Abstract
Background
Studies have linked midlife and late-life cardiovascular risk factors (CVRFs) to cognitive function, yet little is known about CVRF exposure in early adulthood and subsequent cognitive function. In addition, most studies rely on single assessments of CVRFs, which may not accurately reflect long-term exposure. We sought to determine the association between cumulative exposure to CVRFs from early to middle adulthood and cognitive function at midlife.
Methods and Results
In a prospective study of 3381 adults (age, 18–30 years at baseline) with 25 years of follow-up, we assessed cognitive function at year 25 (2010–2011) with the Digit Symbol Substitution Test, Stroop Test, and Rey Auditory Verbal Learning Test analyzed with standardized z scores. The primary predictor was 25-year cumulative exposure estimated by areas under the curve for resting systolic and diastolic blood pressures, fasting blood glucose, and total cholesterol. Higher cumulative systolic and diastolic blood pressures and fasting blood glucose were consistently associated with worse cognition on all 3 tests. These associations were significant primarily for exposures above recommended guidelines; cognitive test z scores were between 0.06 and 0.30 points less, on average, for each 1-SD increase in risk factor area under the curve after adjustment for age, race, sex, and education (P<0.05 for all). Fewer significant associations were observed for cholesterol.
Conclusions
Cumulative exposure to CVRFs from early to middle adulthood, especially above recommended guidelines, was associated with worse cognition in midlife. The meaning of this association and whether it warrants more aggressive treatment of CVRFs earlier in life require further investigation.
Keywords: blood pressure, cholesterol, cognition, glucose, risk factors
Exposure to cardiovascular risk factors (CVRFs), including elevated levels of blood pressure, lipids, and fasting blood glucose (FBG), during early adulthood is associated with adverse cardiovascular outcomes in later life.1–4 The longitudinal relationships of these early adult risk factors with cognitive function are unknown despite a robust body of evidence linking midlife and late-life CVRFs to late-life cognitive function.5
Although accumulating data from observational studies suggest that CVRFs may be modifiable risk factors for cognitive impairment,6,7 randomized, controlled trials targeting the treatment of these conditions, including hypertension, dyslipidemia, and diabetes mellitus, have reported mixed results.8–10 Most studies (both trials and observational) have focused on risk relationships during middle adult to late-life periods,11–13 and the nature of this association during earlier life stages has not been defined. Because the neuropathology associated with cognitive impairment and dementia often develops over decades,14 determination of CVRF effects over the life course, starting in early adulthood, could inform the design of more targeted and effective interventions for healthy cognitive aging.
Because individual profiles of CVRFs vary throughout the life course,15,16 single measures of exposure (often many years earlier) may not reflect the longitudinal variation and cumulative burden associated with elevated CVRF level. Summary measures of cumulative exposure that capture both the duration and intensity could more accurately estimate the effects of these risk factors over several decades. To determine the association between CVRFs early over the life course and cognitive function in midlife, we investigated the cumulative effects of systolic (SBP) and diastolic (DBP) blood pressures, FBG levels, and total cholesterol levels measured from early adulthood to midlife. We hypothesize that greater cumulative exposure to CVRFs is associated with worse cognitive performance in midlife.
Methods
Study Population
We studied participants enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) Study, an investigation of the development of and risk factors for cardiovascular disease. Young adults between 18 and 30 years of age were recruited from population-based samples of 4 US cities (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). A total of 5115 participants were enrolled between 1985 and 1986, with roughly equal sample sizes by sex, age (18–24 and 25–30 years), race (white, black), and education (high school or less, more than high school) at each site. Participants completed follow-up examinations every 2 to 5 years for 25 years: 1987 to 1988 (year 2), 1990 to 1991 (year 5), 1992 to 1993 (year 7), 1995 to 1996 (year 10), 2000 to 2001 (year 15), 2005 to 2006 (year 20), and 2010 to 2011 (year 25; n=3499). At each examination, participants provided written informed consent, and study protocols were reviewed by institutional review boards at each study site, the CARDIA Coordinating Center at the University of Alabama, Birmingham, and the University of California, San Francisco. Further details of study recruitment and design are available.17,18
For this study, we assessed the 3381 participants who completed the year 25 visit with evaluation of CVRFs over time (at least 2 time points required; 98% of the cohort had both baseline and year 25 measures of CVRFs, and 93% had at least two-thirds of the possible CVRF measures over time) and at least 1 of the 3 cognitive assessments at year 25. Compared with those in this analytic cohort, the participants without a cognitive assessment were younger, more likely to be male and black, less educated, and more likely to smoke (P<0.01 for all).
CVRF Measurement
Before each clinic examination, participants were asked to fast and to abstain from smoking or heavy physical activity for at least 12 hours before the visit. Certified technicians collected 3 measures of resting blood pressure at 1-minute intervals using a Hawksley random-zero sphygmomanometer (WA Baum Co, Copaigue, NY) at baseline and years 2, 5, 7, 10, and 15. At years 20 and 25, a digital blood pressure monitor (Omron HEM-907XL; Online Fitness, Santa Monica, CA) was used. The oscillometric values were calibrated to the random-zero values after a study of both devices at year 20. In this analysis, SBP and DBP measures were calculated as the average of the second and third measurements.
Fasting total cholesterol was measured enzymatically on the Abbot Spectrum (using Hitachi 917-R1 cholesterol reagent) at baseline and years 5, 7, 10, 15, 20, and 25 by the Northwest Lipid Research Laboratory at the University of Washington (Seattle) as previously described.19 FBG was measured at baseline with the hexokinase ultraviolet method by American Bio-Science Laboratories (Van Nuys, CA) and at subsequent clinic examinations at years 7, 10, 15, 20, and 25 with hexokinase coupled to glucose-6-phosphate dehydrogenase by Linco Research (St. Louis, MO).
Cognitive Function Assessment
CARDIA technicians who underwent formal training and certification administered a battery of 3 cognitive tests at the year 25 examination that included the Digit Symbol Substitution Test (DSST), the Stroop Test, and the Rey Auditory Verbal Learning Test (RAVLT). DSST assesses attention, working memory, psychomotor speed, and executive function, with higher scores indicating better performance, with a range of 0 to 133.20 The Stroop Test of executive function uses 3 subtests. We calculated an interference score by subtracting the score on subtest II from subtest III, with a higher interference score indicating worse performance.21,22 RAVLT is a test of verbal memory with a range of 0 to 15. Scores on the delayed test were used, with higher scores indicating better performance.23,24 For ease of interpretation, all cognitive test scores were transformed into standardized z scores, with positive values indicating better performance and negative values indicating worse performance.
Covariates
At each examination, weight and height were measured, and body mass index was calculated as weight in kilograms divided by height in meters squared. Demographic characteristics, cigarette smoking (in years), and alcohol use were based on self-report. Diabetes mellitus at year 25 was defined as fasting plasma glucose ≥126 mg/dL, oral glucose tolerance test ≥200 mg/dL, glycosylated hemoglobin ≥6.5%, or use of diabetes medications. Incident cardiovascular events over 25 years of follow-up were defined by patient self-report and adjudicated review of hospitalization records.
Statistical Analysis
Our primary predictors were areas under the curve (AUCs) for 4 CVRFs. We estimated the mean curve for each CVRF using linear mixed models for each race and sex group, using a linear spline in age with knots at 30 and 40 years of age corresponding to decades of the life span, for face validity and to ensure adequate numbers of observations in each of the 3 age ranges defined by the knots. We then calculated participant-specific CVRF curves as best linear unbiased predictions based on the mixed models. Next, we calculated AUCs over the interval from baseline to the year 25 visit. Additionally, to determine differences between early adult and early midlife effects, we also calculated AUCs as subsets of the follow-up interval before and after 35 years of age.
We then used linear regression to assess the independent associations of the AUCs with cognitive function assessed at the year 25 visit. For each test, we first estimated the unadjusted association and then estimated the association controlling for age at year 25, race/ ethnicity, sex, and education. In additional models, we also controlled for year 25 body mass index, diabetes mellitus, and smoking, as well as baseline CVRF level. In a sensitivity analysis, we adjusted for incident cardiovascular events, including myocardial infarction, coronary revascularization, stroke, peripheral artery disease, and congestive heart failure. We also estimated associations for models excluding participants with incident cardiovascular events.
To distinguish whether any association with cognitive function was attributable to CVRF level above or below recommended guidelines, we divided the overall AUC for each participant and CVRF into 2 regions separated by a horizontal line at the recommended (normal) guidelines defined by American Heart Association criteria for ideal cardiovascular health: SBP <120 mm Hg, DBP <80 mm Hg, FBG <100 mg/dL, and total cholesterol <200 mg/dL25. We then calculated the area in each of these regions. For curves always remaining below the guideline value, the area in the upper region is zero.
All analyses were completed with SAS version 9.3, and significance testing was 2-sided with the significance level set at P<0.05.
Results
At year 25, participant mean age was 50.2±3.6 years, 46.4% were black, 43.6% were male, and 83.9% had greater than a high school education (Table 1). At year 25, mean CVRF level was SBP (mean±SD) of 119.7±16.2 mm Hg, DBP of 74.8±11.2 mm Hg, FBG of 99.5±28.7 mg/dL, and total cholesterol of 192.1±36.7 mg/dL. Mean year 25 cognitive score was 8.3±3.3 for RAVLT, 69.9±16.2 for DSST, and 22.7±10.9 for the Stroop interference score (Table 2). Over the follow-up period, 92 participants had an incident cardiovascular event, including myocardial infarction (n=32), coronary revascularization (n=32), stroke (n=31), peripheral artery disease (n=4), and congestive heart failure (n=18).
Table 1.
Demographic and Risk Characteristics of the 3381 CARDIA Participants
| Demographics | |
| Age at baseline, y | 25.1±3.6 |
| Age at year 25, y | 50.2±3.6 |
| Male, n (%) | 1475 (43.6) |
| Black, n (%) | 1567 (46.4) |
| Education, y | 15.5±2.5 |
| More than high school education, n (%) | 2837 (83.9) |
| Cardiovascular risk factors | |
| Systolic blood pressure, mm Hg | |
| Baseline | 109.9±10.8 |
| Year 25 | 119.7±16.2 |
| Time-weighted average | 111.8±9.3 |
| Diastolic blood pressure, mm Hg | |
| Baseline | 68.4±9.4 |
| Year 25 | 74.8±11.2 |
| Time-weighted average | 71.2±6.7 |
| Fasting blood glucose, mg/dL | |
| Baseline | 82.0±10.9 |
| Year 25 | 99.5±28.7 |
| Time-weighted average | 88.8±11.1 |
| Total cholesterol, mg/dL | |
| Baseline | 177.4±33.1 |
| Year 25 | 192.1±36.7 |
| Time-weighted average | 182.4±26.1 |
Values are mean±SD when appropriate. CARDIA indicates Coronary Artery Risk Development in Young Adults.
Table 2.
Cognitive Test Scores at Year 25
| n | Mean±SD | Range | |
|---|---|---|---|
| Rey Auditory Verbal Learning Test–Delayed, words | 3288 | 8.3±3.3 | 0.0 to 15.0 |
| Digit Symbol Substitution Test, symbols | 3350 | 69.9±16.2 | 8.0 to 125.0 |
| Stroop Interference, points | 3329 | 22.7±10.9 | −23.0 to 127.0 |
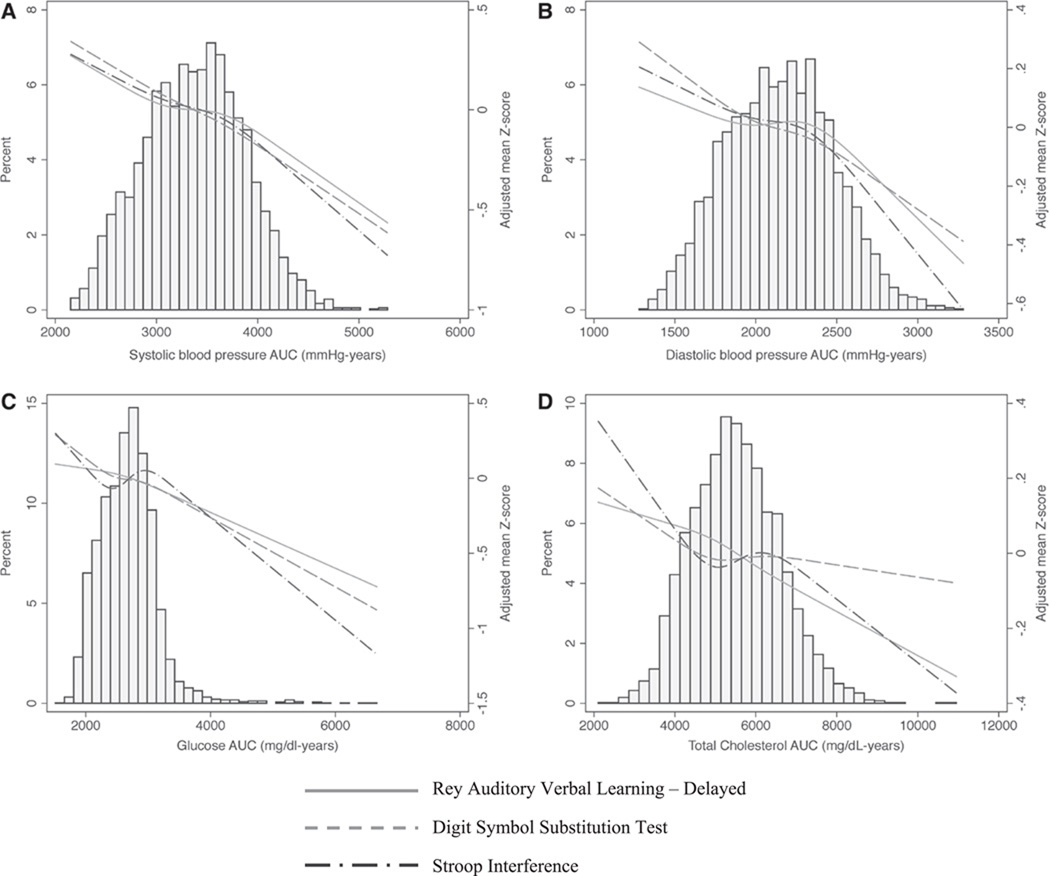
In most cases, the overall cumulative CVRF AUCs were associated with worse cognitive function (Figure 1). Cumulative levels of both SBP and DBP (per 1-SD increase in AUC) over the follow-up were significantly associated with worse performance on all 3 cognitive tests in unadjusted models (Table 3). In models adjusted for age, sex, race, and education, the cumulative effects of SBP remained negatively associated with cognitive function (RAVLT=−0.09, 95% confidence interval [CI], −0.15 to −0.03; DSST=−0.12, 95% CI, −0.18 to −0.06; Stroop=−0.11, 95% CI, −0.17 to −0.05), and cumulative levels of DBP were significantly associated with worse performance on DSST and Stroop (DSST=−0.07, 95% CI, −0.12 to −0.02; Stroop=−0.09, 95% CI, −0.14 to −0.03) but not RAVLT (RAVLT=−0.05, 95% CI, −0.11 to 0.0). Additional adjustment for diabetes mellitus, smoking, and body mass index led to similar results.
Figure 1.
Distribution of cardiovascular risk factor area under the curve (AUC) and association with year 25 cognitive function adjusted for age, sex, race, and education.
Table 3.
Unadjusted Association of Cumulative Exposure to Overall, Normal, and Elevated Cardiovascular Risk Factors and Cognitive Function at Midlife Among 3381 CARDIA Participants
| Standardized Difference (95% CI) | |||
|---|---|---|---|
| Rey Auditory Verbal Learning–Delayed* |
Digit Symbol Substitution Test* |
Stroop Interference* | |
| Systolic blood pressure | |||
| Overall | −0.14 (−0.17 to −0.10)† | −0.21 (−0.24 to −0.18)† | −0.13 (−0.16 to −0.10)† |
| Normal levels | −0.07 (−0.11 to −0.03)† | −0.15 (−0.19 to −0.11)† | −0.06 (−0.10 to −0.02)‡ |
| Elevated levels | −0.74 (−0.92 to −0.57)† | −0.77 (−0.94 to −0.59)† | −0.73 (−0.91 to −0.55)† |
| Diastolic blood pressure | |||
| Overall | −0.12 (−0.16 to −0.09)† | −0.19 (−0.22 to −0.16)† | −0.13 (−0.16 to −0.09)† |
| Normal levels | −0.07 (−0.11 to −0.03))† | −0.15 (−0.19 to −0.11)† | −0.08 (−0.12 to −0.04)† |
| Elevated levels | −0.78 (−1.01 to −0.55)† | −0.71 (−0.94 to −0.47)† | −0.75 (−0.98 to −0.51)† |
| Fasting blood glucose | |||
| Overall | −0.09 (−0.13 to −0.06)† | −0.16 (−0.19 to −0.12)† | −0.09 (−0.13 to −0.06)† |
| Normal levels | −0.05 (−0.09 to .00)§ | −0.13 (−0.18 to −0.09)† | −0.04 (−0.09 to 0.01) |
| Elevated levels | −0.19 (−0.26 to −0.12)† | −0.21 (−0.28 to −0.14)† | −0.20 (−0.27 to −0.13)† |
| Total cholesterol | |||
| Overall | −0.03 (−0.06 to 0.01) | −0.05 (−0.09 to −0.02)‡ | −0.03 (−0.07 to 0.00) |
| Normal levels | −0.02 (−0.03 to 0.06) | −0.05 (−0.09 to 0.00)§ | −0.03 (−0.08 to 0.01) |
| Elevated levels | −0.11 (−0.16 to −0.05)† | −0.06 (−0.12 to −0.01)§ | −0.04 (−0.09 to 0.02) |
CARDIA indicates Coronary Artery Risk Development in Young Adults; and CI, confidence interval.
Per 1-SD increase in area under the curve.
P<0.001.
P<0.01.
P<0.05.
The cumulative effects of FBG followed a pattern similar to that of blood pressure level, with AUC associated with worse performance on RAVLT, Stroop, and DSST in unadjusted models (Table 3). These associations remained significant after adjustment for age, sex, race, and education (RAVLT=−0.07, 95% CI, −0.11 to −0.02; DSST=−0.08, 95% CI, −0.12 to −0.04; Stroop=−0.07, 95% CI, −0.12 to −0.03). When we excluded those participants with diabetes mellitus (at year 25, n=462), this association was no longer significant (RAVLT=−0.10, 95% CI, −0.21 to 0.01; DSST=0.03, 95% CI, −0.08 to 0.14; Stroop=0.0, 95% CI, −0.12 to 0.11). For total cholesterol, the pattern was not as consistent. Cumulative exposure to cholesterol was associated with worse performance on DSST but not RAVLT or Stroop (Table 3). After multivariable adjustment, the overall effects of cholesterol remained statistically significantly associated with worse performance on RAVLT (RAVLT=−0.06, 95% CI, −0.10 to −0.02; DSST= 0.0, 95% CI, −0.04 to 0.04; Stroop=−0.02, 95% CI, −0.07 to 0.02), and additional adjustment for diabetes mellitus, smoking, and body mass index did not appreciably alter the findings.
For each risk factor, further adjustment for baseline CVRF level did not appreciably change the association between AUC effects and cognition. We also investigated interactions between race, CVRF AUC, and cognitive function and found no consistent pattern of effects (for interactions between CVRFs and race: P≥0.27 for all on RAVLT, P≥0.05 for all on DSST, P≥0.16 for all on Stroop). In an additional sensitivity analysis in which we adjusted for incident cardiovascular events, the associations between CVRF AUCs and cognitive function remained statistically significant, but effect sizes were reduced (see the Table in the online-only Data Supplement). The associations were similar for models that excluded participants with incident cardiovascular events.
To determine whether the cumulative effects of CVRFs differed in early adulthood (<35 years of age) compared with early middle age (≥35 years of age), we evaluated models that separately assessed the contribution of each cumulative risk factor before and after 35 years of age. In most cases, if the 25-year cumulative effect of a CVRF was significant, then cumulative effects of the risk factor both before and after 35 years of age were significant (P<0.05 for all). For example, the 25-year cumulative effect of FBG contributed significantly to the variance of each cognitive test, and the effects of FBG both before and after 35 years of age were significant. For DBP, only the cumulative effects after 35 years of age were significant.
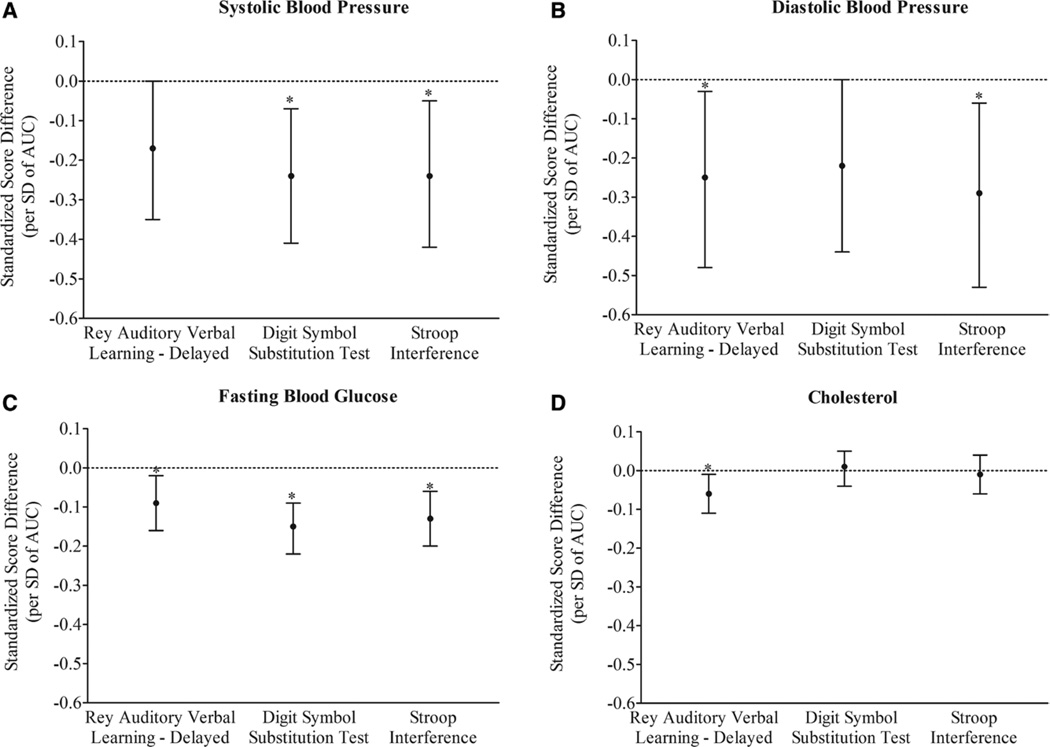
We next investigated whether the associations between CVRFs and cognitive function were attributable to exposure levels above recommended guidelines by estimating the effects of normal (below American Heart Association guidelines) and elevated (above American Heart Association guidelines) cumulative CVRF exposures. Between 30% and 48% of participants had elevated cumulative CVRF exposures (SBP=1635 [48.4%], DBP=1013 [30.0%], FBG=1026 [30.4%], cholesterol=1444 [42.7%]). Cumulative exposures to CVRFs in both normal and elevated ranges were significantly associated with cognitive function in unadjusted models (Table 3), but after adjustment for age, sex, race, and education, only the effects of cumulative exposures to elevated levels of CVRFs remained significant. Cumulative exposure to SBP level above recommended guidelines was significantly associated with worse cognitive function on DSST and Stroop (DSST=−0.24, 95% CI, −0.41 to −0.07; Stroop=−0.24, 95% CI, −0.42 to −0.05; Figure 2A) but was borderline significant for RAVLT (−0.17, 95% CI, −0.35 to 0.0). The cumulative effect of elevated DBP level was associated with worse performance on RAVLT and Stroop (RAVLT=−0.25, 95% CI, −0.48 to −0.03; Stroop=−0.29, 95% CI, −0.53 to −0.06; Figure 2B) but was borderline significant for DSST (−0.22, 95% CI, −0.44 to 0.0). For elevated FBG level, cumulative exposure was negatively associated with performance on RAVLT, DSST, and Stroop (RAVLT=−0.09, 95% CI, −0.16 to −0.02; DSST=−0.15, 95% CI, −0.22 to −0.09; Stroop=−0.13, 95% CI, −0.20 to −0.06, Figure 2C), and for cholesterol level above recommended guidelines, the cumulative effect was significant for worse performance on RAVLT (−0.06, 95% CI, −0.11 to −0.01) and not on DSST or Stroop (DSST=0.01, 95% CI, −0.04 to 0.05; Stroop=−0.01, 95% CI, −0.06 to 0.04; Figure 2D).
Figure 2.
Cumulative linear association of exposure to elevated levels of cardiovascular risk factors with cognitive function at midlife adjusted for age, sex, race, and education. AUC indicates area under the curve. *P<0.05.
Discussion
In this study, we found that CVRFs in early to middle adulthood were associated with worse cognitive performance in midlife. In particular, greater cumulative exposure to these measures in levels above recommended guidelines over 25 years was consistently associated with worse cognitive performance on executive function, processing speed, and verbal memory.
The observed association between CVRFs and cognitive function in this study is supported by findings from other studies of older adult populations. Previous investigations have focused primarily on the association between CVRF exposures and cognitive function after 50 years of age without considering the contribution of early adult exposures.26,27 The most consistent findings have been between midlife CVRFs, including elevated blood pressure and FBG, and late-life cognition.6,7,11 A number of studies have also reported associations between late-life exposures and late-life cognitive impairment, but the results have been less consistent.5 For example, in a large cohort study of older adults, the association between diabetes mellitus and dementia was stronger for midlife diabetes mellitus compared with late-life diabetes mellitus.28 In other studies, decreases in levels of cholesterol and blood pressure in late life were associated with an increased risk of cognitive impairment and dementia, suggesting that the relationship with some CVRFs closer to the time of dementia onset may reflect metabolic dysregulation resulting from impaired neurodegenerative processes.29,30 Our study has important implications for understanding the role of CVRFs across the life course and suggests that even at subclinical levels, the cumulative effects of CVRFs beginning in early adulthood are associated with cognitive function at middle age.
The mechanisms by which CVRFs in early adulthood affect cognitive function are unclear. Cumulative exposure to high level of CVRFs could increase the risk of subclinical ischemia and cause cerebrovascular damage, especially of a subcortical nature. Longitudinal magnetic resonance imaging studies in older adults have demonstrated that CVRFs may accelerate the risk of structural brain changes, including both atrophy and infarcts,31–33 and subcortical regions of the brain may be especially affected, leading to the impairment in executive function that we observed in this study.34 Our findings of an association between CVRFs and poor cognitive function are consistent with other studies of cognitively normal populations.33,35 CVRF exposures have been associated with increased markers of inflammation and oxidative stress, which in turn can cause neuronal damage.36–38 In addition, neuropathological studies suggest a relationship between elevated CVRF levels and amyloidogenic pathology.39–41 CVRFs and the vascular damage associated with elevated levels of these risk factors may interact with amyloid pathways, disrupting amyloid clearance and production and increasing amyloid deposition and plaques.42,43 Genetic risk factors could also link the effects of CVRFs to the risk of cognitive impairment.44,45
To the best of our knowledge, this study is one of the first to investigate the effects of early-life CVRFs on cognitive function in midlife, and its strengths include a well-characterized cohort with >25 years of follow-up data. In addition, because there were repeated measures of CVRFs, we were able to evaluate the cumulative effect of CVRFs (using AUCs), which could more accurately capture longitudinal exposure. Assessment of AUCs may be especially useful for our cohort, a younger, healthy study population for which clinical outcomes such as hypertension, dyslipidemia, and diabetes mellitus may not be as relevant. There are, however, a number of limitations to consider. This was a biracial cohort of black and white adults, and the results may not be as generalizable to other race/ethnic groups. Furthermore, we did not have measures of cognitive function at baseline. Because cognitive function was measured at only 1 time point, year 25, we were unable to determine the relationship of these risk factors with change in cognition.
Although the association between CVRF AUCs and midlife cognitive performance was significant in this study, the effect size for each risk factor was small. However, studies that have investigated differences in midlife cognitive function by other risk factors like apolipoprotein E genotype have reported similar modest effects.46 It is unclear whether the effects observed in this study merit reconsideration of currently accepted approaches for life course management of CVRFs or direct intervention during early adulthood. Because of the robust connection between cardiovascular health and brain health, the conceptual framework developed for cardiovascular health promotion may provide an apt model for cognitive aging.47 In particular, the American Heart Association corresponding recommendations for primordial, primary, and secondary cardiovascular disease prevention at different stages of the life course may be especially relevant for brain health promotion,25 and a recent study from CARDIA has suggested that in young adults a greater number of ideal cardiovascular parameters, which include both lifestyle behaviors and risk factors, may be associated with better midlife cognitive performance.48
Much of the public health debate on cognitive health has focused on early-life development or late-life dementia prevention. Our results not only support a role for early adult CVRFs but also suggest that duration of exposure could be an important factor in determining the risk of cognitive impairment. Elevated but subclinical CVRF levels may be potential modifiable risk factors for accelerated cognitive aging. Although it is unclear whether treatment is warranted, this subgroup, particularly those with multiple elevated CVRFs, may represent a critical target group for early prevention. Current evidence indicates that reducing midlife CVRFs could decrease the risk of late-life dementia,49,50 but if the neuronal damage associated with cumulative CVRF exposure begins even before midlife, an expanded focus on earlier stages of the life course may be necessary to effectively reduce CVRF levels and to affect the public health burden of cognitive impairment. Further understanding of the longitudinal effects of risk factors that takes into account both temporality and duration of exposure may help inform the optimal timing and strategies needed to prevent cognitive decline. Additional long-term investigations that examine the effects of CVRFs and their treatment, coupled with biomarker and magnetic resonance imaging data in younger populations, are required to fully determine the implications for effective population-based interventions over the life course.
Supplementary Material
Clinical Perspective.
A robust body of evidence indicates that markers of cardiovascular health are critical modifiable risk factors for cognitive impairment and dementia. The association between midlife cardiovascular risk factors (CVRFs) and late-life cognitive function is well characterized, but the role of young adult CVRFs in cognitive aging is unclear. Furthermore, few studies have considered the cumulative nature of CVRF exposures. In this large, prospective cohort of >3000 black and white young adults, cumulative exposure to CVRFs over 25 years, including systolic and diastolic blood pressures, fasting blood glucose, and total cholesterol, were associated with worse midlife executive function, processing speed, and verbal memory. These associations were notably significant for cumulative exposure to elevated but subclinical levels of CVRFs. To the best of our knowledge, this is the first study to demonstrate a relationship between cumulative CVRF exposure in early adulthood and cognitive impairment in middle age. Although changes in treatment guidelines for CVRFs may not be warranted at this stage, the findings suggest that young adults with elevated CVRFs may be a promising target group for early intervention. This study provides a novel perspective on the relationship between cardiovascular health and cognitive aging, with implications for future research and policies related to the role of primary prevention across the life course.
Acknowledgments
Sources of Funding
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content.
Disclosures
Dr Yaffe has served on data safety monitoring boards for Takeda, Inc, on a study sponsored by the National Institutes of Health, and as a consultant for Novartis Inc.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.113.004798/-/DC1.
The other authors report no conflicts.
References
- 1.Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, Chen W, Srinivasan SR, Daniels SR, Kähönen M, Laitinen T, Taittonen L, Berenson GS, Viikari JSA, Raitakari OT. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood/clinical perspective. Circulation. 2010;122:2514–2520. doi: 10.1161/CIRCULATIONAHA.110.966465. [DOI] [PubMed] [Google Scholar]
- 2.Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study) J Am Coll Cardiol. 2011;58:2396–2403. doi: 10.1016/j.jacc.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pletcher MJ, Bibbins-Domingo K, Lewis CE, Wei GS, Sidney S, Carr JJ, Vittinghoff E, McCulloch CE, Hulley SB. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med. 2008;149:91–99. doi: 10.7326/0003-4819-149-2-200807150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pletcher MJ, Bibbins-Domingo K, Liu K, Sidney S, Lin F, Vittinghoff E, Hulley SB. Nonoptimal lipids commonly present in young adults and coronary calcium later in life: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Ann Intern Med. 2010;153:137–146. doi: 10.1059/0003-4819-153-3-201008030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Midlife vascular risk factors and Alzheimer’s disease: evidence from epidemiological studies. J Alzheimers Dis. 2012;32:531–540. doi: 10.3233/JAD-2012-120802. [DOI] [PubMed] [Google Scholar]
- 6.Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 8.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease, II: review of human trials and recommendations. Arch Neurol. 2011;68:1385–1392. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staessen JA, Thijs L, Richart T, Odili AN, Birkenhäger WH. Placebo-controlled trials of blood pressure-lowering therapies for primary prevention of dementia. Hypertension. 2011;57:e6–e7. doi: 10.1161/HYPERTENSIONAHA.110.165142. [DOI] [PubMed] [Google Scholar]
- 10.Morris JK, Burns JM. Insulin: an emerging treatment for Alzheimer’s disease dementia? Curr Neurol Neurosci Rep. 2012;12:520–527. doi: 10.1007/s11910-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joas E, Bäckman K, Gustafson D, Ostling S, Waern M, Guo X, Skoog I. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796–801. doi: 10.1161/HYPERTENSIONAHA.111.182204. [DOI] [PubMed] [Google Scholar]
- 12.Mielke MM, Zandi PP, Shao H, Waern M, Östling S, Guo X, Björkelund C, Lissner L, Skoog I, Gustafson DR. The 32-year relationship between cholesterol and dementia from midlife to late life. Neurology. 2010;75:1888–1895. doi: 10.1212/WNL.0b013e3181feb2bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reijmer YD, van den Berg E, Dekker JM, Nijpels G, Stehouwer CD, Kappelle LJ, Biessels GJ. Development of vascular risk factors over 15 years in relation to cognition: the Hoorn Study. J Am Geriatr Soc. 2012;60:1426–1433. doi: 10.1111/j.1532-5415.2012.04081.x. [DOI] [PubMed] [Google Scholar]
- 14.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushnick MR, Panton LB. Lipid and lipoprotein concentrations in Americans: ethnicity and age. In: Moffatt RJ, Stamford B, editors. Lipid Metabolism and Health. Boca Raton, FL: CRC Press; 2006. p. 315. [Google Scholar]
- 16.Whincup PH, Cook DG, Geleijnse JM. A life course approach to blood pressure. In: Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease Epidemiology. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 17.Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, Jacobs DR, Jr, Liu K, Orden S, Pirie P. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Control Clin Trials. 1987;8(suppl):68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 18.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Jacobs DR, Liu K, Williams OD, Hilner JE, Perkins LL, Marcovina SM, Hulley SB. Seven-year trends in plasma low-density-lipoprotein- cholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6:235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III) San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 21.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643. [Google Scholar]
- 22.MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg SJ, Ryan JJ, Prifitera A. Rey Auditory-Verbal Learning Test performance of patients with and without memory impairment. J Clin Psychol. 1984;40:785–787. doi: 10.1002/1097-4679(198405)40:3<785::aid-jclp2270400325>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 25.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force, Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 26.Carmelli D, Swan GE, Reed T, Miller B, Wolf PA, Jarvik GP, Schellenberg GD. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology. 1998;50:1580–1585. doi: 10.1212/wnl.50.6.1580. [DOI] [PubMed] [Google Scholar]
- 27.Elias MF, D’Agostino RB, Elias PK, Wolf PA. Neuropsychological test performance, cognitive functioning, blood pressure, and age: the Framingham Heart Study. Exp Aging Res. 1995;21:369–391. doi: 10.1080/03610739508253991. [DOI] [PubMed] [Google Scholar]
- 28.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solomon A, Kareholt I, Ngandu T, Winblad B, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Serum cholesterol changes after midlife and late-life cognition. Neurology. 2007;68:751–756. doi: 10.1212/01.wnl.0000256368.57375.b7. [DOI] [PubMed] [Google Scholar]
- 30.Stewart R, Xue QL, Masaki K, Petrovitch H, Ross GW, White LR, Launer LJ. Change in blood pressure and incident dementia. Hypertension. 2009;54:233–240. doi: 10.1161/HYPERTENSIONAHA.109.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuorinen M, Solomon A, Rovio S, Nieminen L, Kåreholt I, Tuomilehto J, Soininen H, Kivipelto M. Changes in vascular risk factors from midlife to late life and white matter lesions: a 20-year follow-up study. Dement Geriatr Cogn Disord. 2011;31:119–125. doi: 10.1159/000323810. [DOI] [PubMed] [Google Scholar]
- 32.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, Mosley TH., Jr Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68(suppl):S74–S87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishtala A, Preis SR, Beiser A, Devine S, Hankee L, Seshadri S, Wolf PA, Au R. Midlife cardiovascular risk impacts executive function: Framingham Offspring Study. [Accessed November 19, 2013];Alzheimer Dis Assoc Disord. doi: 10.1097/WAD.0b013e3182a715bc. [published online ahead of print August 29, 2013]. http://journals.lww.com/alzheimerjournal/pages/articleviewer.aspx?year=9000&issue=00000&article=99676&type=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K, Lindquist K, Schwartz AV, Vitartas C, Vittinghoff E, Satterfield S, Simonsick EM, Launer L, Rosano C, Cauley JA, Harris T. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011;77:1351–1356. doi: 10.1212/WNL.0b013e3182315a56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJH. The role of lipoproteins and inflammation in cognitive decline: do they interact? Neurobiol Aging. 2012;33:196.e1–196.e12. doi: 10.1016/j.neurobiolaging.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 39.Lesser GT, Beeri MS, Schmeidler J, Purohit DP, Haroutunian V. Cholesterol and LDL relate to neuritic plaques and to APOE4 presence but not to neurofibrillary tangles. Curr Alzheimer Res. 2011;8:303–312. doi: 10.2174/156720511795563755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knopman DS, Roberts R. Vascular risk factors: imaging and neuropathologic correlates. J Alzheimers Dis. 2010;20:699–709. doi: 10.3233/JAD-2010-091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging. 2012;33:1979–1987. doi: 10.1016/j.neurobiolaging.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Norden AG, van Dijk EJ, de Laat KF, Scheltens P, Olderikkert MG, de Leeuw FE. Dementia: Alzheimer pathology and vascular factors: from mutually exclusive to interaction. Biochim Biophys Acta. 2012;1822:340–349. doi: 10.1016/j.bbadis.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bender AR, Raz N. Age-related differences in episodic memory: a synergistic contribution of genetic and physiological vascular risk factors. Neuropsychology. 2012;26:442–450. doi: 10.1037/a0028669. [DOI] [PubMed] [Google Scholar]
- 46.Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacco RL. Achieving ideal cardiovascular and brain health: opportunity amid crisis. Circulation. 2011;123:2653–2657. doi: 10.1161/CIR.0b013e318220dec1. [DOI] [PubMed] [Google Scholar]
- 48.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Jr, Zhu N, Lloyd-Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.