Abstract
The oral microbiota was compared between Romanian adolescents with a high prevalence of caries and no dental care and Swedish caries-active and caries-free adolescents in caries prevention programs and with a low prevalence of caries. Biofilm samples were analyzed by FLX+ pyrosequencing of the V1 to V4 hypervariable regions of the 16S rRNA gene and polymerase chain reaction (PCR)/quantitative PCR (qPCR) for Streptococcus mutans and Streptococcus sobrinus. Sequences obtained blasted to 9 phyla, 66 genera, and 401 human oral taxa (HOT) in the 16S rRNA Human Oral Microbiome Database, of which 295 were represented by ≥20 sequences. The Romanian adolescents had more sequences in Firmicutes and fewer in Actinobacteria phyla and more sequences in the genera Bacteroidetes [G-3], Porphyromonas, Abiotrophia, Filifactor, Peptostreptococcaceae [11][G-4], Pseudoramibacter, Streptococcus, and Neisseria and fewer in Actinomyces, Selenomonas, Veillonella, Campylobacter, and TM7 [G-1] than the Swedish groups. Multivariate modeling employing HOT, S. sobrinus and S. mutans (PCR/qPCR), and sugar snacks separated Romanian from Swedish adolescents. The Romanian adolescents’ microbiota was characterized by a panel of streptococci, including S. mutans, S. sobrinus, and Streptococcus australis, and Alloprevotella, Leptotrichia, Neisseria, Porphyromonas, and Prevotella. The Swedish adolescents were characterized by sweet snacks, and those with caries activity were also characterized by Prevotella, Actinomyces, and Capnocytophaga species and those free of caries by Actinomyces, Prevotella, Selenomonas, Streptococcus, and Mycoplasma. Eight species including Streptococcus mitis and Streptococcus species HOT070 were prevalent in Romanian and Swedish caries-active subjects but not caries-free subjects. In conclusion, S. mutans and S. sobrinus correlated with Romanian adolescents with caries and with limited access to dental care, whereas S. mutans and S. sobrinus were detected infrequently in Swedish adolescents in dental care programs. Swedish caries-active adolescents were typically colonized by Actinomyces, Selenomonas, Prevotella, and Capnocytophaga. Hence, the role of mutans streptococci as a primary caries pathogen appears less pronounced in populations with prevention programs compared to populations lacking caries treatment and prevention strategies.
Keywords: oral microbiota, pyrosequencing, adolescents, Sweden, Romania, mutans streptococci
Introduction
The oral cavity harbors one of the most complex microbiomes in the body (Dewhirst et al. 2010). The oral microbiota is stable and in harmony with the host, unless disturbed by medication, disease, low pH (Marsh 2003), or significant changes in diet (David et al. 2014). Dental caries, one of the most prevalent diseases worldwide (Petersen et al. 2005), is associated with dysbiosis of the tooth-colonizing microbiota, characterized by the accumulation of aciduric and acidophilic bacteria (Takahashi and Nyvad 2011).
Dental caries results from the demineralization of tooth tissues by acids produced from the bacterial fermentation of dietary carbohydrates. The net outcome is modified by inherent host resistance and susceptibility and lifestyle factors, such as oral hygiene practices, diet, and fluoride exposure. The prevalence of dental caries, oral hygiene, use of fluoride, snacking habits, and access to dental care differ greatly between different parts of the world (www.who.int). In some countries, such as the Scandinavian countries, long-standing population and individual caries preventive measures have resulted in a low mean incidence of caries (Hugoson and Koch 2008), whereas other countries have an increased or maintained high incidence of caries. Romania is a European country with the latter caries trend (Funieru et al. 2014). Even in communities with a low incidence of caries, however, approximately 15% to 20% of the population is caries active in spite of preventive efforts (Hugoson and Koch 2008).
Individual bacterial species have been associated with dental caries in several studies, including mutans streptococci, aciduric non–mutans streptococci, lactobacilli, actinomyces, bifidobacteria, and Scardovia (Ruoff 1991; Mantzourani et al. 2009; Takahashi and Nyvad 2011; Tanner et al. 2011). A number of studies have also evaluated the complexity of the oral microbiota in dental caries (Kanasi et al. 2010; Crielaard et al. 2011; Ma et al. 2015), but results differ between studies, suggesting that the composition of the caries-associated microbiota has not been definitively identified. DNA-based methods, such as the next-generation FLX+ and Illumina pyrosequencing methods combined with curated gene databases, such as the Human Oral Microbiome Database (HOMD; www.HOMD.org) (Chen et al. 2010), are comprehensive methods to analyze the microbiota and provide data to clarify the composition of the caries-associated microbiome.
The aim of the present study was to evaluate the tooth microbiota in adolescents with a high prevalence of caries and who never had access to dental care and the microbiota of another population who had systematic dental care and preventive measures from early childhood and who remained caries free or had a low prevalence of caries. This study used microbial data from FLX+ pyrosequencing with the long read option with species/taxa identifications from the 16S rRNA HOMD. To optimize detection sensitivity of the key caries pathogens Streptococcus mutans and Streptococcus sobrinus, these taxa were also assayed directly by polymerase chain reaction (PCR).
Materials and Methods
Ethics
The study was approved by the Regional Ethical Review Board in Umeå, Sweden (Dnr 2012-111-31M) and regional authorities in Romania. All participants and their caregivers gave informed consent to participate.
Participant Recruitment
In Sweden, 17-y-old adolescents (n = 64) who were caries free with no signs of the disease (n = 28) or had high caries risk and activity (n = 36) (Söderström et al. 2014) were recruited from a Public Dental Health Care Clinic in Umeå, Sweden. These adolescents had access to dental care and preventive measures since early childhood (http://www.socialstyrelsen.se/nationalguidelines). Twelve caries-free and 12 caries-active Swedish adolescents were randomly selected for microbial analysis.
In Romania, schoolchildren who lived in a rural village in the northwestern part of the country with only permanent teeth (aged 14–15 y; n = 14) were selected for microbial analysis. The Romanian population had limited access to dental care, and any treatment was directed only to relieve pain. Information was collected during 2012 and 2013 for both groups.
Caries Scoring and Questionnaire
Initial and cavitated carious lesions were measured in dental clinics using standard equipment by 1 Swedish dentist and 1 Swedish-trained final-year dental student. Radiographs were taken only in the Swedish cohort. The sum of decayed, missing, and filled surfaces (DMFS) was calculated. Information on general health status, oral hygiene habits, and dietary habits was obtained by a questionnaire.
Sample Collection, DNA Isolation, PCR, and Counts of Mutans Streptococci
Biofilm samples were collected from all supragingival tooth surfaces using sterilized toothpicks. Pooled plaque samples were stored in TE buffer (10 mM Tris, 1 mM EDTA, pH 7.6) at −80 °C. Tooth biofilm DNA was extracted using the Gen Elute Bacterial Genomic DNA kit (Sigma-Aldrich, St. Louis, MO, USA) with the addition of lysozyme and mutanolysin as described (Lif Holgerson et al. 2011). The quality and quantity of DNA were evaluated using a Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) to meet the standard set by the sequencing facility, namely, an OD 260/280 ratio of approximately 1.8 and an OD 260/230 ratio of approximately 2.0. PCR and qPCR of S. mutans and S. sobrinus were performed (Yano et al. 2002) using the KAPA2G Robust HotStart PCR Ready Mix (2′) kit (Kapa Biosystems, Boston, MA, USA).
Pyrosequencing and Sequence Analyses
Sequencing of DNA from the biofilm samples was performed using the Lib-L kit on an FLX+ Titanium platform (Roche Applied Science, Indianapolis, IN, USA) at GATC Biotech AG (Konstanze, Germany). The V1 to V4 hypervariable regions of the 16S rRNA gene were amplified using the forward primer 27F with an adaptor sequence and sample-specific barcode oligonucleotide tags and the reverse primer 805R. Sequences obtained were processed using QIIME (version 1.8.0, QIIME.org), and HOMD human oral taxa (HOT) at a ≥98.5% similarity level were determined (Appendix).
Statistical Analyses
Normally distributed variables were presented as means with 95% confidence intervals (CIs) and differences between means tested with analysis of variance or an unpaired t test. For nonnormally distributed variables, medians with ranges were calculated, and the Kruskal-Wallis analysis of variance by rank and the Jonckheere trend test with the order Sweden caries free < Sweden caries active < Romania high caries prevalence were used. HOT prevalences were highly skewed, with >50% of the subjects lacking detection. Therefore, the mean prevalence and detection frequency of taxa in the 3 caries groups are presented, together with median prevalences among those with detected taxa. The χ2 test was used to test differences in group frequency distributions. For comparisons between HOT, P ≤ 0.008 (accounting for multiple testing by the false discovery rate) was considered statistically significant, and for other variables, P < 0.05 was considered statistically significant.
Rarefaction curves were calculated to compare microbial richness among the samples, and principal coordinates analysis (PCoA) was performed to compare the phylogenetic diversity (β diversity) using QIIME. Multivariate principal component analysis (PCA) and partial least squares (PLS) regression (SIMCA P+, version 12.0; Umetrics AB, Umeå, Sweden) were used to explore the clustering of subjects and taxa associated with caries status, respectively.
Results
Characteristics of the Romanian and Swedish adolescents studied are presented in the Table. Children in the Romanian group were approximately 2.5 y younger than in the Swedish cohort and had, on average, 1 tooth less than the Swedish adolescents. Other characteristics of the Romanian participants were a markedly higher prevalence of caries, even compared with the Swedish caries-active group, and a higher prevalence of S. sobrinus (P < 0.001) and S. mutans (P = 0.021) by PCR (Table). S. mutans was detected in 86% of the Romanian group, and 50% of Romanian adolescents had S. mutans with S. sobrinus by PCR compared to 48% with S. mutans and 0% with S. mutans with S. sobrinus in the Swedish groups.
Table.
Characteristics of the 3 Study Groups.
| Romanian Group |
Swedish Groups |
|||
|---|---|---|---|---|
| High Prevalence of Caries (n=14) | Caries Active (n = 12) | Caries Freec (n = 11) | P Value among Groups | |
| Male gender,a % | 43 | 50 | 54 | 0.840 |
| Age,b mean (95% CI), y | 14.4 (14.1–14.6) | 17 | 17 | <0.001 |
| Teeth,b mean (95% CI), n | 27 (26–28) | 28 (27–28) | 28 (28–29) | 0.018 |
| Caries status | ||||
| DMFS,b mean (95% CI) | 20.1 (13.3–26.8) | 7.5 (5.7–9.3) | 0 | <0.001 |
| Streptococcus mutans | ||||
| PCR positive,a % | 85.7 | 50.0 | 45.5 | 0.069d |
| DNA,b mean (95% CI), pg/µL | 96 (60–252) | 41 (26–108) | 65 (35–165) | 0.774 |
| Streptococcus sobrinus | ||||
| PCR positive,a % | 50.0 | 0 | 0 | 0.001 |
| DNA,b mean (95% CI), pg/µL | 10 (1–20) | 0 | 0 | 0.024 |
| S. mutans and S. sobrinus | ||||
| PCR positive,a % | 50.0 | 0 | 0 | 0.001 |
CI, confidence interval; DMFS, decayed, missing, and filled surfaces; PCR, polymerase chain reaction.
Differences in subject distributions were tested with the χ2 test among groups.
Differences between group means were tested with analysis of variance. Adjusting for the number of teeth did not alter the relation between groups.
FLX+ Titanium sequencing failed for 1 sample in the Swedish caries-free group.
The prevalence in the merged Swedish groups was 48%, and testing of the Romanian group versus the merged Swedish groups resulted in P = 0.021.
Pyrosequencing of Oral Samples
Blasting of 942,788 quality-filtered sequences from 37 adolescents (1 sample failed) at ≥98.5% threshold identified 401 HOT (species/phylotypes) in the HOMD. Of these, 295 taxa were represented by ≥20 sequences. Using the latter cutoff, the mean number of species/phylotypes by subject was 170 (95% CI, 160–180; range, 111–230), of phyla was 9 (mean, 8; range, 5–9), and of genera was 66 (mean, 44; range, 28–60) (Appendix Table 1). The dominant phyla (Firmicutes, Bacteroidetes, Fusobacteria, and Actinobacteria) accounted for 95.2% of all sequences, Proteobacteria for 4.2%, and the remaining phyla or divisions (Spirochaetes, TM7, Tenericutes, and SR1) for <1% of the sequences.
The majority of all sequences (83.9%) were classified into 9 genera (Streptococcus, Prevotella, Leptotrichia, Actinomyces, Fusobacterium, Capnocytophaga, Corynebacterium, Campy-lobacter, and Porphyromonas), each of which comprised >1% of all sequences. The remaining taxa were in 57 genera that each represented <1% of all sequences. The group median prevalences for phyla and genera are presented in Appendix Table 1. The top 15 ranked species by prevalence were Streptococcus mitis, Corynebacterium matruchotii, Prevotella nigrescens, S. mitis bv2, Alloprevotella tannerae, Leptotrichia wadei, Streptococcus cristatus, Leptotrichia hofstadii, Actinomyces gerencseriae, Prevotella oris, Streptococcus sp. HOT071, Fusobacterium nucleatum subsp. polymorphum, Prevotella sp. HOT317, Actinomyces sp. HOT448, and Streptococcus sanguinis (all ≥2% of all sequences) (Appendix Table 2).
Core Microbiome
Twenty-four species were detected in all adolescents (5 in Actinomyces, 5 in Streptococcus, 3 in Prevotella, 3 in Fusobacterium, and 1 each in Alloprevotella, Campylobacter, Capnocytophaga, Corynebacterium, Eikenella, Granulicatella, Lachnoanaerobaculum, and Veillonella), and 78 species were found in 90% of the adolescents (Appendix Table 3).
Microbiome Profile by Caries Group
The species richness differed 2.7 times between the samples (Fig. 1A) and was higher in Romanian adolescents with a high prevalence of caries than either the caries-active or caries-free Swedish groups (Fig. 1B). Univariate analyses confirmed that a significantly higher number of the 295 HOT were in the Romanian high caries group than in the caries-active and caries-free Swedish groups (median, 188, 166, and 154, respectively; PGROUP = 0.027; PTREND = 0.009).
Figure 1.
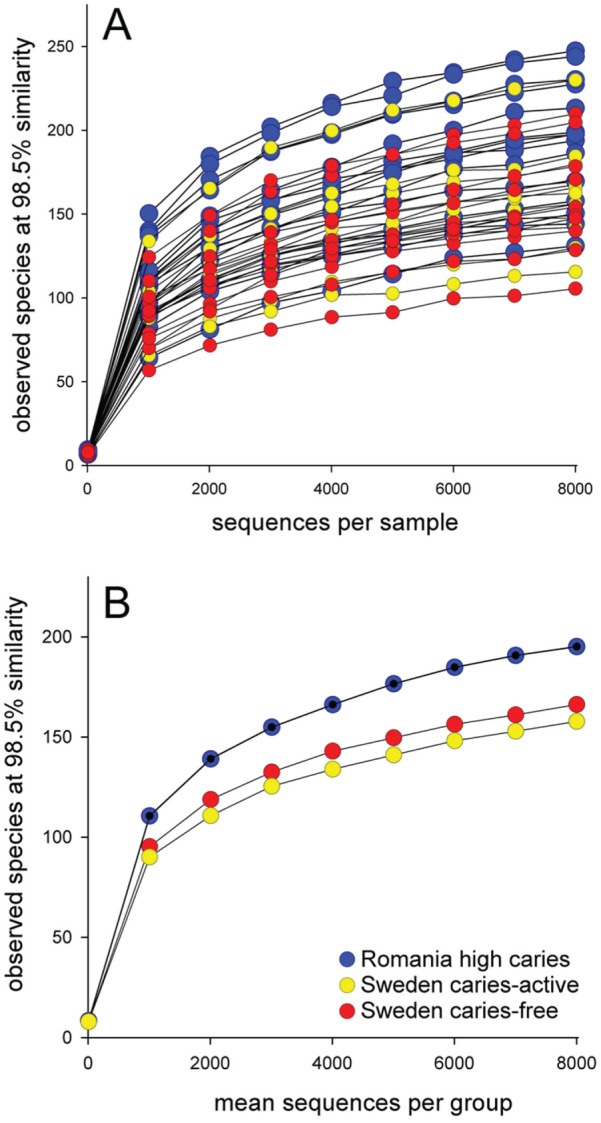
Rarefaction curves. (A) Species richness, the number of types of sequences in a sample, in individual Romanian adolescents (blue lines) with a high prevalence of caries (n = 14), Swedish adolescents with caries activity (yellow lines, n = 12), and caries-free Swedish adolescents (red lines, n = 11). (B) Mean number of types of sequences in the 3 groups.
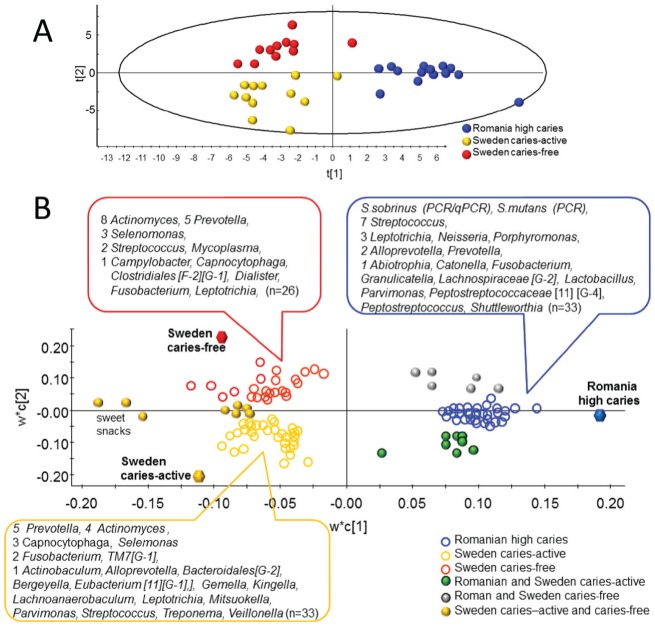
PCoA modeling of all HOT separated Romanian adolescents from most of the Swedish adolescents (Fig. 2A). Group separation was more distinct in the PCA score plot using the 295 HOT represented by ≥20 sequences, S. sobrinus and S. mutans detected by PCR/qPCR, and intake of sugar snacks (Fig. 2B).
Figure 2.
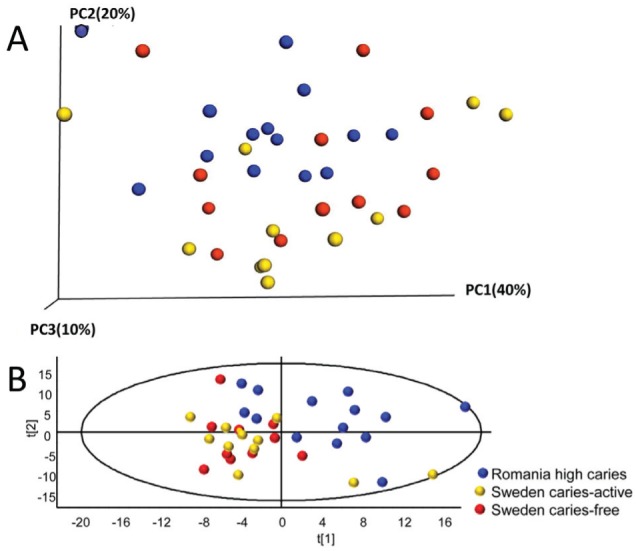
Caries group separation by principal coordinates analysis (PCoA) and principal component analysis (PCA). (A) PCoA plot displaying the distribution among the 37 samples based on USEARCH clustering of the 942,774 sequences at 98.5% similarity against the Human Oral Microbiome Database (β diversity). (B) PCA modeling displaying subject clustering using the 295 human oral taxa (HOT) with ≥20 cluster sequences, Streptococcus sobrinus and Streptococcus mutans by polymerase chain reaction (PCR), and intake of sweet snacks. Blue dots for Romanian adolescents with a high prevalence of caries (n = 14), yellow dots for Swedish adolescents with caries activity (n = 12), and red dots for caries-free Swedish adolescents (n = 11). The scores t1 and t2 are the new partial least squares (PLS) regression–created variables summarizing the x-variables. The oval circle illustrates the tolerance ellipse based on the Hotelling T2 distribution; any observation located outside of the ellipse would be an outlier.
The number of phyla or genera detected did not differ between the 3 caries groups (PGROUP = 0.233 and 0.121, respectively), but the distribution of sequences among phyla and genera did. Romanian adolescents had, compared to Swedish caries-active and caries-free adolescents, a significantly higher proportion of sequences in Firmicutes (median, 36.5%, 18.1%, and 22.6%, respectively; PTREND = 0.015) and tended to have a lower proportion in Actinobacteria (median, 5.8%, 26.6%, and 19.1%, respectively; PTREND = 0.015) (Appendix Table 1). At the genus level, Romanian adolescents had, compared to the Swedish groups, significantly (PGROUP ≤ 0.008) more sequences in the genera Porphyromonas (PTREND = 0.001), Abiotrophia (PTREND = 0.002), Peptostreptococcaceae [11][G-7] (PTREND = 0.011), Pseudoramibacter (PTREND = 0.003), Streptococcus (PTREND = 0.027), and Neisseria (PTREND = 0.004) but significantly fewer in Actinomyces (PTREND = 0.005), Selenomonas (PTREND < 0.001), and Campylobacter (PTREND = 0.001) (Appendix Table 1). Significant increasing trends (PTREND ≤ 0.008)—but group differences did not reach significance—were found for Bacteroidetes [G-3] (PGROUP = 0.022), Filifactor (PGROUP = 0.027), and Peptostreptococcaceae [11][G-4] (PGROUP = 0.020), and decreasing trends were found for Veillonella (PGROUP = 0.015) and TM7 [G-1] (PGROUP = 0.013).
Multivariate PLS modeling with the 295 HOT, S. sobrinus and S. mutans by PCR, and intake of sweet snacks as the block of independent variables, and caries groups as the block of dependent variables, separated the subjects into 3 distinct clusters corresponding to the 3 caries categories (Fig. 3A). This model had an explanatory power (R2) of 43.0% and a predictive power (Q2) of 29.5%. The PLS loading plot identified S. sobrinus by PCR/qPCR and S. mutans by PCR and 30 HOT uniquely associated with Romanian adolescents with a high prevalence of caries, that is, streptococci (Streptococcus australis, S. cristatus, S. sanguinis, Streptococcus sinensis, S. sobrinus, Streptococcus sp. HOT074, and Streptococcus sp. HOT431) and a few species/phylotypes in Allopre-votella, Leptotrichia, Neisseria, Por-phyromonas, and Prevotella (Fig. 3B, Appendix Table 4). Further, 8 taxa that were associated with the Romanian high caries group were also associated with the Swedish caries-active, but not the caries-free, group. These were Dialister pneumosintes, Gemella haemolysans, Leptotrichia shahii, Pepto-streptococcaceae [11][G-2] sp. HOT091, Porphyromonas catoniae, Prevotella sp. HOT301, S. mitis, and Streptococcus sp. HOT070. Swedish adolescents, with no difference between caries-free and caries-active subjects, were characterized by the intake of sweet products (chocolate, sweets, and ice cream) and Actinomyces sp. HOT525, Capnocyto-phaga sp. HOT 336, F. nucleatum subsp. animalis, Prevotella maculosa, and P. nigrescens. The tooth microbiota of Swedish caries-active adolescents was characterized by 5 species in Prevotella, 4 in Actinomyces, 3 in Capnocytophaga and Selenomonas, and a few in Fusobacterium and TM7 [G-1], while that of Swedish caries-free adolescents was characterized by 8 species in Actinomyces, 5 in Prevotella, 3 in Selenomonas, and a few in Streptococcus and Mycoplasma. While S. mutans and S. sobrinus were detected by PCR (Table) and by pyrosequencing (Appendix Table 2), the sensitivity of detection was higher by PCR and disease associations greater with the PCR data.
Figure 3.
Partial least squares (PLS) regression of the microbiota associated with the caries groups. The PLS model used caries grouping as the y-variable and the 295 human oral taxa (HOT) with ≥20 cluster sequences, Streptococcus sobrinus and Streptococcus mutans by polymerase chain reaction (PCR)/quantitative PCR (qPCR), and intake of sweet snacks (3 variables) as the independent variable block. (A) The PLS scatter loading plot illustrating the clustering of Romanian adolescents with a high prevalence of caries (blue dots, n = 14) versus Swedish caries-active (yellow dots, n = 12) and caries-free (red dots, n = 11) adolescents. The scores t1 and t2 are the new PLS-created variables summarizing the x-variables. The oval circle illustrates the tolerance ellipse based on the Hotelling T2 distribution; any observation located outside of the ellipse would be an outlier. (B) The PLS loading plot illustrating variables associated with each of the 3 caries groups. w*c[1] and w*c[2] represent loading for the 2 first components. The HOT with Variable Importance in Projection (VIP) values are shown in Appendix Table 4.
Discussion
This study compared the dental biofilm composition of Romanian adolescents with a high prevalence of caries with that of Swedish adolescents with or without caries. S. sobrinus and S. mutans were strongly associated with increasing caries status as reported earlier (Takahashi and Nyvad 2011), and although a major difference in microbial composition was associated with the study population, a core microbiome of 24 taxa was identified. A significant feature of this study is that the Romanian adolescents had minimal dental care and thus could be considered to represent the microbiota of dental caries unaltered by oral hygiene practices or fluoride exposure. Most Swedish adolescents brushed their teeth daily with a fluoride-containing toothpaste and had dietary and oral hygiene counseling, topical fluoride treatments, and regular visits to a dental clinic since early childhood. Thus, the conditions of developing countries transforming to a Westernized lifestyle and countries that have practiced caries prevention for decades are mirrored in the study populations. Factors that could influence the oral microbiota of the Romanian adolescents were more severe caries and cavities involving dentin (Jiang et al. 2014) and generally no tooth brushing, suggesting that their biofilms were more mature. The increased caries and infrequent tooth brushing are consistent with their living in a village of lower socioeconomic status (Do 2012). The Romanian adolescents were younger and had, on average, 1 fewer tooth than the Swedish adolescents, although it is unclear whether these characteristics would have impacted results as much as the differences in caries, oral hygiene, and socioeconomic status between the populations.
Culture-independent methods have contributed to the understanding of the complex microbiota in oral diseases (Paster et al. 2001; Kanasi et al. 2010; Zaura 2012). Most such studies target the 16S rRNA gene, and those employing multiplex sequencing techniques target the V1 to V2, V3 to V4, or V5 to V6 hypervariable regions in 400- to 450-bp stretches, which can limit the taxonomic resolution to the genus level. The present study used sequences in 16S rRNA spanning over 4 variable regions (V1–V4), which can improve the taxonomic resolution significantly and allow species- and phylotype (HOT)–level identifications. While speciation is also possible using microarrays or cloning and sequencing techniques, these approaches are limited by the panel of primers used in microarrays and the number of colonies selected for sequencing in clonal analyses.
In the present study, the greatest species diversity was in the Romanian adolescents with the highest prevalence of caries, as observed in some studies (Luo et al. 2012; Thomas et al. 2012) but not others (Gross et al. 2010; Simón-Soro et al. 2013). While the increased microbial diversity may be due to a caries-associated ecological shift, it may also reflect the children’s environment including the age and size of biofilm samples since the Romanian subjects were generally untreated and had abundant dental plaque.
Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Fusobacteria are the most abundant phyla, and Streptococ-cus, Actinomyces, Prevotella, Fusobacterium, Leptotrichia, and Corynebacterium are the dominant genera in dental biofilm or saliva (Wade 2013). These phyla and genera were also abundant in the present study but with similar proportions of Bacteroidetes and Firmicutes. Notably, 24 species were found in all 37 subjects (core microbiome), namely, species in the Streptococcus group (S. cristatus, S. mitis, S. mitis bv2, and S. sanguinis) and Actinomyces (A. gerencseriae, Actinomyces naeslundii, and Actinomyces oris), some Prevotella and Fusobacterium, and single species of Alloprevotella, Campylobacter, Capnocytoph-aga, Corynebacterium, Eikenella, Granulicatella, Lachnoan-aerobaculum, and Veillonella). The concept of a core microbiome was recently questioned (Li et al. 2013), but the present and other studies (Nasidze et al. 2009; Zaura et al. 2009) support the hypothesis of a core microbiome at least in populations with a common ancestry (Mason et al. 2013).
Most caries studies using pyrosequencing targeted young children with a primary dentition (Gross et al. 2010; Ling et al. 2010). Thus, the current study expands the knowledge to adolescents with permanent teeth. Comparisons between the present and published studies should bear in mind that the structure of the tooth-coating protein pellicle (Sønju Clasen et al. 1997) and colonizing bacteria (Crielaard et al. 2011) differ between primary and permanent dentitions. Still, several of the genera, particularly Actinomyces, Prevotella, Leptotrichia, and Granulicatella, that were associated with caries in the present study cohort are reported to be overrepresented in dental caries in young children (Gross et al. 2010; Ling et al. 2010; Jiang et al. 2013). The presence of Capnocytophaga sp. HOT335, F. nucleatum subsp. nucleatum, and selected Streptococcus species in caries-free adolescents is consistent with previous reports (Gross et al. 2010; Ling et al. 2010; Jiang et al. 2013; Simón-Soro et al. 2013). However, 3 Neisseria (including Neisseria flavescens) and 3 Porphyromonas (including P. catoniae) species were prevalent in the Romanian caries group, which is in contrast to findings by Crielaard et al. (2011).
The detection of S. mutans and S. sobrinus was higher using PCR than pyrosequencing, as had been expected due to the use of species-specific primers for PCR rather than the more inclusive primers targeting the V1 to V4 regions of 16S rRNA used for pyrosequencing. Both S. mutans and S. sobrinus were highly associated with caries, with a strikingly high prevalence of S. sobrinus alone and S. mutans with S. sobrinus in the Romanian adolescents. However, 15% of the Romanian and 30% of the Swedish caries-active adolescents had no detectable mutans streptococci despite having active caries, which is consistent with the ecological plaque hypothesis that suggests that acid for caries initiation may come from any or all of several acidogenic species, including the lactobacilli, bifidobacteria, and species with weaker acid production, such as non–mutans streptococci and Actinomyces species (Takahashi and Nyvad 2011).
We conclude that S. sobrinus, S. mutans, and other Streptococcus species, particularly Streptococcus sp. HOT074 and Streptococcus sp. HOT431, characterized the microbiota of Romanian adolescents with limited access to dental care, whereas L. shahii, P. catoniae, and Streptococcus sp. HOT070 were present in both the Romanian and Swedish caries groups. In contrast, S. mutans was less frequent and S. sobrinus was rarely detected among Swedish adolescents who had regular dental care and individual anticaries regimens since early childhood. The Swedish participants were typically colonized with species in the Actinomyces, Selenomonas, Prevotella, and Capnocytophaga genera. These findings suggest that the role of mutans streptococci as a primary caries pathogen is less pronounced in populations exposed to preventive programs, whereas both S. mutans and S. sobrinus are prevalent in populations without routine caries treatment and prevention strategies. The latter populations are generally characterized by lower socioeconomic status, lack of oral hygiene and fluoride exposure from toothpaste, and restoration of cavities, which likely explain the differences seen between the Romanian and Swedish groups. Nonetheless, systematic, long-term dental care appears to affect the microbiota in a population over time.
Author Contributions
I. Johansson, E. Witkowska, B. Kaveh, contributed to conception, design, and data analysis, drafted and critically revised the manuscript; P. Lif Holgerson, contributed to data acquisition and analysis, drafted and critically revised the manuscript; A.C.R. Tanner, contributed to data analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
Carina Öhman and Agneta Rönnlund are acknowledged for laboratory work and Dr. Mara Campeanu and Dr. Karin Sunnegårdh-Grönberg for supporting data collection in Romania and Sweden, respectively. The authors acknowledge input from Dr. Floyd Dewhirst in data analysis and interpretation.
Footnotes
The present study was supported by grants from The Swedish Patent Revenue Foundation and TUA, the County Council of Västerbotten, Sweden, and by grant DE-016937 from the National Institute of Dental and Craniofacial Research at the National Institutes of Health, USA.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Chen T, Yu WH, Izard J, Baranova OV, Lakshmanan A, Dewhirst FE. 2010. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford). 2010:baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, Keijser BJ. 2011. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics. 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, Erdman SE, Alm EJ. 2014. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 15(7):R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol. 192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do LG. 2012. Distribution of caries in children variations between and within populations. J Dent Res. 91(6):536–543. [DOI] [PubMed] [Google Scholar]
- Funieru C, Twetman S, Funieru E, Dumitrache AM, Sfeatcu RI, Baicus C. 2014. Caries experience in schoolchildren in Bucharest, Romania: the PAROGIM study. J Public Health Dent. 74(2):153–158. [DOI] [PubMed] [Google Scholar]
- Gross EL, Leys EJ, Gasparovich SR, Firestone ND, Schwartzbaum JA, Janies DA, Asnani K, Griffen AL. 2010. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J Clin Microbiol. 48(11):4121–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugoson A, Koch G. 2008. Thirty year trends in the prevalence and distribution of dental caries in Swedish adults (1973-2003). Swed Dent J. 32(2):57–67. [PubMed] [Google Scholar]
- Jiang W, Ling Z, Lin X, Chen Y, Zhang J, Yu J, Xiang C, Chen H. 2014. Pyrosequencing analysis of oral microbiota shifting in various caries states in childhood. Microb Ecol. 67(4):962–969. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang J, Chen H. 2013. Pyrosequencing analysis of oral microbiota in children with severe early childhood dental caries. Curr Microbiol. 67(5):537–542. [DOI] [PubMed] [Google Scholar]
- Kanasi E, Dewhirst FE, Chalmers NI, Kent R, Jr., Moore A, Hughes CV, Pradhan N, Loo CY, Tanner AC. 2010. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 44(5):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nasidze I, Quinque D, Li M, Horz HP, André C, Garriga RM, Halbwax M, Fischer A, Stoneking M. 2013. The saliva microbiome of Pan and Homo. BMC Microbiol. 13:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lif Holgerson P, Harnevik L, Hernell O, Tanner AC, Johansson I. 2011. Mode of birth delivery affects oral microbiota in infants. J Dent Res. 90(10):1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Kong J, Jia P, Wei C, Wang Y, Pan Z, Huang W, Li L, Chen H, Xiang C. 2010. Analysis of oral microbiota in children with dental caries by PCR-DGGE and barcoded pyrosequencing. Microb Ecol. 60(3):677–690. [DOI] [PubMed] [Google Scholar]
- Luo AH, Yang DQ, Xin BC, Paster BJ, Qin J. 2012. Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Dis. 18(6):595–601. [DOI] [PubMed] [Google Scholar]
- Ma C, Chen F, Zhang Y, Sun X, Tong P, Si Y, Zheng S. 2015. Comparison of oral microbial profiles between children with severe early childhood caries and caries-free children using the human oral microbe identification microarray. PLoS One. 10(3):e0122075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzourani M, Gilbert SC, Sulong HN, Sheehy EC, Tank S, Fenlon M, Beighton D. 2009. The isolation of bifidobacteria from occlusal carious lesions in children and adults. Caries Res. 43(4):308–313. [DOI] [PubMed] [Google Scholar]
- Marsh PD. 2003. Are dental diseases examples of ecological catastrophes? Microbiology. 149(Pt 2):279–294. [DOI] [PubMed] [Google Scholar]
- Mason MR, Nagaraja HN, Camerlengo T, Joshi V, Kumar PS. 2013. Deep sequencing identifies ethnicity-specific bacterial signatures in the oral microbiome. PLoS One. 8(10):e77287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasidze I, Li J, Quinque D, Tang K, Stoneking M. 2009. Global diversity in the human salivary microbiome. Genome Res. 19(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. 2001. Bacterial diversity in human subgingival plaque. J Bacteriol. 183(12):3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. 2005. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 83(9):661–669. [PMC free article] [PubMed] [Google Scholar]
- Ruoff KL. 1991. Nutritionally variant streptococci. Clin Microbiol Rev. 4(2):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón-Soro A, Tomás I, Cabrera-Rubio R, Catalan MD, Nyvad B, Mira A. 2013. Microbial geography of the oral cavity. J Dent Res. 92(7):616–621. [DOI] [PubMed] [Google Scholar]
- Söderström U, Johansson I, Sunnegårdh-Grönberg K. 2014. A retrospective analysis of caries treatment and development in relation to assessed caries risk in an adult population in Sweden. BMC Oral Health. 14:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sønju Clasen AB, Hannig M, Skjørland K, Sønju T. 1997. Analytical and ultrastructural studies of pellicle on primary teeth. Acta Odontol Scand. 55(6):339–343. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nyvad B. 2011. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 90(3):294–303. [DOI] [PubMed] [Google Scholar]
- Tanner AC, Kent RL, Jr., Holgerson PL, Hughes CV, Loo CY, Kanasi E, Chalmers NI, Johansson I. 2011. Microbiota of severe early childhood caries before and after therapy. J Dent Res. 90(11):1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RZ, Zijnge V, Ciçek A, de Soet JJ, Harmsen HJ, Huysmans MC. 2012. Shifts in the microbial population in relation to in situ caries progression. Caries Res. 46(5):427–431. [DOI] [PubMed] [Google Scholar]
- Wade WG. 2013. The oral microbiome in health and disease. Pharmacol Res. 69(1):137–143. [DOI] [PubMed] [Google Scholar]
- Yano A, Kaneko N, Ida H, Yamaguchi T, Hanada N. 2002. Real-time PCR for quantification of Streptococcus mutans. FEMS Microbiol Lett. 217(1):23–30. [DOI] [PubMed] [Google Scholar]
- Zaura E. 2012. Next-generation sequencing approaches to understanding the oral microbiome. Adv Dent Res. 24(2):81–85. [DOI] [PubMed] [Google Scholar]
- Zaura E, Keijser BJ, Huse SM, Crielaard W. 2009. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 9:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.