Abstract
Adjuvants to the traditional therapy of inflammatory bowel disease (IBD) have been studied to enhance the efficacy of the treatment and improve patients’ quality of life. Omega-3 polyunsaturated fatty acids (ω3FA) have been associated with attenuation of the inflammatory responses in IBD, possibly acting as substrates for anti-inflammatory eicosanoid production, similar to prostaglandins and leukotrienes. ω3FA also act as substrates for the synthesis of resolvins, maresins and protectins, indispensable in resolving inflammation processes. These acids may influence the development or course of IBD by: reducing oxidative stress, production of tumor necrosis factor-α and proinflammatory cytokines; working as chemopreventive agents; and decreasing the expression of adhesion molecules. There are numerous controversies in the literature on the effects of ω3FA in the prevention or treatment of IBD, but their effects in reducing inflammation is incontestable. Therefore, more studies are warranted to elucidate the pathophysiological mechanisms and establish the recommended daily intake to prevent or induce remission in IBD patients.
Keywords: Ulcerative colitis, Crohn’s disease, omega-3 polyunsaturated fatty acids
Introduction
The immune system prevents against infection involving inflammatory processes resulting in a response to trauma or microbial infections and it is related to the process completion in order to extinguish the stimulus or to remove the tissue damage. Many diseases such as cardiovascular disorders, Alzheimer’s disease, rheumatoid arthritis, cancer and inflammatory bowel disease (IBD) are caused by inflammatory processes and the course of the pathology continues because of the inappropriate or excessive responses that accompany them chronically [1,2].
Under homeostasis the gastrointestinal system represents a perfect balance between the host and the microbiota in a complex and dynamic process, with important role in the mucosal immunity. When this balance is lost the consequences can result in the increase in intestinal permeability and bacterial translocation across the intestinal mucosa, leading to a local and systemic immune activation implicated in many different diseases including IBD. The two main forms of IBD include Crohn’s disease (CD) and ulcerative colitis (UC) [3-7].
When a patient develops IBD, he acquires the disability of recognition of pattern recognition receptors (PRRs) as Toll-like receptors (TLR), on epithelial and immune cells in the intestine. This leads to the incapacity of differentiating between pathogenic and commensal bacteria (macrophages and dendritic cells on recognition of commensal microbiota modify their status to an activated phenotype) and consequently, extends the activation of nuclear factor kappa B (NFκB), a pro-inflammatory transcription factor which triggers overproduction of inflammatory cytokines, such as tumor necrosis factor (TNF) -α and interleukins (IL) -1β, -6, -12 and -23 (Fig. 1). Processed antigens are presented to naïve CD4 T-cells and the natural killer T cells produce IL-13, strongly associated with the epithelial cell barrier disruption. Circulating T cells bind to colonic endothelial cells through the mucosal vascular adhesion molecule 1, whose production increases in the inflamed intestine. This is accompanied by upregulation of inflammatory chemokines and consequent recruitment of circulating leukocytes that leads to the perpetuation of the inflammation. The chronic inflammatory process involves modifications on the bowel habits, pain, bleeding, and increases the risk for bowel cancer [8-11].
Figure 1.
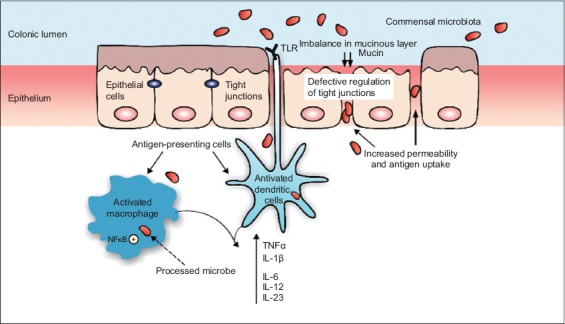
Pathophysiology of inflammatory bowel disease. The imbalance in mucinous layer allows higher permeability of the intestinal epithelium leading to an increase in the uptake of antigens. Dendritic cells and macrophages recognize commensal microbiota and become activated. Activation of nuclear factor kappa B (NFκB) increases the synthesis of proinflammatory cytokines as tumor necrosis factor (TNF)-α, interleukin (IL) -6, -12, -23, and -1β. Modified from Ordas et al [10]
TLR, toll-like receptor
UC and CD may affect adults and young population driving to a prolonged course and recurrence, affecting education, capacity for work and quality of life. The care IBD patient should have is a challenge due to the heterogeneous nature of the disease and the lack of consensus in many areas of practice. IBD management is usually conducted by pharmacotherapy but patients should be approached in different ways to have a follow up to match their needs and improve their quality of life. This should be done by a multidisciplinary team and the treatment should go beyond the use of conventional therapies.
Several substances as corticosteroids, thiopurines and biologic agents are available and antibiotics, probiotics, and nutritional supplements can be used as supportive therapy. Thus, the use of alternative therapies as omega-3 polyunsaturated fatty acids (PUFA) (n-3 or ω3 FA) could bring important benefits to the IBD patient [8,12-15].
Methods
This review was based on a survey of articles in order to bring relevant information about the use of ω3FA. We used the following databases: PubMed, Medline, Scielo, Scopus and Lilacs. A retrospective search was carried out to identify relevant clinical trials or epidemiological studies and reviews limited to indexed scientific articles involving humans and animals.
Influence of diet on IBD patients
There is a genetic predisposition to the development of IBD but its increasing incidence in developing countries suggests that environmental factors, such as diet, are also critical components of susceptibility to the occurrence of the disease. Authors have shown that highest consumption of red meat, saturated fat, refined carbohydrates, and food additives as well the low amount of dietetic fibers, fruits, vegetables and antioxidants had increased risk of developing IBD. Dietary compounds as protein, linoleic acid (ω6FA) and digestible carbohydrates may contribute to the pathogenesis because they cause intestinal microbiota modifications leading to an increase in intestinal permeability, and inflammation processes augment [16-25].
Several authors have shown that, in addition to modifications in the food choices, the use of ω3FA may bring benefits because may influence the development or course of IBD [16,26-30]. Normally, the recommended intake of ω3FA is 1.6 g/day for men and 1.1 g/day for women. This intake can come from the regular food consumption or from supplementation with fish or olive oil or use of emulsions consisting of coconut oil, soy, olive oil or fish. Literature shows converging opinions about a daily recommendation but authors agree that 500 mg/day of eicosapentaenoic acid/docosahexaenoic acid could bring health benefits. Di Nicolantonio et al [31] suggested 2 servings of fatty fish per week for the general population. There is no consensus on ω3FA dietary recommendations for IBD patients.
ω3FA
ω3FA belong to a lipid class called PUFA. This family includes lipids with two or more double bonds considered to be essential nutrients because the body does not have the capacity to produce them endogenously. They can be found in significant proportions in different food sources, as in linseed, nuts and fish. Examples of these acids are α linoleic acid with a chain with 18 carbon atoms and 3 double bonds (C18:3n-3), eicosapentaenoic acid (C20:5n-3), and docosahexaenoic acid (Fig. 2) (C22:6 n-3) [32-33].
Figure 2.
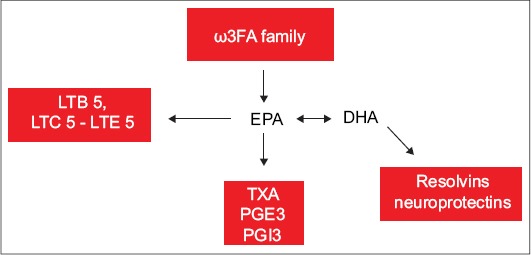
Synthesis of eicosanoids from ω3 FA: 3 series prostanoids TXA3, PGE3 and PGI3 and 5 series leukotrienes LTB5, LTC5-LTE5. Modified from Barbalho et al [33] and Din et al [37]
ω3 FA, omega 3 fatty acid; TXA3, thromboxane A3; PGE3, prostaglandin E3; PGI3 and 5, prostaglandin I3 and 5; LTB5, leukotriene B5; LTC5-LTE5, leukotriene C5-leukotriene E5
While saturated fatty acids are related to insulin resistance, higher levels of triglycerides, weight gain, increase in the adipocyte size and increase in adipose tissue inflammation, ω3FA improve blood lipid levels, reduce weight and attenuate inflammation processes implicated in cardiovascular diseases and other inflammatory diseases. They can also improve neural function and sensitivity to acetylcholine, balance the membrane fluidity and decrease post-exercise inflammation leading to adaptations to exercises such as decreasing aspects of fatigue and improving peripheral neuromuscular function [32,34-36].
Pathophysiological data
The interest in the use of ω3 FA has grown tremendously in the last years. They are substrates for inflammatory and anti-inflammatory eicosanoid production, such as prostaglandins and leukotrienes, and so they have been used to the prevention of different inflammatory diseases in animals and humans (Fig. 3). One possibility to explain the beneficial effects of ω3FA is the competition that avoids conversion of arachidonic acid to pro-inflammatory eicosanoids such as prostaglandins, leukotrienes and lipoxins through the cyclooxygenase or lipoxygenase enzymes. Eicosapentaenoic acid and docosahexaenoic acid can replace arachidonic acid and inhibit pro-inflammatory mediator production. They may also inhibit inflammation acting in leukocyte chemotaxis, adhesion molecule expression and leukocyte-endothelial adhesive interactions, and suppressing the production of other inflammatory cytokines, and T-helper 1 lymphocyte reactivity. Furthermore, ω3FA are substrates to the synthesis of resolvins, maresins and protectins, indispensable in resolving inflammation processes [26,37-41].
Figure 3.
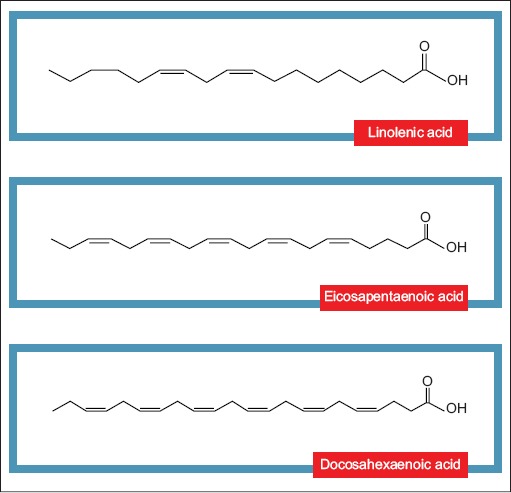
Structure of omega 3 fatty acids: first double bond at the third carbon molecule from the methyl end of the chain. There are three possibilities for names: C18:3n-3, C20:5n-3 and C22:6n-3omega, respectively linolenic acid, eicosapentanoic acid and docosahexanoic acid
The beginning of inflammation is important for the body to make the defense against trauma or microorganism infection, and so is the finalization of the process. If this does not occur, the organism will develop a disease. In this duet, i.e. the beginning and the end of inflammation process, the same lipid substances are involved. Thus, the use of eicosapentaenoic acid and docosahexaenoic acid may be promising in minimizing or preventing inflammatory diseases such as IBD [42-44]. Both animal and clinical studies show that these acids may have a potential role in the treatment of IBD. Besides, patients see them as both safe and natural [30]. IBD patients may exhibit a deficiency in essential fatty acids, and ω3FA supplements may benefit IBD patients by inhibiting natural cytotoxicity (by changing arachidonic acid metabolites) and/or improving oxidative stress. The anti-inflammatory actions of ω3FA may also be associated with their ability to change the composition of the cell membrane and the ability to activate the anti-inflammatory transcription peroxisome proliferator activated receptor (PPAR) γ [26,30,45-48].
There is evidence that the gastrointestinal mucosa is highly responsive to long-chain PUFA such as ω3. The intake of ω3FA can be helpful in the treatment of UC and CD as it can alleviate the symptoms and help the recovery of the mucosal due to its anti-inflammatory properties. Reasons probably are related to the reduction in the intestinal production of the precursor of pro-inflammatory cytokines (leukotrienes and prostaglandins) of odd series. Furthermore, there is evidence that these acids may reduce the protein expression of intestinal NFκB p65 related to apoptotic cells [28,41,48-52].
ω3FA may influence the membrane-cytoskeletal structure and function in CD4+ T cells leading to the reduction in the inflammation processes [22]. TLR and nucleotide-binding oligomerization domain proteins (NOD) have a critical role in the detection of microbial infection and induction of inflammatory and immune responses. When both TLR and NOD are activated, there is activation of NFκB which stimulates synthesis of pro-inflammatory cytokines. Studies are associating TLR4 and NOD signaling in multi-layered IBD, interfering in pathogen-associated molecular patterns leading to acute and chronic intestinal inflammation [10,49,53,54].
Results of clinical studies
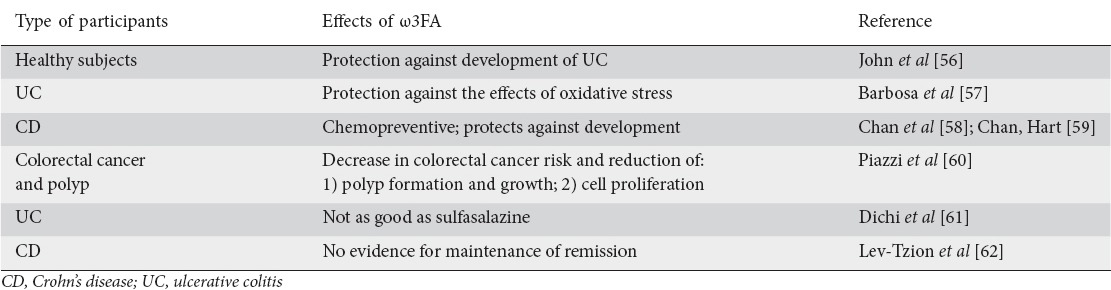
Many authors have studied the role of omegas in the prevention, treatment and maintenance of remission of inflammatory diseases such as IBD. Pearl et al [55] studied colonic mucosa biopsies from 69 UC patients and found that inflamed mucosa had higher levels of arachidonic, docosapentaenoic and docosahexaenoic acids and lower levels of linoleic, α-linolenic and eicosapentaenoic acids compared with the control group. The severity of inflammation was positively associated with the levels of arachidonic, docosapentaenoic and docosahexaenoic acid and negatively associated with levels of linoleic, α-linolenic and eicosapentaenoic acids suggesting that there are modifications in fatty acid metabolism in the inflamed gut mucosa. These modifications can offer novel targets for intervention and nutritional strategies should also be considered. Table 1 summarizes the studies showing the effects of ω3FA in different types of participants; some show an important role of ω3FA in the course of CD, UC and reduction of colorectal cancer and polyp, while others provided inconclusive or negative results [56-62].
Table 1.
Effects of the use of omega 3 fatty acids (ω3FA) in different type of participants
The controversial findings on the relationship between ω3FA and IBD (as seen in Table 1) may be due to a number of reasons: 1) different forms of ω3FA have different effects when compared to the native form found in fish; 2) genetic differences in ω3FA receptors may interfere with the responsiveness to fatty acid supplementation; 3) modifications in G-protein receptors and PPAR-α (considered to be a dietary lipid sensor); and 4) differences or problems in their methodology (insufficient number of patients, short period of study, heterogeneity of disease, lifestyle and other aspects of the studied population). These aspects may interfere in the efficacy of ω3FA in controlling symptoms, inflammation and remission in IBD patients [8,16,30,63,64].
ω3FA in animal models
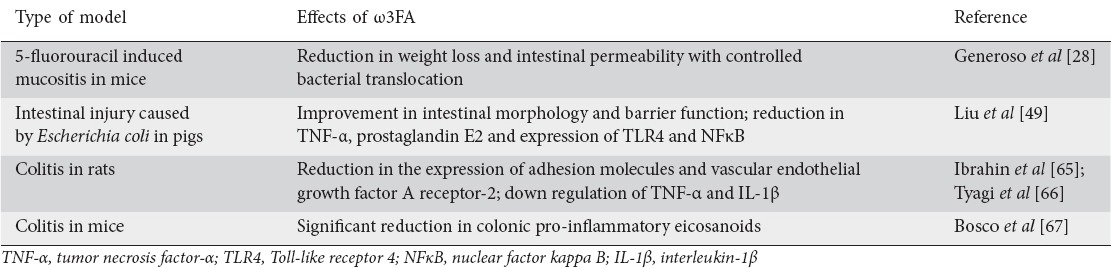
Literature is also rich in studies using ω3FA in different types of animal models. Table 2 presents some studies showing that these acids may reduce weight loss and intestinal permeability, improve the intestinal morphology and barrier function and decrease the synthesis of inflammatory markers as TNF-α, IL-6, IL-1β, prostaglandin E2 and expression of TLR4 and NFκB [28,49,65-67].
Table 2.
Effects of the use of omega 3 fatty acids (ω3FA) in animal models
ω3FA and pain
ω3FA are shown to regulate pain, depending on the amount of intake and subsequent cellular distribution. When a large amount of ω3FA was administered, reduced thermal hyperalgesia was observed compared with a group that received a large amount of linoleic acid, suggesting that there is a dose-dependent association between these acids and pain control. Pain relief was observed in several pathologies, including IBD, possibly because ω3FA reduce proinflammatory cytokine and eicosanoid production. The use of ω3FA can also block the activity of mitogen-activated protein kinase related to the modulation of central sensitization induced by inflammatory and neuropathic pain. Linolenic acid declines the production of lysophosphatidic acid that is strongly related to the development of neuropathic pain [68-73]. It has been hypothesized that the effects of docosahexaenoic acid in pain control are due to its anti-inflammatory effect via suppression of the arachidonic acid cascade; inhibition of voltage-gated sodium channels; and promotion of the agonistic action toward transient receptor potential vanilloid 1 (related to inflammation processes and calcium channels inhibition). They also found that docosahexaenoic acid reduces pain indirectly through the release of an endogenous opioid peptide β-endorphin and not only because it acts on the opioid receptor [74,75]. Other studies showed that increased consumption of ω3FA and decreased consumption of ω6FA can modify endocannabinoid production in humans thereby suggesting that their derivatives could have physical and/or psychological pain modulating properties [76,77].
Concluding remarks
IBD is considered a public health problem owing to the high cost it incurs for the Public Health System and the burden it has on the patients’ quality of life. Several studies show that ω3FA lead to the production of resolvins, protectins and maresins which attenuate the inflammatory processes possibly benefiting IBD patients. However, there are many controversies over the ω3FA effects on IBD, and results of the studies should be interpreted with caution due to the enormous variability in the size of the samples, the amount of ω3FA administered and the methodology employed. Studying the pharmacology of ω3FA will help establish their real effects, thus bringing new possibilities to the treatment of inflammatory diseases. More research is warranted to fully elucidate how these acids influence IBD and to define the daily amount recommended to help prevent or induce remission of IBD.
Biography
University of Marília and Food Technology School (FATEC); University Hospital, UNIMAR; Diagnostic Center in Gastroenterology, Brazil
Footnotes
Conflict of Interest: None
References
- 1.Stark AK, Sriskantharajah S, Hessel EM, Okkenhaug K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr Opin Pharmacol. 2015;23:82–91. doi: 10.1016/j.coph.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerra I, Bermejo F. Biosimilars in inflamatory bowel disease: Management and care. Rev Esp Enferm Dig. 2015;107:389. [PubMed] [Google Scholar]
- 3.Nee J, Feuerstein JD. Optimizing the care and health of women with inflammatory bowel disease. Gastroenterol Res Pract. 2015;2015:435820. doi: 10.1155/2015/435820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elia PP, Tolentino YF, Bernardazzi C, de Souza HS. The role of innate immunity receptors in the pathogenesis of inflammatory bowel disease. Mediators Inflamm. 2015;2015:936193. doi: 10.1155/2015/936193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade ME, Araújo RS, de Barros PA, et al. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin Nutr. 2015;34:1080–1087. doi: 10.1016/j.clnu.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Victoria CR, Sassaki LY, Nunes HRC. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009;46:20–25. doi: 10.1590/s0004-28032009000100009. [DOI] [PubMed] [Google Scholar]
- 7.Nguyena DL, Leea JG, Parekha NK, Samarasenaa J, Bechtoldb ML, Chang K. The current and future role of endomicroscopy in the management of inflammatory bowel disease. Ann Gastroenterol. 2015;28:331–336. [PMC free article] [PubMed] [Google Scholar]
- 8.Tabbaa M, Golubic M, Roizen MF, Bernstein AM. Docosahexaenoic acid, inflammation, and bacterial dysbiosis in relation to periodontal disease, inflammatory bowel disease, and the metabolic syndrome. Nutrients. 2013;5:3299–3310. doi: 10.3390/nu5083299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal world congress of gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 10.Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 11.Cheifetz AS. Management of active Crohn disease. J Am Med Association. 2013;309:2150–2158. doi: 10.1001/jama.2013.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. doi: 10.2147/DDDT.S11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prüfer J, Schuchardt M, Tölle M, et al. Harmful effects of the azathioprine metabolite 6-mercaptopurine in vascular cells: induction of mineralization. PLoS One. 2014;9:e101709. doi: 10.1371/journal.pone.0101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shores DR, Binion DG, Freeman BA, Baker PR. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:2192–2204. doi: 10.1002/ibd.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 16.Dixon LJ, Kabi A, Nickerson KP, McDonald C. Combinatorial effects of diet and genetics on inflammatory bowel disease pathogenesis. Inflamm Bowel Dis. 2015;21:912–922. doi: 10.1097/MIB.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40:754–760. doi: 10.1136/gut.40.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russel MG, Engels LG, Muris JW, et al. Modern life in the epidemiology of inflammatory bowel disease: A case-control study with special emphasis on nutritional factors. Eur J Gastroenterol Hepatol. 1998;10:243–249. doi: 10.1097/00042737-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Frolkis A, Dieleman LA, Barkema HW, et al. Environment and the inflammatory bowel diseases. Can J Gastroenterol. 2013;27:e18–e24. doi: 10.1155/2013/102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung MK, Park MY. Nutritional modulators of ulcerative colitis: clinical efficacies and mechanistic view. World J Gastroenterol. 2013;19:994–1004. doi: 10.3748/wjg.v19.i7.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeffer-Gik T, Levine A. Dietary clues to the pathogenesis of Crohn’s disease. Dig Dis. 2014;32:389–394. doi: 10.1159/000358143. [DOI] [PubMed] [Google Scholar]
- 22.Hou TY, McMurray DN, Chapkin RS. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.03.091. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farzaei MH, Rahimi R, Abdollahi M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr Pharm Biotechnol. 2015;16:196–210. doi: 10.2174/1389201016666150118131704. [DOI] [PubMed] [Google Scholar]
- 24.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault M-C, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol. 2010;105:2195–2201. doi: 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 25.Ananthakrishnan AN, Khalili H, De Silva PS. Higher dietary fiber intake is associated with lower risk of Crohn’s disease but not ulcerative colitis – a prospective study. Gastroenterology. 2010;142(Suppl 1):S-148. [Google Scholar]
- 26.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Lorente-Cebrián S, Costa AG, Navas-Carretero S, et al. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem. 2015;71:341–349. doi: 10.1007/s13105-015-0395-y. [DOI] [PubMed] [Google Scholar]
- 28.Generoso SV, Rodrigues NM, Trindade LM, et al. Dietary supplementation with omega-3 fatty acid attenuates 5-fluorouracil induced mucositis in mice. Lipids Health Dis. 2015;14:54. doi: 10.1186/s12944-015-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin FP, Lichti P, Bosco N, et al. Metabolic phenotyping of an adoptive transfer mouse model of experimental colitis and impact of dietary fish oil intake. J Proteome Res. 2015;14:1911–1919. doi: 10.1021/pr501299m. [DOI] [PubMed] [Google Scholar]
- 30.Farrukh A, Mayberry JF. Is there a role for fish oil in inflammatory bowel disease? World J Clin Cases. 2014;2:250–252. doi: 10.12998/wjcc.v2.i7.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiNicolantonio JJ, Niazi AK, McCarty MF, O’Keefe JH, Meier P, Lavie CJ. Omega-3s and cardiovascular health. Ochsner J. 2014;14:399–412. [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis EJ, Radonic PW, Wolever TM, Wells GD. 21 days of mammalian omega-3 fatty acid supplementation improves aspects of neuromuscular function and performance in male athletes compared to olive oil placebo. J Int Soc Sports Nutr. 2015;12:28. doi: 10.1186/s12970-015-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbalho SM, Bechara MD, Quesada K, Goulart RA. Omega 3 fatty acid and the resolution of inflammatory processes. Medicina. 2011;44:234–240. [Google Scholar]
- 34.Duivenvoorde LP, van Schothorst EM, Swarts HM, et al. A difference in fatty acid composition of isocaloric high-fat diets alters metabolic flexibility in male C57BL/6JOlaHsd mice. PLoS One. 2015;10:e0128515. doi: 10.1371/journal.pone.0128515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duivenvoorde LP, van Schothorst EM, Derous D, et al. Oxygen restriction as challenge test reveals early high-fat-diet-induced changes in glucose and lipid metabolism. Pflugers Arch. 2015;467:1179–1193. doi: 10.1007/s00424-014-1553-8. [DOI] [PubMed] [Google Scholar]
- 36.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. J Am Med Assoc. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 37.Din JN, Newby DE, Flapan AD. Omega 3 fatty acids and cardiovascular disease-fishing for a natural treatment. BMJ. 2004;328:30–35. doi: 10.1136/bmj.328.7430.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gdula-Argasińska J, Czepiel J, Woźniakiewicz A, et al. n-3 Fatty acids as resolvents of inflammation in the A549 cells. Pharmacol Rep. 2015;67:610–615. doi: 10.1016/j.pharep.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 39.White PJ, Mitchell PL, Schwab M, et al. Transgenic ω-3 PUFA enrichment alters morphology and gene expression profile in adipose tissue of obese mice: Potential role for protectins. Metabolism. 2015;64:666–676. doi: 10.1016/j.metabol.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Shevalye H, Yorek MS, Coppey LJ, et al. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J Neurophysiol. 2015;114:199–208. doi: 10.1152/jn.00224.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arisue A, Shimojima N, Tomiya M, et al. Effect of an omega-3 lipid emulsion in reducing oxidative stress in a rat model of intestinal ischemia–reperfusion injury. Pediatr Surg Int. 2012;28:913–918. doi: 10.1007/s00383-012-3144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiala M, Halder RC, Sagong B, et al. ω-3 Supplementation increases amyloid-βphagocytosis and resolvin D1 in patients with minor cognitive impairment. FASEB J. 2015;29:2681–2689. doi: 10.1096/fj.14-264218. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Hjorth E, Vedin I, et al. Effects of n-3 FA supplementation on the release of pro-resolving lipid mediators by blood mononuclear cells: the OmegAD study. J Lipid Res. 2015;56:674–681. doi: 10.1194/jlr.P055418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki H, Sasaki T, Ueda T, Arita M. Resolvins as regulators of the immune system. Sci World J. 2010;10:818–831. doi: 10.1100/tsw.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salem N, Jr, Eggersdorfer M. Is the world supply of omega-3 fatty acids adequate for optimal human nutrition? Curr Opin Clin Nutr Metab Care. 2015;18:147–154. doi: 10.1097/MCO.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 46.Kris-Etherton PM, Innis S Ammerican Dietetic Assocition Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107:1599–1611. [PubMed] [Google Scholar]
- 47.Uchiyama K, Nakamura M, Odahara S, et al. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1696–1707. doi: 10.1002/ibd.21251. [DOI] [PubMed] [Google Scholar]
- 48.Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. 2011;187:1957–1969. doi: 10.4049/jimmunol.1101305. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Chen F, Odle J, Lin X, Jacobi SK, Zhu H, Wu Z. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr. 2012;142:2017–2024. doi: 10.3945/jn.112.164947. [DOI] [PubMed] [Google Scholar]
- 50.Gurzell EA, Wiesinger JA, Morkam C, Hemmrich S, Harris WS, Fenton JI. Is the omega-3 index a valid marker of intestinal membrane phospholipid EPA+DHA content? Prostaglandins Leukot Essent Fatty Acids. 2014;91:87–96. doi: 10.1016/j.plefa.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loeschke K, Ueberschaer B, Pietsch A, et al. n-3 fatty acids only delay early relapse of ulcerative colitis in remission. Dig Dis Sci. 1996;41:2087–2094. doi: 10.1007/BF02093614. [DOI] [PubMed] [Google Scholar]
- 52.Hawthorne AB, Daneshmend TK, Hawkey CJ, et al. Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut. 1992;33:922–928. doi: 10.1136/gut.33.7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parlato M, Yeretssian G. NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int J Mol Sci. 2014;15:9594–9627. doi: 10.3390/ijms15069594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Pearl DS, Masoodi M, Eiden M, et al. Altered colonic mucosal availability of n-3 and n-6 polyunsaturated fatty acids in ulcerative colitis and the relationship to disease activity. J Crohns Colitis. 2014;8:70–79. doi: 10.1016/j.crohns.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 56.John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:602–606. doi: 10.1097/MEG.0b013e3283352d05. [DOI] [PubMed] [Google Scholar]
- 57.Barbosa DS, Cecchini R, El Kadri MZ, Rodríguez MA, Burini RC, Dichi I. Decreased oxidative stress in patients with ulcerative colitis supplemented with fishoil omega-3 fatty acids. Nutrition. 2003;19:837–842. doi: 10.1016/s0899-9007(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 58.Chan SS, Luben R, Olsen A, et al. Association between high dietary intake of the n-3 polyunsaturated fatty acid docosahexaenoic acid and reduced risk of Crohn’s disease. Aliment Pharmacol Ther. 2014;39:834–842. doi: 10.1111/apt.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan SS, Hart AR. Commentary: the association between high dietary intake of docosahexaenoic acid and reduced risk of Crohn’s disease-authors’ reply. Aliment Pharmacol Ther. 2014;39:1332. doi: 10.1111/apt.12755. [DOI] [PubMed] [Google Scholar]
- 60.Piazzi G, D’Argenio G, Prossomariti A, et al. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int J Cancer. 2014;135:2004–2013. doi: 10.1002/ijc.28853. [DOI] [PubMed] [Google Scholar]
- 61.Dichi I, Frenhane P, Dichi JB, et al. Comparison of omega-3 fatty acids and sulfasalazine in ulcerative colitis. Nutrition. 2000;16:87–90. doi: 10.1016/s0899-9007(99)00231-2. [DOI] [PubMed] [Google Scholar]
- 62.Lev-Tzion R, Griffiths AM, Leder O, Turner D. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014;2:CD006320. doi: 10.1002/14651858.CD006320.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoshi T, Wissuwa B, Tian Y, et al. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+channels. Proc Natl Acad Sci USA. 2013;110:4816–4821. doi: 10.1073/pnas.1221997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marion-Letellier R, Savoye G, Beck PL, Panaccione R, Ghosh S. Polyunsaturated fatty acids in inflammatory bowel diseases: a reappraisal of effects and therapeutic approaches. Inflamm Bowel Dis. 2013;19:650–661. doi: 10.1097/MIB.0b013e3182810122. [DOI] [PubMed] [Google Scholar]
- 65.Ibrahim A, Aziz M, Hassan A, et al. Dietary α-linolenic acid-rich formula reduces adhesion molecules in rats with experimental colitis. Nutrition. 2012;28:799–802. doi: 10.1016/j.nut.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 66.Tyagi A, Kumar U, Reddy S, et al. Attenuation of colonic inflammation by partial replacement of dietary linoleic acid with α-linolenic acid in a rat model of inflammatory bowel disease. Br J Nutr. 2012;108:1612–1622. doi: 10.1017/S0007114511007197. [DOI] [PubMed] [Google Scholar]
- 67.Bosco N, Brahmbhatt V, Oliveira M, et al. Effects of increase in fish oil intake on intestinal eicosanoids and inflammation in a mouse model of colitis. Lipids Health Dis. 2013;12:81. doi: 10.1186/1476-511X-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tokuyama S, Nakamoto K. Unsaturated fatty acids and pain. Biol Pharm Bull. 2011;34:1174–1178. doi: 10.1248/bpb.34.1174. [DOI] [PubMed] [Google Scholar]
- 69.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wagner K, Vito S, Inceoglu B, Hammock BD. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014;113-115:2–12. doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pérez J, Ware MA, Chevalier S, Gougeon R, Bennett GJ, Shir Y. Dietary fat and protein interact in suppressing neuropathic pain-related disorders following a partial sciatic ligation injury in rats. Pain. 2004;111:297–305. doi: 10.1016/j.pain.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Mirnikjoo B, Brown SE, Kim HF, Marangell LB, Sweatt JD, Weeber EJ. Protein kinase inhibition by omega-3 fatty acids. J Biol Chem. 2001;276:10888–10896. doi: 10.1074/jbc.M008150200. [DOI] [PubMed] [Google Scholar]
- 73.Seung Kim HF, Weeber EJ, Sweatt JD, Stoll AL, Marangell LB. Inhibitory effects of omega-3 fatty acids on protein kinase C activity in vitro. Mol Psychiatry. 2001;6:246–248. doi: 10.1038/sj.mp.4000837. [DOI] [PubMed] [Google Scholar]
- 74.Nakamoto K, Nishinaka T, Mankura M, Fujita-Hamabe W, Tokuyama S. Antinociceptive effects of docosahexaenoic acid against various pain stimuli in mice. Biol Pharm Bull. 2010;33:1070–1072. doi: 10.1248/bpb.33.1070. [DOI] [PubMed] [Google Scholar]
- 75.Nakamoto K, Nishinaka T, Ambo A, Mankura M, Kasuya F, Tokuyama S. Possible involvement of β-endorphin in docosahexaenoic acid-induced antinociception. Eur J Pharmacol. 2011;666:100–104. doi: 10.1016/j.ejphar.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 76.Ramsden CE, Zamora D, Makriyannis A, et al. Diet-induced changes in n-3- and n-6-derived endocannabinoids and reductions in headache pain and psychological distress. J Pain. 2015;16:707–716. doi: 10.1016/j.jpain.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trépanier MO, Hopperton KE, Orr SK, Bazinet RP. N-3 polyunsaturated fatty acids in animal models with neuroinflammation: An update. Eur J Pharmacol. 2015 doi: 10.1016/j.ejphar.2015.05.045. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


