Abstract
Rate of immune reconstitution (IR) directly correlates with the number of hematopoietic stem cells (HSC) infused and is particularly delayed in patients undergoing cord blood (CB) transplantation (CBT). Methods to increase the number of CB natural killer (NK) cells have the potential to improve IR after CBT. NK cells are the first lymphocyte population to recover after HSC transplant and are central to preventing early relapse and infection. CB NK cells are low in number and are known to be incomplete in maturation and require activation for effective function. Here, we report a clinically relevant ex vivo expansion method that increases the number of activated CB NK cells. We report a multi-log increase in NK cell number when CB mononuclear cells (CBMC) are co-cultured with interleukin (IL)-2 and IL-15 resulted. Furthermore, NK cells expressing activating receptors and adhesion molecules responsible for cytotoxicity increased throughout culture while inhibitory receptor expression remained low. Additionally, cytotoxic function against various malignancies was significantly enhanced in cultured NK cells but not CD3+CD56+ cells. These data suggest that ex vivo expansion and activation of CB NK cells is a clinically feasible and relevant approach to prevent early infection and relapse after CBT.
Introduction
NK cells are one part of the innate immune system that eliminates malignant and virally infected cells through cytolytic killing and cytokine secretion. The receptors that regulate NK cell function may be categorized on the basis of their ligand specificity for major histocompatibility complex class I (MHC-I) and related molecules [1]. In humans, one of the most important groups of receptors responsible for NK cell function are killer cell Ig-like receptors (KIRs). KIRs are expressed at the surface of NK cells and recognize human leukocyte antigen (HLA) class I molecules [2]. The KIR ligands expressed on target cells, or lack thereof, determine the response of NK cells, resulting in either tolerance or cytolytic killing of the target. However, overall NK cell responses are dependent on a balance of signals generated through both activating and inhibitory receptors. Expression of various combinations of these NK cell receptors creates a diverse repertoire of effector cells.
NK cells play a crucial role in early IR after HCT because they are the first lymphocyte subset to recover [3, 4]. Thus, methods to increase the number of CB NK cells have the potential to prevent early relapse, infection and graft versus host disease (GvHD), as well as facilitate engraftment following CBT [5, 6]. Studies have shown that CB contains a higher percentage of NK cells than adult peripheral blood (PB) [7, 8]. Although NK cells in CB are reported to have lower cytotoxic function than PB, cytotoxicity can be significantly increased by activation with a cytokine cocktail, often containing IL-2 or IL-15 [7, 9–14]. Alternatively, NK cell cytotoxic function has also been augmented by the use of chimeric antigen receptor or artificial antigen presenting feeder cells [15–18]. Yet cytolytic function of NK cells has typically only been assessed by the use of the K562 cell line, a chronic myelogenous leukemia known to be NK cell sensitive. Determining the cytotoxic potential of NK cells against other leukemia and lymphomas is warranted.
In haploidentical HCT, selecting a donor based on KIR ligand mismatch shows a significant survival advantage. However, the effect of KIR ligand mismatch in CBT remains controversial. Two retrospective studies on the effects of KIR ligand incompatibility in unrelated CBT report conflicting results. The Eurocord study showed a favorable effect of KIR ligand mismatching on relapse incidence and leukemia-free survival, whereas the Minneapolis study showed no effect on these end points and a detrimental effect on incidence of GvHD [19]. While the KIR profile is similar in both CB and PB NK cells, studies have indicated that CB NK cells have lower KIR expression than PB [12]. While current studies have demonstrated that CB NK cell have heterogeneous KIR profiles, most studies have focused on freshly isolated NK cells [20]. Few studies have examined KIR profiles in NK cells before and after culture [12–14]. Additional studies in the field of NK cells, their receptors and their ligands may aid in determining the role of KIR-ligand mismatching after CBT.
With over 20,000 CBTs performed since 1988, CB is a widely accepted alternative source of HSC for transplantation and has emerged as an accessible source of NK cells that can be easily purified, and have the potential for multi-log fold expansion [11, 21]. We isolated and then expanded CB NK cells using IL-2, IL-15 and OKT3 culture conditions. NK cells were co-cultured with CD56− leukocytes, as they require the presence of other cells, specifically dendritic cells, to proliferate [22]. While CD3+CD56+ cells are also expanded during culture, previous studies have described conflicting results regarding their cytotoxic potential [11, 23]. In adult PB, NK-like T cells are reported to be diverse and capable of cytotoxic function. However, the T cell diversity of CB CD3+CD56+ cells has not been well described. Here we evaluate CB NK and CD3+CD56+ cell cytotoxic function toward several leukemia and lymphoma target cell lines, as well as examine activating and inhibitory receptor profiles of these cells before and after culture. Our results demonstrate that CB NK cells but not CD3+CD56+ cells can be successfully expanded ex vivo to become anti-tumor effector cells. Furthermore, we compare the expression profiles of activating and inhibitory receptors of CB NK cells to PB NK cells to determine whether the changes in expression were unique to the cell source or a result of the expansion method.
Materials and Methods
Isolation and Culture of CBMC and PBMC
Culture conditions for the expansion of NK cells were optimized using CB mononuclear cells (CBMCs) (St. Louis Cord Blood Bank, St. Louis, MO, USA). Peripheral blood mononuclear cells (PBMC) were collected from healthy donors with approval from Institutional Review Board and written informed consent from donors (St. Jude Children’s Research Hospital Blood Donor Center, Memphis, TN, USA). After ficoll density centrifugation using Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA, USA) according to the manufacturers instructions, 1×107 CBMCs and PBMCs were set aside and the remaining cells were then enriched for the CD56+ population using an AutoMACS CD56 microbead kit (Mitenyi Biotech, Auburn, CA, USA). As previously described, the enriched CD56+ cells were then mixed at a 1:1 ratio with CD56− cells to permit NK cell proliferation (i.e. CB or PB MIX samples) [24]. All samples were plated at a density of 1–2×106 cells/mL of CellGenix Stem Cell Growth Media (SCGM) containing 5% human serum (Lonza, Walkersville, MD, USA), 100 U/mL penicillin, 100 μg/mL streptomycin (Life Technologies, Carlsbad, CA, USA), 500 IU/mL IL-2 (SJCRH, Memphis, TN, USA), 10ng/mL IL-15 (CellGenx, Portsmouth, NH, USA) and 10 ng/mL OKT3 (BioLegend, San Diego, CA, USA). Media was changed 5 days post isolation using the above media without OKT3, and then every 3 days thereafter for up to 28 days post isolation.
Target Cell Line Culture
K562 (chronic myelogenous leukemia, CML), Jurkat (T cell Acute Lyphoblastic leukemia, T-ALL), MV4-11 (mixed lineage leukemia, MLL) and RS4:11 (MLL) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Life Technologies) containing 10% fetal bovine serum (FBS, ThermoScientific, Rockford, IL, USA), 100 U/mL penicillin and 100 μg/mL streptomycin. HDLM2 (Hodgkin’s lymphoma) cells were cultured in DMEM containing 20% FBS and pen/strep. Target cells were split 1:10 every 3 days.
Phenotypic Analysis by Flow Cytometry
Immunofluorescence Staining
Briefly, cells were washed once with 2% FBS in PBS, blocked with Human FcR (BD Biosciences, San Jose, CA, USA) for 15 minutes, followed by 15 minutes staining using an antibody cocktail or matching isotype control cocktail. Cells were then washed 3 times. Flow Cytometry was performed using a Millipore Guava easyCyte 8HT flow cytometer with the Millipore InCyte 2.2.2 software (Billerica, MA, USA). For the CD107a phenotypic panel, flow cytometry was performed on a BD FACSAria III using the BD FACSDiva 7.0 software. A minimum of 30,000 events was collected and data was analyzed using FlowJo 9.7 (Tree Star, Ashland, OR, USA).
Natural Killer Cell Phenotypic Panel
NK (CD3−CD14−CD56+) and CD3+CD56+ (CD3+CD14−CD56+) cell proliferation during culture was evaluated by flow cytometry for the expression of CD3 PerCP-Cy5.5, CD45 FITC, and CD56 APC (BD Bioscience) and absence of CD14 PE-Cy7 (BioLegend), CD19 PE, CD20 PE (BD Bioscience) on days 0, 7, 14, 21 and 28 following isolation.
Killer Ig-like Receptor Phenotypic Panel
KIR phenotypic analysis of NK (CD3−CD14−CD45+CD56+) and CD3+CD56+ (CD3+CD14− CD45+CD56+) cells by flow cytometry was performed on days 0, 14 and 21 post isolation. Samples were assessed for expression of CD3 APC-Cy7, CD4 PerCP, CD8 PE, ITGAL/CD11a PE (BD Bioscience), CD14 PE-Cy7 (BioLegend), CD45 FITC, CD56 APC, FasR/CD95 PerCP-Cy5.5 (BD Bioscience), FasL/CD178 AlexaFluor488 (AbD Serotec, Raleigh, NC, USA), DNAM1/CD226 FITC, 2B4/CD244 PE, TRAIL/CD253 PE, NKG2D/CD314 PerCP-Cy5.5 (BD Bioscience), NKp30/CD337 PE-Cy5, NKp44/CD336 PE-Cy5, NKp46/CD335 PE-Cy5 (BeckmanCoulter, Miami, FL, USA), KIR2DL1/CD158a FITC/PE (R&D systems, Minneapolis, MN, USA), KIR2DL2/3/CD158b FITC/PE, KIR3DL1/NKB1 FITC/PE (BD Bioscience), NKG2A/CD159a PE (BeckmanCoulter) and NTBA/SLAMF6 FITC (R&D Systems).
CD107a Phenotypic Panel
NK (CD3−CD14−CD45+CD56+) and CD3+CD56+ (CD3+CD14−CD45+CD56+) cells were analyzed by flow cytometry for the expression of CD3 BV605, CD4 BV786, CD8 BV510, CD45 FITC, CD56 APC, CD107a PE (BD Bioscience) and CD14 PE-Cy7 (BioLegend).
Cytotoxicity Co-Culture Assays
BADTA Cytotoxicity Assay
Cytotoxicity of samples was assessed on the day of isolation and at days 7, 14 and 21 post isolation against both NK cell sensitive (K562, Jurkat) and NK cell resistant (MV4-11, RS4;11, HDLM2) target cancer cell lines. Target cells were labeled with the DELFIA BADTA reagent according to manufacturer’s instructions (PerkinElmer, Waltham, MA, USA), washed and then co-cultured with effector cell samples (CBMC or MIX) at a ratio of 10:1 (effector to target) for 2 hours at 37°C. Each co-culture was performed in triplicate. Supernatants were then incubated with the DELFIA Europium detection solution (PerkinsElmer), and fluorescence was measured on a Wallac Victor 2 Counter Plate Reader (PerkinsElmer). Cytotoxicity was calculated using the following formula: % Cell Lysis = 100 × [(experimental release − spontaneous release)/(maximal release − spontaneous release)]
CD107a Assay
Expression of CD107a on the surface of both NK and CD3+CD56+ cells was assessed at day of isolation and at days 14 and 21 following co-culture with the above target cancer cell lines. 2×105 effector cells were co-cultured with or without 2×105 target cells per well in a 96-well plate for 2 hours at 37°C. Cells were then stained with the above CD107a phenotypic panel and analyzed by flow cytometry.
Statistical Analysis
Data is presented as the mean ± the standard error of the mean (SEM). Statistical significance was assessed using a two-tailed nonparametric t-test when comparing two groups, or a one-way ANOVA using the Tukey’s post hoc test when comparing more than 2 groups (Graphpad Prism 5.0, La Jolla, CA, USA). P values below 0.05 were considered statistically significant. *p<0.05, **p<0.01, ***p<0.001.
Results
CB NK and CD3+CD56+ Cells Proliferate Ex Vivo
CBMC and CB MIX samples were cultured for up to 28 days after isolation. We found that both NK and CD3+CD56+ cell populations within each sample type had a significant multi-log fold increase up to 21 days of culture as determined by flow cytometry (Figure 1A). Samples cultured for 28 days had a decrease in overall total cell numbers and fold decrease between day 21–28 (data not shown), indicating the optimal culturing period for CB NK and CD3+CD56+ cells is between 0–21 days after isolation. While CD3+CD56+ cells (solid lines) had a greater fold increase than NK cell (broken lines) populations, we determined that overall cell numbers were lower for CD3+CD56+ cells than NK cells. When examining overall frequencies of the lymphocyte population, NK cells were at a greater frequency than the CD3+CD56+ cell population, in both the CBMC and CB MIX populations before and after culture (Figure 1B).
Figure 1. CB NK and CD3+CD56+ Cells Proliferate Ex Vivo.
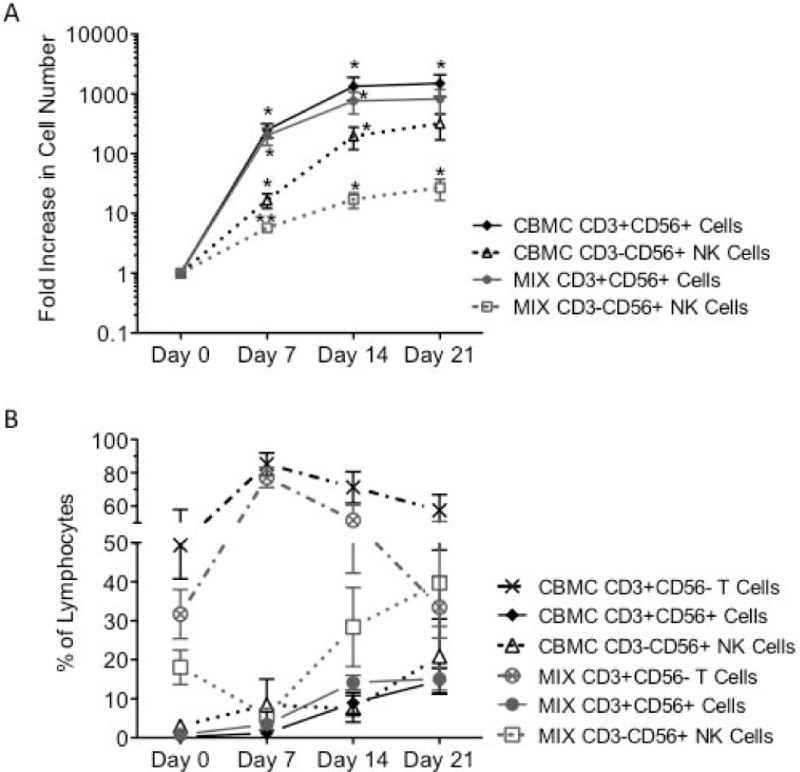
Freshly isolated CBMC or CB CD56+ enriched (MIX) cells were cultured for 21 days with IL-2, IL-15 and 0KT3. NK (- - -; CBMC Δ/MIX □) and CD3+CD56+ cell (–; CBMC ◆/MIX •) proliferation was then assessed for phenotypic expression of CD3+/−CD14−CD19−CD20−CD45+CD56+ by flow cytometry (A, N=10). The frequency of CD3+CD56− T cells (-- - --; CBMC ×/MIX ⊗) CD3+CD56+ cells (–; CBMC ◆/MIX •) and NK cells (- - -; CBMC Δ/MIX □) within the lymphocyte population was also assessed during culture (B, N=8). Depicted are means ± SEM. (*p<0.05, **p<0.01)
Enriched CB CD56+ Cell Samples are More Cytotoxic to K562 After Culture
After establishing culture conditions that significantly expand NK cells, we next examined their function by assaying cytotoxic function toward K562, a CML and NK cell-sensitive target cell line. Cytolytic potential of samples were assessed at day of isolation, as well as days 7, 14, 21 and 28, by co-culturing samples (effector) with K652 (target) at a variety of effector: target ratios, including 40:1, 20:1, 10:1, 5:1 and 1:1 (Supplemental Figure 1 and Figure 2). Both CBMC and CB MIX samples were not cytotoxic on the day of isolation. However, CB MIX samples (white bars) were significantly more cytotoxic at days 7–21 when compared to day of isolation, while CMBC samples (gray bar) were only significantly cytotoxic after 14–21 days of culture (Figure 2). Furthermore, the CB MIX samples were significantly more cytotoxic than the CBMC samples throughout culture. After 21 days of culture, cytotoxicity decreased between days 21–28 (Supplemental Figure 1), which confirmed that optimal culturing time period is up to 21 days.
Figure 2. Enriched CB CD56+ Cell Samples are More Cytotoxic to K562 After Culture.
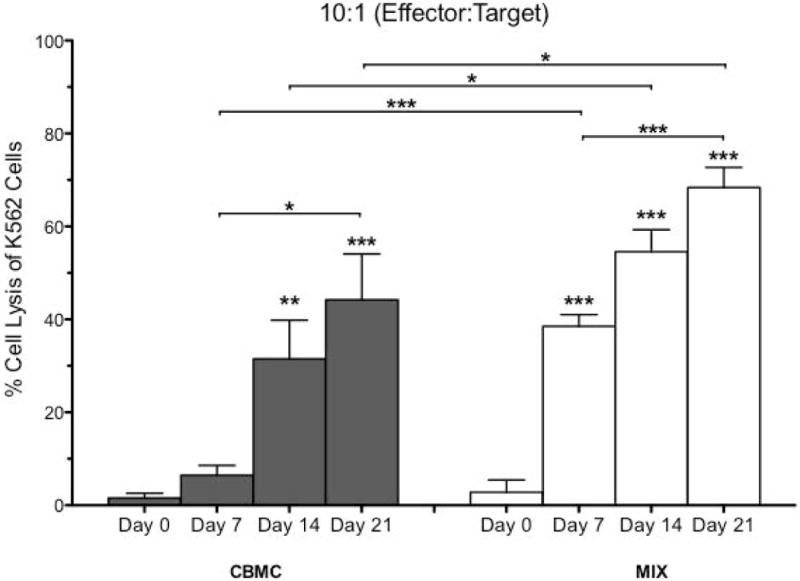
CB samples were assessed for cytotoxic function towards K562, a CML cell line sensitive to NK cells using a BADTA cytotoxicity assay. Samples were co-cultured for 2 hours with K562 at effector: target ratios of 10:1. Samples were performed in triplicate. Depicted are means ± SEM (N=8). Significance was assessed by comparing cultured samples to freshly isolated, day 0 samples, or between groups as indicated. (*p<0.05, **p<0.01, ***p<0.001)
CB CD56+ Cells Expressing Activating Receptors Increase During Culture
Cytotoxicity of NK cells is influenced by the regulation of various activating and inhibitory receptors and ligands. Thus we performed an extensive analysis by flow cytometry to determine receptor and ligand expression during culture. NK cell receptors, including activating, co-activating, inhibitory and adhesion receptors, as well as other receptors responsible for cytotoxicity were evaluated in both CB and PB NK and CD3+CD56+ cell populations during culture.
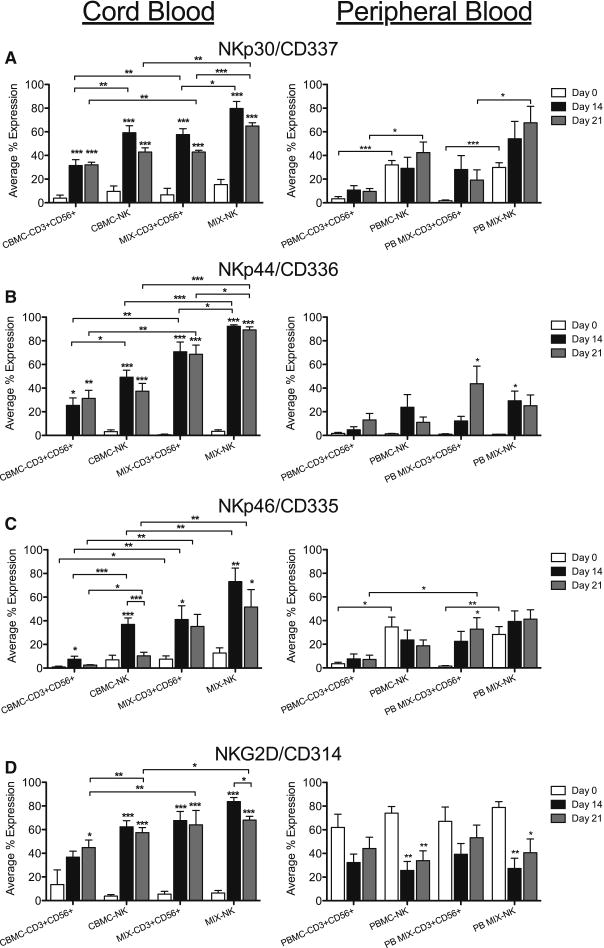
We first examined activating receptor expression, on the day of isolation and days 14 and 21 in culture for CB and PB. The percent of NK and CD3+CD56+ cells expressing NKp30/CD337, NKp44/CD336 and NKp46/CD335 (white bars) was very low for freshly isolated CB samples (Figures 3A–C). However, there was a significant increase in the percent of NK and CD3+CD56+ cells in all CB samples after 14–21 days of culture (black, gray), that expressed natural cytotoxicity receptors (NCRs), NKp30/CD337 and NKp44/CD336 and NKG2D/CD314 (Figures 3A, B, D, left panels) while NKp46/CD335 (Figure 3C) was expressed in significantly more CB NK and CD3+CD56+ cells at day 14 (black). We also found that the number of NK and CD3+CD56+ cells expressing these receptors was significantly higher in CB MIX samples compared to CBMC cultures and, the percent of NK cells expressing activating receptors was significantly more than CD3+CD56+ cells. Previously, Rujkijyanont et al. showed that ex vivo expansion of PB NK and CD3+CD56+ cells resulted in increased expression of activating receptors but not inhibitory KIRs [24]. Here, we report a similar increase in expression of activating receptors during culture in CB samples, with a greater percent increase in CB compared to PB samples during culture.
Figure 3. CB CD56 Cells Expressing Activating Receptors Increase During Culture.
NK and CD3+CD56+ cells from CB and PB were gated on CD3+/−CD14−CD45+CD56+ expression then evaluated for expression of activating receptors NK/CD337 (A), NKp44/CD336 (B), NKp46/CD335 (C) and NKG2D/CD314 (D) on days 0, 14 and 21. Depicted are means ± SEM (CBMC, N=8; MIX/PBMC/PB MIX, N=4). Significance was assessed by comparing cultured samples to freshly isolated, day 0 samples or between groups as indicated. (*p<0.05, **p<0.01, ***p<0.001)
Activating Co-Receptor and Adhesion Receptor Expression in CD56+ Cells During Culture
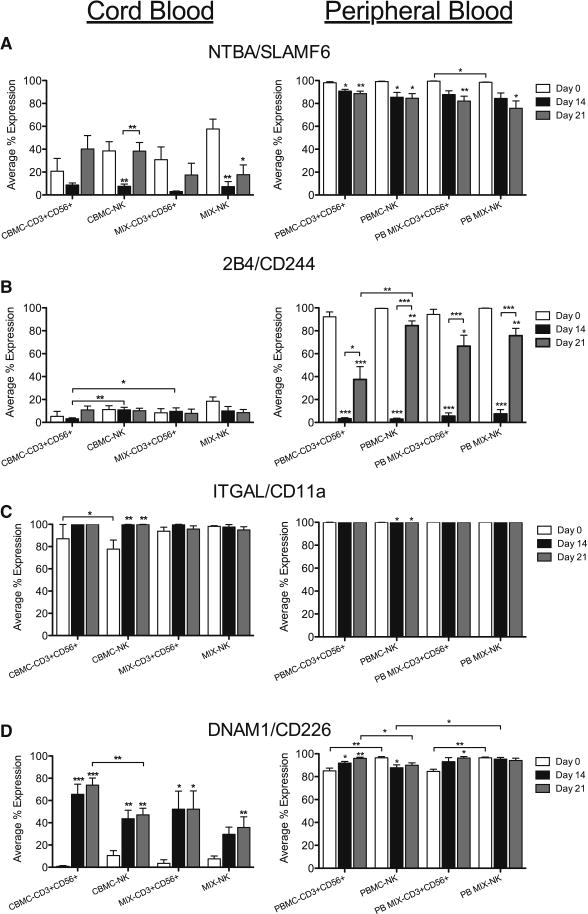
Concurrently, the expression of the activating co-receptors NTBA/SLAMF6 and 2B4/CD244 were also explored in CB and PB. Unlike the activating receptors, NTBA/SLAMF6 was expressed in NK and CD3+CD56+ cells from freshly isolated CB and PB, however it was significantly lower in CB than PB (Figure 4A). The percent of cells expressing NTBA/SLAMF6 decreased between day 0 and 14 in culture, but increased again between days 14–21 in all CB samples, while the percent of PB CD56+ cells expressing NTBA/SLAMF6 significantly declined during culture (Figure 4A). Additionally, 2B4/CD244 expression did not significantly change during culture for CB samples, while the majority of PB CD56+ cells expressed 2B4/CD244 on day 0, which then significantly declined during culture (Figure 4B).
Figure 4. Activating Co-Receptor and Adhesion Receptor Expression in CD56+ Cells During Culture.
NK and CD3+CD56+ cells from CB and PB were gated on CD3+/−CD14−CD45+CD56+ expression then evaluated for expression of activating co-receptors, NTBA/SLAMF6 (A) and 2B4/CD244 (B), and adhesion molecules, ITGAL/CD11a (C) and DNAM1/CD226 (D), on days 0, 14 and 21. Depicted are means ± SEM (CBMC, N=8; MIX/PBMC/PB MIX, N=4). Significance was assessed by comparing cultured samples to freshly isolated, day 0 samples or between groups as indicated. (*p<0.05, **p<0.01, ***p<0.001)
As adhesion receptors are important molecules that contribute to lytic synapse formation, and subsequent cytolytic function, we examined both ITGAL/CD11a and DNAM1/CD226 expression in NK cell subsets. In both CBMC and CB MIX samples, the majority of NK and CD3+CD56+ cells expressed ITGAL/CD11a (Figure 4C) on the day of isolation (white) and by days 14–21 (black, gray) in culture, majority of all CB NK and CD3+CD56+ cells expressed this adhesion molecule. In contrast, freshly isolated CB samples had very little expression of DNAM1/CD226 in either NK or CD3+CD56+ cells compared to PB (Figure 4D). However, the percent of CD56+ cells that expressed DNAM1/CD226 on days 14 and 21 was significantly increased compared to day 0 CB samples, whereas the majority of PB samples expressed DNAM1/CD226 throughout culture. Furthermore, more CD3+CD56+ cells expressed DNAM1/CD226 than NK cells in the CBMC samples.
CD56+ Cells Express Inhibitory Receptors During Culture
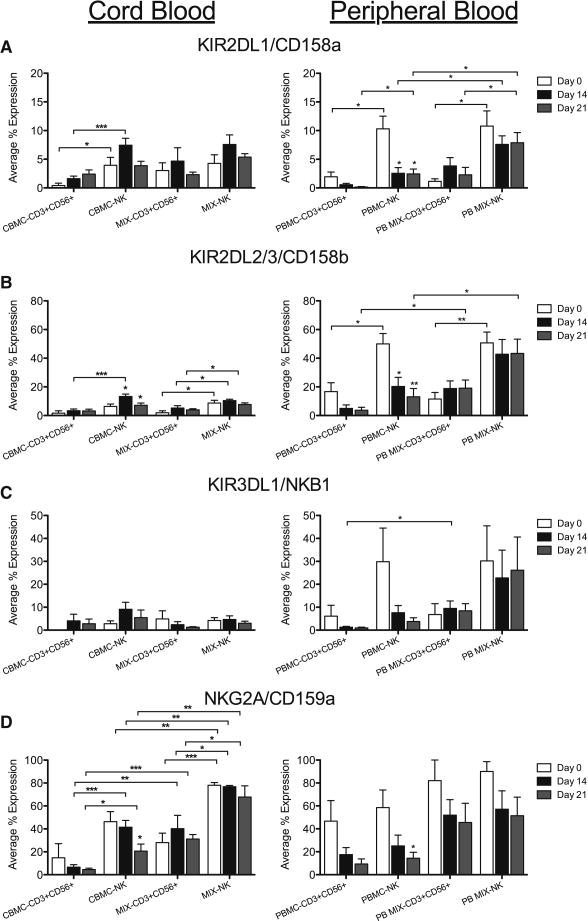
Cytotoxic function is due to a combination of activating and inhibitory receptor signaling. Therefore we assessed KIR inhibitory receptors on the CD56+ subsets of our samples. The percent of CD56+ cells that expressed KIR2DL/1/CD158a, KIR2DL2/3/CD158b and KIR3DL1/NKB1 was low on day 0 for all CB NK cells and both CB and PB CD3+CD56+ cells, while the percent of PB NK expressing these KIRs was much higher (Figures 5A–C). Unlike the activating receptors, cultured CB samples only showed a slight, but insignificant, increase in the percent of NK and CD3+CD56+ cells that express these inhibitory receptors. In both CBMC and CB MIX samples, there was a higher percentage of NK cells that expressed KIR2DL2/3/CD158b than CD3+CD56+ cells (Figure 5B). In contrast, the percent of CD56+ cells that express NKG2A/CD159a was much higher than the latter inhibitory KIR for both CB and PB samples. While there was no statistical difference in the percent of CB cells that expressed NKG2A/CD159a on the day of isolation or through culture, this inhibitory receptor was expressed in significantly more NK cells than CD3+CD56+ cells, for both CBMC and CB MIX samples. In addition, the CB MIX samples had a higher percent of CD56+ cells that expressed NKG2A/CD159a than the CBMC samples.
Figure 5. CD56+ Cells Express Inhibitory Receptors During Culture.
NK and CD3+CD56+ cells from CB and PB were gated on CD3+/−CD14−CD45+CD56+ expression then evaluated for expression of inhibitory KIR, KIR2DL1/CD158a (A), KIR2DL2/3/CD158b (B) and KIR3DL1/NKB1 (C) and NKG2A/CD159a (D), on days 0, 14 and 21. Depicted are means ± SEM (CBMC, N=8; MIX/PBMC/PB MIX, N=4). Significance was assessed by comparing cultured samples to freshly isolated, day 0 samples or between groups as indicated. (*p<0.05, **p<0.01, ***p<0.001)
Expression of FasR, FasL and TRAIL on CD56+ Cells During Culture
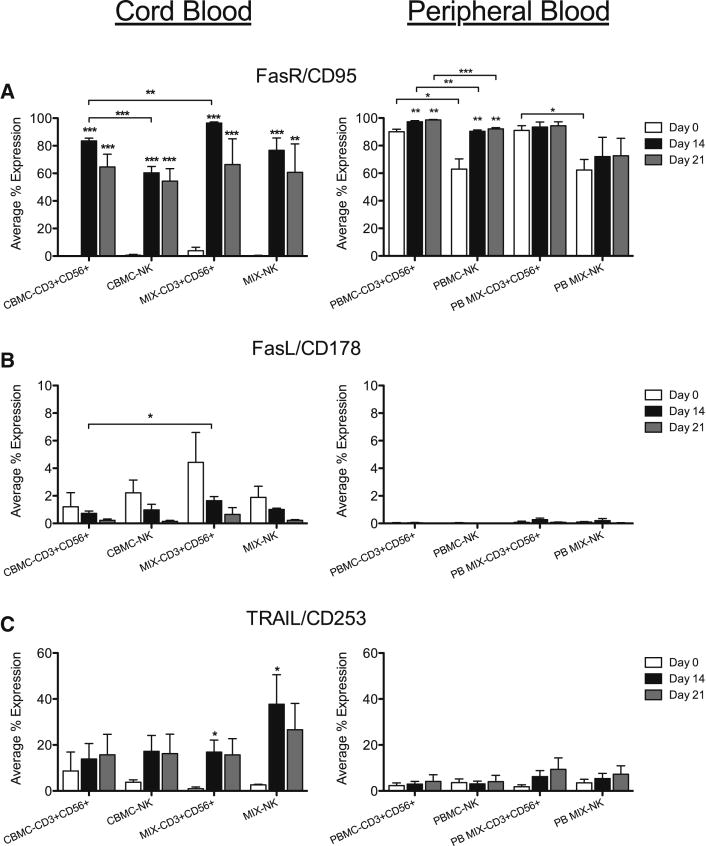
Fas Receptor/CD95 was expressed in the majority of NK and CD3+CD56+ cells in freshly isolated PB samples, while only a very small percentage of CB CD56+ cells expressed FasR/CD95 at day 0. However, significantly more CB samples expressed Fas Receptor after days 14 and 21 in culture compared to day 0 (Figure 6A). FasL/CD178, which may contribute to target cell apoptosis, was expressed in only a few percent of CB and PB cells, and did not significantly change during culture (Figure 6B). TRAIL/CD253, another apoptosis-inducing ligand, was expressed in a small subset of NK and CD3+CD56+ cells in CB and PB (Figure 6C); however, only CB MIX samples had a significant increase in the percent of cells expressing this ligand at day 14 (black).
Figure 6. Expression of FasR, FasL and TRAIL on CD56+ Cells During Culture.
NK and CD3+CD56+ cells from CB and PB were gated on CD3+/−CD14−CD45+CD56+ expression then evaluated for expression of additional receptors, FasR/CD95 (A), FasL/CD178 (B) and TRAIL/CD253 (C), on days 0, 14 and 21. Depicted are means ± SEM (CBMC, N=8; MIX/PBMC/PB MIX, N=4). Significance was assessed by comparing cultured samples to freshly isolated, day 0 samples or between groups as indicated. (*p<0.05, **p<0.01, ***p<0.001)
Ex Vivo Cultured CB NK Cells are More Cytotoxic than CD3+CD56+ Cells Toward Leukemia/Lymphoma Cell Lines
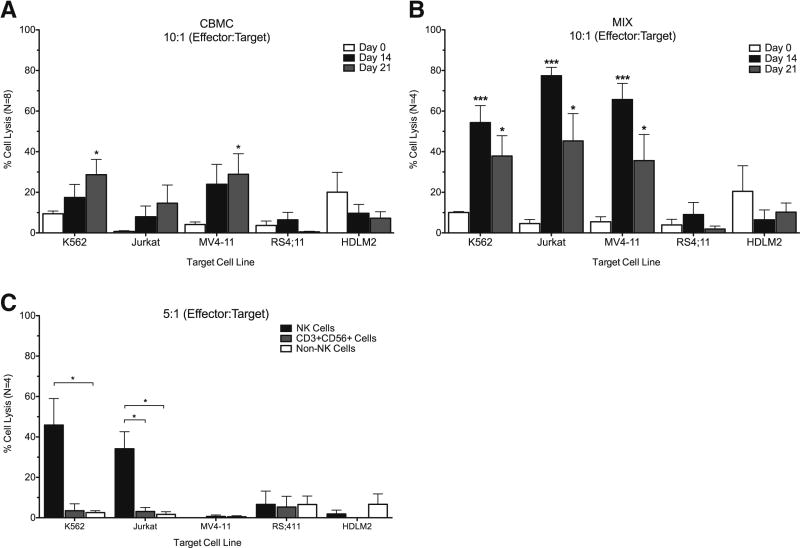
After identifying different receptors that could contribute to the increase in cytotoxic function against the NK cell sensitive, K562 line, we then examined whether these cultured samples also had the ability to lyse a variety of different leukemia and lymphoma cell lines. Cytolytic potential of samples were assessed at day of isolation, as well as at days 14 and 21 by co-culturing samples (effector) with K652, Jurkat (NK cell sensitive), MV4-11, RS4;11 and HDLM2 (NK cell resistant) cell lines. Samples were co-cultured at a ratio of 10:1 which was previously determined in Figure 2 as a moderate ratio which would identify statistical significance while conserving sample. Cultured CBMC samples had a significant increase in cytotoxic function against K562, as previously described, but also MV4-11, NK resistant cells, after 21 days of culture (Figure 7A). In contrast, when we assayed the cytolytic potential of the CB MIX samples which have an enriched CD56+ population, there was a drastic increase in cytotoxic function against all K562, Jurkat and MV4-11 target cells at both days 14 and 21 (Figure 7B, black and gray). We were not able to attain cytotoxic function against RS4;11 or HDLM2, which are known to be NK cell-resistant.
Figure 7. Ex Vivo Cultured CB NK Cells are More Cytotoxic than CD3+CD56+ Cells Toward Leukemia/Lymphoma Cell Lines.
Cytotoxicity of samples was assessed at day of isolation as well as at days 14 and 21 using a BADTA assay. Several cancer cell lines were used as target cells, including K562 (CML) and Jurkat (T-ALL) which are considered NK cell sensitive cell lines, and the MV4-11 (MLL), RS4;11 (MLL/ALL) and HDLM2 (Hodgkin’s lymphoma) cell lines which are considered NK cell resistant. CBMC (A) or CB MIX (B) samples were co-cultured for 2 hours at an effector: target ratio of 10:1. Day 14 CBMC samples were also sorted into NK (CD3−CD14−CD56+) and CD3+CD56+ (CD3+CD14−CD56+) or non-NK (CD56−) cell groups before co-culturing at a ratio of 5:1 with the target cells (C). Samples were performed in triplicate. Depicted are means ± SEM. Significance was assessed by comparing cultured samples to freshly isolated, day 0 samples or between groups as indicated. (*p<0.05, ***p<0.001)
As there was a significant increase in the cytotoxicity of samples enriched with C56+ cells, CB NK and CD3+CD56+ cells were then sorted and assessed for cytotoxicity. CB samples were cultured for 14 days, and then NK, CD3+CD56+ and non-NK cells were sorted and co-cultured with BADTA-labeled target cell lines at a ratio of 5:1. A lower ratio was used in order to test the NK and CD3+CD56+ cells against all target cell lines. Results of this assay demonstrated that NK cells and not CD3+CD56+ cells were responsible for cytotoxic function, as they were significantly more cytotoxic than either the CD3+CD56+ cells or non-NK cells against K562 and Jurkat cells (Figure 7C).
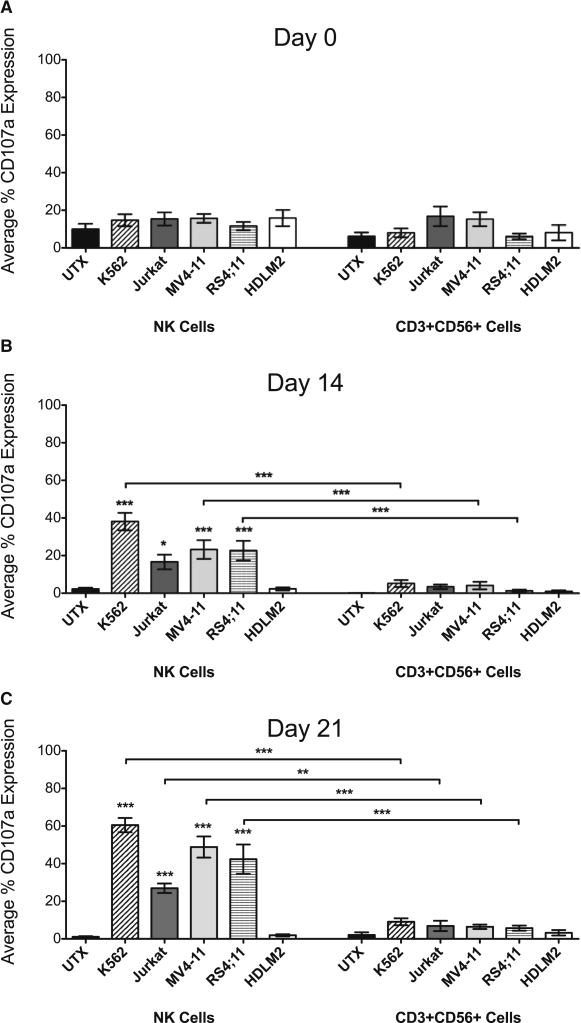
Expression of Surface CD107a Following Target Cell Co-Culture
CD107a is a marker for degranulation when expressed on the surface of NK and T cell subsets, and is an alternative indicator of cytotoxic function. In order to further verify NK cell cytolytic potential in our samples, we also examined CD107a surface expression on CB NK and CD3+CD56+ cells following co-culture with the target cells. On day of isolation there was no difference in the number of cells expressing CD107a between untreated or co-cultured cells for either NK or CD3+CD56+ cells (Figure 8A). However, after culturing for 14 and 21 days, there were significantly more CB NK cells that expressed surface CD107a when co-cultured with K562 (slanted stripe), Jurkat (gray), MV4-11 (light gray) and RS4;11 (horizontal stripe) than untreated NK cells (black) (Figures 8B–C). In addition, NK cells had the highest percent expression of CD107a compared to CD3+CD56+ cells, which expressed significantly less CD107a following co-culture (Figure 8B–C). Only a small percent of CD3+CD56+ cells overall expressed CD107a, independent of either time in culture or co-culture with target cells. These CD107a results further confirm our sorted cytotoxicity assay that only CB NK cells had cytolytic potential.
Figure 8. Expression of Surface CD107a Following Target Cell Co-Culture.
Cytotoxicity of CB samples was assessed at day of isolation (A) as well as at days 14 (B) and 21 (C). CBMC samples were co-cultured 1:1 with K562, Jurkat, MV4-11, RS4;11 or HDLM2 cells, or cultured alone (UTX) for 2 hours. Samples were then analyzed by flow cytometry. CB NK and CD3+CD56+ cells were gated on CD3+/−CD14− CD45+CD56+ expression then evaluated for surface expression of CD107a. Depicted are means ± SEM (N=12). Significance was assessed by comparing co-cultured samples to untreated samples or between groups as indicated. (*p<0.05, **p<0.01, ***p<0.001)
Discussion
Here we report that NK cells can be successfully proliferated ex vivo from CB to become functional effector cells against leukemia. We employed the ex vivo expansion method first developed by Rujkijyanont et al. for PB-derived NK cells. We utilized IL-2, IL-15 and the monoclonal CD3 antibody, OKT3, into our culture media that led to a significant multi-log fold increase in NK cell proliferation after 21 days in culture for both CBMC and CB MIX samples[24]. We also showed a significant increase in CD3+CD56+ cell proliferation during this time in both sample types. Our culture conditions show that NK cells can be feasibly expanded from either CBMC cells or by enriching for CD56+, which would decrease material required for ex vivo expansion in clinical settings.
Upon initial investigation as to the cytotoxic function of the cultured samples, all samples were significantly cytotoxic towards K562 cells, at effector: target ratios of 40:1 down to 5:1. However, as previously described, freshly isolated CB samples did not exhibit cytotoxic function [7, 9, 10]. We also determined that the CB MIX samples containing an enriched CD56+ population were more cytotoxic than the CBMC samples, suggesting that the number of NK cells correlated with the cytotoxic potential of the sample. While the frequency of NK cells in the lymphocyte population also increased in both sample types during culture, they did not become the majority lymphocyte population. This result suggests that not only did the increase in CD56+ cells affect sample cytotoxicity, but also that the NK cell population was activated by the culture method.
As cytotoxicity for all the samples significantly increased throughout culture, we examined the NK cell receptor expression profiles of samples before and after culture. In contrast to the findings by Ayello et al., we observed a significant up regulation in the number of both CB NK and CD3+CD56+ cells that express the activating receptors NKp30/CD337, NKp44/CD336 and NKp46/CD335 and NKG2D/CD314 during culture [23]. Correspondingly to the activating receptors, more CB NK and CD3+CD56+ cells also expressed DNAM1/CD226 after culture, while the majority of these cells expressed ITGAL/CD11a. When surveying inhibitory KIR, we found that only a small number of CB NK and CD3+CD56+ cell express KIR2DL/1/CD158a, KIR2DL2/3/CD158b or KIR3DL1/NKB1 which did not dramatically change by culture. However, many more CB CD56+ cells expressed the inhibitory NKG2A/CD159a, yet its expression was unchanged during culture. These results also conflict with the Ayello et al. findings, which reported an increase of inhibitory receptors on CD3+CD56+ cells, and a decrease of these receptors on NK cells [23]. However, this discrepancy may be due not only to the cytokine cocktail used, but also the culture period, which was only 7 days versus our 14–21 day period. Yet, our findings show similar trends as the Rujkijyanont et al. study of PB-derived NK cells, which utilized the same culture methods [24]. This study found that CD56+ cells from PB also had an increase in activating receptor expression, but not inhibitory KIR expression following culture that resulted in an activating, cytotoxic phenotype [24]. CB NK cells, similar to PB NK cells, had a significant increase in activating receptors during culture. However, there were unique differences between the two NK sources. The activating co-receptors and adhesion receptor expression (NTBA/SLAMF6 and DNAM/CD226) were noticeably lower in CB compared to PB. Furthermore, the lower expression of these receptors in CB samples compared to PB samples after culture suggests that this may be a characteristic inherent to the NK cell source rather than due to the expansion method.
Our phenotypic profiles coincided with our K562 cytotoxicity results. When cytotoxic function was increased there was also a significant increase in adhesion molecules and activating receptor expression, and a relatively lower level of inhibitory KIR expression. These results are encouraging because it demonstrates that the cells being cultured are not hyporesponsive, which is often times found in NK cells that express high levels of activating receptors but lack inhibitory receptors [25–27]. We also examined other receptors that contribute to cell cytotoxicity, but found that neither NK nor CD3+CD56+ cells have high expression of either TRAIL/CD253 or the FasL/CD178, which could also mediate cytolytic function. This indicates that the cytotoxicity of either CB NK or CD3+CD56+ cells may be predominantly granzyme and perforin dependent.
After determining the receptor profiles of both cultured CB NK and CD3+CD56+ cells, we then evaluated the cytotoxic potential against a combination of leukemia and lymphoma cell lines that are either NK cell sensitive or resistant. At a 10:1 effector: target ratio, CBMC samples had moderate cytotoxicity toward K562 and Jurkat (NK cell sensitive) and MV4-11 (NK cell resistant) cell lines. In contrast, the CB MIX samples had significantly more cytotoxicity toward these three cell lines. Again we confirmed the indication that increased CD56+ cell numbers correlated with a greater cytotoxic potential. Also of note, our samples had the ability to lyse one of the NK cell resistant cell lines, MV4-11, which is MLL rearranged and FLT-3 positive, which is promising for both in vivo and clinical studies.
Up to this point, our cytotoxic function studies have only assayed the samples as a whole. We have demonstrated that both CB NK and CD3+CD56+ cell population have significantly proliferated in culture, and that both cell types express the necessary receptor profiles needed for cytolytic function. However, we wanted to identify if either subset or potentially both subsets were carrying out this cytotoxic response. Thus we sorted our samples into CB NK, CD3+CD56+ and non-NK (CD56−) groups and repeated our cytotoxicity studies with our 5 target cell lines. We also monitored degranulation using surface expression of CD107a on both CB NK and CD3+CD56+ cell subsets following co-culture with the target cells. CB NK cells had significantly more CD107a on their surface after co-culture and only NK cells were able to lyse our target cells. In contrast, CB CD3+CD56+ cells had lower CD107a expression, suggesting less degranulation, and failed to have a cytolytic response. These data confirm that only the CB NK cells have cytotoxic potential. These results also suggest that although cultured CB CD3+CD56+ cells have the receptors and adhesion molecules necessary to yield a cytolytic response, they are unable to do so.
The lack of CB CD3+CD56+ cell cytolytic potential may be due to several factors [28]. It was previously described that CB CD3+CD56+ cells are not as potent as CB NK cells [29]. Recent studies of NKT cells of either the T cell receptor (TCR) αβ or γδ lineage in CB have been reported but the function has not been well described [30]. If in fact the CD3+CD56+ cells are TCRγδ, they may not have robust cytotoxic potential, but other effector functions [31]. If the CD3+ CD56+ cells are TCRαβ, they may be non-CD1d restricted as the majority of these cells were found to be CD8+ (data not shown), or MHC class restricted or possess degenerate TCRαβ receptors. Future studies will examine the proliferation and characterization of these CB CD3+ CD56+ cell by performing a single-cell PCR assay to determine the type(s) of CD3+ CD56+ cells present in culture.
Currently, clinical trials are utilizing PB NK cells in combination with HCT with beneficial results [32, 33]. Our study has determined that CB NK cells can also be feasibly propagated ex vivo for potential use in a clinical setting and maintain cytotoxic function through 21 days of culture. We intend to expand upon these studies by investigating the function of these cells in an in vivo mouse model to determine if these cells facilitate engraftment and survival following a CBT and/or have a graft versus leukemia effect in a mouse leukemia model. As CBT numbers rise, the ability to decrease GvHD, delayed IR, infection and relapse is of critical importance. The use of CB NK cells in combination with CBT may be one method to decrease these complications.
Supplementary Material
Samples were assessed for cytotoxic function towards K562, a CML cell line sensitive to NK cells using a BADTA cytotoxicity assay. CB Samples were co-cultured for 2 hours with K562 at effector: target ratios of 40:1 down to 1:1. Samples were performed in triplicate. Depicted are means ± SEM (N=8). Significance was assessed by comparing cultured samples to freshly isolated, day 0 samples. (*p<0.05, **p<0.01, ***p<0.001)
Highlights.
CB CD56+ cells can be feasibly expanded ex vivo using IL-2, IL-15 and OKT3
CB NK & CD3+CD56+ cells have increased activating receptor expression after culture
CB NK & CD3+CD56+ cells have low inhibitory KIR receptor expression after culture
NK cells but not CD3+CD56+ cells gain cytotoxic function after culture
Acknowledgments
We would like to thank Pia Rujkijyanont for technical advice and Jim Houston for performing flow cytometry and sorting of samples.
This work was supported by grants from the National Institutes of Health, National Cancer Institute Cancer Center Support Grant, American Lebanese Syrian Associated Charities, St. Baldrick’s Foundation and Assisi Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: The authors have no financial interests to disclose.
References
- 1.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–60. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 2.Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–77. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merindol N, Charrier E, Duval M, Soudeyns H. Complementary and contrasting roles of NK cells and T cells in pediatric umbilical cord blood transplantation. Journal of Leukocyte Biology. 2011;90:49–60. doi: 10.1189/jlb.0111007. [DOI] [PubMed] [Google Scholar]
- 4.Danby R, Rocha V. Improving Engraftment and Immune Reconstitution in Umbilical Cord Blood Transplantation. Front Immunol. 2014;5:68. doi: 10.3389/fimmu.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clausen J, Wolf D, Petzer AL, Gunsilius E, Schumacher P, Kircher B, et al. Impact of natural killer cell dose and donor killer-cell immunoglobulin-like receptor (KIR) genotype on outcome following human leucocyte antigen-identical haematopoietic stem cell transplantation. Clinical and Experimental Immunology. 2007;148:520–8. doi: 10.1111/j.1365-2249.2007.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggeri L. Effectiveness of Donor Natural Killer Cell Alloreactivity in Mismatched Hematopoietic Transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 7.Dalle J-H, Menezes J, Wagner R, Blagdon M, Champagne J, Champagne MA, et al. Characterization of Cord Blood Natural Killer Cells: Implications for Transplantation and Neonatal Infections. Pediatric Research. 2005;57:649–55. doi: 10.1203/01.PDR.0000156501.55431.20. [DOI] [PubMed] [Google Scholar]
- 8.López MC, Palmer BE, Lawrence DA. Phenotypic differences between cord blood and adult peripheral blood. Cytometry Part B: Clinical Cytometry. 2009;76B:37–46. doi: 10.1002/cyto.b.20441. [DOI] [PubMed] [Google Scholar]
- 9.Xing D, Ramsay AG, Gribben JG, Decker WK, Burks JK, Munsell M, et al. Cord blood natural killer cells exhibit impaired lytic immunological synapse formation that is reversed with IL-2 exvivo expansion. J Immunother. 2010;33:684–96. doi: 10.1097/CJI.0b013e3181e475e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Xu H, Zheng X, Wei H, Sun R, Tian Z. High expression of NKG2A/CD94 and low expression of granzyme B are associated with reduced cord blood NK cell activity. Cell Mol Immunol. 2007;4:377–82. [PubMed] [Google Scholar]
- 11.Carlens SS, Gilljam MM, Chambers BJB, Aschan JJ, Guven HH, Ljunggren HGH, et al. A new method for in vitro expansion of cytotoxic human CD3−CD56+ natural killer cells. Human Immunology. 2001;62:1092–8. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 12.Luevano M, Daryouzeh M, Alnabhan R, Querol S, Khakoo S, Madrigal A, et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Human Immunology. 2012;73:248–57. doi: 10.1016/j.humimm.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Luevano M, Domogala A, Blundell M, Jackson N, Pedroza-Pacheco I, Derniame S, et al. Frozen cord blood hematopoietic stem cells differentiate into higher numbers of functional natural killer cells in vitro than mobilized hematopoietic stem cells or freshly isolated cord blood hematopoietic stem cells. PloS one. 2014;9:e87086. doi: 10.1371/journal.pone.0087086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayello J, van de Ven C, Fortino W, Wade-Harris C, Satwani P, Baxi L, et al. Characterization of Cord Blood Natural Killer and Lymphokine Activated Killer Lymphocytes Following Ex Vivo Cellular Engineering. Biology of Blood and Marrow Transplantation. 2006;12:608–22. doi: 10.1016/j.bbmt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Fujisaki H, Kakuda H, Imai C, Mullighan CG, Campana D. Replicative potential of human natural killer cells. British Journal of Haematology. 2009;145:606–13. doi: 10.1111/j.1365-2141.2009.07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer research. 2009;69:4010. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah N, Martin-Antonio B, Yang H, Ku S, Lee DA, Cooper LJ, et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PloS one. 2013;8:e76781. doi: 10.1371/journal.pone.0076781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willemze R, Rodrigues CA, Labopin M, Sanz G, Michel G, Socié G, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eissens DN, Spanholtz J, van der Meer A, van Cranenbroek B, Dolstra H, Kwekkeboom J, et al. Defining Early Human NK Cell Developmental Stages in Primary and Secondary Lymphoid Tissues. PloS one. 2012;7:e30930. doi: 10.1371/journal.pone.0030930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spanholtz J, Tordoir M, Eissens D, Preijers F, Van Der Meer A, Joosten I, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PloS one. 2010;5:e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–65. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayello J, van de Ven C, Cairo E, Hochberg J, Baxi L, Satwani P, et al. Characterization of natural killer and natural killer-like T cells derived from ex vivo expanded and activated cord blood mononuclear cells: implications for adoptive cellular immunotherapy. Exp Hematol. 2009;37:1216–29. doi: 10.1016/j.exphem.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Rujkijyanont P, Chan WK, Eldridge PW, Lockey T, Holladay M, Rooney B, et al. Ex vivo activation of CD56(+) immune cells that eradicate neuroblastoma. Cancer research. 2013;73:2608–18. doi: 10.1158/0008-5472.CAN-12-3322. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song Y-J, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 26.Della Chiesa M, Falco M, Podesta M, Locatelli F, Moretta L, Frassoni F, et al. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: a role for human cytomegalovirus? Blood. 2012;119:399–410. doi: 10.1182/blood-2011-08-372003. [DOI] [PubMed] [Google Scholar]
- 27.Bjorklund AT, Schaffer M, Fauriat C, Ringden O, Remberger M, Hammarstedt C, et al. NK cells expressing inhibitory KIR for non-self-ligands remain tolerant in HLA-matched sibling stem cell transplantation. Blood. 2010;115:2686–94. doi: 10.1182/blood-2009-07-229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan WK, Rujkijyanont P, Neale G, Yang J, Bari R, Das Gupta N, et al. Multiplex and genome-wide analyses reveal distinctive properties of KIR+ and CD56+ T cells in human blood. Journal of Immunology. 2013;191:1625–36. doi: 10.4049/jimmunol.1300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Q, Grzywacz B, Wang H, Kataria N, Cao Q, Wagner JE, et al. Umbilical cord blood T cells express multiple natural cytotoxicity receptors after IL-15 stimulation, but only NKp30 is functional. Journal of Immunology. 2008;181:4507–15. doi: 10.4049/jimmunol.181.7.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musha N, Yoshida Y, Sugahara S, Yamagiwa S, Koya T, Watanabe H, et al. Expansion of CD56+ NK T and gamma delta T cells from cord blood of human neonates. Clinical and Experimental Immunology. 1998;113:220–8. doi: 10.1046/j.1365-2249.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Ombrain MC, Hansen DS, Simpson KM, Schofield L. gammadelta-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-gamma response to Plasmodium falciparum malaria. Eur J Immunol. 2007;37:1864–73. doi: 10.1002/eji.200636889. [DOI] [PubMed] [Google Scholar]
- 32.Choi I, Yoon SR, Park SY, Kim HJ, S J, Jang YJ, Kang M, et al. Donor-Derived Natural Killer Cells Infused after Human Leukocyte AntigeneHaploidentical Hematopoietic Cell Transplantation: A Dose-Escalation Study. Biology of Blood and Marrow Transplantation. 2014;20:696–704. doi: 10.1016/j.bbmt.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 33.Yoon SR, Lee YS, Yang SH, Ahn KH, Lee JH, Kim DY, et al. Generation of donor natural killer cells from CD34(+) progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study. Bone Marrow Transplantation. 2010;45:1038–46. doi: 10.1038/bmt.2009.304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Samples were assessed for cytotoxic function towards K562, a CML cell line sensitive to NK cells using a BADTA cytotoxicity assay. CB Samples were co-cultured for 2 hours with K562 at effector: target ratios of 40:1 down to 1:1. Samples were performed in triplicate. Depicted are means ± SEM (N=8). Significance was assessed by comparing cultured samples to freshly isolated, day 0 samples. (*p<0.05, **p<0.01, ***p<0.001)