Abstract
Background
Studies have demonstrated that the use of sedation (regardless of type) increases polyp detection rates during colonoscopy. Compared to conscious sedation (CS), propofol sedation (PS) has led to detection of more advanced polyps, yet no apparent difference was found in the overall adenoma detection rate (ADR) in patients undergoing colonoscopy for various reasons. We aimed to assess whether there was a significant difference in the ADR in patients specifically undergoing screening colonoscopies using PS versus CS.
Methods
This is a retrospective analysis of 699 consecutive patients who underwent inpatient screening colonoscopies at one academic inpatient center. The decision to perform endoscopy using PS versus CS was determined on an individual basis by each provider, taking into account various patient parameters.
Results
No significant difference was noted between ADR or location of detected adenomas between the CS and PS groups. When accounting for each variable, only total endoscopy time of less than 20 min resulted in a statistically significant ADR difference between the two sedation groups (CS: 15.6% vs PS: 21.3%, P = 0.038).
Conclusion
ADR in screening colonoscopies is not increased by the use of PS compared to CS. While the use of propofol-based anesthesia is clearly associated with increased patient satisfaction and pain levels, the ADR is not enhanced, and its widespread use in screening colonoscopy sedation should still be investigated.
Keywords: Adenoma detection rate, colonoscopy, propofol sedation, conscious sedation, gastroenterology fellow training
Introduction
Colonoscopy has become accepted as the most effective method of screening the colon in average-risk patients for colorectal cancer (CRC) [1,2]. Detecting and resecting precursor colorectal polyps and adenomas found during colonoscopy has effectively decreased the incidence of CRC [3-5]. However, it is well known that polyps are missed during colonoscopy, resulting in interval cancers in the years following colonoscopy [6]. Among quality parameters, the adenoma detection rate (ADR) remains the most important parameter and the main measure of a quality colonoscopy [7,8]. Some supported factors shown to affect adenoma and polyp detection rates (ADR and PDR) include: adequacy of bowel preparation, cecal intubation rate, withdrawal time, image enhancements (high definition, narrow band imaging), the performing endoscopist independent of patient behaviors, and use of sedation [7,9,10]. Traditionally, sedation has consisted of a benzodiazepine and an opioid. Recently, propofol has been utilized as an alternative option for sedation due to its rapid induction of sedation, faster recovery, lack of active metabolites, and equivalent levels of amnesia [11,12]. Although limited in study design and not translated to ADR, the use of sedation (regardless of type) suggests an increase in PDRs during colonoscopy [13]. Compared to moderate sedation using a benzodiazepine and opioid, deep sedation with propofol has led to detection of more advanced polyps, yet no apparent difference in the overall ADR or PDR in patients undergoing colonoscopy for various reasons [14].
The aim of this study was to assess whether there was a significant difference in the ADR in patients specifically undergoing screening colonoscopies using propofol sedation (PS) versus conscious sedation (CS).
Patients and methods
Study design
This retrospective study was conducted at one academic hospital-based inpatient endoscopy unit where approximately 5000 endoscopic procedures are performed annually. Gastroenterologist-controlled CS was achieved with fentanyl and midazolam with or without diphenhydramine. PS was administered by an experienced anesthesiologist with or without a certified registered nurse anesthetist (CRNA). Fentanyl was administered up to a maximum of 100 mg and midazolam up to a maximum of 10 mg while continuously monitoring cardiorespiratory parameters. The decision to perform endoscopy with or without propofol was determined on an individual basis by each provider, taking into account a patient’s medical history, body habitus, body mass index, concomitant medication use, and success with prior procedures using CS. For purposes of the study, adenomas from the cecum to the distal transverse colon were defined as proximal, and adenomas from the descending colon at the splenic flexure to the rectum were defined as distal. Bowel prep quality was described as fair or poor as determined by the gastroenterologist performing the procedure. Cases with poor prep had significantly poor visualization of all of the mucosal surfaces and were considered unsatisfactory. All colonoscopies were performed using Olympus high definition H180AL endoscopes with high definition flat screen monitors at the same academic teaching institution. The study was approved by the University of Florida College of Medicine institutional review board.
Study sample
The study population consisted of 699 consecutive patients who underwent inpatient screening colonoscopies between from July 1st, 2012 through May 30th, 2013. Patients were included if they met standard guidelines for screening colonoscopy [15]. Patients were excluded if they had a personal history of CRC, history of colon polyps, inflammatory bowel disease, family history of CRC, gastrointestinal bleeding, abdominal bleeding, and other gastrointestinal cancers. Colonoscopies were performed by an experienced interventional gastroenterologist or by the gastroenterology fellows (1st, 2nd or 3rd year of fellowship training) under direct supervision of the same gastroenterology staff included in the study.
Outcomes
Our primary endpoint was the ADR associated with the type of sedation. Secondary objectives included gender and race predilection for sedation type and impact of terminal ileum (TI) intubation on ADR.
Statistical analysis
The data is presented as counts (frequencies) and percentages. Chi-square test was used for comparing categorical variables. Continuous and ordinal variables were described using means ± standard deviations, and analyzed using the non-parametric Wilcoxon Rank-Sum test. We conducted univariate analysis to describe the differences between sedation methods. Multivariable analyses was performed using a Cochran-Mantel-Haenszel (CMH) test to investigate the difference in ADR between medication groups, controlling for age group, provider type, gender, race, insurance status, withdrawal times, total time, and presence of TI intubation. The CMH test was also used to investigate the difference between medication group and insurance type, controlling for race. The Breslow-Day test evaluated the homogeneity of the odds ratios across strata. Additionally, the relationships between TI intubation and prep quality, and provider type were explored using counts and percentages and analyzed using Chi-square tests. These analyses were descriptive in nature and no adjustments have been made for multiple tests. All analysis was completed using SAS® Version 9.3 for Windows (Cary, North Carolina).
Results
Patient characteristics
The mean age of the study population was 58 years. There was a significant statistical difference in race between the CS (N = 391) and PS (N = 398) groups, with 67.3% blacks in the CS group and 53.6% blacks in the PS group (P = 0.001) and insurance type, with 46.5% non-private/other versus the 38.8% non-private/other, respectively (P = 0.039). There was also a significant difference in the medication groups by level of training for year 1 fellows (P = 0.0075) (Table 1). Significant difference was noted in “time to reach cecum” and “total time” of colonoscopy (P < 0.0001 and P = 0.036, respectively) (Table 1). There were no statistical differences between the two sedation groups in terms of age, gender, bowel prep quality, TI intubation rate, and colon withdrawal time (Table 1).
Table 1.
A Demographic patient characteristics and procedural outcome by medication group
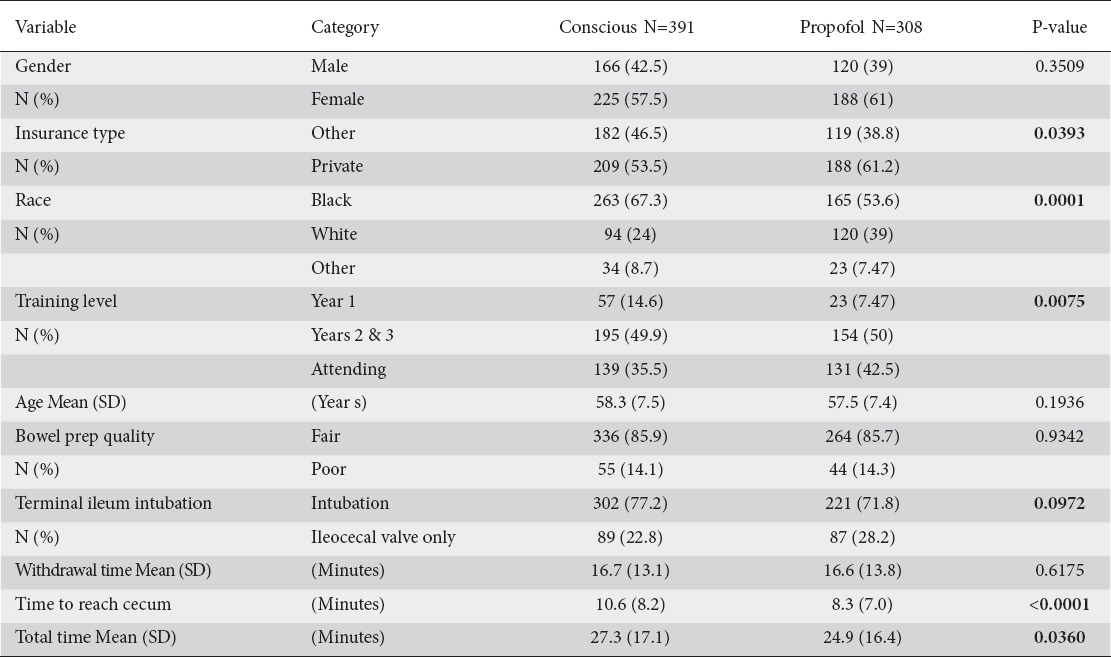
No significant difference was noted between ADRs or location of detected adenomas between the CS and PS groups [Table 2]. Because patient assignment to each of the sedation groups was not randomized, a multivariate analysis was performed using the CMH test to determine whether any of the confounding variables contributed specifically to the ADRs. When accounting for each variable, only total endoscopy time of less than 20 min resulted in a statistically significant ADR difference between the two sedation groups (CS: 15.6% vs PS: 21.3%, P = 0.038) [Table 3]. Using CS as the reference standard, the odds ratio for ADRs does not differ when PS was used (OR: 0.7; 95% CI: 0.5-1.0). No significant difference was noted between the sedation groups regarding TI intubation (Table 1B).
Table 2.
Adenoma detection rates between medication groups

Table 3.
Confounding variable adjusted adenoma detection rates between medication groups

Discussion
This study shows that PS was not associated with an overall significant difference in ADR compared to CS. Adjustments for patient characteristics also failed to show an overall advantage for the detection of adenomas associated with PS. As concerns about experienced endoscopists having missed adenoma rates hovering around 25% [6,16], factors affecting ADRs have come into question.
Patient pain remains one of the major reasons for early termination of colonoscopy. The majority of colonoscopies today in the United States are performed under sedation, helping to decrease procedure-related pain and discomfort [17]. Recently, propofol has been considered an alternative option for sedation due to its rapid induction of sedation, faster recovery, lack of active metabolites, and equivalent levels of amnesia [11,12]. When compared to the traditional benzodiazepine and opioid sedation, propofol was associated with a statistically significant improvement in comfort and sedation score, with comparable safety parameters [18]. Use of sedation, regardless of type, during colonoscopies has been shown to increase the likelihood of reaching the cecum and improve PDR [13]. When using the traditional benzodiazepine/opioid regimen, the level of sedation (deep or moderate) demonstrated no significant difference in detection of polyps [14]. However, more advanced lesions (>9 mm) have been found with PS rather than CS [14].
Adequate sedation allows the endoscopist time to focus on the examination and not be distracted by patient incorporation or inability to adequately complete the examination. Most importantly, more complete visualization under colonoscopy due to patient cooperation has led to increased operator satisfaction. Benefits of PS are clear when it comes to patient pain and satisfaction [18]. As our study suggests, PS did not translate to higher ADRs and PDRs. The question remains: do we continue to offer PS without a clear objective quality measure benefit, such as ADR or PDR? The increase in patient satisfaction with PS may help ease any negative public impression of getting a screening colonoscopy, even though our data suggests no tangible benefit to the ADR or PDR with choice of sedation. Widespread use of deep PS would invariably lead to increased medical costs without a tangible benefit in patient outcome (e.g. no benefit to the ADR or PDR with choice of sedation), except for increased patient satisfaction with the examination and maybe a less negative public impression about colonoscopy. In addition, PS is typically administered independent of the endoscopist and with anesthesia assistance. However, studies have shown administration of PS by endoscopists to be safe, and have no statistically significant rates of adverse events as compared to other choices of sedation [19]. If nurses administer PS under the supervision of the endoscopist, one may argue focus is being diverted away from the main role of the endoscopist: the colonoscopy. However, nurse sedation administration under endoscopist supervision may help to lower costs to the patient, as the high price of anesthesiologist propofol administration would not be needed [20]. However, data suggests nurses tend to sedate patients to a greater degree than physicians and are less willing for patients to experience discomfort. In heavily sedated patients, higher degree of air can be insufflated because patients do not report pain. This causes flat polyps to become less apparent to the endoscopist. On the other hand, previous studies have suggested more heavily sedated patients allow the endoscopist more time to aspirate air and inspect the mucosa. Regardless, the current study results show that withdrawal times and ADR in the proximal or distal colon were comparable between the CS and PS groups. Moreover, increased patient satisfaction can also be achieved using less painful insertion techniques or less expensive sedation protocols, thus, lowering medical bills. Further studies are needed to see if more specific polyp location along with actual polypectomy rates vary based on type and level of sedation administered.
Summary Box.
What is already known:
Some factors shown to affect adenoma and polyp detection rates (ADR and PDR) include: adequacy of bowel preparation, cecal intubation rate, withdrawal time, image enhancements, the performing endoscopist independent of patient behaviors, and use of sedation
The use of sedation (regardless of type) increases PDR during colonoscopy
Compared to moderate sedation using a benzodiazepine and opioid, sedation with propofol has led to detection of more advanced polyps, yet no apparent difference in the overall ADR or PDR in colonoscopies undergone for various reasons
What the new findings are:
For screening colonoscopies, the ADR is not increased by the use of propofol sedation compared to sedation with a benzodiazepine (midazolam) and opioid (fentanyl)
Adjustments for patient demographics, trainee level, withdrawal time, total time taken, preparation quality, and terminal ileum intubation failed to show an overall advantage in detecting adenomas when using propofol sedation
When accounting for each variable, only total endoscopy time of less than 20 min resulted in a statistically significant ADR difference between the two sedation groups
Prior studies have shown variable effects of fellow involvement in colonoscopy on ADR and PDR, with some suggesting improvement [21], and others reporting no effect or diminished detection rates [22]. Our study was one of the first to investigate level of training and choice of sedation in regards to ADR. No significant differences were noted in ADR between level of training and sedation type. Further randomized-controlled studies are needed to confirm this initial finding, and also to see if this also remains true in colonoscopies performed for non-screening purposes. Regardless, prior studies have shown most fellows do not improve their ADR after training completion [23], thus, it is imperative all factors investigating quality measures, such as choice of sedation on ADR, be heavily investigated during their training years.
Procedural technique was the same for each endoscopist in both the PS and CS endoscopy subgroups. All bowel preparations were graded fair or poor and withdrawal times were documented for all patients. All colonoscopies were performed using Olympus high definition H180AL endoscopes at the same academic teaching institution. Multiple core quality indicators were included that have not been addressed in prior related studies, such as: ADR, PDR, cecal intubation, time, bowel prep, and withdrawal time. Our study was retrospective, non-randomized, and administration in only a single setting. The unblinded fashion of the study as far as the endoscopist is concerned, might influence the outcomes measures; however, this limitation is unavoidable. Size of adenomas and polys were also not described.
In conclusion our data shows that the detection rate of adenomatous polyps in screening colonoscopies is not increased by the use of PS compared to sedation with a benzodiazepine (midazolam) and opioid (fentanyl). While the use of propofol-based anesthesia is clearly associated with increased patient satisfaction and pain levels, the ADR is not enhanced, and its widespread use in screening colonoscopy sedation should still be investigated.
Biography
University of Florida College of Medicine-Jacksonville, FL; University of South Alabama, Mobile, AL, USA
Footnotes
Conflict of Interest: None
References
- 1.Rex DK. Colonoscopy. Gastrointest Endosc Clin N Am. 2000;10:135–160. [PubMed] [Google Scholar]
- 2.Rex DK, Johnson DA, Anderson JC, et al. AGA Guidelines for Colorectal Cancer Screening 2008. Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim EC, Lance P. Colorectal polyps and their relationship to cancer. Gastroenterol Clin North Am. 1997;26:1–17. doi: 10.1016/s0889-8553(05)70280-6. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1198. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 6.Van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866–2877. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 8.Rizk MK, Sawhney MS, Cohen J, et al. Quality indicators common to all GI endoscopic procedures. Gastrointest Endosc. 2015;81:31–53. doi: 10.1016/j.gie.2014.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European panel of appropriateness of gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 10.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 11.Bryson HM, Fulton BR, Faulds D. Propofol: an update of its use in anesthesia and conscious sedation. Drugs. 1995;50:513–559. doi: 10.2165/00003495-199550030-00008. [DOI] [PubMed] [Google Scholar]
- 12.Reimann FM, Samson U, Derad I, Fuchs M, Schiefer B, Stange EF. Synergistic sedation with low dose midazolam and propofol for colonoscopies. Endoscopy. 2000;32:239–244. doi: 10.1055/s-2000-134. [DOI] [PubMed] [Google Scholar]
- 13.Radaelli F, Meucci G, Sgroi G, et al. Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. 2008;103:1122–1130. doi: 10.1111/j.1572-0241.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 14.Paspatis GA, Tribonias G, Manolaraki MM, et al. Deep sedation compared with moderate sedation in polyp detection during colonoscopy: a randomized controlled trial. Colorectal Dis. 2011;13:e137–e144. doi: 10.1111/j.1463-1318.2011.02555.x. [DOI] [PubMed] [Google Scholar]
- 15.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Rex DK, Chadalawada V, Helper DJ. Wide angle colonoscopy with a prototype instrument: impact on miss rates and efficiency as determined by back-to-back colonoscopies. Am J Gastroenterol. 2003;98:2000–2005. doi: 10.1111/j.1572-0241.2003.07662.x. [DOI] [PubMed] [Google Scholar]
- 17.Bannert C, Reinhart K, Dunkler D, et al. Sedation in screening colonoscopy: impact on quality indicators and complications. Am J Gastroenterol. 2012;107:1837–1848. doi: 10.1038/ajg.2012.347. [DOI] [PubMed] [Google Scholar]
- 18.Koshy G, Nair S, Norkus EP, Hertan HI, Pitchumoni CS. Propofol versus midazolam and meperidine for conscious sedation in GI endoscopy. Am J Gastroenterol. 2000;95:1476–1479. doi: 10.1111/j.1572-0241.2000.02080.x. [DOI] [PubMed] [Google Scholar]
- 19.Rex DK, Deenadayalu VP, Eid E, et al. Gastroenterology. 2009;137:1229–1237. doi: 10.1053/j.gastro.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 20.Position Statement: Nonanesthesiologist administration of propofol for GI endoscopy. American Society for Gastrointestinal Endoscopy (ASGE) Gastrointest Endosc. 2009;70:53–59. doi: 10.1016/j.gie.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Buchner AM, Shahid MW, Heckman MG, et al. Trainee participation is associated with increased small adenoma detection. Gastrointest Endosc. 2011;73:1223–1231. doi: 10.1016/j.gie.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 22.Leffler DA, Kheraj R, Bhansali A, et al. Adenoma detection rates vary minimally with time of day and case rank: a prospective study of 2139 first screening colonoscopies. Gastrointest Endosc. 2012;75:554–560. doi: 10.1016/j.gie.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 23.van Doorn SC, Klanderman RB, Hazewinkel Y, Fockens P, Dekker E. Adenoma detection rate varies greatly during colonoscopy training. Gastrointest Endosc. 2015;82:122–129. doi: 10.1016/j.gie.2014.12.038. [DOI] [PubMed] [Google Scholar]


