Abstract
Hesperidin has been shown to possess a potential inhibitory effect on vascular formation in endothelial cells. However, the fundamental mechanism for the anti-angiogenic activity of hesperidin is not fully understood. In the present study, we evaluated whether hesperidin has anti-angiogenic effects in mouse embryonic stem cell (mES)-derived endothelial-like cells, and human umbilical vascular endothelial cells (HUVECs), and evaluated their mechanism via the AKT/mammalian target of rapamycin (mTOR) signaling pathway. The endothelial cells were treated with several doses of hesperidin (12.5, 25, 50, and 100 μM) for 24 h. Cell viability and vascular formation were analyzed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and tube formation assay, respectively. Alteration of the AKT/mTOR signaling in vascular formation was analyzed by western blot. In addition, a mouse aortic ring assay was used to determine the effect of hesperidin on vascular formation. There were no differences between the viability of mES-derived endothelial-like cells and HUVECs after hesperidin treatment. However, hesperidin significantly inhibited cell migration and tube formation of HUVECs (P<0.05) and suppressed sprouting of microvessels in the mouse aortic ring assay. Moreover, hesperidin suppressed the expression of AKT and mTOR in HUVECs. Taken together, these findings suggest that hesperidin inhibits vascular formation by blocking the AKT/mTOR signaling pathways.
Keywords: hesperidin, vascular formation, AKT/mTOR, HUVECs, mouse embryonic stem cells
INTRODUCTION
Angiogenesis is the formation of a mature blood vessel network through expansion and remodeling of the pre-existing vascular primordium. Blood vessel formation during angiogenesis involves the induction of new sprouts, coordinated and directed endothelial cell migration, and proliferation (1). It also plays an important role in the development of cancer (2). Therefore, the identification of anti-angiogenic agents with novel mechanisms of action is an important strategy for studying angiogenic processes, and discovering potential lead candidates for the development of new cancer drugs. The angiogenic signaling is significantly involved in the proliferation, migration, and invasion of endothelial cells (3) through the activation of several signaling pathways, such as extracellular-signal-regulated kinase (ERK) (4), c-Jun N-terminal kinases (JNK) (5), p38 mitogen-activated protein kinases (MAPK) (6), and AKT (7). Recently, Guru et al. (8) and Yang et al. (9) reported that the AKT/mammalian target of rapamycin (mTOR) signaling pathway plays an important role in hypoxia-inducible factor 1α/vascular endothelial growth factor mediated angiogenesis.
Embryonic stem (ES) cells have been used as a powerful tool for the study of vasculogenesis and angiogenesis, including angioblast differentiation, proliferation, migration, endothelial cell-cell adhesion, and vascular morphogenesis (10–12). In our previous study, the efficacy of mES/embryoid body (EB)-derived endothelial cells was demonstrated to be a useful tool to study endothelial cell biology and developmental processes according to natural products treatment (13,14).
Hesperidin, a flavanone glycoside found abundantly in citrus fruits, possesses a wide range of pharmacological properties, including potential anti-inflammatory and anti-cancer effects (15). It induces cell growth arrest and apoptosis in a large variety of cells, including colon and pancreatic cancer cells (16,17). However, the mechanisms underlying the potential anti-angiogenic activity of hesperidin are not fully understood.
Therefore, the objectives of the present study were to analyze the effects of hesperidin on vascular formation and microvessel sprouting in a mES-derived endothelial cell system. Also, the mechanism for the anti-angiogenic activity of hesperidin was evaluated via the AKT/mTOR signaling pathway analysis in HUVECs.
MATERIALS AND METHODS
Reagents
Hesperidin was purchased from Sigma-Aldrich Co. (St. Louis, Mo, USA). The compound was dissolved in 100% dimethyl sulfoxide (DMSO). A 100 mM/L stock solution of hesperidin was prepared and stored as small aliquots at −20°C until needed. We purchased 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), DMSO, gelatin, horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit antibodies from Sigma-Aldrich Co. Growth factor-reduced Matrigel was purchased from BD Biosciences (San Jose, CA, USA). The phospho-specific antibodies anti-p38, anti-SAPK/JNK, anti-PI3K, anti-AKT, anti- mTOR, anti-p70S6K, and the AKT inhibitor LY294002 were purchased from Cell Signaling Technology (Danvers, MA, USA). The HRP-conjugated β-actin, phospho-ERK, and platelet endothelial cell adhesion molecule (PECAM) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Mouse embryonic cell culture and endothelial differentiation
Mouse D3 ES cells [ATCC Cat. No. CRL-1934, American Type Culture Collection (ATCC), Rockville, MD, USA] were co-cultured with mitomycin C-treated mouse embryonic fibroblasts in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Hyclone, Ogden, UT, USA), 1,000 U/mL leukaemia inhibitory factor (Chemicon, Temecula, CA, USA), and basic ES medium components [50 U/mL penicillin and 50 μg/mL streptomycin (Invitrogen), 1% non-essential amino acids (Invitrogen) and 0.1 mM β-mercaptoethanol (Invitrogen)]. The hanging drop method (20 μL per drop; 1×105 cells/mL) was used to induce differentiation as previously described (14,18). The EBs were formed by incubating the hanging drop cultures for three days. The resulting EBs were transferred onto gelatin-coated chamber slides (Nunc, St. Louis, MO, USA) or 60 mm dishes to allow for attachment. Endothelial cell differentiation was induced in EBs by switching the culture conditions to medium containing endothelial basal medium-2 (EBM-2), 5% FBS, a growth factor cocktail, and ascorbic acid [endothelial growth medium-2 (EGM-2)-MV Bullet Kit; Lonza, Walkersville, MD, USA]. The cell culture and endothelial differentiation conditions for the mES cells followed the protocol in our previous publication (14, 19).
Endothelial cell culture
HUVECs were obtained from ATCC and cultured in EGM-2 supplemented with 10% FBS at 37°C in a 5% CO2 atmosphere. HUVECs at passages three to five were used in the experiments. The commercially available vascular endothelial cell-specific supplement EGM-2 MV Bullet Kit (Lonza) was used (13).
Cell viability assay
Cell viability was assessed by an MTT assay. The growth inhibition activity in cultured mES-derived endothelial-like cells was determined using MTT assays as previously described (14,18). Briefly, cells were seeded at a density of 5,000 cells/well into 96-well plates. On differentiation day 10, the cells were exposed to various concentrations of hesperidin for 24 h. After incubation, MTT solution was added, and the cells were incubated for an additional 4 h. The resulting formazan was dissolved in DMSO, and the absorbance was detected at 570 nm with a VersaMax ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA). The effect of hesperidin on cell viability was calculated as percentages relative to the solvent-treated control. HUVECs (5×103 cells/well) were seeded into a 96-well plate with EGM-2 medium supplemented with 10% FBS. After the incubation for 24 h, the culture medium was removed, and the cells were rinsed twice with phosphate buffered saline (PBS) and then incubated with serum-free medium for 12 h. Following serum starvation, the cells were cultured in fresh 2% FBS medium containing various concentrations of hesperidin at 37°C for 24 h. After the incubation, an MTT solution was added, and the plate was incubated for an additional 4 h. The resulting formazan deposit was dissolved with DMSO, and the absorbance was detected at 570 nm with a VersaMax ELISA microplate reader (Molecular Devices).
Cell cycle analysis
To determine the level of cell cycle arrest following hesperidin exposure for 24 h during the differentiation of mES cells into endothelial cells, the cells were treated with various concentrations (0 to 50 μM) of hesperidin for 24 h. The cells were harvested (trypsinization and centrifugation) and fixed with 70% ethanol overnight at 4°C. After washing, the cells were subsequently stained with 50 μg/mL of propidium iodide (PI) and 50 μg/mL of RNase A for 1 h in the dark and then subjected to flow cytometry analysis in order to determine the percentage of cells at specific cell cycle phase. Flow cytometry analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA). Events were evaluated for each sample and the cell cycle distribution was analyzed using Cell Quest software (Becton Dickinson). The results were presented as the number of cells versus the amount of DNA as indicated by fluorescence signal intensity. All the experiments were conducted three times.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Cells were dissolved using Trizol® (Invitrogen) and total RNA was extracted according to the manufacturer’s protocol. The total cellular RNA was quantified using a NanoDrop spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA). Reverse transcription was performed using 2 μg of purified total RNA and the SuperScript First-Strand Synthesis System (Invitrogen). The synthesized cDNAs were amplified by PCR. The primers used for RT-PCR are listed in Table 1. The thermal cycling parameters were as follows: 5 min at 94°C, 30 amplification cycles (denaturation at 94°C for 30 s, annealing at 55~60°C for 30 s, and extension at 72°C for 30 s), and a final extension at 72°C for 5 min. The amplified products were separated on 1.5% agarose gels. The gels were stained with SYBR® Gold staining solution (Invitrogen) and visualized by UV transillumination (GelDoc™ XR, BioRad Laboratories, Inc., Hercules, CA, USA).
Table 1.
Sequences of oligonucleotide primers used for RT-PCR analysis
| Gene | Primer sequences | Product size (bp) | |
|---|---|---|---|
| PECAM | Forward | 5′-CCATCATGGGAGGTGATGAA-3′ | 278 |
| Reverse | 5′-GATACGCCATGCACCTTCAC-3′ | ||
| GAPDH | Forward | 5′-GGAGCCAAAAGGGTCATCAT-3′ | 212 |
| Reverse | 5′-GTGATGGCATGGACTGTGGT-3′ | ||
Immunocytochemistry
Cells were plated onto confocal dishes and induced to differentiate into endothelial-like cells by incubation in EGM-2 medium for 10 days. After the cells were treated with various concentrations (0 to 50 μM) of hesperidin for 24 h, the cells were fixed with 4% paraformaldehyde. The cells were blocked with blocking solution containing 1% bovine serum albumin (BSA)/PBS for 30 min and then incubated with rat anti-mouse PECAM (1:200) (Santa Cruz) overnight at 4°C. After being washed, the cells were incubated with Alexa Fluor 594-labeled chicken anti-rat IgG (1:1,000) (Invitrogen). After staining, the cover slips were mounted with medium containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA). Confocal laser-scanning microscopy was performed using a Leica TCS SP2 laser-scanning spectral confocal microscope and Carl Zeiss LSM 710 NLO (Carl Zeiss, Oberkochen, Germany). Data were acquired and analyzed with Leica confocal software.
Scratch-wound migration assay
HUVECs were allowed to grow to full confluence in 6-well plates pre-coated with 0.1% gelatin and then incubated with 10 mg/mL mitomycin C (Sigma-Aldrich Co.) at 37°C in a 5% CO2 atmosphere for 2 h to inactivate the HUVECs. Monolayers HUVECs were wounded by scratching with a 0.2-mL pipette tip. Fresh medium containing various concentrations of hesperidin was added. Images were taken with an inverted phase contrast light microscope (Olympus Optical Co., Ltd., Tokyo, Japan) after 24 h incubation. The migrated cells were then counted from three randomly selected fields under an optical microscope at 200× magnification. The migrated cells were quantified by manual counting (DMC advanced program), and the inhibition was calculated as a percentage relative to control.
Tube formation assay with HUVECs on Matrigel
Matrigel (70 μL/well) was added to a 96-well plate and polymerized for 30 min at 37°C. The HUVEC (3×104 cells) were seeded onto each well of the Matrigel-coated 96-well plate and then incubated in 2% FBS-EBM-2 with various concentrations of hesperidin. After 8 h of incubation, the formation of endothelial cell tubular structure was visualized under an inverted microscope and photographed at 40× magnification. Subsequently, tube formation was quantified by calculating the tube length and was expressed as a percentage by normalization with untreated control cells.
Western blot analysis
Cells were treated with hesperidin for 24 h. Harvested cells were lysed in protein extraction solution (Intron Biotechnology, Inc., Gyeonggi, Korea) containing protease inhibitors and phosphatase inhibitors for 10 min at 4°C. The total protein concentration in the supernatants was measured by the Bradford assay. After heating at 95°C for 5 min, total protein samples (40 μg) were subjected to 6~15% SDS-PAGE. The proteins were transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA) at 100 V for 60~100 min. The membranes were incubated with 5% BSA in TBST (TBS with 0.05% Tween 20) for 30 min at room temperature and then with primary antibodies diluted (1:200~1:1,000) in 5% BSA in TBST overnight at 4°C. The membranes were washed three times with TBST and incubated with the corresponding secondary antibodies. Protein bands were detected using an enhanced chemiluminescence detection kit (Intron Biotechnology, Inc.) and an LAS-1000 Imager (Fuji Film Corp., Tokyo, Japan).
Mouse aortic ring assay
The mouse aortic ring assay was performed as previously described (20). Forty-eight-well plates were covered with 150 μL of Matrigel and then incubated at 37°C and 5% CO2 for 30 min. The aortas isolated from mice (Central Laboratory Animal Inc., Seoul, Korea) were cleaned of periadventitial fat and connective tissue and cut into 1~1.5 mm long rings. After rinsing with PBS, the aortas were placed in the Matrigel-covered wells and covered with an additional 200 μL of Matrigel. The artery rings were cultured in 1 mL of EGM without serum for 24 h, and then the medium was replaced with 1 mL of EGM containing supplements with vehicle or hesperidin (25, 50, or 100 μM). The medium was replaced every 2 days with medium that had the same composition as described above. After 7 days, the microvessel growth was measured by taking photographs with an Olympus inverted microscope (40× objective). The length of the capillary was estimated using a phase-contrast microscope by measuring the distance from the cut end of the aortic segment to the approximate middle point of the capillary. The length of the capillary was measured using Adobe Photoshop software (DMC advanced program). Each value represents the average of 3~4 culture samples.
Statistical analysis
The results are expressed as the mean±SD. Statistical significance was determined using a one-way analysis of variance (ANOVA) and Student’s t-test for paired data. A P-value of <0.05 was considered statistically significant. The calculations were performed using SPSS for Windows Version 10.0 (SPSS, Chicago, IL, USA).
RESULTS
The effect of hesperidin on endothelial cell viability
To determine the anti-angiogenic activity of hesperidin, we first evaluated whether hesperidin inhibits the viability of mES-derived endothelial-like cells and HUVECs using the MTT assay. To determine the non-cytotoxic concentration of hesperidin against endothelial cells, the cells were initially treated with hesperidin (0~100 μM) for 24 h. In particular, concentrations of hesperidin greater than 100 μM significantly (P<0.05) decreased cell viability in mES-derived endothelial-like cells (Fig. 1A). Although the viability of HUVECs was decreased by treatment with 100 μM hesperidin, this was not significant when compared to the control cells (Fig. 1B). Therefore, further studies of hesperidin’s biological activities were carried out at less than or equal to 100 μM hesperidin to avoid HUVEC cytotoxicity.
Fig. 1.
The effects of hesperidin on cell viability of mES-derived endothelial-like cells (A) and HUVECs (B). The mES-derived endothelial-like cells and HUVECs were treated with hesperidin (0~100 μM) for 24 h. Cell viability is expressed as the percentage of viable cells cultured in the absence of hesperidin and is expressed as the mean±SD. *P<0.05 compared to control.
Suppression of the endothelial biomarker PECAM expression in cultured mES-derived endothelial-like cells
To further confirm the concentration ranges of hesperidin without cytotoxicity in the differentiated endothelial-like cells, the cells were treated with hesperidin (0~50 μM) for 24 h and the cell cycle was analyzed. The cell cycle of mES-derived endothelial-like cells in the presence of hesperidin was measured using flow cytometry. The cells were harvested 24 h after treatment with hesperidin at various concentrations and analyzed for their cell cycle distributions (sub-G1, G0/G1, S, and G2/M). As shown in Fig. 2, the cell cycle arrest of mES-derived endothelial-like cells was not significantly changed by hesperidin in a concentration-dependent manner. Therefore, further analysis of the biological activities of hesperidin was performed at less than 50 μM in the differentiated endothelial-like cells. To further investigate the relationship between hesperidin’s inhibitory effects on growth and its suppression of endothelial biomarker expression, we examined the expression of PECAM, a representative endothelial biomarker, in mES-derived endothelial-like cells (Fig. 3). Once endothelial cells differentiated from mES cells, they were treated with hesperidin (0~50 μM) for 24 h. The expression of PECAM was easily detected by immunofluorescence (control), but 50 μM of hesperidin suppressed the expression of PECAM in a 2-dimensional (2D) culture (Fig. 3B).
Fig. 2.
The effects of hesperidin on the cell cycle in mES-derived endothelial-like cells. Cells were treated with hesperidin (0~50μM) for 24 h, stained with PI and then analyzed on a FACSCalibur flow cytometer. Quantitation of the PI staining data is presented as the percentages of cell cycle distribution. They are expressed as the mean±SD.
Fig. 3.
The effects of hesperidin on the vascularization of mES-derived endothelial-like cells. The mES cells were differentiated into endothelial cells for 10 days and then treated with hesperidin (0~50 μM) for 24 h. (A) Expression of PECAM mRNA in the mES-derived endothelial-like cells was detected by RT-PCR analysis. GAPDH was used as an internal control. (B) The mES-derived endothelial-like cells were exposed to hesperidin (0 and 50 μM) after 10 days of differentiation for 24 h. The mES-derived endothelial-like cells were stained with an antibody directed against the endothelial cell biomarker PECAM using immunofluorescence. Cell nuclei were stained with DAPI. Magnification 40×.
Inhibition of endothelial cell migration and tube formation by hesperidin
Endothelial cell migration and tube formation are essential steps in angiogenesis. We therefore determined the effects of hesperidin on endothelial cell migration using wound healing migration assays in vitro. As shown in Fig. 4A, C, wound healing by migrating HUVECs was almost complete after 24 h of incubation, but hesperidin treatment inhibited the migration of the endothelial cells in a concentration-dependent manner. In particular, HUVECs migration was significantly suppressed by treatment with 100 μM hesperidin (P<0.01). Furthermore, it is well known that endothelial cells are able to spontaneously form capillary-like networks in a Matrigel in vitro (21). Capillary-like tube formation that depends on maturation of migrated endothelial cells is also involved in the early steps of angiogenesis. To determine whether hesperidin suppresses tube formation, we examined the spontaneous tube formation that occurs upon incubation of HUVECs in Matrigel in the presence of hesperidin. Hesperidin treatment significantly inhibited capillary-like network formation by cultured HUVECs in a concentration-dependent manner (P<0.01) (Figs 4B, C).
Fig. 4.
The effects of hesperidin on the migration and capillary structure formation of HUVECs. (A) Cells were grown to confluency in six-well plates, wounded, and treated with the indicated concentrations of hesperidin. (B) Cells were placed in 96-well plates coated with Matrigel. After 4~8 h in the absence and presence of hesperidin, the tubular structures were photographed. The migrated cells were quantified by manual counting. (C) The calculation of cell number for migrated and tube formations in HUVECs depend on hesperidin treatment. The results are reported as the mean±SD. *P<0.05, **P<0.01 versus control cells.
Suppression of AKT/mTOR signaling by hesperidin in HUVECs
To further understand the molecular basis of the hesperidin-mediated anti-angiogenic activity, we investigated cellular signaling pathways in HUVECs. As shown in Fig. 5A, hesperidin treatment (0~100 μM) in HUVECs did not induce the activation of all MAPK signaling pathways, including ERK, JNK, and p38. It is well known that activation of the AKT/mTOR pathway contributes to migration of endothelial cells in angiogenesis (22). We found that hesperidin treatment suppressed AKT activation, leading to suppression of the activation of mTOR and its downstream effector p70S6K (Fig. 5B). The effects of hesperidin on the AKT signaling pathway were further confirmed using co-treatment with hesperidin and the AKT inhibitor LY294002. As shown in Figs 5C, D, co-treatment with hesperidin and LY294002 resulted in greater suppression of AKT activity than either inhibitor alone.
Fig. 5.
The effect of hesperidin on MAPK and AKT/mTOR signaling. Cells were treated with the indicated concentrations of hesperidin for 24 h and the MAPK (A) and AKT/mTOR (B) protein expression analyzed by western blotting. (C, D) The cells were treated with 50 μM of the AKT inhibitor LY294002 with or without hesperidin (at 0 or 100 μM). β-actin was used as an internal control. The statistical analysis was performed using a Student’s t-test. *P<0.05, **P<0.01 compared to control.
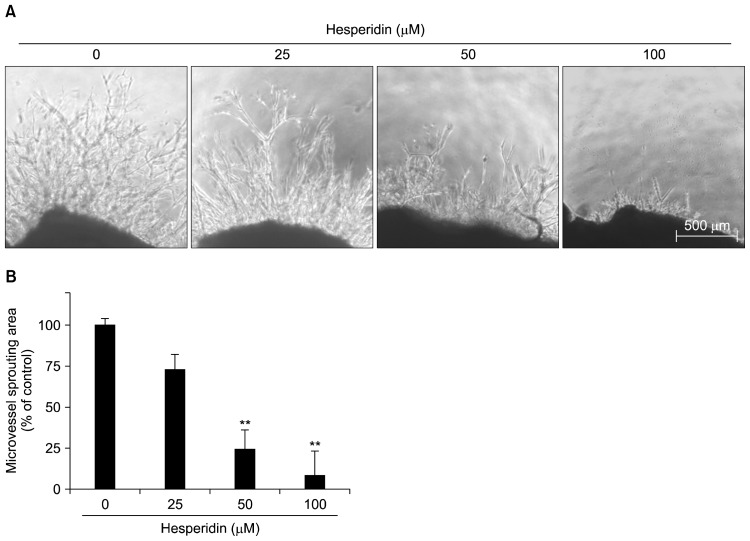
Suppression of capillary sprouting by hesperidin in a mouse aortic ring assay
A mouse aortic ring assay was used to investigate the effect of hesperidin on capillary sprouting/vascular formation. This ex vivo assay system is widely used as an effective tool for evaluating the anti-angiogenic activity of test compounds in a complex system in which endothelial cells, fibroblasts, pericytes, and smooth muscle cells interact (23). As shown in Fig. 6, treatment with hesperidin significantly and dose-dependently suppressed the outgrowth of microvessels from the aortic rings (P<0.01).
Fig. 6.
The effect of hesperidin on microvessel outgrowth arising from mouse aortic rings. Aortic rings isolated from mice were embedded in Matrigel in 48-well plates and then fed medium containing various concentrations of hesperidin for 7 days. Representative photographs of three independent experiments are shown. The microvessel length was measured on day 7 of culture. The values are the means±SD (n=3), and **P<0.01 versus control cells.
DISCUSSION
In the present study, we demonstrated that hesperidin inhibits vascular formation in mES-derived endothelial-like cells both in vitro and in an ex vivo system through decreased AKT/mTOR signaling in HUVECs.
Flavonoids are polyphenolic compounds present in plants with several potentially beneficial effects on human health (24). Accumulated evidence shows that these compounds have multiple modes of anti-cancer activities (25). Phytochemical-mediated anti-angiogenic intervention is an upcoming area of research that promises an effective cancer prevention strategy. Several phytochemicals have been shown to target tumor angiogenesis using in vitro and in vivo model systems (26–29). Angiogenesis is the formation of new blood vessels from the endothelium of the existing vasculature; the inhibition of angiogenesis is associated with a significant delay in tumor growth and migration (30,31). Thus, anti-angiogenic therapy is currently one of the most promising and efficient therapies against cancer (32).
Hesperidin possesses notable anti-proliferative activity in cancer cells (16,17). However, we have demonstrated the anti-angiogenic effect of hesperidin in modest concentration ranges that do not induce cytotoxicity of endothelial cells (Fig. 1). Hesperidin suppressed the expression of PECAM in the mES-derived endothelial-like cells (Fig. 3). This finding suggests that the anti-angiogenic activity of hesperidin in vascular formation could be associated with the suppression of PECAM expression in endothelial cells. Hesperidin also has a potential for anti-angiogenic activity in HUVECs via the suppression of AKT/mTOR signaling (Fig. 5).
There are several kinds of signaling pathways involved in angiogenesis. For example, MAPK signaling is considered one of the critical molecular events in the growth, survival, and migration of vascular endothelial cells in angiogenesis (5,6). Also, the AKT kinases are activated in endothelial cells by a variety of stimuli (33), and they regulate multiple critical steps by phosphorylating different downstream substrates, such as mTOR (34). The mTOR kinases, central regulators of cell metabolism, growth, proliferation, and survival (35,36), are activated during various cellular processes, such as tumor initiation, progression, and angiogenesis. Based on our data, hesperidin did not induce the activation of all MAPK signaling pathways. However, the inhibitory effects of hesperidin were closely associated with the suppression of AKT/mTOR in HUVECs (Fig. 5). This finding may uncover a possible mechanism of action for the anti-angiogenic activity of hesperidin in endothelial cells.
In conclusion, we systematically demonstrated that hesperidin potently inhibits sprouting of microvessels in mES-derived endothelial-like cells in vitro as well as in an ex vivo aortic ring model by blocking the AKT/mTOR signaling pathways. To our knowledge, this is the first report to evaluate the anti-angiogenic activity and mechanism of action of hesperidin in endothelial cells depends on vascular differentiation derived from mES cells. These findings provide an understanding of the mechanisms involved in the modes of action of hesperidin, and help to develop a new drug for the targeting of angiogenesis in cancer therapy.
ACKNOWLEDGEMENTS
This work was supported by Kyungnam University Foundation Grant, No 20150096.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev. 2009;19:476–483. doi: 10.1016/j.gde.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Gordon MS, Mendelson DS, Kato G. Tumor angiogenesis and novel antiangiogenic strategies. Int J Cancer. 2010;126:1777–1787. doi: 10.1002/ijc.25026. [DOI] [PubMed] [Google Scholar]
- 3.Cristi E, Perrone G, Toscano G, Verzì A, Nori S, Santini D, Tonini G, Vetrani A, Fabiano A, Rabitti C. Tumour proliferation, angiogenesis, and ploidy status in human colon cancer. J Clin Pathol. 2005;58:1170–1174. doi: 10.1136/jcp.2004.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, Tabayashi K, Shibuya M, Sato Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19:2138–2146. doi: 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- 5.Meadows KN, Bryant P, Vincent PA, Pumiglia KM. Activated Ras induces a proangiogenic phenotype in primary endothelial cells. Oncogene. 2004;23:192–200. doi: 10.1038/sj.onc.1206921. [DOI] [PubMed] [Google Scholar]
- 6.Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc Res. 2006;69:512–519. doi: 10.1016/j.cardiores.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 8.Guru SK, Pathania AS, Kumar S, Ramesh D, Kumar M, Rana S, Kumar A, Malik F, Sharma PR, Chandan BK, Jaglan S, Sharma JP, Shah BA, Tasduq SA, Lattoo SK, Faruk A, Saxena AK, Vishwakarma RA, Bhushan S. Secalonic acid-D represses HIF-1α/VEGF mediated angiogenesis by regulating the Akt/mTOR/p70S6K signaling cascade. Cancer Res. 2015;75:2886–2896. doi: 10.1158/0008-5472.CAN-14-2312. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Cong H, Han C, Yue L, Dong H, Liu J. 12-Deoxyphorbol 13-palmitate inhibits the expression of VEGF and HIF-1α in MCF-7 cells by blocking the PI3K/Akt/mTOR signaling pathway. Oncol Rep. 2015;34:1755–1760. doi: 10.3892/or.2015.4166. [DOI] [PubMed] [Google Scholar]
- 10.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 11.Risau W, Sariola H, Zerwes HG, Sasse J, Ekblom P, Kemler R, Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988;102:471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Claesson-Welsh L. Embryonic stem cell models in vascular biology. J Thromb Haemost. 2009;7:53–56. doi: 10.1111/j.1538-7836.2009.03427.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim GD, Kim GJ, Seok JH, Chung HM, Chee KM, Rhee GS. Differentiation of endothelial cells derived from mouse embryoid bodies: a possible in vitro vasculogenesis model. Toxicol Lett. 2008;180:166–173. doi: 10.1016/j.toxlet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Kim GD, Bae SY, Park HJ, Bae K, Lee SK. Honokiol inhibits vascular vessel formation of mouse embryonic stem cell-derived endothelial cells via the suppression of PECAM and MAPK/mTOR signaling pathway. Cell Physiol Biochem. 2012;30:758–770. doi: 10.1159/000341455. [DOI] [PubMed] [Google Scholar]
- 15.Benavente-García O, Castillo J. Update on uses and properties of Citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 16.Park HJ, Kim MJ, Ha E, Chung JH. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine. 2008;15:147–151. doi: 10.1016/j.phymed.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 17.Patil JR, Chidambara Murthy KN, Jayaprakasha GK, Chetti MB, Patil BS. Bioactive compounds from Mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J Agric Food Chem. 2009;57:10933–10942. doi: 10.1021/jf901718u. [DOI] [PubMed] [Google Scholar]
- 18.Heuer J, Bremer S, Pohl I, Spielmann H. Development of an in vitro embryotoxicity test using murine embryonic stem cell cultures. Toxicol In Vitro. 1993;7:551–556. doi: 10.1016/0887-2333(93)90064-C. [DOI] [PubMed] [Google Scholar]
- 19.Scholz G, Pohl I, Genschow E, Klemm M, Spielmann H. Embryotoxicity screening using embryonic stem cells in vitro: correlation to in vivo teratogenicity. Cells Tissues Organs. 1999;165:203–211. doi: 10.1159/000016700. [DOI] [PubMed] [Google Scholar]
- 20.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc. 2012;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 21.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 22.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruger EA, Duray PH, Tsokos MG, Venzon DJ, Libutti SK, Dixon SC, Rudek MA, Pluda J, Allegra C, Figg WD. Endostatin inhibits microvessel formation in the ex vivo rat aortic ring angiogenesis assay. Biochem Biophys Res Commun. 2000;268:183–191. doi: 10.1006/bbrc.1999.2018. [DOI] [PubMed] [Google Scholar]
- 24.Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr. 2003;133:3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- 25.Fresco P, Borges F, Diniz C, Marques MP. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26:747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 26.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wähälä K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 27.Cao Y, Cao R, Bråkenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr Biochem. 2002;13:380–390. doi: 10.1016/S0955-2863(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 28.Tosetti F, Ferrari N, De Flora S, Albini A. ‘Angioprevention’: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002;16:2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 29.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Tong YG, Zhang XW, Geng MY, Yue JM, Xin XL, Tian F, Shen X, Tong LJ, Li MH, Zhang C, Li WH, Lin LP, Ding J. Pseudolarix acid B, a new tubulin-binding agent, inhibits angiogenesis by interacting with a novel binding site on tubulin. Mol Pharmacol. 2006;69:1226–1233. doi: 10.1124/mol.105.020537. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 32.Chen SH, Murphy DA, Lassoued W, Thurston G, Feldman MD, Lee WM. Activated STAT3 is a mediator and biomarker of VEGF endothelial activation. Cancer Biol Ther. 2008;7:1994–2003. doi: 10.4161/cbt.7.12.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90:1243–1250. doi: 10.1161/01.RES.0000022200.71892.9F. [DOI] [PubMed] [Google Scholar]
- 34.Cho DH, Choi YJ, Jo SA, Ryou J, Kim JY, Chung J, Jo I. Troglitazone acutely inhibits protein synthesis in endothelial cells via a novel mechanism involving protein phosphatase 2A-dependent p70 S6 kinase inhibition. Am J Physiol Cell Physiol. 2006;291:C317–C326. doi: 10.1152/ajpcell.00491.2005. [DOI] [PubMed] [Google Scholar]
- 35.Hwang M, Perez CA, Moretti L, Lu B. The mTOR signaling network: insights from its role during embryonic development. Curr Med Chem. 2008;15:1192–1208. doi: 10.2174/092986708784310459. [DOI] [PubMed] [Google Scholar]
- 36.Foster KG, Acosta-Jaquez HA, Romeo Y, Ekim B, Soliman GA, Carriere A, Roux PP, Ballif BA, Fingar DC. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem. 2010;285:80–94. doi: 10.1074/jbc.M109.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]