Purpose of review
Hypothalamic alterations, pathological or treatment induced, have major impact on prognosis in craniopharyngioma patients mainly because of consequent hypothalamic obesity. Recent insight in molecular genetics, treatment strategies, risk factors and outcomes associated with hypothalamic obesity provide novel therapeutic perspectives. This review includes relevant publications since 2013.
Recent findings
Recent findings confirm that alterations in posterior hypothalamic areas because of tumour location and/or treatment-related injuries are associated with severe hypothalamic obesity, reduced overall survival and impaired quality of life in long-term survivors of childhood-onset craniopharyngioma. However, eating disorders are observed because of hypothalamic obesity without clear disease-specific patterns. Treatment options for hypothalamic obesity are very limited. Treatment with invasive, nonreversible bariatric methods such as Roux-en-Y gastric bypass is most efficient in weight reduction, but controversial in the paediatric population because of medical, ethical, and legal considerations. Accordingly, treatment in craniopharyngioma should focus on prevention of (further) hypothalamic injury. Presurgical imaging for grading of hypothalamic involvement should be the basis for hypothalamus-sparing strategies conducted by experienced multidisciplinary teams.
Summary
Until a nonsurgical therapeutic option for hypothalamic obesity for paediatric patients is found, prevention of hypothalamic injury should be the preferred treatment strategy, conducted exclusively by experienced multidisciplinary teams.
Keywords: craniopharyngioma, eating disorders, hypothalamus, obesity, quality of life, survival
INTRODUCTION
Energy homeostasis is regulated by a complex neuroendocrine system, of which the hypothalamus is the centre for said regulation. The functional disruption of the hypothalamic network causes hypothalamic obesity [1▪,2,3,4▪]. Recent studies have illuminated how the hypothalamus regulates appetite and satiety [1▪,5]. The disruptions causing hypothalamic obesity include brain tumours, neurosurgery, and/or cranial irradiation.
In this context, craniopharyngioma is a paradigmatic disease comprising different risk factors for hypothalamic obesity. Childhood-onset craniopharyngiomas are rare intracranial embryonal malformations of the sellar region [6▪,7▪]. These tumours show low-grade histological malignancy (WHO °I), frequently affect hypothalamic/pituitary regions and the optic chiasm. Hypothalamic involvement and/or treatment-related lesions to hypothalamic structures result in impaired physical and social functionality [8▪▪,9▪▪,10,11▪] that includes severe neuroendocrine sequelae, mainly hypothalamic obesity, with major negative impact on quality of life in surviving patients [1▪,6▪,8▪▪,9▪▪,10,12,13▪▪,14]. Unfortunately, attempts to control hypothalamic obesity with diet, exercise, and/or pharmacological treatments have not been satisfactory.
The review summarizes novel insights in hypothalamic appetite regulation, pathophysiology, aetiology, clinical characteristics, and treatment modalities for hypothalamic obesity in patients with childhood-onset craniopharyngioma.
Box 1.
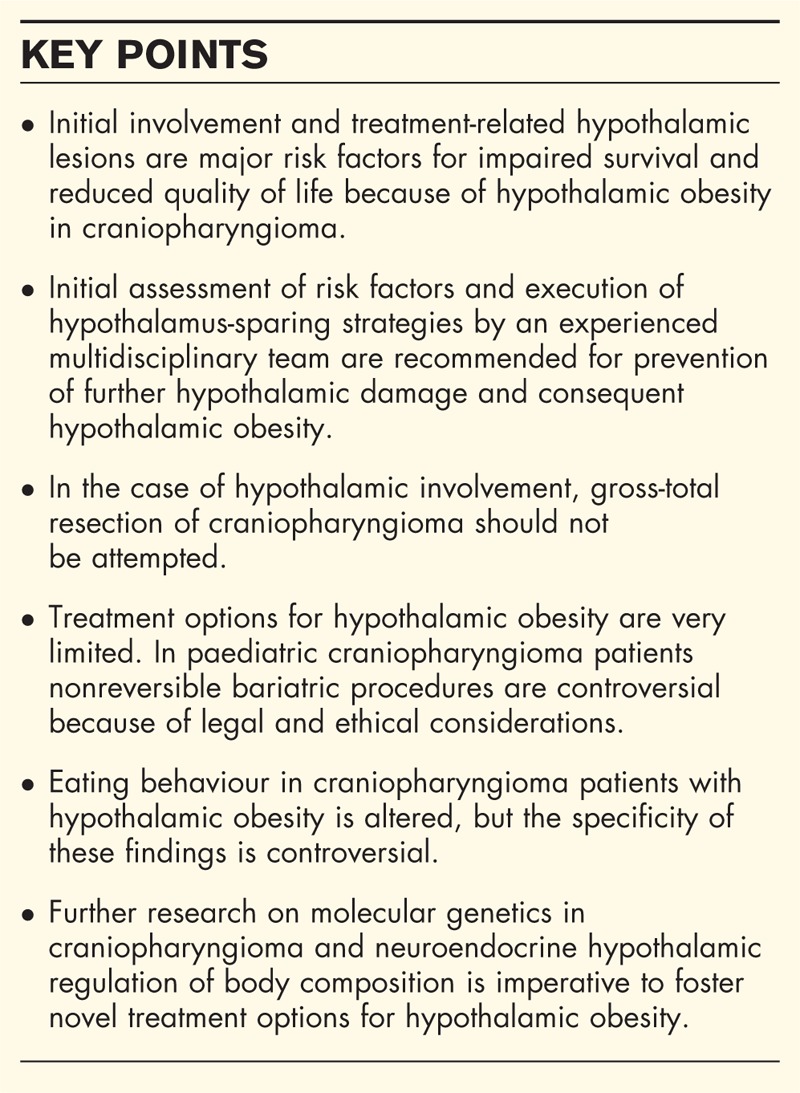
no caption available
EPIDEMIOLOGY, PATHOLOGY, AND CLINICAL PRESENTATION
Craniopharyngiomas are rare, with an incidence of 0.5 to two cases per million persons per year. A bimodal age distribution has been shown, with peak incidence rates in children of ages 5–14 years and adults of ages 50–74 years [15▪,16]. In childhood and adolescence, its histological type is usually adamantinomatous with cyst formation. More than 70% of the adamantinomatous type of craniopharyngioma bear a mutation of the β-catenin gene, which is not detectable in the adult papillary type of craniopharyngioma [17,18▪▪,19,20]. Recent reports [21,22] on molecular findings in adamantinomatous craniopharyngioma and a novel mouse model [23] indicate tentative perspectives on future treatment and prevention of hypothalamic infiltration in adamantinomatous craniopharyngioma [24]. Brastianos et al.[25▪▪] reported on a BRAF mutation in 95% of papillary-type histology, which is not detectable in adamantinomatous craniopharyngioma. This specific finding has important relevance in differential diagnosis of sellar masses [26–28] and is a potential target for pharmaceutical therapy [29].
The main hypothalamic areas involved in energy regulation are the medial hypothalamus, which consists of the ventromedial nucleus, arcuate nucleus, and paraventricular nucleus. Arcuate nucleus neurons generate orexigenic peptides, such as agouti-related protein and neuropeptide Y and secrete anorexigenic peptides-like proopiomelanocortin, a precursor of α-melanocyte-stimulating hormone (α-MSH). α-MSH acts at the melanocortin-4 receptor and reduces appetite and food intake. Afferent signals, including leptin, insulin, ghrelin, and peptide YY, affect the anorexigenic centre of the hypothalamus. Efferent signals from the paraventricular nucleus in turn stimulate the sympathetic nervous system [5,30]).
The diagnosis of childhood craniopharyngioma is often made late – sometimes years after the initial appearance of symptoms – with a clinical picture at the time of diagnosis often dominated by nonspecific manifestations of intracranial pressure [31,32]. Further, primary manifestations are visual impairment (62–84%) and endocrine deficits (52–87%) [9▪▪,33].
Recent reports confirm that initial imaging [12,34▪▪,35,36,37▪,38] and assessment of hypothalamic involvement of craniopharyngioma is essential in estimating prognosis and long-term quality of life [39–41,42▪]. Muller et al.[40,41] observed that initial tumour involvement of mammillary bodies confirmed by imaging served as an independent risk factor for impaired long-term prognosis regardless of chosen treatment strategies. These findings are confirmed by further studies on the pathogenic relevance of hypothalamic structures located at mammillary bodies for the development of hypothalamic obesity [43,44].
TREATMENT STRATEGIES AND HYPOTHALAMIC INJURY
For favourably localized tumours, the preferred treatment of choice, especially at primary craniopharyngioma diagnosis, is an attempt at complete resection with preservation of visual and hypothalamic function [6▪,12,39,45–50]. For unfavourably localized tumours – those too close to or too entangled with the optic chiasm and/or the hypothalamus – a planned limited resection should be performed to preserve integrity of and/or to avoid further damage to the hypothalamic and optic structures [4▪,51▪,52,53,54▪▪,55▪–57▪]. Endoscopic routes provide novel and less traumatic approaches for surgical resection [58▪,59].
The implantation of an intracystic catheter with a subcutaneous reservoir enables the possibility of repeated decompression of the cyst and instillation of sclerosing agents [60▪,61]. Bartels et al.[62] report good tolerability and high efficiency of interferon alpha as the intracystic sclerosing agent.
Irradiation is effective in preventing relapses and progression of residual tumour and is therefore a recommended treatment option in cases of limited surgical perspectives [63▪,64]. Preliminary experiences with proton beam therapy applied to craniopharyngioma are promising, offering a more protective radio-oncological option than conventional external irradiation, especially for tumours localized in the vicinity of the optic chiasm, pituitary gland, or hypothalamus [65▪,66]. Stereotactic irradiation and gamma knife treatment are options in rare cases [67,68▪].
HYPOTHALAMIC OBESITY
Symptoms related to hypothalamic dysfunction, such as obesity, daytime sleepiness, disturbed circadian rhythm, behavioural changes, and imbalances in regulation of thirst, body temperature, heart rate, and/or blood pressure, have been found at diagnosis in 35% of childhood craniopharyngioma patients [6▪,12,69,70,71▪,72▪]. The rate of hypothalamic dysfunction dramatically increases following treatment; in some series up to 65–80% [4▪].
Associated with high morbidity, suprachiasmatic lesions are difficult to treat. Surgical removal of tumour tissue beyond the mammillary bodies risks hypothalamic structures and consequent hypothalamic obesity [39,40,51▪,73▪]. With the aid of imaging studies, recent reports have indicated that the degree of obesity of affected patients is positively correlated with the degree of hypothalamic damage [39,40–42▪]. Taking these considerations into account, novel classifications of presurgical involvement and postsurgical lesions of hypothalamic structures based on MRI have been recently published [40,41,51▪] that might help to establish risk-adapted, that is, hypothalamus-sparing surgical strategies.
Weight gain in childhood craniopharyngioma often occurs years before diagnosis [33], with 12–19% of patients reported to be obese at diagnosis [6▪]. Weight gain occurs despite adequate endocrine replacement of pituitary hormone deficiencies. The hypothalamic disturbance in energy management contributes to obesity and is exacerbated by factors limiting physical activity such as marked daytime sleepiness [74]. The degree of obesity frequently increases early after treatment and rapid weight gain occurs the first 6–12 months after treatment [33]. Following treatment, the prevalence of obesity is higher, reaching up to 55% [75]. Obesity results in increased risks of metabolic syndrome and cardiovascular disease [73▪].
The relation of obesity with hypothalamic damage is obvious in craniopharyngioma [5,30]. It is likely that in cases of suprasellar extension, hypothalamic function will be compromised and will remain compromised to a certain extent when treated surgically or with irradiation. The hypothalamus contains many groups of nerve cell bodies forming distinct nuclei, which have highly diverse molecular, structural, and functional organizations [1▪,5]. The hypothalamus plays a major role in keeping the internal environment stable by synchronizing circadian rhythms. Recent data indicate that a proper balance of the autonomic nervous system is crucial for metabolism [76]. It is well known that adipose tissue is richly innervated by sympathetic nerve fibres that control lipolysis. It now appears that lipogenesis is also controlled by parasympathetic innervation of adipose tissue originating from separate sympathetic and parasympathetic neurons in the periventricular nucleus and suprachiasmatic nucleus (SCN) [1▪,30,76]. Such a high level of differentiation puts the SCN in a key position to balance circadian activity of both branches of the autonomous nervous system [30]. Considering the large proportion of patients with damage to suprasellar structures, it is likely that craniopharyngiomas and/or the effects of treatment damage the SCN. This in turn, affects regulation of central clock mechanisms, which predisposes to alterations in metabolism [74]. Clearly, surgical strategies to preserve hypothalamic integrity are mandatory for the prevention of sequelae such as severe obesity owing to hypothalamic lesions [12].
A study involving self-assessment by nutritional diaries revealed that hypothalamic obesity can occur in patients with childhood craniopharyngioma even when their caloric intake is similar to controls matched for BMI [77]. An analysis of physical activity showed that patients with childhood craniopharyngioma had a markedly lower level of physical activity than BMI-matched healthy controls [77]. Aforementioned marked daytime sleepiness and disturbances of circadian rhythms have been demonstrated in patients with childhood craniopharyngioma and obesity [74], which in turn were correlated with low nocturnal and early morning melatonin levels in saliva [78]. The proposed pathogenic mechanism involves impaired hypothalamic regulation of circadian melatonin rhythms in patients with craniopharyngioma extending to the suprasellar area. Initial experiences with melatonin substitution in patients with childhood craniopharyngioma were promising: melatonin levels normalized and daytime sleepiness and physical activity improved [78].
Polysomnographic studies in patients with childhood craniopharyngioma and severe daytime sleepiness have revealed sleeping patterns typical for hypersomnia and secondary narcolepsy [74], leading to the conclusion that secondary narcolepsy should be taken into consideration as a pathogenic factor in severely obese children and adolescents with craniopharyngioma. Treatment with central-stimulating agents (methylphenidate, modafinil) has had a significantly beneficial effect on daytime sleepiness in these patients [79].
A decreased metabolic rate, in terms of both resting and total energy expenditure, is likely to contribute to weight gain in this population. Adults and paediatric patients with childhood-onset craniopharyngioma were found to have a lower resting energy expenditure (REE) compared with controls that were not explained by differences in body composition [80▪]. This energy intake/REE ratio was significantly lower in those with tumours involving the third ventricle [81]. Further, factors that could potentially contribute to decreased physical activity are neurological and visual deficits [82,83], increased daytime sleepiness [74,78], and psychosocial difficulties [75].
Roemmler-Zehrer and colleagues [84▪] recently analysed the gastrointestinal hormones ghrelin and peptide YY and their effect on satiety in obese craniopharyngioma patients. Their findings support the hypothesis that reduced ghrelin secretion and reduced postprandial suppression of ghrelin and severe obesity leads to disturbed regulation of appetite in craniopharyngioma patients. However, peptide YY levels did not differ between normal weight, obese, and very obese patients. Further potential pathogenic roles of peripheral α-MSH and brain-derived neurotrophic factor in childhood craniopharyngioma obesity have been postulated [85,86].
CHALLENGES IN TREATING HYPOTHALAMIC OBESITY
Owing to the above-reported disturbances in energy expenditure, central sympathetic output, and appetite-regulation, it is clear why craniopharyngioma patients with hypothalamic obesity typically develop morbid obesity that is mainly unresponsive to conventional lifestyle modifications [1▪,6▪,10,12,49,87].
Recent studies on novel pharmaceutical treatment options in craniopharyngioma patients with hypothalamic obesity report mixed results. Based on impairment of sympatho-adrenal activation and epinephrine production manifesting as a reduced hormonal response to hypoglycaemia, treating this disorder with amphetamine derivatives has been suggested [79]. Zoicas et al.[88] treated eight adult patients (six craniopharyngioma) with hypothalamic obesity with glucagon-like peptide 1 (GLP-1) analogues and observed a substantial and sustained weight loss associated with improvements in metabolic and cardiovascular risk profiles. Kalina et al.[89▪] analysed the effect of metformin and fenofibrate treatment on metabolic status in 22 patients with childhood-onset craniopharyngioma. The authors reported a positive effect on dyslipidemia and homeostatic model assessment during short-term follow-up of 6 months. van Santen et al.[90▪] reported a hypothalamic obesity case resulting from treatment of craniopharyngioma in which T3 monotherapy was not effective in increasing REE or brown adipose tissue activity. This may be explained by damage to the ventromedial hypothalamic region, which is a key area in the hypothalamus for T3-mediated brown adipose tissue activation. Whereas substitution therapy with recombinant growth hormone is safe and efficient in promoting normal growth, relevant weight reducing effects are not observed in patients with hypothalamic obesity [91,92].
Childhood craniopharyngioma patients with hypothalamic obesity have a parasympathetic predominance of the autonomic nervous system induced by vagal activation, manifesting as daytime sleepiness and reduced heart rate variability [76]. Parasympathetic stimulation causes insulin secretion by way of direct activation of β cells as well as adipogenesis. As insulin is an anabolic hormone, it is likely to be an important driver of weight gain in hypothalamic obesity. As octreotide is a somatostatin analogue and thus causes reduction in insulin secretion, Lustig et al.[93] used octreotide in a double-blind randomized controlled study in children with hypothalamic obesity, demonstrating moderate reductions in weight gain in which insulin levels during a proof-of-concept oral glucose tolerance test decreased without leading to major changes in glucose tolerance.
Initial experiences with bariatric surgery in severely obese childhood craniopharyngioma patients achieved sufficient tolerability and short-term weight reduction [94,95]. An instant improvement of binge-eating behaviour in patients immediately after laparoscopic adjustable gastric banding was observed, but failed in long-term weight reduction. Nevertheless, weight stabilization could be achieved with regular follow-up monitoring [96]. Treatment with invasive, nonreversible bariatric methods such as Roux-en-Y gastric bypass is most efficient in weight reducing [94] but controversial in the paediatric population because of medical, ethical, and legal considerations [96]. Reports on tolerability and efficacy of deep brain stimulation [97] and gastric pacemaking devices [98] in treatment of craniopharyngioma patients with hypothalamic obesity have not been published.
Despite the availability of these promising therapeutic approaches [99▪], it must be emphasized that currently no generally accepted (pharmacological or bariatric) therapy for hypothalamic obesity in craniopharyngioma has been shown to be effective in randomized studies. Furthermore, structured rehabilitation programs for weight reduction in survivors of craniopharyngioma have no proven long-term effect on long-term weight stabilization [100▪].
However, a purposeful care home environment [101▪] and adequate communication of interdisciplinary decisions with patients’ families [102▪] have been reported to exert beneficial effects on follow-up treatment of craniopharyngioma patients.
EATING BEHAVIOUR
Strong associations between obesity and an obesogenic environment [103] and consequent eating behaviour have been confirmed in children and adolescents [104▪]. Owing to the dearth of studies on eating behaviour in craniopharyngioma patients, Hoffmann et al.[13▪▪] analysed eating behaviour and eating disorders in 101 survivors of childhood craniopharyngioma and 85 BMI-matched healthy controls. Severely obese patients (BMI > 8 SD; N = 9) presented with pathological eating behaviours and more weight problems and eating disorders, as compared with obese (BMI 3–8 SD; N = 44) and normal or overweight patients (BMI < 3 SD; N = 48). However, craniopharyngioma patients with different degrees of obesity showed similar or even less pathological findings as compared with BMI-matched normal controls. The authors conclude that the observed eating disorders are not disease-specific in craniopharyngioma patients.
Using functional MRI, Roth et al.[105▪,106] assessed pre and post-meal responses to visual food cues in craniopharyngioma patients’ brain regions of interest. Following the test meal, BMI-matched controls showed suppression of activation by high-calorie food cues whereas craniopharyngioma patients showed trends toward higher activation. These data support the hypothesis that perception of food cues may be altered in craniopharyngioma patients with hypothalamic obesity, especially after food intake.
Roemmler-Zehrer et al.[107▪] compared eating behaviour in 26 craniopharyngioma patients (four childhood-onset cases) with 26 patients with nonfunctioning pituitary adenoma. Whereas craniopharyngioma patients scored higher in conscious hunger perception, the rate of eating disorders was similar in both groups, supporting the speculation that eating disorders in patients with hypothalamic obesity are not disease specific.
Even though hypothalamic obesity is a frequent sequela in craniopharyngioma, diencephalic syndrome leading to weight loss and cachexia can occur as a rare hypothalamic disturbance of body composition in craniopharyngioma [108,109▪▪]. Hoffmann et al.[109▪▪] analysed the incidence of diencephalic syndrome, its clinical manifestations before and after diagnosis of craniopharyngioma, and outcome in 485 patients recruited in the German Childhood Craniopharyngioma Registry. Only 4.3% of all craniopharyngioma patients presented with a low weight (BMI ≤ 2-SD) at time of diagnosis. Initial significant differences between patients with low weight at diagnosis and normal weight patients at diagnosis are usually observed at 5 years of age. Within the first 2 years after diagnosis, the BMI of diencephalic syndrome patients and normal weight patients converge to a similar level. The authors concluded from their analysis of patients’ histories that diencephalic syndrome at the time of diagnosis does not preclude subsequent weight gain caused by a craniopharyngioma with hypothalamic involvement.
SEQUELAE, PROGNOSIS, AND QUALITY OF LIFE
The standardized overall mortality rate varies between 2.88 and 9.28 in cohort craniopharyngioma studies. Patients with craniopharyngioma have a 3 to 19-fold higher cardiovascular mortality in comparison to the general population; women with craniopharyngioma have an even higher risk [16]. The 20-year overall survival is impaired in patients with hypothalamic involvement of craniopharyngioma [8▪▪,9▪▪]. Hypothalamic obesity has significant negative impact on long-term quality of survival [9▪▪]. Increased daytime sleepiness, fatigue, disturbances of circadian rhythms [74,78,110▪], gastrointestinal and pulmonary complaints (diarrhea, dypnea) [9▪▪], memory deficits [111▪▪,112▪], and neuropsychological imbalances [69,107▪,113–115] are major long-term side-effects in patients with hypothalamic obesity.
Hoffmann et al.[116▪▪] recently reported on nonalcoholic fatty liver disease (NAFLD), a severe, previously underestimated sequela in craniopharyngioma patients with hypothalamic obesity. NAFLD occurred in about 50% of craniopharyngioma patients with hypothalamic obesity and was associated with elevated liver enzymes and homeostatic model assessment index. Over half of all patients (60%) with NAFLD were treated by stimulating agents, exerting considerable liver toxicity. The authors recommended that stimulating agents for treatment of daytime sleepiness in craniopharyngioma should be prescribed judiciously.
CONCLUSION
Hypothalamic involvement of craniopharyngioma and treatment-related lesions of hypothalamic areas are major risk factors for impaired survival, neuropsychological deficits [117▪▪], and reduced quality of life mainly because of hypothalamic obesity and the alterations in eating behaviour associated with hypothalamic obesity. As posttreatment options are very limited for hypothalamic obesity, we recommend hypothalamus-sparing treatment strategies conducted exclusively by experienced multidisciplinary teams and in the context of national or international trials/registries [118▪].
Acknowledgements
The study was supported by the German Childhood Cancer Foundation (Deutsche Kinderkrebsstiftung), Bonn, Germany. The authors are grateful for the help of Margarita Neff-Heinrich (Göttingen, Germany) in proofreading and editing the manuscript.
Disclosure Statement: This manuscript was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Haliloglu B, Bereket A. Hypothalamic obesity in children: pathophysiology to clinical management. J Pediatr Endocrinol Metab 2015; 28:503–513. [DOI] [PubMed] [Google Scholar]; A comprehensive review on the hypothalamic obesity in pediatric patients.
- 2.Bereket A, Kiess W, Lustig RH. Kiess W, Wabitsch M, Maffeis C, Sharma AM, et al. Hypothalamic obesity in children. Metabolic syndrome and obesity in childhood and adolescence 1st ed.Basel, Switzerland: Karger; 2015; 19:13–30. [Google Scholar]
- 3.Müller HL. Evans JJ, Kenning TJ. Craniopharyngioma: pediatric management. Craniopharyngiomas: comprehensive diagnosis, treatment and outcome 1st edMunich, Germany: Elsevier; 2015. 429–458. [Google Scholar]
- 4▪.Daubenbuchel AM, Muller HL. Neuroendocrine disorders in pediatric craniopharyngioma patients. J Clin Med 2015; 4:389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review on the broad range of neuroendocrine disorders in patients with childhood-onset craniopharyngioma.
- 5.Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci 2015; 9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪.Muller HL. Craniopharyngioma. Endocr Rev 2014; 35:513–543. [DOI] [PubMed] [Google Scholar]; Review on the broad range of neuroendocrine disorders in patients with childhood-onset craniopharyngioma.
- 7▪.Muller HL. Craniopharyngioma. Handb Clin Neurol 2014; 124:235–253. [DOI] [PubMed] [Google Scholar]; Comprehensive review on the current state of art in diagnostics, treatment, and follow-up of craniopharyngioma.
- 8▪▪.Daubenbuchel AM, Hoffmann A, Gebhardt U, et al. Hydrocephalus and hypothalamic involvement in pediatric patients with craniopharyngioma or cysts of Rathke's pouch: impact on long-term prognosis. Eur J Endocrinol 2015; 172:561–569. [DOI] [PubMed] [Google Scholar]; The first report on impact of initial hydrocephalus and hypothalamic involvement on long-term prognosis in childhood craniopharyngioma.
- 9▪▪.Sterkenburg AS, Hoffmann A, Gebhardt U, et al. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro Oncol 2015; 17:1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]; The 20-years follow-up analysis of 485 patients with childhood-onset craniopharyngioma showing impaired overall survival in patients with hypothalamic involvement. Relapse and progression rates were similar in regard to different degrees of resection (gross total resection vs. incomplete resection).
- 10.Muller HL. Childhood craniopharyngioma: current status and recent perspectives in diagnostics and treatment. J Pediatr Endocrinol Metab 2015; 28:1–2. [DOI] [PubMed] [Google Scholar]
- 11▪.Karavitaki N. Management of craniopharyngiomas. J Endocrinol Invest 2014; 37:219–228. [DOI] [PubMed] [Google Scholar]; Review on treatment options in craniopharyngioma.
- 12.Muller HL. Paediatrics: surgical strategy and quality of life in craniopharyngioma. Nat Rev Endocrinol 2013; 9:447–449. [DOI] [PubMed] [Google Scholar]
- 13▪▪.Hoffmann A, Postma FP, Sterkenburg AS, et al. Eating behavior, weight problems and eating disorders in 101 long-term survivors of childhood-onset craniopharyngioma. J Pediatr Endocrinol Metab 2015; 28:35–43. [DOI] [PubMed] [Google Scholar]; First report on eating behavior and eating disorders in a large cohort of 101 long-term survivors of childhood-onset craniopharyngioma.
- 14.Cohen M, Bartels U, Branson H, et al. Trends in treatment and outcomes of pediatric craniopharyngioma, 1975–2011. Neuro Oncol 2013; 15:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Olsson DS, Andersson E, Bryngelsson IL, et al. Excess mortality and morbidity in patients with craniopharyngioma, especially in patients with childhood onset: a population-based study in Sweden. J Clin Endocrinol Metab 2015; 100:467–474. [DOI] [PubMed] [Google Scholar]; Population-based analysis of the Swedish Registry on outcome in childhood-onset craniopharyngioma.
- 16.Erfurth EM, Holmer H, Fjalldal SB. Mortality and morbidity in adult craniopharyngioma. Pituitary 2013; 16:46–55. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Barbera JP. Molecular and cellular pathogenesis of adamantinomatous craniopharyngioma. Neuropathol Appl Neurobiol 2015; 41:721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18▪▪.Martinez-Barbera JP, Buslei R. Adamantinomatous craniopharyngioma: pathology, molecular genetics and mouse models. J Pediatr Endocrinol Metab 2015; 28:7–17. [DOI] [PubMed] [Google Scholar]; Review on novel findings in molecular genetics and a mouse model for adamentinomatous craniopharyngioma.
- 19.Preda V, Larkin SJ, Karavitaki N, et al. The Wnt signalling cascade and the adherens junction complex in craniopharyngioma tumorigenesis. Endocr Pathol 2015; 26:1–8. [DOI] [PubMed] [Google Scholar]
- 20.McCabe MJ, Dattani MT. Genetic aspects of hypothalamic and pituitary gland development. Handb Clin Neurol 2014; 124:3–15. [DOI] [PubMed] [Google Scholar]
- 21.Holsken A, Stache C, Schlaffer SM, et al. Adamantinomatous craniopharyngiomas express tumor stem cell markers in cells with activated Wnt signaling: further evidence for the existence of a tumor stem cell niche? Pituitary 2014; 17:546–556. [DOI] [PubMed] [Google Scholar]
- 22.Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A 2011; 108:11482–11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Barbera JP. Biology of human craniopharyngioma: lessons from mouse models. J Endocrinol 2015; 226:T161–T172. [DOI] [PubMed] [Google Scholar]
- 24.Hussain I, Eloy JA, Carmel PW, Liu JK. Molecular oncogenesis of craniopharyngioma: current and future strategies for the development of targeted therapies. J Neurosurg 2013; 119:106–112. [DOI] [PubMed] [Google Scholar]
- 25▪▪.Brastianos PK, Taylor-Weiner A, Manley PE, et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet 2014; 46:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report on BRAF mutations as typical molecular finding in papillary craniopharyngioma.
- 26.Kim JH, Paulus W, Heim S. BRAF V600E mutation is a useful marker for differentiating Rathke's cleft cyst with squamous metaplasia from papillary craniopharyngioma. J Neurooncol 2015; 123:189–191. [DOI] [PubMed] [Google Scholar]
- 27.Schweizer L, Capper D, Holsken A, et al. BRAF V600E analysis for the differentiation of papillary craniopharyngiomas and Rathke's cleft cysts. Neuropathol Appl Neurobiol 2014; 41:733–742. [DOI] [PubMed] [Google Scholar]
- 28.Larkin SJ, Preda V, Karavitaki N, et al. BRAF V600E mutations are characteristic for papillary craniopharyngioma and may coexist with CTNNB1-mutated adamantinomatous craniopharyngioma. Acta Neuropathol 2014; 127:927–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aylwin SJ, Bodi I, Beaney R. Pronounced response of papillary craniopharyngioma to treatment with vemurafenib, a BRAF inhibitor. Pituitary 2015; [Epub ahead of print]. doi: 10.1007/s11102-015-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Choi JH. Pathophysiology and clinical characteristics of hypothalamic obesity in children and adolescents. Ann Pediatr Endocrinol Metab 2013; 18:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen EH, Jorgensen JO, Bjerre P, et al. Acute presentation of craniopharyngioma in children and adults in a Danish national cohort. Pituitary 2013; 16:528–535. [DOI] [PubMed] [Google Scholar]
- 32.Khan RB, Merchant TE, Boop FA, et al. Headaches in children with craniopharyngioma. J Child Neurol 2013; 28:1622–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller HL, Emser A, Faldum A, et al. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J Clin Endocrinol Metab 2004; 89:3298–3305. [DOI] [PubMed] [Google Scholar]
- 34▪▪.Mortini P, Gagliardi F, Bailo M, et al. Magnetic resonance imaging as predictor of functional outcome in craniopharyngiomas. Endocrine 2015. [DOI] [PubMed] [Google Scholar]; Study on MRI and clinical findings 47 childhood-onset craniopharyngioma patients with the aim to identify objective radiological criteria as preoperative prognostic factors of hypothalamic damage. Comparison of results with regard to current grading systems for hypothalamic involbvement/damage.
- 35.Park SW, Jung HW, Lee YA, et al. Tumor origin and growth pattern at diagnosis and surgical hypothalamic damage predict obesity in pediatric craniopharyngioma. J Neurooncol 2013; 113:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi S, Peng J, Pan J, et al. Growth and weight of children with craniopharyngiomas based on the tumour location and growth pattern. J Clinl Neurosci 2013; 20:1702–1708. [DOI] [PubMed] [Google Scholar]
- 37▪.Buchfelder M, Schlaffer S. Imaging of pituitary pathology. Handb Clin Neurol 2014; 124:151–166. [DOI] [PubMed] [Google Scholar]; Review on imaging characteristics and differential diagnosis in sellar masses.
- 38.Friedman DP, Gandhe AR. Evans JJ, Kenning TJ. Imaging of craniopharyngiomas and radiologic differential diagnosis. Craniopharyngiomas: comprehensive diagnosis, treatment and outcome 1st edMunich, Germany: Elsevier; 2015. 59–94. [Google Scholar]
- 39.Elowe-Gruau E, Beltrand J, Brauner R, et al. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab 2013; 98:2376–2382. [DOI] [PubMed] [Google Scholar]
- 40.Muller HL, Gebhardt U, Faldum A, et al. Xanthogranuloma, Rathke's cyst, and childhood craniopharyngioma: results of prospective multinational studies of children and adolescents with rare sellar malformations. J Clin Endocrinol Metab 2012; 97:3935–3943. [DOI] [PubMed] [Google Scholar]
- 41.Muller HL, Gebhardt U, Teske C, et al. Postoperative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur J Endocrinol 2011; 165:17–24. [DOI] [PubMed] [Google Scholar]
- 42▪.Roth CL, Eslamy H, Werny D, et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity (Silver Spring) 2015; 23:1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]; Grading system for imaging on hypothalamic lesions after initial surgery of craniopharyngioma with high-prediction sensitivity for the devlopment of hypothalamic obesity.
- 43.Pascual JM, Prieto R, Carrasco R, Barrios L. Displacement of mammillary bodies by craniopharyngiomas involving the third ventricle: surgical-MRI correlation and use in topographical diagnosis. J Neurosurg 2013; 119:381–405. [DOI] [PubMed] [Google Scholar]
- 44.Gu Y, Zhang X. Mammillary body angle and craniopharyngioma. J Neurosurg 2014; 120:1241–1243.author reply 1243–1245. [DOI] [PubMed] [Google Scholar]
- 45.Rath SR, Lee S, Kotecha RS, et al. Childhood craniopharyngioma: 20-year institutional experience in Western Australia. J Paediatr Child Health 2013; 49:403–408. [DOI] [PubMed] [Google Scholar]
- 46.Lo AC, Howard AF, Nichol A, et al. Long-term outcomes and complications in patients with craniopharyngioma: the British Columbia Cancer Agency experience. Int J Radiat Oncol Biol Phys 2014; 88:1011–1018. [DOI] [PubMed] [Google Scholar]
- 47.Hankinson TC, Palmeri NO, Williams SA, et al. Patterns of care for craniopharyngioma: survey of members of the American Association of Neurological Surgeons. Pediatr Neurosurg 2013; 49:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaidi HA, Chapple K, Little AS. National treatment trends, complications, and predictors of in-hospital charges for the surgical management of craniopharyngiomas in adults from 2007 to 2011. Neurosurg Focus 2014; 37:E6. [DOI] [PubMed] [Google Scholar]
- 49.Muller HL. Childhood craniopharyngioma: treatment strategies and outcomes. Exp Rev Neurother 2014; 14:187–197. [DOI] [PubMed] [Google Scholar]
- 50.Solari D, Cavallo LM, Cappabianca P. Surgical approach to pituitary tumors. Handb Clin Neurol 2014; 124:291–301. [DOI] [PubMed] [Google Scholar]
- 51▪.Flitsch J, Aberle J, Burkhardt T. Surgery for pediatric craniopharyngiomas: is less more? J Pediatr Endocrinol Metab 2015; 28:27–33. [DOI] [PubMed] [Google Scholar]; Review on the risk-adapted surgical treatment strategies.
- 52.Buchfelder M, Schlaffer SM, Lin F, Kleindienst A. Surgery for craniopharyngioma. Pituitary 2013; 16:18–25. [DOI] [PubMed] [Google Scholar]
- 53.Mortini P, Gagliardi F, Boari N, Losa M. Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit Rev Oncol Hematol 2013; 88:514–529. [DOI] [PubMed] [Google Scholar]
- 54▪▪.Ali ZS, Bailey RL, Daniels LB, et al. Comparative effectiveness of treatment options for pediatric craniopharyngiomas. J Neurosurg Pediatr 2014; 13:178–188. [DOI] [PubMed] [Google Scholar]; Comparative study on quality of life in childhood-onset craniopharyngioma after diffferent degrees of resection recommending limited hypothalamic-sparing approaches combined with irradiation for best qualitiy of life outcome.
- 55▪.Ogawa Y, Kawaguchi T, Tominaga T. Outcome and mid-term prognosis after maximum and radical removal of craniopharyngiomas with the priority to the extended transsphenoidal approach: a single center experience. Clin Neurol Neurosurg 2014; 125:41–46. [DOI] [PubMed] [Google Scholar]; Maximum and radical removal through the extended transsphenoidal approach achieved high rate of total removal and good visual outcomes in 42 craniopharyngioma patients analysed in a single centre retrospective study.
- 56▪.Raza SM, Schwartz TH. How to achieve the best possible outcomes in the management of retroinfundibular craniopharyngiomas? World Neurosurg 2014; 82:614–616. [DOI] [PubMed] [Google Scholar]; Report favouring expanded endonasal approaches for subchiasmatic, infundibular, and retroinfundibular craniopharyngioma to achieve best oncologic and quality of life outcomes.
- 57▪.Hoffmann A, Warmth-Metz M, Gebhardt U, et al. Childhood craniopharyngioma: changes of treatment strategies in the trials KRANIOPHARYNGEOM 2000/2007. Klin Padiatr 2014; 226:161–168. [DOI] [PubMed] [Google Scholar]; Comparison of different treatment strategies (gross-total vs. limited resection) in two prospective multicentre studies.
- 58▪.Patel KS, Raza SM, McCoul ED, et al. Long-term quality of life after endonasal endoscopic resection of adult craniopharyngiomas. J Neurosurg 2015. 1–10. [DOI] [PubMed] [Google Scholar]; New surgical endoscopic approaches and imporovement of long-term quality of life.
- 59.Cavallo LM, Solari D, Esposito F, et al. The role of the endoscopic endonasal route in the management of craniopharyngiomas. World Neurosurg 2014; 82:S32–S40. [DOI] [PubMed] [Google Scholar]
- 60▪.Bailey S, Parkes J. Intracystic interferon therapy in childhood craniopharyngioma: who, when and how? Clin Endocrinol 2015; 82:29–34. [DOI] [PubMed] [Google Scholar]; The role of intracystic therapy in the form of interferon alpha is discussed; including when to use this therapeutic option and practical details of its use.
- 61.Zheng J, Fang Y, Cai BW, et al. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database Syst Rev 2014; 9:CD008890. [DOI] [PubMed] [Google Scholar]
- 62.Bartels U, Laperriere N, Bouffet E, Drake J. Intracystic therapies for cystic craniopharyngioma in childhood. Front Endocrinol 2012; 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63▪.Klimo P, Jr, Venable GT, Boop FA, Merchant TE. Recurrent craniopharyngioma after conformal radiation in children and the burden of treatment. J Neurosurg Pediatr 2015; 15:499–505. [DOI] [PubMed] [Google Scholar]; The study highlights the therapeutic dilemma in recurrent craniopharyngioma after irradiation. The authors recommend an attempt at gross-total resection in such cases.
- 64.Greenfield BJ, Okcu MF, Baxter PA, et al. Long-term disease control and toxicity outcomes following surgery and intensity modulated radiation therapy (IMRT) in pediatric craniopharyngioma. Radiother Oncol 2015; 114:224–229. [DOI] [PubMed] [Google Scholar]
- 65▪.Bishop AJ, Greenfield B, Mahajan A, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multiinstitutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys 2014; 90:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study reports on equivalent survival and disease control outcomes in proton beam therapy and conventional intensity modulated radiation therapy. Long-term toxicities still have to be analyzed.
- 66.Bradley JA, Indelicato DJ. Evans JJ, Kenning TJ. The role of proton therapy in the treatment of craniopharyngioma. Craniopharyngiomas: comprehensive diagnosis, treatment and outcome 1st edMunich, Germany: Elsevier; 2015. 347–364. [Google Scholar]
- 67.Harrabi SB, Adeberg S, Welzel T, et al. Long term results after fractionated stereotactic radiotherapy (FSRT) in patients with craniopharyngioma: maximal tumor control with minimal side effects. Radiat Oncol 2014; 9:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68▪.Lee CC, Yang HC, Chen CJ, et al. Gamma Knife surgery for craniopharyngioma: report on a 20-year experience. J Neurosurg 2014; 121 Suppl:167–178. [DOI] [PubMed] [Google Scholar]; The study on 137 craniopharyngioma patients treated with gamma knife surgery comes to the conclusion that gamma knife surgery is a relatively safe treatment option for recurrent or residual tumours especially of small size.
- 69.Fjalldal S, Holmer H, Rylander L, et al. Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. J Clin Endocrinol Metab 2013; 98:3253–3262. [DOI] [PubMed] [Google Scholar]
- 70.Steno J, Bizik I, Steno A, Matejcik V. Craniopharyngiomas and the hypothalamus. J Neurosurg 2013; 119:1646–1650. [DOI] [PubMed] [Google Scholar]
- 71▪.Rosenfeld A, Arrington D, Miller J, et al. A review of childhood and adolescent craniopharyngiomas with particular attention to hypothalamic obesity. Pediatr Neurol 2014; 50:4–10. [DOI] [PubMed] [Google Scholar]; Study on morbidity and mortality in childhood-onset craniopharyngioma showing that hypothalamic obesity is a significant complication of craniopharymngioma associated with increased mortality.
- 72▪.Khan MJ, Humayun KN, Donaldson M, et al. Longitudinal changes in body mass index in children with craniopharyngioma. Horm Res Paediatr 2014; 82:372–379. [DOI] [PubMed] [Google Scholar]; Obesity at presentation, rather than panhypopituitarism either at or after presentation, predicts the risk for long-term obesity in childhood craniopharyngioma.
- 73▪.Erfurth EM. Endocrine aspects and sequel in patients with craniopharyngioma. J Pediatr Endocrinol Metab 2015; 28:19–26. [DOI] [PubMed] [Google Scholar]; Review on endocrine disturbances and their impact on outcome in craniopharyngioma.
- 74.Muller HL. Increased daytime sleepiness in patients with childhood craniopharyngioma and hypothalamic tumor involvement: review of the literature and perspectives. Int J Endocrinol 2010; 2010:519607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muller HL. Consequences of craniopharyngioma surgery in children. J Clin Endocrinol Metab 2011; 96:1981–1991. [DOI] [PubMed] [Google Scholar]
- 76.Cohen M, Syme C, McCrindle BW, Hamilton J. Autonomic nervous system balance in children and adolescents with craniopharyngioma and hypothalamic obesity. Eur J Endocrinol 2013; 168:845–852. [DOI] [PubMed] [Google Scholar]
- 77.Harz KJ, Muller HL, Waldeck E, et al. Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab 2003; 88:5227–5231. [DOI] [PubMed] [Google Scholar]
- 78.Muller HL, Handwerker G, Gebhardt U, et al. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control 2006; 17:583–589. [DOI] [PubMed] [Google Scholar]
- 79.Elfers CT, Roth CL. Effects of methylphenidate on weight gain and food intake in hypothalamic obesity. Front Endocrinol 2011; 2:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80▪.Bomer I, Saure C, Caminiti C, et al. Comparison of energy expenditure, body composition, metabolic disorders, and energy intake between obese children with a history of craniopharyngioma and children with multifactorial obesity. J Pediatr Endocrinol Metab 2015. [DOI] [PubMed] [Google Scholar]; REE is lower in craniopharyngioma patients compared with children with multifactorial obesity regardless of the amount of fat-free mass.
- 81.Holmer H, Pozarek G, Wirfalt E, et al. Reduced energy expenditure and impaired feeding-related signals but not high-energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J Clin Endocrinol Metab 2010; 95:5395–5402. [DOI] [PubMed] [Google Scholar]
- 82.Prieto R, Pascual JM, Barrios L. Optic chiasm distortions caused by craniopharyngiomas: clinical and magnetic resonance imaging correlation and influence on visual outcome. World Neurosurg 2015; 83:500–529. [DOI] [PubMed] [Google Scholar]
- 83.Drimtzias E, Falzon K, Picton S, et al. The ophthalmic natural history of paediatric craniopharyngioma: a long-term review. J Neurooncol 2014; 120:651–656. [DOI] [PubMed] [Google Scholar]
- 84▪.Roemmler-Zehrer J, Geigenberger V, Stormann S, et al. Food intake regulating hormones in adult craniopharyngioma patients. Eur J Endocrinol 2014; 170:627–635. [DOI] [PubMed] [Google Scholar]; Dysregulation of food intake regulating hormones are more pronounced in adult-onsnet craniopharyngioma patients when compared with adult patients with NFPA.
- 85.Roth CL, Elfers C, Gebhardt U, et al. Brain-derived neurotrophic factor and its relation to leptin in obese children before and after weight loss. Metabolism 2013; 62:226–234. [DOI] [PubMed] [Google Scholar]
- 86.Roth CL, Enriori PJ, Gebhardt U, et al. Changes of peripheral alpha-melanocyte-stimulating hormone in childhood obesity. Metabolism 2010; 59:186–194. [DOI] [PubMed] [Google Scholar]
- 87.Muller HL. Childhood craniopharyngioma. Pituitary 2013; 16:56–67. [DOI] [PubMed] [Google Scholar]
- 88.Zoicas F, Droste M, Mayr B, et al. GLP-1 analogues as a new treatment option for hypothalamic obesity in adults: report of nine cases. Eur J Endocrinol 2013; 168:699–706. [DOI] [PubMed] [Google Scholar]
- 89▪.Kalina MA, Wilczek M, Kalina-Faska B, et al. Carbohydrate-lipid profile and use of metformin with micronized fenofibrate in reducing metabolic consequences of craniopharyngioma treatment in children: single institution experience. J Pediatr Endocrinol Metab 2015; 28:45–51. [DOI] [PubMed] [Google Scholar]; Treatment of metabolic sequelae in childhood-onset craniopharyngioma with metformin and micronized fenofibrate attenuates disturbances in a short-term observation.
- 90▪.van Santen HM, Schouten-Meeteren AY, Serlie M, et al. Effects of T3 treatment on brown adipose tissue and energy expenditure in a patient with craniopharyngioma and hypothalamic obesity. J Pediatr Endocrinol Metab 2015; 28:53–57. [DOI] [PubMed] [Google Scholar]; The report contradicts a previous publication showing that T3 monotherapy does not seem to be effective in decreasing hypothalamic obesity in childhood craniopharyngioma.
- 91.Profka E, Giavoli C, Bergamaschi S, et al. Analysis of short- and long-term metabolic effects of growth hormone replacement therapy in adult patients with craniopharyngioma and nonfunctioning pituitary adenoma. J Endocrinol Invest 2014; 38:413–420. [DOI] [PubMed] [Google Scholar]
- 92.Yuen KC, Koltowska-Haggstrom M, Cook DM, et al. Clinical characteristics and effects of GH replacement therapy in adults with childhood-onset craniopharyngioma compared with those in adults with other causes of childhood-onset hypothalamic-pituitary dysfunction. Eur J Endocrinol 2013; 169:511–519. [DOI] [PubMed] [Google Scholar]
- 93.Lustig RH, Hinds PS, Ringwald-Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2003; 88:2586–2592. [DOI] [PubMed] [Google Scholar]
- 94.Bretault M, Boillot A, Muzard L, et al. Clinical review: bariatric surgery following treatment for craniopharyngioma: a systematic review and individual-level data meta-analysis. J Clin Endocrinol Metab 2013; 98:2239–2246. [DOI] [PubMed] [Google Scholar]
- 95.Gatta B, Nunes ML, Bailacq-Auder C, et al. Is bariatric surgery really inefficient in hypothalamic obesity? Clin Endocrinol 2013; 78:636–638. [DOI] [PubMed] [Google Scholar]
- 96.Muller HL, Gebhardt U, Maroske J, Hanisch E. Long-term follow-up of morbidly obese patients with childhood craniopharyngioma after laparoscopic adjustable gastric banding (LAGB). Klin Padiatr 2011; 223:372–373. [DOI] [PubMed] [Google Scholar]
- 97.Ho AL, Sussman ES, Zhang M, et al. Deep brain stimulation for obesity. Cureus 2015; 7:e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zonca P, Hoppe C, Cambal M, Jacobi CA. Gastric stimulation in treatment in type 2 diabetes mellitus. Bratisl Lek Listy 2014; 115:34–37. [DOI] [PubMed] [Google Scholar]
- 99▪.Gump JM, Donson AM, Birks DK, et al. Identification of targets for rational pharmacological therapy in childhood craniopharyngioma. Acta Neuropathol Commun 2015; 3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]; First published transcriptome for adamentinomatous craniopharyngioma and identification of targets for rational therapy.
- 100▪.Sterkenburg AS, Hoffmann A, Gebhardt U, et al. [Childhood craniopharyngioma with hypothalamic obesity: no long-term weight reduction due to rehabilitation programs]. Klin Padiatr 2014; 226:344–350. [DOI] [PubMed] [Google Scholar]; Study on effects of structured rehabilitation programs on long-term weight development in patients with childhood-onset craniopharyngioma.
- 101▪.Meijneke RW, Schouten-van Meeteren AY, de Boer NY, et al. Hypothalamic obesity after treatment for craniopharyngioma: the importance of the home environment. J Pediatr Endocrinol Metab 2015; 28:59–63. [DOI] [PubMed] [Google Scholar]; Case report on the impact of home environment on the treatment efficacy of hypothalamic obesity in childhood-onset craniopharyngioma.
- 102▪.Nemergut DR, Townsend AR. The importance of interdisciplinary communication with patients about complex, chronic illnesses: our experiences as parents of a child with a craniopharyngioma. J Pediatr Endocrinol Metab 2015; 28:3–5. [DOI] [PubMed] [Google Scholar]; Perspectives of parents of a child with craniopharyngioma: importance of interdisciplinary communication.
- 103.Lipek T, Igel U, Gausche R, et al. Obesogenic environments: environmental approaches to obesity prevention. J Pediatr Endocrinol Metab 2015; 28:485–495. [DOI] [PubMed] [Google Scholar]
- 104▪.Obregon AM, Pettinelli PP, Santos JL. Childhood obesity and eating behaviour. J Pediatr Endocrinol Metab 2015; 28:497–502. [DOI] [PubMed] [Google Scholar]; Review on childhood obesity and associated eating behaviour.
- 105▪.Heymsfield SB, Avena NM, Baier L, et al. Hyperphagia: current concepts and future directions proceedings of the 2nd international conference on hyperphagia. Obesity (Silver Spring) 2014; (22 Suppl 1):S1–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report on different manifestations of the clinical symptom hyperphagia in various diseases and current concepts of treatment.
- 106.Roth CL, Aylward E, Liang O, et al. Functional neuroimaging in craniopharyngioma: a useful tool to better understand hypothalamic obesity? Obes Facts 2012; 5:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107▪.Roemmler-Zehrer J, Geigenberger V, Stormann S, et al. Specific behaviour, mood and personality traits may contribute to obesity in patients with craniopharyngioma. Clin Endocrinol 2015; 82:106–114. [DOI] [PubMed] [Google Scholar]; Obesity in craniopharyngioma patients is associated with eating disorders, negative mood alterations and increased anxiety-related personality traits.
- 108.Kilday JP, Bartels U, Huang A, et al. Favorable survival and metabolic outcome for children with diencephalic syndrome using a radiation-sparing approach. J Neurooncol 2014; 116:195–204. [DOI] [PubMed] [Google Scholar]
- 109▪▪.Hoffmann A, Gebhardt U, Sterkenburg AS, et al. Diencephalic syndrome in childhood craniopharyngioma-results of german multicenter studies on 485 long-term survivors of childhood craniopharyngioma. J Clin Endocrinol Metab 2014; 99:3972–3977. [DOI] [PubMed] [Google Scholar]; First report on incidence and clinical course of diencephalic syndrome before and after diagnosis of childhood-onset craniopharyngioma.
- 110▪.Pickering L, Jennum P, Gammeltoft S, et al. Sleep-wake and melatonin pattern in craniopharyngioma patients. Eur J Endocrinol 2014; 170:873–884. [DOI] [PubMed] [Google Scholar]; Study on circadian rhythms and melatonin patters in craniopharyngioma patients.
- 111▪▪.Ozyurt J, Thiel CM, Lorenzen A, et al. Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. J Pediatr 2014; 164:876–881.e874. [DOI] [PubMed] [Google Scholar]; Analysis of neuropsychological outcome in long-term survivors of childhood-onset craniopharyngioma with special regard to hypothalamic involvement.
- 112▪.Ozyurt J, Lorenzen A, Gebhardt U, et al. Remote effects of hypothalamic lesions in the prefrontal cortex of craniopharygioma patients. Neurobiol Learn Mem 2014; 111:71–80. [DOI] [PubMed] [Google Scholar]; Functional magnetic resonance imaging study in childhood-onset craniopharyngioma patients observing remote effects of hypothalamic lesions in the prefrontal cortex.
- 113.Zada G, Kintz N, Pulido M, Amezcua L. Prevalence of neurobehavioral, social, and emotional dysfunction in patients treated for childhood craniopharyngioma: a systematic literature review. PloS One 2013; 8:e76562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crespo I, Santos A, Webb SM. Quality of life in patients with hypopituitarism. Curr Opin Endocrinol Diabetes Obes 2015; 22:306–312. [DOI] [PubMed] [Google Scholar]
- 115.Crespo I, Valassi E, Santos A, Webb SM. Health-related quality of life in pituitary diseases. Endocrinol Metab Clin North Am 2015; 44:161–170. [DOI] [PubMed] [Google Scholar]
- 116▪▪.Hoffmann A, Bootsveld K, Gebhardt U, et al. Nonalcoholic fatty liver disease and fatigue in long-term survivors of childhood-onset craniopharyngioma. Eur J Endocrinol 2015; 173:389–397. [DOI] [PubMed] [Google Scholar]; First report on incidence and risk factors for NAFLD in childhood-onset craniopharyngioma patients.
- 117▪▪.Ozyurt J, Muller HL, Thiel CM. A systematic review of cognitive performance in patients with childhood craniopharyngioma. J Neurooncol 2015; 125:9–21. [DOI] [PubMed] [Google Scholar]; First compehensive metaanalysis of the literature on cognitive performance in childhood craniopharyngioma showing that episodic memory recall in particular is impaired, largely sparing other memory components.
- 118▪.Tallen G, Resch A, Calaminus G, et al. Strategies to improve the quality of survival for childhood brain tumour survivors. Eur J Paediatr Neurol 2015; 19:619–639. [DOI] [PubMed] [Google Scholar]; Report on structure and organiszation of the German Brain Tumor Network (HIT) in which the reference assessment of imaging, neuropathology and surgical approaches in all patients prospectively recruited in the German Craniopharyngioma Registry is provided.


