Abstract
Objective
The amygdala is especially reactive to threatening stimuli, and the degree of reactivity predicts individual differences in the expression of depression and anxiety. Emerging research suggests that emotional neglect during childhood as well as hypercortisolemia may lead to heightened threat-related amygdala reactivity. This raises the possibility that genetic variation affecting hypothalamic-pituitary-adrenal (HPA) axis function contributes to individual differences in amygdala reactivity, both independently and as a function of childhood emotional neglect.
Method
This study assessed whether the mineralocorticoid receptor iso/val polymorphism (rs5522), a functional genetic variant affecting HPA axis function, influenced threat-related amygdala reactivity in 279 individuals in late childhood and early adolescence. The study also explored the extent to which any effects of the genotype on amygdala reactivity were contingent upon previous childhood emotional neglect.
Results
Prior childhood emotional neglect and the val allele were associated with greater amygdala reactivity. Moreover, a significant genotype-by-emotional neglect interaction was observed whereby greater amygdala reactivity in val allele carriers was independent of previous childhood emotional neglect, while greater reactivity in iso homozygotes was revealed only in the context of a history of elevated emotional neglect. At relatively low levels of previous emotional neglect, val carriers had heightened amygdala reactivity relative to iso homozygotes.
Conclusions
These results suggest that relatively greater amygdala reactivity may represent a biological mechanism through which childhood adversity and functional genetic variation in HPA axis responsiveness to stress may mediate risk for psychopathology.
Introduction
The amygdala is critical for learning the emotional significance of stimuli and effecting appropriate changes in behavioral vigilance and physiological arousal in response to environmental challenges (1). It is especially reactive to threatening stimuli, and the magnitude of reactivity is associated with individual differences in the behavioral expression of threat sensitivity as well as the pathophysiology of mood and anxiety disorders (2). Identifying both biological and environmental factors that contribute to variability in threat-related amygdala reactivity is an important step in understanding the etiology of dysfunctional emotional processing and related risk for psychopathology.
Consistent with a wealth of animal research documenting the effects of stress on amygdala structure and function (3), emerging human research has linked acute stress during adulthood, severeforms of childhoodemotional neglect (e.g., institutional rearing), and hypercortisolemia with heightened amygdala reactivity and greater volume (4–10). Circumstantial behavioral and physiological evidence is consistent with these novel neural findings: individuals who were abused or neglected during childhood have an attentional bias toward threatening stimuli (11), childhood abuse is associated with a potentiated fear startle response (12), and stress has been shown to facilitate memory for emotional content but to disrupt memory for neutral content (13). In other studies (14–16), these stress-sensitive startle, attentional, and mnemonic biases were linked with elevated amygdala reactivity. Because the amygdala undergoes rapid development during childhood, researchers have postulated that childhood through early adolescence may be a critical period during which the amygdala is particularly sensitive to stress (3). Collectively, these results suggest that stress exposure, particularly during childhood, promotes heightened amygdala reactivity, which may precipitate the development of psychopathology.
Given the regulatory role of the amygdala in the biological stress response system (17), as well as the associations between stress and amygdala function reviewed above, stress-related genetic variation may independently, and interactively with stress exposure, contribute to individual differences in amygdala reactivity. Alongside numerous regulatory mechanisms, the high-affinity mineralocorticoid receptor and low-affinity glucocorticoid receptor regulate the onset and termination, respectively, of the hypothalamic-pituitary-adrenal (HPA) axis stress response (18). One of the primary functions of the glucocorticoid receptor is to normalize brain activity to prestress levels through negative feedback inhibition of the HPA axis. In contrast, the mineralocorticoid receptor, because of its relatively high affinity and nearly constant occupancy by cortisol, mediates tonic inhibition of the HPA axis under basal and stressful conditions (18). The mineralocorticoid receptor also has a role in behavioral stress response functions, including the appraisal of novel situations and the selection of appropriate responses to deal with challenges (18). Research on these functions, combined with rodent data indicating that mineralocorticoid receptor overexpression in the amygdala reduces corticosterone release and anxiety-like behavior (19), highlights the potentially powerful influence of variability in mineralocorticoid receptor signaling, including that resulting from genetic polymorphisms, on the expression of individual differences in threat-related amygdala reactivity. Moreover, such mineralocorticoid receptor-mediated effects on reactivity may be contingent on the experience of stress.
There is a common missense polymorphism (rs5522) within exon 2 of the human mineralocorticoid receptor gene (NR3C2) that results in the substitution of isoleucine for valine [iso(A)/val(G)]. In vitro data show that the val allele is characterized by reduced cortisol-related transactivation (20, 21). Animal studies have shown that reductions in mineralocorticoid receptor function (i.e., knockout or antagonism) increase basal and stress-evoked HPA axis activity, while enhanced receptor expression reduces HPA axis activity under these circumstances (19, 22, 23). Because the val allele is associated with reduced cortisol-mediated binding that inhibits HPA axis function (20, 21), it is not surprising that it has been associated with heightened stress reactivity as indexed by endocrine, autonomic, perceptual, and subjective self-report measures (20, 21, 24; but see also 25). Moreover, the val allele has been associated with depressive symptoms as well as stress-induced reward learning deficits, which further suggests the relevance of the mineralocorticoid receptor iso/val polymorphism in the emergence of psychopathology (24, 26).
Our main goal in this study was to evaluate direct biological effects of the mineralocorticoid receptor iso/val polymorphism at the level of threat-related amygdala reactivity and to explore potential interactions between the mineralocorticoid receptor iso/val polymorphism and childhood emotional neglect in mediating variability in amygdala reactivity. To this end, 279 individuals in late childhood or early adolescence completed a widely used and well-characterizedblood-oxygen-level-dependent(BOLD) functional magnetic resonance imaging (fMRI) challenge paradigm that reliably elicits amygdala reactivity in both children and adults in normative and clinical populations (27, 28). Additionally, participants completed a questionnaire assessing childhood trauma exposure. From these data, we tested three primary hypotheses. First, based on evidence that emotional neglect and caregiver deprivation are associated with heightened amygdala reactivity to threat (6), we hypothesized that childhood emotional neglect would predict elevated threat-related amygdala reactivity. Second, because the val allele has been associated with lower cortisol-related mineralocorticoid receptor function and heightened HPA axis activity (22), we hypothesized that the val allele would be associated with elevated amygdala reactivity regardless of past emotional neglect history. Third, we predicted a genotype-by-emotional neglect interaction on threat-related amygdala reactivity wherein val carriers would have heightened amygdala reactivity to threat regardless of emotional neglect history because of a constitutional profile mirroring that of individuals who have been emotionally neglected, while iso homozygotes would have elevated amygdala reactivity only in the context of a history of heightened childhood emotional neglect. Alternatively, we hypothesized that the val allele and childhood emotional neglect may compound one another such that val allele carriers would be characterized by extremely heightened amygdala reactivity in the context of previous childhood emotional neglect relative to iso homozygotes.
Method
Participants
Children and adolescents (N=279) from 11 to 15 years old were randomly selected from the general population as part of the ongoing Teen Alcohol Outcomes Study at the University of Texas Health Science Center at San Antonio. This study was designed to investigate how genes, the environment, and neural systems contribute to adolescent psychopathology, with an emphasis on alcohol use disorders. Emotional neglect and fMRI data were available for all 279 participants. Self-report and behavioral data are summarized in Table 1. The study was approved by the institutional review board at the University of Texas Health Science Center at San Antonio.
TABLE 1.
Demographic, Behavioral, and Self-report Variables in a Study of the Association Between Mineralocorticoid receptor Genotype, Childhood Neglect, and Amygdala reactivitya
| Iso/Iso Homozy- gotes (N=193) |
Val Carriers (N=86) | Analysis | Total Sample (N=279) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | χ 2 | df | p | N | % |
|
| |||||||||
| Sex (female) | 95 | 49.22 | 45 | 52.32 | 0.23 | 1 | 0.63 | 140 | 50.18 |
| Race | |||||||||
| Caucasian | 114 | 59.07 | 44 | 51.16 | 1.51 | 1 | 0.22 | 158 | 56.63 |
| Hispanic | 65 | 33.68 | 38 | 44.19 | 2.82 | 1 | 0.09 | 103 | 36.92 |
| Other | 14 | 7.25 | 4 | 4.65 | 0.67 | 1 | 0.41 | 18 | 6.45 |
|
| |||||||||
| Mean | SD | Mean | SD | t | df | p | Mean | SD | |
|
| |||||||||
| Age (years) | 13.64 | 0.99 | 13.48 | 0.98 | 1.29 | 277 | 0.20 | 13.59 | 0.99 |
| Pubertal status (Tanner stage) | 3.56 | 1.00 | 3.36 | 0.96 | 1.55 | 277 | 0.12 | 3.50 | 0.99 |
| Childhood Trauma Questionnaire scores | |||||||||
| Emotional neglect | 8.40 | 3.67 | 7.77 | 2.76 | 1.44 | 277 | 0.15 | 8.21 | 3.43 |
| Emotional abuse | 7.60 | 3.17 | 6.99 | 2.41 | 1.60 | 277 | 0.11 | 7.41 | 2.96 |
| Physical neglect | 6.19 | 1.76 | 6.14 | 1.68 | 0.21 | 277 | 0.84 | 6.17 | 1.73 |
| Physical abuse | 6.30 | 1.94 | 6.10 | 1.73 | 0.79 | 277 | 0.43 | 6.24 | 1.88 |
| Sexual abuse | 5.13 | 0.63 | 5.20 | 0.73 | 0.79 | 277 | 0.43 | 5.15 | 0.66 |
| Face matching accuracy (%) | 98.82 | 5.02 | 99.28 | 2.70 | 0.78 | 255 | 0.44 | 0.99 | 0.04 |
| Face matching reaction time (ms) | 1,369 | 313 | 1,387 | 263 | 0.44 | 255 | 0.66 | 1,374 | 298 |
| Shape matching accuracy (%) | 97.01 | 7.54 | 98.19 | 4.47 | 1.31 | 255 | 0.19 | 0.97 | 0.07 |
| Shape matching reaction time (ms) | 1,223 | 249 | 1,239 | 216 | 0.49 | 255 | 0.63 | 1,228 | 239 |
Test statistics represent results from comparisons between iso homozygotes and val carriers. Behavioral data were available only for 257 participants because of technical difficulties with the equipment used to collect behavioral responses during fMRI.
Self-Report Assessments
The Childhood Trauma Questionnaire was used to quantify lifetime exposure to maltreatment in five categories: emotional, physical, and sexual abuse and emotional and physical neglect (29). Each of the instrument’s subscales has robust internal consistency and excellent convergent validity with a clinician-rated interview of childhood abuse and therapists’ ratings of abuse (30). We focused on the emotional neglect subscale because previous research has associated severe forms of emotional neglect with heightened amygdala reactivity (4, 6). Moreover, there was a substantial amount of variability on this subscale relative to the other subscales in the community sample studied here. The Tanner scale was used to assess pubertal status (31, 32).
Neuroimaging Protocol
Challenge paradigm
A widely used and validated challenge paradigm was employed to elicit robust amygdala reactivity (27, 28). Task details are in the data supplement that accompanies the online edition of this article.
fMRI acquisition parameters
BOLD fMRI data were acquired with a gradient-echo echo planar imaging (EPI) sequence (TR=2,000, TE=25 msec, field of view=20 cm, matrix=64×64) covering 34 interleaved 3 mm-thick axial slices on a Siemens 3-T Trio Scanner. Before collecting fMRI data for each participant, we performed a reference EPI scan that was visually inspected for artifacts and good signal.
Image processing and analysis
After preprocessing steps (see the online data supplement), linear contrasts using canonical hemodynamic response functions estimated a faces (match faces task) > shapes (match shapes task) contrast image for each individual. These contrast images were entered into a second-level random-effects model (one-sample t test) to determine mean task-related responses using a combined voxel-level threshold of p<0.05, family-wise error-corrected whole-brain, and cluster threshold of ≥10 contiguous voxels. BOLD contrast estimates were extracted from functional clusters meeting significance criteria in the right and left anatomical amygdala regions of interest, which were defined using the Brodmann template in the Wake Forest University PickAtlas. By extracting amygdala BOLD parameter estimates from the functional clusters activated by our paradigm rather than clusters specifically correlated with our independent variables of interest, we precluded the possibility of any regression coefficient inflation that may have resulted from capitalizing on the same data twice (33). We have successfully used this conservative strategy in previous studies (27, 34).
DNA Analyses
Genomic DNA from all participants was isolated from buccal cells drawn from Oragene DNA self-collection kits (DNA Genotek, Inc., Kanata, Ont., Canada). Samples were genotyped using the Il-lumina Human 610-Quad BeadChip (see the online data supplement); rs5522 data were extracted based on our a priori hypotheses, and no other polymorphisms were tested. The average call rate was 94.7%. Mineralocorticoid receptor rs5522 did not differ significantly from Hardy-Weinberg equilibrium (p=0.83).
Statistical Analyses
Multiple regression analyses using the Predictive Analytics Software statistical package (PASW or SPSS, version 18, Chicago) examined how emotional neglect and the mineralocorticoid receptor genotype independently and interactively influence threat-related amygdala reactivity. We entered gender and pubertal status in the first step, followed by emotional neglect and mineralocorticoid receptor genotype in the second step, and the interaction of mineralocorticoid receptor genotype and emotional neglect in the last step. Post hoc tests were carried out in two ways (35). First, we used simple slope analyses to assess whether the association between amygdala reactivity and childhood emotional neglect was significant for each genotype group. Second, we used the Johnson-Neyman method to calculate the childhood emotional neglect values at which there were genotype differences in amygdala reactivity. In an attempt to account for background genetic differences associated with ancestral origin, we ran two additional sets of analyses. Ancestry-informative principal component variables, generated using the Eigenstrat program (36), were included in the first step of the regression. In the second, we restricted analyses to individuals reporting Caucasian ancestry (see the online data supplement). All analyses were conducted on BOLD contrast estimates extracted from functional clusters exhibiting a significant main effect of task in the right and left amygdala. All predictor variables were mean centered before the analyses and computation of the interaction term.
Results
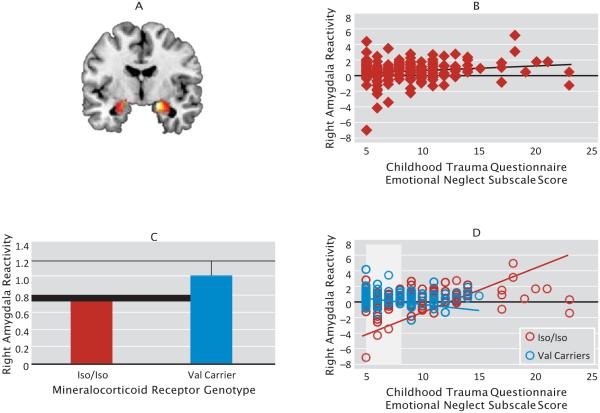
Consistent with previous findings (27, 28), the main effects of task analyses revealed robust amygdala reactivity (Figure 1, panel A). Regression analyses predicting threat-related amygdala reactivity in the entire sample produced a significant overall model for reactivity of the right but not the left amygdala (right: F=4.13, df=5, 273, p=0.001, R2=0.07; left: F=0.95, df=5, 273, p=0.45, R2=0.02). In the right amygdala, greater childhood emotional neglect (b=0.06, SE=0.02, t=3.02, p=0.003; Figure 1B) and val carrier status (b=0.31, SE=0.15, t=2.06, p=0.04; Figure 1C) independently predicted heightened threat-related amygdala reactivity. The interaction of mineralocorticoid receptor genotype and childhood emotional neglect contributed significantly to the model after accounting for these main effects (b=−0.10, SE=0.05, t=−2.06, ΔF=4.26, ΔR2=0.014, p=0.04, not controlling for main effects of genotype or emotional neglect; b=−0.14, SE=0.05, t=−2.92, ΔF=8.51, ΔR2=0.03, p=0.004). As shown in Figure 1D, simple slope analyses indicated a significant positive association between childhood emotional neglect and threat-related amygdala reactivity in iso homozygotes (b=0.08, SE=0.02, t=3.64, p=0.0003) but not in val carriers. Moreover, Johnson-Neyman analyses revealed that iso homozygotes had reduced amygdala reactivity relative to val carriers at low emotional neglect scores (i.e., participants at or below the 60.2 percentile; those scoring 8 or lower on the Childhood Trauma Questionnaire emotional neglect scale; t>1.97 and p<0.05 in all cases). When ancestry-informative principal components were added to the first step of the regression or when the same analyses were conducted for Caucasians only, the main findings were reproduced, except that the main effect of mineralocorticoid receptor genotype was reduced to a trend in analyses with principal components (see the online data supplement). Exploratory analyses using the other Childhood Trauma Questionnaire subscales (emotional abuse, physical neglect, physical abuse, and sexual abuse) revealed no significant overall models (F<1.92 and p>0.09 in all cases).
FIGURE 1. Effects of Childhood emotional Neglect, Mineralocorticoid Genotype, and interaction of Mineralocorticoid Genotype and Childhood emotional Neglect on Threat-related Amygdala reactivitya.
a Panel A shows the left and right amygdala reactivity to the faces > shapes contrast (neurological format): right Montreal Neurological Institute (MNI) coordinates: x=18, y=−6, z=−18; 217 voxels; t=14.74, p<0.001, family-wise error-corrected; left MNI coordinates: x=−16, y=−6, z=−14; 146 voxels, t=10.62, p<0.001, family-wise error-corrected. Panel B shows that childhood emotional neglect is positively associated with amygdala reactivity. Panel C shows that val carriers have elevated threat-related amygdala reactivity relative to iso homozygotes. Panel D shows that childhood emotional neglect is positively associated with amygdala reactivity only in iso homozygotes. The model is plotted with raw data values, although statistics were run with mean-centered values. Highlighted areas indicate regions where the difference between the groups reaches significance. Importantly, removal of the outlier (−7 amygdala reactivity) does not change the results (overall model, F=3.40, df=5, 272, p=0.005; main effect of emotional neglect, p=0.004; main effect of val carrier status, p=0.07; interaction, p=0.04). Moreover, restricting analyses to individuals with a childhood emotional neglect score ≤15 (as no val carriers reported emotional neglect above a score of 15) produced the same pattern of results (overall model, F=3.48, df=5, 263, p=0.005; main effect of emotional neglect, p=0.02; main effect of val carrier status, p=0.03; interaction, p=0.02).
Discussion
We examined how a functional genetic polymorphism affecting HPA axis function and a history of childhood emotional neglect independently and interactively influence amygdala reactivity to threatening stimuli. Three primary findings emerged. First, extending recent research linking extreme forms of emotional neglect (e.g., institutional rearing) to heightened threat-related amygdala reactivity (4, 6), we observed a similar pattern with self-reported emotional neglect in a community sample. Second, we found that the val allele was associated with heightened threat-related amygdala reactivity (however, this effect fell short of significance when we included ancestry-informative principal components but remained significant in the Caucasian-only subsample; see the online data supplement). This effect is consistent with evidence that the val allele of a common mineralocorticoid receptor iso/val polymorphism confers heightened neuroendocrine and autonomic responses to stressors (20, 22, 24). Third, we observed an interaction between the mineralocorticoid receptor genotype and a history of childhood emotional neglect such that a positive association between emotional neglect and threat-related amygdala reactivity was observed only in iso homozygotes, and val carriers had heightened amygdala reactivity relative to iso homozygotes only at relatively low levels of previous childhood emotional neglect.
Childhood Emotional Neglect
Early life stress is one of the strongest predictors of developing emotional, cognitive, and behavioral difficulties (37). However, the mechanisms by which early adversity is associated with such difficulties are poorly understood. This study extends recent findings that extreme forms of childhood emotional neglect are associated with increased amygdala volume (7) and threat-related reactivity (4, 6). Here we show that self-reported history of emotional neglect in children from a community sample is similarly associated with heightened amygdala reactivity, providing initial evidence that milder forms of neglect are also associated with biases in neural function related to an increased risk for psychopathology.
Animal research has shown that a host of early life stressors such as maternal separation are associated with disrupted diurnal HPA axis activity and increased HPA axis and autonomic excitability (3). This evidence is buttressed by limited human research showing that day care (a putative proxy for maternal separation) is associated with similar, albeit less pronounced, differences (38). Moreover, animal models have linked early stressors with increased dendritic branching, the expression of corticotropin-releasing hormone, and neuronal excitability in the amygdala, which are correlated with HPA axis activity and anxiety (3). Consistent with these findings, elevated endogenous cortisol is associated with potentiated amygdala reactivity to emotional facial expressions like those used in the present study (9, 10).
At the cellular level, animal research suggests that stress may potentiate amygdala reactivity to threat by increasing calcium influx in basolateral amygdala neurons, thus increasing excitatory and reducing inhibitory neurotransmission (39). Human studies show that pharmacologically induced elevations of cortisol and norepinephrine together potentiate amygdala reactivity to threatening faces (40). However, pharmacologically administered hydrocortisone alone blunts amygdala reactivity, likely because of glucocorticoid-mediated negative feedback in the context of no other HPA axis activation (40, 41). We speculate that changes in HPA axis function as well as amygdala cytoarchitecture in people with a history of childhood emotional neglect may promote heightened amygdala reactivity to threat. This heightened reactivity may translate to increased vigilance and lay the groundwork for the development of stress-related psychopathologies such as mood and anxiety disorders. Consistent with this speculation, the association between stress and anxiety-related behavior in animals is abolished with the antagonism of excitatory N-methyl-d-aspartic acid neurotransmission in the amygdala (42).
Mineralocorticoid Receptor Genotype Effects and Moderation of Childhood Emotional Neglect
Mineralocorticoid receptor val carriers had heightened amygdala reactivity relative to iso homozygotes. Previous studies have linked the val allele to the loss of cortisol-related mineralocorticoid receptor function and heightened stress reactivity (20, 21, 24; but also see 25). Endogenous cortisol is associated with elevated amygdala reactivity (9, 10), and overexpression of the mineralocorticoid receptor in the amygdala reduces corticosterone release in rodents (19). The relatively potentiated amygdala reactivity in val carriers observed in our study may reflect reduced cortisol-related mineralocorticoid receptor function and heightened basal cortisol levels. The val allele may confer a constitutional profile similar to that observed in maltreated individuals (e.g., heightened basal HPA axis function, blunted cortisol upon awakening, and heightened stress-related endocrine and autonomic responses), and thus similar stress system mechanisms may underlie the relationship between the val allele and heightened amygdala reactivity to threat.
Childhood emotional neglect was positively associated with amygdala reactivity only in iso homozygotes, and genotype groups differed from one another only at relatively low levels of emotional neglect, such that val carriers had elevated amygdala reactivity relative to iso homozygotes. We speculate that the nature of this gene-by-environment interaction may reflect shifts in the neuroendocrine and autonomic profiles of val allele carriers. It is possible that relatively elevated activity of the HPA axis places val carriers closer to a physiological ceiling and thus makes them relatively insensitive to further modulation of amygdala reactivity by previous emotional neglect. The finding of generally greater amygdala reactivity in val carriers relative to iso homozygotes is consistent with this speculation. Additionally, threat-related amygdala reactivity in iso homozygotes may be more sensitive to environmental conditions, for better or worse (43).
The overall effect of task was associated with left and right amygdala reactivity, consistent with a recent metaanalysis (44). However, the emotional neglect and mineralocorticoid receptor genotype findings were lateralized to the right. Evidence suggests that the right amygdala subserves quick and perhaps automatic stimulus detection while the left amygdala provides a more sustained evaluative response (44). This interpretation is consistent with the role of the mineralocorticoid receptor in the initiation of stress response as well as the heightened HPA axis activation conferred by the val allele and observed in individuals with a history of childhood emotional neglect. Accordingly, early emotional neglect and mineralocorticoid receptor genotype may influence the initial phase of threat detection, which may lead to exaggerated responsiveness to mildly threatening and even neutral or positive events.
The amygdala can be divided into anatomically defined ventral (containing the basolateral complex) and dorsal (containing the central nucleus and sublenticular extended amygdala) subregions. A host of animal studies have evaluated the effects of stress and mineralocorticoid receptor expression specifically in the basolateral complex of the amygdala (19, 39). Our exploratory analyses of ventral and dorsal amygdala subregions (see the online data supplement) showed no evidence that either structure was specifically involved in the effects we found in our study. Rather, we found a general effect on activation extending across both the ventral and dorsal subregions. This may reflect a glutamatergic drive of ventral neurons on dorsal neurons, resulting in the generally increased activity seen in the BOLD fMRI signal from the entire amygdala. Additionally, the trend-level effect in the right dorsal but not ventral amygdala may reflect the specific connections between the nuclei of the dorsal subregion (e.g., the central nucleus) and HPA axis targets (e.g., the paraventricular nucleus), which would be particularly influenced by differences in mineralocorticoid receptor signaling.
Emotional neglect alone and in interaction with mineralocorticoid receptor genotype was associated with right amygdala reactivity. However, none of the other Childhood Trauma Questionnaire subscales (emotional abuse, physical neglect, physical abuse, and sexual abuse) were associated with significant effects. As shown in Table 1, emotional neglect was the most endorsed subscale and contained the most variability. Virtually no sexual abuse, physical neglect, or physical abuse was reported in this sample, which may account for the lack of associations with amygdala reactivity and these variables. Moreover, the specificity of this association is not entirely surprising. Previous research on childhood adversity in both animals (e.g., maternal separation) and extreme phenotypes in humans (e.g., institutional rearing) captures experiences best approximated by emotional neglect as opposed to other forms of maltreatment assessed by the Childhood Trauma Questionnaire (3, 4, 6, 7). We speculate that because emotional neglect leaves individuals with fewer support resources to buffer the negative consequences of stress and threat, it may be particularly likely to result in greater and perhaps more generalized sensitivity to threat. This speculation is consistent with data indicating that emotional neglect predicts trauma exposure and contributes to depression and anxiety even when controlling for physical and sexual maltreatment (45). It is further reflected in recent findings that perceived social support may buffer against the expression of anxiety as a function of amygdala reactivity (34).
This is one of the first imaging gene-by-environment interaction studies. It provides an example of how incorporating the environment into imaging genetics research can clarify the relationships between genes and brain function (46). Moreover, it has become increasingly evident in imaging genetics that common single genetic polymorphisms are likely to have small effects, if any, on brain function or behavior (2). By incorporating the environment into such models, we may gain traction in demonstrating relationships between these small effects and behaviorally relevant brain function (46). While our entire model accounted for 7% of the variance in threat-related amygdala reactivity, the genotype-by-emotional neglect interaction only accounted for 1.4% of the variance in amygdala reactivity after controlling for main effects of genotype and emotional neglect. This is a relatively small amount of independent variance; however, when viewed in the context of the overall model it still demonstrates significant predictive utility for questions of basic science and, potentially, even those related to clinical issues (e.g., novel molecular targets). For example, studies reporting associations between amygdala reactivity and 5-HTTLPR genotype have accounted for similar percentages of variance (47); given that those findings have been shown to have clinical relevance (48), the small effects reported here may as well. Moreover, it is possible that even small differences in how the amygdala processes information result in a cascade of neural, hormonal, and physiological changes that affect behavior and clinical risk; such small effects may be particularly pertinent in the context of cellular and nuclear receptors in the HPA axis, which can act as transcription factors to widely influence gene expression (49).
However, the small amount of additional variance also demonstrates the need for other techniques, such as the use of biologically informed multilocus genetic profiles, to better represent underlying molecular differences (50). A genetic profile representing variability in multiple levels of the HPA axis might offer particular utility; for example, polymorphisms that are known to affect stress response (e.g., rs5522) as well as those that have been associated with impaired negative feedback of the HPA axis (e.g., FKBP5 polymorphisms [51]) could be combined into a single genetic profile score that better represents HPA axis function.
Our study is not without limitations. First, while we speculated on the relationship between HPA axis function and amygdala reactivity in the context of a history of childhood emotional neglect and mineralocorticoid receptor genotype, we did not collect neuroendocrine measures (e.g., cortisol) to directly test whether such differences might mediate this relationship. Second, the assessment of emotional neglect relied on retrospective self-report. Given that such retrospective memory may be influenced by other factors that are also associated with amygdala reactivity (e.g., neuroticism), it will be important for future research to replicate these findings with more refined assessments (e.g., public records and interviews). The Childhood Trauma Questionnaire nevertheless possesses excellent reliability and validity relative to other measures (e.g., clinician-rated abuse from interview) (30). Moreover, our results nicely complement animal research and human research in extreme environments (e.g., institutional rearing), lending credence to the findings reported here (4–9). It will be important for researchers to examine whether associations between mineralocorticoid receptor genotype, emotional neglect, and amygdala reactivity predict individual differences in behavior and psychopathology. Prospective research that follows individuals into peak periods of psychopathology development, as the Teen Alcohol Outcomes Study is designed to do, will be particularly valuable (52).
Limitations notwithstanding, this study provides important evidence demonstrating that a common functional polymorphism associated with variability in the responsiveness of the HPA axis moderates the impact of childhood emotional neglect on threat-related amygdala reactivity. If replicated in additional samples, the neural effects described here may represent a biological mechanism mediating stress responsivity and
Supplementary Material
Acknowledgements
Supported by National Institute on Alcohol Abuse and Alcoholism grant R01AA016274 (Dr. Williamson). The authors thank the Teen Alcohol Outcomes Study staff for their assistance conducting the study.
Footnotes
Disclosures
The authors report no financial relationships with commercial interests.
Contributor Information
Ryan Bogdan, Laboratory of NeuroGenetics, Department of Psychology and Neuroscience, Duke University, Durham, N.C.
Douglas E. Williamson, Genetic Epidemiology Program, Department of Psychiatry, University of Texas Health Sciences Center at San Antonio
Ahmad R. Hariri, Laboratory of NeuroGenetics, Department of Psychology and Neuroscience, Duke University, Durham, N.C
References
- 1.Whalen PJ, Phelps EA, editors. The Human Amygdala. Guilford; New York: 2009. [Google Scholar]
- 2.Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 3.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin J, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, Rutter M, Sonuga-Barke EJS. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 5.Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JY, Ackerman JP, Pine DS, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10:34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Marle HJF, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 8.van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Maheu FS, Mazzone L, Merke DP, Keil MF, Stratakis CA, Pine DS, Ernst M. Altered amygdala and hippocampus function in adolescents with hypercortisolemia: a functional magnetic resonance imaging study of Cushing syndrome. Dev Psychopathol. 2008;20:1177–1189. doi: 10.1017/S0954579408000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. J Abnorm Psychol. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- 11.Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, Ressler KJ. Childhood abuse is associated with increased startle reactivity in adulthood. Depress Anxiety. 2009;26:1018–1026. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem. 2007;14:861–868. doi: 10.1101/lm.743507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoury-Malhame ME, Reynaud E, Soriano A, Michael K, Salgado-Pineda P, Zendjidjian X, Gellato C, Eric F, Lefebvre MN, Rouby F, Samuelian JC, Anton JL, Blin O, Aubert-Khalfa S. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. doi: 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Murty VP, Ritchey M, Adcock RA, LaBar KS. fMRI studies of successful emotional memory encoding: a quantitative metaanalysis. Neuropsychologia. 2010;48:3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- 16.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joels M, Karst H, Derijk R, Dekloet E. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 2008;31:1–7. doi: 10.1016/j.tins.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Mitra R, Ferguson D, Sapolsky RM. Mineralocorticoid receptor overexpression in basolateral amygdala reduces corticosterone secretion and anxiety. Biol Psychiatry. 2009;66:686–690. doi: 10.1016/j.biopsych.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 19.DeRijk RH, Wust S, Meijer OC, Zennaro MC, Federenko IS, Hellhammer DH, Giacchetti G, Vreugdenhil E, Zitman FG, de Kloet ER. A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. J Clin Endocrinol Metab. 2006;91:5083–5089. doi: 10.1210/jc.2006-0915. [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen N, Kumsta R, Entringer S, de Kloet ER, Zitman FG, DeRijk RH, Wüst S. Functional mineralocorticoid receptor gene variation influences the cortisol awakening response after dexamethasone. Psychoneuroendocrinology. 2010;35:339–349. doi: 10.1016/j.psyneuen.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Derijk R, Vanleeuwen N, Klok M, Zitman F. Corticosteroid receptor-gene variants: modulators of the stress-response and implications for mental health. Eur J Pharmacol. 2008;585:492–501. doi: 10.1016/j.ejphar.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Gass P, Reichardt HM, Strekalova T, Henn F, Tronche F. Mice with targeted mutations of glucocorticoid and mineralocorticoid receptors: models for depression and anxiety? Physiol Behav. 2001;73:811–825. doi: 10.1016/s0031-9384(01)00518-2. [DOI] [PubMed] [Google Scholar]
- 23.Bogdan R, Perlis RH, Fagerness J, Pizzagalli DA. The impact of mineralocorticoid receptor iso/val genotype (rs5522) and stress on reward learning. Genes Brain Behav. 2010;9:658–667. doi: 10.1111/j.1601-183X.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouma EMC, Riese H, Nolte IM, Oosterom E, Verhulst FC, Ormel J, Oldehinkel AJ. No associations between single nucleotide polymorphisms in corticoid receptor genes and heart rate and cortisol responses to a standardized social stress test in adolescents: the TRAILS study. Behav Genet. 2010;41:253–261. doi: 10.1007/s10519-010-9385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuningas M, de Rijk RH, Westendorp RGJ, Jolles J, Eline Slagboom P, van Heemst D. Mental performance in old age dependent on cortisol and genetic variance in the mineralocorticoid and glucocorticoid receptors. Neuropsychopharmacology. 2006;32:1295–1301. doi: 10.1038/sj.npp.1301260. [DOI] [PubMed] [Google Scholar]
- 26.Carre JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nsq101. Epub ahead of print, Dec 22, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein D. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 29.Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the Childhood Trauma Interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. 1995;152:1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- 30.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viviani R. Unbiased ROI selection in neuroimaging studies of individual differences. Neuroimage. 2010;50:184–189. doi: 10.1016/j.neuroimage.2009.10.085. [DOI] [PubMed] [Google Scholar]
- 33.Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49:651–656. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- 35.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 36.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunnar MR, Kryzer E, Van Ryzin MJ, Phillips DA. The rise in cortisol in family day care: associations with aspects of care quality, child behavior, and child sex. Child Dev. 2010;81:851–869. doi: 10.1111/j.1467-8624.2010.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karst H, Nair S, Velzing E, Rumpff-van Essen L, Slagter E, Shinnick-Gallagher P, Joels M. Glucocorticoids alter calcium conductances and calcium channel subunit expression in basolateral amygdala neurons. Eur J Neurosci. 2002;16:1083–1089. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- 39.Kukolja J, Schlapfer TE, Keysers C, Klingmuller D, Maier W, Fink GR, Hurlemann R. Modeling a negative response bias in the human amygdala by noradrenergic-glucocorticoid interactions. J Neurosci. 2008;28:12868–12876. doi: 10.1523/JNEUROSCI.3592-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henckens MJAG, van Wingen GA, Joels M, Fernandez G. Time-dependent effects of corticosteroids on human amygdala processing. J Neurosci. 2010;30:12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamec R, Blundell J, Burton P. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiol Behav. 2005;86:75–91. doi: 10.1016/j.physbeh.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 42.Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27:1247–1258. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene-environment interactions. Trends Cogn Sci. 2011;15:651–656. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri A, Kolachana B, Nardini M, Weinberger DR, Scarabino T. Variation of human amygdala response during threatening stimuli as a function of 5 HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 47.Gillihan SJ, Rao H, Brennan L, Wang DJ, Detre JA, Sankoorikal GM, Brodkin ES, Farah MJ. Serotonin transporter genotype modulates the association between depressive symptoms and amygdala activity among psychiatrically healthy adults. Psychiatry Res. 2011;193:161–167. doi: 10.1016/j.pscychresns.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 49.Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology. 2011;36:1940–1947. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 51.Somerville LH, Fani N, McClure-Tone EB. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev Neuropsychol. 2011;36:408–428. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.