Abstract
Background
Hospital readmissions for heart failure (HF) contribute to increased morbidity and resource burden. Predictors of hospitalization and patterns of cardiovascular events over the lifetime of patients with HF have not been elucidated.
Methods and Results
We examined recurrent hospitalizations, cardiovascular events, and survival among newly discharged (April 1999–March 2001) patients with HF in the Enhanced Feedback For Effective Cardiac Treatment phase 1 study. During 10-year follow-up, we examined all new cardiovascular hospitalizations and selected predictors of readmission. Among 8543 patients (mean age, 77.4±10.5 years; 51.6% women) followed for 22 567 person-years, 60.7% had ischemic etiology, and 67.3% had HF with reduced ejection fraction (left ventricular ejection fraction ≤45% versus >45% [HF with preserved ejection fraction]). Overall, 10-year mortality was 98.8%, with 35 966 hospital readmissions occurring over the lifetime of the cohort. Adjusted hazards ratios (HRs) for first cardiovascular hospitalization were 1.36 for ischemic HF (95% CI, 1.28–1.44; P<0.001), 1.10 for HF with reduced ejection fraction (95% CI; 1.00–1.20; P=0.045), and 1.00 for men (95% CI, 0.94–1.06; P=0.979). On repeated-events time-to-event analysis, ischemic HF was a predictor of cardiovascular (HR, 1.24; 95% CI, 1.18–1.29), HF (HR, 1.20; 95% CI, 1.13–1.27), and coronary heart disease (HR, 2.01; 95% CI, 1.81–2.24) hospitalizations (all P<0.001). Of all recurrent HF hospitalizations, 26.8% occurred in the first and 39.8% in the last deciles of cohort survival duration. Similarly, 29.7% and 52.3% of all cardiovascular readmissions occurred in the first and last deciles of the cohort survival duration, respectively.
Conclusions
Among newly discharged patients with HF, cardiovascular events were clustered at early postdischarge and prefatal time periods, and were increased among those with ischemic etiology.
Keywords: heart failure, hospital readmission, cardiovascular diseases, myocardial ischemia, epidemiology, health services research, prognosis, population
There is increasing interest in the high rates of repeat hospital visits and readmissions for heart failure (HF).1 Repeat hospitalizations contribute significantly to the increased costs of health care for patients with HF,2,3 and the issue of readmissions has been identified as a priority problem for the healthcare system.4 As an ambulatory care-sensitive condition, hospital readmissions for HF have also been deemed indicators of reduced quality of care.5,6
Despite the importance of readmissions in the HF population, many challenges remain. First, the important predictors of readmission among patients with HF have not been determined.7,8 Second, from a methodological standpoint, most prior studies examined occurrence of the first readmission and did not account for all hospitalizations occurring over the lifetime of the patient after the initial HF hospital discharge.9 Although HF is commonly classified, based on underlying left ventricular (LV) systolic function, as HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF), it is unknown whether this classification is predictive of repeated hospitalizations and death occurring over the lifetime of the patient. Furthermore, it is unknown whether classification based on ischemic or nonischemic etiology is predictive of hospitalization and cardiovascular events over the lifetime of patients with HF.10
In this study, we examined a patient cohort discharged after being newly hospitalized for HF and followed them over their lifetime for all cardiac and noncardiac hospitalizations that occurred until death. These patients were originally enrolled in phase 1 of the Enhanced Feedback For Effective Cardiac Treatment (EFFECT) study and have been followed for up to 10 years. We examined patterns of hospitalization and recurrent cardiovascular events and the association of sex, presence of HFrEF versus HFpEF, and ischemic versus nonischemic etiology on hospitalizations in this population-based cohort.
Methods
Study Population
We evaluated patients with HF who were newly admitted to the hospital in Ontario, Canada, in phase 1 of the EFFECT study, which is described in detail elsewhere.11 Briefly, these patients were identified by examining the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) from April 1999 to March 2001 for those given a primary or most responsible diagnosis of HF as indicated by the International Classification of Diseases, Ninth Revision, code 428.x. In the EFFECT phase 1 study, primary chart records were abstracted by highly trained nurse-abstractors from 86 hospital corporations for clinical information, and only patients who met the Framingham criteria for HF12 were included. In this study, we examined mortality among all patients subsequent to the index EFFECT admission and readmissions among those who survived to index hospital discharge. Approval from the ethics review board was obtained from all participating institutions before the study.
Data Sources
Using the patients’ unique, encrypted health card number, we linked the EFFECT data to the hospitalization information contained in the CIHI-DAD and examined all subsequent hospitalizations using International Classification of Diseases, Ninth Revision, Clinical Modification, and International and Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Canada, codes. Cardiovascular hospitalizations included those for HF, coronary artery disease, and other cardiac disease as the primary or most responsible diagnosis. Other cardiac disease hospitalizations included admissions for arrhythmia; myocardial, pericardial, and endocardial diseases; cerebrovascular disease; hypertensive disease; pulmonary vascular disease; rheumatic heart disease; shock; syncope; sudden cardiac death; valvular heart disease; and other unspecified cardiac diseases. All other reasons for hospitalization were assigned as noncardiovascular. In the CIHI-DAD, each hospitalization has 1 primary diagnosis. However, multiple new conditions could be diagnosed during the course of a single hospitalization. Therefore, we also identified all secondary conditions in any of the 16 CIHI-DAD diagnosis fields that were new conditions contributing to the hospital stay. Together, these conditions (primary and new secondary diagnoses) were classified as hospitalization-based cardiovascular or noncardiac events. To exclude readmissions for day procedures, hospitalizations with a length of stay of ≤1 day were not included as an event.
Mortality was determined by linkages with the Registered Persons Database for vital statistics. All hospitalizations and invasive procedures were examined in each patient with HF until death occurred or until the date of last available follow-up.
Study Definitions
We defined the lifetime of the newly admitted patient with HF to begin on initial hospitalization for HF.11 HFrEF or HFpEF was defined by LV ejection fraction (LVEF) of ≤45% versus >45% based on previously defined thresholds.13 Ischemic HF was defined by prior coronary artery disease, unstable angina, myocardial infarction, percutaneous coronary intervention, or coronary artery bypass graft surgery. Morbid outcomes were defined as either hospitalizations (ie, each admission contributes 1 primary diagnosis) or hospitalization-based events (ie, 1 admission could generate multiple events if new secondary diagnoses are coded). Secondary diagnoses of chronic, preexisting conditions were not included; thus, analysis of hospitalization-based events accounted for multiple de novo conditions that could arise during a single hospital admission.
Statistical Analysis
Continuous variables are reported as mean±SD and compared using Student t test. Categorical variables were compared between groups using the χ2 statistic. A descriptive analysis of hospitalizations was initially performed by counting the number of hospitalizations occurring during follow-up. In these analyses, each hospitalization was categorized as due to HF, coronary heart disease, other cardiac, or noncardiovascular based on the primary diagnosis in the CIHI-DAD. The effect of clinical categories on time to first hospitalization was assessed using adjusted Cox proportional hazards models, accounting for patients clustered within hospitals.14 These models were adjusted for sex, ischemic versus nonischemic etiology, and HFrEF versus HFpEF and censored at death or on last follow-up date of March 31, 2009.15 Patients without documented LVEF were included in the regression analyses as a separate category (eg, unknown LVEF). We also adjusted for the following covariates based on a previously validated model16: age, serum sodium level, hemoglobin level, cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, hepatic cirrhosis, dementia, cancer, systolic blood pressure, respiratory rate, and blood urea nitrogen. We constructed survival curves adjusted for these covariates using the corrected group prognosis method.17 The proportional hazards assumption was tested for Cox regression models using a Kolmogorov-Smirnov supremum-type test on 1000 resamplings of the cumulative sums of martingale residuals.18
Repeat hospitalization outcomes were examined using Cox regression analysis for recurrent events, accounting for the possibility of multiple readmissions occurring over time in the same patient.14 To further explore the patterns of hospitalizations over the lifetime of the HF cohort, we divided the lifetime (after the index HF discharge) of each patient into deciles and counted the number of hospitalizations occurring in each time fraction of the survival duration. Similarly, the lifetime of each patient was divided into tertiles (early, middle, late), and hospitalizations occurring in each of the 3 periods were examined for all new hospital-based events. In sensitivity analyses, we treated hospitalizations and mortality as competing events and jointly modeled the cause-specific hazard functions of these 2 outcomes.19 P<0.05 was considered statistically significant. Analyses were performed using SAS version 9.2 (SAS Institute) stati stical software.
Results
Study Cohort
We examined 8543 patients with HF (mean age, 77.4±10.5 years) of whom 60.7% had ischemic HF, 67.3% had HFrEF, and 51.6% were women. The baseline characteristics of the study cohort are shown in Table 1. Patients with ischemic HF were younger than those with nonischemic HF and had significantly higher rates of hypertension, hyperlipidemia, diabetes, and smoking and greater comorbidity burden. Among the subcohort with LV functional assessment performed (42.5%), those with HFpEF were older and had higher prevalence of hypertension and atrial fibrillation than those with HFrEF. Although women were older and had more hypertension, they also had lower rates of chronic obstructive pulmonary disease than men.
Table 1.
Baseline Characteristics
| Characteristic | Ischemic | Nonischemic | P Value |
|---|---|---|---|
| No. patients | 5185 | 3358 | |
| Age, y | 77.0±9.9 | 77.9±11.3 | <0.001 |
| Hypertension | 2601 (50.2) | 1475 (43.9) | <0.001 |
| Hyperlipidemia | 1260 (24.3) | 257 (7.7) | <0.001 |
| Diabetes | 2020 (39.0) | 981 (29.2) | <0.001 |
| Smoking history | 1991 (38.4) | 1096 (32.6) | <0.001 |
| Cerebrovascular disease | 1012 (19.5) | 485 (14.4) | <0.001 |
| Peripheral vascular disease | 885 (17.1) | 310 (9.2) | <0.001 |
| Dialysis | 75 (1.4) | 48 (1.4) | 0.948 |
| Chronic obstructive lung disease | 978 (18.9) | 575 (17.1) | 0.042 |
| Atrial fibrillation | 1633 (31.5) | 955 (28.4) | 0.003 |
| HFrEF | HFpEF | ||
|---|---|---|---|
| No. patients | 2448 | 1190 | |
| Age, y | 74.6±10.4 | 77.9±9.8 | <0.001 |
| Hypertension | 1187 (48.5) | 667 (56.1) | <0.001 |
| Hyperlipidemia | 583 (23.8) | 178 (15.0) | <0.001 |
| Diabetes | 926 (37.8) | 384 (32.3) | 0.001 |
| Smoking history | 1085 (44.3) | 433 (36.4) | <0.001 |
| Cerebrovascular disease | 398 (16.3) | 207 (17.4) | 0.388 |
| Peripheral vascular disease | 405 (16.5) | 140 (11.8) | <0.001 |
| Dialysis | 23 (0.9) | 13 (1.1) | 0.622 |
| Chronic obstructive lung disease | 381 (15.6) | 225 (18.9) | 0.001 |
| Atrial fibrillation | 690 (28.2) | 448 (37.6) | <0.001 |
| Men | Women | ||
|---|---|---|---|
| No. patients | 4133 | 4410 | |
| Age, y | 75.2±10.6 | 79.4±9.9 | <0.001 |
| Hypertension | 1811 (43.8) | 2265 (51.4) | <0.001 |
| Hyperlipidemia | 843 (20.4) | 674 (15.3) | <0.001 |
| Diabetes | 1554 (37.6) | 1447 (32.8) | <0.001 |
| Smoking history | 2012 (48.7) | 1075 (24.4) | <0.001 |
| Cerebrovascular disease | 714 (17.3) | 783 (17.8) | 0.56 |
| Peripheral vascular disease | 737 (17.8) | 458 (10.4) | <0.001 |
| Dialysis | 70 (1.7) | 53 (1.2) | 0.056 |
| Chronic obstructive lung disease | 859 (20.8) | 694 (15.7) | <0.001 |
| Atrial fibrillation | 1238 (30.0) | 1350 (30.6) | 0.508 |
Data are presented as mean±SD or n (%). HFrEF indicates heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction.
Time to Death
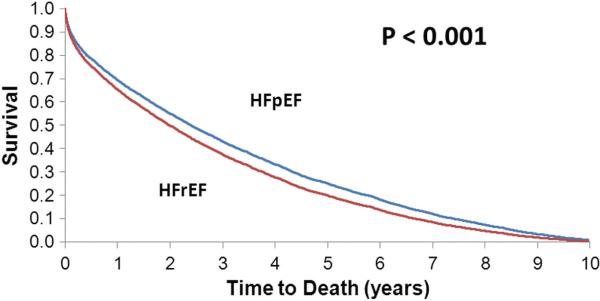
We examined 22 567 person-years of follow-up. Overall, 10-year mortality was 98.8% with a median survival of 1.75 years. Benchmark survival rates of 25%, 10%, and 5% occurred after 4.25, 6.94, and 8.34 years of follow-up, respectively. The adjusted hazard ratio [HR] for mortality was 1.17 (95% CI, 1.08–1.26; P<0.001) for HFrEF compared with HFpEF (Figure 1). The adjusted HR for ischemic versus nonischemic HF was 1.07 (95% CI, 1.02–1.13; P=0.004). Compared with patients with LV function testing performed, those with unknown LVEF had shorter median survival (1.52 versus 2.16 years, P<0.001) and HF hospitalization-free survival (2.76 versus 3.19 years, P=0.01). There was an increased risk of death among men (adjusted HR, 1.06; 95% CI, 1.01–1.11; P=0.019) compared with women.
Figure 1.
Adjusted survival curve by left ventricular function: HFrEF versus HFpEF. HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Analysis of Time to First Hospitalization
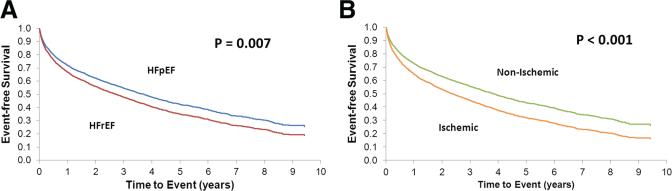
HFrEF was associated with an increased age- and sex-adjusted risk of first hospitalization for HF and coronary heart disease (Table 2). Ischemic HF was associated with the largest risks of hospitalization for HF, coronary heart disease, and cardiovascular disease (Table 2). HF hospitalization-free survival curves are shown in Figure 2A for HFrEF versus HFpEF and Figure 2B for ischemic versus nonischemic HF. For patients with HFrEF adjusted HRs for first HF hospitalization were 1.16 (95% CI, 1.04–1.30; P=0.008) and 1.47 (95% CI, 1.26–1.72; P<0.001) for first coronary heart disease hospitalization (Table 2). There were no differences in adjusted rehospitalization outcomes among men versus women (Table 2). In sensitivity analyses, we accounted for competing risks in which the effects of sex, ischemic versus nonischemic status, and HFrEF versus HFpEF on the cause-specific hazard function for the readmission outcomes were estimated. Results similar to those aforementioned were observed.
Table 2.
Association Between Sex, Ischemic Etiology, and HFrEF versus HFpEF on Time to First Readmission
| Men vs Women |
Ischemic vs Nonischemic |
HFrEF vs HFpEF |
||||
|---|---|---|---|---|---|---|
| Unadjusted Analysis | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Hospitalizations | ||||||
| Cardiovascular | 1.07 (1.02–1.13) | 0.008 | 1.39 (1.33–1.46) | <0.001 | 1.13 (1.03–1.24) | 0.011 |
| HF | 1.08 (1.01–1.15) | 0.016 | 1.33 (1.24–1.42) | <0.001 | 1.20 (1.08–1.34) | 0.001 |
| CHD | 1.12 (1.03–1.23) | 0.008 | 2.34 (2.13–2.58) | <0.001 | 1.47 (1.28–1.68) | <0.001 |
| Other cardiac disease | 1.13 (1.05–1.21) | <0.001 | 1.29 (1.20–1.39) | <0.001 | 1.12 (0.99–1.27) | 0.078 |
| Noncardiovascular | 0.98 (0.93–1.02) | 0.322 | 1.07 (1.02–1.13) | 0.004 | 0.98 (0.91–1.05) | 0.488 |
| Death | 1.03 (0.99–1.08) | 0.117 | 1.12 (1.07–1.17) | <0.001 | 1.15 (1.07–1.23) | <0.001 |
| Adjusted Analysis | HR*† (95% CI) | P Value | HR*‡ (95% CI) | P Value | HR*§ (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Hospitalizations | ||||||
| Cardiovascular | 1.00 (0.94–1.06) | 0.979 | 1.36 (1.29–1.43) | <0.001 | 1.10 (1.00–1.20) | 0.057 |
| HF | 1.02 (0.95–1.09) | 0.627 | 1.29 (1.20–1.39) | <0.001 | 1.16 (1.04–1.30) | 0.008 |
| CHD | 1.03 (0.94–1.14) | 0.520 | 2.30 (2.07–2.57) | <0.001 | 1.47 (1.26–1.72) | <0.001 |
| Other cardiac disease | 1.04 (0.96–1.12) | 0.355 | 1.25 (1.16–1.35) | <0.001 | 1.06 (0.93–1.21) | 0.368 |
| Noncardiovascular | 0.98 (0.92–1.03) | 0.387 | 1.01 (0.96–1.07) | 0.667 | 0.97 (0.89–1.05) | 0.420 |
| Death | 1.06 (1.01–1.11) | 0.021 | 1.07 (1.02–1.13) | 0.006 | 1.17 (1.08–1.26) | <0.001 |
HFrEF indicates heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazards ratio; HF, heart failure; CHD, coronary heart disease; EFFECT, Enhanced Feedback For Effective Cardiac Treatment study.
EFFECT-HF risk score covariates: age, serum sodium level, hemoglobin level, cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, hepatic cirrhosis, dementia, cancer, systolic blood pressure, respiratory rate, and blood urea nitrogen.
HRs for men vs women (referent) adjusted for ischemic vs nonischemic, HFrEF vs HFpEF, hospital-level clustering, and EFFECT-HF risk score covariates.
HRs for ischemic vs nonischemic HF (referent) adjusted for HFrEF vs HFpEF, men vs women, hospital-level clustering, and EFFECT-HF risk score covariates.
HRs for HFrEF vs HFpEF (referent) adjusted for ischemic vs nonischemic, men vs women, hospital-level clustering, and EFFECT-HF risk score covariates.
Figure 2.
A, Adjusted heart failure hospitalization-free survival: HFrEF versus HFpEF. B, Adjusted heart failure hospitalization-free survival: ischemic versus nonischemic heart failure. HFpEF indicates heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Analyses of Repeat Hospitalizations
The rates of repeat hospitalizations per 100 person-years of follow-up are shown in Table 3. Accounting for multiple possible hospitalizations per patient, those with ischemic HF were far more likely than those with nonischemic HF to experience repeat cardiovascular, HF, and coronary heart disease hospitalizations. There were smaller differences in repeat hospitalizations when compared by HFrEF versus HFpEF and by sex.
Table 3.
Hospital Readmission Rate per 100 Person-Years of Follow-up
| Hospitalization Type | Ischemic | Nonischemic | HFrEF | HFpEF | Men | Women |
|---|---|---|---|---|---|---|
| Cardiovascular | 96.7 | 62.1 | 83.8 | 72.9 | 84.4 | 80.9 |
| HF | 49.9 | 33.3 | 42.9 | 34.9 | 45.0 | 41.3 |
| CHD | 24.5 | 8.7 | 19.4 | 14.7 | 18.1 | 17.4 |
| Other cardiac disease | 29.9 | 23.0 | 28.9 | 28.1 | 27.8 | 26.5 |
| Noncardiovascular | 154.0 | 137.0 | 137.3 | 132.2 | 149.9 | 146.0 |
For all comparisons above, age and sex-adjusted P<0.001 for ischemic vs nonischemic and HFrEF vs HFpEF and age-adjusted P<0.001 for men vs women. HFrEF indicates heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HF, heart failure; CHD, coronary heart disease.
Repeated-events Cox regression analysis (Table 4) demonstrated attenuated effect sizes compared with the analysis of time to first events shown in Table 2. Despite this, ischemic HF etiology remained predictive of cardiovascular readmissions over the post-HF onset lifetime, with a 24% increased risk after multivariable adjustment. Ischemic etiology also increased the risk of HF hospitalizations by 20% and doubled the risk of coronary heart disease hospitalizations after multivariable adjustment.
Table 4.
Repeated-Events Cox Regression Analysis
| Cardiovascular vs Noncardiovascular Hospitalizations |
||||
|---|---|---|---|---|
| Cardiovascular |
Noncardiovascular |
|||
| Predictor Variable | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Men | 1.00 (0.96–1.04) | 0.962 | 1.04 (1.00–1.09) | 0.080 |
| Ischemic (vs nonischemic) | 1.24 (1.18–1.29) | <0.001 | 1.01 (0.97–1.05) | 0.707 |
| HFrEF (vs HFpEF) | 1.03 (0.96–1.10) | 0.418 | 0.91 (0.85–0.98) | 0.007 |
| Type of Cardiovascular Hospitalization |
||||
|---|---|---|---|---|
| Heart Failure |
Coronary Heart Disease |
|||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Men | 1.00 (0.94–1.05) | 0.892 | 1.00 (0.93–1.09) | 0.920 |
| Ischemic (vs nonischemic) | 1.20 (1.13–1.27) | <0.001 | 2.01 (1.81–2.24) | <0.001 |
| HFrEF (vs HFpEF) | 1.05 (0.96–1.15) | 0.254 | 1.12 (0.98–1.27) | 0.109 |
Covariates in the multivariable model are age, sex, ischemic vs nonischemic etiology, HFrEF vs HFpEF, unknown left ventricular ejection fraction, serum sodium level, hemoglobin level, cerebrovascular disease, diabetes, chronic obstructive pulmonary disease, hepatic cirrhosis, dementia, cancer, systolic blood pressure, respiratory rate, and blood urea nitrogen. HR indicates hazards ratio; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction.
Timing of Hospitalizations Relative to Initial Discharge and Death
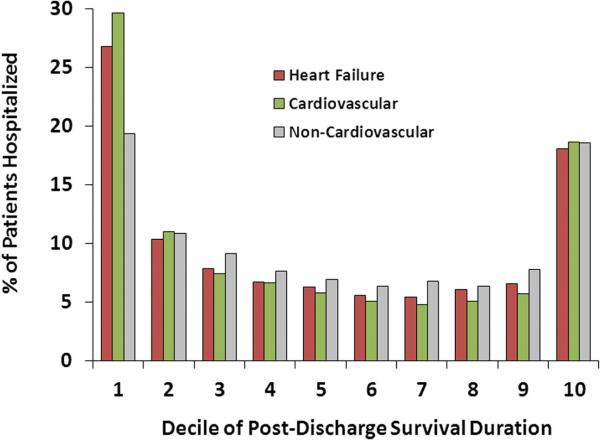
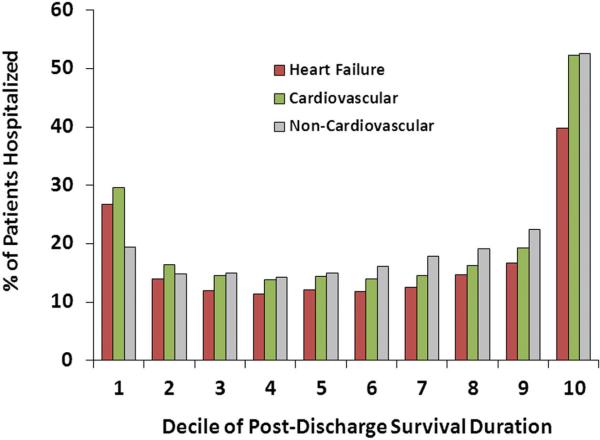
Repeat hospitalizations for HF occurred in 61.3% of patients, whereas cardiovascular events occurred in 66.5% of patients within the first year after discharge. Among patients who were rehospitalized for HF or for cardiovascular disease, the largest proportion of initial readmissions occurred within the first and last deciles of the postdischarge survival duration (Figure 3), where each decile was a median of 63 days in length. Considering any rehospitalization, accounting for multiple hospitalizations per patient, the greatest proportion of hospital admissions occurred in the decile of survival time just before death followed by the immediate postdischarge period (Figure 4). Noncardiovascular hospitalizations demonstrated an early peak for the first readmission and prominent increases in the last decile for the first or any readmission.
Figure 3.
First readmission timing over the lifespan of patients with heart failure.
Figure 4.
Timing of any readmission over the lifespan of patients with heart failure.
Timing of Repeat Hospitalization-Based Events
Repeat hospitalization-based events occurred more frequently before death. In the 18 months before death, there were 6924 hospitalizations with a new diagnosis of recurrent HF: 14.2%, 19.4%, and 47.5% of the entire cohort experienced recurrent HF events during the periods of 12 to 18 months, 6 to 12 months, and <6 months before death, respectively. In a similar time frame, there were 9314 hospitalizations with recurrent cardiovascular disease events in the last 18 months before death, with the majority of events occurring near the end of the lifespan: 20.8% at 12 to 18 months, 26.2% at 6 to 12 months, and 62.1% within 6 months before death. Noncardiovascular events (total, 6924) also showed a similar time course, with 16.7%, 20.7%, and 43.7% of patients given a diagnosis within 12 to 18 months, 6 to 12 months, and <6 months before death, respectively.
Examining the number of repeat hospitalization-based events that occurred in the first (early), second (middle), and third (late) tertiles of the total survival duration, cardiovascular causes (primarily recurrent HF) were predominant in all 3 time periods. Frequency distribution of events were very consistent in those with 1, 2, and ≥3 admissions occurring over the lifetime after the index HF hospitalization.
Discussion
We conducted a comprehensive, long-term analysis of hospitalizations of newly discharged patients with HF who were followed until nearly the entire study cohort had died. During 10-year follow-up, ≈99% of the cohort died, and the median survival was 1.8 years. We found that patients with ischemic HF demonstrated increased mortality, experienced earlier readmission for cardiovascular disease, and exhibited greater risk of repeat hospitalizations over their lifetime. Over the lifetime, survival was marginally, but statistically significantly higher among women and patients with HFpEF. Patients with HFrEF were more likely to experience marginally increased risk of early cardiovascular readmissions, with an increase in hospitalizations for HF and coronary artery disease. Those with ischemic HF were significantly more likely to experience repeat cardiovascular disease admissions of all subtypes.
Cardiovascular readmissions occurred frequently, and these repeat hospitalizations were largely due to episodes of recurrent HF. Examining the first readmission, 66.5% were hospitalized for cardiovascular disease, and 61.3% were readmitted for HF within the first year. Most first readmissions occurred in the initial and final deciles of the cohort's lifespan. When all hospitalizations were examined, cardiovascular disease and recurrent HF comprised the majority of events in the early, middle, and late periods of the survival duration. Repeat hospitalizations occurred most frequently within a few months (ie, final lifespan decile) before death. In multivariable analysis, repeat cardiovascular admissions occurred more frequently among younger patients and those with ischemic HF. When we further dissected cardiovascular events, ischemic HF remained the only significant predictor of repeat admissions for HF and cardiovascular disease. LVEF and sex did not predict repeat HF, coronary heart disease, or cardiovascular readmissions.
This study contributes new data by examining the full range of outcomes over the lifetime of patients in a population-based setting, exploring outcomes by HF subtype, and examining time to first rehospitalization versus recurrent events. The cross-sectional associations were consistent with previously published studies that found a greater frequency of women, older age, hypertension, and atrial fibrillation among patients with HFpEF.10,20 The present findings also support prior community-based epidemiological studies that reported greater risks of coronary heart disease-related deaths in men than in women and in patients with HFrEF than in patients with HFpEF.21,22 The distribution of cardiovascular versus noncardiovascular readmissions was consistent with a long-term HF follow-up study from Olmstead County, Minnesota.23 However, the present study extends prior literature by examining events occurring over the lifetime and the prognostic implications of HF subtypes, particularly classification based on ischemic versus nonischemic HF and LV systolic function.
A novel aspect of the present study was the examination of patterns of hospitalization and cardiovascular events occurring over the post-HF lifetime, with death occurring in nearly all patients during the study. Prior reports evaluating mortality found few differences or nonsignificant trends in short- or near-term events when contrasted by HFpEF versus HFrEF or by sex.24,25 Prior studies comparing ischemic and nonischemic HF did not explore the impact on hospitalizations, were limited to shorter follow-up duration, or were performed in a selected subset of enrollees.26,27 Some studies comparing ischemic versus nonischemic HF reported no differences in death or hospitalizations when limited to near-term follow-up.28 The present analysis extends the literature by examining a broad range of events occurring over the lifetime of patients with HF and by identifying significant mortality and morbidity differences in extended follow-up.
Methodologically, we also examined readmissions using an approach that accounted for repeat events. Unlike prior studies of HF morbidity that focused on occurrence of the first event without regard for the possibility of multiple readmissions per patient, results differed when we examined multiple events occurring over the lifetime. Ischemic HF was a ubiquitous predictor of repeat hospitalizations, time to first hospitalization, and death, which may be attributable to the potentially destabilizing impact of coronary ischemia.7,29 Although patients with HFrEF were more likely to be rehospitalized early, this increased risk was not observed for repeat events over the lifetime relative to those with HFpEF. The reduction in risk of repeat events among those with HFrEF over the lifetime may be attributed in part to the availability of medical therapies, which can reduce hospitalizations among those with reduced LV systolic function. In contrast, there are few efficacious pharmacological therapies that have demonstrably reduced repeat hospitalizations in patients with HFpEF.
The present study has important implications for HF care because repeat hospitalizations reflect a progressive illness where the cumulative effects of increasing morbidity may eventually result in heightened mortality risk.30,31 The findings suggest that determining the presence of ischemia is of critical importance because it was the most robust predictor of repeat HF and cardiovascular admissions and may be amenable to antiischemic therapeutic interventions. As reported in the STICH (Surgical Treatments for Ischemic Heart Failure) trial, judicious use of coronary revascularization procedures may have beneficial effects on reducing hospitalizations,32 which are prominent outcomes over the lifetime of patients with HF. Before the policy of broad use of revascularization interventions is enacted, however, further studies are required to better characterize patient subsets that would most benefit from medical therapy or intervention.33 Although the initial readmission often occurred early after the index HF discharge, cardiovascular causes were prominent reasons for hospitalization in early, middle, and late phases of the survival duration. Thus, the results suggest that interventions to reduce hospitalizations should (1) be provided early after HF discharge, (2) be sustained over the patient's lifetime, and (3) consider both cardiac and noncardiac aspects of care.
The study had several strengths, including complete, extended follow-up for a wide range of outcome events and all patient deaths recorded in a cohort where almost all patients died. Although this study provided new insights into the HF syndrome, there were several limitations. LV function, laboratory abnormalities, and concomitant comorbidities were evaluated at the index admission, and we did not account for changes that may have occurred during follow-up. However, our group has shown previously that adjustment for covariates at baseline predicted long-term risk even after stratification by LVEF.34 Crossover of some patients who were initially nonischemic to an ischemic categorization may have occurred, potentially attenuating differences in outcomes between those with ischemic and nonischemic HF. As with all epidemiological studies, there may be additional impacts of regional variations in care and outcomes, and thus, confirmatory studies in other jurisdictions are needed. Finally, our examination of morbidity was limited to hospitalization events, and clinical worsening that was treated purely in the ambulatory setting was not evaluated. However, symptomatic worsening that requires hospitalization increases mortality risk in a graded manner30,31 and has substantial implications from the standpoint of the healthcare system.
In conclusion, among patients with HF who were discharged from the hospital, readmissions for recurrent HF and cardiovascular disease most often occurred in the early months postdischarge or in the late months before death. Over the lifetime of patients with HF, recurrent hospital-based events were often attributable to cardiovascular disease and were frequently related to HF. Although patients with HFrEF were marginally more likely to experience earlier rehospitalization for HF and coronary heart disease when examined over the lifetime, ischemic HF was the most potent predictor of repeat hospitalizations and earlier readmission to the hospital for all cardiovascular causes in the population. Identification of patients whose HF is attributable to ischemic heart disease coupled with appropriate therapeutic interventions may improve survival and reduce the burden of this leading cause of hospitalization and healthcare costs.
CLINICAL PERSPECTIVE.
Hospital readmissions for heart failure (HF) contribute to increased morbidity and resource burden. However, the predictors of hospitalization and patterns of cardiovascular events over the lifetime of patients with HF have not been elucidated. In this study, we examined recurrent hospitalizations, cardiovascular events, and survival over extended follow-up among patients with HF who were newly discharged. Among 8543 patients followed for 22 567 person-years, 60.7% had HF of ischemic etiology and HF with reduced ejection fraction (left ventricular ejection fraction ≤45% versus >45% [HF with preserved ejection fraction]) was present in 67.3%. Over the 10-year follow-up period, 98.8% of the cohort died, and 35 966 hospital readmissions occurred. Cardiovascular readmissions occurred frequently and were largely due to episodes of recurrent HF. Within the first year postdischarge, 66.5% of patients were rehospitalized for cardiovascular disease, and 61.3% were readmitted for HF. There was a preponderance of readmissions in the months comprising the initial and final deciles of the lifespan of the cohort. Of all recurrent HF hospitalizations, 26.8% occurred in the first and 39.8% in the last deciles of cohort survival duration. Similarly, 29.7% and 52.3% of all cardiovascular readmissions occurred in the first and last deciles of the cohort survival duration, respectively. The presence of ischemic HF etiology was a ubiquitous predictor of the first cardiovascular readmission, recurrent hospitalizations for cardiovascular, HF, and coronary heart disease on repeated-events analysis. Although presence of HF with reduced ejection fraction was associated with a shorter time to first recurrent HF or coronary heart disease readmission, it was not a predictor of repeat hospitalizations.
Acknowledgments
Sources of Funding
This study was supported by operating grant MOP 114937 from the Canadian Institutes of Health Research (CIHR), Career Investigator awards from the Heart and Stroke Foundation of Ontario (to Drs Tu and Austin), a Canada Research Chair in Health Services Research (to Dr Tu), and a CIHR Clinician-Scientist Award (to Dr Lee). The Institute for Clinical Evaluative Sciences is supported in part by a grant from the Ontario Ministry of Health and Long Term Care. The opinions, results, and conclusions are those of the authors, and no endorsement by the Ontario Ministry of Health and Long Term Care or by the Institute for Clinical Evaluative Sciences is intended or should be inferred.
Footnotes
Disclosures
None.
References
- 1.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Chen LM, Jha AK, Guterman S, Ridgway AB, Orav EJ, Epstein AM. Hospital cost of care, quality of care, and readmission rates: penny wise and pound foolish? Arch Intern Med. 2010;170:340–346. doi: 10.1001/archinternmed.2009.511. [DOI] [PubMed] [Google Scholar]
- 4.Tu JV, Nardi L, Fang J, Liu J, Khalid L, Johansen H. National trends in rates of death and hospital admissions related to acute myocardial infarction, heart failure and stroke, 1994–2004. CMAJ. 2009;180:E118–E125. doi: 10.1503/cmaj.081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 6.Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139:72–77. doi: 10.1016/s0002-8703(00)90311-9. [DOI] [PubMed] [Google Scholar]
- 7.Adams KF, Jr, Dunlap SH, Sueta CA, Clarke SW, Patterson JH, Blauwet MB, Jensen LR, Tomasko L, Koch G. Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol. 1996;28:1781–1788. doi: 10.1016/S0735-1097(96)00380-4. [DOI] [PubMed] [Google Scholar]
- 8.Flaherty JD, Bax JJ, De Luca L, Rossi JS, Davidson CJ, Filippatos G, Liu PP, Konstam MA, Greenberg B, Mehra MR, Breithardt G, Pang PS, Young JB, Fonarow GC, Bonow RO, Gheorghiade M, Acute Heart Failure Syndromes International Working Group Acute heart failure syndromes in patients with coronary artery disease early assessment and treatment. J Am Coll Cardiol. 2009;53:254–263. doi: 10.1016/j.jacc.2008.08.072. [DOI] [PubMed] [Google Scholar]
- 9.Collins SP, Schauer DP, Gupta A, Brunner H, Storrow AB, Eckman MH. Cost-effectiveness analysis of ED decision making in patients with non-high-risk heart failure. Am J Emerg Med. 2009;27:293–302. doi: 10.1016/j.ajem.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu JV, Donovan LR, Lee DS, Wang JT, Austin PC, Alter DA, Ko DT. Effectiveness of public report cards for improving the quality of cardiac care: the EFFECT study: a randomized trial. JAMA. 2009;302:2330–2337. doi: 10.1001/jama.2009.1731. [DOI] [PubMed] [Google Scholar]
- 12.Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O'Donnell CJ, Nam BH, Larson MG, D'Agostino RB, Vasan RS. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA, Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 14.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer Verlag; New York, NY: 2000. [Google Scholar]
- 15.Lawless JF. Statistical Models and Methods for Lifetime Data. 2nd ed. John Wiley & Sons; New York, NY: 2003. [Google Scholar]
- 16.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 17.Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Galbraith PD, Knudtson ML, APPROACH (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease) Investigators Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale–based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 19.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 21.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DS, Gona P, Albano I, Larson MG, Benjamin EJ, Levy D, Kannel WB, Vasan RS. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, Roger VL. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB, OPTIMIZE-HF Investigators and Hospitals Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 25.Lee WY, Capra AM, Jensvold NG, Gurwitz JH, Go AS. Gender and risk of adverse outcomes in heart failure. Am J Cardiol. 2004;94:1147–1152. doi: 10.1016/j.amjcard.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 26.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB, OPTIMIZE-HF Investigators and Hospitals Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–854. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 27.Atchley AE, Iskandrian AE, Bensimhon D, Ellis SJ, Kitzman DW, Shaw LK, Pagnanelli RA, Whellan DJ, Gardin JM, Kao A, Abdul-Nour K, Ewald G, Walsh MN, Kraus WE, O'Connor CM, Borges-Neto S, HF-ACTION Trial Nuclear Ancillary Study Investigators Relationship of technetium-99m tetrofosmin-gated rest single-photon emission computed tomography myocardial perfusion imaging to death and hospitalization in heart failure patients: results from the nuclear ancillary study of the HF-ACTION trial. Am Heart J. 2011;161:1038–1045. doi: 10.1016/j.ahj.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchihashi M, Tsutsui H, Kodama K, Kasagi F, Setoguchi S, Mohr M, Kubota T, Takeshita A. Medical and socioenvironmental predictors of hospital readmission in patients with congestive heart failure. Am Heart J. 2001;142:E7. doi: 10.1067/mhj.2001.117964. [DOI] [PubMed] [Google Scholar]
- 29.Frazier CG, Alexander KP, Newby LK, Anderson S, Iverson E, Packer M, Cohn J, Goldstein S, Douglas PS. Associations of gender and etiology with outcomes in heart failure with systolic dysfunction: a pooled analysis of 5 randomized control trials. J Am Coll Cardiol. 2007;49:1450–1458. doi: 10.1016/j.jacc.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 30.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 31.Lee DS, Austin PC, Stukel TA, Alter DA, Chong A, Parker JD, Tu JV. “Dose-dependent” impact of recurrent cardiac events on mortality in patients with heart failure. Am J Med. 2009;122:162–169. doi: 10.1016/j.amjmed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O'Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL, STICH Investigators Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, Drozdz J, Farsky PS, Feldman AM, Doenst T, Michler RE, Berman DS, Nicolau JC, Pellikka PA, Wrobel K, Alotti N, Asch FM, Favaloro LE, She L, Velazquez EJ, Jones RH, Panza JA, STICH Trial Investigators Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko DT, Alter DA, Austin PC, You JJ, Lee DS, Qiu F, Stukel TA, Tu JV. Life expectancy after an index hospitalization for patients with heart failure: a population-based study. Am Heart J. 2008;155:324–331. doi: 10.1016/j.ahj.2007.08.036. [DOI] [PubMed] [Google Scholar]