Summary
The regulators of Mycobacterium tuberculosis DNA replication are largely unknown. Here, we demonstrate that in synchronously replicating M. tuberculosis, MtrA access to origin of replication (oriC) is enriched in the post-replication (D) period. The increased oriC binding results from elevated MtrA phosphorylation (MtrA~P) as evidenced by reduced expression of dnaN, dnaA and increased expression of select cell division targets. Overproduction of gain-of-function MtrAY102C advanced the MtrA oriC access to the C period, reduced dnaA and dnaN expression, interfered with replication synchrony and compromised cell division. Overproduction of wild-type (MtrA+) or phosphorylation-defective MtrAD56N did not promote oriC access in the C period, nor affected cell cycle progression. MtrA interacts with DnaA signaling a possibility that DnaA helps load MtrA on oriC. Therefore, oriC sequestration by MtrA~P in the D period may normally serve to prevent untimely initiations and that DnaA-MtrA interactions may facilitate regulated oriC replication. Finally, despite the near sequence identity of MtrA in M. smegmatis and M. tuberculosis, the M. smegmatis oriC is not MtrA-target. We conclude that M. tuberculosis oriC has evolved to be regulated by MtrA and that cell cycle progression in this organisms are governed, at least in part, by oscillations in the MtrA~P levels.
Keywords: Mycobacterium, Cell cycle, Tuberculosis, Response Regulator, Synchronous replication
Introduction
Mediation of DNA replication at the origin of replication (oriC) by the DnaA initiator protein is an essential aspect of the cell duplication process in eubacteria and is followed by DNA segregation and cell division, the latter of which includes the FtsZ-catalyzed septal-ring assembly, septum synthesis, and cell separation steps. Much of our understanding of the regulation of the chromosomal DNA replication process comes from Gram-positive (Bacillus subtilis) and Gram-negative (Escherichia coli, Caulobacter cresentus) model organisms. However, the regulatory mechanisms operated in these bacteria are not necessarily similar (reviewed in (Skarstad & Katayama, 2013)). For example, E. coli and B. subtilis exhibit multi-fork replication, i. e, grow with overlapping replication cycles in nutrient-rich media, whereas C. cresentus, which shows a dimorphic lifestyle, does not exhibit multi-fork replication. E. coli uses several regulatory mechanisms that control either the availability of oriC or the levels and activities of DnaA for ensuring one replication event per cell cycle. These include the following: the sequestration of oriC by the negative regulator SeqA, the regulatory inactivation of DnaA (RIDA) by the combined action of Hda and the DNA polymerase subunit DnaN clamp, the quenching of active pools of DnaA by datA sequence enriched with DnaA boxes, the stimulation of DnaA-ATP hydrolysis at datA locus in a manner dependent upon the activity of integration-host factor, and the regulation of dnaA transcription [reviewed in (Skarstad & Katayama, 2013)]. B. subtilis, does not have SeqA system, but uses the DnaA-interacting proteins Soj, SirA, and YabA for modulating DnaA activity (Murray & Errington, 2008, Noirot-Gros et al., 2006, Wagner et al., 2009) and the transcriptional regulator and oriC-binding protein Spo0A for inhibiting initiation of DNA replication at the onset of sporulation (Castilla-Llorente et al., 2006). C. cresentus uses response regulator (RR) CtrA-dependent cascade type of regulation to sequester oriC. CtrA-mediated regulation is, however, dependent on its proteolytic stability and phosphorylation status (Domian et al., 1997, Quon et al., 1998). Interestingly, C. cresentus DnaA levels, unlike its counterparts, are unstable and oscillate as a function of the cell cycle, thus contributing to the regulation of replication (Gorbatyuk & Marczynski, 2005). Finally, other RR such as ArcA in E. coli and HP1021 in Helicobacter pylori have also been reported to interact with oriC and interfere with the replication initiation process in vitro (Donczew et al., 2015, Lee et al., 2001).
It is unknown how the DNA replication process in Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), is regulated. M. tuberculosis is a slow-grower with an average doubling time of 24 hours (h). TB accounts for nearly 1. 5 million deaths per year and one-third of the global population are latently infected with M. tuberculosis. It is known that M. tuberculosis successfully shifts between active and clinically asymptomatic latent (persistent) growth states in response to immune pressure (Smith, 2003). Although the doubling times of latent M. tuberculosis in humans cannot be assessed and are expected to be high, immune-restrained M. tuberculosis replicate during persistent growth in mice lungs (Gill et al., 2009). Intuition is that the replication and cell cycle processes must be tightly controlled during latency and reactivation. Published data showed that the M. tuberculosis dnaA-dnaN intergenic region serves as oriC (Qin et al., 1999), and M. tuberculosis DnaA ATP-hydrolysis activity is required for its rapid oligomerization on oriC, a result different from that seen in E. coli. Additionally, DnaA defective for ATP-hydrolysis is non-functional in vivo (Madiraju et al., 2006) and synchronously replicating M. tuberculosis do not show hyper-initiation (Nair et al., 2009). Finally, Rv1985c gene product has been shown to bind M. tuberculosis oriC at the AT-rich region in vitro and prevent oriC duplex unwinding when added prior to the addition of DnaA (Kumar et al., 2009), however, the biological significance of these findings have not been evaluated. Recent cell cycle studies with M. smegmatis, a rapid grower with a doubling time of 3 h and a nonpathogen, indicate that M. smegmatis does not exhibit multi-fork replication (Santi et al., 2013, Trojanowski et al., 2015) and that their cell cycle organization is distinct compared with well-known model organisms (Santi et al., 2013).
MtrA is the essential RR component of the MtrAB histidine-aspartate two-component response regulatory system (2CRS) of M. tuberculosis (Zahrt & Deretic, 2000). Earlier studies showed that MtrA is poorly phosphorylated in vitro (Friedland et al., 2007, Rajagopalan et al., 2010); D56 residue is important for MtrA phosphorylation and replacement of D with N residue abolished MtrA phosphorylation ability (Fol et al., 2006); phosphorylated MtrA (MtrA~P) binds oriC in vitro at four different locations, designated as MtrA-boxes F2, F3, F4 and F5 (Rajagopalan et al., 2010). The arrangement of the MtrA- and DnaA- boxes on oriC is non-overlapping (Rajagopalan et al., 2010). It is shown that promoters (P) for dnaA, secreted antigen 85B and cell wall mycolyl transferase (fbpB), and essential cell wall hydrolase, ripA are MtrA-targets (Fol et al., 2006, Plocinska et al., 2012, Rajagopalan et al., 2010). Other studies showed that M. tuberculosis multiplication upon infection depends, in part, on the optimal ratios of phosphorylated to nonphosphorylated MtrA (Fol et al., 2006). While these data connect MtrA levels/phosphorylation activity to cell cycle (i.e. replication and cell division) and possibly other processes, the biological significance of MtrA binding to oriC and the roles of MtrA~P, if any, on oriC replication are unknown. The present study was undertaken to evaluate the roles of MtrA in M. tuberculosis DNA replication. Characterization of the MtrA-oriC interactions under synchronous replication conditions led us to conclude that MtrA~P functions as a regulator of oriC replication. These studies also showed that MtrA interacts with DnaA and that M. smegmatis oriC is not MtrA target. Our studies suggest that the M. tuberculosis oriC has evolved to be regulated by MtrA and that the MtrA-mediated regulation of DNA replication is different from that known in other organisms.
Results
MtrA~P binds preferentially to oriC
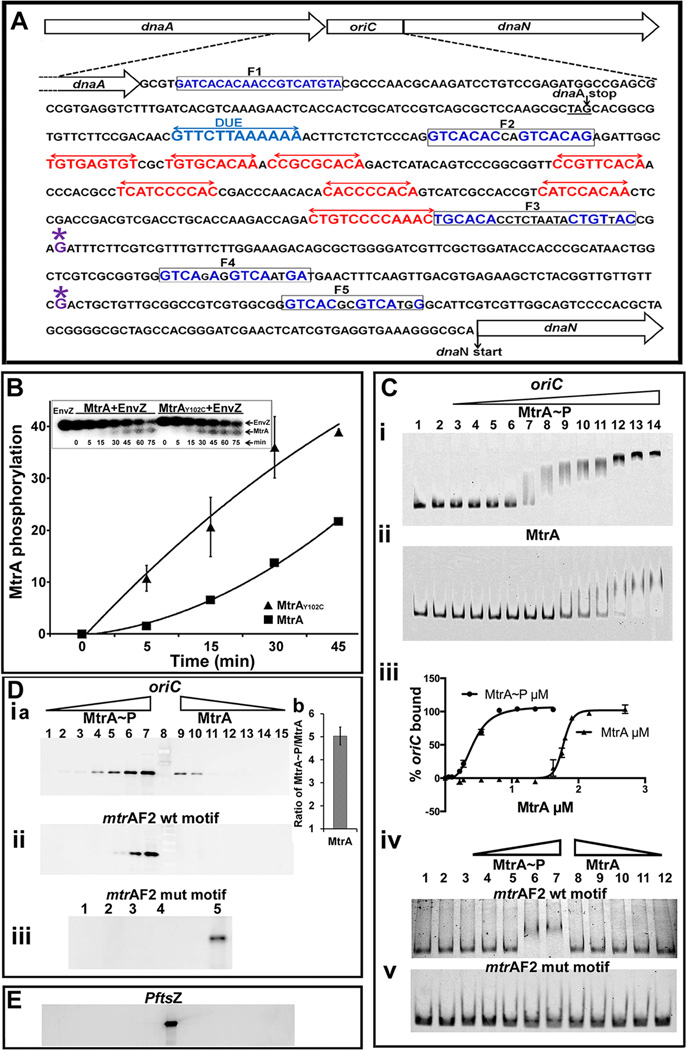
Two series of experiments were performed to evaluate MtrA~P binding to oriC (Fig. 1 A). First, MtrA or MtrA~P binding to full-length oriC was detected by electrophoretic gel-mobility shift assay (EMSA). MtrA~P was produced by incubating MtrA with E. coli EnvZ kinase and ATP or 32P-ATP as described (Fol et al., 2006). Incubation of MtrA with EnvZ led to a time dependent increase in MtrA phosphorylation (Fig. 1 B). EMSA experiments clearly showed that MtrA~P bound oriC better than MtrA (Fig. 1 C-i, ii). Following densitometry, the fraction of free oriC was calculated, the total bound oriC was determined and a plot of the percent bound oriC versus in put MtrA concentration was prepared (Fig. 1 C-iii). It should be noted that the fraction of bound oriC includes both stable and unstable MtrA~P-oriC complexes in the reaction. These data revealed a sigmoidal curve typical of cooperative binding (affinity of MtrA~P to oriC increases upon binding of some MtrA~P), with a Hill coefficient of 3.2 (± 0.22), and apparent dissociation constant KD of 410 nM (± 0.014). The oriC fragment contains 4 MtrA-binding sites (Fig. 1 A) and a perfect cooperative binding is expected to give a Hill coefficient of 4. Thus, the measured 3. 2 Hill coefficient number is indicative of highly cooperative system where all MtrA binding sites in oriC are occupied interdependently by MtrA~P. The measured KD could be a large overestimate as it was based on the assumption that the recombinant MtrA is fully active and is efficiently phosphorylated.
Fig. 1.
(A) M. tuberculosis oriC region and oriC sequence with DnaA- and MtrA- boxes. The dnaA-dnaN intergenic region of M. tuberculosis containing DnaA-boxes (red double arrows), MtrA-motifs (boxed) and DUE sequence (blue double arrows) is shown. For clarity, only the DnaA-boxes defined based on our earlier DMS footprinting data are shown (Madiraju et al., 2006). Although not shown, DNaseI footprinting data located two additional boxes in the 3’ end of oriC, presumably these are low-affinity DnaA- boxes (Zawilak et al., 2004). Presumptive transcription start sites at −110 (downstream of F4 box) and −258 (downstream of F3 box) relative to dnaN start codon based on M. bovis BCG sequence are marked with ‘*’ above the ‘G’ residue (Salazar et al., 2003). (B) Kinetics of MtrA phosphorylation. Autophosphorylated EnvZ was incubated with MtrA or MtrAY102C, samples at indicated time periods were removed, resolved by SDS-PAGE, autoradiography was performed, signals were quantitated by densitometry on a Bio-Rad Molecular imager and data plotted. Inset shows a representative SDS-PA gel autoradiograph image of radiolabeled MtrA and EnvZ proteins. (C) EMSA showing MtrA binding to oriC (panels i, ii) and DNA fragments containing MtrA F2 WT and mutant sequences (panels iv, v). Lanes 1 and 2 (panels i– v) are controls wherein DNA alone (lane 1) or that incubated with EnvZ in the absence of MtrA (lane 2) were resolved. MtrA-mCherry~P was used at 0.054, 0.108, 0.216, 0.27, 0.54, 0.81, 1.35, 1.62, 1.89, 2.16 and 2.7 µM (panels C-i) and MtrA-mCherry at 0.216, 0.27, 0.54, 0.81, 1.08, 1.35, 1.62, 1.75, 1.8, 1.9, 2.16 and 2.7 µM (panels C-ii). Binding data were quantified and the percent oriC bound was calculated (panel iii). MtrA-binding to F2 WT (panel iv) and mutant sequence (panel v) were performed at 0.27, 0.54, 1.08, 2.16 and 2.7 µM protein concentration. (D) Solid phase DNA-binding assays. Streptavidin magnetic beads conjugated with biotinylated oriC (panel i) or MtrA F2 box sequences (panels ii, iii) were incubated with MtrA and processed as described in methods section. MtrA proteins were used at 0.0365, 0.0735, 0.147, 0.294, 0.588, 1.176, 2.32 µM. MtrA~P or MtrA bound to oriC was determined by densitometry and MtrA~P/MtrA calculated for 1.176, 2.32 µM (Panel D-i b). Binding experiments with MtrA-F2 mutant box sequence were performed in duplicate at 1.176 µM (panel D-iii, lanes 1 and 2) and 2.32 µM MtrA (panel D-iii, lanes 3 and 4) respectively, as other concentrations did not show any binding. (E) Binding assays were performed with PftsZ and immunoblotted with α-MtrA as described above. All binding conditions were similar to oriC (D-i). Note: Lanes ‘8’ in D-i a and E and ‘5’ in D-iii are positive controls wherein streptavidin beads conjugated to oriC or MtrA F2 box, respectively, were incubated with 1. 176 µM MtrA~P.
On the other hand, nonphosphorylated MtrA showed inefficient binding and MtrA-oriC complexes were relatively unstable (Fig. 1 C, compare ii with i). The MtrA-oriC complexes were detected at relatively high protein concentrations, i.e. 1.60 µM (lane 9) with apparent KD of 1.7 µM (± 0.008). The observed poor binding of MtrA to oriC is consistent with our published footprinting data (Rajagopalan et al., 2010). Similar binding pattern was also noted for other targets (see below). EMSA studies with DNA-fragments bearing individual MtrA-boxes revealed modest MtrA~P binding to the F2 box (Fig. 1 C-iv) and that the binding was abolished when the F2 box was replaced with a mutant sequence demonstrating the specificity of MtrA-oriC interactions (Fig. 1 C-v, see EMSA methodology section for mutant F2 box sequence). No detectable MtrA binding to oriC DNA fragments containing the individual F3, F4 or F5 boxes was detected (data not shown). Presumably, F2 box with GTCACA sequence is the preferred motif and is likely accessed first by MtrA~P in vivo followed by other MtrA-boxes. It should be noted that the F2 MtrA-box is located between the AT-rich DNA unwinding element (DUE) and a DnaA-box (Fig. 1 A) and is conserved in diverse Mycobacterium spp. Also, the loss of the GTCACA motif abolished autonomous replication activity of oriC plasmids (Rajagopalan et al., 2010). Further studies with more sensitive assays are required for evaluating the kinetics, order and consequences of MtrA-binding to oriC MtrA-boxes in the presence and absence of DnaA.
Next, we performed solid-phase binding experiments wherein biotinylated oriC coupled to streptavidin magnetic beads was incubated with varying concentrations of MtrA or MtrA~P, magnetically separated, resolved by SDS-PAGE, immunoblotted and band signal intensities corresponding to MtrA quantitated by densitometry (Fig. 1 D-i a). Again, distinct differences between MtrA and MtrA~P processed samples were noted. For example, bands corresponding to MtrA~P were detected at lower protein concentrations as compared to MtrA (compare lanes 3–7 with 9–13). The calculated ratio of bound MtrA~P to MtrA revealed a five-fold difference in signal intensity (Fig. 1 D-i b). MtrA or MtrA~P did not bind PftsZ under these conditions indicating that oriC is a specific target (Fig. 1 E). Finally, consistent with the EMSA data, MtrA~P bound to DNA fragment bearing F2 WT sequence, but not to that containing mutant sequence (Fig. 1 D-ii, iii). Together, these data emphasize that MtrA~P binds better than MtrA to oriC and F2 box.
MtrAY102C binds oriC and DNA fragments bearing individual MtrA-boxes
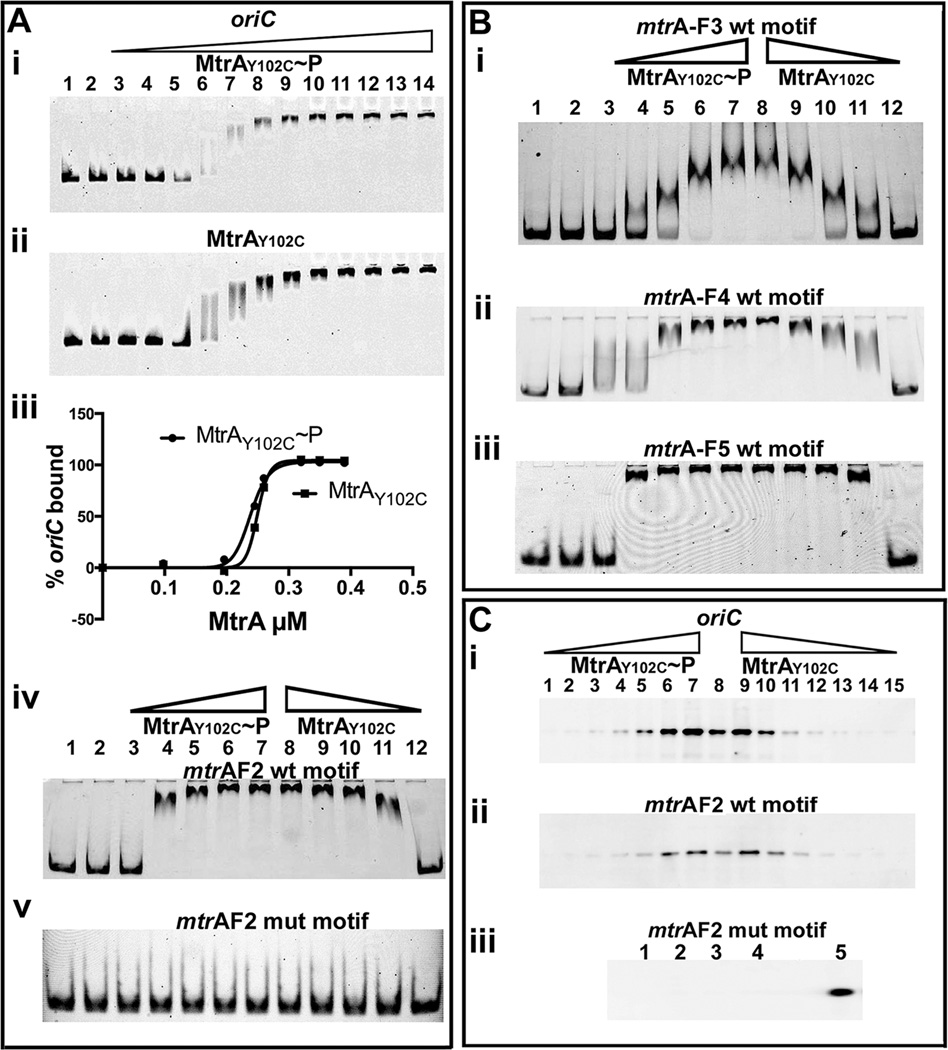
MtrA crystal structure data revealed that the amino-terminal regulatory and carboxy-terminal effector domains are oriented in such a way that extensive contacts between these two lock the regulatory domain in inactive conformation leading to decreased activation (Friedland et al., 2007). This could in turn lead to inefficient target binding. These data also revealed that Y102 residue is oriented away at outward position and hydrogen bonded with D190 of the DNA-binding domain, thereby bridging the stability of domains (Friedland et al., 2007). In an effort to identify MtrA proteins exhibiting enhanced phosphorylation activities, we characterized several MtrA mutant proteins and found that MtrAY102C is phosphorylation-competent (Al Zayer et al., 2011, Plocinska et al., 2012), acts as a gain-of-function (GoF) protein that functions in the absence of MtrB (Plocinska et al., 2012). We then investigated MtrAY102C interaction with oriC. In vitro phosphorylation experiments revealed that MtrAY102C exhibits higher rates and yields of phosphorylation as compared to MtrA (Fig. 1 B). EMSA studies showed that MtrAY102C bound oriC with and without phosphorylation similarly with apparent of KD of 0.23 µM (± 0.002) (Fig. 2 A- i–iii). MtrAY102C bound DNA fragments bearing F2 (Fig. 2 A–iv) but not mutant box (Fig. 2 A-v); also unlike the situation with MtrA~P, it bound F3, F4 and F5 boxes (Fig. 2 B i–iii). Data from solid phase DNA binding experiments were also consistent with EMSA data (Fig. 2 C- i–iii). Altered DNA binding properties of MtrAY102C are likely due to Y102C change that destabilizes the MtrA inter-domain orientation thereby promoting active conformation of the regulatory domain. A consequence could be enhanced rate of phosphorylation (Fig. 1 B) and target binding (Fig. 2). The ability of MtrAY102C to bind DNA fragments bearing individual MtrA-boxes further supports the notion that MtrA phosphorylation promotes oriC binding and that MtrA-boxes in oriC are occupied under optimal MtrA~P conditions.
Fig. 2.
(A) EMSA showing MtrAY102C binding to oriC and F2 box sequences. Lanes 1 and 2 (panels i, ii, iv and v) are MtrA controls wherein DNA was incubated with (lane 1) or without (lane 2) EnvZ and electrophoresed. MtrAY102C~P (panel A-i) or MtrAY102C (panel A-ii) was used at 0.098, 0.196, 0.245, 0.294, 0.343, 0.392, 0.784, 1.76, 1.96, 2.45, 2.94 and 3.43 µM. Binding data were quantified and plotted (panel- iii). MtrAY102C binding to MtrA F2 WT (panel iv) and mutant box (panel v) fragments were assayed at 0.196, 0.392, 0.784, 1.176 and 1.96 µM in ascending (lanes 3 to 7) and descending (8 to 12) order of protein concentration, respectively. (B) MtrAY102C binding to MtrA-boxes F3, F4 and F5. All experimental conditions including the controls are as described in ‘A’. (C) Solid-phase DNA binding assay: MtrAY102C~P or MtrAY102C binding to biotinylated oriC (panels i) or MtrA F2 fragments (panels ii,iii) was determined as described under Fig. 1 D at 0.049, 0.098, 0.196, 0.392, 0.784, 1.568, 3.136 µM concentrations. Data shown are in increasing order of protein concentration for MtrAY102C~P and decreasing order for MtrAY102C. MtrAY102C~P binding to F2 mutant MtrA-box was assayed at 1.568 (lanes 1 and 2) and 3.136 µM (lanes 3 and 4), respectively. Note: Lanes ‘8’ in C-i/ii and ‘5’ in C-iii are positive controls wherein streptavidin beads conjugated to oriC or MtrA F2 box, respectively, were incubated with 1. 568 µM MtrA~P.
MtrA binding to oriC is associated with modulation of dnaN transcription
dnaN is located downstream of oriC (Fig. 1 A). The nucleotide sequences of oriC and dnaN of M. tuberculosis and the closely related vaccine strain M. bovis BCG are nearly identical; also M. tuberculosis oriC is functional in M. bovis BCG (Qin et al., 1999, Salazar et al., 1996). Primer extension studies identified M. bovis BCG dnaN promoter in the oriC 3’ end with two transcription start sites designated as T1 and T2 at nucleotides −110 and −258 relative to dnaN start codon (Salazar et al., 2003). Although M. tuberculosis PdnaN is not determined, given the conserved organization of oriC and flanking regions of M. tuberculosis and M. bovis BCG, we assumed that M. tuberculosis PdnaN is located in its oriC (see Fig. 1 A ‘*’ above G residues for presumptive T1 and T2 sites). Accordingly, we considered a possibility that one consequence of MtrA binding to oriC is the modulation of dnaN transcription. Hence, we evaluated dnaN transcription by qRT-PCR relative to 16S rRNA under steady-state growth conditions in M. tuberculosis strains producing either normal (control) or elevated levels of wild-type (WT) MtrA (MtrA+; Rv78), MtrAY102C (RvY102C) or phosphorylation-defective MtrAD56N (Rv129, (Fol et al., 2006), see Table S2 for strains and plasmids) and the data were normalized relative to control. The dnaN transcription showed a significant reduction in RvY102C, but was modestly decreased in Rv78 and elevated in Rv129 (Fig. S1). Earlier studies revealed that MtrAD56N does not bind DNA fragment bearing MtrA-boxes (Al Zayer et al., 2011). The F3 MtrA- box and one of the promoters of dnaN overlap (see Fig. 1A). Perhaps, the compromised affinity of MtrAD56N towards the F3 MtrA-box in the Rv129 background resulted in increased dnaN transcription. Nonetheless, these data support a notion that MtrAY102C overproduction leading to aberrant MtrA~P is associated with reduction in dnaN transcription.
MtrA accesses oriC after the DNA synthesis period of cell cycle
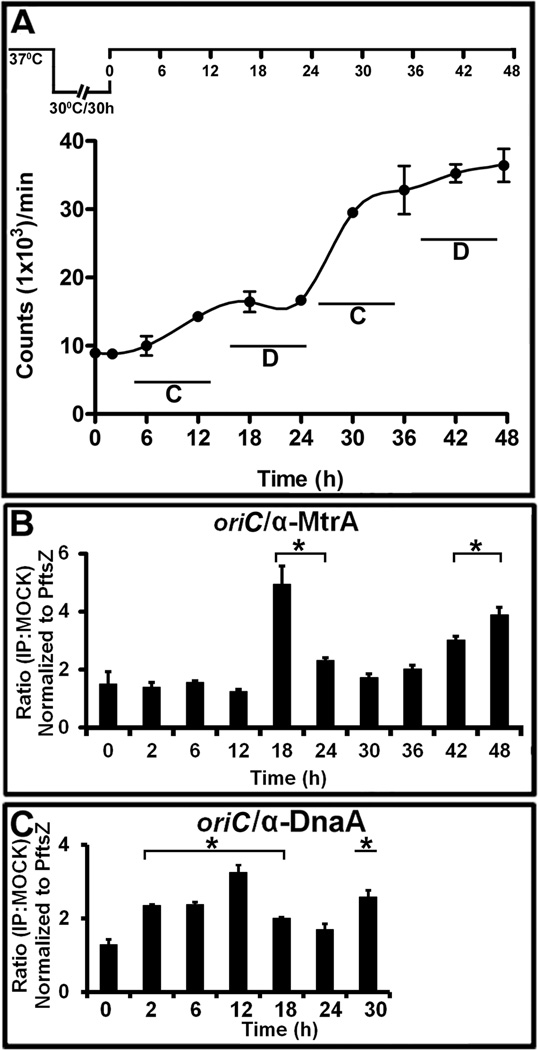
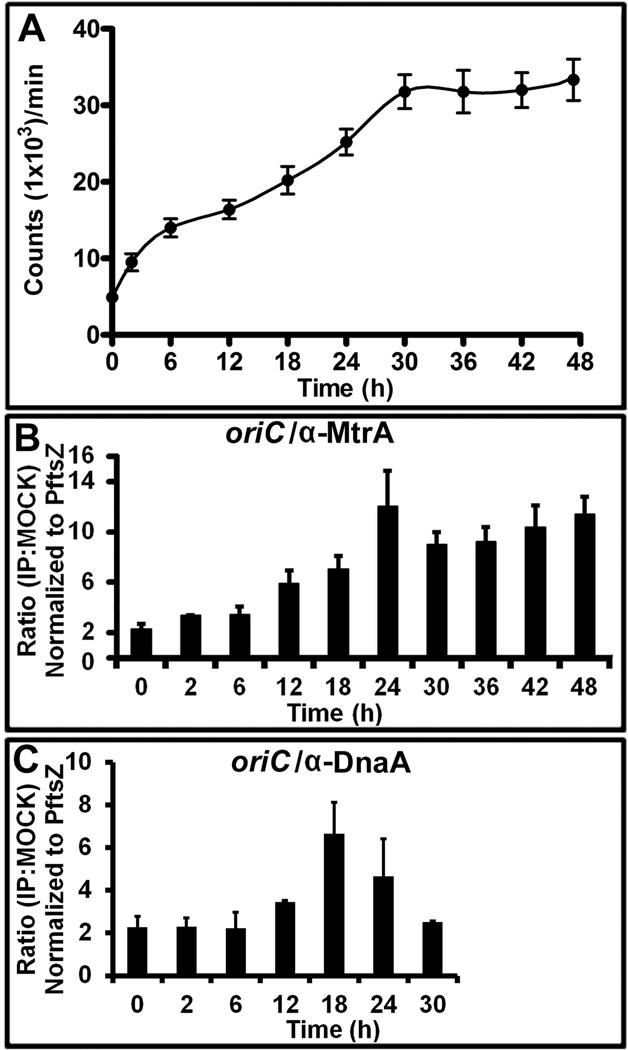
To determine the biological consequences of MtrA interactions with oriC, we first sought to determine the specific timing of their interactions during the cell cycle process. To this end, we used synchronously replicating cultures for evaluating DNA replication and for determining the MtrA-occupancy of oriC by ChIP (see below). We recently engineered and characterized three dnaA cold-sensitive (Mtb dnaAcos) strains (Nair et al., 2009) and evaluated their ability to show synchronous replication following the earlier published protocols (Dick et al., 1998, Lim et al., 1999, Wayne, 1977). These studies revealed that the engineered Mtb dnaAcos strains are cold-sensitive for replication initiation at nonpermissive (30°C) temperature, but resume synchronous replication after a lag of 2 to 4 h upon shift to a permissive (37°C) temperature (Nair et al., 2009). One of the strains, Mtb dnaAcos115, referred to as dnaAcos hereafter, was characterized further. Consistent with the published report (Nair et al., 2009), we observed synchronous replication following temperature shift from 30°C to 37°C and found that the typical DNA synthesis period (a round of replication leading to the doubling of the initial counts) lasted for 12 h, whereas the inter-replication period was approximately 10 to 12 h (Fig. 3 A). For clarity, DNA synthesis period was defined as the C period and the time interval between two replication cycles as the D period (see Fig. 3 A). The C period includes the initiation (i.e. the initial interactions between DnaA and oriC leading to the formation of the initiation complex) and the DNA chain-elongation steps. Thus, the D period in this study is different than the traditional one defined for E. coli cell cycle, which is the time between the end of chromosome replication and cell division (Cooper & Helmstetter, 1968). DNA incorporation studies revealed a 2 h lag (pre-replication period) in the first cycle of replication, which is presumably due to a slow build up of DnaA and possibly other cofactors necessary for the assembly of M. tuberculosis orisome. CFU analysis revealed the doubling of the cell number by 30 h (Fig. S 2). It is unknown if DNA segregation is initiated and completed within the D period and/or overlaps with the C period of second cycle, but it is likely that cell division initiated in the first cycle is completed during the second cycle of replication. These latter results are similar to the situation reported with M. smegmatis single-cell-dynamics studies (Santi et al., 2013).
Fig. 3.
The MtrA and DnaA occupancy of oriC in synchronously replicating cultures. (A) DNA synthesis was measured as 3H-uracil incorporation, normalized to OD600=1 and presented as counts per minute (CPM) on the Y-axis. The X-axis shows the time periods when samples were processed. The C and D periods are marked for clarity. A typical dnaAcos synchronization plan is also shown at the top of this panel. (B) ChIP-PCR assay showing the MtrA occupancy of oriC during the cell cycle. The ChIP assay was performed at the indicated time points with α-MtrA followed by PCR of oriC (MtrA target) and PftsZ (non-target). The ratio of IP to the mock signal was determined for each time point, normalized against the PftsZ promoter value and shown on the Y-axis. The p-values were calculated by Student’s unpaired t-test and * denotes a p-value ≤0.05 for samples showing significant enrichment of 2 and above. (C) ChIP-PCR assay showing the DnaA occupancy of oriC during the cell cycle. All experimental conditions and details as described in ‘B’ except that DnaA antibodies were used to process samples.
MtrA ChIP-PCR for oriC followed by normalization to the control target, PftsZ (Rajagopalan et al., 2010), revealed significant (i.e., normalized ratio of 2 and above with p-values below <0.05) oriC enrichment at 18 and 24 h (i.e., in cells with no ongoing DNA replication) and again after 42 and 48 h, but none in the C period (Fig. 3 B). ChIP-PCR experiments with DnaA-antibodies revealed, as expected, the DnaA enrichment of oriC in the C period with detectable binding in the D period (Fig. 3 C, see 12 and 18 h).
oriC sequestration in the D period by MtrA is not due to changes in the mtrA transcript and protein levels
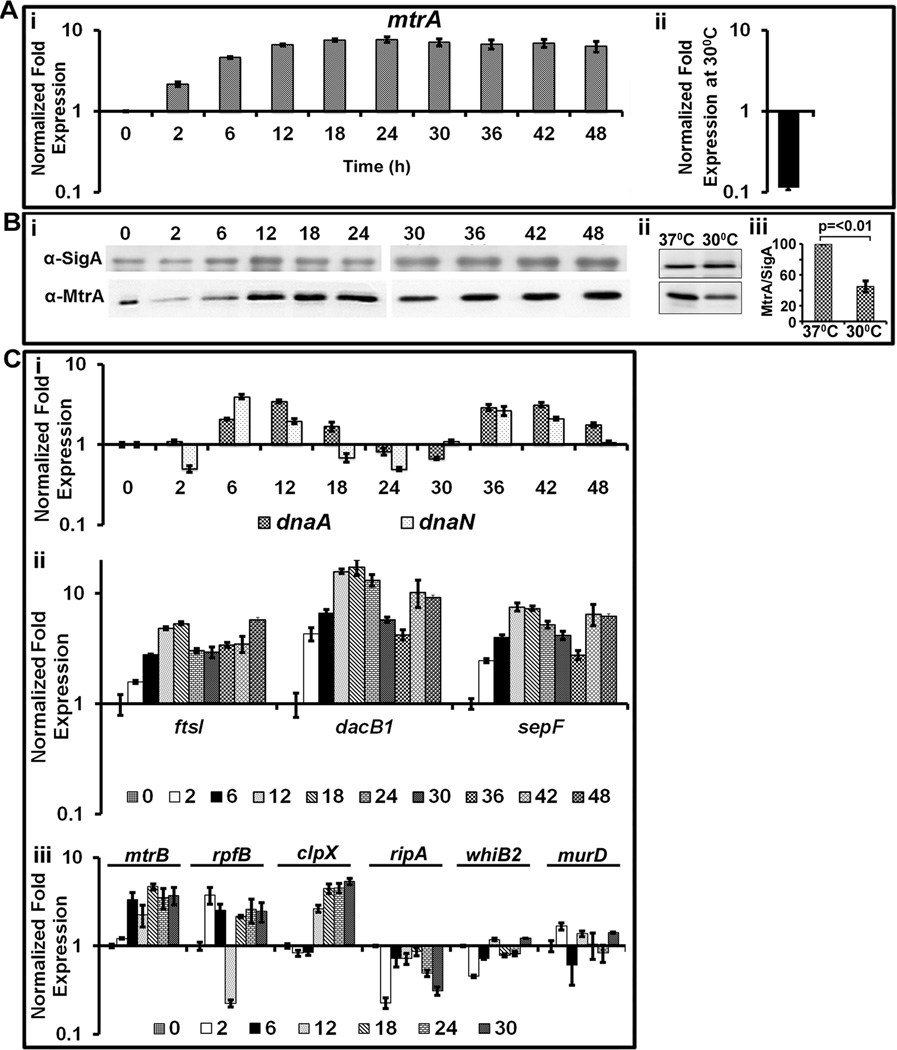
Several experiments were carried out to understand why MtrA occupancy of oriC was enriched in the D period. First, we measured the mtrA transcript levels by qRT-PCR in dnaAcos at various periods after initiation of synchronous replication, normalized to 16S rRNA and determined fold expression relative to time ‘0’ (Fig. 4 A). As can be seen, mtrA transcription was reduced at non-permissive temperature, i.e. 30°C (Fig. 4 A- ii), but steadily increased up to 6 h and remained fairly high thereafter (Fig. 4 A). These results suggest that mtrA transcription is high in actively replicating cells. As a control, we measured dosR RR expression and found that it remained low in both C and D periods (Fig. S 3). Next, we evaluated by immunoblotting the intracellular MtrA levels relative to the house-keeping SigA protein (Fig. 4 B-i). Consistent with the mtrA transcription profiles, MtrA protein levels were low at 30°C compared to 37°C (Fig. 4 B – ii, iii), gradually increased up to 12 h, thereafter were high and remained fairly constant (Fig. 4 B -i). We conclude from these data that oriC enrichment by MtrA in the D period is not due to changes in the mtrA transcript and protein levels.
Fig. 4.
Evaluation of the mtrA transcript, protein and select target expression as a function of cell cycle. (A) qRT-PCR analysis of mtrA transcript levels: Panel i: Total RNA from synchronously replicating cultures of M. tuberculosis dnaAcos at the indicated time points were extracted and the expression levels of 16S rRNA and mtrA were determined. Data shown are normalized to 16SrRNA and fold expression relative to 30°C is shown. Panel ii shows mtrA expression levels in dnaAcos grown at 30°C for 30 h and normalized against that actively growing at 37°C. (B) Immunoblots showing MtrA and SigA: Total proteins were extracted from synchronously replicating dnaAcos samples at indicated time periods, probed with α-MtrA and α-SigA to determine expression levels of MtrA during cell cycle. Note: Two micrograms of lysates were loaded for each time point except for 0 h sample, for which 5 µg of lysate was loaded to detect MtrA as its concentration was reduced under non-growing conditions. Panel ii- immunoblots showing MtrA and SigA in Mtb dnaAcos grown at 30°C for 30 h and actively growing cultures at 37°C. Panel iii: MtrA and SigA band intensities of panel ii were determined, the MtrA/SigA ratio and the p-value were determined from three independent experiments. (C) qRT-pCR analysis of select MtrA-targets normalized to that of 16S rRNA. Panel i: expression profiles of dnaN and dnaA; Panel ii: expression profiles of ftsI, dacB1 and sepF; Panel iii; expression profiles of targets rpfB, ripA, whiB2, clpX and non-targets murD and mtrB.
Next, we considered whether increased oriC enrichment in the D period is in part due to increased MtrA phosphorylation. To address this issue, we aimed to measure MtrA~P in both C and D periods by two independent measures. First, we attempted to detect and distinguish the MtrA and MtrA~P forms using Phos-tag acrylamide gels among other methods, but met with limited success. Because a consequence of RR phosphorylation is the modulation of target gene expression, as an alternative measure for MtrA~P status, we evaluated the expression levels of MtrA-targets, dnaN (see above Fig. S 1), dnaA (Fol et al., 2006), ripA (Plocinska et al., 2012) and others (identified based on our MtrA ChIP-Seq studies; see below), during synchronous replication. The dnaN transcription, relative to 16S rRNA, showed a sharp increase between 6 and 12 h followed by return to basal levels and decreased thereafter. Another burst of dnaN transcription occurred between 30 to 42 h and decreased thereafter (Fig. 4 C-i). A similar trend was also noted with the dnaA expression (Fig. 4 C-i). Thus, the periodic dnaN and dnaA expression show inverse correlation with the MtrA-occupancy of oriC. Like oriC, the immediate 5’ upstream region of dnaA contains MtrA binding sites [(Li et al., 2010), see also Fig. S 4A]. ChIP-PCR studies with MtrA antibodies revealed PdnaA enrichment in both C and D periods, with modestly elevated levels at 12 and 18 h (Fig. S 4B) indicating that MtrA binds PdnaA. We infer from these data that increased expression levels of dnaA and dnaN are required during the C period and that MtrA~P likely functions as a transcriptional repressor of dnaA and dnaN.
The above data lead to a hypothesis that MtrA activity, hence MtrA~P, is temporal. To validate this hypothesis, we evaluated the expression profiles of select MtrA-targets involved in other aspects of cell cycle, i.e. cell division. We recently performed MtrA ChIP-Seq in M. tuberculosis producing MtrAY102C background, elucidated the comprehensive MtrA-regulon (Madiraju and Rajagopalan, unpublished data), and selected dacB1, sepF, pbpB, ripA, rpfB, whiB2 and clpX as potential MtrA-targets for this study. In addition, expression profiles of non-targets murD and mtrB were measured. The nucleotide sequences upstream of the selected targets contained distinct MtrA-binding motifs (Table S3). EMSA with select target genes confirmed MtrA~P binding to their upstream regions (Fig. S 5, note inefficient binding of MtrA to these targets). QRT—PCR data revealed that although ftsI, dacB1 and sepF were expressed in both C and D periods, their expression was elevated in the D period (Fig. 4 C-ii); other targets, i.e. rpfB, ripA, clpX and whiB2 did not show such changes (Fig. 4 C-iii). We conclude that expression of not all MtrA-targets is temporal and that MtrA~P pools, although maintained in both the C and D periods, are elevated in the D period.
DnaA protein acts as a transcriptional regulator and since the dnaA transcript levels were reduced in the D period (Fig. 4 C-i), the possibility that decreased dnaA expression in the D period contributes to increased ftsI, dacB1, and sepF expression remains open. This possibility implies that DnaA acts as a transcriptional repressor of the above genes. To address this concern, expression levels of these targets in M. tuberculosis overexpressing dnaA were measured. Little or no changes to the expression levels of mtrA, ftsI, dacB1 and sepF and a modest 2.5 fold reduction of whiB2 was observed (Fig. S 6), thus alleviating the concern that the observed changes in the MtrA-target expression are due to changes in the dnaA expression levels, but likely due to increased MtrA activity.
Untimely MtrA~P access to oriC interferes with oriC replication
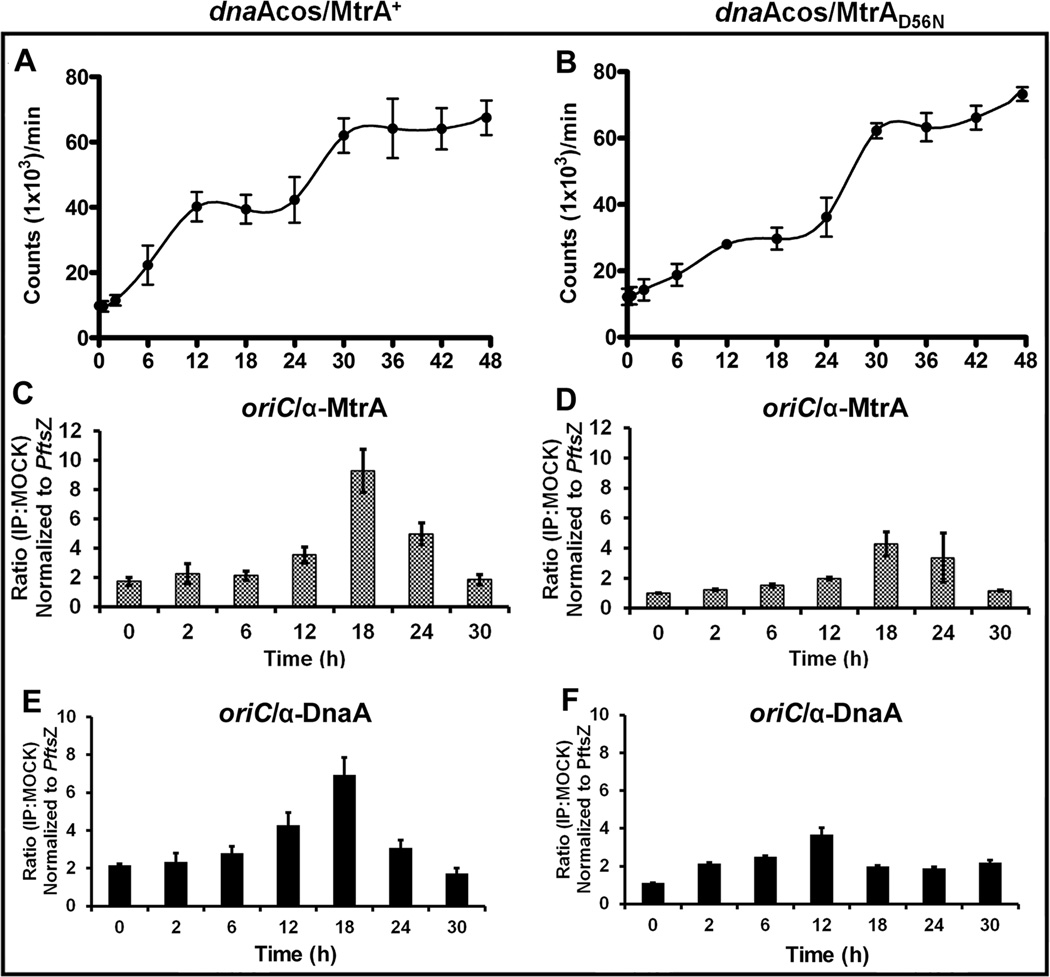
Next, we evaluated the consequences associated with the untimely MtrA~P access to oriC in the C period. Towards this end, we wished to create a M. tuberculosis strain producing the GoF MtrAY102C as the sole source for MtrA in the dnaAcos background by recombineering approach ((van Kessel & Hatfull, 2008), but met with limited success (not shown); presumably, unregulated MtrA~P owing to MtrAY102C production is toxic to M. tuberculosis growth and viability. As an alternative, we created and characterized a dnaAcos meropdiploid expressing mtrA from the tetracycline-inducible promoter (Ehrt et al., 2005). Addition of anhydrotetracycline (atc) at the time of initiation of synchronous replication led to elevated mtrA transcript and protein levels in both the C and D periods (Fig. S 7 A/B). The DNA synthesis pattern was, however, different from that seen with the dnaAcos (Fig. 5 A). A steady increase in DNA synthesis up to 30 h corresponding to nearly three rounds of DNA replication was noted; no additional increase in DNA synthesis beyond 30 h was observed (Fig. 5 A- compare with Fig. 3 A). Also, no lag or pre-replication period was detected under these conditions. The distinct inter-replication period, as seen with the dnaAcos, was absent (compare Fig. 5 A with 3A). These data suggest that elevated MtrAY102C allows untimely initiations. CFU analysis showed cell doubling by 30 h (Fig. S 8 -A).
Fig. 5.
DNA synthesis and oriC occupancy of MtrA and DnaA in dnaAcos/MtrAY102C. The inducer ‘atc’ was added at the time of initiation of synchronous replication (0 h) to overproduce MtrAY102C. (A). DNA synthesis determined as described under Fig. 3A. (B). ChIP-PCR showing the MtrA occupancy of oriC performed as described for Fig. 3B. (C) ChIP-PCR showing the DnaA occupancy of oriC as described for Fig. 3C.
ChIP-PCR analysis with MtrA antibodies revealed significant oriC enrichment from 12 h onwards, but detectable oriC enrichment (IP:Mock ratios 2 and high) also occurred at 2 and 6 h (Fig. 5 B) indicating that MtrAY102C accessed oriC, albeit modestly, in the C period and affected replication synchrony. These results indicate that DNA synthesis under MtrAY102C overproduction conditions is asynchronous and leads to eventual DNA synthesis arrest. OriC-DnaA ChIP experiments revealed that DnaA accessed oriC at all time periods, but increased access resulting in elevated enrichment was noted from 12 h onwards (Fig. 5 C compare with Fig. 3 C). These results are similar to those for MtrAY102C occupancy of oriC (Fig. 5 B). Perhaps, MtrA and DnaA interact (see below) and such interactions impact their associations with oriC (see below).
To examine whether the observed effects are specific to MtrAY102C, we overexpressed in the dnaAcos background either WT mtrA (dnaAcos/MtrA+) or phosphorylation-defective mtrA (dnaAcos/MtrAD56N). Addition of atc increased their expression levels to similar extents (Fig. S 9 A/B). The DNA synthesis profiles, however, revealed differences between the two. For example, the dnaAcos/MtrA+ showed the completion of two replication cycles by 12 h followed by a distinct D period and another burst of replication after 24 h (Fig. 6 A, see incorporation levels). Pre-replication or lag period, as observed for dnaAcos (Fig. 3 A) was not observed. Interestingly, the DNA synthesis pattern of the dnaAcos/MtrAD56N was similar to dnaAcos (Fig. 6 B). Together, these data indicate that MtrA overproduction can promote DNA synthesis in the C period provided that MtrA is competent for phosphorylation. Next, we examined the MtrA-occupancy of oriC. Similar to dnaAcos, maximal oriC-enrichment by MtrA was noted at 12, 18 and 24 h with both dnaAcos/MtrA+ and dnaAcos/MtrAD56N (Fig. 6 C and D, compare with Fig. 3 B). Despite stimulation of DNA synthesis upon MtrA overproduction (dnaAcos/MtrA+), significant oriC enrichment was not observed in the C period emphasizing that MtrA access to oriC is regulated. DnaA-occupancy of oriC in the dnaAcos/MtrA+ revealed oriC enrichment in both C and D periods with increased levels in the D period (Fig. 6 E). The dnaAcos/MtrAD56N DnaA-occupancy of oriC data were similar to that seen with dnaAcos (Fig. 6 F). CFU analysis revealed cell doubling by 30 h in both cases (Fig. S 8 -B and C). We infer from these data that overproduction of GoF MtrAY102C, which likely leads to elevated MtrA~P pools, promotes oriC access in the C period and promotes untimely initiations.
Fig. 6.
Characterization of dnaAcos/MtrA+ and dnaAcos/MtrAD56N. DNA synthesis in dnaAcos/MtrA+ (A) and dnaAcos/MtrAD56N (B). All experimental details are as described under Fig. 3A. MtrA occupancy of oriC in dnaAcos/MtrA+ (C) and dnaAcos/MtrAD56N (D). ChIP-PCR was performed following IP with MtrA antibodies as described under Fig. 3B. DnaA occupancy of oriC in dnaAcos/MtrA+ (E) and dnaAcos/MtrAD56N (F). ChIP-PCR was performed following IP with DnaA antibodies as described under Fig. 3C.
Aberrant MtrA~P impacts the MtrA-target expression
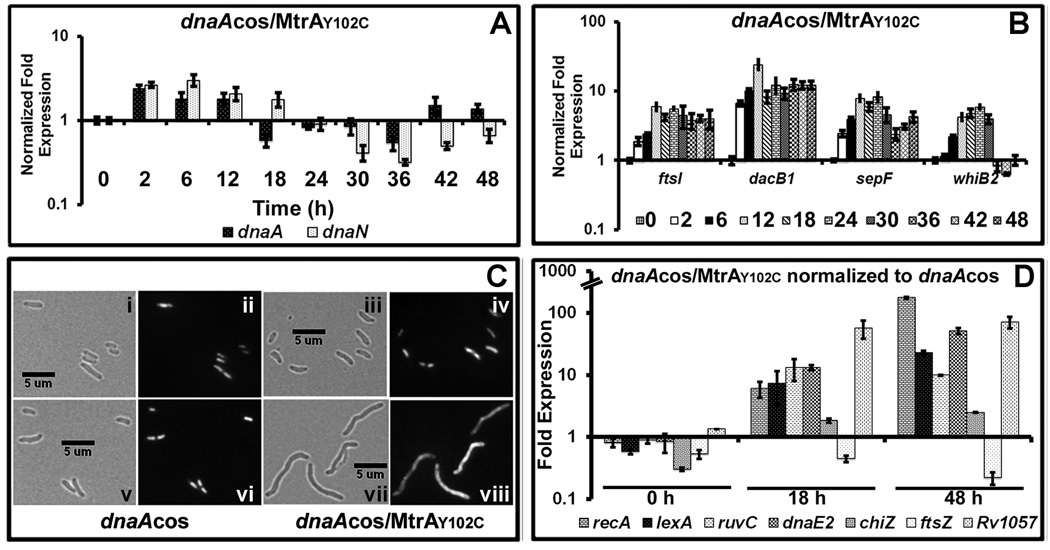
Next, we evaluated the MtrA-target expression in the dnaAcos/MtrAY102C during synchronous replication essentially as described for Fig. 4. The dnaA and dnaN expression levels were high till 12 h, but reduced significantly at 18 h and beyond (Fig. 7 A and compare with Fig. 4 C-i). While the reduced dnaA and dnaN expression levels correlate well with the blocked DNA synthesis, their expressions levels remained high till 12 h. Both oriC and PdnaA contain DnaA- and MtrA- boxes [see Fig. 1 A, S 4, (Fol et al., 2006, Li et al., 2010, Rajagopalan et al., 2010)]. Perhaps, MtrA ability to access these targets is governed by other proteins, e.g. DnaA, thereby delaying the MtrA~P-mediated transcriptional repression. We also found that the ftsI, dacB1, sepF and whiB2 expression in the dnaAcos/MtrAY102C was aberrant (Fig. 7 B, compare with Fig. 4 C-ii, note that sepF expression pattern was different indicating a complex regulation of its expression). Thus, elevated MtrA~P overproduction leads to aberrant target expression.
Fig. 7.
Characterization of dnaAcos/MtrAY102C: (A) qRT-PCR analysis of select MtrA targets. Total RNA was extracted from dnaAcos/mtrAY102C at indicated time points and fold expression relative to 16S rRNA was calculated as described in Fig. 4A. (B) qRT-PCR analysis of select cell-division targets ftsI, dacB1, sepF and whiB2 relative to 16S rRNA. (C) Cell morphology of dnaAcos and dnaAcos/MtrAY102C: The dnaAcos (panels i, ii, v, vi) and dnaAcos/MtrAY102C (panels iii, iv, vii, viii) cultures were visualized by microscopy at 0 h (i– iv) and 48 (v– viii) h after initiation of synchronous replication. Brightfield (i, iii, v, vii) and respective fluorescence images obtained following propidium iodide staining (ii, iv, vi, viii) are shown. (D) qRT-PCR analysis of SOS target gene expression. Total RNA from synchronously replicating cultures of M. tuberculosis dnaAcos and dnaAcos/MtrAY102C at indicated time points was extracted and the expression levels of recA, lexA, ruvC, dnaE2, chiZ ftsZ and Rv1057 were determined. Data were normalized to 16SrRNA and fold expression relative to 30°C was determined. Final fold expression was calculated by normalizing dnaAcos/MtrAY102C expression at 0, 18 and 48 h to the respective time points of dnaAcos.
Production of GoF MtrAY102C is associated with altered shape and induction of the SOS response
The cell morphology of dnaAcos and dnaAcos/MtrAY102C were similar at 0 h (see Fig. 7 C panels i/ii vs iii/iv). However, the dnaAcos/MtrAY102C cells at 48 h were elongated when compared to dnaAcos (Fig. 7 C, compare panel vii with v). Nucleoid staining by propidium iodide revealed that elongated cells contained unresolved nucleoids (Fig. 7 C, compare panel viii with vi). The cell morphology of dnaAcos/MtrA+ and dnaAcos/MtrAD56N was similar to that noted with the dnaAcos (data not shown). Blocked DNA synthesis leading to altered cell shape also implies stress and possible induction of the SOS response. The LexA-regulon and induction of the SOS response in M. tuberculosis have been well investigated (Rand et al., 2003, Smollett et al., 2012). Evaluation of the expression of select targets involved in the SOS response which are under LexA-RecA control, e.g. recA, lexA, ruvC, dnaE2, chiZ and LexA-dependent, but RecA-independent, e.g. Rv1057, in dnaAcos/MtrAY102C relative to dnaAcos revealed their upregulation at 18 and 48 h (Fig. 7 D). In contrast, ftsZ, which is not a member of the LexA-regulon, was not significant changed. Together, these results support the notion that the dnaAcos/MtrAY102C cells are stressed.
MtrA interacts with DnaA
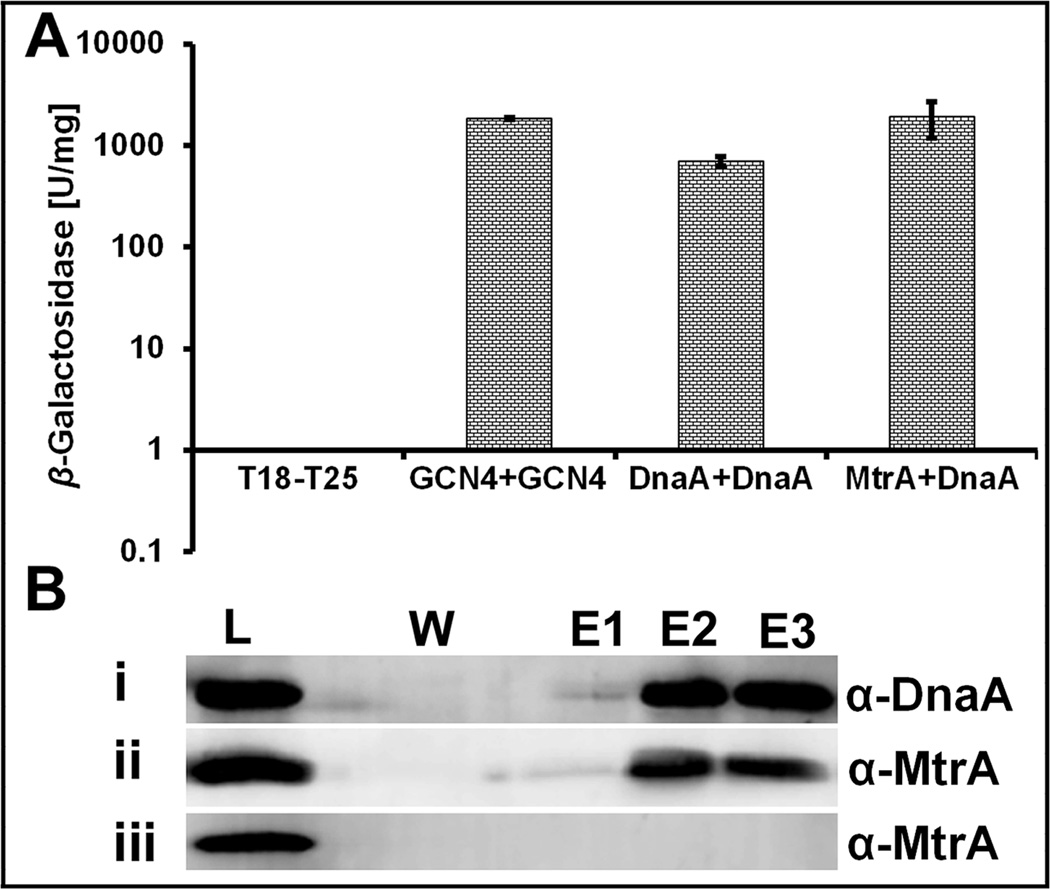
Given the effects of MtrA~P on oriC replication and regulation of dnaA transcription, we considered a possibility that MtrA interacts with DnaA. Consistent with this prediction, E. coli based bacterial-2-hybrid assays revealed pair-wise interactions between MtrA-DnaA and DnaA-DnaA, similar to the positive control GCN4-GCN4. The β-galactosidase activities of the DnaA-DnaA and MtrA-DnaA pairs were comparable to the positive control (Fig. 8 A). We also found interactions between MtrAD56N and DnaA (not shown). To further validate these interactions in mycobacterial host, we performed pull-down assays with recombinant M. smegmatis producing M. tuberculosis DnaA-His protein (see methodology for details). M. smegmatis lysate carrying DnaA-His was mixed with that overproducing MtrA, allowed to bind Ni-NTA resin, washed, the bound proteins were eluted and immunoblotted with DnaA and MtrA antibodies for detecting DnaA and MtrA, respectively. The fractions containing DnaA also contained MtrA indicating interactions between these two proteins (Fig. 8 B, panels i and ii). Controls showed that MtrA per se did not bind to the Ni-NTA resin (Fig. 8 B, panel iii). Together, these data confirm interactions between MtrA and DnaA.
Fig. 8. BACTH and Pull-down assays.
(A) BACTH analysis showing the interaction between MtrA and DnaA in E. coli. Strength of interactions is presented as β-galactosidase units per milligram protein as described under methods. GCN4-GCN4 is positive control. Mean ± SD values from three independent experiments are shown. (B) Immunoblots showing pull-down of DnaA and MtrA proteins. M. smegmatis lysates containing DnaA-His and MtrA were mixed and processed for pull-down reaction on Ni-NTA resin as described in text. Eluted proteins were examined following immunoblotting with DnaA (panel i) and MtrA (panel ii) antibodies. Pull-down reaction with M. smegmatis MtrA lysate was performed and eluted samples were probed with MtrA antibodies (panel iii). W refers to the sample collected after washing the resin 8× with buffer containing 20 mM imidazole whereas E1, E2 and E3 are elutions with 300 mM imidazole. L= load. Note - panel iii lane corresponding to W does not contain MtrA although wash sample 1 contained MtrA (data not shown).
MtrA does not bind M. smegmatis oriC
We reported earlier that among the four oriC MtrA-boxes, only the F2-box is conserved in M. smegmatis (Rajagopalan et al., 2010). This, combined with the near sequence identity of MtrA proteins and the conserved mtrAB regions, raises a question as to whether the M. smegmatis oriC is MtrA target and if so, whether the MtrA-mediated effects on oriC replication in M. smegmatis are similar to M. tuberculosis. The M. smegmatis counterpart of M. tuberculosis dnaAcos is not available. Nonetheless, to evaluate MtrA role in oriC replication, a series of experiments were carried out. First, we determined the M. smegmatis oriC plasmid transformation frequency, a measure for oriC plasmid replication. We found that M. smegmatis oriC plasmids with WT (Qin et al., 1997)) or mutant F2 box sequences (pEBM9, see Table S2) showed a similar transformation efficiency (~0.3 × 103 transformants/µg DNA). The respective oriC plasmids were also recovered from these transformants (data not shown). These results stand in stark contrast to the situation reported with M. tuberculosis oriC plasmids because oriC plasmids with mutations in the MtrA-boxes could neither be transformed nor recovered (Rajagopalan et al., 2010).
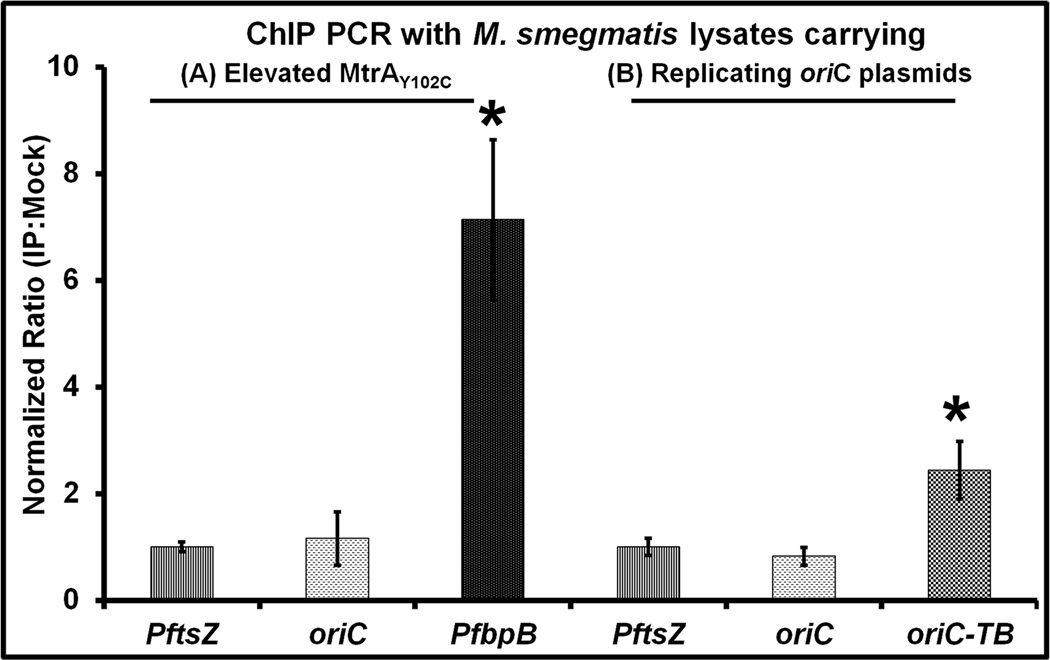
Second, we performed oriC ChIP-PCR experiments using MtrA antibodies with lysates prepared from M. smegmatis producing either normal (data not shown) or elevated MtrA levels and normalized data to PftsZ (Fig. 9 A). No oriC enrichment was detected (see Fig. 9 A). MtrA antibodies, however, enriched the MtrA-target promoters PfbpB (Fig. 9 A) and PripA (not shown, see (Plocinska et al., 2012)) under the same conditions indicating that the failure to enrich oriC is not due to technical limitations. OriC enrichment also did not occur with M. smegmatis containing extra copies of M. smegmatis oriC on a plasmid [Fig. 9 B, (Qin et al., 1999, Qin et al., 1997)], but significant oriC enrichment occurred with M. smegmatis harboring E. coli-Mycobacterium shuttle vector containing M. tuberculosis oriC sequence (Fig. 9 B, see oriC-TB lane). The use of shuttle plasmids was necessary for the maintenance of M. tuberculosis oriC sequences in M. smegmatis as the M. tuberculosis oriC is not functional in M. smegmatis (Qin et al., 1999). Thus, selective enrichment of M. tuberculosis oriC (Fig. 9 B, see lane oriC-TB), M. smegmatis PfbpB (Fig. 9A) and pripA (data not shown) by ChIP with MtrA antibodies in M. smegmatis lysates supports the notion that M. smegmatis oriC is not MtrA target, whereas M. tuberculosis oriC is a target. Finally, replacement of M. smegmatis oriC on the chromosome with oriC carrying mutant F2-box sequence by homologous recombination did not lead to any measurable defects in growth and viability under the experimental conditions investigated here (data not shown). Taken together, these results suggest that MtrA does bind M. smegmatis oriC.
Fig. 9.
ChIP-PCR studies with M. smegmatis lysates. (A) M. smegmatis producing MtrAY102C were processed for ChIP studies with MtrA antibodies essentially as described for Fig. 3C. Following the reversal of cross-links, PCR was performed for MtrA-targets oriC and PfbpB and the non-target PftsZ. (B) ChIP-PCR with M. smegmatis lysates carrying the M. smegmatis oriC (pMQ131) (Qin et al., 1997) and M. tuberculosis oriC (pMQ219) (Qin et al., 1999) in replicating plasmids.
Discussion
Although the whole genome sequence of M. tuberculosis was published over 14 years ago (Cole et al., 1998), the potential regulators of M. tuberculosis oriC replication have not been defined. Our studies connect the essential MtrA RR (Zahrt & Deretic, 2000) to the regulation of oriC replication and reveal that MtrA exerts regulatory effects on oriC DNA replication (see below). Furthermore, the MtrA-mediated regulatory effects are linked to its phosphorylation state (see below).
MtrA~P: negative regulator of oriC replication
oriC DNA binding experiments (Figs. 1, 2) combined with our earlier published footprinting data (Rajagopalan et al., 2010) support the notion that MtrA~P binds cooperatively to its binding sites in oriC. We showed that in synchronously replicating cells producing normal levels of MtrA~P, MtrA sequesters oriC (Fig. 3), represses dnaA and dnaN transcription and promotes ftsI, dacB1 and sepF transcription [Fig. 4 C, see also (Fol et al., 2006, Li et al., 2010)] in the D period. Our results also revealed that mtrA transcript and protein levels were similar in the C and D periods (Fig. 4). We infer from these data that pools of MtrA~P, hence MtrA~P to MtrA ratio, are elevated in the D period. Overproduction of GoF MtrAY102C advanced MtrA~P to the C period and enabled MtrA access to oriC (Fig. 5 B); this untimely oriC access, although stimulated DNA synthesis during the initial period (see below), led to eventual DNA synthesis blockage, defects in cell division, repression of dnaA and dnaN transcription (Figs. 5 A, 7). Together, these results are consistent with a hypothesis that MtrA~P acts as a negative regulator of oriC replication wherein it sequesters oriC and represses dnaA and dnaN transcription to prevent untimely initiations in replicating cells. The concept that pools of MtrA~P are elevated in the D period is not unreasonable because phosphorylation of MtrB sensor kinase (also referred to as activation), which is necessary for MtrA~P and MtrA-target expression, is promoted upon MtrB septal association (Plocinska et al., 2012). Presumably, viable septa necessary for promoting MtrB activation are abundant during or after the replication cycle, and once activated, MtrB~P promotes MtrA~P thereby elevating the ratios of MtrA~P to MtrA. This could in turn lead to oriC sequestration, repression of dnaA and dnaN transcription, stimulation of the expression of MtrA-targets ftsI, sepF, dacB1 critical for septum synthesis and cell division and optimal cell cycle progression; all of these events are impacted upon MtrAY102C overproduction. Thus, DNA replication and cell cycle progression in M. tuberculosis are governed, in part by oscillations in the MtrA phosphorylation levels.
Despite DnaA association with oriC, reinitiation did not take place in the D period (Fig. 3 C). One reason for this could be that the DnaA oligomerization state in the C and D periods is different, hence DnaA-oriC complexes in the D period are not proficient at initiating new rounds of replication. One caveat to this argument is that the nucleotide-bound states of DnaA during M. tuberculosis cell cycle progression are unknown. While both DnaA.ATP and DnaA.ADP forms bind oriC with similar affinity, only DnaA competent to bind and hydrolyze ATP is proficient for the formation of oligomeric complexes and oriC unwinding in vitro (Kumar et al., 2009, Madiraju et al., 2006, Yamamoto et al., 2002), and possibly for replication initiation in vivo (Madiraju et al., 2006). This makes us think that at least a majority of the DnaA bound to oriC in the C period is the DnaA.ATP form, hence competent for replication initiation. On the other hand, the non-overlapping arrangement of DnaA and MtrA boxes, the location of DUE relative to F2 box (Fig. 1A), combined with the observed interactions between DnaA and MtrA (Fig. 8) leads to an alternate hypothesis that DnaA helps load MtrA on oriC in the D period, and that MtrA binding to oriC and DnaA limits subsequent DnaA oligomerization, organization of DnaA.ATP-initiation complex competent for replication and or oriC unwinding in the D period. These possibilities are not mutually exclusive.
Our results showing that elevated ratios of MtrA~P to MtrA result in the repression of dnaA transcription are consistent with a recent report showing that PdnaA activity is increased in the lpqB mutant background containing decreased levels of MtrA~P (Nguyen et al., 2010). It is pertinent to note that dnaA transcription is shown to be elevated in M. tuberculosis overexpressing MtrA+ upon infection in monocyte-derived macrophages (Fol et al., 2006). Presumably, the regulation of dnaA transcription upon infection is rather complex, possibly involving the activities of hitherto uncharacterized regulators (Galagan et al., 2013).
MtrA~P: positive regulator of oriC replication
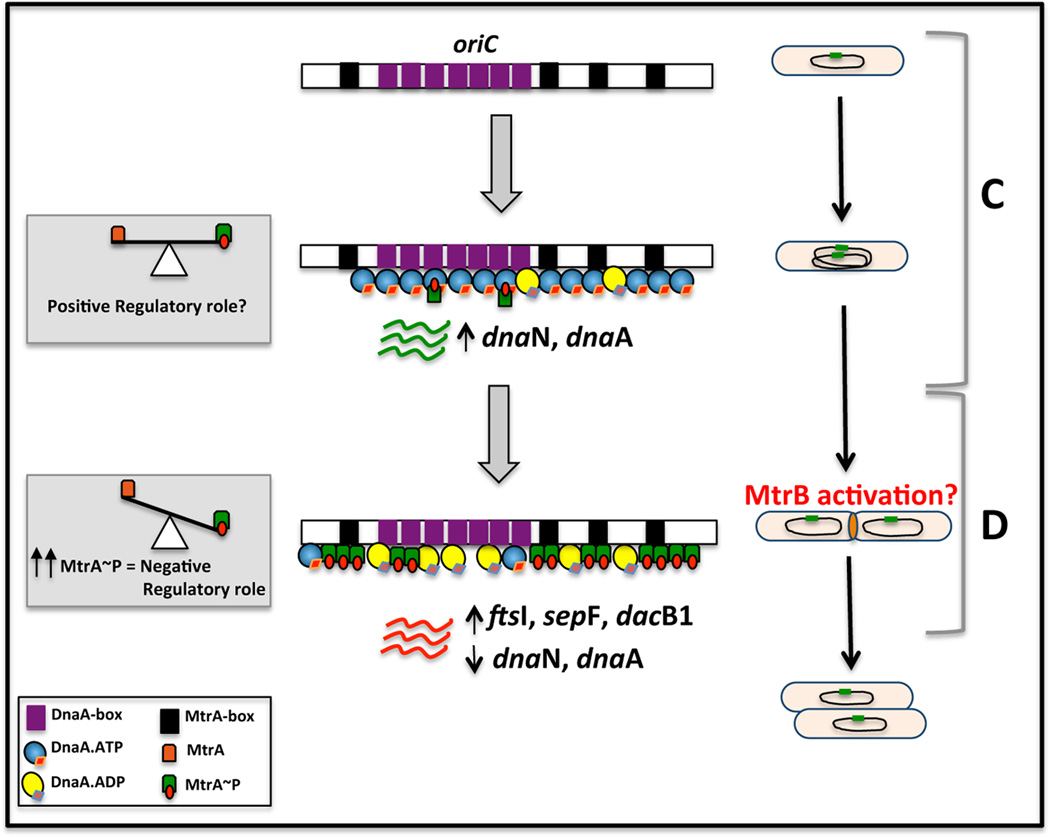
Stimulation of DNA synthesis without significant oriC enrichment upon MtrA+ overproduction (Fig. 6 A/C, compare with panel B/D) signals a possibility that MtrA~P exerts a positive regulatory effect on oriC replication. Our results showing little or no pre-replication period under MtrA+ overproduction conditions (Fig. 7 A/B) and severely compromised oriC plasmid transformation efficiency of oriC MtrA-box mutant plasmids (Rajagopalan et al., 2010) are in partial agreement of a concept that MtrA promotes oriC replication. Overproduction of GoF MtrAY102C also promoted DNA synthesis and suppressed the lag or pre-replication period (Fig. 5 A). While the precise mechanisms as to how MtrA~P exerts positive regulatory effect are unknown, it is likely that MtrA interactions with DnaA are important in this regard. One possibility is that MtrA remains associated with DnaA at the end of the D period despite its dissociation from oriC owing to a reduction in the MtrA~P pools, and facilitates increased DnaA oligomerization on oriC and the DnaA-mediated oriC initiation complex formation, perhaps analogous to the situation seen with DiaA protein of E. coli (Keyamura et al., 2007) and HobA of H. pylori (Zawilak-Pawlik et al., 2007). Another possibility is that MtrA/DnaA interactions promote the stability of DnaA, if any, thereby increase the intracellular pools of DnaA necessary for initiation. A consequence would be suppression of pre-replication period and stimulation of oriC replication. Nonetheless, we think that MtrA is an auxiliary factor of the M. tuberculosis replisome machinery in normally replicating cells, and that MtrA~P acts as a negative regulator in the D period and a positive regulator in the C period. A cartoon showing the MtrA~P mediated regulatory effects on oriC replication is shown (Fig. 10).
Fig. 10.
Cartoon showing the MtrA-mediated regulatory effects on oriC replication and cell cycle progression: M. tuberculosis oriC with MtrA-boxes (black) and DnaA-boxes (magenta) is shown to the left and cell cycle progression with C and D periods are shown to the right. Note the non-overlapping arrangement of the DnaA- and MtrA-binding sites in oriC implies that the binding of MtrA~P and DnaA to their respective binding sites proceed independently. MtrA activity, i.e. the ratio of MtrA to MtrA~P, is shown in equilibrium in the C period whereas shown altered with increased MtrA~P in the D period. MtrB sensor kinase activation is proposed occur in the D period, thereby trigger elevated MtrA~P. Accordingly, the transcriptions of dnaA and dnaN are shown elevated in the C period and reduced in the D period. Also, increased transcription of ftsI, dacB1, sepF are shown in the D period. Because the ATPase activity of DnaA is required for its rapid oligomerization on oriC (Madiraju et al., 2006) and oriC unwinding (Kumar et al., 2009), binding of both DnaA.ATP and DnaA.ADP forms to oriC in the C period are shown. Although significant oriC enrichment by MtrA does not occur in the C period, MtrA~P may also associate with DnaA. MtrA access to oriC in the D period is either direct and or could be aided by DnaA. A consequence of the negative regulatory role of MtrA~P (marked in the D period) would be the prevention of hyper-replication and promotion of regulated cell cycle progression. Reduction in MtrA~P pools at the end of D period could release MtrA from oriC; but MtrA~P may remain associate with DnaA to promote orisome assembly and another cycle of DnaA-mediated oriC replication. This positive regulatory role of MtrA~P could also involve interactions with other components of replisome machinery. However, the molecular details as to how these events occur are unknown. Nonetheless, the presumptive positive regulatory role is marked in the C period.
OriC sequestration and repression of dnaA and dnaN transcription in nonreplicating cells are likely the control mechanisms operating for limiting reinitiation in the D period. What then are the mechanisms for controlling reinitiation events in the C period? RIDA and datA like control mechanisms as described for E. coli and other bacteria (Katayama et al., 2010, Skarstad & Katayama, 2013) have not yet been described in mycobacteria. We propose that MtrA levels and phosphorylation activity are in part, responsible for controlling reinitiations in the C period. MtrB is the cognate sensor kinase that phosphorylates MtrA (Al Zayer et al., 2011, Plocinska et al., 2012). Thus, understanding the signals promoting MtrB activation, hence MtrA~P, during cell cycle will aid in unraveling the regulatory mechanisms impacting replication initiation. PdnaA, which contains MtrA- and DnaA- boxes, could exert another layer of control (Figs. S4 and 1 A), by acting as a sink to quench the active pools of DnaA remaining after initiation. Further studies are required to address these issues.
It is intriguing that M. smegmatis oriC is not a MtrA-target (Fig. 9). Also, the absence of F2-box did not affect M. smegmatis oriC plasmid transformation efficiency, growth and viability (see results). These data imply that either MtrA does not bind the lone F2 box of M. smegmatis oriC in vivo or the binding if any, has no biological consequences under the experimental conditions tested here. This begs two important but related questions: First, why is that the M. tuberculosis oriC evolved to be regulated by the MtrAB 2CRS? M. tuberculosis is a successful pathogen that can shift from an active multiplicative state to a chronic state in response to immune pressure. While the factors governing these processes are largely unknown, changes in the MtrA levels and its phosphorylation status are known to impact M. tuberculosis proliferation upon infection (Fol et al., 2006). Perhaps, regulation of the DnaA-mediated oriC replication along with other processes by the MtrA~P in response to immune/ environmental pressure is an adaptation strategy that M. tuberculosis uses for its optimal survival upon infection. Second, how is M. smegmatis oriC replication regulated? We suspect that either other yet to be identified proteins/factors and or MtrA interactions with DnaA and possibly other unidentified replisome components, contribute to the regulation of M. smegmatis oriC replication.
The MtrA-mediated regulation described here shares both similarities and differences with the known regulators in other organisms. For example, MtrA distinguishes itself from other regulators in that, unlike E. coli SeqA, the oriC sequestration process by MtrA is delayed until the D period (Skarstad & Katayama, 2013), and unlike Spo0A (Castilla-Llorente et al., 2006) and CtrA (Quon et al., 1996, Quon et al., 1998) RRs, MtrA targets dnaN and dnaA, in addition to affecting the expression of other genes (Fig. 4). On the other hand, like the B. subtilis Soj, SirA, and YabA proteins (Murray & Errington, 2008, Noirot-Gros et al., 2006, Wagner et al., 2009), and HP1021 of H. pylori (Donczew et al., 2015) MtrA interacts with DnaA. The dnaN gene location, which is often adjacent to oriC, is well conserved in several bacteria (Gao et al., 2013), and the E. coli and C. cresentus model organisms discussed here are exceptions. Also, the MtrAB 2CRS is conserved in high G+C rich actinobacteria such as Corynebacterium glutamicum, C. diphtheria and Streptomyces sps (Hoskisson & Hutchings, 2006). Thus, the MtrA-mediated regulation of oriC replication described here may extend to other eubacterial members having similar oriC organization and/or MtrA-like regulatory proteins.
Methods
Bacterial strains, culture conditions and molecular biology details
M. tuberculosis and M. smegmatis mc2155 strains (Table S2) were propagated at 37°C in Middlebrook 7H9 broth supplemented with oleic acid-albumin-dextrose-catalase (OADC) or ADC, respectively as described (Plocinska et al., 2012). Actively growing dnaAcos cultures were kept at 30°C for 30 h prior to shifting to 37°C for initiating synchronous replication as previously described (Nair et al., 2009). Where needed, dnaAcos strains carrying plasmids expressing mtrA and its mutant derivatives were induced with 50 ng/mL atc added at the time of initiation of synchronous replication. Bacterial growth was assayed by measuring changes in absorbance at 600 nm, and viability by determining colony-forming units. The sequences of oligonucleotide primers used for cloning are given in Table S1. For some experiments, M. smegmatis transformed with oriC plasmids were plated for determining transformation efficiency as described (Qin et al., 1999, Qin et al., 1997).
MtrA-mCherry and MtrAY102C proteins
Recombinant plasmid overexpressing his-mtrA--mCherry was created in three steps. First, mCherry coding region was amplified from pEB6 as XbaI-SwaI fragment using primers mCher_F_XbaI_lnkr and MR316_R_SwaI (Table S1). Second, gfp gene in the mtrA-gfp construct was replaced with mCherry (Table S2). Finally, the mtrA-mCherry coding region was amplified using primers MVM409F and mCher_R_XhoI (Table S1) and cloned into pET19b plasmid as NdeI-XhoI fragment for producing his-tagged protein. His-MtrA-mCherry was purified on Ni-NTA resin. Overproduction and purification of MtrAY102C-MBP was as described (Plocinska et al., 2012).
Chromatin immunoprecipitation (ChIP)-PCR
Formaldehyde cross-linked cultures of M. tuberculosis and M. smegmatis were used to perform ChIP-PCR using α-MtrA, α-DnaA or α-FtsZ antibodies, as described previously. The band intensities of each target were normalized to the non-target PftsZ and genes with normalized values of 2 and above were considered as potential targets of MtrA or DnaA (Plocinska et al., 2012, Rajagopalan et al., 2010).
DNA synthesis and Western analysis
DNA synthesis was quantitated by measuring the incorporation of 3H-uracil into alkali-stable DNA of uniformly labeled cells from samples collected in triplicate following initiation of synchronous replication as described (Nair et al., 2009). For Western analysis, cellular lysates of samples collected prior to and at various periods after the initiation of synchronous replication were prepared by bead beating and processed for immunoblotting with α-MtrA and α-SigA antibodies. Data were quantified using the volumetric analysis tool of the QuantityOne software and the MtrA/SigA ratios were determined as described (Fol et al., 2006, Plocinska et al., 2012).
Microscopy
Mtb dnaAcos and dnaAcos/MtrAY102C at 0 and 48 h after the initiation of synchronous replication were fixed in 4% paraformaldehyde for 24 h, washed 3 times with PBS followed by incubation with propidium iodide stain (Molecular Probes) for 30 min at room temperature and visualized by brightfield and fluorescent imaging as described (Chauhan et al., 2006).
Electrophoretic Mobility Shift Assay (EMSA)
A 526-bp fragment containing full-length oriC and 110-bp fragments bearing WT or mutant F2, F3, F4 and F5 MtrA-box motifs were amplified using 6-carboxyfluorescein (FITC)-labeled primers (Table S1) and WT (pMQ219) and mutant (pEBM12 and pEBM13) oriC plasmids (Table S2) as the templates. Mutant MtrA F2 box motif sequence TATATACcaTATATAT was as described (Rajagopalan et al., 2010). EMSA assays were performed with recombinant MtrA-mCherry (MtrA) and MtrAY102C-MBP preparations as described (Plocinska et al., 2012). Briefly, EnvZ was first autophosphorylated in a buffer containing 50 mM Tris-HCl, pH 7.5, 50 mM KCl, 20 mM MgCl2, 1 mM DTT, 1 mM ATP at 37°C for 5 min prior to using in MtrA and MtrAY102C transphosphorylation reactions. Next, FITC-labeled DNA (200 fmol) was incubated with increasing concentrations of phosphorylated or non-phosphorylated MtrA/MtrAY102C along with poly(dI/dC) and sheared salmon sperm DNA for 15 min. The DNA-protein complexes were resolved in 5% polyacrylamide gels at 4°C, gels were scanned with a Molecular Imager (Fx) and data were analyzed using QuantityOne software (Plocinska et al., 2012, Rajagopalan et al., 2010). The percent oriC bound was calculated by quantifying free DNA in each well after appropriate background correction and subtraction from control (no MtrA) lanes (QuantityOne, BioRad). Apparent KD and Hill-coefficient numbers were calculated using Prism 6 (Graphpad) software.
Solid phase oriC binding assays
Biotinylated oriC was PCR amplified using primers MVM257B/MVM1004 and template pMQ219 whereas biotinylated WT and mutant F2 MtrA-box sequences were amplified using primers MVM257B/MMtrF2R and templates pMQ219 and pMMR87, respectively (Rajagopalan et al., 2010). Approximately 10 µg biotinylated DNA and M270 Streptavidin magnetic beads were incubated with rocking for 3 h in buffer containing 5mM Tris-HCl, pH 7.5; 0.5 mM EDTA and 1M NaCl as per manufacturer’s protocol (Invitrogen). Beads were magnetically separated from unbound DNA, washed and 2 µl beads were incubated with indicated concentrations of MtrA or MtrAY102C in a 20 µl final volume at 37°C for 15 min. At the end of incubation, beads were collected, washed with 1× EMSA buffer, mixed with SDS-PAGE sample buffer and resolved by SDS-PAGE. Immunoblotting with α-MtrA was performed and the amount of bound MtrA was determined. As a control, PftsZ was amplified using biotinylated oligo (ftsZP_F) and ftsZP_R and processed as described above. MtrA~P/MtrA ratios were calculated for 1.176 and 2.32 µM following densitometric analysis of data from two independent experiments.
RNA extraction and qRT-PCR
Extraction of total RNA from guanidine thiocyanate-fixed cells of M. tuberculosis followed by quantitative Real-Time PCR (qRT-PCR) for select genes were performed as described (Plocinska et al., 2012, Rajagopalan et al., 2010). QRT-PCR was performed in triplicate from three independent experiments in Bio-Rad iQ™5 iCycler Real-Time PCR detection system using FAM-labeled 2× iQ SYBR Supermix (Bio-rad, Cat# 1708880) as described. The primers used for qRT-PCR are listed in Table S1. The threshold cycle (Ct) value for each gene of interest was normalized to the Ct value of 16S rRNA, and the fold expression was calculated (fold change = 2−Δ(ΔCt)) using the iQ™5 optical system software.
Protein-protein interaction assays: (i) Bacterial two hybrid (BACTH) assay
BACTH assays were performed using BATCH system kit (Euromedex) as described previously (Plocinski et al., 2012). The MtrA and DnaA proteins were expressed as C- terminal fusions to Bordetella pertussis T18 or T25 adenylate cyclase fragments in the E. coli BTH101 strain (Tables S2). The co-transformants were spotted on minimal media agar supplemented with 0.004% X-gal, 100 µg/ml Amp and 50 µg/ml Km (Karimova et al., 2005). The interaction strength was determined by measuring the extent of β-galactosidase activity in the broth-grown cultures (Plocinski et al., 2012). Beta-galactosidase activity of at least 5-fold or higher than that measured for E. coli BTH101 strain carrying single gene and empty vector was considered indicative of a positive interaction (Karimova et al., 2005). E. coli BTH101 transformants containing pKT25-GCN4 and pUT18C-GCN4 served as positive controls for complementation.
(2) Pull-down assays
Pull-down assays were performed to show interactions between DnaA and MtrA. Recombinant M. smegmatis bearing (pMMR41) or (pMG78) plasmids were induced with 0.2% acetamide for 6 hr to overproduce DnaA-His and MtrA respectively. The cell pellets were washed twice in 1× PBS, resuspended in lysis buffer containing 25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1mM EDTA, 0.5% NP-40, 2.5% glycerol, 1mM PMSF and zirconia beads, beaten for 10× 30 sec and spun at 13, 200 rpm for 10 min to collect supernatant. Five hundred µL each of DnaA-His and MtrA lysates was mixed, pre-incubated at 4°C for 2 h followed by incubation with Ni-NTA resin for I h at 4°C. The Ni-NTA resin containing DnaA-His and MtrA was loaded onto spin column, washed 8× with 250 µL of lysis buffer, eluted with 50 µL buffer containing 300 mM imidazole and the collected fractions were analyzed by immunoblotting following SDS-PAGE.
Supplementary Material
Acknowledgements
We thank Drs. Dorota Stankowska for help with RvY102C and Naresh Arora with M. smegmatis ChIP experiments, Drs. Rao Lella and Susan Howard for helpful discussions, Dr. Sabine Ehrt for pTet plasmid system. This work was supported by NIH grants AI 084734 (MM) and AI 048417 (MR).
Footnotes
All authors declare ‘no potential conflict of interest’.
References
- Al Zayer M, Stankowska D, Dziedzic R, Sarva K, Madiraju MV, Rajagopalan M. Mycobacterium tuberculosis mtrA merodiploid strains with point mutations in the signal-receiving domain of MtrA exhibit growth defects in nutrient broth. Plasmid. 2011;65:210–218. doi: 10.1016/j.plasmid.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla-Llorente V, Munoz-Espin D, Villar L, Salas M, Meijer WJ. Spo0A, the key transcriptional regulator for entrance into sporulation, is an inhibitor of DNA replication. EMBO J. 2006;25:3890–3899. doi: 10.1038/sj.emboj.7601266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Lofton H, Maloney E, Moore J, Fol M, Madiraju MV, Rajagopalan M. Interference of Mycobacterium tuberculosis cell division by Rv2719c, a cell wall hydrolase. Mol Microbiol. 2006;62:132–147. doi: 10.1111/j.1365-2958.2006.05333.x. [DOI] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Cooper S, Helmstetter CE. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968;31:519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dick T, Lee BH, Murugasu-Oei B. Oxygen depletion induced dormancy in Mycobacterium smegmatis. FEMS Microbiol Lett. 1998;163:159–164. doi: 10.1111/j.1574-6968.1998.tb13040.x. [DOI] [PubMed] [Google Scholar]
- Domian IJ, Quon KC, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- Donczew R, Makowski L, Jaworski P, Bezulska M, Nowaczyk M, Zakrzewska-Czerwinska J, Zawilak-Pawlik A. The atypical response regulator HP1021 controls formation of the Helicobacter pylori replication initiation complex. Mol Microbiol. 2015;95:297–312. doi: 10.1111/mmi.12866. [DOI] [PubMed] [Google Scholar]
- Ehrt S, Guo XV, Hickey CM, Ryou M, Monteleone M, Riley LW, Schnappinger D. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 2005;33:e21. doi: 10.1093/nar/gni013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fol M, Chauhan A, Nair NK, Maloney E, Moomey M, Jagannath C, Madiraju MV, Rajagopalan M. Modulation of Mycobacterium tuberculosis proliferation by MtrA, an essential two-component response regulator. Mol Microbiol. 2006;60:643–657. doi: 10.1111/j.1365-2958.2006.05137.x. [DOI] [PubMed] [Google Scholar]
- Friedland N, Mack TR, Yu M, Hung LW, Terwilliger TC, Waldo GS, Stock AM. Domain orientation in the inactive response regulator Mycobacterium tuberculosis MtrA provides a barrier to activation. Biochemistry. 2007;46:6733–6743. doi: 10.1021/bi602546q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, Minch K, Peterson M, Lyubetskaya A, Azizi E, Sweet L, Gomes A, Rustad T, Dolganov G, Glotova I, Abeel T, Mahwinney C, Kennedy AD, Allard R, Brabant W, Krueger A, Jaini S, Honda B, Yu WH, Hickey MJ, Zucker J, Garay C, Weiner B, Sisk P, Stolte C, Winkler JK, Van de Peer Y, Iazzetti P, Camacho D, Dreyfuss J, Liu Y, Dorhoi A, Mollenkopf HJ, Drogaris P, Lamontagne J, Zhou Y, Piquenot J, Park ST, Raman S, Kaufmann SH, Mohney RP, Chelsky D, Moody DB, Sherman DR, Schoolnik GK. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature. 2013;499:178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Luo H, Zhang CT. DoriC 5.0: an updated database of oriC regions in both bacterial and archaeal genomes. Nucleic Acids Res. 2013;41:D90–D93. doi: 10.1093/nar/gks990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009;15:211–214. doi: 10.1038/nm.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk B, Marczynski GT. Regulated degradation of chromosome replication proteins DnaA and CtrA in Caulobacter crescentus. Mol Microbiol. 2005;55:1233–1245. doi: 10.1111/j.1365-2958.2004.04459.x. [DOI] [PubMed] [Google Scholar]
- Hoskisson PA, Hutchings MI. MtrAB-LpqB: a conserved three-component system in actinobacteria? Trends Microbiol. 2006;14:444–449. doi: 10.1016/j.tim.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Karimova G, Dautin N, Ladant D. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J Bacteriol. 2005;187:2233–2243. doi: 10.1128/JB.187.7.2233-2243.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T, Ozaki S, Keyamura K, Fujimitsu K. Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC. Nat Rev Microbiol. 2010;8:163–170. doi: 10.1038/nrmicro2314. [DOI] [PubMed] [Google Scholar]
- Keyamura K, Fujikawa N, Ishida T, Ozaki S, Su'etsugu M, Fujimitsu K, Kagawa W, Yokoyama S, Kurumizaka H, Katayama T. The interaction of DiaA and DnaA regulates the replication cycle in E. coli by directly promoting ATP DnaA-specific initiation complexes. Genes Dev. 2007;21:2083–2099. doi: 10.1101/gad.1561207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Farhana A, Hasnain SE. In-vitro helix opening of M. tuberculosis oriC by DnaA occurs at precise location and is inhibited by IciA like protein. PLoS One. 2009;4:e4139. doi: 10.1371/journal.pone.0004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Han JS, Jeon Y, Hwang DS. The arc two-component signal transduction system inhibits in vitro Escherichia coli chromosomal initiation. J Biol Chem. 2001;276:9917–9923. doi: 10.1074/jbc.M008629200. [DOI] [PubMed] [Google Scholar]
- Li Y, Zeng J, Zhang H, He ZG. The characterization of conserved binding motifs and potential target genes for M. tuberculosis MtrAB reveals a link between the two-component system and the drug resistance of M. smegmatis. BMC Microbiol. 2010;10:242. doi: 10.1186/1471-2180-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A, Eleuterio M, Hutter B, Murugasu-Oei B, Dick T. Oxygen depletion-induced dormancy in Mycobacterium bovis BCG. J Bacteriol. 1999;181:2252–2256. doi: 10.1128/jb.181.7.2252-2256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju MV, Moomey M, Neuenschwander PF, Muniruzzaman S, Yamamoto K, Grimwade JE, Rajagopalan M. The intrinsic ATPase activity of Mycobacterium tuberculosis DnaA promotes rapid oligomerization of DnaA on oriC. Mol Microbiol. 2006;59:1876–1890. doi: 10.1111/j.1365-2958.2006.05068.x. [DOI] [PubMed] [Google Scholar]
- Murray H, Errington J. Dynamic control of the DNA replication initiation protein DnaA by Soj/ParA. Cell. 2008;135:74–84. doi: 10.1016/j.cell.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Nair N, Dziedzic R, Greendyke R, Muniruzzaman S, Rajagopalan M, Madiraju MV. Synchronous replication initiation in novel Mycobacterium tuberculosis dnaA cold-sensitive mutants. Mol Microbiol. 2009;71:291–304. doi: 10.1111/j.1365-2958.2008.06523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HT, Wolff KA, Cartabuke RH, Ogwang S, Nguyen L. A lipoprotein modulates activity of the MtrAB two-component system to provide intrinsic multidrug resistance, cytokinetic control and cell wall homeostasis in Mycobacterium. Mol Microbiol. 2010;76:348–364. doi: 10.1111/j.1365-2958.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- Noirot-Gros MF, Velten M, Yoshimura M, McGovern S, Morimoto T, Ehrlich SD, Ogasawara N, Polard P, Noirot P. Functional dissection of YabA, a negative regulator of DNA replication initiation in Bacillus subtilis. Proc Natl Acad Sci U S A. 2006;103:2368–2373. doi: 10.1073/pnas.0506914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plocinska R, Purushotham G, Sarva K, Vadrevu IS, Pandeeti EV, Arora N, Plocinski P, Madiraju MV, Rajagopalan M. Septal Localization of the Mycobacterium tuberculosis MtrB Sensor Kinase Promotes MtrA Regulon Expression. J Biol Chem. 2012;287:23887–23899. doi: 10.1074/jbc.M112.346544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plocinski P, Arora N, Sarva K, Blaszczyk E, Qin H, Das N, Plocinska R, Ziolkiewicz M, Dziadek J, Kiran M, Gorla P, Cross TA, Madiraju M, Rajagopalan M. Mycobacterium tuberculosis CwsA interacts with CrgA and Wag31 and the CrgA-CwsA complex is involved in peptidoglycan synthesis and cell shape determination. J Bacteriol. 2012 doi: 10.1128/JB.01005-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin MH, Madiraju MV, Rajagopalan M. Characterization of the functional replication origin of Mycobacterium tuberculosis. Gene. 1999;233:121–130. doi: 10.1016/s0378-1119(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Qin MH, Madiraju MV, Zachariah S, Rajagopalan M. Characterization of the oriC region of Mycobacterium smegmatis. J Bacteriol. 1997;179:6311–6317. doi: 10.1128/jb.179.20.6311-6317.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- Quon KC, Yang B, Domian IJ, Shapiro L, Marczynski GT. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci U S A. 1998;95:120–125. doi: 10.1073/pnas.95.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan M, Dziedzic R, Al Zayer M, Stankowska D, Ouimet MC, Bastedo DP, Marczynski GT, Madiraju MV. Mycobacterium tuberculosis origin of replication and the promoter for immunodominant secreted antigen 85B are the targets of MtrA, the essential response regulator. J Biol Chem. 2010;285:15816–15827. doi: 10.1074/jbc.M109.040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand L, Hinds J, Springer B, Sander P, Buxton RS, Davis EO. The majority of inducible DNA repair genes in Mycobacterium tuberculosis are induced independently of RecA. Mol Microbiol. 2003;50:1031–1042. doi: 10.1046/j.1365-2958.2003.03765.x. [DOI] [PubMed] [Google Scholar]
- Salazar L, Fsihi H, de Rossi E, Riccardi G, Rios C, Cole ST, Takiff HE. Organization of the origins of replication of the chromosomes of Mycobacterium smegmatis, Mycobacterium leprae and Mycobacterium tuberculosis and isolation of a functional origin from M. smegmatis. Mol Microbiol. 1996;20:283–293. doi: 10.1111/j.1365-2958.1996.tb02617.x. [DOI] [PubMed] [Google Scholar]
- Salazar L, Guerrero E, Casart Y, Turcios L, Bartoli F. Transcription analysis of the dnaA gene and oriC region of the chromosome of Mycobacterium smegmatis and Mycobacterium bovis BCG, and its regulation by the DnaA protein. Microbiology. 2003;149:773–784. doi: 10.1099/mic.0.25832-0. [DOI] [PubMed] [Google Scholar]
- Santi I, Dhar N, Bousbaine D, Wakamoto Y, McKinney JD. Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria. Nature communications. 2013;4:2470. doi: 10.1038/ncomms3470. [DOI] [PubMed] [Google Scholar]
- Skarstad K, Katayama T. Regulating DNA replication in bacteria. Cold Spring Harbor perspectives in biology. 2013;5:a012922. doi: 10.1101/cshperspect.a012922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 2003;16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smollett KL, Smith KM, Kahramanoglou C, Arnvig KB, Buxton RS, Davis EO. Global analysis of the regulon of the transcriptional repressor LexA, a key component of SOS response in Mycobacterium tuberculosis. J Biol Chem. 2012;287:22004–22014. doi: 10.1074/jbc.M112.357715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski D, Ginda K, Pioro M, Holowka J, Skut P, Jakimowicz D, Zakrzewska-Czerwinska J. Choreography of the Mycobacterium replication machinery during the cell cycle. mBio. 2015;6:e02125–e02114. doi: 10.1128/mBio.02125-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel JC, Hatfull GF. Efficient point mutagenesis in mycobacteria using single-stranded DNA recombineering: characterization of antimycobacterial drug targets. Mol Microbiol. 2008;67:1094–1107. doi: 10.1111/j.1365-2958.2008.06109.x. [DOI] [PubMed] [Google Scholar]
- Wagner JK, Marquis KA, Rudner DZ. SirA enforces diploidy by inhibiting the replication initiator DnaA during spore formation in Bacillus subtilis. Mol Microbiol. 2009;73:963–974. doi: 10.1111/j.1365-2958.2009.06825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne LG. Synchronized replication of Mycobacterium tuberculosis. Infect Immun. 1977;17:528–530. doi: 10.1128/iai.17.3.528-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Muniruzzaman S, Rajagopalan M, Madiraju MV. Modulation of Mycobacterium tuberculosis DnaA protein-adenine-nucleotide interactions by acidic phospholipids. Biochem J. 2002;363:305–311. doi: 10.1042/0264-6021:3630305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt TC, Deretic V. An essential two-component signal transduction system in Mycobacterium tuberculosis. J Bacteriol. 2000;182:3832–3838. doi: 10.1128/jb.182.13.3832-3838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilak A, Kois A, Konopa G, Smulczyk-Krawczyszyn A, Zakrzewska-Czerwinska J. Mycobacterium tuberculosis DnaA initiator protein: purification and DNA-binding requirements. Biochem J. 2004;382:247–252. doi: 10.1042/BJ20040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilak-Pawlik A, Kois A, Stingl K, Boneca IG, Skrobuk P, Piotr J, Lurz R, Zakrzewska-Czerwinska J, Labigne A. HobA--a novel protein involved in initiation of chromosomal replication in Helicobacter pylori. Mol Microbiol. 2007;65:979–994. doi: 10.1111/j.1365-2958.2007.05853.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.