the StepOnePlus system from Life technologies (Grand Island, NY) using a two-step TaqMan Gene Expression Kit (Life technologies, Grand Island, NY). The gene expression levels in 4-, 8-, and 12-day treated samples was measured using the probes for CYP and AOEs obtained from Life technologies. The relative fold expression for tested probes was calculated using 2−ΔΔCt method by employing actin as the housekeeping gene.
Western blot analysis
Total cell protein pellet obtained using the Allprep DNA/RNA/protein kit was resuspended in 5% SDS and protein concentration was determined using the BCA protein assay kit. Equal amount of protein (up to 30μg) from 12-day treated groups of U937 cells was separated on 10% SDS-PAGE gel and transferred to polyvinylidene fluoride membrane. Transferred blots were blocked with the blocking buffer and incubated overnight at 4°C with the primary antibody. The dilution for primary antibodies used for this study were: CYP2E1 (1:500), CYP3A4 (1:400), catalase (1:1000), SOD1 (1:1000), SOD2 (1:1000), PRDX6 (1:1000), and β-actin (1:1000). Next day, the blots were washed and incubated with secondary antibody (1:10,000) for 1 hour. The fluorescence intensity of blots was measured and quantified using the LI-COR Odyssey® imager.
ROS and cell viability measurement by flow cytometry
Levels of ROS production in treated cells was analyzed using previously described method with DCFDA dye using a kit (Jin et al., 2011). Briefly, at the end of treatment paradigms, cells were collected and washed twice with phosphate buffer saline (PBS). Subsequently, cells were incubated at room temperature for 30 minutes with DCFDA (1–5 μM) and Ghost dye (1μl/ml) following instructions provided by manufacturer. Following incubation, cells were washed and emission for ROS and cell viability were measured using flow cytometer. Mean fluorescent intensity (MFI) was measured and analyzed for the different treatment groups, and are represented as 100% for vehicle-treated control cells.
Cell viability measurement using XTT assay
The cytotoxic effects of chronic treatment of U937 cells with ART and/or ethanol were measured using the XTT cell viability kit. The assay is based on the principle that metabolically active (live) cells reduce the tetrazolium salt XTT into a highly colored, water soluble, formazan dye. This color change was analyzed by measuring the absorbance at 450 nm using the Cytation 5 Multi-Mode spectrophotometer from BioTek Instruments Inc. (Winooski, VT). Following instructions provided by manufacturer, changes in U937 cell viability for treated groups were determined following normalization to vehicle-treated control cells (treated as 100%).
Statistical analysis
The effect of chronic ethanol, ART, and ethanol+ART treatments on U937 cells was compared to the vehicle-treated control cells employing the independent t-test analysis. Two-way ANOVA was done to determine whether they interact synergistically or additively by ethanol- and ART treatments. All analyses were performed using IBM SPSS version 21. Results obtained from control-vehicle treated cells were treated as 100% to quantify data from treated cells. A p value of ≤0.05 was considered significant for this study. *p≤0.05; ** p≤0.01.
Results
In the following sections quantitative RTPCR are performed from 4-, 8-, and 12-day treated U937 cells, and Western blot are performed from 12-day treated cells. In addition, since changes in cell viability were noticed as early as after 4 days of chronic treatment with ethanol±ART using XTT assay, the ROS and cell viability measurements using flow cytometer are presented for the 4-day treated U937 cells.
Effect of chronic ethanol and/or ART treatments on CYP2E1 expression
Chronic treatment with ethanol±ART had a significant effect on the expression of major ethanol metabolizing cytochrome P450 enzyme, CYP2E1 (Fig. 2). At mRNA level, transcription of CYP2E1 was significantly upregulated following 8 days of treatment in the ethanol+ART treated monocytic cells (p≤0.05). On the other hand, CYP2E1 protein level was increased in the ART- (~2 fold) and ethanol-treated (~5 fold) U937 cells after 12 days of treatment (p≤0.05). Although higher than vehicle-treated control cells (~5 fold), the increased CYP2E1 expression in ethanol+ART treated cells was found to be near significant owing to a high inter-experiment variability (p=0.07). No additive/synergistic interaction between the effect of ART and ethanol treatments on CYP2E1 expression was observed.
Figure 2.
Changes in mRNA and protein expressions of CYP2E1 in U937 cells treated with vehicle control, ART, ethanol, and ethanol+ART. CYP2E1 mRNA level was measured after 4-, 8-, 12-day treatments and CYP2E1 protein expression was measured following 12-day treatment of U937 cells. Representative Western blot for CYP2E1 and β-actin are presented. p≤0.01 (**); p≤0.05 (*).
Effect of chronic ethanol and/or ART treatments on expressions of antioxidant enzymes
To evaluate the effects of chronic ethanol±ART treatments on the expression of AOEs in U937 cells, levels of major AOEs including catalase, SOD1, SOD2, and PRDX6 were examined after 12-day treatment. The transcription of PRDX6 (Fig. 3A) following 8-day treatment with ethanol was observed to be significantly reduced (by 30%) in U937 cells (p≤0.05). Following 12-day treatment, ethanol (p≤0.05) and ethanol+ART (p≤0.01) treated U937 cells showed significantly decreased PRDX6 protein levels by 40±13% and 48±9%, respectively. Similarly, the mRNA level for cytosolic superoxide dismutase, SOD1 (Fig. 3B), was found to be significantly reduced (20–30%) following 12-day treatment with ethanol and ethanol+ART compared to vehicle-treated cells. Subsequently, significant downregulation (~40%–50%) in protein expression of SOD1 was observed in all three 12-day treatment groups (ART, ethanol, and ethanol+ART) compared to control treated U937 cells (p≤0.05).
Figure 3.
Changes in mRNA and protein expressions of major antioxidant enzymes in U937 cells treated with vehicle control, ART, ethanol, and ethanol+ART. The mRNA level was measured after 4-, 8-, 12-day treatments and protein expression was measured following 12-day treatment of U937 cells. Representative Western blot for antioxidant enzyme and β-actin are presented. A) PRDX6 B) SOD1 C) Catalase D) SOD2. p≤0.01 (**); p≤0.05 (*).
In contrast, expression of catalase (Fig. 3C) was found to be significantly affected (~30%) only at the mRNA level following 8-day treatment with either ethanol or ART. Transcription of catalase gene at other time points (4-day and 12-day) and protein levels measured after 12-day treatment remain unchanged compared to control cells (p>0.05). Similarly, expression of SOD2 (Fig. 3D), the mitochondrial superoxide dismutase enzyme, was minimally affected by the treatments, compared to control, at both mRNA and protein levels in U937 cells (p>0.05). Overall, attenuation of AOEs expression was observed to be mainly an ethanol-driven response in chronically treated U937 cells.
Effect of chronic ethanol and/or ART treatment on CYP3A4 expression
Chronic treatment of U937 cells with ethanol±ART was found to significantly decrease the expression of major drug metabolizing CYP3A4 enzyme (Fig. 4). Transcription of CYP3A4 gene was observed to be significantly downregulated after 4-day treatment with ethanol (by ~60%) and 12-day treatment with ART (by ~40%), ethanol (by ~80%), and ethanol+ART (by ~75%). After 12-day treatment, chronic ART treatment resulted in appreciable decrease in CYP3A4 protein expression, by ~38±17%, in U937 cells (p>0.05). On the other hand, the level of CYP3A4 protein was significantly reduced by ~74±15% and ~90±8% in ethanol (p≤0.01) and ethanol+ART (p≤0.01) treated cells, respectively, after 12 days of treatment. The combined effect observed in ethanol+ART treated cells was partially additive, in part, due to marked decrease in CYP3A4 levels by ethanol.
Figure 4.
Changes in mRNA and protein expressions of CYP3A4 in U937 cells treated with vehicle control, ART, ethanol, and ethanol+ART. CYP3A4 mRNA level was measured after 4-, 8-, 12-day treatments and CYP3A4 protein expression was measured following 12-day treatment of U937 cells. Representative Western blot for CYP3A4 and β-actin are presented. p≤0.01 (**); p≤0.05 (*).
Increased ROS production following chronic treatment with ethanol and/or ART
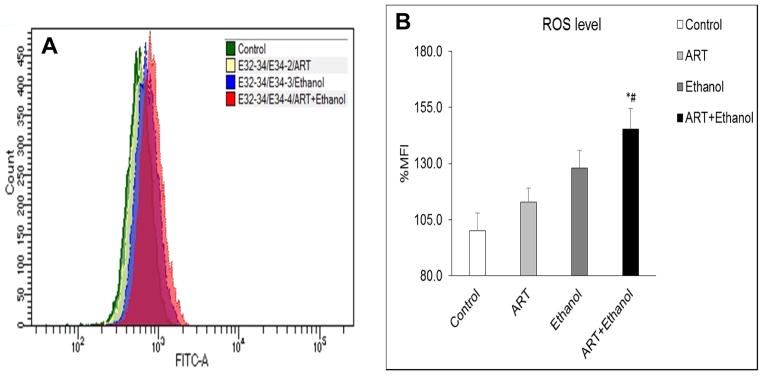
The effects of chronic treatment with ART, ethanol, and ethanol+ART on ROS production in U937 cells was analyzed using flow cytometry. Figure 5A represents the raw data obtained from the flow cytometry, whereas Figure 5B represents the analysis of these data as % MFI in various treatments. Following 4-day treatment, compared to vehicle-control, while ART treated cells displayed appreciable increase in ROS production (12±6%; p>0.05), the ethanol (28±8%; p=0.06) and ethanol+ART treated U937 cells (45±9%; p≤0.05) showed marked increase in intracellular ROS levels (Fig. 5A and 5B). In combination, an additive effect of ART and ethanol treatments on ROS production was observed in ethanol+ART treated U937 cells. Moreover, the ethanol+ART treated U937 cells displayed significant increase in ROS production compared to ART alone treated cells (#, p≤0.05).
Figure 5.
Effect of chronic (4-day) treatment with vehicle control, ART, ethanol, and ethanol+ART on production of reactive oxygen species (ROS) in U937 cells. A) Representative histograms for ROS-mediated fluorescence generated following treatment with DCFDA dye. B) Bar graph representing quantified ROS levels following ART, ethanol, and ethanol+ART treatments compared to vehicle treated control U937 cells. MFI: mean fluorescence intensity. p≤0.05 (#; compared to ART); p≤0.05 (*; compared to vehicle-control).
Decreased viability of U937 cells after chronic treatment with ethanol and/or ART
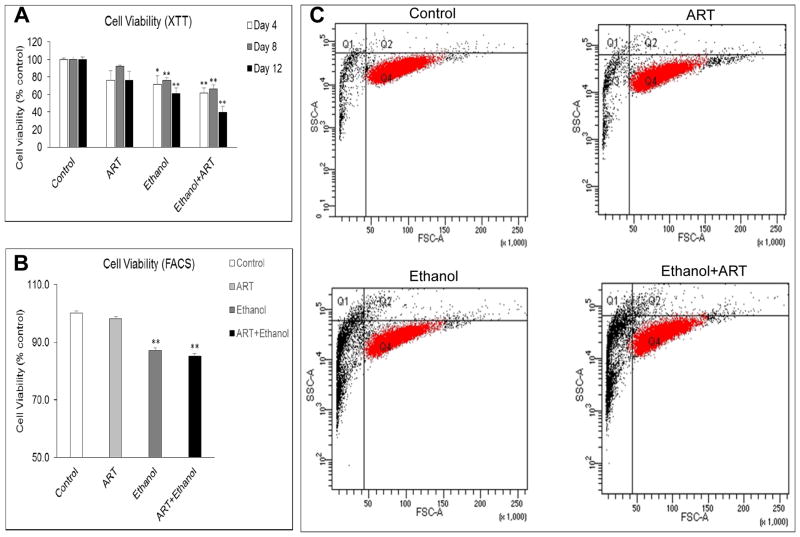
Chronic treatment of U937 cells with ethanol and ethanol+ART was associated with significant changes in cell viability. Analysis of results from XTT assay revealed a significant reduction in cell viability following 4-day (29±10%), 8-day (24±3%), and 12-day (39±6%) chronic treatment with ethanol compared to control cells (Fig. 6A). In comparison to vehicle-treated cells, the ethanol+ART treated U937 monocytic cells demonstrated a reduced cell viability at all three time points: 4-day (38±5%; p≤0.05), 8-day (34±4%; p≤0.01), and 12-day (60±7%; p≤0.01). At Day 12, although ART treatment showed slight decrease in cell viability (24±10%), and ethanol showed significant decrease (40±6%), the combination showed additive effects (60±7%) on cell viability in U937 cells (Fig. 6A). In addition, the cell viability measurements by flow cytometry using the Ghost dye confirmed the increase in cell death for 4-day ethanol and ethanol+ART treated U937 cells (p≤0.01) compared to vehicle-treated control cells (Fig. 6B and 6C). Figure 6B represents the analysis of raw data obtained in Figure 6C. Overall, the decrease in cell viability observed under chronic treatment conditions with ART and ethanol was observed to be additive, with ethanol treatments demonstrating statistically significant effects. Furthermore, cell images obtained at 10X magnification represent the changes observed after 4-day of chronic treatment with vehicle-control, ART, ethanol, and ethanol+ART (Fig. 7). As expected day 0 had relatively lower number of cells (0.2*106/mL) compared with day 5 (~3–4*106/mL). While ART treatment did not have a visible effect on cell morphology, both ethanol and ethanol+ART treatments altered cell morphology and decreased cell numbers compared to the control cells, suggesting an increased cellular toxicity by ethanol and ethanol+ART treatments. These results are in agreement with the results (ROS and cell viability) obtained by XTT and flow cytometry.
Figure 6.
Changes in cell viability observed in vehicle control, ART, ethanol, and ethanol+ART treated U937 cells. A) Cell viability measurement following 4-, 8-, and 12-day treatments with ethanol±ART using XTT assay. B) Cell viability measurement following 4-day treatment with ethanol±ART using ghost dye (flow cytometer). C) Representative dot plot for viable cells (red) in 4-day treatment with ethanol±ART. p≤0.01 (**); p≤0.05 (*).
Figure 7.
Representative microscopic images (10X) of U937 cells before (Day 0) and after 4-day treatment (Day 5) with vehicle-control, ART, ethanol, and ethanol+ART. The bar in the inset represents 100 μM. As expected Day 0 has relatively lower number of cells (0.2*106/mL) compared with Day 5 (~3–4*106/mL).
Discussion
Our previous studies have highlighted the effect of acute exposure to ethanol on CYPs, AOEs, and oxidative stress, as well as role of CYP2E1 in oxidative stress mediated cytotoxicity in U937 monocytic cells (Jin et al., 2013, Jin et al., 2011). The present work demonstrates the effects of chronic exposure of ethanol in the absence and presence of DRV/RTV, which are highly prescribed PIs in first line ART regimen, in U937 monocytic cells. Chronic ethanol treatment, similar to previous observations in hepatic cells (Jimenez-Lopez and Cederbaum, 2005, Yun et al., 2014), was found to significantly enhance the expression of CYP2E1. Interestingly, contrary to acute ethanol treatment mediated upregulation, chronic treatment with ethanol, in both the absence and presence of ART, was found to decrease the expression of CYP3A4 in U937 cells. These changes were associated with unaltered or reduced expression of major AOEs in chronic ethanol and/or ART treated cells. Moreover, these changes were associated with enhanced ROS production and cell death by ethanol and ART treatments. This is the first report of the effects of the chronic exposures of ethanol and/or ART on regulation of CYPs and AOEs, which are associated with the increased ROS production and cytotoxicity in monocytic cells.
CYP2E1-mediated ethanol metabolism is a well-established mechanism for ROS production in chronic alcohol users, especially in the liver (Cederbaum, 2012, Comporti et al., 2010, Lieber, 1997). Inhibition of the CYP2E1 enzyme has been shown to abolish ethanol metabolism mediated generation of ROS and tissue damage (Chen et al., 2014). While our previous works have focused on effects of acute ethanol treatment (Jin et al., 2013, Jin et al., 2011), the present study demonstrate the ethanol-mediated induction in CYP2E1 in the absence and presence of ART in monocytes following chronic treatment. While ART and ethanol treatments demonstrated moderate and high induction in CYP2E1 expression, respectively, the effect of combined treatments (ethanol+ART) did not translate into an additive/synergistic response on CYP2E1 expression. This observation is consistent with the fact that CYP2E1 is mainly regulated by post-translational stabilization of CYP2E1 protein by alcohol (Roberts et al., 1995) with no existing report suggesting ART mediated changes in CYP2E1 expression. More importantly, the ethanol-mediated CYP2E1 induction has been reported in conjunction with other known effects of ethanol for instance, enhanced ROS production in liver and other extra-hepatic cells (Sharma et al., 2012). Our previous report also suggests the role of alcohol-mediated production of ROS following induction of CYP2E1 in U937 cells (Jin et al., 2013). In agreement with the existing studies that suggest ethanol-induced oxidative stress and tissue injury (Jimenez-Lopez and Cederbaum, 2005), enhanced cell death was observed in chronic ethanol±ART treated U937 cells. The current finding also correlated with our recent data from monocytes collected from HIV+ alcohol users in which significant induction of CYP2E1, in association with enhanced oxidative stress, was observed in comparison to ethanol users (unpublished observations).
Several intracellular antioxidant defenses are involved in detoxification of ROS resulting in cellular protection (Kalyanaraman, 2013). SOD, for instance, is the chief enzyme responsible for quenching of the superoxide anion in to hydrogen peroxide and oxygen (Abreu and Cabelli, 2010). Catalases, on the other hand are responsible for conversion of hydrogen peroxide into water and oxygen (De Duve and Baudhuin, 1966). PRDX6 acts as a peroxidase and is known to reduce hydrogen peroxide, fatty-acid hydroperoxides, and phospholipid hydroperoxides (Manevich and Fisher, 2005). Since phospholipid peroxidation is a typical feature of ethanol-induced oxidative stress (Adachi et al., 2004), the role of PRDX6 is critical in minimizing the ethanol-induced damage to membranes and restoring the membrane integrity by reducing hydroperoxides (Manevich et al., 2007). Perhaps, to compensate for the ethanol-associated increase in ROS production, acute exposure to ethanol was associated with induction in activity of AOEs including SOD and catalase (Jin et al., 2011). In the present study, however, chronic ethanol and/or ART treatments were associated with significant reduction/unchanged expression of AOEs indicating a failure of the antioxidant defense mechanisms to counter the enhanced ROS production by ethanol and/or ART exposures. We rationalize that the failure to counter excessive ROS production, and resulting enhanced cellular oxidative stress, results in the significant cell death observed in chronically-treated U937 cells.
The CYP3A family, especially CYP3A4, is accountable for metabolism of approximately 45% clinically used drugs including all the PIs (Zanger and Schwab, 2013). Moreover, being a strong inhibitor of CYP3A4, RTV-boosted HIV therapy has been widely used to achieve optimum efficacy, while minimizing the dose of co-administered PIs (Hull and Montaner, 2011). By inhibiting CYP3A4 enzyme, RTV increases the bioavailability and efficacy of PI including DRV (Santos et al., 2015). In fact, higher intracellular accumulation of RTV in peripheral blood mononuclear cells was observed when dosed with DRV (D’avolio et al., 2013). While literature review also indicates a RTV-based induction in CYP enzymes, including the CYP3A isoform (Foisy et al., 2008), results from the present study demonstrates a significant reduction in CYP3A4 expression following chronic treatment with ART, ethanol, and ethanol+ART. Our finding suggests drastically reduced drug-metabolizing ability in ethanol±ART U937 cells leading to accumulation of these drugs and eventually drug-mediated cytotoxicity. Similar observations reported in hepatic cells treated with ethanol and RTV-boosted PI bolster our current findings that chronic treatment with ethanol renders monocytic cells susceptible to cytotoxicity, perhaps due to a lack of ART clearance capacity in ethanol-treated cells (Hu et al., 2015). Similarly, another report suggests an increased oxidative stress following treatment with PIs (Lagathu et al., 2007). Taken together, the lack of major drug metabolizing enzyme CYP3A4 can be expected to produce significant drug-drug interactions and perhaps life-threatening toxicity in HIV-infected alcohol users.
Since alcohol consumption is highly prevalent amongst HIV-infected population (Alcohol and HIV/AIDS, 2010) and alcohol is known to exacerbate HIV pathogenesis and disease progression (Baum et al., 2010, Lucas et al., 2002), the observed chronic effects of ethanol±ART in U937 monocytic cells have significant clinical implications. Chronic ethanol administration in non-human primates has already been shown to enhance simian immunodeficiency virus replication and progression of disease (Bagby et al., 2006). Importantly, in a recently published article, we have demonstrated, for the first time, an alcohol-induced upregulation of CYP expression and increased oxidative stress in monocytes collected from HIV-infected individuals (Ande et al., 2015b). The data from this study suggests that alcohol-induced oxidative stress in HIV-infected alcohol users is mediated perhaps through an increased expression of CYP2E1 that is consistent with a decreased level of plasma alcohol in these individuals. Moreover, in this study the levels of AOEs (SOD1 and catalase) were reduced in HIV-infected alcohol users compared with alcohol users providing a rationale for the increased oxidative stress observed in these subjects. These results provide further evidence with regards to an interaction between alcohol and HIV infection in myeloid lineage cells and, therefore, underscores the significance of the present study. An increased oxidative stress by ethanol±ART in monocytic cells can be rationalized to directly impact viral replication. The fact that oxidative stress has been shown to increase viral replication in in vitro studies (Schreck et al., 1991) and use of antioxidants is associated with decreased HIV pathogenesis (Hummelen et al., 2010), our findings pose significant clinical implications for HIV-infected alcohol users who are on ART medication.
Similarly, in smoking model, we have demonstrated that an enhanced nicotine metabolism, perhaps through CYP pathway, is associated with increased HIV replication in HIV-infected smokers (Ande et al., 2015a, Earla et al., 2014). Our recent work also highlights the significance of unchanged/downregulated levels of AOE, along with enhanced oxidative stress, with respect to increased viral replication in HIV positive smokers (Ande et al., 2015a). Furthermore, in our in vivo studies we have established the role of CYP pathway in nicotine-mediated generation of oxidative stress in U937 monocytic as well as SVGA astrocytic cells (Jin et al., 2012, Ande et al., 2012). Taken together, our in vitro and ex vivo studies have suggested the potential role of CYP and oxidative stress pathways in smoking-mediated HIV pathogenesis. Thus, extending the rationale to other drug abuse models, the examination of potential role of these pathways in alcohol-mediated changes in HIV systems is critical.
In conclusion, the current observations concurrent with our recent findings (Jin et al., 2013) rationalize the involvement of CYP2E1 in facilitating oxidative stress mediated HIV replication in monocytes by ethanol and/or ART exposures (Baruchel and Wainberg, 1992). In addition, downregulated/unchanged expression of AOEs, especially PRDX6 and SOD1 are expected to increase cytosolic superoxide concentration, thereby promoting HIV replication and transmission in monocytes (Kameoka et al., 1993). Such alterations in cellular antioxidant capacity can be rationalized to severely impact viral pathogenesis and treatment outcomes. Future studies evaluating the chronic effects of ethanol±ART in HIV-infected primary macrophages are expected to reveal the direct impact of ethanol±ART on viral replication. We are currently in the process of recruiting patients in USA to further examine the impact of alcohol abuse on the efficacy of ART and HIV pathogenesis by employing a longitudinal design of study. This study is expected to expand our understanding of the role of CYPs and AOEs in governing alcohol-mediated changes in HIV replication. Confirming the involvement of upregulated CYP2E1 and downregulated AOEs in mediating increased oxidative stress and subsequent viral replication would provide novel targets for treating HIV-infected alcohol users. While CYP2E1 is known to mediate the deleterious effects of ethanol, owing to the inherent cytotoxicity of present CYP2E1 inhibitors like diallyl sulfide, chemically blocking this enzyme in chronic studies is futile. Therefore, efforts are currently underway to synthesize/characterize pharmacologically safer CYP2E1 inhibitors with potential clinical use in HIV-infected alcohol users.
Acknowledgments
We acknowledge the Department of Molecular Sciences, UTHSC, for the use of flow cytometry and Dr. Charles R. Yates for the access to LI-COR instrument for protein imaging. We also thank Dr. Narasimha M. Midde for his critical comments while manuscript preparation. This work was supported by the NIH grant AA022063.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- Abreu IA, Cabelli DE. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim Biophys Acta. 2010;1804:263–74. doi: 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Adachi J, Matsushita S, Yoshioka N, Funae R, Fujita T, Higuchi S, Ueno Y. Plasma phosphatidylcholine hydroperoxide as a new marker of oxidative stress in alcoholic patients. J Lipid Res. 2004;45:967–71. doi: 10.1194/jlr.M400008-JLR200. [DOI] [PubMed] [Google Scholar]
- Alcohol and HIV/AIDS. Alcohol Research: current reviews. Vol. 33. NIAAA; 2010. p. 3. [Google Scholar]
- Ande A, Earla R, Jin M, Silverstein PS, Mitra AK, Kumar A, Kumar S. An LC-MS/MS method for concurrent determination of nicotine metabolites and the role of CYP2A6 in nicotine metabolite-mediated oxidative stress in SVGA astrocytes. Drug Alcohol Depend. 2012;125:49–59. doi: 10.1016/j.drugalcdep.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande A, Mcarthur C, Ayuk L, Awasom C, Achu PN, Njinda A, Sinha N, Rao PS, Agudelo M, Nookala AR, Simon S, Kumar A, Kumar S. Effect of Mild-to-Moderate Smoking on Viral Load, Cytokines, Oxidative Stress, and Cytochrome P450 Enzymes in HIV-Infected Individuals. PLoS One. 2015a;10:e0122402. doi: 10.1371/journal.pone.0122402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ande A, Sinha N, Rao PSS, Mcarthur CP, Ayuk L, Achu PN, Njinda A, Kumar A, Kumar S. Enhanced oxidative stress by alcohol use in HIV+ patients: possible involvement of cytochrome P450 2E1 and antioxidant enzymes. AIDS Research and Therapy. 2015b;12 doi: 10.1186/s12981-015-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back D, Sekar V, Hoetelmans RM. Darunavir: pharmacokinetics and drug interactions. Antivir Ther. 2008;13:1–13. [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30:1781–90. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Baruchel S, Wainberg MA. The role of oxidative stress in disease progression in individuals infected by the human immunodeficiency virus. J Leukoc Biol. 1992;52:111–4. doi: 10.1002/jlb.52.1.111. [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai SH, Sales S, Page JB, Campa A. Alcohol Use Accelerates HIV Disease Progression. Aids Research and Human Retroviruses. 2010;26:511–518. doi: 10.1089/aid.2009.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol. 2011;267:109–23. doi: 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM. The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS. 2014;28:2175–87. doi: 10.1097/QAD.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol E, Alfano M, Biswas P, Poli G. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. J Leukoc Biol. 2006;80:1018–30. doi: 10.1189/jlb.0306150. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667–85. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Zhang CL, Zhao XL, Xie KQ, Zeng T. Inhibition of cytochrome P4502E1 by chlormethiazole attenuated acute ethanol-induced fatty liver. Chem Biol Interact. 2014;222C:18–26. doi: 10.1016/j.cbi.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Collins DR, Lubow J, Lukic Z, Mashiba M, Collins KL. Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4+ T Lymphocytes. PLoS Pathog. 2015;11:e1005054. doi: 10.1371/journal.ppat.1005054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comporti M, Signorini C, Leoncini S, Gardi C, Ciccoli L, Giardini A, Vecchio D, Arezzini B. Ethanol-induced oxidative stress: basic knowledge. Genes Nutr. 2010;5:101–9. doi: 10.1007/s12263-009-0159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’avolio A, Simiele M, Calcagno A, Siccardi M, Larovere G, Agati S, Baietto L, Cusato J, Tettoni M, Sciandra M, Trentini L, Di Perri G, Bonora S. Intracellular accumulation of ritonavir combined with different protease inhibitors and correlations between concentrations in plasma and peripheral blood mononuclear cells. J Antimicrob Chemother. 2013;68:907–10. doi: 10.1093/jac/dks484. [DOI] [PubMed] [Google Scholar]
- De Duve C, Baudhuin P. Peroxisomes (microbodies and related particles) Physiol Rev. 1966;46:323–57. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Earla R, Ande A, Mcarthur C, Kumar A, Kumar S. Enhanced nicotine metabolism in HIV-1-positive smokers compared with HIV-negative smokers: simultaneous determination of nicotine and its four metabolites in their plasma using a simple and sensitive electrospray ionization liquid chromatography-tandem mass spectrometry technique. Drug Metab Dispos. 2014;42:282–93. doi: 10.1124/dmd.113.055186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–59. doi: 10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Baca LM, Weiser B, Burger H, Kalter DC, Meltzer MS. The macrophage in the persistence and pathogenesis of HIV infection. AIDS. 1989;3:475–95. doi: 10.1097/00002030-198908000-00001. [DOI] [PubMed] [Google Scholar]
- Guha D, Klamar CR, Reinhart T, Ayyavoo V. Transcriptional Regulation of CXCL5 in HIV-1-Infected Macrophages and Its Functional Consequences on CNS Pathology. J Interferon Cytokine Res. 2015;35:373–84. doi: 10.1089/jir.2014.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidelines for the Use of Antiretroviral Agents in HIV-1-Adults and Adolescents. 2014 Available at: https://aidsinfo.nih.gov/guidelines.
- Harman AN, Nasr N, Feetham A, Galoyan A, Alshehri AA, Rambukwelle D, Botting RA, Hiener BM, Diefenbach E, Diefenbach RJ, Kim M, Mansell A, Cunningham AL. HIV Blocks Interferon Induction in Human Dendritic Cells and Macrophages by Dysregulation of TBK1. J Virol. 2015;89:6575–84. doi: 10.1128/JVI.00889-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Han H, Lau MY, Lee H, Macveigh-Aloni M, Ji C. Effects of combined alcohol and anti-HIV drugs on cellular stress responses in primary hepatocytes and hepatic stellate and kupffer cells. Alcohol Clin Exp Res. 2015;39:11–20. doi: 10.1111/acer.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in HIV therapy. Ann Med. 2011;43:375–88. doi: 10.3109/07853890.2011.572905. [DOI] [PubMed] [Google Scholar]
- Hummelen R, Hemsworth J, Reid G. Micronutrients, N-acetyl cysteine, probiotics and prebiotics, a review of effectiveness in reducing HIV progression. Nutrients. 2010;2:626–51. doi: 10.3390/nu2060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Lopez JM, Cederbaum AI. CYP2E1-dependent oxidative stress and toxicity: role in ethanol-induced liver injury. Expert Opin Drug Metab Toxicol. 2005;1:671–85. doi: 10.1517/17425255.1.4.671. [DOI] [PubMed] [Google Scholar]
- Jin M, Ande A, Kumar A, Kumar S. Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis. 2013;4:e554. doi: 10.1038/cddis.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Arya P, Patel K, Singh B, Silverstein PS, Bhat HK, Kumar A, Kumar S. Effect of alcohol on drug efflux protein and drug metabolic enzymes in U937 macrophages. Alcohol Clin Exp Res. 2011;35:132–9. doi: 10.1111/j.1530-0277.2010.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Earla R, Shah A, Earla RL, Gupte R, Mitra AK, Kumar A, Kumar S. A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV + smokers. J Neuroimmune Pharmacol. 2012;7:289–99. doi: 10.1007/s11481-011-9283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson F. Drug-drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition. J Intern Med. 2010;268:530–9. doi: 10.1111/j.1365-2796.2010.02301.x. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244–57. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameoka M, Kimura T, Ikuta K. Superoxide enhances the spread of HIV-1 infection by cell-to-cell transmission. FEBS Lett. 1993;331:182–6. doi: 10.1016/0014-5793(93)80322-l. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kameoka M, Ikuta K. Amplification of superoxide anion generation in phagocytic cells by HIV-1 infection. FEBS Lett. 1993;326:232–6. doi: 10.1016/0014-5793(93)81797-4. [DOI] [PubMed] [Google Scholar]
- Kramski M, Schorcht A, Johnston AP, Lichtfuss GF, Jegaskanda S, De Rose R, Stratov I, Kelleher AD, French MA, Center RJ, Jaworowski A, Kent SJ. Role of monocytes in mediating HIV-specific antibody-dependent cellular cytotoxicity. J Immunol Methods. 2012;384:51–61. doi: 10.1016/j.jim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–31. [PubMed] [Google Scholar]
- Kumar S, Kumar A. Differential effects of ethanol on spectral binding and inhibition of cytochrome P450 3A4 with eight protease inhibitors antiretroviral drugs. Alcohol Clin Exp Res. 2011;35:2121–7. doi: 10.1111/j.1530-0277.2011.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Rao PS, Earla R, Kumar A. Drug-drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol. 2015;11:343–55. doi: 10.1517/17425255.2015.996546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagathu C, Eustace B, Prot M, Frantz D, Gu Y, Bastard JP, Maachi M, Azoulay S, Briggs M, Caron M, Capeau J. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir Ther. 2007;12:489–500. [PubMed] [Google Scholar]
- Lieber CS. Cytochrome P-4502E1: Its physiological and pathological role. Physiological Reviews. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–74. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38:1422–32. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Reddy KS, Shuvaeva T, Feinstein SI, Fisher AB. Structure and phospholipase function of peroxiredoxin 6: identification of the catalytic triad and its role in phospholipid substrate binding. J Lipid Res. 2007;48:2306–18. doi: 10.1194/jlr.M700299-JLR200. [DOI] [PubMed] [Google Scholar]
- Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-drug interactions. J Neuroimmune Pharmacol. 2006;1:323–39. doi: 10.1007/s11481-006-9034-2. [DOI] [PubMed] [Google Scholar]
- Qazi NA, Morlese JF, Pozniak AL. Lopinavir/ritonavir (ABT-378/r) Expert Opin Pharmacother. 2002;3:315–27. doi: 10.1517/14656566.3.3.315. [DOI] [PubMed] [Google Scholar]
- Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem. 1995;270:29632–5. doi: 10.1074/jbc.270.50.29632. [DOI] [PubMed] [Google Scholar]
- Santos JR, Llibre JM, Berrio-Galan D, Bravo I, Miranda C, Perez-Alvarez S, Perez-Alvarez N, Paredes R, Clotet B, Molto J. Monotherapy with boosted PIs as an ART simplification strategy in clinical practice. J Antimicrob Chemother. 2015;70:1124–9. doi: 10.1093/jac/dku509. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Saurabh K, Yadav S, Jain SK, Parmar D. Ethanol induced induction of cytochrome P450 2E1 and activation of mitogen activated protein kinases in peripheral blood lymphocytes. Xenobiotica. 2012;42:317–26. doi: 10.3109/00498254.2011.624648. [DOI] [PubMed] [Google Scholar]
- Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res. 2014;12:85–96. doi: 10.2174/1570162x12666140526114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlich ER, Wu WC, Normolle DP, Barratt-Boyes SM. Macrophages and Myeloid Dendritic Cells Lose T Cell-Stimulating Function in Simian Immunodeficiency Virus Infection Associated with Diminished IL-12 and IFN-alpha Production. J Immunol. 2015 doi: 10.4049/jimmunol.1500683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Izadkhashti A, Price RW, Mallon PW, De Meulder M, Timmerman P, Gisslen M. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res Hum Retroviruses. 2009;25:457–61. doi: 10.1089/aid.2008.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JW, Son MJ, Abdelmegeed MA, Banerjee A, Morgan TR, Yoo SH, Song BJ. Binge alcohol promotes hypoxic liver injury through a CYP2E1-HIF-1alpha-dependent apoptosis pathway in mice and humans. Free Radic Biol Med. 2014;77:183–94. doi: 10.1016/j.freeradbiomed.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]