Abstract
Background:
Mechanical ventilation is one of the supporting treatments that are used for different reasons. To reduce patients’ inconvenience caused due to using tracheal tube and ventilator, sedation is routinely used. Using scales for the sedation, for example, Richmond Agitation Sedation Scale (RASS), may reduce dose of sedation and length of mechanical ventilation.
Materials and Methods:
This study is a randomized clinical trial on 64 patients selected from three intensive care units (ICUs) in Isfahan, Iran. Through random allocation, 32 patients were assigned to each of the study and control groups. In the control group, patients’ level of consciousness and the amount of drug consumption in every shift, based on physician order, were recorded. In the study group, RASS score was recorded every hour and sedation was administered based on that. The purpose of the study was to investigate of application of RASS for drug consumption until weaning of the patient from the ventilator. Independent t-test with significance level of 0.05 was used.
Results:
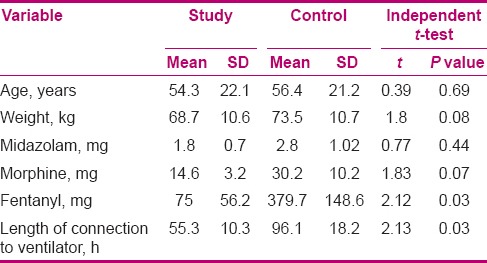
Results showed no significant difference in the mean consumption of midazolam and morphine after intervention, but there was a significant difference in fentanyl (P = 0.03) consumption (379 μg in the control group vs 75 μg in the study group) between groups after the intervention. The mean duration of being connected to the ventilator was significantly less in the study group (P = 0.03).
Conclusions:
Application of RASS by nurses leads to a decrease in sedation consumption, connection to ventilator, and length of stay in the hospital.
Keywords: Intensive care unit, Richmond Agitation Sedation Scale, sedation, ventilation
INTRODUCTION
Mechanical ventilation is one of the supportive treatments used for various reasons such as the need to control patients’ respiration during surgery or treatment of severe head injuries and maintaining oxygenation when patients’ ventilation is inadequate. Despite numerous benefits in using such mechanical ventilation machines, their long-term use may lead to several problems such as ventilator-associated pneumonia (VAP), respiratory complications, gastrointestinal hemorrhage, cardiovascular complications, and bed sore.[1] Meanwhile, patients’ fight with the ventilator is one of the common complications of this supportive treatment. Using a tracheal tube and a ventilator can lead to patients’ stimulation, anxiety, pain, and discomfort that result in many physiological and psychological complications.[2]
However, to reduce patients’ discomfort, sedation is routinely administrated with the goal of providing comfort to the patient.[3] Patients’ sedation with sedatives, narcotics, and tranquilizers can lead to facilitation of mechanical ventilation and treatment, decrease in patients’ physical and psychological discomfort, and anxiety control.[4,5] On the other hand, in spite of having all the above-mentioned advantages, patients’ sedation has also some problems, and has thus been reported as an issue in treating these patients.
Studies show that using a high amount of sedatives to sedate the patient can lead to respiratory system depression and, consequently, prolonged patients’ connection to the ventilator, an increase in medication costs, medication dependency, respiratory infection, and therefore finally leading to high hospitalization costs.[6] Lesser consumption of such medications can also lead to anxiety, hyperactivity, pain, hypertension, and tachycardia and has unpredictable effects on patients’ function.[7] With regard to the aforementioned points, striking a balance in the management of patients’ anxiety and pain to control the side effects and using a medication by which no respiratory depression occurs is one of the most challenging medical and nursing cares. Meanwhile, using a specific and accurate program that can detect and reduce these complications and is accepted by all is among the main problems in injection of such medications. Striking a balance in this regard or prediction and reduction of patients’ anxiety, restlessness, and pain without patients’ deep sedation are all controversial.[8,9] Some scales have been designed in this context. Research shows that using such scales of sedation and their scoring system can reduce the length of ventilation, and the incidence of pain and hospital infections.[10] There are some scales such as patients’ comfort scale (CS), which was designed in 1992 to investigate patients’ stimulation and reaction to the environment of ICUs by assessing respiration, muscle tone, and other physical parameters. Ramsay scale, designed in 1974, is the first scale to assess patients’ comfort and it only measures patients’ stimulation. Another scale is Richmond Agitation Sedation Scale (RASS) that assesses patients’ sedation.[4] Meanwhile, research showed that application of RASS is more clear and convenient and takes lesser time, leads to patients’ connection to the ventilator for a shorter time, and shorter duration of stay in the ICU, compared to other scales.[11,12] But some other studies show that application of this scale does not result in patients’ recovery, and moreover, it increases the risk of patients’ early and unsuccessful extubation and incidence of stress disorder.[10] Goodwin et al. (2012), in a study conducted in Germany on using sedation to have the body organs function at their best, showed that using RASS leads to sedation in intubated patients, and a reduction in medication consumption and their complications including delirium.[13] Payen et al. (2007), in a study conducted in 44 ICUs in France on the correct practice of sedating the patients connected to ventilator in the ICU, showed that using scales such as RASS led to reduced need for the sedative (43% vs 72%).[14] On the contrary, Albert and Adam (2006) reported that using RASS had a lesser effect on the number of days of hospitalization of the patient and the mechanical ventilation.[15] Back et al. (2008) reported in Australia that using RASS had no notable effect on ventilation, hospitalization length, and patients’ mortality.[16] With regard to the existing controversy about the efficiency of RASS in different studies and nurses’ need to use various evaluation scales for a better quality of care, the researchers thought that conducting the present study to investigate the efficacy of RASS and sedation of the patients, connected to ventilators, with the cooperation of the physicians and nurses is essential. Its effect on the length of mechanical ventilation, type and dosage of medications for patients’ sedation was also investigated in the present study.
MATERIALS AND METHODS
This is a two-group clinical trial in which the effect of sedation on the results of RASS application among the patients connected to ventilator, as well as its effect on the length of mechanical ventilation, type and dosage of the consumed sedative among the patients in the study group were evaluated and measured. A total of 64 patients were selected by random allocation from three ICUs in Al-Zahra hospital in Isfahan, Iran during 2013–2014. After getting permission from the nursing and midwifery school, and handing her letter of introduction to the authorities of Al-Zahra hospital and explaining the research project to the head nurses of ICUs in Al-Zahra hospital, the researcher entered the selected ICUs. Firstly, necessary explanations about the research and correct application of RASS were given to all nurses in the wards by the researcher and an anesthesiologist, and the obscure points were clarified. Then, following the ethical considerations including explanation of the method, maintaining the confidentiality of patients’ information, and obtaining consent from patients’ accompanying persons in all three ICUs in Al-Zahra hospital, the subjects were selected by convenient sampling. After the subjects meeting the inclusion criteria were selected, they were assigned to study and control groups through random allocation by use of random numbers table until there were 32 subjects in each group. Subjects’ demographic characteristics were recorded by the researcher. In the control group, patients’ glasgow coma scale score was calculated and recorded, and the sedatives such as midazolam, morphine, and fentanyl, based on physician's order (PRN if needed) in each shift, were given [Table 1]. The criterion for PRN was nurses’ and physicians’ personal judgments without use of RASS.
Table 1.
Basic dosage of sedation consumption[20]

Validity and reliability of this scale in Iran were established by Tadrisi et al. in Bagiatollah University of Medical Sciences among 120 patients (α =95%).[12] Before conducting the research, its validity and reliability were investigated among 10 patients in a pilot study under the supervision of the research supervisor in Al-Zahra hospital, and after obtaining his and the anesthesiologist’ approval, the research was conducted on 64 patients.
In the study group, the score of RASS was checked and recorded in different shifts every hour by the researcher or her co-worker and sedation was administrated accordingly.
The type and dosage of sedatives were recorded and compared in each group. To score RASS, three sequential steps of observation, reaction to auditory stimulation, and reaction to physical stimulations were used [Table 2]. Firstly, the patient was observed and scored from 0 to +4 if conscious [rows 1–4 in Table 2]; but if unconscious, he/she was called loudly for several times, and if there was a response, he/she was scored from −1 to −3 [rows 5–7 in Table 2]. At the last step, which was the step of painful stimulation, the patient's sternum was pressed hard and the patient status was scored from −4 to −5 [rows 8–9 in Table 2]. After determination of RASS score, the medication dosage was determined with the goal of maintaining the patient in sedation scores between −1 and 0. If the patient obtained scores +1–+4, it showed inefficiency of the ventilator or patient's inappropriate conditions or inefficiency of sedation. In such a case, firstly the ventilator and other equipments were checked, changeable causes (position and hypoxia) were modified, the environment was modified, and verbal assurance was given to the patient. If patient's restlessness remained steady, the researcher informed the anesthesiologist about the patient's condition and the anesthesiologist increased the primary dosage of sedation or changed the medication based on patient's obtained RASS score. Ultimately, the patient was assessed by RASS every hour to check whether the patient had obtained the favorite score (from −1 to 0) or not. Dosage and type of medication were changed based on the low or high score of the patient, and medication was administered under the supervision of the researcher or an anesthesiologist.
Table 2.
Richmond Agitation Sedation Scale
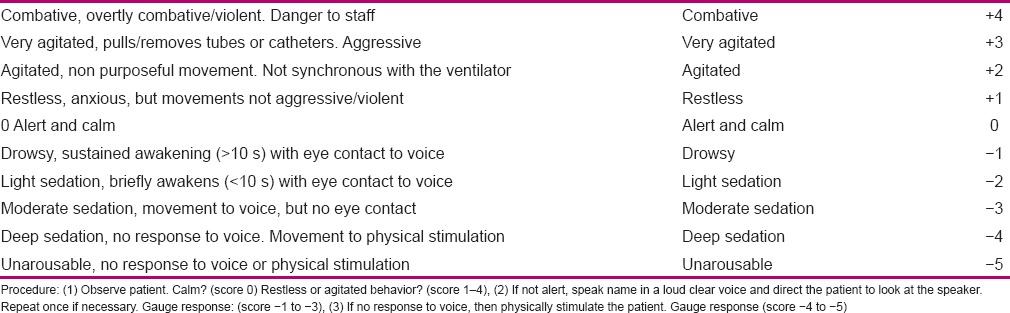
The patients who scored negative scores less than −1 to −5 seemingly had higher sedation and, consequently, their medication was reduced based on the physician's order to reach the favorite score. In the study group, patients’ constant monitoring was done by the researcher based on RASS, and any changes were reported to the anesthesiologist. Dosage of sedation differed based on the patients’ condition. For the patients who received scores 0 to −1, the level of sedation was appropriate and medication was continued without any change. Using this scale in the study group was continued for 24 h a day from patients’ intubation to disconnection from the ventilator. The condition was investigated in the morning and evening shifts by the researcher and in the night shift by a trained co-worker, and was compared with that of the control group. In all working shifts, trained nurses cooperated with the researcher. Data were analyzed by using SPSS version 18. Independent and paired t-test, with a significance level of 0.05, was used for comparisons.
RESULTS
Chi-square test with regard to sex (P = 0.78) and independent t-test with regard to age (P = 0.69) showed no significant difference between the two groups. In the study and control groups, subjects’ age ranged 18–84 years with mean (SD) of 54.3 (22.1) years and 19–89 years with mean (SD) of 56.4 (21.2) years, respectively.
There was no significant difference in weight between the two groups (P = 0.08). Results showed that the consumption of midazolam and morphine was not significantly different, but the mean consumption of fentanyl showed a significant difference (P = 0.03) between the two groups. Mean length of connection to ventilator was significantly lower in the study group compared to the control group. As the consumed medication dosages and subjects’ weight were not identical in the two groups, to control the confounding effect of these variables, analysis of covariance (ANCOVA) was adopted, and then again, the mean lengths of patients’ connection to ventilator were compared between the two groups. By controlling these variables, we found that administration of medication based on RASS was effective on the length of patients’ connection to the ventilator. The researchers found that using this scale led to a reduction in patients’ stay in ICU in the study group, compared to the control group [Table 3].
Table 3.
Comparison of mean age, weight, medications, and length of connection to ventilator in the two groups
DISCUSSION
This clinical trial showed that using RASS was effective on patients’ length of ventilation and consumption of some sedatives.
The two groups of the present study showed no significant difference in demographic characteristics. Rosaria et al. (2012), in a study on using RASS in cancer patients and giving them sedatives based on this scale as well as evaluating their level of satisfaction and quality of life, showed no significant difference in subjects’ demographic characteristics between the two groups, possibly due to random allocation of the subjects to the two groups.[17] Tadrisi et al. (2009), in a study on precise determination of validity and reliability of RASS, showed that consumption of medications was less in the study group compared to the control group, which is in line with the present study findings, possibly due to similar methodology and the type of consumed sedatives.[12] Degrado et al., in a study with the goal of evaluating patients with RASS and prescription of sedation based on this scale, showed that there was no significant difference between the study and control groups. They also reported that consumption of sedatives was significantly higher in the study group compared to the control group.[18] The difference between their results and those of the present study can be due to different diagnoses of patients in the two groups and the reasons for taking sedatives, as well as the different methods used in the studies as this test was conducted mostly among the patients with high respiratory problems or distress that could have itself increased the score of RASS and, consequently, consumption of sedatives. Although Payen et al. (2007), in a study with the goal of pain assessment and sedation in ICU patients conducted during a week, reported no significant difference in subjects’ demographic characteristics between the two groups, they reported a significant decrease in consumption of midazolam and fentanyl after using the scales from the second to the sixth days (P < 0.05). They also claimed that consumption of morphine increased but that of fentanyl decreased in the study group. The reason of lower consumption of midazolam and increased consumption of morphine in the study group can be due to different methods used and the patients’ diagnoses.[14] Tanios et al., in a study on evaluation of the barriers in use of sedation protocols, showed that use of scales including RASS led to less connection of the patients to the ventilator and their stay in ICU as well as a reduction in consumption of the sedatives. The similarity of their results with those of the present study could be attributed to similar methods used, patients’ random allocation to two groups, and similar patients’ diagnoses.[10] Goodwin et al. (2012), in a study on functioning of body organs at their best, investigated the use of scales including RASS in patients after coronary artery bypass graft surgery and acute respiratory distress syndrome to intermittently take sedatives and its association with disconnection of patients from the ventilator, and concluded that these scales led to a reduction in patients’ connection to the ventilator and their stay in ICU.[13] Kapila et al. (2008), in a study conducted in Australia on ICU patients’ sedation, used scales including RASS on 192 patients hospitalized in ICU and reported temporary consumption of sedatives due to use of these scales. Their results showed that use of the scales led to lower treatment costs and less consumption of sedatives including propofol and midazolam.[19] The reason for lower consumption of midazolam can be the consumption of other sedatives based on diagnosis of the anesthesiologist or patients’ different diagnoses and signs, compared to the present study.
CONCLUSION
The results showed that use of RASS diminished the length of mechanical ventilation, but had no effect on the consumption of some sedatives. Application of this scale in nursing interventions, in addition to speeding up patients’ recovery, can improve the ability of the nurses in evaluating patients and making decisions about them.
ACKNOWLEDGMENTS
This article was derived from a master thesis of Farzaneh Toghyani with project number 392464, Isfahan University of Medical Sciences, Isfahan, Iran. We appreciate From Clinical Research Development of Center. The researchers appreciate all who helped in the present study, including the staff of the studied wards and vice-chancellery for research of nursing and midwifery school in Isfahan University of Medical Sciences (research project No. 392464), as well as all the patients and their families for their cooperation.[20]
Footnotes
Source of Support: Vice-chancellor for research, Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Curtis N, Kimberly V. Patient-Focused Sedation and Analgesia in the ICU. Chest. 2008;133:552–65. doi: 10.1378/chest.07-2026. [DOI] [PubMed] [Google Scholar]
- 2.Noyal M, Sujatha S, Tarun K, Ashok S, Subhash C. Ventilator-associated pneumonia: A review. Eur J Intern Med. 2010;21:360–8. doi: 10.1016/j.ejim.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Peck M, Down J. Use of sedatives in the critically ill. Anaesth Intensive Care Med. 2010;11:12–5. [Google Scholar]
- 4.Ma P, Liu J, Xi X, Du B, Yuan X, Lin H, et al. Practice of sedation and the perception of discomfort during mechanical ventilation in Chinese intensive care units. J Crit Care. 2010;25:451–7. doi: 10.1016/j.jcrc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Ashkenazy S, Dekyser F. Assessment of the reliability and validity of the Comfort Scale for adult intensive care patients. Hear Lung. 2011;40:44–51. doi: 10.1016/j.hrtlng.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Hawks S, Brandon D, Uhl T. Nurse perception of Bispectral Index monitoring as an adjunct to sedation scale assessment in the critically ill paediatric patient. Intensive Crit Care Nurs. 2013;29:92–6. doi: 10.1016/j.iccn.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Hofhuis JG, Langevoort G, Rommes JH, Spronk PE. Sleep disturbances and sedation practices in the intensive care unit-A postal survey in the Netherlands. Intensive Crit Care Nurs. 2012;28:141–9. doi: 10.1016/j.iccn.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Roberts RJ, de Wit M, Epstein SK, Didomenico D, Devlin JW. Predictors for daily interruption of sedation therapy by nurses: A prospective, multicenter study. J Crit Care. 2010;25:83–9. doi: 10.1016/j.jcrc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Jarzyna D, Jugquist C, Pasero C, Willens J, Nisbet A, Oakes L. American Society for Pain Management Nursing Guidelines on Monitoring for Opioid-Induced Sedation and Respiratory Depression. Pain Manag Nurs. 2011;12:118–45. doi: 10.1016/j.pmn.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Tanios M, Dewit M, Epstein S, Devlin J. Perceived barriers to the use of sedation protocols and daily sedation interruption: A multidisciplinary survey. J Crit Care. 2009;24:66–73. doi: 10.1016/j.jcrc.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Randen I, Torunn I. Sedation practice in three Norwegian ICUs. A survey of intensive care nurses’ perceptions of personal and unit practice. Intensive Crit Care Nurs. 2010;26:270–2. doi: 10.1016/j.iccn.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, et al. Richmond agitation-sedation scale validity and reliability in intensive care unit adult patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin H, Lewin J, Mirski M. Coperative sedation optimizing comfort while maximizing systemic and neurological function. Crit Care. 2012;16:217. doi: 10.1186/cc11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payen JF, Chanques G, Mantz J, Hercule C, Auriant I, Leguillou JL, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patient. Anesthesiology. 2007;106:687–95. doi: 10.1097/01.anes.0000264747.09017.da. [DOI] [PubMed] [Google Scholar]
- 15.Adam C, Rosser D, Manji M. Impact of introducing a sedation management guideline in intensive care. Anaesthesia. 2006;61:260–3. doi: 10.1111/j.1365-2044.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- 16.Bucknall T, Manias E, Presneill J. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Crit Care Med. 2008;36:1444–50. doi: 10.1097/CCM.0b013e318168f82d. [DOI] [PubMed] [Google Scholar]
- 17.Benitez-Rosario MA, Castillo-Padrós M, Garrido-Bernet B, Ascanio-León B. Quality of Care in Palliative Sedation: Audit and Compliance Monitoring of a Clinical Protocol. J Pain Symptom Manage. 2012;44:532–41. doi: 10.1016/j.jpainsymman.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Degrado JR, Anger KE, Szumita PM, Pierce CD, Massaro AF. Evaluation of a local ICU sedation guideline on goal-directed administration of sedatives and analgesics. J Pain Res. 2011;4:127–34. doi: 10.2147/JPR.S18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reschreiter H, Matt M, Kapila A. Sedation practice in the intensive care unit. A UK national survey. Crit Care. 2008;12:R152. doi: 10.1186/cc7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viencent J, Abraham E, Moore F, Mitchell K. Text book of critical care, 6 edition, Philadelphia, volume 1; 2011. p. 9, volume 2. p.1496.


