Abstract
Background
Wingless-type MMTV integration site family member 5A (Wnt5a) has been documented to either overexpress or be lost in several malignancies. Our study aimed to investigate the expression and clinical significance of Wnt5a protein in triple-negative breast cancer (TNBC).
Material/Methods
By using immunohistochemistry, Wnt5a expression was evaluated in 90 TNBC specimens. The association between Wnt5a expression and clinic-pathological factors was assessed by using the chi-square test. The survival analysis of patients was conducted by using the Kaplan-Meier and log-rank tests. Cox regression was utilized for the univariate and multivariate analyses of prognostic factors.
Results
Results showed that negative Wnt5a expression correlated with positive lymph node metastasis (LNM) (P=0.007) and Ki67 proliferation (P=0.002). Patients with negative Wnt5a expression had significantly poorer recurrence-free survival (RFS) than patients with positive Wnt5a expression (P=0.008). Multivariate Cox regression analysis revealed that decreased Wnt5a expression was an independent prognostic factor for RFS (P=0.014).
Conclusions
Negative Wnt5a protein expression might contribute to the tumor progression and poor prognosis of TNBC and might be a new therapy target in TNBC.
MeSH Keywords: Prognosis, Tissue Array Analysis, Triple Negative Breast Neoplasms
Background
Breast cancer is the second most common cancer in humans and is the most common malignant tumor in females. Breast cancer accounts for approximate 23% of new cancer cases and 14% of total cancer mortality [1]. Triple-negative breast cancer (TNBC), which is characterized by a lack of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) according to immunohistochemical (IHC) and/or fluorescent in situ hybridization, results is a special type of breast cancer and accounts for 12–20% of all breast cancer occurrences [2]. Compared with the other 3 subtypes of breast cancers, TNBC presents an aggressive biological behavior and poor prognosis [3]. Given that TNBC is insensitive to mammography screening, patients may be diagnosed at a higher grade [4]. Compared with the other 3 subtypes, TNBC are prone to distant recurrence and death within 5 years of diagnosis [5]. Thus, identifying new biomarkers to predict poor prognosis is important.
Wnt protein is a large secreted protein family that participates in numerous aspects of cellular processes. Wnt signaling can be divided into 2 parts: the canonical βn-catenin-dependent signaling pathway and the non-canonical catenin-independent signaling pathway. Wnt5a is an important member of the non-canonical Wnt pathway. Wnta5a is often dysfunctional, and its protein is recognized as either tumor-suppressive or tumor-promoting [6]. Wnt5a expression is elevated in melanoma, and its high expression is associated with poor prognosis in melanoma [7,8]. Moreover, Wnt5a expression is found to be positive in gastric cancer, and its high expression is associated with bad outcomes [9]. However, in certain types of cancer, the loss or decreased expression of Wnt5a is associated with unfavorable prognosis [10,11].
Such controversial Wnt5a results are also involved in breast cancer. Sue et al. [12] found that Wnt5a is up-regulated in primary breast cancers compared with normal breast tissues. Furthermore, the up-regulation of Wnt5a is crucial for the macrophage-induced invasion of invasive breast cancer [13]. Klemm et al. found that, compared with MCF7 cell line, Wnt5a has higher expression in MDA-MB-231 cell line [14]. Moreover, in breast cancer patients with brain metastasis, Wnt5a is highly expressed in brain tissues [14]. However, the loss or low expression of Wnt5a also has poor prognostic value in breast cancer [15–18]. In MCF7 and 4T1cell lines, the silencing of Wnt5a may lead to increased cell invasiveness [19].
However, the clinical significance of Wnt5a protein expression in TNBCs remains unclear. In the present study, we aimed to detect the clinical significance of Wnt5a expression in TNBCs.
Material and Methods
Patients and tissue specimens
A total of 425 paraffin-embedded samples of breast-invasive ductal carcinomas were obtained from the Department of Pathology of the Affiliated Tumor Hospital of Harbin Medical University. The number of TNBCs in tissue microarrays (TMA) was 90. Informed consents were obtained from the subjects, and the study was performed with the approval of the Ethics Committee of Harbin Medical University. The patients had complete medical records since 2006. Clinical records were obtained from the department providing follow-up care. The patients were all females. The median age of the TNBC patients was 49 years old (range from 34 years to 69 years). Each sample was routinely tested for ER, PR, HER2, Ki67, and p53 by immunohistochemistry. IHC markers were assayed in paraffin-embedded formalin-fixed tissue stained with hematoxylin and eosin by using antibodies to the proteins ER, PR, and Her-2 (Zhongshan Bio Co., China). Samples that showed nuclear staining for ER or PR in more than 1% of the cells were considered ER-positive or PR-positive, respectively [20]. Positive staining for HER2 was defined on the basis of the percentage of tumor cells and the intensity of membrane staining. HER2 was scored from 0 to 3+ on the basis of the method recommended for the Dako Hercep test. Tumors were recognized as positive for HER2 if immunostaining was scored as 3+ or when the HER-2 fluorescence in situ hybridization amplification ratio was greater than 2.2 (21). Ki67 and p53 staining cells were counted and expressed as a percentage. Low expression was identified as Ki67 <14% [22] and p53 <25% [23].
Follow-up
Patients were followed up for at least 3 months and up to 82 months (median 75 months). All patients received a definite pathological diagnosis, and none received any therapy before surgery. Recurrence-free survival (RFS) was used as the assessment for prognostic analyses. RFS was defined as the time interval from the date of surgery to the date of local, regional, and distant recurrences and breast cancer death.
Tumor phenotype classification
According to the Scarff-Bloom-Richardson system, the invasiveness of cancers can be determined on the basis of the extent of cell mitosis frequency, tubule formation, and nuclear pleomorphism. Invasiveness in patients was classified as low grade (grade I), moderate grade (grade II), and high grade (grade III). Grades I and II were grouped together. The occurrence of lymph node metastases (LNM) and tumor size (negative: <2 cm; positive: ≥2 cm) were also recorded for each patient.
The molecular subtypes of breast cancer were classified according to the criteria established in the St. Gallen International Breast Cancer Conference 2011 (24): luminal A type: ER and/or PR-positive and HER2-negative and Ki67 low (<14%); luminal B type: ER- and/or PR-positive, HER2-negative, and Ki67 high (≥14%); (HER2-positive) ER- and/or PR-positive, HER2-overexpressed or amplified, and any Ki67; HER2-positive type: ER- and PR-negative, and HER2-overexpressed or -amplified; TNBC type: ER-negative, PR-negative, and HER2-negative.
Tissue microarray construction
By using a punching machine, the selected area of the paraffin block was punched out, and a 3-mm tissue core was placed onto a recipient block. The selected tissue was then extracted. More than 2 tissue cores were extracted to minimize extraction bias. All breast invasive ductal carcinomas and corresponding normal breast sample blocks were cut with a microtome to 44μm and arrayed as triplicates. Each tissue core was assigned a unique TMA location number linked to a database containing other clinicopathological data.
Immunohistochemistry
Tissue sections were dried at 70°C for 3 h. After deparaffinization and hydration, tissue sections were washed in phosphate-buffered saline (PBS; 3×3 min). The washed sections were treated with 3% H2O2 in the dark for 5 min to 20 min. After washing with distilled water, sections were washed with PBS (3×5 min). Antigen retrieval was performed in citrate buffer (pH6.0). Each section was then treated with 300 ml to 500 ml of Wnt5a mouse monoclonal antibodies (Abcam, USA; a dilution of 1:200) solution at 4°C overnight. After washing with PBS (3×5 min), each section was incubated with 300 ml to 500 ml of secondary antibody at room temperature for 30 min. After washing with PBS (3×5 min), each section was treated with 300 ml to 500 ml of diaminobenzidine (DAB) working solution at room temperature for 3 min to 10 min, and then washed with distilled water. Wnt5a were all cytoplasmically stained. In our study, Wnt5a was expressed in most tumor cells (75%); therefore, only staining intensity was calculated in the statistical analyses. According to staining intensity (negative=0, weak=1, moderate=2, and strong=3), we recognized 0,1 as Wnt5a-negative and 2,3 as Wnt5a-positive [18]. The staining on each sample was scored independently by 2 pathologists without knowledge of the clinicopathological findings.
Statistical analysis
Data analysis was performed using SPSS 20.0 statistical software (SPSS Inc., Chicago, USA). The chi-square test was used to examine any differences in categorical variables. Kaplan-Meier survival curves were analyzed by log-rank statistics. The influences of different variables on TNBC were assessed by Cox regression analyses including univariate and multivariate analysis. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were recorded to evaluate the relative risk of TNBC. Statistical significance was assumed when P<0.05.
Results
Decreased Wnt5a expression is common in TNBC tissues
Breast TMA was used to evaluate Wnt5a expression in TNBC tissues and was compared with normal breast tissues. The rate of decreased Wnt5a- expression accounts for 61.1% of breast cancer tissues in this study (Figure 1).
Figure 1.
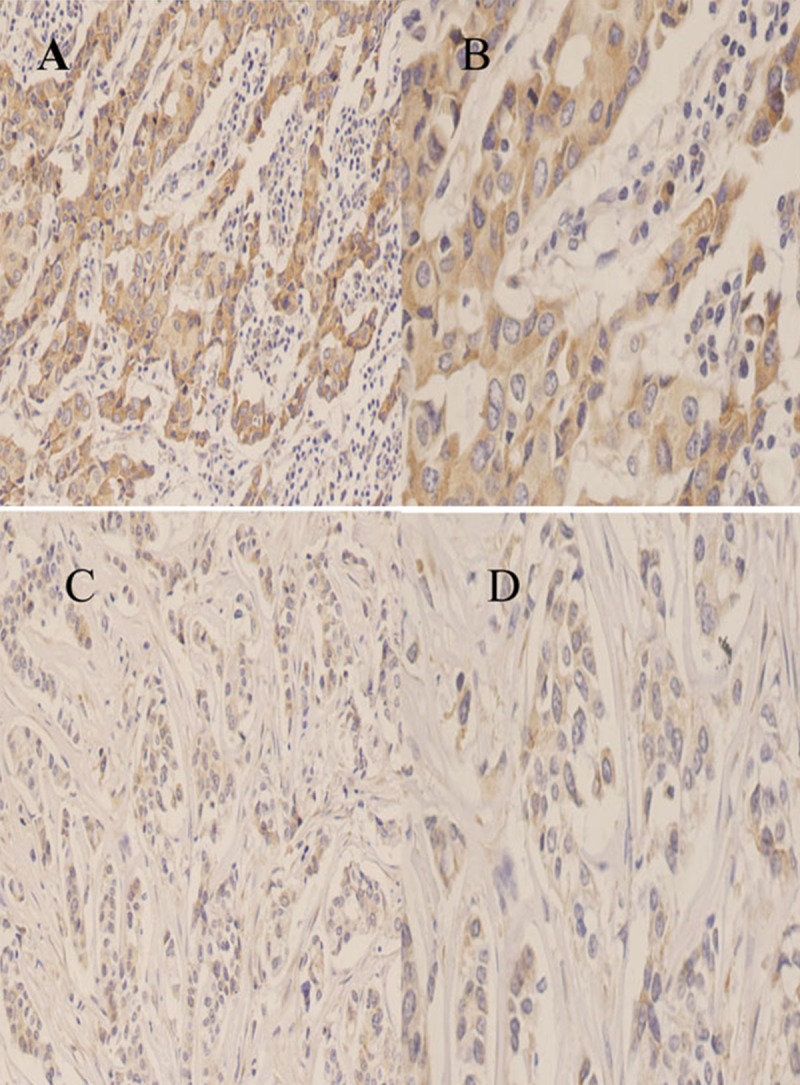
Immunohistochemical staining of Wnt5a protein in breast cancer tissues. (A) High expression in breast cancer tissue(200×); (B) High expression in breast cancer tissue (400×); (C) Low expression in breast cancer tissue (200×); (D) Low expression in breast cancer tissue (400×).
Negative Wnt5a protein expression is associated with lymph node metastasis and Ki67 proliferation
We also analyzed the association between Wnt5a expression level and various clinicopathological factors (Table 1). We found that negative Wnt5a expression was significantly associated with positive LNM (P=0.07) and positive Ki67 status (P=0.02). However, no difference was found between Wnt5a expression and other clinicopathological features.
Table 1.
Correlation between Wnt5a expression with different clinic-pathological parameters.
| Characteristic | Total no. | Positive expression | Negative expression | χ2 | P value | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| All | 90 | 35 | 38.9 | 55 | 61.1 | ||
| Age | 0.083 | 0.773 | |||||
| <50 | 48 | 18 | 37.5 | 30 | 62.5 | ||
| ≥50 | 42 | 17 | 40.5 | 25 | 59.5 | ||
| Grade | 0.380 | 0.537 | |||||
| I+II | 25 | 11 | 44.0 | 14 | 56.0 | ||
| III | 65 | 24 | 36.9 | 41 | 63.1 | ||
| Tumor size | 2.019 | 0.155 | |||||
| <2 cm | 33 | 16 | 48.5 | 17 | 51.5 | ||
| ≥2 cm | 57 | 19 | 33.3 | 38 | 66.7 | ||
| LNM | 7.317 | 0.007 | |||||
| Negative | 31 | 18 | 58.1 | 13 | 41.9 | ||
| Positive | 59 | 17 | 28.8 | 42 | 71.2 | ||
| TNM stage | 1.278 | 0.528 | |||||
| I | 18 | 5 | 27.8 | 13 | 72.2 | ||
| II | 44 | 19 | 43.2 | 25 | 56.8 | ||
| III | 28 | 11 | 39.3 | 17 | 60.7 | ||
| P53 status | 0.713 | 0.399 | |||||
| Negative | 49 | 21 | 42.9 | 28 | 57.1 | ||
| Positive | 41 | 14 | 34.1 | 27 | 65.9 | ||
| Ki67 status | 9.997 | 0.002 | |||||
| Negative | 38 | 22 | 57.9 | 16 | 42.1 | ||
| Positive | 52 | 13 | 25.0 | 39 | 75.0 | ||
Deceased Wnt5a expression is correlated with poor patient survival
To study whether Wnt5a expression is associated with RFS, we utilized Kaplan Meier survival analysis and log-rank test. The Kaplan Meier survival curves are shown in Figure 2. Among the 90 patients, patients with negative -Wnt5a- expression showed significantly poorer outcome (68.543±3.121 months) in terms of RFS than patients with positive-Wnt5a-expression (75.143±2.869) (P=0.008, log-rank test).
Figure 2.
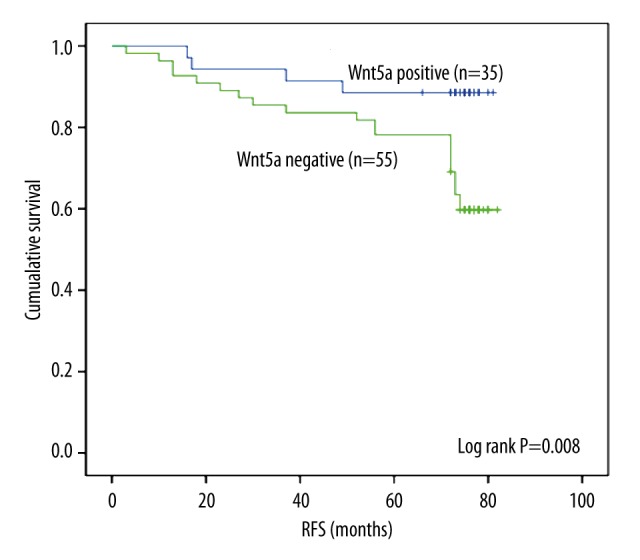
Kaplan-Meier curves for Wnt5a expression in relation to overall survival in 90 breast cancer patients. Patients with negative Wnt5a expression presented a significantly poorer prognosis than those with positive Wnt5a expression (P=0.008, log-rank test).
Both univariate and multivariate survival analyses were used to evaluate the relationship between Wnt5a expression and clinicopathological characteristics on prognosis (Table 2). By using Cox regression analysis, the univariate analyses of RFS showed negative Wnt5a expression (HR=3.779, 95% CI=1.302–10.967) and LNM (OR=3.581, 95% CI=1.233–10.401) as a significant poor prognosis factor. No other features were discovered to be associated with prognosis. By using a Cox regression model, multivariate analysis was conducted on the same set of patients for Wnt5a expression and for pathological factors of survival time. By using multivariate analysis, we discovered that the negative expression of Wnt5a (HR=3.779, 95% CI=1.302–10.967) was an independent prognostic predictor (Table 2).
Table 2.
Univariate and multivariate Cox regression analysis for RFS.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | |
| Age (≥50 vs. <50) | 0.970 | (0.448, 2.097) | ||
| Grade (III vs. II+I) | 1.817 | (0.685, 4.820) | ||
| Tumor size (≥2 cm vs. <2 cm) | 1.111 | (0.504, 2.452) | ||
| LNM (Positive vs. Negative) | 3.581 | (1.233, 10.401) | ||
| TNM stage (III vs. II+I) | 2.135 | (0.641, 7.113) | ||
| P53 (Positive vs. Negative) | 1.789 | (0.822, 3.897) | ||
| Ki67 (Positive vs. Negative) | 2.100 | (0.883, 4.995) | ||
| Wnt5a expression (Negative vs. Positive) | 3.779 | (1.302, 10.967) | 3.779 | (1.302, 10.967) |
Discussion
The loss of Wnt5a has been reported to act as a negative prognostic factor in breast cancer [17,18]. Moreover, Janna et al. [18] found that the loss of Wnt5a expression is associated with TNBC, but they did not clearly state the function of this expression in TNBC. The purpose of our study was to determine whether the loss of Wnt5a presents a pivotal prognostic value in TNBC.
In our study, 55 of the 90 TNBC patients were Wnt5a-negative (accounting for approximately 61.1%). In TNBC, the loss rate of Wnt5a is higher than that (44% [17] and 40.4% [18]) in unclassified breast cancer. In the present study, we found that negative Wnt5a expression in the cytoplasm of cancer cells is associated with lymph metastasis (P=0.07). A previous study [25] indicated that in the MDA-MB-231 cell line, which is a TNBC cell line, the re-expression of Wnt5a could inhibit cell migration. Moreover, in the 4T1 cell line, which expresses very low Wnt5a, breast cancer cell metastasis to the lung can be inhibited when the Wnt5a protein is re-expressed. This finding is similar to that in the current study. In MMTV-PyVmT and MMTV-PyVmT: Wnt5a−/− tumors, the absence of Wnt5a enhances the tumor growth [26]. Moreover, in the absence of Wnt5a, Ki67 staining illustrates a significant elevation in proliferation [26], which is consistent with our results. In the present study, negative Wnt5a was associated with Ki67 proliferation (P=0.002). Although statistically insignificant, the finding showed a trend wherein negative Wnt5a was associated with large tumor size. We speculate that the loss of Wnt5a might result in tumor growth via Ki67 proliferation.
Both univariate and multivariate analyses found that patients with low level of Wnt5a expression presented significantly poorer RFS rates than patients with higher Wnt5a expression. Thus, the loss of Wnt5a expression is an independent unfavorable survival factor for patients with TNBC. That finding is different from the research conducted by Säfholm et al., which found that reconstitution of Wnta5a signaling in 4T1 mouse breast cancer cells (which lack ER, PR, and HER2) could impair migration and invasion, and that recombinant Wnt5a- and Wnt5a-derived Foxy-5 peptide may be a potential therapeutic strategy for triple-negative breast cancer [27]. Additionally, LNM is an independent poor survival factor in univariate analysis, but under multivariate analysis, it had no significant association with the prognosis of TNBC. Therefore, we speculated that TNBC risk was influenced by confounding factors, but the genetic factor was the decisive factor.
The results of this study suggest that down-regulated Wnt5a predicted a poor prognosis of TNBC. However, our study has numerous deficiencies. Firstly, this was a case-only study, and there was no control group. Secondly, gene-gene and gene-environmental interactions were not considered in our study. TNBC onset is influenced by genetic and environmental factors, and a single gene cannot determine the development of it. Finally, there was only 1 ethnicity in this study. Gene expression level was distinguished in different ethnicity. All of the limitations will make the inaccurate of our results. Based on the above, the result of our study was insufficient to provide an effective therapy method for TNBC. Thus, we should further study the function mechanism of Wnt5a in TNBC.
Conclusions
Our study shows that the loss of Wnt5a is associated with lymph node metastasis and Ki67 proliferation in TNBC. Low level of Wnt5a protein was correlated with poorer survival rate in patients with TNBC. Our results suggest that Wnt5a might serve as a tumor suppressor gene in TNBC and that Wnt5a might be a potential new drug target in breast cancer treatment.
Footnotes
Source of support: This project was supported by grants from the National Natural Science Fund (81172498), funds from The Affiliated Tumor Hospital of Harbin Medical University (JJZ2010-04), the ‘Wu Liande’ Fund of Harbin Medical University (WLD-QN1118), and the Special Fund of the Translational Medical Research between China and Russia (CR201402)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–28. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 3.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–18. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 4.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–72. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 6.McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–14. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weeraratna AT, Jiang Y, Hostetter G, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 8.Jenei V, Sherwood V, Howlin J, et al. A t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc Natl Acad Sci USA. 2009;106:19473–78. doi: 10.1073/pnas.0909409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurayoshi M, Oue N, Yamamoto H, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–48. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 10.Dejmek J, Dejmek A, Safholm A, et al. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 2005;65:9142–46. doi: 10.1158/0008-5472.CAN-05-1710. [DOI] [PubMed] [Google Scholar]
- 11.Liu XH, Pan MH, Lu ZF, et al. Expression of Wnt-5a and its clinicopathological significance in hepatocellular carcinoma. Dig Liver Dis. 2008;40:560–67. doi: 10.1016/j.dld.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Lejeune S, Huguet EL, Hamby A, et al. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res. 1995;1:215–22. [PubMed] [Google Scholar]
- 13.Pukrop T, Klemm F, Hagemann T, et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci USA. 2006;103:5454–59. doi: 10.1073/pnas.0509703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemm F, Bleckmann A, Siam L, et al. beta-catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis. 2011;32:434–42. doi: 10.1093/carcin/bgq269. [DOI] [PubMed] [Google Scholar]
- 15.Dejmek J, Leandersson K, Manjer J, et al. Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin Cancer Res. 2005;11:520–28. [PubMed] [Google Scholar]
- 16.Leris AC, Roberts TR, Jiang WG, et al. WNT5A expression in human breast cancer. Anticancer Res. 2005;25:731–34. [PubMed] [Google Scholar]
- 17.Jonsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409–16. [PubMed] [Google Scholar]
- 18.Sand-Dejmek J, Ehrnstrom R, Berglund P, et al. The prognostic significance of Wnt-5a expression in primary breast cancer is extended to premenopausal women. PLoS One. 2013;8:e70890. doi: 10.1371/journal.pone.0070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai J, Guan H, Fang L, et al. MicroRNA-374a activates Wnt/beta-catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123:566–79. doi: 10.1172/JCI65871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–22. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 22.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung SY, Kim HY, Nam BH, et al. Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat. 2010;120:627–37. doi: 10.1007/s10549-010-0780-8. [DOI] [PubMed] [Google Scholar]
- 24.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Crossman DK, Mitchell EH, et al. WNT5A inhibits metastasis and alters splicing of Cd44 in breast cancer cells. PLoS One. 2013;8:e58329. doi: 10.1371/journal.pone.0058329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roarty K, Baxley SE, Crowley MR, et al. Loss of TGF-beta or Wnt5a results in an increase in Wnt/beta-catenin activity and redirects mammary tumour phenotype. Breast Cancer Res. 2009;11:R19. doi: 10.1186/bcr2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Safholm A, Tuomela J, Rosenkvist J, et al. The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin Cancer Res. 2008;14:6556–63. doi: 10.1158/1078-0432.CCR-08-0711. [DOI] [PubMed] [Google Scholar]


