Abstract
Despite evidence for heritable variation in cannabis involvement and the discovery of cannabinoid receptors and their endogenous ligands, no consistent patterns have emerged from candidate endocannabinoid (eCB) genetic association studies of cannabis involvement. Given interactions between eCB and stress systems and associations between childhood stress and cannabis involvement, it may be important to consider childhood adversity in the context of eCB-related genetic variation. We employed a system-level gene-based analysis of data from the Comorbidity and Trauma Study (N = 1,558) to examine whether genetic variation in 6 eCB genes (anabolism: DAGLA, DAGLB, NAPEPLD, catabolism: MGLL, FAAH, binding: CNR1; SNPs N = 65) and childhood sexual abuse (CSA) predicts cannabis dependence symptoms. Significant interactions with CSA emerged for MGLL at the gene-level (p = .009), and for rs604300 within MGLL (ΔR2 = .007, p < .001), the latter of which survived SNP-level Bonferroni correction and was significant in an additional sample with similar directional effects (N = 859; ΔR2 = .005, p = .026). Furthermore, in a third sample (N = 312), there was evidence that rs604300 genotype interacts with early life adversity to predict threat-related basolateral amygdala habituation, a neural phenotype linked to the eCB system and addiction (ΔR2 = .013, p = .047). Rs604300 may be related to epigenetic modulation of MGLL expression. These results are consistent with rodent models implicating 2-arachidonoylglycerol (2-AG), an endogenous cannabinoid metabolized by the enzyme encoded by MGLL, in the etiology of stress adaptation related to cannabis dependence, but require further replication.
Keywords: cannabis, endocannabinoid, childhood abuse, MGLL, amygdala
Endocannabinoid Variants and Childhood Adversity Predict Cannabis Dependence Symptoms and Amygdala Habituation
After alcohol, cannabis is the second most widely used recreational drug in developed nations (Degenhardt & Hall, 2012). In the United States, 45% of adults report using cannabis at some point in their lives, with 12% using in the past 12 months (Substance Abuse and Mental Health Services Administration, 2012). Cannabis dependence was formerly determined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; 4th ed., text rev.; American Psychiatric Association, 2000)1 by the endorsement of at least 3 of the following 6 criteria within a 12-month period: tolerance, use in larger quantities or for longer than intended, repeated unsuccessful attempts to quit or cut back, giving up important activities, spending excessive time acquiring or using cannabis, and recurrent use despite physical and/or emotional problems. Estimates suggest that 1.3% of the U.S. population meets the criteria for DSM-IV-TR cannabis dependence at some point during their lives (Stinson, Ruan, Pickering, & Grant, 2006). Among users, 9% qualify for dependence; of daily users, that number rises to roughly 25-50% (Hall & Degenhardt, 2009; Lopez-Quintero et al., 2011).
Genetic Basis for Cannabis Dependence
Even though twin studies suggest thatapproximately 50-60% of variance in cannabis use disorders (abuse and dependence) is attributable to additive genetic influences (Verweij et al., 2010), molecular genetic studies have had limited success identifying variants implicated in the emergence of problematic cannabis involvement. For instance, a genome-wide association study (GWAS) of cannabis dependence, performed in the replication sample used in the current study, failed to find any genome-wide significant (i.e., p < 5E-8) associations (Agrawal et al., 2011). While GWAS is a powerful tool for SNP discovery, it is fairly agnostic with regard to the putative mechanisms underlying the behavior under study, equally weighting, arguably unduly, variants in all gene systems. Those interested in hypothesis-driven data analyses have frequently reverted to candidate gene approaches, which, with rare exception, have yielded null or nonreplicable results (Agrawal & Lynskey, 2009; Flint & Munafò, 2013).
Challenges with A Priori Selection of Candidate Variants
Traditional candidate gene analyses, historically conducted in modestly sized samples of less than 500 participants, relied on a handful of genotypes selected for their purported functional effects on behavior. Reliance on such restricted sets of variants was primarily due to an oversimplification of genotype-phenotype relationships. For instance, if positive reinforcement is a core feature of substance use and is related to the release of dopamine, then variants associated with differential dopamine function (e.g., rs1800497 in DRD2/ANKK1) must be of importance to cannabis dependence (Maldonado & Rodriquez de Forseca, 2002). While the hypothesis that dopaminergic mechanisms, even specifically DRD2 receptors, are at play is fairly reasonable, a recent meta-analysis suggests no association between this polymorphism and cannabis dependence (Deng et al., 2015). Prior limitations with annotation of the human genome, compounded by the prohibitive costs associated with large-scale genotyping, likely relegated the implementation of early genetic association studies to a limited number of handpicked variants across genes and/or gene-tagging single nucleotide polymorphisms (SNPs). However, recent improvements in feasibility, customizability, and affordability of genome-wide arrays, the practicality of imputing, and the efficiency of data analysis (particularly with software packages such as PLINK; Purcell et al., 2007; Chang et al., 2015) have rendered some custom candidate gene panels obsolete. The availability of high density arrays with superior gene coverage allow for the study of candidate gene systems, an approach more amenable to hypothesis-testing.
Modern Methods for Gene-Based Analyses
With the increasing accessibility of genome-wide data, there has been a corresponding surge of novel methods that leverage its high dimensionality. Typically, because approximately 1 million SNPs are examined individually, GWAS incur a high cost for multiple comparisons (p < 5E-8). In response, and consistent with the polygenic basis underlying behavioral traits (Plomin, Haworth, & Davis, 2009), recent research has begun to examine aggregate genetic influence and effects across genes and biological systems (Holmans, 2010; Neale & Sham, 2004; Purcell et al., 2009; Ramanan, Shen, Moore, & Saykin, 2012; Wang, Li, & Bucan, 2007; Wang, Li, & Hakonarson, 2010). Such gene- and system-level association analyses not only reduce the likelihood of false negatives by lowering the threshold for significance, but are also more compatible with the resolution (i.e., downstream neural, behavioral, and self-report measures) at which clinical, behavioral, and neural genetics research is conducted (Dudbridge, Gusnanto, & Koeleman, 2006; Nikolova, Ferrell, Manuck, & Hariri, 2011; Plomin et al., 2009).
When there is ample evidence for the involvement of a given gene or system but little prior knowledge regarding specific polymorphisms within it, methods have been introduced that average across associations for all variants within the gene/system, typically regardless of function, to create a summary statistic (Holmans, 2010; Li, Gui, Kwan, & Sham, 2011; Liu et al., 2010; Neale & Sham, 2004; Ramanan et al., 2012; Wang et al., 2007, 2010). Numerous approaches have been proposed to mine GWAS data in this manner. For instance, the Versatile Gene-Based Association Analysis (VEGAS; Liu et al., 2010) software package utilizes p-values for each SNP from a typical GWAS, assigns them to one of 17,787 autosomal genes, and creates a sum statistic representing gene-based association whose empirical p-value is calculated via simulations. This approach has successfully identified genes associated with cannabis dependence when single-SNP GWAS have failed (Agrawal et al., 2014).
While the above represents a data-driven approach for assignment of SNPs to genes/systems, other methods allow investigators to ascribe SNPs to a gene set and conduct set-based association tests. Using such an approach, one study linked variants in the norepinephrine, glutamatergic, GABAergic, and corticotropin-releasing hormone systems to alcohol dependence (Reimers, Riley, Kalsi, Kertes, & Kendler, 2012). The present study uses a similar approach to examine the relationship between all SNPs in genes within the endocannabinoid system and cannabis dependence. Due to the relatively recent characterization of cannabinoids' actions in the brain and the limited exploration of individual SNPs in this system, prior robust association findings are limited, making system-level and gene-based analyses an appealing alternative to traditional single-SNP or additive multilocus candidate gene approaches (e.g., Nikolova, Ferrell, Manuck, & Hariri, 2011).
The Endocannabinoid System and Cannabis Involvement
In this study, we propose that genes comprising the endocannabinoid system may play a particularly important role in the etiology of cannabis dependence, especially in the context of childhood stress. In the past 25 years, receptors for exogenous cannabinoids such as Δ9-tetrahydrocannabinol (THC) in Cannabis, along with endogenous ligands, have been identified (for a review, see De Petrocellis & Di Marzo, 2009). The endogenous cannabinoid, or “endocannabinoid” (eCB), system plays a critical role in reward, anxiety, and stress responsiveness (Hill, McLaughlin, et al., 2010; Solinas, Goldberg, & Piomelli, 2008). The molecular aspects of the eCB system have been extensively reviewed elsewhere (see Bisogno, 2008; Bisogno, Ligresti, & Di Marzo, 2005; Di Marzo, 2011; Di Marzo, 2009; Piomelli, 2003; Wilson & Nicoll, 2002) and are briefly described below (see also Table 1).
Table 1. Endocannabinoid System Genes.
| Gene | Protein | Role | Rationale |
|---|---|---|---|
| CNR1 | CB1 | Receptor | primary endocannabinoid receptor |
| CNR2 | CB2 | Receptor | secondary endocannabinoid receptor |
| FAAH | FAAH | Catabolism | responsible for the breakdown of AEA and a small percentage of the breakdown of 2-AG |
| MGLL | MAGL | Catabolism | responsible for ∼85% of the breakdown of 2-AG |
| DAGLA | DAGL-α | Anabolism | with DAGL-β, responsible for the hydrolosis of DAGs to 2-AG |
| DAGLB | DAGL-β | Anabolism | with DAGL-α, responsible for the hydrolosis of DAGs to 2-AG |
| NAPEPLD | NAPE-PLD | Anabolism | enzyme in the most direct synthesis pathway of AEA |
Note. CB1 = cannabinoid type 1 receptor; CB2 = cannabinoid type 2 receptor; AEA = anandamide; FAAH = fatty acid amide hydrolase-1; 2-AG = 2-arachidonoylglycerol; MAGL = monoacylglycerol lipase; DAGL-α = sn-1-selective diacylglycerol lipase- α; DAGs = diacylglycerols; DAGL-β = sn-1-selective diacylglycerol lipase- β; NAPE-PLD = N-acyl-phosphatidylethanolamine-selective phosphodiesterase.
Unlike most transmitter systems, endocannabinoid receptors have two major ligands, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), as well as a host of other putative ligands (e.g., noladin, virodhamine). AEA and 2-AG are synthesized postsynaptically and released “on-demand,” presumably by an unknown endocannabinoid membrane transporter. Anandamide is synthesized directly from the phospholipid precursor N-arachidonoyl phosphatidylethanolamine (NAPE) by N-acyl-phosphatidylethanolamine-selective phosphodiesterase (NAPE-PLD); alternative indirect synthesis routes exist but have not yet been fully characterized. The other eCB ligand, 2-AG, is formed by the hydrolysis of diacylglycerols (DAGs) by sn-1-selective diacylglycerol lipases (DAGLs). Once released into the synapse, eCB ligands bind to cannabinoid type 1 (CB1) and type 2 (CB2) receptors. As with eCB ligands, other putative receptors exist (e.g., GPR55, TRPV1) but have yet to be well characterized. AEA is broken down primarily by fatty acid amide hydrolase-1 (FAAH), while 2-AG is mostly catabolized by monoacylglycerol lipase (MAGL).
Given that eCB receptors bind the psychoactive component, THC, in cannabis as well as endogenous ligands similar in structure to THC, it is a promising candidate system for probing genetic vulnerability to cannabis dependence. Chronic cannabis users, for example, show global decreases in CB1 receptor availability (Ceccarini et al., 2013). Correspondingly, animal models have persuasively documented that the psychoactive effects of cannabis are exerted via their interface with these eCB receptors in the central nervous system, particularly CB1 (Lichtman & Martin, 1997; Wiley et al., 2014). For instance, impairment of short term spatial memory is a frequently observed outcome of acute THC administration (Wise et al., 2012), and CB1 receptor antagonism in the hippocampus attenuates this memory disruption, while elevation of AEA and 2-AG levels via simultaneous FAAH and MAGL blockade recapitulates this phenotype (Wise, Thorpe, & Lichtman, 2009). Despite this strong preclinical evidence, much of the extant literature on specific genes and polymorphisms within the eCB system influencing cannabis phenotypes (e.g., Filbey, Schacht, Myers, Chavez, & Hutchison, 2010; Haughey, Marshall, Schacht, Louis, & Hutchison, 2008; Schacht, Selling, & Hutchison, 2009) remains unreplicated or has yielded null results (Agrawal & Lynskey, 2009). Furthermore, variation in eCB genes along the anabolic and catabolic pathways has been largely neglected.
The Role of Environment
Independent lines of research linking the eCB system to stress responsiveness (Gunduz-Cinar, Hill, McEwen, & Holmes, 2013), and early life adversity to cannabis dependence risk (Duncan et al., 2008), suggest that it is important to consider the environment with regard to eCB-related genetic risk for cannabis dependence. The eCB system is modulated by stress and has reciprocal interactions with the hypothalamic-pituitary-adrenal (HPA) axis, a central regulator of stress responsiveness. Non-human animal models have demonstrated that stress exposure increases the eCB ligand 2-AG while decreasing AEA levels, with pharmacologic evidence that these changes mediate effects of stress on HPA axis activation (Hill et al., 2009; Patel, Kingsley, Mackie, Marnett, & Winder, 2009; Rademacher et al., 2008). Moreover, there is accumulating preclinical evidence that, unlike adult-onset stressors, chronic stress exposure during early life and adolescence results in sustained stress-related effects on eCB signaling and gene expression that do not recover following enrichment during adulthood (Buwembo, Long, & Walker, 2013; El Rawas et al., 2011; Lee & Hill, 2013; Malone, Kearn, Chongue, Mackie, & Taylor, 2008; Reich, Mihalik, Iskander, Seckler, & Weiss, 2013; Sciolino et al., 2010). These sustained differences in the eCB system may leave individuals vulnerable to later psychopathology, including cannabis dependence.
Independent of genetic considerations, environmental factors are thought to contribute to 40-50% of variation in problematic cannabis use (Verweij et al., 2010). Abuse, particularly during childhood, has been reliably associated with substance use problems in both adolescence and adulthood (see Simpson & Miller, 2002, for review). Childhood sexual abuse (CSA), in particular, is associated with increased adolescent and adult substance use and misuse, with one study suggesting a 2-fold increased hazard of cannabis use disorders in individuals exposed to CSA (Duncan et al., 2008). Similarly, a longitudinal study found that, even after controlling for covariates (including socioeconomic status, other childhood adversity, parental separation, and parental history of offending), exposure to CSA was significantly predictive of alcohol and other substance use disorders by age 16 to 18 years (Fergusson, Horwood, & Lynskey, 1996). Furthermore, risk for substance use and disorders is elevated in twins exposed to CSA relative to their genetically related unexposed co-twin, underscoring the importance of environmental experience (Myers & Prescott, 2000; Nelson et al., 2006).
Given interactions between the eCB and stress regulatory systems, as well as documented main effects of early life adversity on cannabis use disorders and HPA-axis and eCB-related function, it is possible that eCB-related genetic variation only confers risk for cannabis involvement in the context of stress exposure. Such Gene × Environment interactions (GxE) can emerge in a variety of forms. For instance, genetic effects might be potentiated or exacerbated in the context of environmental exposure. As an example, Meyers and colleagues found that the G allele of ADH1B rs1229984, which is associated with decreased conversion of alcohol to acetaldehyde and increased problematic alcohol use, has a stronger influence on heavy drinking and alcohol dependence in individuals with a prior history of CSA (Meyers et al., 2013). In contrast, certain genotypes might suppress risk or protection afforded by the environment. For instance, a synonymous polymorphism within CNR1 (rs1049353) has been found to attenuate the pathogenic effects of childhood physical abuse on later vulnerability to anhedonia (Agrawal, Nelson, et al., 2012; but see also Pearson et al., 2013). In that study, carriers of the minor allele were protected from the pathogenic effects of childhood abuse on anhedonia.
Endocannabinoids and the Basolateral Amygdala
In addition to guiding GxE research, neuroscience can be used to disentangle the neural mechanisms through which genetic variation and the environment promote individual differences in behavior (Caspi & Moffitt, 2006). Cross-species research on the effects of stress on the eCB system as well as pharmacologic eCB manipulation suggest that individual differences in amygdala function, and in particular its diminished response to repeated threat-related stimuli (i.e., habituation), may play a key role in mediating associations between the eCB system, stress exposure, and behavior (Ramikie & Patel, 2012). First, stress-induced endocannabinoid changes (e.g., reduced AEA, increased 2-AG) within the basolateral amygdala (BLA) facilitate HPA axis activation and increase anxiety (Rademacher et al., 2008). Moreover, chronic early life stress results in sustained eCB differences within the BLA that may provide a lasting environmental signature conferring vulnerability to cannabis dependence symptoms (Lee & Hill, 2013; Sciolino et al., 2010). Second, THC and CB1 agonist administration reduce subjective anxiety, blunt threat-related amygdala function, and facilitate fear extinction (Gruber, Rogowska, & Yurgelun-Todd, 2009; Phan et al., 2008; Rabinak et al., 2013). Importantly, knockout and pharmacologic manipulation studies in rodents suggest that eCB signaling is critical for fear extinction, but not conditioning, which is consistent with recent genetic work in humans linking an eCB polymorphism (rs324420 in FAAH) to amygdala habituation as well as stress-related negative emotionality (Gunduz-Cinar, MacPherson, et al., 2013). Collectively, these independent lines of research suggest that the effects of eCB-related genetic variation and stress exposure may exert behavioral effects by influencing amygdala habituation to threat-related stimuli. Such differences may lead to cannabis dependence symptoms through self-medication of cannabinoid signaling, impulsivity, and/or the removal of negative affect, consistent with recent theories of the transition to dependence (Koob & Volkow, 2010).
The Present Study
Based on prior evidence (a) supporting the role of the eCB system and related genes in cannabis dependence, (b) that childhood adversity confers risk for substance use disorders, and (c) of interactions between the eCB system and neural systems underlying threat and stress responsivity, we first examined whether CSA moderates associations between genetic variation across the eCB system and cannabis dependence symptoms (Sample 1 N = 1,558). Second, we attempted to replicate any interactions with sexual/physical abuse in an independent sample (Sample 2 N = 859). Third, in light of evidence that the eCB system plays a prominent role in fear extinction and amygdala-driven stress reactivity, we examined whether any variants associated with cannabis dependence symptoms in the context of CSA predicted early life stress (ELS)-related differences in amygdala habituation (Sample 3 N = 312). Independent and dependent variables used across studies are described in Table 2. In addition to traditional single-SNP analyses within the eCB system, we employed gene-based analyses in our discovery sample that aggregated effects across SNPs within a gene.
Table 2. Participant Characteristics and Variables Across Studies.
| Study | Subjects | Genotype Groups | Childhood Adversity Measure | Outcome |
|---|---|---|---|---|
| CATS | Opioid-dependent cases and neighborhood controls who ever used cannabis (N = 1558) | GG, AG, AAa | Number of types of childhood sexual abuse (i.e., unwanted exposure, touching, threats, vaginal sex, oral sex, anal sex) endorsed | DSM-IV cannabis dependence symptoms among cannabis users |
| SAGE | Alcohol-dependent cases and non-dependent controls (N = 859) | GG, AXb | Whether ever experienced childhood physical or sexual abuse (yes = 1, no = 0) | DSM-IV cannabis dependence symptoms (nonusers coded as “0”) |
| DNS | Young adult college students (N = 312) | GG, AXb | Occurrence and frequency of emotional, physical, and sexual abuse, as wellas emotional and physical neglect | Right and left amygdala habituation to emotional faces |
Note. CATS = Comorbidity and Trauma Study; SAGE = Study of Addiction: Genetics and Environment; DNS = Duke Neurogenetics Study
Analyses using carrier coding, as in the other studies, yielded consistent results.
Heterozygotes and A allele homozygotes treated as one group.
Methods
Comorbidity and Trauma Study (CATS; N = 1,558)
Australian adults of European ancestry who completed the Comorbidity and Trauma Study (CATS) and had data on childhood sexual abuse and cannabis use were considered for analyses (N = 1,621). Of these participants, 96.1% reported having ever used cannabis; as such, those who did not report using cannabis were excluded from subsequent analyses, leaving a final N of 1,558 (Table 3). CATS is a case-control study of opioid-dependent individuals (primarily heroin, n = 1,189), aged 18 or older, who were recruited from clinics in the greater Sydney region at which they received opioid substitution therapy (for additional details: Nelson et al., 2013, 2014; Shand, Degenhardt, Slade, & Nelson, 2011) Neighborhood controls (n = 369) who had little or no lifetime history of recreational opioid use were recruited from socially disadvantaged neighborhoods in geographic proximity to locations where cases had been recruited. Participants were excluded for recent suicidal intent and current psychosis. Institutional Review Board (IRB) approval was obtained from University of New South Wales, Washington University School of Medicine, QIMR Berghofer Medical Research Institute, and Sydney area health service ethics committees.
Table 3. Sample Demographics.
| Variable | CATS | SAGE | DNS |
|---|---|---|---|
| N | 1558 | 859 | 312 |
| Age | 36.11 (8.92) | 37.39 (10.90) | 19.71 (1.23) |
| Female (%) | 42.2% | 52.3% | 51.6% |
| Can. Dep. Sx | 2.95 (2.15) | 1.23 (2.00) | N/A |
| Cannabis Dependent (%) | 55.5% | 23.6% | 1.6% |
| CSA/CA/CTQ | 1.57 (2.04)a | 0.21 (N/A)b | 31.18 (6.68)c |
Note. CATS = Comorbidity and Trauma Study; SAGE = Study of Addiction: Genetics and Environment; DNS = Duke Neurogenetics Study; CSA = childhood sexual abuse; CA = childhood abuse (physical or sexual); CTQ = Childhood Trauma Questionnaire.
Average number of types of CSA reported, out of 6.
Proportion reporting any CA.
Minimum score for CTQ is 25, indicating no childhood trauma.
Measures
A modified version of the Semi-Structured Assessment for the Genetics of Alcoholism was used to assess DSM-IV psychiatric diagnosis (SSAGA-OZ; Bucholz et al., 1994; Heath et al., 2011). CSA was defined using 6 items that assessed unwanted exposure to another person's genitals, touching of breasts and/or genitalia, threats regarding sexual activity, and attempting to or having vaginal, oral, or anal sex, all prior to age 18. Items were averaged and then log-transformed to create a continuous CSA score (log-transformed: M = 0.09, SD = 0.11; untransformed: M = 0.26, SD = 0.34; raw: M = 1.57, SD = 2.04; distribution: 0 n = 806, 1 n = 184, 2 n = 114, 3n = 111, 4 n = 114, 5 n = 127, 6 n = 102). Our outcome variable of interest, cannabis dependence symptoms, was defined as the number of lifetime DSM-IV dependence symptoms reported2 (Table 3; M = 2.95, SD = 2.15). The distributions of dependence symptoms across genotype and CSA groups are given in the Supplement (Table S1).
Gene and SNP selection and gene-level testing
Details regarding genotyping using a GWAS array are available in supplemental text. SNPs (MAF > .05; call rate > 95%) within six endocannabinoid-related genes (CNR1, FAAH, MGLL, DAGLA, DAGLB, and NAPEPLD), ±10kbps to include both promoter and flanker regions, were extracted into a gene set using PLINK (v1.07; http://pngu.mgh.harvard.edu/purcell/plink/; Purcell et al., 2007). CNR2 was not included in our analysis due to lack of coverage on the array. The resulting 79 SNPs were then pruned for independence (50-SNP window shifted 5 SNPs at each step, linkage disequilibrium r2 threshold = .80), which resulted in a pruned set of 65 independent SNPs (excluding CNR2), ranging from 3 (NAPEPLD) to 24 (MGLL) within each gene (Table S2).
The procedure for set-based testing in PLINK was modified and adapted for these analyses3 (Perlis et al., 2008; Purcell et al., 2007). In the modified set-based approach used here, a null distribution was formed via 100,000 label-swapping permutations, in which the outcome phenotype (dependence symptoms), CSA score, and covariates of no interest were permuted together. A participant's full genome was left intact to preserve the linkage structure across individual SNPs during permutations.
Once the relevant data were permuted, statistical tests were performed for each permuted dataset. Ordinary least squares regression was used to test whether the interaction between CSA and each SNP within each gene was associated with cannabis dependence symptoms. Analyses of main effects were conducted in the same manner and were controlled for in interaction testing. Case status (opioid dependent vs. non-dependent), sex, age quintile, and three ancestrally informative principal components were entered as covariates for all analyses. In accordance with recommendations for GxE analyses, additional covariates were entered representing the interactions between the SNP and each covariate of no interest as well as CSA score and each covariate of no interest (Keller, 2014). In order to restrict the gene-level test statistic to only potentially informative SNPs and thus minimize the influence of gene size and within-gene linkage disequilibrium patterns on significance, the maximum number of SNPs within each gene passing the nominal uncorrected significance threshold of p < .05 in a single permutation was used as the number of SNPs (NmaxSNPs) across which the SNP-level ΔR2 values were averaged to form gene-level statistics (Gene-Stats). For example, the gene MGLL contained 24 SNPs, but the maximum number of nominally significant SNPs obtained in any one of the 100,000 permutations was 14, so only the 14 highest ΔR2 values in the original analysis and each permutation were averaged to obtain the gene-level -statistic. In PLINK, in contrast, an arbitrary SNP cutoff across all genes would have been pre-specified.
The gene-level empirical p-value was defined as the proportion of times the original Gene-Stat exceeded a permuted Gene-Stat. Bonferroni correction was then used to adjust for the 6 genes under study, with a final p-threshold p < .0083 (0.05/6). As a corollary to these gene-based analyses, individual SNPs were also tested for significance using a significance threshold of p < .0008 (α=.05/65 SNPs). All statistical analyses were coded in Python (v.2.7.6) using the Numerical Python (“NumPy”, v.1.7.1), StatsModels (v.0.5.0), and Python Data Analysis (“pandas”, v.0.12.0) libraries. Follow-up analyses of individual SNPs were conducted using the PROCESS (Hayes, 2013) and MODPROBE (Hayes & Matthes, 2009) macros in SPSS (v.22).
Study of Addiction: Genetics and Environment (SAGE; N = 859)
European-American4 participants with genotypic and interview data were obtained from the Study of Addiction: Genetics and Environment (SAGE; Bierut et al., 2010). SAGE was funded as part of the Gene Environment Association Studies (GENEVA) initiative supported by the National Human Genome Research Institute (dbGaP study accession phs000092.v1.p1). For this study, alcohol dependent and control participants were recruited from three large, complementary datasets ascertained for alcohol (Collaborative Study of the Genetics of Alcoholism; COGA; Foroud et al., 2000; T. Reich et al., 1998), nicotine (Collaborative Study of the Genetics of Nicotine Dependence; COGEND; Bierut et al., 2007) and cocaine (Family Study of Cocaine Dependence; FSCD; Bierut, Strickland, Thompson, Afful, & Cottler, 2008) dependence. Childhood abuse data were only collected in FSCD and from a subset of COGA participants, constraining our analyses to 859 participants (Table 3). Genotyping details are available in the Supplement. The top SNP from CATS, rs604300 in MGLL (call rate = 100%, HWE p = 0.60, MAF = 0.11), was the only variant examined for the purposes of replication within SAGE and was coded as major allele homozygotes (GG) and A allele carriers (AA/AG) due to the rareness of A allele homozygosity (n = 8). The Institutional Review Board at each contributing institution reviewed and approved the protocols for genetic studies under which all participants were recruited.
Measures
All three participating studies (COGA, COGEND, and FSCD) used the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994). Childhood abuse (CA) was dichotomously coded into participants reporting no physical or sexual abuse (n = 549) or at least one instance of physical or sexual abuse, defined as physical injury by or forced sexual contact with a relative or someone other than a family member before the age of 16 (n = 310). Physical abuse was included due to the smaller sample and limited sexual abuse of the sample. Of our final participants, 65.7% reported having ever used cannabis. As in CATS, dependence symptoms were defined as the number of lifetime DSM-IV dependence symptoms reported (Table 3; M = 1.22, SD = 2.00); due to the relatively small sample size, individuals who had never used cannabis were included in these analyses and coded as “0” dependence symptoms. Distributions of cannabis dependence symptoms across genotype and CA groups are provided in the Supplement (Table S3).
Statistical analyses
Ordinary least squares regression was used to test the association of cannabis dependence symptoms with the interaction between rs604300 genotype and CA. Sex, age quartiles, one ancestrally informative principal component, and study of origin (FSCD or COGA) were included as covariates. As in CATS, additional covariates were entered representing the interactions between rs604300 and each covariate of no interest as well as CA and each covariate of no interest (Keller, 2014).
Duke Neurogenetics Study (DNS; N = 312)
The Duke Neurogenetics Study (DNS) assesses a wide range of behavioral, experiential, and biological phenotypes among young-adult (aged 18-22) college students (Gorka, Knodt, & Hariri, 2014; Corral-Frías et al., 2015). European-American participants who completed the ongoing DNS for whom overlapping fMRI threat-related amygdala reactivity and genetic data were available as of January 6, 2014, were included in analyses (N = 325). Participants provided informed written consent prior to participation and were in good general health and free of DNS exclusion criteria: (1) medical diagnosis of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease or lifetime psychotic symptoms; (2) use of psychotropic, glucocorticoid or hypolipidemic medication, and (3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension). Current DSM-IV Axis I and select Axis II disorders (i.e., Antisocial Personality Disorder and Borderline Personality Disorder) were assessed with the electronic Mini International Neuropsychiatric Interview (Sheehan, 1998) and Structured Clinical Interview for the DSM-IV Axis II (SCID-II; First, Gibbon, Spitzer, Williams, & Benjamin, 1997). These disorders are not exclusionary, as the DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology. Participants were excluded (n = 13) for quality issues in data collection: a large number of movement outliers in fMRI data (n = 3), inadequate signal in our regions of interest (n = 6), and poor behavioral performance (n = 4). This resulted in a final sample for analysis of 312 (Table 3). Details regarding genotyping are available in the Supplement. The top SNP from CATS, rs604300 in MGLL (call rate = 100%, HWE p = 0.75, MAF = 0.10), was the only variant examined for the purposes of these analyses; due to its low minor allele frequency and the resulting small number of minor homozygotes in DNS (n = 2), AA/AG participants were combined to form a minor allele carrier group (n = 61).
Self-report measures
Participants completed a battery of self-report questionnaires to assess past and current experiences and behavior. For the present analyses, the Childhood Trauma Questionnaire: Short Form (CTQ-SF; Bernstein et al., 2003) was used to measure ELS. The CTQ-SF is a 28-item, retrospective screening tool used to detect the occurrence and frequency of emotional, physical, and sexual abuse, as well as emotional and physical neglect before the age of 17. The CTQ has high test-retest reliability (i.e., coefficients ranging from .79 to .86; Bernstein & Fink, 1998) and internal consistency (i.e., coefficients ranging from .66 to .92; Bernstein & Fink, 1998; Scher, Stein, Mccrearyp, & Forde, 2001), and it correlates with both a clinician-rated interview of childhood abuse and independent therapists' ratings of abuse (Bernstein & Fink, 1998; Bernstein et al., 2003). Though a sexual abuse subscore was available, only 3.8% (n = 12) of the final sample reported any form of sexual abuse. As such, an aggregate score of all CTQ-SF questions was used as an index of ELS. As in CATS, this measure was log-transformed to reduce skew (log-transformed: M = 1.49, SD = 0.08; untransformed: M = 31.18, SD = 6.68, where a score of 25 indicates no endorsement of any stressor). These scores are comparable to those obtained in other community (e.g., metropolitan Memphis, Tennessee area, N = 1,007, M = 31.7; Scher et al., 2001) and college samples (e.g., UCSD; N = 949, M = 35.2; Wright et al., 2001). DSM-IV diagnoses of cannabis dependence were available for participants; however, only 1.6% of the sample met diagnostic criteria, so an analysis of cannabis dependence symptomatology was not feasible (Table 3).
BOLD fMRI corticolimbic reactivity paradigm
A widely used and reliable challenge paradigm was employed to elicit amygdala reactivity. The paradigm consists of 4 task blocks requiring face-matching interleaved with 5 control blocks requiring shape-matching. In each face-matching trial within a block, participants view a trio of faces derived from a standard set of facial affect pictures (Ekman & Friesen, 1975; expressing angry, fearful, surprised, or neutral emotions) and select which of 2 faces presented on the bottom row of the display matches the target stimulus presented on the top row. Each emotion-specific block (e.g., fearful facial expressions only) consists of 6 individual trials, balanced for gender of the face. Block order is pseudo-randomized across participants. Each of the 6 face trios is presented for 4 seconds with a variable inter-stimulus interval of 2-6 seconds; total block length is 48 seconds. In the shape-matching control blocks, participants view a trio of geometric shapes (i.e., circles, horizontal and vertical ellipses) and select which of 2 shapes displayed on the bottom matches the target shape presented on top. Each control block consists of 6 different shape trios presented for 4 seconds with a fixed inter-stimulus interval of 2 seconds, comprising a total block length of 36 seconds. The total paradigm is 390 seconds in duration. Reaction times and accuracy are recorded through an MR-compatible button box.
Details regarding BOLD fMRI acquisition and analysis may be found in the Supplement. Briefly, amygdala habituation to threat-related stimuli, our outcome variable of interest in this analysis, was calculated as the linear decrease in reactivity over successive face-matching blocks (i.e., block 1 > block 2 > block 3 > block 4). BOLD paramete estimates from clusters within the left and right basolateral amygdala ROIs exhibiting a main effect for the habituation contrast were extracted using the VOI tool in SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and exported for regression analyses. Extracting parameter estimates from clusters activated by our fMRI paradigm, rather than those specifically correlated with our independent variables of interest, precludes the possibility of any correlation coefficient inflation that may result when an explanatory covariate is used to select a region of interest. We have successfully used this strategy in prior studies (Bogdan, Williamson, & Hariri, 2012). The distribution of this outcome measure across genotype and ELS groups is reported in the Supplement (Table S4).
Statistical analyses
Ordinary least squares regression was used to test the association of rs604300, ELS, and threat-related amygdala habituation. To maintain variability but constrain the influence of extreme outliers, prior to analyses all variables were Winsorized (i.e., outliers more than ± 3 standard deviations from the mean were set at ± 3 standard deviations from the mean; habituation n = 1; ELS n = 2). Sex and two ancestrally informative principal components were entered as covariates for all analyses. As in CATS and SAGE, additional covariates were entered representing the interactions between rs604300 and each covariate of no interest as well as ELS and each covariate of no interest (Keller, 2014).
Results
CATS
No nominally significant gene-level or SNP-level main effects on cannabis dependence emerged. Of the 6 eCB genes tested, only MGLL demonstrated a significant interaction with CSA predicting symptoms of cannabis dependence (Table 4; Figure S1; Gene-Stat = 0.002 using top 14 SNPs; 6 of 24 SNPs nominally significant; p = .0085. Inspection of individual SNPs across the entire eCB gene set revealed one SNP, rs604300 within MGLL, that survived SNP-level Bonferroni correction (Table 5; Table S5; Figure 1; bGxE = -4.37, 95% CI [-6.69, -2.05], ΔR2 = .007, ΔF(1,1527) = 13.69, p = .0002). Within the full rs604300 model, and consistent with prior literature (Duncan et al., 2008), there was an overall main effect of CSA on cannabis dependence symptoms, such that increasing exposure to CSA was associated with a greater number of dependence symptoms (bE = 2.73, 95% CI [1.72, 3.73], p < .001). However, this effect was only present in G allele homozygotes (bE = 3.68, 95% CI [2.56, 4.81], p < .001); there was no association between CSA and cannabis dependence symptoms in heterozygotes (bE = -0.69, 95% CI [-2.75, 1.38], p = .515), and a negative relationship between CSA and cannabis dependence symptoms in A allele homozygotes (bE = -5.06, 95% CI [-9.30, -0.81], p = .020). Post-hoc Johnson-Neyman tests revealed that the association between genotype and cannabis dependence symptoms was significant at mean-centered log-transformed CSA values of 0.11 and above, corresponding to the endorsement of 1.69 or more forms of childhood sexual abuse. There was no main effect of rs604300 genotype on cannabis dependence symptoms (bG = -0.14, 95% CI [-0.37, 0.08], p = .218), nor was there evidence that genotype was associated with CSA (bG = 0.002, 95% CI [-0.01, 0.01], p = .745).
Table 4. Gene-Level Analysis Results in CATS.
| Gene | NmaxSNPS | Gene-Stat | Within-Gene P |
|---|---|---|---|
| CNR1 | 11 | .0006 | .605 |
| DAGLA | 9 | .0002 | .926 |
| DAGLB | 4 | .0006 | .348 |
| FAAH | 5 | .0010 | .167 |
| MGLL | 14 | .0024 | .009 |
| NAPEPLD | 3 | .0009 | .178 |
Note. NmaxSNPS = maximum number of nominally significant (p < 0.05) SNPs obtained in permutations; Gene-Stat = average of the NmaxSNPSmost significant SNP ΔR2 values; Within-Gene P = empirical p-value obtained for each gene.
Table 5. SNP-Level Analysis Significant Results in CATS.
| Gene | SNP | A1 | A2 | MAF | HWE P | T | ΔR2 | P (uncorr.) |
|---|---|---|---|---|---|---|---|---|
| FAAH | rs4660928 | A | C | .28 | .41 | -1.97 | .002 | .049 |
|
| ||||||||
| MGLL | rs604300 | A | G | .11 | .79 | -3.70 | .007 | < .001 |
| rs507961 | A | G | .21 | .94 | -3.23 | .006 | .001 | |
| rs497897 | A | G | .10 | .38 | -3.01 | .005 | .003 | |
| rs13066225 | A | G | .18 | .50 | 2.54 | .004 | .011 | |
| rs664910 | G | A | .31 | .72 | -2.37 | .003 | .018 | |
| rs782446 | C | A | .22 | 1.00 | -2.11 | .002 | .035 | |
Note. A1 = sample minor allele; A2 = sample major allele; MAF = minor allele frequencies; HWE P = Hardy-Weinberg test statistics.
Figure 1.
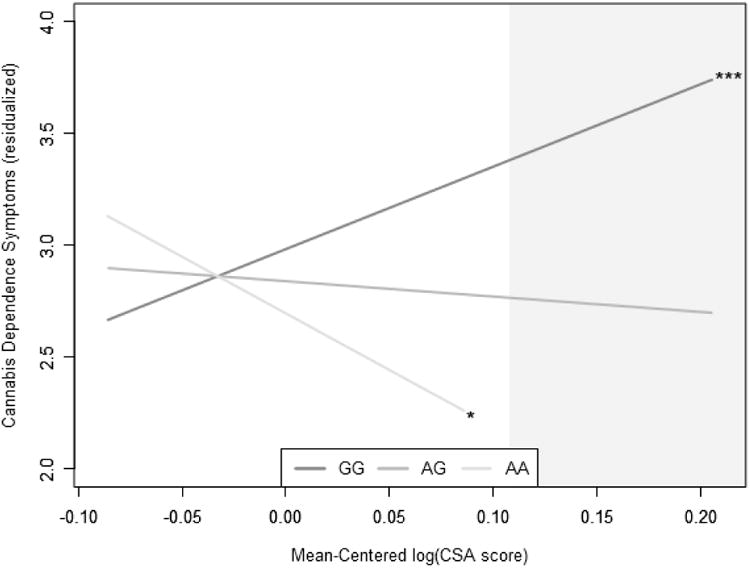
Interaction of rs604300 with childhood sexual abuse to predict cannabis dependence symptoms in CATS. X-axis denotes the log of a participant's CSA score, while y-axis indicates the residualized number of DSM-IV cannabis dependence symptoms endorsed by a participant. Shaded area indicates Johnson-Neyman region of significance. For raw data, see Figure S2. CSA = childhood sexual abuse.
* p < .05. ** p < .01. *** p < .001.
SAGE
The interaction between rs604300 genotype and CA was significantly associated with cannabis dependence symptoms in SAGE (Table S6; Figure 2; bGxE = -0.75, 95% CI [-1.42, -0.09], ΔR2 = .005, ΔF(1,837) = 4.95, p = 0.026). As in CATS, CA was associated with greater endorsement of DSM-IV cannabis dependence criteria (bE = 0.98, 95% CI [0.72, 1.24], p < .001), but this effect was observed only in G homozygotes (bE = 1.13, 95% CI [0.84, 1.42], p < .001), with no association between CA and cannabis dependence symptoms among carriers of the minor A allele (bE = 0.38, 95% CI [-0.22, 0.98], p = .215). Genotype did not predict CA (bG = -0.02, 95% CI [-0.10, 0.61], p = .640), but, unlike in CATS, there was a significant main effect of genotype on cannabis dependence symptoms, such that A allele carriers had relatively greater symptoms (bG = 0.32, 95% CI [0.02, 0.63], p = .039), though post-hoc tests indicated this effect was significant only among participants reporting no CA (bG = 0.59, 95% CI [0.20, 0.98], p = .003)5.
Figure 2.
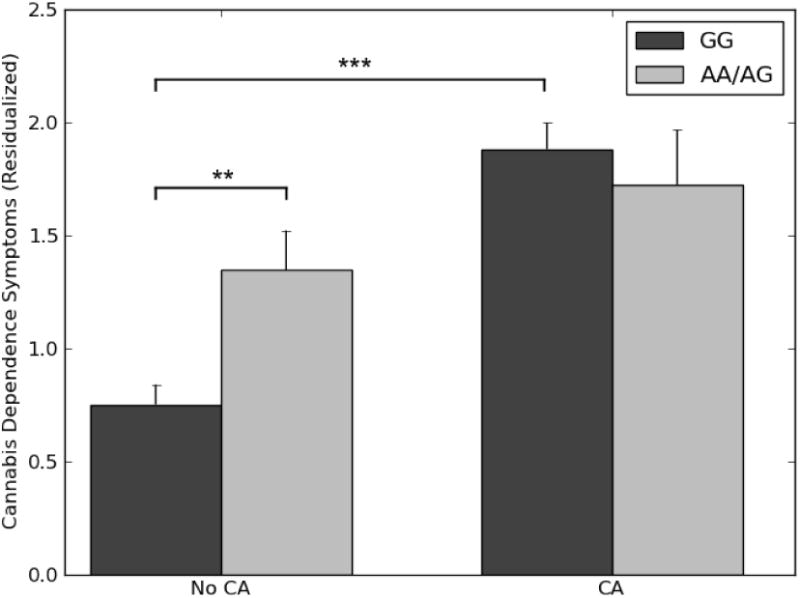
Interaction of rs604300 with childhood abuse to predict cannabis dependence symptoms in SAGE. X-axis denotes whether or not a participant experienced abuse before the age of 16, while y-axis indicates residualized number of DSM-IV cannabis dependence symptoms endorsed by that participant. Error bars represent the SEM. For raw data, see Figure S3. CA = childhood abuse.
* p < .05. ** p < .01. *** p < .001.
DNS
Consistent with prior work, we found evidence of threat-related amygdala habituation, whereby amygdala activation decreased across blocks (Figure 3a). A Genotype × ELS interaction predicted threat-related habituation in the right, but not left, basolateral amygdala (Right: Table S7; Figure 3b; bGxE = 0.77, 95% CI [0.01, 1.53], ΔR2 = .013, ΔF(1,299) = 3.97, p = .047; Left: bGxE = -0.05, 95% CI [-0.70, 1.70], ΔR2 < .001, ΔF(1, 299) = 0.01, p = 0.915). A allele carriers, who were protected against the effects of CA on cannabis dependence symptoms within CATS and SAGE, displayed heightened right basolateral amygdala habituation in the context of increased ELS (bE = 0.82, 95% CI [0.14, 1.51], p = .018); G homozygotes, who were vulnerable to cannabis dependence in the context of childhood abuse in the prior two samples, did not exhibit a relationship between ELS and amygdala habituation (bE = -0.20, 95% CI [-0.28, 0.40], p = .744). Post-hoc Johnson-Neyman tests revealed that the association between genotype and amygdala habituation was significant at mean-centered log-transformed CTQ values of -0.06 (i.e., raw score of 26.68, where the minimum possible score is 25) and below. This pattern is consistent with SAGE, in which, among individuals reporting no CA, A allele carriers had relatively higher cannabis dependence symptoms. The main effects of ELS and genotype on habituation were not significant, and, as in both CATS and SAGE, genotype was not associated with ELS (all ps > 0.10).
Figure 3.
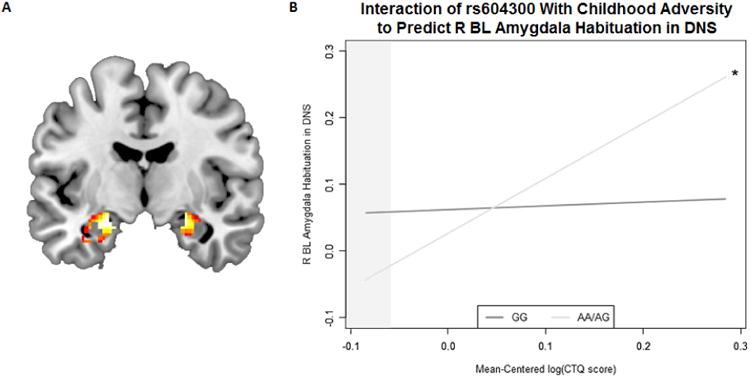
Main effects of fMRI task and interactions with rs604300 to predict threat-related amygdala habituation. A) Bilateral threat-related basolateral amygdala habituation (block 1 > block 2 > block 3 > block 4) across all participants. Right hemisphere: Montreal Neurological Institute (MNI) coordinates = 24, -8, and -16 (t = 9.25, P < .05 FWE), cluster size = 103 voxels. Left hemisphere: MNI coordinates = -22, -8, and -16 (t = 9.00, P < .05 FWE), cluster size = 161 voxels. B) Graph of the interaction between the most significant SNP from the Comorbidity and Trauma Study (CATS), rs604300 in MGLL, with childhood adversity to predict habituation to threat-related stimuli in the right basolateral amygdala in the Duke Neurogenetics Study (DNS). X-axis denotes the log of a participant's score on the Childhood Trauma Questionnaire—Short Form (CTQ), while y-axis indicates amount of neural reactivity to socially-relevant stimuli early in the course of the task relative to later in the task. Shaded area indicates Johnson-Neyman region of significance. For raw data, see Figure S4. R BL = right basolateral.
* p < .05. ** p < .01. *** p < .001.
Post-hoc Structural Equation Modelling
Given the replicated associations between rs603600 and childhood adversity predicting cannabis dependence symptoms and the biological extension of that interaction to predict right basolateral amygdala habituation, a post-hoc structural equation model (SEM) was tested in an opioid-dependent subset of CATS (n = 1,182) that integrated a dichotomous measure of using cannabis to control mood6 (see Supplement for additional methodological details; Figure 4). The rs604300 × CSA interaction significantly predicted using cannabis to control mood (bGxE = -2.04, 95% CI [-3.78, -0.20], p = .025), which, in turn, significantly predicted cannabis dependence symptoms (bM = 0.84, 95% CI [0.71, 0.96], p < .001). The rs604300 × CSA interaction was specific to cannabis: it did not predict the use of other types of substances, or of substances in general, to control mood (all ps> 0.20). The SEM overall demonstrated good fit (relative χ2< 0.001, RMSEA < 0.001, WRMR = 0.40, CFI = 1.00). The indirect pathway from the GxE interaction to cannabis dependence symptoms through using cannabis to control mood was also significant (bIND = -1.71, 95% CI [-3.26, -0.21], p = .029). Analyses of indirect effects within genotype groups revealed that the indirect effect of CSA on cannabis dependence symptoms through using cannabis to control mood was significant only among G allele homozygotes (GG: bIND = 1.85, 95% CI [1.17, 2.47], p < .001; AA/AG: bIND = 0.13, 95% CI [-1.24, 1.27], p = .851). These results are consistent with the proposed hypothesis of differential sensitization to stress across rs604300 genotypes leading to stress-related coping with cannabis and thus susceptibility to dependence.
Figure 4.
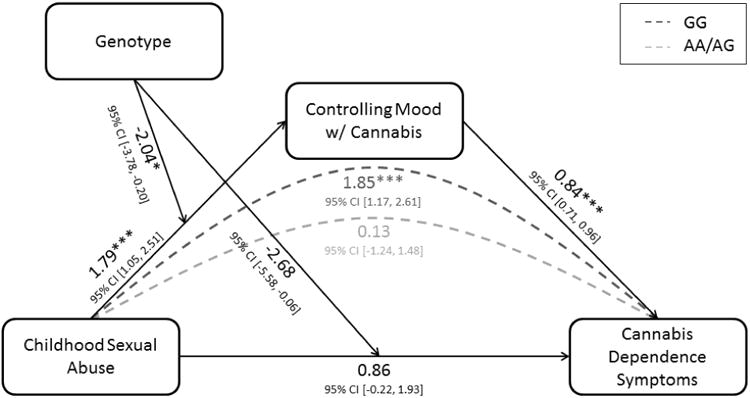
Full structural equation model predicting the effect of childhood sexual abuse (CSA) on cannabis dependence symptoms through using cannabis to control mood, as moderated by rs604300 genotype. The dashed lines represent the indirect effects of CSA on dependence symptoms through the mediator for each genotype group.
* p < .05. ** p < .01. *** p < .001.
Epigenetic Annotation
The WashU EpiGenome Browser (http://epigenomegateway.wustl.edu/browser/; Zhou et al., 2011, 2013, in press) was used to annotate the epigenetic landscape surrounding rs604300. As described in detail in the Supplement, we found evidence that this SNP resides just downstream of epigenetic histone modifications (i.e., H3K4me1, H3K27ac) within a functional enhancer that likely upregulates gene expression. There was evidence of tissue specificity wherein enrichment is highest in neural tissue. Moreover, there was evidence that methylation and histone modification in this epigenetically-regulated enhancer region of MGLL may play role in MGLL transcription (Figure 5 Figure S8). As such, it is possible that environmentally-mediated individual differences in methylation and histone modification-related enhancement of this region may produce differential MGLL expression in the brain. Rs604300 is located near this epigenetically-regulated enhancer; while we are unable to test for genotype-dependent differences in methylation and histone modification with the data available to us, this annotation suggests that rs604300 genotype itself or a SNP in LD with it may impact MGLL expression by affecting epigenetic modification within this MGLL enhancer.
Figure 5.
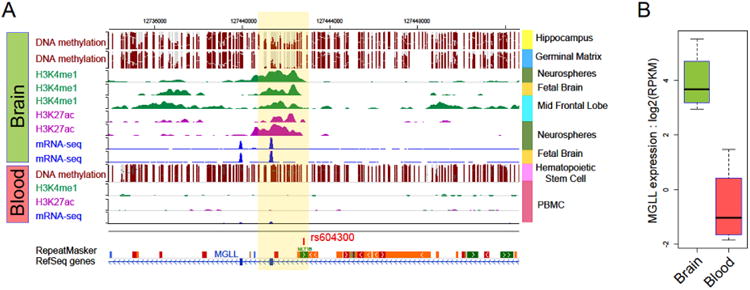
Methylation and histone-mediated epigenetic signatures and MGLL mRNA expression in brain and blood samples. A) There is little methylation in neurospheres in this region accompanied by H3k4me1 and H3K27ac enrichment. Such enrichment, would serve to enhance MGLL gene expression. In contrast, in blood cells, this region is heavily methylated with no H3k4me1 and H3K27ac enrichment. B) These epigenetic markers of methylation and histone-modification-related enhancement may serve to drive MGLL expression in the brain while suppressing it in blood. Additional details may be found in the Supplement.
Discussion
Despite the fundamental link between cannabis and the eCB system, human genetics studies have not reliably linked eCB genetic polymorphisms to cannabis-related outcomes (Agrawal & Lynskey, 2009; Verweij et al., 2012). Here, we found evidence that rs604300 genotype in MGLL moderates the relationship between CSA and cannabis dependence symptoms. Those who carried two copies of the major G allele were at increased risk for cannabis dependence as a function of increasing exposure to CSA events. This finding was not surprising, as CSA has been widely documented as a potent contributor to the etiology of a host of addictive behaviors, including cannabis dependence (Duncan et al., 2008; Fergusson et al., 1996; Myers & Prescott, 2000; Nelson et al., 2006; Simpson & Miller, 2002). What was particularly intriguing was that rs604300 minor A allele carriers did not demonstrate increased vulnerability to cannabis dependence symptoms in the presence of CSA exposure. This alludes to the potential buffering effects of the A allele in rs604300 on the pathogenic effects of CSA. This is highly congruent not only with one prior study of eCB genotypes and their interplay with childhood trauma to predict anhedonia (Agrawal, Nelson, et al., 2012), but also complies with observations in rodent paradigms documenting the role of eCBs in stress adaptation (e.g., Gunduz-Cinar, MacPherson, et al., 2013; Hill & Gorzalka, 2006; Hill & McEwen, 2010; Hill, Patel, et al., 2010; Patel, Roelke, Rademacher, Cullinan, & Hillard, 2004; Viveros, Marco, & File, 2005). Importantly, our finding of increased amygdala habituation as a function of ELS in minor A allele carriers, but not in GG individuals, reinforces the possibility that MGLL rs604300 genotype may play a key role in decoupling the neurobiological link between ELS and mental health outcomes in later life. Such increased amygdala habituation to threatening stimuli may be reflective of an adaptive response that could potentially promote fear extinction when a threat is no longer imminent and, speculatively, a decreased reliance on substances for affect regulation. In support of this hypothesis, a post-hoc SEM demonstrated that the pathway from CSA to cannabis dependence symptoms was partially mediated by using cannabis to control one's mood in GG individuals only. Notably, however, this interpretation remains speculative and must be tempered by our inability to temporally disentangle using marijuana to change one's mood from cannabis dependence symptoms.
In interpreting these results, it is important to note that, consistent with prior behavioral, psychiatric, and neuro-genetics studies (Duncan & Keller, 2011; Flint & Munafo, 2013; Hibar et al., 2015), the effects reported in this study are small, explaining only 0.5-1.3% of variation in our outcome phenotypes. As such, these findings alone will not be practically informative at an individual level. Additionally, evidence suggests that many published GxE results may represent type-I errors, leading to low replication rates (Duncan & Keller, 2011). Interactions between genes and environment, relative to main effects of genotype, are particularly susceptible to increased rates of false positives due to low prior probabilities of interaction, reduced variance of the interaction term, and the potential for unmodeled nonlinearity (see Dick et al., 2015, for review). However, within the current study, the convergence of evidence across three samples implicating rs604300 in individual differences in both cannabis dependence symptoms and a psychiatrically relevant neural phenotype in the context of childhood adversity pinpoints a target within the endocannabinoid system worthy of future replication and extension.
Sensitivity to Stress and Vulnerability to Problematic Cannabis Use: The Importance of 2-AG and MAGL
A majority of research evaluating the role of eCB signaling in stress responsiveness has focused on CB1-AEA activity (Gunduz-Cinar, Hill, et al., 2013; Gunduz-Cinar, MacPherson, et al., 2013). However, emerging rodent research also suggests that 2-AG, which is increased following stress exposure, may be particularly important for recovery from stress's anxiogenic effects (Patel et al., 2009). Consistent with this idea, selective MAGL inhibitors acutely reduce anxiety (Busquets-Garcia et al., 2011; Kinsey, O'Neal, Long, Cravatt, & Lichtman, 2011), with evidence that chronic MAGL inhibition prevents the development of stress-related anxiety, potentially by blocking stress-induced long-term depression of inhibitory signaling in the BLA (Sumislawski, Ramikie, & Patel, 2011).
Interestingly, MAGL inhibition also mimics THC, particularly its anxiolytic and antidepressant-like properties (Wiley et al., 2014), and attenuates cannabis withdrawal in rodents (Schlosburg et al., 2009). Moreover, much like stress, chronic exposure to THC in rodents is associated with desensitization of CB1 receptors and reduction in AEA and 2-AG. Consistent with the potential importance of MAGL in problematic cannabis use, two independent prior cannabis dependence linkage studies have identified the chromosomal region on 3p where MGLL resides (Agrawal et al., 2008; Hopfer et al., 2007). Thus, there is considerable evidence that MAGL, in concert with FAAH and CB1, plays a critical role in the behavioral experiences associated with THC, particularly its effects on mood.
Childhood stress, particularly sexual abuse, is amongst the most prominent risk factors for problems related to mood and anxiety as well as cannabis dependence (Duncan et al., 2008; Lindert et al., 2014). The persistent use and misuse of drugs in individuals exposed to CSA, as well as to childhood physical abuse, may reflect coping behavior (Bujarski et al., 2012; Potter, Vujanovic, Marshall-Berenz, Bernstein, & Bonn-Miller, 2011; Vilhena-Churchill & Goldstein, 2014; Walsh et al., 2014), and this relationship may be susceptible to eCB-regulated modulation in the BLA. Speculatively, individuals with increased MAGL function, corresponding to reduced availability of 2-AG, may be more prone to poorer stress recovery, which could lead to stress-induced elevations in anxiety and resulting emotion regulation with cannabis. Conversely, if the minor A allele of rs604300 is associated with MAGL reductions, then this stress-adaptive state, as reflected by enhanced amygdala habituation in the context of prior stress exposure, might result in stress adaptation, and downstream, with less problematic cannabis use. This also fits well with our pathway analysis, as well as with rodent studies showing the involvement of the eCB in extinction of aversive memories (de Bitencourt, Pamplona, & Takahashi, 2013).
A challenge associated with this interpretation is that the functional consequences of rs604300 are not understood. Our examination of gene expression data (publically available at: http://www.braineac.org/) suggest that rs604300 genotype is not associated with global differences in MGLL expression (Hardy, Trabzuni, & Ryten, 2009; Ryten, Trabzuni, & Hardy, 2009). However, if rs604300-related reductions in MGLL expression only occur in the context of childhood adversity, then its main effects may not be observable in curated expression datasets from the general population. Given the exquisite plasticity of eCB signaling in the context of stress, we speculate that the action of rs604300 on cannabis dependence may be epigenetic in nature. While intronic, rs604300 is located just downstream of an enhancer site that is heavily influenced by epigenetic modification (see Supplement). As such, it is possible that rs604300 may result in epigenetically-dependent differences in expression. Future epigenetic research in the context of early life adversity with this locus may be promising.
Limitations
While the interaction between rs604300 genotype and CA reached significance in SAGE, there are several caveats to interpreting this replication. First, due to the low prevalence of CSA (20.0%), childhood abuse was defined in SAGE as exposure to sexual or physical abuse. This is in contrast to CATS, where individuals reported experiencing 1.57 CSA events on average. These substantial differences in childhood abuse experiences across samples could have resulted in the regions of significance differing across studies (i.e., genotype effects only at higher levels of CSA in CATS and only among the no-CA group in SAGE and low ELS group in DNS). Furthermore, due to heterogeneous measurement of CA across the studies contributing to the SAGE sample, CA was dichotomized (present versus absent), thus resulting in a loss of power when compared to the continuous measure used in CATS. Relatedly, as the minor allele is rare, AA and AG individuals were combined in SAGE. As shown in Figures S5 and S6, restricting the sample to CSA alone or examining AA and AG individuals separately did not alter the nature of the interaction; however, we did not consider these findings to be reliable due to the modest sample size (Table S3). Conversely, assuming a dominant model in CATS (i.e., examining AA/AG individuals together) or using a combined measure of childhood physical and sexual abuse resulted in little change to the nature or significance of the interaction (dominant model: bGxE = -4.62, 95% CI [-7.01, -2.22], ΔR2 = .008, ΔF(1,1527) = 14.29, p = .0002; combined CSA and CPA: bGxE = -3.18, 95% CI [-6.23, -0.13], ΔR2 = .002, ΔF(1,1527) = 4.18, p = .041).
A second important distinction between CATS and SAGE relates to the distribution of cannabis dependence symptoms. Due to study design (i.e. opioid dependent cases and controls from high risk neighborhoods), the rate of lifetime cannabis use in CATS was 96.1%, and a majority of the participants reported experiencing cannabis dependence symptoms. Despite the oversampling for alcohol dependence in SAGE, rates of cannabis use and endorsement of dependence symptoms were lower, presumably due to the control group, which was not environmentally matched to the alcoholic cases. This necessitated the inclusion of never-users of cannabis in the replication analyses; however, restricting analyses to ever users produced a similar, albeit non-significant, pattern of results (Figure S7). The distinction between the phenotypes used in CATS and SAGE is nontrivial. By including never-users in SAGE, the effects of genotype and environment and their interplay on later, more problematic stages of cannabis involvement (i.e. dependence symptoms) cannot be disentangled from their effects on cannabis use. As a corollary, the high rate of cannabis use in CATS precluded any study of contributors to its variance. Therefore, we can only conclude that the interaction between rs604300 and childhood abuse may be related to both onset of cannabis use as well as misuse. This is consistent with twin epidemiological studies that document a high degree of genetic overlap between these stages of cannabis involvement (Agrawal et al., 2012).
Third, we were unable to link individual differences in amygdala habituation as a result of rs604300 and childhood trauma interplay to cannabis dependence. The extent of problematic cannabis use is limited in this college-attending sample – only 48.4% of the EA participants report using cannabis even once in their lifetime, and, of those, only 23.7% report using it >10 times, with 4 individuals meeting criteria for cannabis dependence. Because THC administration reduces anxiety in rodents and amygdala reactivity to threatening stimuli, it is possible that individuals who show prolonged amygdala response (i.e., decreased amygdala habituation), may be more sensitive to the coping-related effects of cannabis and thus more likely to develop problematic cannabis usage (Phan et al., 2009; Koob & Volkow, 2010). Evidence that fear extinction and drug-seeking extinction rely on similar neural pathways also suggests that individuals who can more readily adapt to threatening stimuli may also be more able to extinguish substance use (Peters, Kalivas, & Quirk, 2009). On the other hand, it is also entirely possible that increased amygdala habituation might lead to more exploratory behavior and exposure to substances, but not necessarily increased problematic use (Lissek et al., 2005).
Fourth, while we conducted a system-level analysis, our gene-based testing did not reach statistical significance after Bonferroni correction. The single SNP that did survive correction, rs604300, is unlikely to be the only important variant within the eCB system. The balance between AEA (metabolized by FAAH) and 2-AG may be paramount to understanding eCB system contributions to psychiatric disorders and related brain function. A particularly fruitful future approach may be to examine evidence for epistasis using system-level analytic methods that do not rely on SNP-level priors or on the additive nature of the current approach (e.g., random forest regression trees; for review, see Upstill-Goddard, Eccles, Fliege, & Collins, 2013).
Summary
Limitations notwithstanding, this study finds evidence across two samples that the minor A allele of rs604300 within MGLL exerts protective effects against childhood abuse-related increases in cannabis dependence. We further extend these findings to show that rs604300 genotype interacts with early life stress to predict amygdala habituation, providing a neural mechanism for future study in the context of cannabis dependence symptoms. Consistent with the proposed hypothesis that cannabis dependence symptoms may arise from using cannabis to cope with stress, a post-hoc structural equation model showed that reporting that one uses cannabis to alter one's mood indirectly linked the interaction between rs604300 and childhood sexual abuse to cannabis dependence symptoms only among G allele homozygotes. These findings await further replication and validation from independent studies.
This study highlights the importance of considering the role of genotype in stress adaptation or resilience. In addition to its clearly demonstrated impact on energy regulation/obesity and pain modulation (André & Gonthier, 2010; Iversen & Chapman, 2002; Li, Jones, & Persaud, 2011; Lynch & Ware, 2015), there is considerable research underway exploring the therapeutic role of eCB signaling in treatment of mental health problems (Hill & Gorzalka, 2009), including addiction (e.g., Pava & Woodward, 2012). For example, MAGL inhibition attenuates the effects of withdrawal in opioid-, nicotine-, and THC-dependent rodents (Muldoon et al., 2015; Ramesh et al., 2011, 2013; Schlosburg et al., 2009). If eCB signaling is associated with individual differences in stress adaptation, then medications directed at enhancing these effects may be a valuable resource in the treatment arsenal. However, manipulation of the eCB system is challenging and prior failed clinical trials documenting serious adverse psychiatric events, including suicide, in cardiovascular risk patients treated with CB1 antagonist, rimonabant, serves as a cautionary tale (Boekholdt & Peters, 2010; Topol et al., 2010).
Supplementary Material
General Scientific Summary.
This study suggests that genetic variation within the endocannabinoid system confers differential susceptibility to cannabis dependence symptoms in the context of early life adversity. These effects may arise through associations with threat-related brain function and substance-related coping.
Acknowledgments
Funding: CEC is supported by National Science Foundation predoctoral grant DGE-1143954. AA acknowledges support from R01DA23668 and K02DA32573. TW, BZ, and DL are supported by American Cancer Society grant RSG-14-049-01-DMC, NIH grants R01HG007354, R01HG007175, and R01ES024992. LD is supported by an Australian National Health and Medical Research Council (NHMRC) research fellowship (1041472). ARH receives support through National Institute on Drug Abuse grants DA033369 and DA031579. RB receives support from the Klingenstein Third Generation Foundation and the National Institutes of Health (NIA R01-AG045231).
Funding support for the Comorbidity and Trauma Study (CATS) was provided by the National Institute on Drug Abuse (R01 DA17305); GWAS genotyping services at the Center for Inherited Disease Research (CIDR) at The Johns Hopkins University were supported by the National Institutes of Health [contract N01-HG-65403]. The National Drug and Alcohol Research Centre at the University of New South Wales is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvements Grants Fund.
Funding support for the Study of Addiction: Genetics and Environment (SAGE) was provided through the NIH Genes, Environment and Health Initiative [GEI] (U01 HG004422). SAGE is one of the genome-wide association studies funded as part of the Gene Environment Association Studies (GENEVA) under GEI. Assistance with phenotype harmonization and genotype cleaning, as well as with general study coordination, was provided by the GENEVA Coordinating Center (U01 HG004446). Assistance with data cleaning was provided by the National Center for Biotechnology Information. Support for collection of datasets and samples was provided by the Collaborative Study on the Genetics of Alcoholism (COGA; U10 AA008401), the Collaborative Genetic Study of Nicotine Dependence (COGEND; P01 CA089392), and the Family Study of Cocaine Dependence (FSCD; R01 DA013423, R01 DA019963). Funding support for genotyping, which was performed at the Johns Hopkins University Center for Inherited Disease Research, was provided by the NIH GEI (U01HG004438), the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the NIH contract “High throughput genotyping for studying the genetic contributions to human disease” (HHSN268200782096C).
The Duke Neurogenetics Study is supported by Duke University and National Institute on Drug Abuse grant DA033369.
Footnotes
The DSM-5 no longer diagnoses cannabis dependence. Instead, the 6 DSM-IV dependence criteria were combined with 3 abuse criteria (use in hazardous situations, failure to fulfil major role obligations and social/interpersonal problems due to recurrent use), as well as withdrawal and craving to define cannabis use disorders in individuals experiencing 2 or more of these problems in a 12 month period. However, as the data used in this study precede DSM-5 by several years, we utilize the DSM-IV definitions.
DSM-IV cannabis dependence symptoms: tolerance, use in larger quantities or for longer than intended, repeated unsuccessful attempts to quit or cut back, giving up important activities, spending excessive time acquiring or using cannabis, and recurrent use despite physical and/or emotional problems.
The set-based testing procedure in PLINK v 1.07 does not allow for tests of GxE interactions and uses an averaging procedure that is sensitive to differences in gene size, which was undesirable for the eCB gene set, in which the number of tagged SNPs within each gene, post-pruning for quality assurance and linkage disequilibrium, ranged from 3 to 24.
Only participants of European ancestry were selected in our replication and follow-up samples in order to be consistent with the ancestral composition of CATS.
A similar pattern was observed in CATS, wherein the A allele was associated with greater cannabis dependence symptoms in participants reporting no CSA, but this effect was not significant (bG = 0.24, 95% CI [-0.06, 0.54], p = .120).
The question of interest (“Have you frequently used [cannabis] to control your mood or to chase another drug?”) was asked in a section of the interview only completed by participants meeting criteria for opioid dependence. See Supplement for full context.
Theodore Beauchaine served as the Guest Editor for this manuscript.
Disclosure of interest: LJB is listed as an inventor on Issued U.S. Patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. EDC is employed by 23andMe.
Contributor Information
Caitlin E. Carey, Department of Psychology, Washington University in St. Louis
Arpana Agrawal, Department of Psychiatry, Washington University School of Medicine.
Bo Zhang, Department of Genetics, Washington University School of Medicine.
Emily D. Conley, 23andMe, Mountain View, California
Louisa Degenhardt, National Drug and Alcohol Research Centre, University of New South Wales Australia.
Andrew C. Heath, Department of Psychiatry, Washington University School of Medicine
Daofeng Li, Department of Genetics, Washington University School of Medicine.
Michael T. Lynskey, Institute of Psychiatry, Kings College London
Nicholas G. Martin, QIMR Berhofer Medical Research Institute
Grant W. Montgomery, QIMR Berghofer Medical Research Institute
Ting Wang, Department of Genetics, Washington University School of Medicine.
Laura J. Bierut, Department of Psychiatry, Washington University School of Medicine
Ahmad R. Hariri, Department of Psychology and Neuroscience, Duke University
Elliot C. Nelson, Department of Psychiatry, Washington, University School of Medicine
Ryan Bogdan, Department of Psychology, Washington University in St. Louis.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th, text rev. Washington, DC: 2000. [Google Scholar]
- Agrawal A, Lynskey MT. Candidate genes for cannabis use disorders: Findings, challenges and directions. Addiction. 2009;104(4):518–32. doi: 10.1111/j.1360-0443.2009.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Bucholz KK, Kapoor M, Almasy L, Dick DM, et al. Bierut LJ. DSM-5 cannabis use disorder: A phenotypic and genomic perspective. Drug and Alcohol Dependence. 2014;134:362–9. doi: 10.1016/j.drugalcdep.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Hinrichs A, Grucza R, Saccone SF, Krueger R, et al. Bierut LJ. A genome-wide association study of DSM-IV cannabis dependence. Addiction Biology. 2011;16(3):514–8. doi: 10.1111/j.1369-1600.2010.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Nelson EC, Littlefield AK, Bucholz KK, Degenhardt L, Henders AK, et al. Lynskey MT. Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Archives of General Psychiatry. 2012;69(7):732–40. doi: 10.1001/archgenpsychiatry.2011.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Pergadia ML, Saccone SF, Lynskey MT, Wang JC, Martin NG, et al. Madden PAF. An autosomal linkage scan for cannabis use disorders in the nicotine addiction genetics project. Archives of General Psychiatry. 2008;65(6):713–21. doi: 10.1001/archpsyc.65.6.713. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Verweij KJH, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, et al. Lynskey MT. The genetics of addiction-a translational perspective. Translational Psychiatry. 2012 May;2:e140. doi: 10.1038/tp.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André A, Gonthier MP. The endocannabinoid system: Its roles in energy balance and potential as a target for obesity treatment. The International Journal of Biochemistry & Cell Biology. 2010;42(11):1788–801. doi: 10.1016/j.biocel.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14(8):746–54. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyamina A, Kebir O, Blecha L, Reynaud M, Krebs MO. CNR1 gene polymorphisms in addictive disorders: A systematic review and a meta-analysis. Addiction Biology. 2011;16(1):1–6. doi: 10.1111/j.1369-1600.2009.00198.x. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A retrospective self-reportmanual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27(2):169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, et al. Rice JP. A genome-wide association study of alcohol dependence. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(11):5082–7. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, et al. Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Human Molecular Genetics. 2007;16(1):24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Strickland JR, Thompson JR, Afful SE, Cottler LB. Drug use and dependence in cocaine dependent subjects, community-based individuals, and their siblings. Drug and Alcohol Dependence. 2008;95(1-2):14–22. doi: 10.1016/j.drugalcdep.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T. Endogenous cannabinoids: Structure and metabolism. Journal of Neuroendocrinology, 20 Suppl. 2008;1(10):1–9. doi: 10.1111/j.1365-2826.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: Biochemical aspects. Pharmacology, Biochemistry, and Behavior. 2005;81(2):224–38. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Boekholdt SM, Peters RJG. Rimonabant: Obituary for a wonder drug. Lancet. 2010;376(9740):489–90. doi: 10.1016/S0140-6736(10)61080-X. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. American Journal of Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. Journal of Studies on Alcohol and Drugs. 1994;55(2):159. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Bujarski SJ, Feldner MT, Lewis SF, Babson KA, Trainor CD, Leen-Feldner E, et al. Bonn-Miller MO. Marijuana use among traumatic event-exposed adolescents: Posttraumatic stress symptom frequency predicts coping motivations for use. Addictive Behaviors. 2012;37(1):53–9. doi: 10.1016/j.addbeh.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biological Psychiatry. 2011;70(5):479–86. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Buwembo A, Long H, Walker CD. Participation of endocannabinoids in rapid suppression of stress responses by glucocorticoids in neonates. Neuroscience. 2013;249:154–61. doi: 10.1016/j.neuroscience.2012.10.057. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene – environment interactions in psychiatry: Joining forces with neuroscience. Nature Reviews: Neuroscience. 2006 Jul;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K. [(18)F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addiction Biology. 2013:1–11. doi: 10.1111/adb.12116. [DOI] [PubMed] [Google Scholar]
- Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience. 2015;4(1):7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Frías NS, Nikolova YS, Michalski LJ, Baranger DAA, Hariri AR, Bogdan R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychological Medicine. 2015:1–13. doi: 10.1017/S0033291715000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bitencourt RM, Pamplona FA, Takahashi RN. A current overview of cannabinoids and glucocorticoids in facilitating extinction of aversive memories: Potential extinction enhancers. Neuropharmacology. 2013;64:389–95. doi: 10.1016/j.neuropharm.2012.05.039. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. An introduction to the endocannabinoid system: From the early to the latest concepts. Best Practice & Research Clinical Endocrinology & Metabolism. 2009;23(1):1–15. doi: 10.1016/j.beem.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Deng XD, Jiang H, Ma Y, Gao Q, Zhang B, Mu B, et al. Liu Y. Association between DRD2/ANKK1 TaqIA polymorphism and common illicit drug dependence: Evidence from a meta-analysis. Human Immunology. 2015;76(1):42–51. doi: 10.1016/j.humimm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacological Research. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoid signaling in the brain: Biosynthetic mechanisms in the limelight. Nature Neuroscience. 2011;14(1):9–15. doi: 10.1038/nn.2720. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, et al. Sher KJ. Candidate gene-environment interaction research: Reflections and recommendations. Perspectives on Psychological Science. 2015;10(1):37–59. doi: 10.1177/1745691614556682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F, Gusnanto A, Koeleman BPC. Detecting multiple associations in genome-wide studies. Human Genomics. 2006;2(5):310–7. doi: 10.1186/1479-7364-2-5-310. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3500180&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AE, Sartor CE, Scherrer JF, Julia D, Heath AC, Nelson EC, et al. Bucholz KK. The association between cannabis abuse and dependence and childhood physical and sexual abuse: Evidence from an offspring of twins design. Addiction. 2008;103(6):990–997. doi: 10.1111/j.1360-0443.2008.02210.x.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Unmasking the face. Englewood Cliffs, NJ: Spectrum-Prentis Hall; 1975. [Google Scholar]
- El Rawas R, Thiriet N, Nader J, Lardeux V, Jaber M, Solinas M. Early exposure to environmental enrichment alters the expression of genes of the endocannabinoid system. Brain Research. 2011;1390:80–9. doi: 10.1016/j.brainres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. Childhood sexual abuse and psychiatric disorder in young adulthood: II. Psychiatric outcomes of childhood sexual abuse. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(10):1365–74. doi: 10.1097/00004583-199610000-00024. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2010;35(4):967–75. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) American Psychiatric Press, Inc; 1997. [Google Scholar]
- Flint J, Munafò MR. Candidate and non-candidate genes in behavior genetics. Current Opinion in Neurobiology. 2013;23(1):57–61. doi: 10.1016/j.conb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Edenberg HJ, Goate A, Rice J, Flury L, Koller DL, et al. Reich T. Alcoholism susceptibility loci: Confirmation studies in a replicate sample and further mapping. Alcoholism: Clinical and Experimental Research. 2000;24(7):933–945. doi: 10.1111/j.1530-0277.2000.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Gorka AX, Knodt AR, Hariri AR. Basal forebrain moderates the magnitude of task-dependent amygdala functional connectivity. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: An FMRI study. Drug and Alcohol Dependence. 2009;105(1-2):139–53. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: Mediating protection and recovery from stress. Trends in Pharmacological Sciences. 2013;34(11):637–44. doi: 10.1016/j.tips.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, et al. Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular Psychiatry. 2013;18(7):813–23. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374(9698):1383–91. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JRZ, et al. Oldehinkel AJ. Differential susceptibility in youth: Evidence that 5-HTTLPR × positive parenting is associated with positive affect “for better and worse”. Translational Psychiatry. 2011;1(9):e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Trabzuni D, Ryten M. Whole genome expression as a quantitative trait. Biochemical Society Transactions. 2009;37(Pt 6):1276–7. doi: 10.1042/BST0371276. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: Influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. Addiction. 2008;103(10):1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x.Marijuana. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. 2013 doi: 10.1017/SJP.2021.46. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41(3):924–36. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, et al. Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: Findings and implications. Biological Psychiatry. 2011;70(6):513–8. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Neural control of chronic stress adaptation. Frontiers in Behavioral Neuroscience. 2013 Aug;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. Medland SE. Common genetic variants influence human subcortical brain structures. Nature. 2015;8 doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Increased sensitivity to restraint stress and novelty-induced emotionality following long-term, high dose cannabinoid exposure. Psychoneuroendocrinology. 2006;31(4):526–36. doi: 10.1016/j.psyneuen.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS & Neurological Disorders Drug Targets. 2009;8(6):451–8. doi: 10.2174/187152709789824624. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19839936. [DOI] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34(5):791–7. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TTY, Gray JM, et al. Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9406–11. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34(13):2733–45. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: From synaptic signaling to behavioral output. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(45):14980–6. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmans P. Advances in genetics. 1st. Vol. 72. Elsevier Inc; 2010. Statistical methods for pathway analysis of genome-wide data for association with complex genetic traits; pp. 141–79. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Lessem JM, Hartman CA, Stallings MC, Cherny SS, Corley RP, et al. Crowley TJ. A genome-wide scan for loci influencing adolescent cannabis dependence symptoms: Evidence for linkage on chromosomes 3 and 9. Drug and Alcohol Dependence. 2007;89(1):34–41. doi: 10.1016/j.drugalcdep.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L, Chapman V. Cannabinoids: A real prospect for pain relief? Current Opinion in Pharmacology. 2002;2:50–55. doi: 10.1016/s1471-4892(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC. Gene × environment interaction studies have not properly controlled for potential confounders: The problem and the (simple) solution. Biological Psychiatry. 2014;75(1):18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, O'Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacology, Biochemistry, and Behavior. 2011;98(1):21–7. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie CC, Doheny KF, Mirel DB, Pugh EW, Bierut LJ, Bhangale T, et al. Weir BS. Quality control and quality assurance in genotypic data for genome-wide association studies. Genetic Epidemiology. 2010;34(6):591–602. doi: 10.1002/gepi.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TTY, Hill MN. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience. 2013;249:106–14. doi: 10.1016/j.neuroscience.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Li C, Jones PM, Persaud SJ. Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas. Pharmacology & Therapeutics. 2011;129(3):307–20. doi: 10.1016/j.pharmthera.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Li MX, Gui HS, Kwan JSH, Sham PC. GATES: A rapid and powerful gene-based association test using extended Simes procedure. American Journal of Human Genetics. 2011;88(3):283–93. doi: 10.1016/j.ajhg.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced antinociception in rats. Pharmacology Biochemistry and Behavior. 1997;57(1-2):7–12. doi: 10.1016/S0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Lindert J, von Ehrenstein OS, Grashow R, Gal G, Braehler E, Weisskopf MG. Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: Systematic review and meta-analysis. International Journal of Public Health. 2014;59(2):359–72. doi: 10.1007/s00038-013-0519-5. [DOI] [PubMed] [Google Scholar]
- Lissek S, Baas JMP, Pine DS, Orme K, Dvir S, Rosenberger E, Grillon C. Sensation seeking and the aversive motivational system. Emotion. 2005;5(4):396–407. doi: 10.1037/1528-3542.5.4.396. [DOI] [PubMed] [Google Scholar]
- Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, et al. Macgregor S. A versatile gene-based test for genome-wide association studies. American Journal of Human Genetics. 2010;87(1):139–45. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2011;115(1-2):120–30. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Ware M. Cannabinoids for the treatment of chronic non-cancer pain: An updated systematic review of randomized controlled trials. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology. 2015 doi: 10.1007/s11481-015-9600-6. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodriguez de Fonseca F. Cannabinoid addiction: Behavioral models and neural correlates. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(9):3326–3331. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Kearn CS, Chongue L, Mackie K, Taylor DA. Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience. 2008;152(1):265–72. doi: 10.1016/j.neuroscience.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Shmulewitz D, Wall MM, Keyes KM, Aharonovich E, Spivak B, et al. Hasin D. Childhood adversity moderates the effect of ADH1B on risk for alcohol-related phenotypes in Jewish Israeli drinkers. Addiction Biology. 2013:1–10. doi: 10.1111/adb.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon PP, Chen J, Harenza JL, Abdullah RA, Sim-Selley LJ, Cravatt BF, et al. Damaj MI. Inhibition of monoacylglycerol lipase enzyme reduces nicotine withdrawal. British Journal of Pharmacology. 2015;172(3):869–82. doi: 10.1111/bph.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: An epidemiological and cotwin analysis. Archives of General Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Neale BM, Sham PC. The future of association studies: Gene-based analysis and replication. American Journal of Human Genetics. 2004;75(3):353–62. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Lynskey MT, Bucholz KK, Madden PAF, Statham DJ, Martin NG. Childhood sexual abuse and risks for licit and illicit drug-related outcomes: A twin study. Psychological Medicine. 2006;36(10):1473–83. doi: 10.1017/S0033291706008397. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Lynskey MT, Heath AC, Wray N, Agrawal A, Shand FL, et al. Montgomery GW. ANKK1, TTC12, and NCAM1 polymorphisms and heroin dependence: Importance of considering drug exposure. JAMA Psychiatry. 2013;70(3):325–33. doi: 10.1001/jamapsychiatry.2013.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Lynskey MT, Heath AC, Wray N, Agrawal A, Shand FL, et al. Montgomery GW. Association of OPRD1 polymorphisms with heroin dependence in a large case-control series. Addiction Biology. 2014;19(1):111–21. doi: 10.1111/j.1369-1600.2012.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova YS, Ferrell RE, Manuck SB, Hariri AR. Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2011;36(9):1940–7. doi: 10.1038/npp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34(13):2699–709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145(12):5431–8. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: Implications for alcohol dependence and future directions for research. Alcohol (Fayetteville, NY) 2012;46(3):185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JF, Fergusson DM, Horwood LJ, Miller AL, Sullivan PF, Youfang LE, Kennedy MA. Increased risk of major depression by childhood abuse is not modified by CNR1 genotype. Am J Med Genet B Neuropsychiatr Genet. 2013;0(2):224–226. doi: 10.1002/ajmg.b.32124.Increased. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Purcell S, Fagerness J, Kirby A, Petryshen TL, Fan J, Sklar P. Family-based association study of lithium-related and other candidate genes in bipolar disorder. Archives of General Psychiatry. 2008;65(1):53–61. doi: 10.1001/archgenpsychiatry.2007.15. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & Memory. 2009;16:279–288. doi: 10.1101/lm.1041309.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28(10):2313–9. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4(11):873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CMA, Davis OSP. Common disorders are quantitative traits. Nature Reviews Genetics. 2009;10(12):872–8. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- Potter CM, Vujanovic Aa, Marshall-Berenz EC, Bernstein A, Bonn-Miller MO. Posttraumatic stress and marijuana use coping motives: The mediating role of distress tolerance. Journal of Anxiety Disorders. 2011;25(3):437–43. doi: 10.1016/j.janxdis.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. Sham PC. PLINK: A tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WSV, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54(1):108–16. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Ramanan VK, Shen L, Moore JH, Saykin AJ. Pathway analysis of genomic data: Concepts, methods, and prospects for future development. Trends in Genetics : TIG. 2012;28(7):323–32. doi: 10.1016/j.tig.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Gamage TF, Vanuytsel T, Owens RA, Abdullah RA, Niphakis MJ, et al. Lichtman AH. Dual inhibition of endocannabinoid catabolic enzymes produces enhanced antiwithdrawal effects in morphine-dependent mice. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2013;38(6):1039–49. doi: 10.1038/npp.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, et al. Lichtman AH. Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. The Journal of Pharmacology and Experimental Therapeutics. 2011;339(1):173–85. doi: 10.1124/jpet.111.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramikie TS, Patel S. Endocannabinoid signaling in the amygdala: Anatomy, synaptic signaling, behavior, and adaptations to stress. Neuroscience. 2012;204:38–52. doi: 10.1016/j.neuroscience.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Mihalik GR, Iskander aN, Seckler JC, Weiss MS. Adolescent chronic mild stress alters hippocampal CB1 receptor-mediated excitatory neurotransmission and plasticity. Neuroscience. 2013;253:444–54. doi: 10.1016/j.neuroscience.2013.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich T, Edenberg H, Goate A, Williams J, Rice J, Van Eerdewegh P, et al. Begleiter H. Genome-wide search for genes affecting the risk for alcohol dependence. American Journal of Medical Genetics. 1998;81(3):207–215. [PubMed] [Google Scholar]
- Reimers MA, Riley BP, Kalsi G, Kertes DA, Kendler KS. Pathway based analysis of genotypes in relation to alcohol dependence. The Pharmacogenomics Journal. 2012;12(4):342–8. doi: 10.1038/tpj.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryten M, Trabzuni D, Hardy J. Genotypic analysis of gene expression in the dissection of the aetiology of complex neurological and psychiatric diseases. Briefings in Functional Genomics & Proteomics. 2009;8(3):194–8. doi: 10.1093/bfgp/elp028. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Selling RE, Hutchison KE. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: An exploratory analysis. Psychopharmacology. 2009;203(3):511–7. doi: 10.1007/s00213-008-1397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher, Stein B, Mccrearyp DR, Forde DR. The Childhood Trauma Questionnaire in a community sample: Psychometric properties and normative data. Journal of Traumatic Stress. 2001;14(4) doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Carlson BLa, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. The AAPS Journal. 2009;11(2):342–52. doi: 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Bortolato M, Eisenstein SA, Fu J, Oveisi F, Hohmann AG, Piomelli D. Social isolation and chronic handling alter endocannabinoid signaling and behavioral reactivity to context in adult rats. Neuroscience. 2010;168(2):371–86. doi: 10.1016/j.neuroscience.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand FL, Degenhardt L, Slade T, Nelson EC. Sex differences amongst dependent heroin users: Histories, clinical characteristics and predictors of other substance dependence. Addictive Behaviors. 2011;36(1-2):27–36. doi: 10.1016/j.addbeh.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;50(Suppl 2):22–23. quiz 34–57. [PubMed] [Google Scholar]
- Simpson TL, Miller WR. Concomitance between childhood sexual and physical abuse and substance use problems. Clinical Psychology Review. 2002;22(1):27–77. doi: 10.1016/S0272-7358(00)00088-X. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR, Piomelli D. The endocannabinoid system in brain reward processes. British Journal of Pharmacology. 2008;154(2):369–83. doi: 10.1038/bjp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: Prevalence, correlates and co-morbidity. Psychological Medicine. 2006;36(10):1447–60. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Administration. National Survey on Drug Use and Health. Washington, D.C: 2012. [Google Scholar]
- Sumislawski JJ, Ramikie TS, Patel S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: A potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2011;36(13):2750–61. doi: 10.1038/npp.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol EJ, Bousser MG, Fox KAA, Creager MA, Despres JP, Easton JD, et al. Bhatt DL. Rimonabant for prevention of cardiovascular events (CRESCENDO): A randomised, multicentre, placebo-controlled trial. Lancet. 2010;376(9740):517–23. doi: 10.1016/S0140-6736(10)60935-X. [DOI] [PubMed] [Google Scholar]
- Upstill-Goddard R, Eccles D, Fliege J, Collins A. Machine learning approaches for the discovery of gene-gene interactions in disease data. Briefings in Bioinformatics. 2013;14(2):251–60. doi: 10.1093/bib/bbs024. [DOI] [PubMed] [Google Scholar]
- Verweij KJH, Zietsch BP, Liu JZ, Medland SE, Lynskey MT, Madden PAF, et al. Martin NG. No association of candidate genes with cannabis use in a large sample of Australian twin families. Addiction Biology. 2012;17(3):687–90. doi: 10.1111/j.1369-1600.2011.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJ, Zietsch BP, Lynskey MT, Medland SE, Neale MC, Martin NG, et al. Vink JM. Genetic and environmental influences on cannabis use initiation and problematic use: A meta-analysis of twin studies. Addiction. 2010;105(3):417–430. doi: 10.1111/j.1360-0443.2009.02831.x.Genetic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhena-Churchill N, Goldstein AL. Child maltreatment and marijuana problems in young adults: Examining the role of motives and emotion dysregulation. Child Abuse & Neglect. 2014;38(5):962–72. doi: 10.1016/j.chiabu.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacology, Biochemistry, and Behavior. 2005;81(2):331–42. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Walsh K, Elliott JC, Shmulewitz D, Aharonovich E, Strous R, Frisch A, et al. Hasin D. Trauma exposure, posttraumatic stress disorder and risk for alcohol, nicotine, and marijuana dependence in Israel. Comprehensive Psychiatry. 2014;55(3):621–30. doi: 10.1016/j.comppsych.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Bucan M. Pathway-based approaches for analysis of genomewide association studies. American Journal of Human Genetics. 2007;81(6):1278–83. doi: 10.1086/522374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. Analysing biological pathways in genome-wide association studies. Nature Reviews Genetics. 2010;11(12):843–54. doi: 10.1038/nrg2884. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Walentiny DM, Wright MJ, Beardsley PM, Burston JJ, Poklis JL, et al. Vann RE. Endocannabinoid contribution to Δ9-tetrahydrocannabinol discrimination in rodents. European Journal of Pharmacology. 2014;737:97–105. doi: 10.1016/j.ejphar.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll Ra. Science. 5568. Vol. 296. New York, N.Y: 2002. Endocannabinoid signaling in the brain; pp. 678–82. [DOI] [PubMed] [Google Scholar]
- Wise LE, Long KA, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH, States U. Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS Chemical Neuroscience. 2012;3:369–378. doi: 10.1021/cn200130s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34(9):2072–80. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KD, Asmundson GJG, Ph D, Mccreary DR, Scher C, Hami S, Stein MB. Factorial validity of the childhood trauma questionnaire in men and women. Depression and Anxiety. 2001;13:179–183. [PubMed] [Google Scholar]
- Zhou X, Li D, Zhang B, Lowdon RF, Madden PAF, Smirnov I, et al. Wang T. Epigenomic annotation of genetic variants using the Roadmap EpiGenome Browser. Nature Biotechnology. doi: 10.1038/nbt.3158. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Lowdon RF, Li D, Lawson HA, Madden PAF, Costello JF, Wang T. Exploring long-range genome interactions using the WashU Epigenome Browser. Nature Methods. 2013;10(5):375–6. doi: 10.1038/nmeth.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Maricque B, Xie M, Li D, Sundaram V, Martin EA, et al. Wang T. The Human Epigenome Browser at Washington University. Nature Methods. 2011;8(12):989–90. doi: 10.1038/nmeth.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


