Abstract
The anti-obesity effects of starter (Leuconostoc mesenteroides+Lactobacillus plantarum) fermented kimchi on 3T3-L1 adipocyte were studied using naturally fermented kimchi (NK), a functional kimchi (FK, NK supplemented with green tea), and FK supplemented with added starters (FKS). Oil red O staining and cellular levels of triglyceride (TG) and glycerol were used to evaluate the in vitro anti-obesity effects of these kimchis in 3T3-L1 cells. The expressions of adipogenesis/lipogenesis-related genes of peroxisome proliferator-active receptor (PPAR)-γ, CCAAT/enhance-binding protein (C/EBP)-α, and fatty acid synthase (FAS) were determined by RT-PCR. Kimchis, especially FKS, markedly decreased TG levels and increased levels of intracellular glycerol and lipid lipolysis. In addition, FKS also reduced the mRNA levels of PPAR-γ, C/EBP-α, and FAS, which are related to adipogenesis/lipogenesis in 3T3-L1 cells. These results suggest the anti-obesity effects of FKS were to due to enhanced lipolysis and reduced adipogenesis/lipogenesis in 3T3-L1 adipocytes.
Keywords: kimchi, starters, antiobesity, 3T3-L1 cells
INTRODUCTION
Kimchi is a traditional Korean fermented vegetable dish that has well-known beneficial effects, which include antioxidant, antiaging, anticancer, antiobesity, antiatherosclerotic, and antidiabetic effects (1). It was reported that kimchi showed antiobesity effect in 3T3-L1 (2,3) and high fat diet induced obese mice (2–5). We prepared a functional kimchi with previously described anticancer and anti-obesity effects that included the following ingredients: mustard leaves, Chinese peppers, mushrooms, sea tangle juice, bamboo salt (baked 3 times) (6), and green tea, the latter of which has an anti-obesity effect (2). This research focused on improving the health benefits, especially the anti-obesity effects, of functional kimchi by adding starters of Leuconostoc mesenteroides and Lactobacillus plantarum isolated from kimchi (7).
Usually, kimchi is produced by natural fermentation at low temperatures (~5°C), Leuconostoc mesenteroides, Lactobacillus plantarum, and Weissella koreensis are the major lactic acid bacteria found in kimchi (8). The major lactic acid bacteria (LAB) in kimchi are Leuconostoc mesenteroides and Lactobacillus plantarum, which predominate during the early and late stages of fermentation, respectively (9). Leuconostoc sp. and Lactobacillus sp. (the kimchi-derived LAB) have various physiological activities (7) and suppress the progress of obesity (10,11). The use of Leu. mesenteroides as a kimchi starter affects the fermentation process, including the production of metabolites, such as free sugars, amino acids, and organic acids, and the profiles of kimchi LAB communities (12). Starter kimchis inoculated initially with Leu. mesenteroides have better kimchi qualities, microflora, and greater health benefits than nonstarter kimchis (13). Leu. mesenteroides is a heterofermentative LAB and produces lactic acid, acetic acid, carbon dioxide, and ethanol, which increase kimchi quality and enhance taste (14). On the other hand, Lab. plantarum has greater resistance to human gastric acid and bile acid than animal-derived LABs (15).
In this study, we focused on antiobesity effects as assessed by Oil red O staining, levels of triglyceride (TG) and glycerol in 3T3-L1 cells, and the mRNA expressions of adipogenesis/lipogenesis related genes of functional kimchi containing Leu. mesenteroides and Lab. plantarum as starters, and compared these with those of naturally fermented kimchi (NK) and functional kimchi (FK, NK supplemented with green tea).
MATERIALS AND METHODS
Preparation of kimchi and extracted samples
Production of NK, FK, and functional kimchi with starters (FKS) began with the preparation of baechu cabbage by salting the cabbage in salt water (10%) for 10 h, and this was then followed by adding mixtures of subingredients, such as red peppers, radish, and crushed garlic, all of which were purchased in a local market (L-mart, Busan, Korea). The recipes used to produce NK, FK, and FKS are provided in Table 1 (7). The FKS recipe was same as the FK recipe with the exception of the starter addition.
Table 1.
Ingredient ratios of naturally fermented kimchi (NK), functional kimchi (FK), and functional kimchi with probiotic starters (FSK)
| Ingredient | NK | FK | FKS |
|---|---|---|---|
| Baechu cabbage | 100 | 100 | 100 |
| Red pepper powder | 3.5 | 2.5 | 2.5 |
| Crushed garlic | 1.4 | 2.8 | 2.8 |
| Crushed ginger | 0.6 | 0.6 | 0.6 |
| Anchovy juice | 2.2 | – | – |
| Sugar | 1.0 | 1.0 | 1.0 |
| Radish | 13.0 | 11.0 | 11.0 |
| Green onion | 2.0 | 2.0 | 2.0 |
| Green tea | – | 5 | 5 |
| Chinese pepper | – | 0.1 | 0.1 |
| Pear | – | 2.8 | 2.8 |
| Mushroom and sea tangle juice | – | 5.0 | 5.0 |
| Salt | normal | Na:K=3:7 | Na:K=3:7 |
| Mixed starter (Leu. mesenteriodes+Lac. plantarum) | – | – | 106 CFU/g |
| Final salt concentration (%) | 2.5 | 2.2 | 2.2 |
Mixed LAB starters (Lactobacillus plantarum KCCM 11352P and Lecuconostoc mesenteroides KCCM 11353P) were isolated from kimchi, and previously demonstrated their probiotic effects such as antioxidative, pro-apoptotic, and anti-inflammatory effects, and enhancing quality of kimchi (7). Mixed LAB starters were added to seasoning compound of subingredients at 106 CFU/g. All ingredients were admixed and fermented at 5°C for 3 weeks until the kimchi had ripened properly (~pH 4.3). All kimchi samples were freeze-dried (Sanwon Freezing Engineering Co., Busan, Korea) to produce kimchi powders for the study.
Portions of freeze-dried kimchi (20 g) were extracted twice using 400 mL (1:20, w/v) of methanol by stirring for 24 h at room temperature. Methanol extracts were filtered using Toyo filter paper (No: 5A) and then concentrated in vacuo at 37°C using a Rotary Evaporator (EYELA, Miyagi, Japan). Samples were stored at −20°C until required (7).
3T3-L1 adipocyte culture and sample treatment
3T3-L1 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM containing a high concentration of glucose supplemented with 10% bovine calf serum and penicillin/streptomycin in six-well culture plates in triplicate (n=3). Confluent cells were then cultured in adipocyte differentiation cocktail medium [3-isobutyl-1-methylxanthine (IBMX, 5 mM), dexamethasone (1 mM), and insulin (10 mg/mL) in DMEM supplemented with 10% fetal bovine serum (FBS)] for 2 days, and feeding medium [DMEM supplemented with 10% FBS and insulin (10 mg/mL)] was then added and cells were cultured in a CO2 incubator at 37°C for ~6 days when adipocyte differentiation was complete. Cells were treated with 100 μg/mL of the extracted kimchi samples for 24 h as previously described (3).
Oil red O staining assay
Intracellular lipid accumulations were determined using an Oil red O assay. Briefly, treated 3T3-L1 cells were fixed with 3.7% formaldehyde solution for 1 h at 37°C, and then washed 3 times with phosphate buffered saline. Oil red O staining solution was prepared as follows; 0.5 g of Oil red O powder (Sigma, St. Louis, MO, USA) was dissolved in 100 mL of isopropanol to make a stock solution, which was diluted with water (6:4) and filtered. Staining was performed at 25°C for 1 h, and plates were then washed twice in ddH2O and observed and photographed under a microscope (CK Microscope, Olympus, Tokyo, Japan) (16).
Triglyceride (TG) and glycerol contents
TG and glycerol contents were determined using a commercial TG assay and glycerol assay kit (Cayman, Ann Arbor, MI, USA) (17).
Reverse transcription polymerase chain reaction (RT-PCR) assay
Total RNA was isolated from kimchi sample treated cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacture’s recommendations, and centrifuged at 12,000 g for 15 min at 25°C. Samples were then extracted with chloroform, and then isopropanol was added to supernatants (1:1, v/v), and the RNA so obtained was pelleted by centrifugation (12,000 g for 15 min at 4°C). Pellets were washed with ethanol and RNA was solubilized using diethyl pyrocarbonate-treated RNase-free water and quantified by measuring absorbance at 260 nm using a UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan). Equal amounts of RNA (1 μg) were then reverse transcribed using a master mix [1×reverse transcriptase buffer, 1 mM dNTPs, 500 ng of oligodT18 primers (Invitrogen), 140 U of moloney murine leukemia virus (MMLV) (Invitrogen) reverse transcriptase, and 40 U of RNase inhibitor] for 45 min at 42°C. PCR was then conducted in an automatic thermocycler (Bioneer, Daejeon, Korea) over 25 amplification cycles (94°C for 60 s, 54°C for 60 s, and 72°C for 30 s) followed by a final 7-min extension at 72°C. PCR products were separated on 2% agarose gels and visualized by EtBr staining (Invitrogen). GAPDH was used for normalization. Gene expressions were quantified using ImageJ software (National Institutes of Health, Maryland, MD, USA) (7).
The genes primer sequences used were as follows: PPARγ 5′-GAG ATG CCA TTC TGG CCC ACC AAC TTC GG-3′ (forward), 5′-TAT CAT AAA TAA GCT TCA ATC GGA TGG TTC-3′ (reverse), C/EBPα 5′-TGC TGG AGT TGA CCA GTG ACA A-3′ (forward), 5′-AAA CCA TCC TCT GGG TCT CC-3′ (reverse), FAS 5′-GCA CCT GCA GAT CCT TTG AT-3′ (forward), 5′-GTC CCG GCA TTC AGA ATA GT-3′ (reverse), GAPDH 5′-CGG AGT CAA CGG ATT TGG TC-3′ (forward), 5′-AGC CTT CTC CAT GGT GGT GA-3′ (reverse).
Statistical analysis
Results are presented as means±SDs. The significances of differences between group mean values were assessed using one-way ANOVA and Duncan’s multiple range tests. Statistical significance was accepted for P-values<0.05, and the analysis was conducted using the SAS ver. 9.1 statistical software package (SAS Institute Inc., Cary, NC, USA) (7).
RESULTS AND DISCUSSION
Effect of kimchi samples on Oil red O staining in 3T3-L1 adipocytes
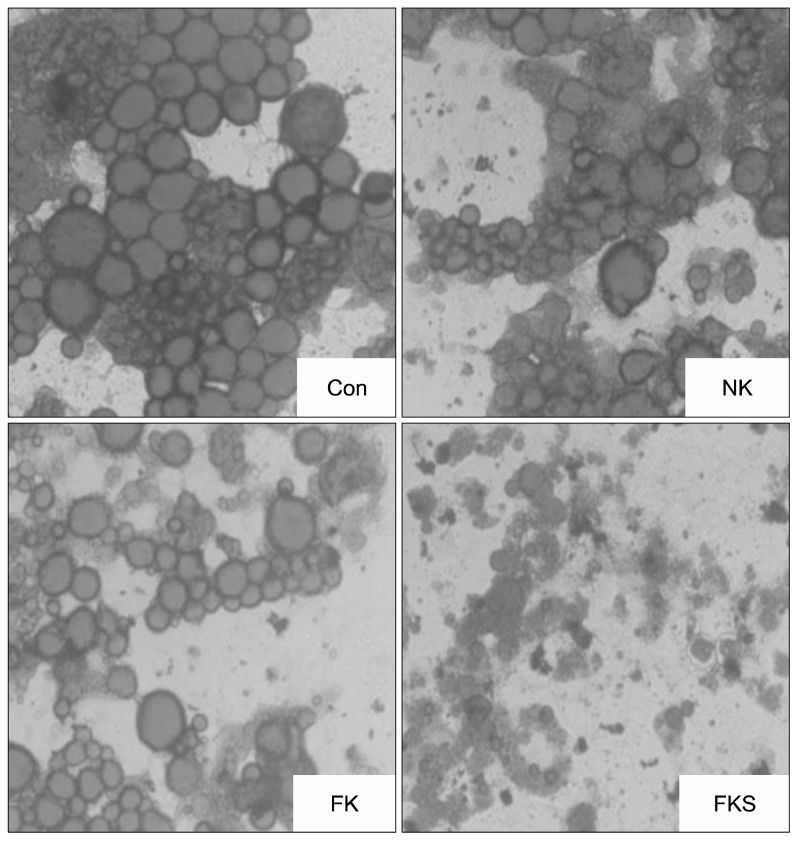
As shown in Fig. 1, treatment-naive control cells (the control group) contained many globular lipid droplets. However, NK and FK treated cells showed fewer and smaller lipid droplets, and FKS treatment significantly enhanced these effects and showed lysis of the globular lipid droplets. Leu. mesenteroides and Lab. plantarum in FKS have acid and bile tolerance, antioxidative effects, and anti-proliferative effects on HT-29 human colorectal cancer cells (18). Also, anti-obesity effect of the above starters on 3T3-L1 cells and animals were previously reported (4,19,20). Lee et al. (21) reported Leu. mesenteroides fermented products reduce lipid accumulation in 3T3-L1 cells. Accordingly, we suggest Leu. mesenteroides and Lab. plantarum starters regulate the fatty acid and amino acid compositions of kimchi which might also have led to reduction of lipid accumulation in 3T3-L1 adipocytes.
Fig. 1.
Effect of kimchi samples on Oil red O staining in 3T3-L1 adipocytes. Con, control; NK, naturally fermented kimchi; FK, functional kimchi; FKS, functional kimchi with starters.
Effect of kimchi samples on TG and glycerol levels in 3T3-L1 adipocytes
As shown in Table 2, control cells contained 0.48±0.09 mg/dL of TG, but TG levels in the three treatments were lower [NK (0.34±0.05 mg/dL), FK (0.30±0.09 mg/dL) and FKS (0.27±0.04 mg/dL)]. NK decreased TG versus the control (P<0.05), but FK and FKS reduced TG levels more than NK; even if significance among the kimchi-treated groups was not shown.
Table 2.
Effects of treating 3T3-L1 adipocytes with kimchis on triglyceride and glycerol levels
| Groups | Levels | |
|---|---|---|
|
| ||
| Triglyceride (mg/dL) | Glycerol (μg/mL) | |
| Control | 0.48±0.09a | 12.9±2.0c |
| NK | 0.34±0.05b | 17.0±3.3b |
| FK | 0.30±0.09b | 19.1±2.2ab |
| FKS | 0.27±0.04b | 21.4±1.5a |
Results are presented as means±SDs. Different letters (a–c) indicate means were significantly different (P<0.05) compared with treatment naïve controls by Duncan’s multiple range tests. NK, naturally fermented kimchi; FK, functional kimchi (kimchi containing green tea; FKS, functional kimchi produced using probiotic starters.
TG hydrolysis releases glycerol and free fatty acid from adipocytes and causes lipolysis (22). In the present study, cells in the control group contained 12.9±2.0 μg/mL of glycerol. All three sample groups contained significantly higher glycerol levels in comparison with the control group [NK (17.0±3.3 μg/mL), FK (19.1±2.2 μg/mL), and FKS (21.4±1.5 μg/mL) (P<0.05)]. These results show NK had greater lipolytic activity than the control, and that FK and FKS had even more lipolytic activity.
Kimchi has antiobesity effects in 3T3-L1 cells and mice (9), and kimchi supplemented with green tea has been reported to have a greater antiobesity effect than kimchi without green tea (2). Green tea contains polyphenols, especially epigallocatechin gallate, and these have been found to reduce total TG accumulation in 3T3-L1 pre-adipocytes during their differentiation to adipocytes (23) and to boost glycerol release due to the up-regulation of lipid lipolysis (24). Lab. plantarum has also been reported to boost glycerol release from 3T3-L1 adipocytes and to reduce TG levels in 3T3-L1 adipocytes significantly (19). In addition, kimchi fermented with Leuconostoc sp. reduced TG serum levels in high fat diet (HFD) induced obese C57BL/6N mice (11), and Cui et al. (4) reported starter (Leu. mesenteroides) fermented kimchi exhibited antiobesity effects in HFD-induced obese mice. In this study, the TG levels of kimchi-treated groups were lowered by the addition of ingredients such as green tea and probiotic starters to kimchi. Park et al. (19) showed the TG levels were significantly reduced by adding Lab. plantarum. Also, glycerol levels of kimchi-treated groups were significantly increased in this study. Thus, we may infer that FK had stronger lipolytic effect than NK because FK contained green tea, and FKS had an even greater lipolytic effect because of the use of a probiotic starter mix.
Kimchi attenuated the mRNA levels of PPAR-γ, C/EBPα, and FAS in 3T3-L1 adipocytes
Adipogenesis is regulated by transcriptional activators, such as IGF-1 (insulin-like growth factor-1), C/EBPα, C/EBPβ, C/EBPδ, PPAR-γ, and FAS (24). PPAR-γ activation may enhance C/EBPα activity and modulate pre-adipocyte differentiation and promote adipogenesis and lipogenesis (25), and increase body weight gain and hepatic TG levels in mice (26).
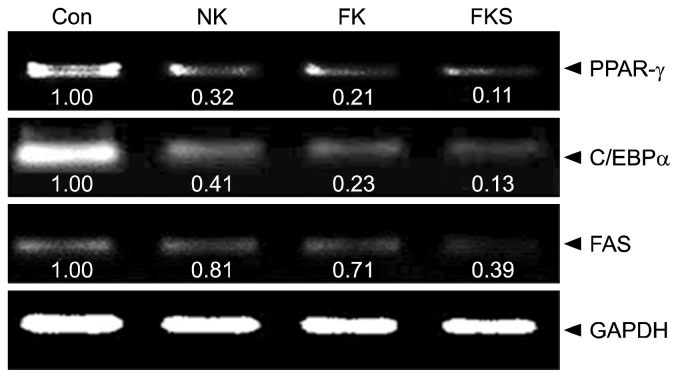
In the present study, treatment with kimchi samples markedly suppressed adipogenesis- and levels of lipogenesis related factors. As shown in Fig. 2, the FKS group expressed PPAR-γ at the lowest level (0.11 times that of the control), followed by the FK group (0.21 times) and the NK group (0.32 times). Cells treated with FKS had lowest C/EBPα expression (0.13 times that of the control), followed by the FK group (0.23 times) and the NK group (0.41 times). In addition, the FKS group had lowest FAS expression (0.39 times that of the control), followed by the FK group (0.71 times) and the NK group (0.81 times).
Fig. 2.
Effect of kimchi samples on the mRNA expressions of PPAR-γ, C/EBPα, and FAS in 3T3-L1 adipocytes. The PCR products were quantified and normalized GAPDH (internal control). Band intensities were measured using a densitometer and expressed as folds versus treatment naïve controls. Fold increase=gene expression/GAPDH×control value (control fold increase=1). NK, naturally fermented kimchi; FK, functional kimchi; FKS, functional kimchi produced using prebiotic starters.
Lab. plantarum has been reported to reduce adipogenesis and lipogenesis by inhibiting the activations of PPAR-γ, C/EBPα, and FAS in 3T3-L1 adipocytes (19,20), Leuconostoc sp. and Lactobacillus sp. have been reported to suppress the development of obesity (10,11), and starter (Leu. mesenteroides)-fermented kimchi has been reported to regulate adipogenesis- and lipogenesis-related genes (e.g., PPARγ, C/EBPα, and FAS) in obese mice (4). The use of a kimchi starter has been shown to affect metabolite production and kimchi LAB communities during fermentation (12). In another study, LAB starters were not only found to affect the microbial environment during fermentation but to become the dominant species in finished kimchi (12,13). In the present study, FKS was found to contain more LABs than NK or FK (FKS: 4.8×108 CFU/g, NK: 3.9×107 CFU/g, FK: 3.3×107 CFU/g after 3 weeks fermentation at 5°C), which showed that the starter kimchi contained more LAB. We also found the taste and quality of FKS was better than that of NK, presumably due to improved regulation during fermentation (data not shown). Therefore, abundant LAB might have inhibited adipogenesis in 3T3-L1 cells and improved taste because of health-beneficial LAB and fermentation products of LAB. These observations suggest that LABs contribute to the health benefits of kimchis by suppressing adipocyte differentiation by PPAR-γ, C/EBPα, and FAS-based adipogenesis/lipogenesis.
In conclusion, NK exhibited anti-obesity effects, but functional kimchi, which contained green tea, had greater anti-obesity effects than NK, and FKS had even better anti-obesity effects than FK. These findings indicate the selection of better subingredients and use of proven pro-biotic LAB starters are essential for promoting the anti-obesity efficacy of kimchi.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Park KY, Rhee SH. Functional foods from fermented vegetable products: Kimchi (Korean fermented vegetables) and functionality. In: Shi J, Ho CT, Shahidi F, editors. Asian Functional Foods. CRC Press Inc; Boca Raton, FL, USA: 2005. pp. 341–380. [Google Scholar]
- 2.Choi WY. PhD Dissertation. Pusan National University; Busan, Korea: 2001. Development of green tea added kimchi and its chemopreventive and antiobesity effects. [Google Scholar]
- 3.Lee KY. MS Thesis. Pusan National University; Busan, Korea: 2015. Survey on middle and high school students kimchi intake patterns and studies on the development of kimchi for adolescent. [Google Scholar]
- 4.Cui M, Kim HY, Lee KH, Jeong JK, Hwang JH, Yeo KY, Ryu BH, Choi JH, Park KY. Antiobesity effects of kimchi in diet-induced obese mice. J Ethnic Foods. 2015;2:137–144. doi: 10.1016/j.jef.2015.08.001. [DOI] [Google Scholar]
- 5.Sheo HJ, Seo YS. The effects of dietary Chinese cabbage kimchi juice on the lipid metabolism and body weight gain in rats fed high-calories-diet. J Korean Soc Food Sci Nutr. 2004;33:91–100. doi: 10.3746/jkfn.2004.33.1.091. [DOI] [Google Scholar]
- 6.Kim HY, Song JL, Chang HK, Kang SA, Park KY. Kimchi protects against azoxymethane/dextran sulfate sodium-induced colorectal carcinogenesis in mice. J Med Food. 2014;17:833–841. doi: 10.1089/jmf.2013.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bong YJ. MS Thesis. Pusan National University; Busan, Korea: 2014. Probiotic effects of kimchi lactic acid bacteria (LAB) and increased health functional of baechu kimchi by LAB starters. [Google Scholar]
- 8.Jung JY, Lee SH, Jin HM, Hahn Y, Madsen EL, Jeon CO. Metatranscriptomic analysis of lactic acid bacterial gene expression during kimchi fermentation. Int J Food Microbiol. 2013;163:171–179. doi: 10.1016/j.ijfoodmicro.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Park KY, Jeong JK, Lee YE, Daily JW., III Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J Med Food. 2014;17:6–20. doi: 10.1089/jmf.2013.3083. [DOI] [PubMed] [Google Scholar]
- 10.Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, Sung MK, McGregor RA, Choi MS. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8:e59470. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rico CW, Shin JH, Um IC, Kang MY. Cholesterol-lowering action and antioxidative effects of microbial gum in C57BL/6N mice fed a high fat diet. Biotechnol Bioprocess Eng. 2011;16:167–172. doi: 10.1007/s12257-010-0122-z. [DOI] [Google Scholar]
- 12.Jung JY, Lee SH, Lee HJ, Seo HY, Park WS, Jeon CO. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int J Food Microbiol. 2012;153:378–387. doi: 10.1016/j.ijfoodmicro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Jung JY, Lee SH, Jeon CO. Kimchi microflora: history, current status, and perspectives for industrial kimchi production. Appl Microbiol Biotechnol. 2014;98:2385–2393. doi: 10.1007/s00253-014-5513-1. [DOI] [PubMed] [Google Scholar]
- 14.Yazdi FT, Behbahani BA, Mohebbi M, Mortazavi A, Ghaitaranpour A. Effect of temperature on microbial changes during kimchi fermentation. Sci J Microbiol. 2013;2:9–14. [Google Scholar]
- 15.Higashikawa F, Noda M, Awaya T, Nomura K, Oku H, Sugiyama M. Improvement of constipation and liver function by plant-derived lactic acid bacteria: a double-blind, randomized trial. Nutrition. 2010;26:367–374. doi: 10.1016/j.nut.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 17.Lee MS, Kim CT, Kim IH, Kim Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytother Res. 2009;23:1088–1091. doi: 10.1002/ptr.2737. [DOI] [PubMed] [Google Scholar]
- 18.Lee KH, Bong YJ, Lee HA, Kim HY, Park KY. Probiotic effects of Lactobacillus plantarum and Leuconostoc mesenteroides isolated from kimchi. J Korean Soc Food Sci Nutr. In press. [Google Scholar]
- 19.Park DY, Ahn YT, Huh CS, Jeon SM, Choi MS. The inhibitory effect of Lactobacillus plantarum KY1032 cell extract on the adipogenesis of 3T3-L1 cells. J Med Food. 2011;14:670–675. doi: 10.1089/jmf.2010.1355. [DOI] [PubMed] [Google Scholar]
- 20.Park JE, Oh SH, Cha YS. Lactobacillus plantarum LG42 isolated from gajami sik-hae inhibits adipogenesis in 3T3-L1 adipocyte. BioMed Res Int. 2013;2013:460927. doi: 10.1155/2013/460927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YJ, Yu SY, Lee JS, Kim MD, Lee DW, Kim KJ, Lee OH. Anti-adipogenic and anti-oxidant activities of mugwort and pine needles fermented using Leuconostoc mesenteroides 1076. Food Biotechnol. 2014;28:79–95. doi: 10.1080/08905436.2014.895945. [DOI] [Google Scholar]
- 22.Moussalli C, Downs RW, May JM. Potentiation by glucose of lipolytic responsiveness of human adipocytes. Diabetes. 1986;35:759–763. doi: 10.2337/diab.35.7.759. [DOI] [PubMed] [Google Scholar]
- 23.Kao YH, Hiipakka RA, Liao S. Modulation of obesity by a green tea catechin. Am J Clin Nutr. 2000;72:1232–1234. doi: 10.1093/ajcn/72.5.1232. [DOI] [PubMed] [Google Scholar]
- 24.Cornelius P, MacDougald OA, Lane MD. Regulation of adipocyte development. Annu Rev Nutr. 1994;14:99–129. doi: 10.1146/annurev.nu.14.070194.000531. [DOI] [PubMed] [Google Scholar]
- 25.Farmer SR. Regulation of PPARγ activity during adipogenesis. Int J Obes (Lond) 2005;29:S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 26.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]