Abstract
Background
This study aimed to determine the role of miR-129-5p in irradiation-induced autophagy in breast cancer cells and to investigate its downstream regulation in autophagy-related radiosensitivity.
Material/Methods
Relative miR-129-5p expression in breast cancer cell lines MCF-7, MDA-MB-231, BT474, and BT549, and in 1 non-tumorigenic breast epithelial cell line, MCF-10A, was compared. The effect of miR-129-5p on irradiation-induced autophagy and radiosensitivity of the cancer cells was explored. The regulative effect of miR-129-5p on HMGB1 and the functional role of this axis in autophagy and radiosensitivity were also studied.
Results
Ectopic expression of miR-129-5p sensitized MDA-MD-231 cells to irradiation, while knockdown of miR-129-5p reduced radiosensitivity of MCF-7 cells. MiR-129-5p overexpression inhibited irradiation-induced autophagy. HMGB1 is a direct functional target of miR-129-5p in breast cancer cells. MiR-129-5p may suppress autophagy and decrease radioresistance of breast cancer cells by targeting HMGB1.
Conclusions
The miR-129-5p/HMGB1 axis can regulate irradiation-induced autophagy in breast cancer and might be an important pathway in regulating radiosensitivity of breast cancer cells.
MeSH Keywords: Autophagy; Breast Neoplasms; HMGB1 Protein; MicroRNAs; Radiotherapy, Adjuvant
Background
Breast carcinoma is the most common female malignancy and the second leading cause of mortality from all cancers in women [1,2]. Radiotherapy is a major adjuvant treatment for most patients receiving breast-conserving surgery. It helps to lower the risk of recurrence in the remaining breast tissue or in the nearby lymph nodes, and contributes to reduced overall mortality [3]. However, successful radiotherapy greatly depends on tumor radiosensitivity and the tolerance of normal tissues [4]. In some subtypes of breast cancer, the rate of biochemical/clinical relapse of patients receiving radiotherapy remains high [4]. Therefore, understanding the molecular mechanisms involved in the radioresistance helps provide useful information for improvement of therapeutic strategies.
Autophagy is a natural destructive mechanism of cells, which is characterized as degrading and recycling of cellular components for new cell formation [5]. Autophagy may also be induced in cancer cells in response to stresses, such as starvation, growth factor deprivation, and hypoxia [6,7]. There is mounting evidence that autophagy contributes to increased chemoresistance and radioresistance in breast cancer [8,9]. Some studies also reported that miRNAs can modulate chemotherapy and radiotherapy via autophagy [10,11]. Specifically, miR-25 can regulate chemoresistance-associated autophagy in breast cancer cells [8]. MiR-200c can inhibit autophagy and decrease radioresistance in breast cancer cells by targeting UBQLN1 (the gene for Ubiquilin-1) [11]. Decreased miR-129-5p is observed in breast cancer, which stimulates the epithelial-mesenchymal transition of breast cancer cells [12,13]. However, whether it is involved in radiosensitivity of breast cancer has not yet been determined. High-mobility group box-1 protein (HMGB1) is a protein that promotes both chemoresistance and radioresistance in breast cancer [14,15].
This study investigated the regulative role of miR-129-5p over HMGB1 in breast cancer and explored the function of the miR-129-5p-HMGB1 axis in irradiation-induced autophagy and radiosensitivity of breast cancer cells.
Material and Methods
Cell culture
Immortalized human breast epithelial cell line MCF-10A and breast cancer cell lines MCF-7 (ATCC® HTB22™), MDA-MB-231 (ATCC® HTB26™), BT549 (ATCC® HTB122™), and BT474 (ATCC® HTB-20™) were obtained from the American Type Culture Collection (ATCC). The cells were cultured using medium described in a previous study [11]. All cells were cultured in humidified air containing 5% CO2 at 37°C.
Reagent and cell transfection
MiR-129-5p mimics, anti-miR-129-5p, HMGB1 siRNA, and the scrambled negative controls were purchased from RiBoBio (Shanghai, China). A GV144-HMGB1 expression plasmid without the 3′UTR sequence was purchased from GeneChem (Shanghai, China). 3-methyladenine (3-MA) and bafilomycin A1 (Baf. A1) were purchased from Sigma-Aldrich (St Louis, MO, USA). To overexpress miR-129-5p or HMGB1, the cells cultured in 6-well plates were transfected with 100 nM miR-129-5 mimics or 200 ng GV144-HMGB1, respectively. To knockdown endogenous HMGB1, cells were transfected with 100 nM HMGB1 siRNA. Transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Clonogenic assay was performed 48 h after transfection to assess cell survival. In some studies, MCF-7 cells and MDA-MB-231 cells were treated with 3-MA (5 mM) or Baf. A1 (100 nM) 1 h before radiation, for a duration of 24 h.
Screening of MDA-MB-231 cells with stable GFP-LC3-expression
Briefly, recombinant lentiviral particles containing GFP-LC3 were obtained from Genepharma (Shanghai, China). MDA-MB-231 cells were infected with the lentiviral particles in the presence of 8μg/ml Polybrene (Sigma-Aldrich). The cells were sorted using fluorescence-activated cell sorting and further screened using geneticin (Gibco, Grand Island, NY, USA). The cells with stable expression of GFP-LC3 were cultured in 400 μg/ml geneticin.
QRT-PCR analysis
Total miRNAs were extracted from cell samples using the miRVana miRNA Isolation Kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The expression of miR-129-5p was quantified using TaqMan MicroRNA Assay Kit (Applied Biosystems, Foster City, CA, USA), with U6 snRNA used as the endogenous control.
TRIzol reagent (Invitrogen) was used to extract total RNA in the cell samples, according to the manufacturer’s instructions. Then, the cDNA was reversely transcribed using the PrimeScript RT reagent kit (TaKaRa, Dalian, China). The PCR primers for HMGB1 were: forward, 5′-ACATCCAAAATCTTGATCAGTTA-3′, reverse, 5′-AGGACAGACTTTCAAAATGTTT-3′. GAPDH was used as the internal control with the following primers: forward, 5′-GGGAAGCTTGTCATCAATGG-3′, reverse, 5′-CATCGCCCCACTTGATTTTG-3′. QRT-PCR analysis was performed using SYBR Premix Ex Taq II (TaKaRa) with an ABI 7500 Real-Time PCR system (Applied Biosystems). The results were calculated using the 2−Δ ΔCT methods.
Clonogenic assay
Cells were plated in 6-well plates and then exposed to the indicated dose at a dose rate of 5 Gy/min by using a linear accelerator (Varian 2300EX, Varian, Palo Alto, CA, USA). The plates were further incubated for 10 to 14 days and then the cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet for 20 min. Colonies with more than 50 cells were counted under a light microscope. Survival fraction was defined as the number of colonies/ (cells inoculated × plating efficiency). Radiation survival curve was derived from multi-target single-hit model: SF=1-(1-exp(-x/D0))^N.
Western blot analysis
Cell samples were lysed using a lysis buffer (Beyotime, Shanghai, China) supplemented with proteinase and phosphatase inhibitor cocktails (Sigma-Aldrich). The protein concentration was determined using a BCA protein assay kit (Beyotime). The proteins were separated on 10% SDS PAGE gel and then transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk for 1 h and then incubated with primary antibodies overnight at 4°. The primary antibodies used were rabbit anti-LC3B (L7543, 1:1000, Sigma-Aldrich), anti-SQSTM1/p62 (ab91526, 1:1000, Abcam, Cambridge, MA, USA), anti-cleaved PARP (#9546, 1:2000, Cell Signaling, Danvers, MA, USA), anti-HMGB1 (ab77302, 1:1000; Abcam), and anti-β-actin (ab189073, 1:1000; Abcam). After washing 3 times, the membranes were incubated in HRP-conjugated secondary antibodies for another 1 h at room temperature. Then, the protein signals were detected using the ECL Western blotting substrate (Promega, Madison, WI, USA).
Dual luciferase reporter assay
Because online prediction showed there are 2 possible binding sites between miR-129-5p and 3′UTR of HMGB1 mRNA, 2 pairs of 3′UTR sequence of HMGB1 containing the wild-type or mutant miR-129-5p binding site were chemically synthesized and cloned into the downstream of the luciferase gene of pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega). The recombinant vectors were named as pmirGLO-HMGB1-WT1, pmirGLO-HMGB1-WT2, pmirGLO-HMGB1-MUT1, and pmirGLO-HMGB1-MUT2, respectively. Insertion and mutation were verified using sequencing. MDA-MB-231 cells were co-transfected with 200 ng luciferase reporter vector and 100 nM miR-129-5p mimics or the negative controls. Luciferase activity was examined 24 h after the transfection using the Dual-Luciferase Assay kit (Promega) according to the manufacturer’s instructions.
Statistical analysis
Data are presented in the form of mean ± standard deviation based on at least 3 independent studies. Comparison was performed using the t test. All statistical analyses were performed using SPSS 18.0 software (SPSS, Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
Results
miR-129-5p sensitized breast cancer cells to irradiation, while irradiation induced-autophagy weakened radiosensitivity
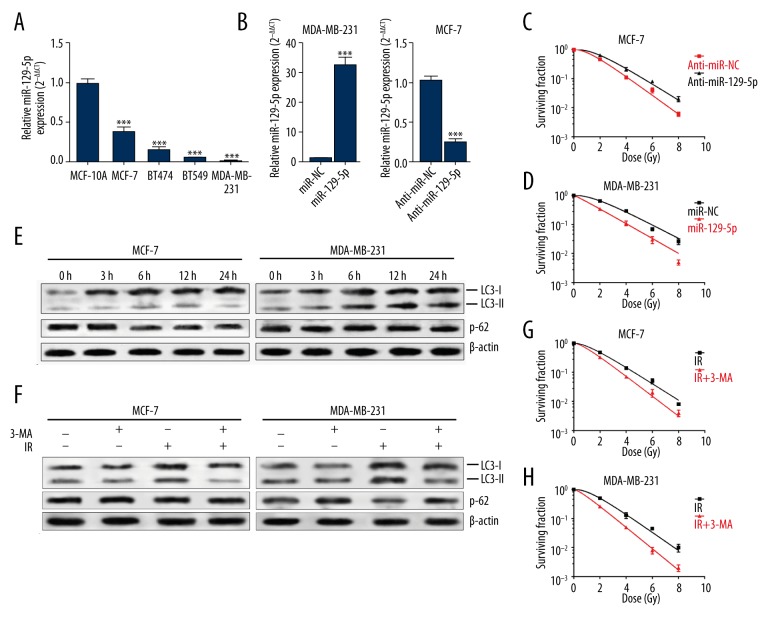
Decreased miR-129-5p can promote epithelial-mesenchymal transition and is associated with poor prognosis in breast cancer [12]. By performing qRT-PCR analysis, we compared miR-129-5p expression in 1 non-tumorigenic breast epithelial cell line and in 4 breast cancer cell lines. Results showed that miR-129-5p expression was significantly lower in MCF-7, MDA-MB-231, BT549, and BT474 cells than in MCF-10A cells (Figure 1A). Then, we enforced miR-129-5p expression in MDA-MB-231 cells and knocked down its expression in MCF-7 cells (Figure 1B). Knockdown of miR-129-5p significantly increased survival fraction of MCF-7 cells (Figure 1C), while overexpression of miR-129-5p substantially lowered the survival fraction of MDA-MB-231 cells exposed to irradiation (Figure 1D).
Figure 1.
MiR-129-5p sensitized breast cancer cells to irradiation, while irradiation induced-autophagy weakened radiosensitivity. (A) QRT-PCR analysis of the relative miR-129-5p expression in 4 breast cancer cell lines (MCF-7, MDA-MB-231, BT549, and BT474) and 1 non-tumorigenic breast epithelial cell line (MCF-10A). (B) QRT-PCR analysis of relative miR-129-5p expression in MDA-MB-231 cells transfected with miR-129-5p mimic or MCF-7 cells transfected with anti-miR-129-5p. (C, D) The survival fraction of MCF-7 cells (with or without miR-129-5p knockdown) (C) and MDA-MB-231 cells (with or without miR-129-5p overexpression) (D) after exposure to indicated doses. Colonies containing >50 cells were counted via microscopic inspection. (E) MCF-7 and MDA-MB-231 cells were exposed to 6-Gy irradiations. Autophagosome formation over time was detected via Western blot using antibodies against LC3 and p62. (F) Western blot analysis of autophagy in MCF-7 and MDA-MB-231 cells with or without treatment with 3-MA (5 mM) after irradiation (6-Gy) for 24 h. (G, H) The survival fraction of MCF-7 and MDA-MB-231 cells with 6-Gy exposure alone or with combined irradiation and 3-MA (5-mM) treatment. * p<0.05, ** p<0.01, *** p<0.001.
Autophagy may promote or alleviate cytotoxic effects of irradiation, depending on the type of cancer cells and the environmental stress [16–18]. Previous studies reported that autophagy might reduce cytotoxic effects of irradiation in breast cancer cells [11]. Consistent with previous studies, we also observed enhanced expression of LC3-II and reduced protein level of p62 in MCF-7 and MDA-MB-231 cells after irradiation (Figure 1E), which suggest irradiation activated autophagy. Then we used 3-MA, which blocks autophagosome formation and functions as an autophagy inhibitor, to further verify irradiation-activated autophagy. MCF-7 and MDA-MB-231 cells treated with 3-MA had significantly inhibited LC3-II expression and reduced degradation of p62 after irradiation (Figure 1F). In addition, 3-MA also promoted radiosensitivity of both MCF-7 and MDA-MB-231 cells (Figure 1G, 1H). These results suggest that autophagy is activated after irradiation and acts as a protective response in breast cancer cell survival.
MiR-129-5p inhibited irradiation-induced autophagy and promoted cell death
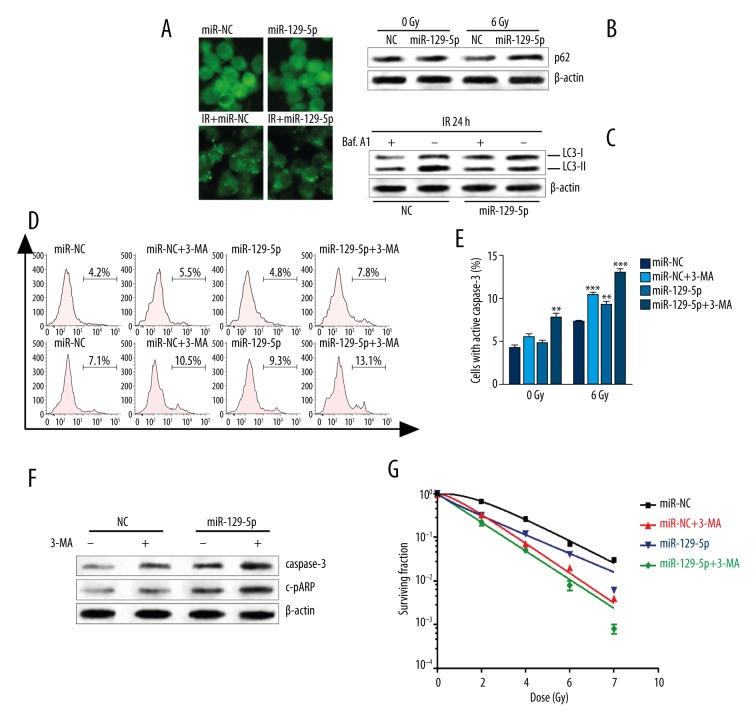
In MDA-MB-231 cells with stable GFP-LC3 expression, miR-129-5p overexpression significantly decreased the lipidation of LC3 after irradiation (Figure 2A). miR-129-5p overexpression also inhibited p62 degradation induced by irradiation (Figure 2B). To investigate the stage in which miR-129-5p was involved in the autophagy process, MDA-MB-231 cells were treated with Baf. A1, an inhibitor of the late phase of autophagy through preventing maturation of autophagic vacuoles [19]. By 24 h after irradiation, the Baf. A1-treated negative control group showed significantly increased accumulation of LC3-II. However, miR-129-5p overexpression substantially attenuated the response (Figure 2C). Therefore, miR-129-5p may inhibit autophagy, beginning in early autophagosome formation.
Figure 2.
MiR-129-5p inhibited irradiation-induced autophagy and promoted cell death. (A) MDA-MB-231 cells stably expressing GFP-LC3 were established. The cells were then transfected with miR-129-5p mimics. At 48 h after transfection, the cells were irradiated (6-Gy) and incubated for another 24 h. Then the accumulation of GFP-LC3 puncta was captured using a fluorescence microscope. (B) Western blot analysis of p62 in MDA-MB-231 cells 48 h after indicated transfection. (C) At 48 h after transfection, MDA-MB-231 cells were treated with Baf. A1 (100 nM) for 1 h and then exposed to 6-Gy irradiation. The expression of LC3-I/-II was analyzed 24 h after irradiation. (D) MDA-MB-231 cells with or without miR-129-5p overexpression were treated with 3-MA (5-mM) for 1 h, followed by irradiation (6-Gy). At 24 h after irradiation, the cells with active caspase-3 were detected using flow cytometry. (E) Quantification of the proportion of cells with active caspase-3 is shown in Figure D. (F) Cleaved caspase-3 (c-caspase-3) and cleaved PARP (c-PARP) protein expression were further detected using Western blot. (G) The survival fractions of cells with the indicated treatment. The data are presented as mean ± standard deviation from 3 independent experiments. * p<0.05, ** p<0.01, *** p<0.001.
We investigated whether miR-129-5p-enhanced radiosensitivity was dependent on autophagy inhibition. Neither 3-MA treatment nor miR-129-5p overexpression alone changed the rate of apoptotic MDA-MB-231 cells without irradiation (Figure 2D, 2E). However, 3-MA treatment and miR-129-5p overexpression increased apoptosis rate (Figure 2D, 2E). In the irradiation group, both 3-MA treatment and miR-129-5p overexpression promoted cell apoptosis (Figure 2D, 2E). Subsequent Western blot analysis showed that 3-MA treatment or miR-129-5p overexpression enhanced the irradiation-induced activation of caspase-3 and PARP (Figure 2F). In addition, 3-MA treatment and miR-129-5p overexpression significantly decreased survival fraction of MDA-MB-231 cells exposed to irradiation (Figure 2G).
MiR-129-5p inhibited irradiation-induced autophagy by targeting HMGB1
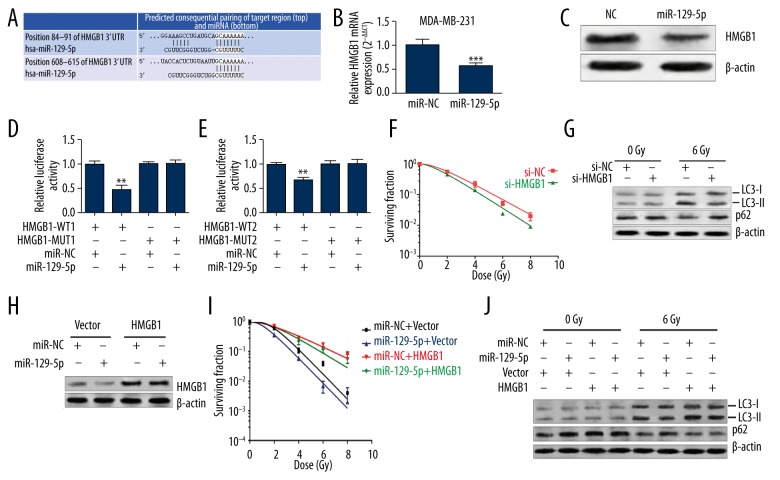
To investigate the downstream regulation of miR-129-5p in irradiation-induced autophagy, the potential target genes were searched for using online databases (TargetScan 6.2). A previous study reported that HMGB1 is a potential target of miR-129-5p in glioma cells [20]. In fact, HMGB1 is abundantly expressed in human breast carcinoma [21] and is associated with poor response to chemotherapy in breast cancer [22]. Online prediction also showed there are 2 possible binding sites between miR-129-5p and HMGB1 (Figure 3A); therefore, we decided to focus on the association between them. Ectopic expression of miR-129-5p significantly reduced HMGB1 expression at mRNA (Figure 3B) and protein (Figure 3C) levels in MDA-MB-231 cells. In addition, miR-129-5p can also suppress the relative luciferase activity of the reporter carrying either one of the wild-type of predicted binding sites of HMGB1 3′UTR, but had no suppressive effects on the reporters carrying mutant sequences (Figure 3D, 3E).
Figure 3.
MiR-129-5p inhibited irradiation-induced autophagy by targeting HMGB1. (A) Predicted binding sites between miR-129-5p and 3′-UTR of HMGB1. (B, C) QRT-PCR (B) and Western blot (C) analysis of HMGB1 expression in MDA-MB-231 cells with or without miR-129-5p overexpression. (D, E) Relative luciferase activity of MDA-MB-231cells after co-transfection with pmirGLO-HMGB1-WT1 or pmirGLO-HMGB1-MUT1 and miR-129-5p mimics or the negative control (D) or after co-transfection with pmirGLO-HMGB1-WT2 or pmirGLO-HMGB1-MUT2 and miR-129-5p mimic or the negative control. (F) Survival fraction of MDA-MB-231 cells with or without knockdown of HMGB1. (G) Western blot analysis of LC3 and p62 activity in MDA-MB-231 cells (with or without knockdown of HMGB1) 24 h after irradiation. (H) Western blot analysis of HMGB1 expression in MDA-MB-231 cells co-transfected with GV144 empty vector or GV144-HMGB1 and miR-129-5p mimics or the negative control. (I) Survival fraction MDA-MB-231 cells with transfection indicated in Figure H. (J) The activity of irradiation-induced autophagy in MDA-MB-231 cells with transfection indicated in Figure H. * p<0.05, ** p<0.01, *** p<0.001.
Finally, we studied the functions of HMGB1 in radiosensitivity. MDA-MB-231 cells with HMGB1 knockdown had reduced survival fraction after irradiation (Figure 3F). HMGB1 knockdown also suppressed irradiation-induced autophagy (Figure 3G), suggesting miR-129-5p can suppress irradiation-induced autophagy by directly targeting HMGB1. To further explore the regulative role of miR-129-5p-HMGB1 axis in radiosensitivity, we constructed an HMGB1 expression plasmid without 3′UTR (Figure 3H). MiR-129-5p could not reverse the increased survival fraction (Figure 3I) and enhanced autophagy (Figure 3J) after irradiation due to HMGB1 overexpression. Therefore, these results further confirmed that HMGB1 is a functional target of miR-129-5p.
Discussion
MiR-129-5p has recently been reported to be a tumor suppressor in breast cancer. It is usually downregulated in breast cancer tissues compared with the paired adjacent normal breast tissues [13]. Functional studies showed that it has great ability to suppress cell mobility and migration in BT549 and MDA-MB-231 cells. In addition, a recent study found that overexpression of miR-129-5p reversed epithelial-mesenchymal transition, whereas depletion of miR-129-5p induced epithelial-mesenchymal transition in breast cancer cells [12]. In fact, increased epithelial-mesenchymal transition is usually associated with decreased radiosensitivity in cancer [23,24]. Therefore, it is possible that miR-129-5p is involved in regulation of radiosensitivity. However, there is no previous study reporting the association between miR-129-5p and radiosensitivity in breast cancer. In the present study we found that miR-129-5p overexpression could sensitize MDA-MB-231 cells to irradiation, while knockdown of miR-129-5p could reduce radiosensitivity of MCF-7 cells. Therefore, we decided to further investigate the underlying mechanisms.
Autophagy is an evolutionarily conserved cellular process that helps maintain cellular homeostasis through protein degradation and organelle turnover [25]. Although this mechanism may suppress tumorigenesis, its activation during the later stages of tumor progression usually acts as a protective mechanism against stressful conditions [26]. A recent study reported that autophagy contributes to resistance of breast cancer cells to ionizing radiation [27]. Inhibition of autophagy can overcome radioresistance in the cancer cells through multiple mechanisms, such as suppression of TAK1 activation [9] and Met inhibition [28]. Several studies also found that miRNAs are involved in regulation of radiosensitivity of cancer cells through autophagy [29]. For example, miR-200c can inhibit autophagy and enhances radiosensitivity in breast cancer cells by targeting UBQLN1 [11]. MiR-101 can sensitize human nasopharyngeal carcinoma cells to radiation by targeting stathmin 1 [30]. MiR-216a can enhance the radiosensitivity of pancreatic cancer cells by inhibiting beclin-1-mediated autophagy [31]. In the present study we confirmed that autophagy induced by irradiation functions as a survival mechanism in breast cancer cells. We also observed that miR-129-5p can enhance radiosensitivity through promoting apoptosis.
In breast cancer, HMGB1 can mediate cancer progression and chemotherapy resistance [14]. Downregulation of HMGB1 can break telomere homeostasis, suppress the repair of DNA damage, and thus increase the radiosensitivity of human breast cancer cells [15]. Therefore, HMGB1 might be a critical gene modulating chemo- and radio-sensitivity of breast cancer cells. However, how it is regulated and how it is involved in radiosensitivity of breast cancer is not quite clear. One study reported that miR-200c can target HMGB1, thereby inhibiting metastasis of breast cancer cells [32]. In fact, a recent study reported that HMGB1 is a potential target of miR-129-5p in glioma cells [20]. By performing dual luciferase assay and Western blot analysis, we verified 2 binding sites between 3′UTR of HMGB1 and miR-129-5p. Enforced miR-129-5p expression significantly suppressed HMGB1 expression at the mRNA and protein levels. We also observed that HMGB1 knockdown substantially enhanced radiosensitivity and suppressed irradiation-induced autophagy in breast cancer cells. In contrast, overexpression of miR-129-5p had limited effect on HMGB1 (without 3′UTR)-induced higher level of autophagy and lower response to irradiation. These findings show that miR-129-5p can suppress autophagy and enhance radiosensitivity of breast cancer cells by targeting HMGB1.
Conclusions
The miR-129-5p/HMGB1 axis can regulate irradiation-induced autophagy in breast cancer and might be an important pathway regulating radiosensitivity of breast cancer cells.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Shioi Y, Kashiwaba M, Inaba T, et al. Long-term complete remission of metastatic breast cancer, induced by a steroidal aromatase inhibitor after failure of a non-steroidal aromatase inhibitor. Am J Case Rep. 2014;15:85–89. doi: 10.12659/AJCR.890023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sio TT, Joliat GR, Jrebi N. Multidisciplinary approach to uncommon, widely metastatic breast cancer. Eur Rev Med Pharmacol Sci. 2014;18:846–50. [PubMed] [Google Scholar]
- 4.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 5.Buyuklu M, Kandemir FM, Ozkaraca M, et al. Protective effect of curcumin against contrast induced nephropathy in rat kidney: What is happening to oxidative stress, inflammation, autophagy and apoptosis? Eur Rev Med Pharmacol Sci. 2014;18:461–70. [PubMed] [Google Scholar]
- 6.Lozy F, Karantza V. Autophagy and cancer cell metabolism. Semin Cell Dev Biol. 2012;23:395–401. doi: 10.1016/j.semcdb.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jezierska A, Matysiak W, Motyl T. ALCAM/CD166 protects breast cancer cells against apoptosis and autophagy. Med Sci Monit. 2006;12(8):BR263–73. [PubMed] [Google Scholar]
- 8.Tan Q, Wang H, Hu Y, et al. Src/STAT3-dependent heme oxygenase-1 induction mediates chemoresistance of breast cancer cells to doxorubicin by promoting autophagy. Cancer Sci. 2015;106:1023–32. doi: 10.1111/cas.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han MW, Lee JC, Choi JY, et al. Autophagy inhibition can overcome radioresistance in breast cancer cells through suppression of TAK1 activation. Anticancer Res. 2014;34:1449–55. [PubMed] [Google Scholar]
- 10.Wang Z, Wang N, Liu P, et al. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget. 2014;5:7013–26. doi: 10.18632/oncotarget.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Q, Liu T, Yuan Y, et al. MiR-200c inhibits autophagy and enhances radiosensitivity in breast cancer cells by targeting UBQLN1. Int J Cancer. 2015;136:1003–12. doi: 10.1002/ijc.29065. [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Zhao Y, Sun XH, et al. Down-regulation of miR-129-5p via the Twist1-Snail feedback loop stimulates the epithelial-mesenchymal transition and is associated with poor prognosis in breast cancer. Oncotarget. 2015;6(33):34423–36. doi: 10.18632/oncotarget.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang QY, Tang J, Zhou CX, Zhao Q. [The down-regulation of miR-129 in breast cancer and its effect on breast cancer migration and motility]. Sheng Li Xue Bao. 2012;64:403–11. in Chinese. [PubMed] [Google Scholar]
- 14.Amornsupak K, Insawang T, Thuwajit P, et al. Cancer-associated fibroblasts induce high mobility group box 1 and contribute to resistance to doxorubicin in breast cancer cells. BMC Cancer. 2014;14:955. doi: 10.1186/1471-2407-14-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke S, Zhou F, Yang H, et al. Downregulation of high mobility group box 1 modulates telomere homeostasis and increases the radiosensitivity of human breast cancer cells. Int J Oncol. 2015;46:1051–58. doi: 10.3892/ijo.2014.2793. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Yan W, He X, et al. miR-375 inhibits autophagy and reduces viability of hepatocellular carcinoma cells under hypoxic conditions. Gastroenterology. 2012;143:177–87.e8. doi: 10.1053/j.gastro.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Bristol ML, Di X, Beckman MJ, et al. Dual functions of autophagy in the response of breast tumor cells to radiation: cytoprotective autophagy with radiation alone and cytotoxic autophagy in radiosensitization by vitamin D 3. Autophagy. 2012;8:739–53. doi: 10.4161/auto.19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Yang Y, Yang X, et al. Autophagy and its function in radiosensitivity. Tumour Biol. 2015;36:4079–87. doi: 10.1007/s13277-015-3496-x. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto A, Tagawa Y, Yoshimori T, et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Huang JQ, Zhang X, Shen LF. MiR-129-2 functions as a tumor suppressor in glioma cells by targeting HMGB1 and is down-regulated by DNA methylation. Mol Cell Biochem. 2015;404:229–39. doi: 10.1007/s11010-015-2382-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brezniceanu ML, Volp K, Bosser S, et al. HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J. 2003;17:1295–97. doi: 10.1096/fj.02-0621fje. [DOI] [PubMed] [Google Scholar]
- 22.Ladoire S, Penault-Llorca F, Senovilla L, et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy. 2015;11:1878–90. doi: 10.1080/15548627.2015.1082022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang L, Graham PH, Hao J, et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng J, Li P, Zhang Q, et al. A radiosensitivity gene signature in predicting glioma prognostic via EMT pathway. Oncotarget. 2014;5:4683–93. doi: 10.18632/oncotarget.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carew JS, Kelly KR, Nawrocki ST. Autophagy as a target for cancer therapy: new developments. Cancer Manag Res. 2012;4:357–65. doi: 10.2147/CMAR.S26133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 27.Chaachouay H, Ohneseit P, Toulany M, et al. Autophagy contributes to resistance of tumor cells to ionizing radiation. Radiother Oncol. 2011;99:287–92. doi: 10.1016/j.radonc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Lin CI, Whang EE, Donner DB, et al. Autophagy induction with RAD001 enhances chemosensitivity and radiosensitivity through Met inhibition in papillary thyroid cancer. Mol Cancer Res. 2010;8:1217–26. doi: 10.1158/1541-7786.MCR-10-0162. [DOI] [PubMed] [Google Scholar]
- 29.Min W, Wang B, Li J, et al. The expression and significance of five types of miRNAs in breast cancer. Med Sci Monit Basic Res. 2014;20:97–104. doi: 10.12659/MSMBR.891246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q, Liu T, Zhang T, et al. MiR-101 sensitizes human nasopharyngeal carcinoma cells to radiation by targeting stathmin 1. Mol Med Rep. 2015;11:3330–36. doi: 10.3892/mmr.2015.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Shi H, Lin S, et al. MicroRNA-216a enhances the radiosensitivity of pancreatic cancer cells by inhibiting beclin-1-mediated autophagy. Oncol Rep. 2015;34:1557–64. doi: 10.3892/or.2015.4078. [DOI] [PubMed] [Google Scholar]
- 32.Chang BP, Wang DS, Xing JW, et al. miR-200c inhibits metastasis of breast cancer cells by targeting HMGB1. J Huazhong Univ Sci Technolog Med Sci. 2014;34:201–6. doi: 10.1007/s11596-014-1259-3. [DOI] [PubMed] [Google Scholar]