Abstract
Objective
Evidence supports an inverse association of childhood socioeconomic status (SES) with systemic inflammation in adulthood. However, it remains to be determined whether this association is dependent on exposure to stressful life experiences.
Methods
We predicted that the combination of a high number of recent negative life events and low childhood SES would be associated with the highest levels of both circulating interleukin (IL)-6 and lipopolysaccharide-stimulated production of IL-6. We tested this prediction among a community sample of 459 adults (47% male, mean age: 42.8, SD=7.3 years).
Results
Inverse associations were found between childhood and adult SES indices with circulating IL-6 levels (r values between −0.07 to −0.16, p<0.05) but not stimulated IL-6 levels (r values between −0.007 to 0.07, p>0.05). The number of recent negative life events (M=2.43, SD=2.34) was not significantly related to subjective childhood SES and other SES indices (r values <0.06, p>0.10)). Multivariate linear regression analyses revealed a significant association between the interaction of subjective childhood SES and recent negative life events and circulating IL-6 (β =−0.09, t(404)= −1.98, p=0.049) and a marginally significant association with stimulated levels of IL-6 (β =−0.10, t(365)= −1.94, p=0.054), whereas these covariate-adjusted models revealed no main effects for subjective SES or recent negative life events.
Conclusions
The relationship between childhood SES and IL-6 appears to be moderated by recent life events, such that individuals with a relatively low childhood SES exhibit an inflammatory phenotype in the context of a high number of recent negative life events.
Keywords: inflammation, interleukin-6, childhood SES, negative life events, life stress
Introduction
Childhood socioeconomic status (SES) is associated with susceptibility to a range of chronic diseases in adulthood. Two reviews of studies examining this relationship concluded that individuals with lower SES in childhood were at elevated risk of premature mortality, regardless of their SES during adulthood (1–2). More specifically, the experience of living in unfavorable socioeconomic circumstances as a child, independently of adult SES, increases vulnerability to infectious and respiratory diseases, some cancers, and cardiovascular disease in adulthood (2–3). Thus, it appears that there is something specific about socioeconomic exposures during childhood that confers risk for disease later in life.
To date, research documenting the association of childhood SES with adult health has largely utilized either single objective markers of SES (e.g., parental education), or composites of objective indicators of SES at different time periods in childhood (e.g., 4). Despite growing evidence from the adult literature that subjective indices of social standing contribute to the prediction of health risk independently of more traditional objective markers of education or income (e.g., 5–6), to date, few studies have examined whether subjective measures of childhood SES relate to health outcomes.
Subjective measures of socioeconomic status may provide an opportunity for individuals to cognitively average a broad array of socioeconomic indicators, only some of which are commonly assessed (7). Alternatively, they may capture more subtle components of social rank that are only loosely tied to objective measures of socioeconomic status. Although few studies have examined the role of subjective childhood SES in shaping adult health, initial evidence suggests that subjective measures of childhood SES associate with biobehavioral responses to threat. One study found that retrospective perceptions of parental social standing in early life, a subjective indicator of childhood SES, relate to amygdala reactivity to threatening faces, and this relationship was not explained by objective indicators of childhood SES (8). In a similar manner, low subjective childhood SES is associated with greater social distress and reduced activity of the right ventrolateral prefrontal cortex (rPLC), a region of the brain critically involved in self-control, in response to social exclusion (9).
These findings raise important questions regarding how childhood socioeconomic factors affect health and vulnerability to disease decades later. The biological embedding of early experience model (10–12), based on human and animal evidence, proposes that early life experiences shape Hypothalamic-Pituitary-Adrenal (HPA) axis function and reactivity across the lifespan. The central premise of this model is that stress experienced in childhood programs the long term functioning of biological stress regulatory systems and thus contributes to enduring individual differences in stress responsivity. This early biological programming is proposed to result in the development of a phenotype that is behaviorally reactive and biologically prone to systemic inflammation. Behaviorally, early life challenge promotes heightened vigilance for threat, increased distrust of others, impaired self-regulation and unhealthy lifestyle choices (13). Biologically, stress regulatory systems that are proposed to be programmed by early life experiences, e.g., HPA axis, moderate levels of systemic inflammation and thus may contribute to vulnerability to inflammatory diseases in adulthood.
Growing evidence shows an association of objective indices of childhood SES with inflammation in adulthood (e.g., 4); however, not all children who experience socioeconomic adversity go on to develop inflammatory disease. This raises the possibility that factors in later life moderate the impact of early SES on systemic inflammation. Based on the biological embedding of childhood adversity model (11), one such factor may be exposure to stressful life events. In humans, prior research has demonstrated that recent negative life events associate with susceptibility to colds, independently of perceived social stress and dispositional negative affect (14). With respect to inflammation, past research shows an association of low childhood SES with higher circulating markers of inflammation in adulthood (4,15), but whether these relationships are moderated by recent life stress has not yet been explored. It is possible that recent negative life events exacerbate the impact of low childhood SES on inflammatory outcomes in adulthood.
Accordingly, the purpose of the present study was to examine whether the interaction between childhood SES and the frequency of recent negative life events in adulthood significantly associates with circulating levels of the proinflammatory cytokine interleukin-6 (IL-6), as a marker of systemic inflammation, and stimulated levels of IL-6 as a measure of immune competence upon exposure to an endotoxin. Consistent with the biological embedding of childhood adversity model (11), we predicted that low childhood SES would be associated with heightened circulating and stimulated levels of IL-6 primarily among individuals with the highest recent exposure to negative life events.
Methods
Participants were drawn from Phase II of the Adult Health and Behavior project (AHAB-II), which assessed behavioral and biological traits among middle-aged community volunteers. The University of Pittsburgh Institutional Board approved the study and all participants provided informed consent in accordance with its regulations. Participants were recruited between March 2008 and October 2011 through mass mailings of recruitment letters to individuals randomly selected from voter registration lists and other public domain lists.
To be eligible to participate in AHAB-II, individuals had to be working at least 25 hours per week, (a substudy involving this cohort was focused on the association between occupational stress and coronary heart disease risk), speak English as their first language, and be between 30 and 54 years of age. Individuals were excluded if they a) had a history of CVD, schizophrenia or bipolar disorder, chronic hepatitis, renal failure, neurological disorder, lung disease requiring drug treatment, or Stage 2 Hypertension (systolic blood pressure/diastolic blood pressure ≥160/100 mm Hg); b) excessively consumed alcohol (≥ 5 portions 3–4 times per week); c) used fish oil supplements (because of requirements for another substudy); d) were prescribed medications with autonomic effects or used insulin, glucocorticoid, antiarrhythmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight loss medications; e) were pregnant; f) had less than eighth grade reading skills; or g) were shift workers. Participants signed a formed consent when enrolled. Depending on extent of participation in visits and compliance with protocol, participants received up to US $ 410.
From the initial sample of 494 participants (81% Caucasian, 53% female, mean age 42.77), analyses were conducted on 457 participants (80% Caucasian, 53% female, mean age 42.75) who had reliable measures of circulating IL-6 and did not have chronic inflammatory disease (N = 1) or take medications known to impact immune function (Total N = 36: cold medications/antihistamines N = 22, inhaled corticosteroids N = 11, immunosuppressants N = 1, allergy shot within prior 2 weeks N = 1, steroids N = 1).
Measures
Objective Childhood SES
Participants completed the SC childhood interview, a retrospective measure that assesses multiple indicators of childhood socioeconomic status (16). For ages 5, 10 and 15, participants were asked to report whether their parents owned their home (0=no, 1=yes), the number of bedrooms in the home, the number of siblings and adults living in the home (used to calculate the number of bedrooms/child, 0= <1, 1=1+), and the number of vehicles owned by the family (0=0–1, 1=2+). We created a childhood SES index for each of these time points by standardizing and summing scores across the 3 indicators (4). This measure was inter-related across time-points (rs>0.42, p<0.001). In order to derive a measure of material wealth across childhood and to reduce the likelihood of Type 1 error in subsequent analyses, we created a material wealth composite by averaging scores from the three time-points (M=2.30, SD=0.65).
Parental Education
Participants reported the level of education that both their mother and father achieved before the participant turned 18 years on a scale from 1–8 (0= no high school diploma, 1=G.E.D, 2=high school diploma, 3=technical training, 4=some college, no degree, 5= Associates degree, 6= Bachelors degree, 7= Masters degree, 8= M.D. or Ph.D.; M=5.80, SD=1.60). Level of education reported for each parent was recoded into four categories (1=High school diploma or lower, 2=some college, 3= bachelors degree, 4= graduate degree or professional degree). Maternal (M=2.68, SD=1.11) and paternal (M=2.13, SD=1.22) education scores were standardized and averaged to compute aggregate parental education (M=2.44, SD=0.96).
Subjective Childhood SES
Participants used nine-rung ladders to indicate their perception of each parent’s SES during their childhood/adolescence relative to the rest of the United States (8, 17–18). Participants were told that those at the top of the ladder had the most money, the most education and the most respected jobs, while those at the bottom were the worst off, with the least money, the least education, and the least respected jobs or no job. Maternal (M=5.78, SD=1.90) and paternal (M=5.94, SD=2.0) subjective SES scores were standardized and averaged to compute aggregate perceived parental social standing scores (M=5.84, SD=1.83)
Adult Subjective Social Status (SSS)
Using the same ladder measure described previously, participants indicated where they felt they currently stood relative to the rest of the United States (M=6.08, SD=1.50) (18).
Adult Objective SES
As a measure of adult socioeconomic status, participants reported their highest level of academic attainment on a scale from 0–8 (0= no high school diploma, 1=G.E.D, 2=high school diploma, 3=technical training, 4=some college, no degree, 5= Associates degree, 6= Bachelors degree, 7= Masters degree, 8= M.D. or Ph.D.; M=5.80, SD=1.60). As with parental education, education was recoded into four categories (1=High school diploma or lower, 2=some college, 3= bachelors degree, 4= graduate degree or professional degree) (M=2.99, SD=0.91).
Negative Life Events
We used the life events checklist (LEL) to measure frequency of recent negative life events (19). Participants were given a list of major life events (e.g., moving, divorce, having a child, death of a relative, financial loss) and were asked to indicate a) whether the event occurred in the past year and, b) if it did, to rate the impact that event had on their psychological state on a 5-point Likert scale ranging from very good to very bad. As a measure of negative life events, we summed the number of events rated as having a moderately bad to very bad impact with scores ranging from 0–18 (M=2.43, SD=2.34). We identified 3 participants who indicated extreme exposure to negative life events, all falling > 4 standard deviations above the mean. All analyses were run first excluding these 3 individuals and then confirmed in the full sample.
Serum IL-6
IL-6 levels were determined using a high sensitivity quantitative sandwich enzyme immunoassay kit (R&D Systems) run according to manufacturer’s directions. The assay standard range is 0.156–10pg/mL. IL-6 levels were extrapolated from a standard curve with linear regression from a log-linear curve. All samples were run in duplicate and the average inter- and intra- assay coefficient of variation was <10%. Natural log transformation was applied to normalize raw score distributions of the IL-6 values.
Stimulated IL-6 production
Whole blood was collected in heparin-treated Vacutainer tubes and stimulated with lipopolysaccharide (LPS serotype 026:B6, Sigma) at a final concentration of 2 ug/mL without antibiotics in polypropylene tubes under sterile conditions (stimulated sample). Control samples, containing whole blood without LPS were run in parallel to measure unstimulated levels of IL-6 production. The samples were incubated at 37° c with 5% CO2 for 24 hours. Following incubation, the tubes were centrifuged at 1000 g for 10 minutes. Supernatants were collected and frozen at −80° c until the study was complete. At the end of the study, LPS stimulated and unstimulated samples were assayed using a multiplex analysis system (BD Biosciences, San Jose, CA). All reagents, working standards and standards were prepared according to the manufacturer’s specifications, and all samples and standards were run in duplicate using a Bio-plex reader (Luminex 100™). Stimulated levels of IL-6 were determined using the Bio-plex manager software (Bio-Rad corporation, Hercules, CA) interpolating from the standard curve (Logistic -5PL curve fit). We included pooled plasma controls on all plates and inter- and intra-assay coefficients of variability were less than 10%.Again, natural log transformation was applied to normalize raw score distribution of stimulated IL-6 values.
Additional Variables
A number of variables were assessed that might explain associations between childhood SES and inflammation. These variables included age, sex, race, body mass index (BMI: kg/m2), physical activity (kcal/week), as assessed with the Paffenbarger physical activity questionnaire (20), current use of cigarettes, and neuroticism. Lastly, while blood draws and demographic questionnaires were completed at Visit 1, the measure of negative life events was collected at Visit 6. To account for variance in the days between Visit 1 and Visit 6 (range: 28–177, M=81.04, SD=26.63), we included days between visits as a covariate in our analyses.
Data Analysis
Continuous covariates were centered before being used in analyses. Race/ethnicity was recoded into White (1) and other (2). Participant sex was coded as Male (1) and Female (2). Initial Pearson product-moment correlation analyses were performed to determine bivariate associations between subjective and objective childhood SES, demographic variables, BMI, adult SES, number of negative life events, neuroticism and circulating and stimulated levels of IL-6. Next, linear regression analyses were conducted examining the associations of objective and subjective measures of childhood SES with adult circulating and stimulated IL-6. In these models, race, sex, mean-centered age, mean-centered BMI, and number of days between visits were entered in the first step, current SES (mean-centered education level), and mean-centered adult subjective social status were entered in the second step, and mean-centered childhood SES was entered in the third step. Next, we examined whether the interaction between objective and subjective measures of childhood SES and the occurrence of recent negative life events associated with circulating and stimulated levels of IL-6. Here, sex, race, mean-centered age, mean-centered BMI and days between visits were entered in the first step, current SES (education) and mean-centered adult subjective social status were entered in step 2, followed by mean-centered measures of childhood SES and negative life events in step 3, and their cross products in step 4. We also considered the possibility that associations of childhood SES and recent negative life events with IL-6 outcomes are quadratic in light of some evidence that individuals at the extremes of high and low stress may be at increased health risk (21).
In separate models, we examined whether any observed effects or interactions between subjective childhood SES and negative life events were independent of health behaviors that can impact inflammatory outcomes, and neuroticism which could influence retrospective recall of childhood SES. Participants were categorized according to their current use of cigarettes (0=never smoked or smoked in the past, 1= current smokers) and physical activity (assessed by the Paffenbarger physical activity questionnaire) was measured in kcal/week. The α level for assumption of statistical significance of the planned comparisons was set at 0.05.
Results
Demographic characteristics of the sample are displayed in Table 1. Circulating IL-6 was associated with BMI (r=0.41, p<0.001), age (r=0.18, p=0.001), and physical activity (r=−0.11, p=0.02). When compared with non-White participants, White participants had lower levels of IL-6 (r=−0.15, p=0.002). Stimulated IL-6 was associated with sex, with males exhibiting larger stimulated production of IL-6 (r=−0.27, p=0.001), and negatively associated with age (r=−0.13, p=0.01). All of the SES measures (both childhood and adult) were significantly related (rs>0.12, ps<0.05; See Table 2). Circulating and stimulated levels of IL-6 were not significantly related (r=−0.05, p=0.31). Table 2 lists bivariate correlations of measures of SES with demographics, physical activity, number of negative life events, and circulating and stimulated levels of IL-6.
Table 1.
Descriptive Statistics
| N | Mean or % (N) | SD | Range | |
|---|---|---|---|---|
| Race (% White) | 429 | 81% (347) | ||
| Female | 429 | 53% (227) | ||
| Age (years) | 429 | 42.76 | 7.31 | 30–54 |
| Physical Activity (kcal/week) | 429 | 2,845 | 2,224 | 84–15,220 |
| Smoking (current smokers) | 429 | 17% (72) | ||
| BMI kg/m2 | 429 | 26.97 | 5.31 | 17.50–49.60 |
| Adult SES | 429 | 2.99 | 0.91 | 0–4 |
| Parental Education | 420 | 2.44 | 0.96 | 0–4 |
| Adult Subjective Social Status | 429 | 6.08 | 1.50 | 0–10 |
| Objective Childhood SES | 427 | 2.30 | .65 | 0–3 |
| Subjective Childhood SES | 419 | 5.84 | 1.83 | 1–10 |
| Number of negative events in past 12 months | 429 | 2.43 | 2.34 | 0–18 |
| Circulating IL-6 (pg/mL) | 429 | 1.12 | .91 | .06–9.8 |
| Stimulated IL-6 (pg/mL) | 386 | 52.979 | 37.670 | 2,510–254,583 |
Adult SES (education level): 1: high school diploma or lower, 2: some college, 3: bachelors degree, 4: graduate or professional degree. Parental Education level: 1: high school diploma or lower, 2: some college, 3: bachelors degree, 4: graduate or professional degree. Adult Subjective Social Status: Participants used nine-rung ladders to indicate their perception of their SES relative to the rest of the United States.
Objective Childhood SES: Composite of parental homeownership, number of bedrooms per child, and number of vehicles owned by family at ages 5, 10, and 15. Higher numbers reflect higher objective childhood SES. Subjective Childhood SES: Participants used nine-rung ladders to indicate their perception of each parent’s SES during their childhood/adolescence relative to the rest of the United States.
Table 2.
Bivariate correlations of SES measures with variables of interest.
| Subjective Childhood SES |
Objective Childhood SES |
Parental Education |
Adult SSS |
Adult SES |
|
|---|---|---|---|---|---|
| Age2 | −.01 | −.05 | −.09 | .03 | −.12* |
| Sex (Male =1, Female=2)1 | −.09 | −.08 | −.09 | −.19** | −.15** |
| Race (White=1, other=2)1 | −.07 | −.30** | −.06 | −.24** | −.25** |
| Subjective childhood SES2 | 1.0 | .27** | .32** | .36** | .12* |
| Objective childhood SES2 | .27** | 1.0 | .24** | .12** | .15** |
| Parental Education2 | .32** | .24** | 1.0 | .17** | .21** |
| Adult Subjective Social Status (SSS)2 | .36** | .12** | .17** | 1.0 | .39** |
| Adult SES2 | .12* | .15** | .21** | .39** | 1.0 |
| Number of negative life events2 | −.04 | −.002 | −.02 | −.06 | −.04 |
| BMI2 | −.01 | −.12* | −.06 | −.13** | −.22** |
| Physical Activity2 | .02 | .03 | .004 | .12* | .14** |
| Cigarette Use (0=non-smoker, 1=smoker)1 | .08 | −.01 | .02 | −.13** | −.11* |
| Neuroticism2 | .01 | −.04 | −.02 | −.13** | −.06 |
| Circulating IL-62 | −.10* | −.16** | −.07* | −.12* | −.15** |
| Stimulated IL-62 | .03 | .04 | −.007 | .02 | .07 |
p <.05;
p <.001;
Spearman Rank Order Correlations;
Pearson product-moment correlations.
The subjective childhood measure of SES was related to parental education (r=0.30, p<0.001), and the childhood material wealth composite (r= 0.30, p=0.001). Together these variables captured 14% of the variance of subjective childhood SES.
Objective Childhood SES and circulating and stimulated IL-6
We first examined associations of objective markers of childhood SES and life events with markers of inflammation in adulthood controlling for demographic variables, BMI, and days between visits. We did not observe a main effect of objective childhood SES on circulating IL-6 levels (β=−0.07, t(414)= −1.43, p=0.15) or on stimulated levels of IL-6 (β =−0.02, t(371)= −0.03, p=0.97).
Subjective Childhood SES and circulating and stimulated IL-6
Controlling for demographic variables, BMI and days between visits, subjective childhood SES was marginally associated with circulating IL-6 (β=0.08, t(422)= −1.88, p=0.061), with low subjective childhood SES associating with higher circulating IL-6. Similar to objective childhood SES, subjective childhood SES was not significantly associated with stimulated levels of IL-6 (β=−0.05, t(382)=−.1.09, p=0.27).
Negative life events and circulating and stimulated IL-6
The occurrence of recent negative life events was not associated with circulating or stimulated levels of IL-6 (β= −0.03, t(424)= −0.57, p=0.56 and β =−0.02, t(383)= −0.31, p=0.76, respectively).
Objective Childhood SES×recent negative life events and circulating and stimulated IL-6
Next, we tested whether the interaction between frequency of recent negative life events and objective childhood SES associated with circulating and stimulated levels of IL-6. Demographic characteristics, BMI, and number of days between the blood draw and measurement of negative life events were entered into step 1, adult SES (education level) and adult subjective social status in step 2, objective childhood SES and negative life events in step 3, and their interaction term in step 4. In these models, objective childhood SES and negative life events were not significantly associated with circulating or stimulated IL-6 (circulating: β= −0.01, t(414)= −0.11, p=0.91 and β= −0.02, t(414)= −0.36 p=0.72 ; stimulated: β= −0.09, t(371)= −1.69, p=0.09 and β= −0.06, t(371)= −1.16, p=0.25), respectively). Furthermore, the interaction between objective SES in childhood and recent negative life events was not significantly associated with circulating or stimulated IL-6 levels (β=−.07, t(394)= −1.46 p=0.15; β= −0.02, t(371)= −0.35, p=0.73), respectively).
Subjective childhood SES × recent negative life events and circulating and stimulated levels of IL-6
Our primary hypothesis focused on the association between the interaction of subjective childhood SES with recent negative life events and circulating and stimulated IL-6. In these models demographic characteristics, BMI, and number of days between the blood draw and measurement of negative life events were entered into step 1, adult SES (education level) adult subjective status, subjective childhood SES and negative life events in step 2, and their interaction term in step 3. There was no evidence of a significant quadratic relationship between subjective childhood SES or recent negative life events and either measure of IL-6. Hence, our findings focus on the observed linear relationships.
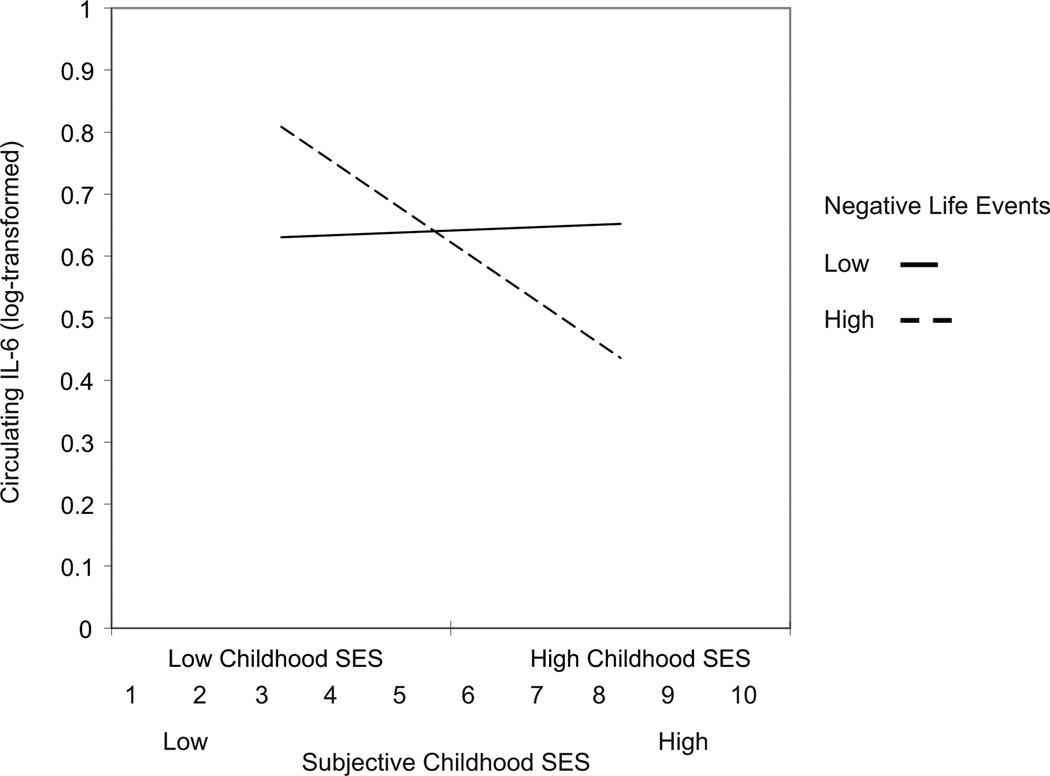
In the linear regression model, subjective childhood SES and recent negative life events were not significantly associated with circulating IL-6 (β =−0.07, t(404)= −1.44, p=0.15 and β= −0.02, t(404)= −0.43, p=0.67). The interaction between subjective childhood SES and recent negative life events was significantly associated with circulating levels of IL-6 (β =−0.09, t(404)= −1.98, p=0.049; see Figure 1). Simple slope analyses revealed that among people who reported a higher number of negative life events (1 standard deviation above mean), low subjective childhood was associated with higher levels of circulating IL-6 (b=−0.05, t=−3.61, p <0.001) compared to individuals who reported higher subjective childhood SES and higher negative life events. However, there was no significant difference in circulating IL-6 as a function of subjective childhood SES for individuals with lower negative life events (1 standard deviation below mean) (b=0.003, t=0.21 p=0.83).
Figure 1.
Interaction between recent negative life events and subjective childhood SES in predicting log-transformed circulating levels of IL-6. Plotted values represent
predicted scores 2 standard deviations above and below the mean of subjective childhood SES.
A similar interactive pattern of results was observed on analyses of stimulated production of IL-6. Subjective childhood SES and recent negative life events were not significantly associated with stimulated levels of IL-6 (β =−0.08, t(364)= −1.45, p=0.15 and β =−0.09, t(364)= −1.83, p=0.07, respectively). However, the interaction between these factors was marginally associated with stimulated levels of IL-6 (β =−0.10, t(365)= −1.93, p=0.054). Simple slope analyses revealed a significant relationship between subjective childhood SES and stimulated levels of IL-6 for individuals who experienced a higher number of negative life events (1 standard deviation above the mean) (b =−0.12, t(364)= −2.75, p=0.010). Again, the relationship between subjective childhood SES and stimulated levels of IL-6 was not significant for those who reported a low number of negative life events (1 standard deviation below the mean) (b =0.04, t(364)=0.83 p=0.41).
Health behaviors, Neuroticism and Objective Childhood SES
We then examined whether consideration of current cigarette use, physical activity, neuroticism and objective childhood SES as covariates would affect the observed pattern of results. The association between the interaction of subjective childhood SES and negative life events with circulating levels of IL-6 became marginally significant with the addition of these covariates (β =−0.08, t(398)= −1.82, p=0.068). The interaction term in parallel models was marginally associated with stimulated levels of IL-6 (β =−0.12, t(356)= −1.81, p=0.071). Statistics for these fully adjusted regression models are presented in Tables 3 and 4. None of these additional covariates were significantly associated with circulating or stimulated IL-6 in these models.
Table 3.
Linear Regression with Childhood SES and negative life events predicting circulating levels of IL-6.
| β | B | SE | t | p | |
|---|---|---|---|---|---|
| Model 1: | |||||
| Age | .12 | .006 | .002 | 2.65 | .01** |
| Sex (Male =1, Female=2) | .05 | .03 | .03 | 1.10 | .27 |
| Race (1=White, 2=other) | .01 | .01 | .04 | .28 | .78 |
| Days between visits | .05 | .001 | .001 | .99 | .32 |
| BMI | .39 | .03 | .003 | 8.47 | <.001 |
| Current SES | −.04 | −.01 | .01 | −.66 | .51 |
| Subjective Social Status | −.03 | −.01 | .01 | −.61 | .54 |
| Subjective childhood SES | −.07 | −.02 | .02 | −1.44 | .15 |
| Negative life events | −.03 | −.01 | .02 | −.43 | .67 |
| Subjective childhood ses × Negative life events | −.08 | −.03 | .02 | −1.97 | .05* |
| Constant | .57 | .09 | 6.54 | <.001 | |
| R2=.22 | |||||
| F (10,415)=10.29, p<.001 | |||||
| Model 2: | |||||
| Age | .14 | .01 | .002 | 2.90 | .004** |
| Sex (Male =1, Female=2) | .04 | .03 | .03 | .93 | .35 |
| Race (1=White, 2=other) | .02 | .02 | .04 | .44 | .66 |
| Days between visits | .04 | .001 | .001 | .80 | .42 |
| BMI | .39 | .03 | .003 | 8.26 | <.001** |
| Current SES | −.03 | −.01 | .01 | −.55 | .59 |
| Subjective Social Status | −.02 | −.004 | .01 | −.33 | .74 |
| Objective Childhood SES | .001 | −.001 | .004 | .01 | .99 |
| Neuroticism | .08 | .001 | .001 | 1.65 | .10 |
| Cigarette Use | −.004 | −.01 | .04 | −.12 | .90 |
| Physical Activity | −.04 | <.001 | .001 | −.76 | .45 |
| Subjective childhood SES | −.08 | −.03 | .02 | −1.54 | .12 |
| Negative life events | −.04 | −.01 | .02 | −.74 | .46 |
| Subjective Childhood SES × negative life events | −.08 | −.03 | .02 | −1.83 | .07† |
| Constant | .58 | .09 | 6.45 | <.001 | |
| R2=.23 | |||||
| F (14,412)=8.28, p<.001 |
Table 4.
Linear Regression with Childhood SES and negative life events predicting stimulated levels of IL-6.
| β | B | SE | t | p | |
|---|---|---|---|---|---|
| Model 1: | |||||
| Age | −.08 | −.01 | .004 | −1.55 | .12 |
| Sex (Male =1, Female=2) | −.27 | −.31 | .06 | −5.21 | <.001** |
| Race (1=White, 2=other) | .04 | .06 | .08 | .71 | .48 |
| Days between visits | −.13 | −.003 | .001 | −2.54 | .01* |
| BMI | .04 | .004 | .01 | .77 | .44 |
| Current SES | .04 | .01 | .02 | .70 | .48 |
| Subjective Social Status | −.01 | −.004 | .02 | −.18 | .86 |
| Subjective childhood SES | −.07 | −.04 | .03 | −1.25 | .21 |
| Negative life events | −.05 | −.04 | .03 | −1.23 | .22 |
| Subjective childhood SES × Negative life events | −.08 | −.06 | .03 | −1.94 | .054† |
| Constant | 11.325 | .17 | 67.47 | <.001 | |
| R2=.11 | |||||
| F (10,375)=4.64, p<.001 | |||||
| Model 2: | |||||
| Age | −.10 | −.01 | .004 | −1.88 | .06† |
| Sex (Male =1, Female=2) | −.27 | −.31 | .06 | −5.01 | <.001** |
| Race (1=White, 2=other) | .01 | .02 | .08 | .27 | .78 |
| Days between visits | −.12 | −.003 | .001 | −2.32 | .02* |
| BMI | .03 | .003 | .01 | .54 | .59 |
| Current SES | .03 | .01 | .02 | .57 | .56 |
| Subjective Social Status | −.01 | −.01 | .02 | −.24 | .82 |
| Objective Childhood SES | −.10 | −.01 | .01 | −1.42 | .16 |
| Neuroticism | −.10 | −.002 | .001 | −1.69 | .09† |
| Cigarette Use | .01 | .02 | .08 | .21 | .83 |
| Physical Activity | −.04 | <.001 | .001 | −.60 | .55 |
| Subjective childhood SES | −.03 | −.02 | .03 | −.49 | .63 |
| Negative life events | −.03 | −.02 | .03 | −.75 | .45 |
| Subjective Childhood SES × negative life events | −.09 | −.06 | .03 | −1.81 | .07† |
| Constant | 11.32 | .18 | 65.72 | <.001 | |
| R2=.12 | |||||
| F (14,370)=3.51, p<.001 |
p <0.05;
p <0.001;
p <0.08
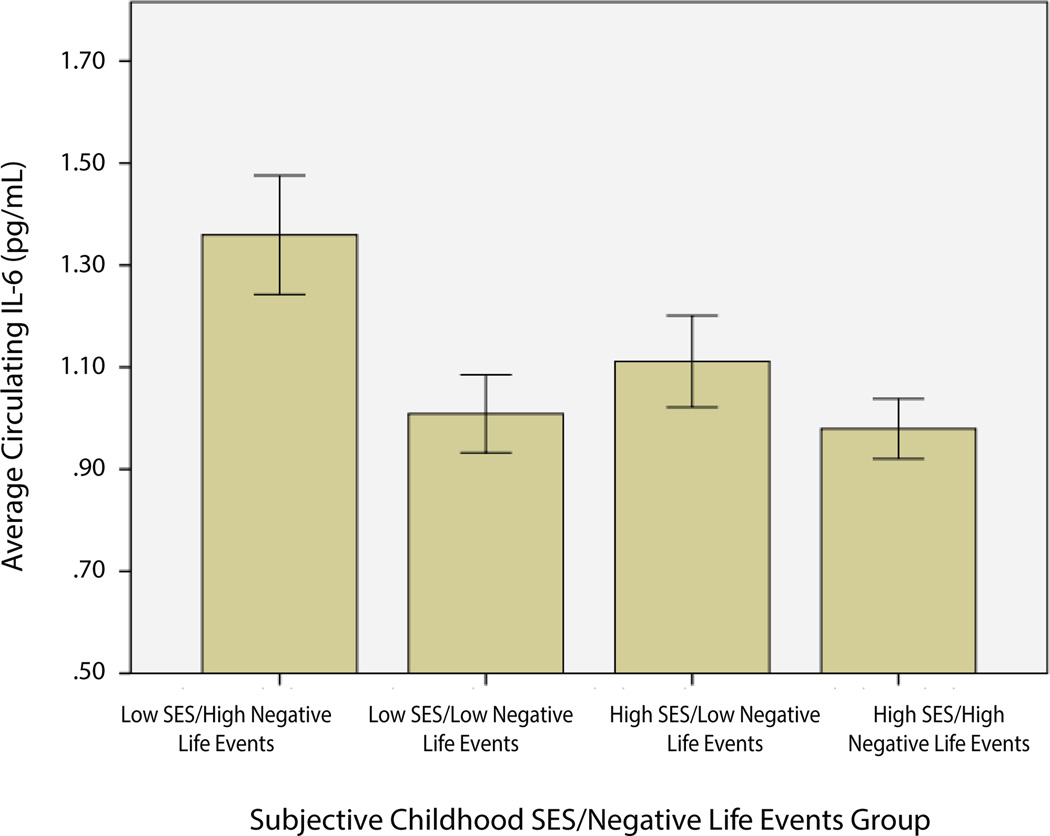
Comparison of circulating IL-6 means among individuals as a function of childhood SES and life event exposure
Finally, we examined differences between average levels of circulating IL-6 between groups of individuals based on their childhood SES and report of recent negative life events. Here, we used a median split for both of these variables to create four groups: low childhood SES/low negative life events, low childhood SES/high negative life events, high childhood SES/low negative life events, and high childhood SES/high negative life events. Comparison of means showed significantly higher levels of circulating IL-6 among individuals in the low childhood SES/high negative life events group (M=1.39, SD=1.32 pg/mL) when compared with the other three groups (M=1.06, SD=0.79 pg/mL), t(88)= −2.12, p=0.037 (See Figure 2). Levels among the 3 comparison groups did not statistically differ (F(2,306) =0.54, p=0.58). We reran all analyses including the 3 individuals with extreme numbers of negative life events and the pattern of findings did not change.
Figure 2.
Average circulating IL-6 levels (pg/mL) as a function of childhood
SES/negative life event group. Error bars represent ±1 SE.
Discussion
In a sample of healthy, mid-life adults we found a marginally significant main effect of subjective childhood SES on circulating IL-6. This was in line with past work documenting similar relationships between measures of objective childhood SES and circulating levels of IL-6 (4). Extending this work, we found that the main effect of subjective childhood SES was qualified by an interaction with the occurrence of recent negative life events, with individuals who reported lower childhood SES and who had experienced higher numbers of recent life events showing higher levels of circulating IL-6 when compared to their counterparts with lower levels of recent stress. Interestingly, the same interaction was marginally associated with stimulated levels of IL-6. These findings indicate that the experience of recent negative life event stress may act as a moderator of the relationship between childhood SES and inflammatory measures.
One way in which childhood SES may affect markers of inflammation in adulthood is by shaping biobehavioral responses to stress or stressful events. Growing evidence suggests that socioeconomic exposures in early life affect neural and behavioral responses to stress. Individuals who report low childhood SES show increased amygdala response to threatening faces (8), are more likely to interpret ambiguous situations as threatening (22), and exhibit greater physiological arousal in anticipation of threat and in response to psychological stress (23–24). This heightened vigilance and arousal affects the autonomic nervous system and the HPA axis which in turn shape the magnitude of the inflammatory response (25). Furthermore, prior research suggests that the experience of stress in early life can diminish the sensitivity of immune cells to the inhibitory signals of glucocorticoids (26). These modulations of the immune system may serve an adaptive purpose in early life environments that are characterized by increased exposure to risk; however, to the extent that these adaptations persist into adulthood, they could contribute to risk for inflammatory disease (27–28). In contrast, individuals reared in high SES environments may find themselves better equipped to cope with stressors in adulthood and this could in part explain the pattern of our findings. In line with this, a statistically significant difference in average levels of circulating IL-6 between low and high childhood SES individuals only emerged in the context of a high number of recent negative life events, while high childhood SES individuals who reported a low number of recent negative life events did not have significantly lower levels than low childhood SES individuals who also had low number of negative life events.
Overall, the findings we report here are consistent with prior research and theory, with individuals who reported lower childhood socioeconomic environments, which are associated with heightened vigilance and increased physiological arousal in response to stress, exhibiting higher circulating IL-6 and tendency to mount higher stimulated IL-6 responses compared to high childhood SES individuals in the context of high, but not low, recent life stress in adulthood. For individuals who may be chronically exposed to such stressors, this predisposition towards inflammation might enhance susceptibility to inflammatory disease in aging.
Prior work has documented a relationship between objective childhood SES and circulating levels of IL-6 (e.g., 4). In the current study, we did not observe a significant relationship between these variables. This could be in part because we assessed material wealth at three time-points (ages 5, 10 and 15), whereas previous studies have measured material wealth every year from birth to 18 years of age. Further, the observed interactions between childhood SES and negative life events were significant only when using a subjective measure of childhood SES.
Subjective measures of SES may afford individuals the opportunity to consider components of their childhood environment that are not captured by traditional indicators of SES. For example, dichotomous measures of car and homeownership may not reflect the condition of the family car, or the size, security, or amenities available in a home. An individual’s retrospective subjective report of their childhood SES may capture these nuances in a way that objective measures fail to. Further, subjective measures of childhood SES likely reflect the degree to which individuals feel poorer when compared with peers. Given our tendency as humans to make social comparisons to relevant others, measures of relative status may be more informative than measures of objective wealth.
In this research, subjective childhood SES was independently related to parental education and objective SES across childhood, however, the magnitude of these associations was only moderate, suggesting that objective indicators may only partially reflect the variance captured in the subjective measure. As such, subjective childhood SES may be capturing multiple dimensions of childhood environments, each of which are uniquely important in shaping health and disease risk across the lifespan. Furthermore, the fact that the reported interaction did not approach significance with objective measures of SES, provides further evidence that subjective experiences related to one’s relative social status may be independently associated with well-being and disease risk and may contribute to socioeconomic gradients of health beyond more objective indicators of childhood or adults SES.
Another avenue through which socioeconomic exposures of early childhood may affect systemic inflammation in later life is by influencing health behaviors. Lower parental education negatively impacts dietary habits, increases likelihood of smoking, and associates with decreased physical activity and higher BMI (29). These behaviors are associated with increased systemic inflammation and risk for inflammatory diseases (30). Importantly, these behaviors have previously accounted for a portion of the association between childhood SES and inflammation in adulthood (1,2). Here, the observed relationships between subjective childhood SES and negative life events on circulating and stimulated IL-6 were independent of BMI, health behaviors, and neuroticism. Lastly, cigarette use, physical activity and neuroticism were not independently associated with circulating or stimulated IL-6. However, it remains possible that unmeasured lifestyle factors could contribute to observed associations.
Limitations
There are limitations of the current study that must be noted. First, the cross-sectional design does not allow for causal inferences regarding the interactive patterns we observed between subjective childhood SES, negative life events, and circulating and stimulated IL-6. Furthermore, the retrospective and subjective assessment of childhood SES is a limitation of this research as it could involve recall bias. It is possible that individual characteristics such as neuroticism influence retrospective recall of childhood socioeconomic status and recent life events. To address this possibility, we included neuroticism in our secondary regression models and it did not appear to alter the pattern of results. Moreover, the subjective measure of childhood SES was significantly related to material wealth across childhood and parental education. Future longitudinal research is warranted to collect subjective and objective measures of SES during childhood in order to provide validation of this subjective measure of childhood SES. Further, longitudinal studies will allow for a better understanding of how these measures inform behavioral, emotional and physiological responses to stressful events and how these, in turn, impact inflammatory mediators and consequently affect health. In addition, this novel interaction should be replicated in future investigations.
It is important to note that the report of recent negative life events does not necessarily associate with occurrence of negative life events at time-points across the life-span. Our findings suggest that low childhood SES shapes a reactive inflammatory phenotype that translates to higher levels of IL-6 in the context of recent life stress. Longitudinal research is warranted to examine whether these effects are similar in the context of ongoing or chronic life stress.
The findings from this investigation focus on one dimension of early life experiences, namely a subjective report of early life experiences related to socioeconomic status. It is important to acknowledge that there is evidence documenting similar associations between other forms of early life adversity (e.g. physical abuse) and systemic levels of inflammation (e.g. 31). Unfortunately, the available data from this research does not allow us to examine whether similar patterns would emerge upon consideration of interactive effects of other dimensions of early life adversity and recent negative life events. As such, this will be an important direction of future research.
Lastly, it should be noted that while the clinical significance of elevated levels of systemic inflammation is relatively clear, the health implications of individual differences in the stimulated production of cytokines remains to be determined. Stimulated levels of inflammatory cytokine production is assumed to be a proxy for the ability of the body’s immune cells to mount an acute inflammatory response in the face of injury or when dealing with an invading pathogen. As such, it is generally believed that magnitude of pro-inflammatory cytokine response to pathogens is critical for health; insufficient response may leave the organism vulnerable to infection, whereas excessive response can increase risk for inflammatory diseases, however more research is needed to determine whether the magnitude of inflammatory responses is related to clinical outcomes (32).
Conclusions
In conclusion, we provide novel evidence that the experience of negative life events moderates the relationship between childhood SES and inflammatory measures among adults. Specifically, low subjective childhood SES and high frequency of recent negative life events associates with the inflammatory phenotype that has been previously associated with low childhood SES (i.e. elevated levels of markers of systemic inflammation). Further, we found evidence of a marginally significant relationship between the interaction of these factors and stimulated production of IL-6.
Our findings are consistent with a growing body of research suggesting that physiological and behavioral responses to stressors are shaped in early life, possibly in response to childhood environments. Specific to the immune system, the proinflammatory phenotype associated with low childhood SES may be useful in conferring early survival advantage in the context of a childhood environment characterized by increased exposure to pathogenic risk. However, this phenotype could be problematic to long-term health and survival, given its association with increased risk for inflammatory disease (26–28). Here, our findings indicate that low childhood SES may act as a vulnerability factor in the context of recent life stress, perhaps in part because of response patterns that are programmed as a function of childhood socioeconomic experiences. Consequently, the combination of low childhood SES and high levels of recent life stress (i.e. a high number of negative life events) may impart an inflammatory phenotype associated with increased disease risk.
Acknowledgments
This work was supported by National institutes of Health grant PO1 HL040962 (SBM)
Acronyms
- BMI
Body Mass Index
- IL-6
Interleukin-6
- SES
Socioeconomic Status
- HPA Axis
Hypothalamic Pituitary Adrenal Axis
- LPS
Lipopolysaccharide
- SSS
Subjective Social Status
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 2.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66(4):553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- 4.Carroll JE, Cohen S, Marsland AL. Early childhood Socioeconomic Status is associated with circulating Interleukin-6 among Mid-life Adults. Brain Behav and Immun. 2011;25:1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Operario D, Adler NE, Williams DR. Subjective social status: reliability and predictive utility for global health. Psychol Health. 2004;19:237–246. [Google Scholar]
- 6.Cohen S, Alper CM, Doyle WJ, Adler NE, Treanor JJ, Turner RB. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol. 2008;27:268–274. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- 7.Singh-Manoux A, Adler NE, Marmot MG. Subjective social status: its determinants and association with measures of ill-health in the Whitehall II study. Soc Sci Med. 2003;56:1321–1333. doi: 10.1016/s0277-9536(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 8.Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB. Potential neural embedding of parental social standing. Social Cogn Affect Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanagisawa K, Masui K, Furutani K, Nomura M, Yoshida H, Ura M. Family socioeconomic status modulates the coping-related neural response of offspring. Soc Cogn Affect Neurosci. 2012;8:617–622. doi: 10.1093/scan/nss039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller GE, Chen E, Parker K. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving towards a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 13.Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychol Sci. 2010;21:31–37. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S, Tyrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 15.Phillips JE, Marsland AL, Flory JD, Muldoon MF, Cohen S, Manuck SB. Parental education is related to C-reactive protein among female middle-aged community volunteers. Brain Behav Immun. 2009;23:677–683. doi: 10.1016/j.bbi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S. SC Childhood Interview. 2010 Retrieved from http://www.psy.cmu.edu:16080/~scohen/ [Google Scholar]
- 17.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychology. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 18.Goodman E, Huang B, Schafer-Kalkhoff T, Adler NE. Perceived socioeconomic status: a new type of identity that influences adolescents’ self-rated health. Journal Adolesc Health. 2007;41:479–487. doi: 10.1016/j.jadohealth.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Tyrell DA, Smith AP. Life events, perceived stress, negative affect and susceptibility to the common cold. J Pers Soc Psychol. 1993;64:131–140. doi: 10.1037//0022-3514.64.1.131. [DOI] [PubMed] [Google Scholar]
- 20.Paffenbarger RS, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 21.Seery MD, Holman AE, Silver RC. Whatever does not kill us: Cumulative lifetime adversity, vulnerability and resilience. J Pers Soc Psychol. 2010;99:1025–1041. doi: 10.1037/a0021344. [DOI] [PubMed] [Google Scholar]
- 22.Chen E, Matthews KA. Socioeconomic differences in social information processing and cardiovascular reactivity. Ann N Y Acad Sci. 1999;896:417–419. doi: 10.1111/j.1749-6632.1999.tb08158.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen E, Matthews KA. Cognitive appraisal bias: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann Behav Med. 2001;23:101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- 24.Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. J Pers. 2004;72:1365–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- 25.Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neurosci and Biobehav R. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U.S.A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller GE, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 29.Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Pub Health. 2005;5:7–13. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med. 2012;43:611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Molec Life Sci. 2004;61:2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]