Abstract
A non-protein amino acid, L-ornithine (Orn), has been shown to stimulate the urea cycle and tissue protein synthesis in the liver. The purpose of the current study was to assess whether Orn affects the mammalian target of rapamycin (mTOR) complex 1 (mTORC1) pathway, which is involved in protein synthesis. Primary cultured cells isolated from Wistar rat liver were incubated in an amino acid-free medium, followed by addition of Orn for 3 h. The cell lysate was subjected to immunoblotting to evaluate the phosphorylation of downstream targets of mTORC1, including p70S6K, S6, and 4EBP1. To assess the involvement of mTORC1 for the effect of Orn, the cells were pretreated with the mTOR inhibitor rapamycin before the addition of Orn and the cell lysate was subjected to immunoblotting. We next examined whether the effects of Orn were exerted in vivo. Orn was orally administered to 18 h food-deprived rats, the blood and the livers were collected at 1 and 3 h after administration for immunoblotting. Orn treatment for primary cultured cells for 3 h enhanced the phosphorylation of p70S6K, S6, and 4EBP1. In addition, rapamycin blocked the effects of Orn completely (p70S6K and S6) or partially (4EBP1). The oral administration of Orn to the rat also augmented the phosphorylation of mTORC1 downstream targets notably in S6 at 1 h. Our findings demonstrate that Orn has the potential to induce the phosphorylation of downstream targets of mTORC1 in the rat liver. This may be mediated by the augmentation of mTORC1 activity.
Keywords: L-ornithine, mTORC1, rapamycin
INTRODUCTION
L-ornithine (Orn) is a non-protein amino acid and an essential component of the urea cycle, the ammonia-detoxifying system in the liver. Among foods, the Asiatic clam (corbiculidae), tuna, and cheese contain high amounts of Orn (1–3). In Japan, Orn is commonly used in dietary supplements and functional foods. Administration of Orn is well known to stimulate the urea cycle (4,5). The urea cycle acts to dispose of excess of nitrogen by converting ammonia to urea. Orn is also synthesized endogenously from amino acids including L-arginine, especially in the liver. In pharmacological therapy, Orn is used to decrease blood ammonia levels and reduce the symptoms of hepatic encephalopathy associated with liver cirrhosis (6). In addition, many human studies have indicated several beneficial effects of Orn, including attenuation of fatigue evoked by exercise or alcohol consumption (7,8), stimulation of growth hormone release (9), and improvements in sleep disturbance (10,11). Furthermore, a report indicated that the addition of Orn to a basal diet induced the blood growth hormone level and the rate of protein synthesis in the liver and gastrocnemius muscle (12). These observations have led to the extensive use of Orn for hepatoprotection. However, the molecular mechanisms leading to increased protein synthesis are not well known.
The mammalian target of rapamycin (mTOR), a member of the phosphatidylinositol 3 kinase family of enzymes, plays a central role as a nutrient, energy, and redox sensor mainly in skeletal muscle and the liver (13,14). mTOR is assembled in two subunit complexes; mTOR complex 1 (mTORC1) and mTOR complex 2. Both complexes are composed of different components and affect their activation and functions each other (15). mTORC1 signaling regulates protein synthesis, cell growth, proliferation, and autophagy. In protein synthesis, mTORC1 effects through the mRNA binding step of translation initiation and the phosphorylation of translational repressor factors such as 70-kDa ribosomal protein S6 kinase (p70S6K), ribosomal protein S6 (S6), and eukaryotic initiation factor 4E binding protein 1 (4EBP1) (16,17). mTORC1 is also the major regulator of cell growth, proliferation, and autophagy.
Various metabolic factors can modulate mTORC1 signaling (18). Of these, L-leucine (Leu) and insulin are well known to promote protein synthesis through the activation of mTORC1 signaling. In addition, recent evidence demonstrated that another amino acid, L-citrulline, is a similar potent regulator of mTORC1 signaling (19).
Considering the fact that L-citrulline is synthesized endogenously mainly from Orn with carbamoyl phosphate, and constitutes one of the significance reactions in the urea cycle, the stimulatory effect of Orn on protein synthesis is predicted to be involved in the mTORC1 pathway. Therefore, the aim of our study was to investigate whether Orn is able to promote liver protein synthesis through the stimulation of mTORC1 pathway in vitro, and also to compare mTORC1 activation by oral administration of Orn to food-deprived rats.
MATERIALS AND METHODS
Animals
Male Wistar rats (Japan SLC, Shizuoka, Japan) were purchased at 7 weeks of age and allowed to acclimate to their new surroundings for at least 1 week. Rats ate laboratory rat chow, AIN-93G (Clea Japan, Tokyo, Japan), and drank tap water ad libitum. Rats were housed individually in cages in a room maintained at 20~25°C with 40~70% relative humidity on a 12-h lighting cycle. All animal experiments were approved by the Kirin Company Ethics Committee for Animal Experimentation and performed in accordance with the guidelines for the care and use of laboratory animals of the Kirin Company.
Isolation and primary culture of rat hepatic cells
Rat liver parenchymal cells were isolated by a collagenase perfusion method, as previously described (20). The yield was 6.8×107 cells/liver on average. Isolated hepatic cells were plated in collagen-coated six-well plates (35 mm in diameter) at a density of 5×105 cells/well in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 0.1 mg/mL streptomycin. After overnight incubation at 37°C in a humidified atmosphere of 5% CO2 to allow attachment to the plates, the cells were rinsed with phosphate-buffered saline to remove all unattached and dead cells.
Cell treatment
Primary cultured cells were starved in an amino acid-free medium based on DMEM. After incubation for 20 min in the medium, the cells were further incubated for 3 h in the amino acid-free medium with 0, 5, 10, or 20 mM Orn or 20 mM Leu, and then lysed for immunoblotting. For the time course experiments, the cells were incubated in the amino acid-free medium with 0 and 20 mM Orn for 1 and 3 h after the 20-min amino acid starvation. To evaluate the involvement of mTORC1, the cells were incubated with amino acid-free medium with or without 1 μM mTOR inhibitor rapamycin, followed by addition of 0 or 20 mM Orn. After 3 h incubation, the cells were collected for immunoblotting. All experiments were performed in triplicate.
Oral amino acid administration and sample collection
Rats were randomly divided into 4 groups (N=5 for each group). After fasting for 18 h, water or 1.74 g/kg body weight Orn hydrochloride was administered by gavage without anesthesia at a volume of 25 mL/kg. The blood and the livers were collected 1 or 3 h after administration, and then the livers were excised and rinsed in ice-cold saline. The liver homogenates were prepared using a Potter-Elvehjem homogenizer in 10 volumes of 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid at pH 7.4 containing 100 mM KCl, 2 mM ethylene glycol-bis(β-aminoethylether)-N,N,N′,N′-tetraacetic acid, 1 mM dithiothreitol, and Halt protease and phosphatase inhibitor cocktail (Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s recommendation. After centrifugation at 10,000 g for 10 min at 4°C, the supernatants were aliquoted and stored at −80°C until use.
Protein concentration measurement
Protein concentrations of the samples were determined by the Lowry assay using bovine serum albumin as a standard (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Immunoblotting
The lysates (20 μg) were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes, and then probed with the appropriate antibodies against phospho-mTOR (Ser2448), mTOR, phospho-p70S6K (Thr389), p70S6K, phospho-S6 (Ser235/236), S6, phospho-4EBP1 (Thr37/46), and 4EBP1 (all from Cell Signaling Technology, Inc., Danvers, MA, USA) to assess their phosphorylation states. Anti-rabbit secondary antibody conjugated to horseradish peroxidase (Cell Signaling Technology, Inc.) and an ECL prime western blotting detection system (GE Healthcare, Amersham, Buckinghamshire, UK) were used for detection. The bands intensities were quantified and processed by LAS-3000 fluorescence imaging system (Fujifilm, Tokyo, Japan). The phosphorylation states were corrected against the respective total protein levels.
Plasma-free amino acid and growth hormone analysis
Plasma was acidified and deproteinized with perchloric acid (final concentration of 0.45 N), and the supernatants were collected after centrifugation. Before the amino acid analysis, samples were diluted 8-fold with 0.1 N hydrochloric acid and prepared with O-phthalaldehyde derivatives. Urea cycle amino acid, Orn, citrulline, and arginine, were measured using a commercially available amino acid analyzer (Shimadzu, Kyoto, Japan).
Plasma growth hormone, insulin, and glucose levels were determined using respective commercial kits according to the manufacturer’s instructions (Shibayagi, Gunma, Japan; Morinaga, Kanagawa, Japan; Wako, Osaka, Japan).
Statistical analysis
All data are expressed as mean±standard error of the mean. The Student’s t-test was used for comparison between two groups, and the analysis of variance followed by the Dunnett’s test was used for more than two groups. A P-value of <0.05 was considered statistically significant.
RESULTS
Orn induces the phosphorylation of mTORC1 downstream targets in primary rat hepatic cells
We first investigated whether Orn augments the phosphorylation of several downstream targets of mTORC1, p70S6K, S6, and 4EBP1, in primary cultures of rat hepatic cells. Orn significantly upregulated the phosphorylation levels of p70S6K, S6, and 4EBP1 but not mTOR at 20 mM (Fig. 1). These levels of phosphorylation were almost the same as that of Leu at 20 mM. Significant elevation of 4EBP1 phosphorylation was detected at 5 mM and dose dependency was also observed; this was not apparent for the other factors measured (Fig. 1).
Fig. 1.
Phosphorylation levels of mTOR (A), p70S6K (B), S6 (C), and 4EBP1 (D) in primary cultured rat hepatic cells treated with Orn or Leu for 3 h. Cell lysates (20 μg) were subjected to immunoblotting with specific antibodies. Ratios of phosphorylated proteins to respective total proteins were compared to judge the phosphorylation states. Inserts show the representative immunoblot images. Upper images show phosphorylated proteins and lower show total proteins. Right and left images were cut out from the same membrane. Values are mean±standard error of the mean, *P<0.05, **P<0.01 compared with the control (Dunnett’s test).
The incubation time of 3 h was better than that of 1 h
To elucidate the time point at which Orn exerts its function, the cells were incubated with Orn for 1 and 3 h. Orn treatment for 3 h resulted in much greater levels of p70S6K and 4EBP1 phosphorylation than that obtained from a 1 h, and the phosphorylation levels of S6 were increased only at 3 h (Fig. 2).
Fig. 2.
Phosphorylation levels of mTOR (A), p70S6K (B), S6 (C), and 4EBP1 (D) in primary cultured rat hepatic cells treated with Orn (20 mM) for 1 or 3 h. Cell lysates (20 μg) were subjected to immunoblotting with specific antibodies. Phosphorylation levels were normalized against total protein. Inserts show the representative immunoblot images. Upper images show phosphorylated proteins and lower show total proteins. Values are mean±standard error of the mean, *P<0.05, **P<0.01 compared with the respective control.
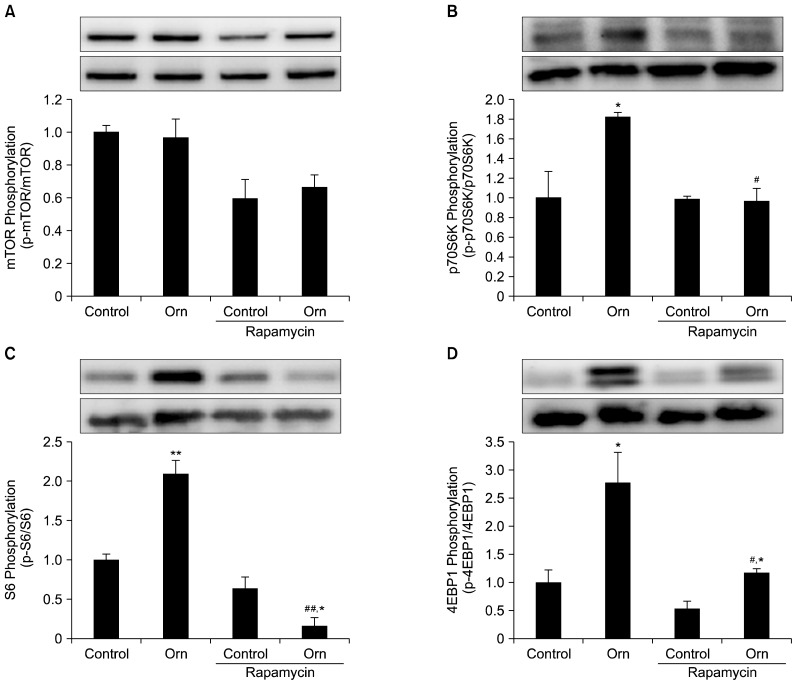
The effect of Orn was inhibited by the mTOR inhibitor rapamycin
Rapamycin is a representative mTOR inhibitor (21). We examined whether the effect of Orn was deleted with the treatment of rapamycin. The cells were incubated with rapamycin for 20 min prior to Orn treatment and incubated for 3 h in the presence of rapamycin and Orn. The rapamycin treatment completely inhibited p70S6K and S6 phosphorylation, and partially inhibited the phosphorylation of 4EBP1 (Fig. 3). These results indicate that Orn augmented the phosphorylation of these factors probably via the mTORC1 activation.
Fig. 3.
Phosphorylation levels of mTOR (A), p70S6K (B), S6 (C), and 4EBP1 (D) in primary cultured rat hepatic cells treated with Orn (20 mM) for 3 h in the presence or absence of rapamycin (1 μM). Cell lysates (20 μg) were subjected to immunoblotting with specific antibodies. Phosphorylation levels were normalized against total protein. Inserts show the representative immunoblot images. Upper images show phosphorylated proteins and lower show total proteins. Values are mean±standard error of the mean, *P<0.05, **P<0.01 compared with the respective control, #P<0.05, ##P<0.01 compared with the Orn without rapamycin.
Orn administration to the rat augmented the phosphorylation of mTORC1 downstream targets
To evaluate whether Orn stimulates the mTORC1 pathway in vivo as well as in vitro, the phosphorylation of mTOR and its downstream targets in the rat liver was examined. Blood and liver were collected from rats at 1 and 3 h after Orn administration via oral gavage. Levels of plasma parameters were analyzed. Oral Orn treatment led to 17.5- and 20.1-fold increases in plasma Orn levels at 1 and 3 h, respectively (Table 1). With regard to other urea cycle amino acids, citrulline levels were decreased by 0.9- and 0.8-fold, respectively, while arginine levels were not significantly different. In addition, Orn administration increased glucose concentration only at 1 h. There were no significant differences in plasma growth hormone and insulin levels, but an increasing trend in growth hormone was shown after Orn administration (P<0.1).
Table 1.
Plasma levels of urea cycle amino acids, growth hormone, insulin, and glucose in rats following oral administration of Orn
| 1 h | 3 h | |||
|---|---|---|---|---|
|
|
|
|||
| Control | Orn | Control | Orn | |
| Amino acids (μM) | ||||
| Orn | 26.7±1.9 | 466.0±47.4** | 28.6±1.7 | 575.7±37.0** |
| Arg | 63.1±2.8 | 72.6±3.2 | 74.4±2.9 | 65.6±3.6 |
| Cit | 72.9±1.1 | 64.3±2.4* | 75.6±2.3 | 58.3±2.1** |
| Growth hormone (pg/mL) | 78.5±11.1 | 139.4±40.5 | 78.1±0.65 | 108.1±10.9 |
| Insulin (ng/mL) | 3.91±0.14 | 3.93±0.49 | 3.74±0.65 | 3.39±0.79 |
| Glucose (mg/dL) | 129.0±7.4 | 167.3±5.7** | 127.4±8.5 | 130.2±5.9 |
Orn, L-ornithine; Arg, L-arginine; Cit, L-citrulline.
Values are mean±standard error of the mean,
P<0.05,
P<0.01 compared with the control.
Orn administration promoted the phosphorylation of p70S6, S6, and 4EBP1 at 1 h. Among these factors, S6 showed the largest effect (6.9-fold compared with the control group). Orn enhanced the phosphorylation of these factors at 3 h, but the effects were much lower than those of 1 h (Fig. 4).
Fig. 4.
Phosphorylation levels of mTOR (A), p70S6K (B), S6 (C), and 4EBP1 (D) in rat liver homogenate. Liver samples were collected 1 or 3 h after oral administration of Orn (1.74 g/kg body weight). Liver homogenates (20 μg) were subjected to immunoblotting with specific antibodies. Phosphorylation levels were normalized against total protein. Inserts show the representative immunoblot images. Upper images show phosphorylated proteins and lower show total proteins. Values are mean±standard error of the mean, *P<0.05, **P<0.01 compared with the control.
DISCUSSION
Recent reports have highlighted the ability of Orn to modulate ammonia metabolism in the liver and also various physiological functions such as anti-fatigue (6,7). Its effect on promoting tissue protein synthesis has also been shown (12). Recently, citrulline, a urea cycle amino acid that is metabolized from Orn, was reported as a potent regulator of the mTORC1 pathway following protein synthesis (19). mTORC1 is a master regulator that controls protein synthesis (16,17). These facts prompted us to consider that Orn promotes tissue protein synthesis via the mTORC1 pathway, similarly to citrulline. The present study was designed to evaluate the hypothesis that Orn stimulates the mTORC1 pathway. We performed Orn treatment on primary cultured rat hepatocyte cells and examined the effects of oral Orn administration in rats.
In primary rat cells cultured in an amino acid-free state, Orn treatment affected the phosphorylation state of typical mTORC1 downstream targets, p70S6K, S6, and 4EBP1 (Fig. 1). This result suggested that Orn has the potential to promote the phosphorylation of the mTORC1 downstream targets. Furthermore, 20 mM Leu treatment had an equivalent effect on the phosphorylation state of these factors as the same concentration of Orn. Therefore, Orn may also be a potent mTORC1 activator, similar to Leu.
We next investigated the appropriate induction time of Orn. Cells were incubated with 20 mM Orn for 1 and 3 h, and the phosphorylation state was evaluated. The effects of Orn at 3 h on phosphorylation were much greater than those at 1 h (Fig. 2). Therefore, we regarded that the incubation time of 3 h was better than that of 1 h in our experimental setting. In a previous report using cultured rat hepatoma H4IIE cells, p70S6K phosphorylation reached a peak at 20 min after the addition of Leu (22). Orn requires a longer time period to induce the effect compared with Leu. We speculated that the mechanisms of Orn stimulation of the mTORC1 pathway are not consistent with that of Leu. Stimulation of mTORC1 downstream targets by Orn was observed in a time- and dose-dependent manner, as shown in Fig. 1 and 2.
Primary cultured cells were treated with rapamycin prior to stimulation with Orn. The activation of p70S6K and S6 phosphorylation by Orn was strongly inhibited by rapamycin, and phosphorylation of 4EBP1 was restricted (Fig. 3). Rapamycin influenced the phosphorylation state of mTORC1 downstream factors; however, the extent of inhibition was different. Previous studies have shown that S6 activity is completely inhibited by rapamycin, but 4EBP1 is only partially inhibited depending on the cell type or duration of rapamycin treatment (23). Thus the restricted inhibition of 4EBP1 shown in the present study was due to the potential of rapamycin. These results suggest that the potential activity of Orn is mainly associated with mTORC1 activation.
We next examined whether the effects of Orn observed in primary cultured cells are also exerted in vivo. Previous reports have demonstrated that overnight fasting almost completely reduced the phosphorylation of mTOR downstream targets and subsequent protein synthesis in both skeletal muscle and the liver (16,24,25). In addition, Norton et al. showed that peak mTOR signaling responses occur shortly after consumption of a meal (26). Rats were starved for 18 h before the experiment began and dissected 1 and 3 h after Orn administration. In various previous reports investigating the effect of Leu, 1.35 g/kg body weight of Leu was administered (19,27,28). We calculated the Orn hydrochloride dosage for 1.74 g/kg body weight as equivalent molar to 1.35 g/kg body weight of Leu.
Orn administration had an obvious stimulatory effect on the phosphorylation of p70S6, S6, and 4EBP1 in the rat liver, particularly after 1 h. This suggests that Orn stimulates the phosphorylation of mTORC1 targets by oral administration as well as in in vitro cell exposure using rat primary cells. A previous report indicated that citrulline, which is metabolized from Orn, is able to promote the phosphorylation of 4EBP1 but not that of S6 (19). In our experiment, S6 phosphorylation was promoted more predominantly than that of 4EBP1. Further, plasma citrulline levels were not increased after Orn administration. These results may suggest that the effect of Orn on mTORC1 signaling was not exerted through its metabolite, citrulline. Another mechanism behind the effects of Orn on mTORC1 pathway was thought to be through growth hormone release, because it was reported that Orn has the potential to stimulate growth hormone release (9). Orn administration tended to increase plasma growth hormone levels (P<0.1, Table 1). Growth hormone is an anabolic hormone that is secreted from the pituitary gland in a pulsatile pattern, as observed in peripheral blood (29). In addition, growth hormone treatment in hepatoma cells activated protein synthesis through the mTORC1 pathway (30). Considering these facts, the effect of Orn observed in vivo may be mediated by the indirect action of increased blood growth hormone levels besides the direct action of Orn itself. In in vitro experiments, the stimulation of mTOR downstream targets was observed after 3 h incubation with Orn (Fig. 2). In contrast, the stimulation was observed in a shorter time in the in vivo experiments (Fig. 4). Considering the time that was required for absorption, in vivo experiments usually need a longer time to exhibit the effect of Orn. The obvious reason of this discrepancy was not clear, but the indirect effects of Orn can be one of the speculated possibilities.
In this study, we were unable to assess the direct relation between Orn and mTOR. The phosphorylation states of mTOR itself were not different by Orn in primary cultured cell and rat liver. Indirect elucidation using the mTOR inhibitor suggested the involvement of mTOR to effect Orn. One possibility would be that the evaluation time from Orn treatment was inappropriate for detecting the mTOR phosphorylation based on the results of Leu on mTOR phosphorylation (Fig. 1). Further studies using specific inhibitors of proposed factors that regulate mTOR activation such as MAPK or PI3K would extend our understanding of the contribution of Orn to the mTORC1 downstream targets.
In conclusion, this is the first study to report the effects of Orn on the mTORC1 downstream targets in the rat liver. The results demonstrate that Orn augments the phosphorylation of representative downstream targets of mTORC1, including p70S6K, S6, and 4EBP1, in amino acid-free conditions in rat primary hepatoma cell and rat liver. Based on the mTOR inhibitor experiments, these effects are likely mediated by the mTOR signaling pathway. The effect of Orn on promoting tissue protein synthesis may be explained by this mechanism.
Footnotes
AUTHOR DISCLOSURE STATEMENT
Kirin Company, Limited, and its affiliate, to which the authors belong, have been released food products containing Orn and Orn bulk powder. The authors declare that no other financial support or compensation has been received for the study.
REFERENCES
- 1.Uchisawa H, Sato A, Ichita J, Matsue H, Ono T. Influence of low-temperature processing of the brackish-water bivalve, Corbicula japonica, on the ornithine content of its extract. Biosci Biotechnol Biochem. 2004;68:1228–1234. doi: 10.1271/bbb.68.1228. [DOI] [PubMed] [Google Scholar]
- 2.Antoine FR, Wei CI, Littell RC, Quinn BP, Hogle AD, Marshall MR. Free amino acids in dark- and white-muscle fish as determined by O-phthaldialdehyde precolumn derivatization. J Food Sci. 2001;66:72–77. doi: 10.1111/j.1365-2621.2001.tb15584.x. [DOI] [Google Scholar]
- 3.Frau M, Massanet J, Rosselló C, Simal S, Cañellas J. Evolution of free amino acid content during ripening of Mahon cheese. Food Chem. 1997;60:651–657. doi: 10.1016/S0308-8146(97)00051-4. [DOI] [Google Scholar]
- 4.Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 5.Krebs HA, Hems R, Lund P. Accumulation of amino acids by the perfused rat liver in the presence of ethanol. Biochem J. 1973;134:697–705. doi: 10.1042/bj1340697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staedt U, Leweling H, Gladisch R, Kortsik C, Hagmüller E, Holm E. Effects of ornithine aspartate on plasma ammonia and plasma amino acids in patients with cirrhosis. A double-blind, randomized study using a four-fold crossover design. J Hepatol. 1993;19:424–430. doi: 10.1016/S0168-8278(05)80553-7. [DOI] [PubMed] [Google Scholar]
- 7.Sugino T, Shirai T, Kajimoto Y, Kajimoto O. L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism. Nutr Res. 2008;28:738–743. doi: 10.1016/j.nutres.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Kokubo T, Ikeshima E, Kirisako T, Miura Y, Horiuchi M, Tsuda A. A randomized, double-masked, placebo-controlled crossover trial on the effects of L-ornithine on salivary cortisol and feelings of fatigue of flushers the morning after alcohol consumption. Biopsychosoc Med. 2013;7:6. doi: 10.1186/1751-0759-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demura S, Yamada T, Yamaji S, Komatsu M, Morishita K. The effect of L-ornithine hydrochloride ingestion on human growth hormone secretion after strength training. Adv Biosci Biotechnol. 2010;1:7–11. doi: 10.4236/abb.2010.11002. [DOI] [Google Scholar]
- 10.Horiuchi M, Kanesada H, Miyata T, Watanabe K, Nishimura A, Kokubo T, Kirisako T. Ornithine ingestion improved sleep disturbances but was not associated with correction of blood tryptophan ratio in Japanese Antarctica expedition members during summer. Nutr Res. 2013;33:557–564. doi: 10.1016/j.nutres.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Miyake M, Kirisako T, Kokubo T, Miura Y, Morishita K, Okamura H, Tsuda A. Randomised controlled trial of the effects of L-ornithine on stress markers and sleep quality in healthy workers. Nutr J. 2014;13:53. doi: 10.1186/1475-2891-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tujioka K, Yamada T, Aoki M, Morishita K, Hayase K, Yokogoshi H. Dietary ornithine affects the tissue protein synthesis rate in young rats. J Nutr Sci Vitaminol (Tokyo) 2012;58:297–302. doi: 10.3177/jnsv.58.297. [DOI] [PubMed] [Google Scholar]
- 13.Marshall S. Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional perspective of diabetes, obesity, and cancer. Sci STKE. 2006;2006:re7. doi: 10.1126/stke.3462006re7. [DOI] [PubMed] [Google Scholar]
- 14.Patti ME, Kahn BB. Nutrient sensor links obesity with diabetes risk. Nat Med. 2004;10:1049–1050. doi: 10.1038/nm1004-1049. [DOI] [PubMed] [Google Scholar]
- 15.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol. 2013;203:563–574. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun. 2004;313:429–436. doi: 10.1016/j.bbrc.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Reiter AK, Anthony TG, Anthony JC, Jefferson LS, Kimball SR. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int J Biochem Cell Biol. 2004;36:2169–2179. doi: 10.1016/j.biocel.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Kwon G, Marshall CA, Pappan KL, Remedi MS, McDaniel ML. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes. 2004;53:S225–S232. doi: 10.2337/diabetes.53.suppl_3.S225. [DOI] [PubMed] [Google Scholar]
- 19.Le Plénier S, Walrand S, Noirt R, Cynober L, Moinard C. Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: a common activation pathway? Amino Acids. 2012;43:1171–1178. doi: 10.1007/s00726-011-1172-z. [DOI] [PubMed] [Google Scholar]
- 20.Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206:700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- 21.Dutcher JP. Mammalian target of rapamycin (mTOR) inhibitors. Curr Oncol Rep. 2004;6:111–115. doi: 10.1007/s11912-004-0022-5. [DOI] [PubMed] [Google Scholar]
- 22.Shigemitsu K, Tsujishita Y, Miyake H, Hidayat S, Tanaka N, Hara K, Yonezawa K. Structural requirement of leucine for activation of p70 S6 kinase. FEBS Lett. 1999;447:303–306. doi: 10.1016/S0014-5793(99)00304-X. [DOI] [PubMed] [Google Scholar]
- 23.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshizawa F, Endo M, Ide H, Yagasaki K, Funabiki R. Translational regulation of protein synthesis in the liver and skeletal muscle of mice in response to refeeding. J Nutr Biochem. 1995;6:130–136. doi: 10.1016/0955-2863(95)00018-U. [DOI] [Google Scholar]
- 25.Yoshizawa F, Kimball SR, Vary TC, Jefferson LS. Effect of dietary protein on translation initiation in rat skeletal muscle and liver. Am J Physiol. 1998;275:E814–E820. doi: 10.1152/ajpendo.1998.275.5.E814. [DOI] [PubMed] [Google Scholar]
- 26.Norton LE, Layman DK, Bunpo P, Anthony TG, Brana DV, Garlick PJ. The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. J Nutr. 2009;139:1103–1109. doi: 10.3945/jn.108.103853. [DOI] [PubMed] [Google Scholar]
- 27.Sans MD, Tashiro M, Vogel NL, Kimball SR, D’Alecy LG, Williams JA. Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. J Nutr. 2006;136:1792–1799. doi: 10.1093/jn/136.7.1792. [DOI] [PubMed] [Google Scholar]
- 28.Anthony JC, Anthony TG, Layman DK. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J Nutr. 1999;129:1102–1106. doi: 10.1093/jn/129.6.1102. [DOI] [PubMed] [Google Scholar]
- 29.D’Agata R, Vigneri R. Circadian variations of human growth hormone serum levels. Longitudinal study. Ann Endocrinol (Paris) 1971;32:383–387. [PubMed] [Google Scholar]
- 30.Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab. 2007;292:E1647–E1655. doi: 10.1152/ajpendo.00674.2006. [DOI] [PubMed] [Google Scholar]