Abstract
OBJECTIVE
Little information is available regarding doses of ionizing radiation from medical imaging in the growing population of children undergoing therapy for cancer who are at risk of developing second cancers. The purpose of our study was to estimate the potential excess lifetime cancer incidence and mortality associated with thallium bone imging in pediatric patients.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of pediatric patients treated between August 1991 and December 2003 for newly diagnosed osteosarcoma who underwent 201Tl imaging as part of the treatment protocol. According to age at diagnosis and doses of 201Tl, we estimated the excess cancer incidence and cancer mortality for boys and girls at 5 and 15 years old.
RESULTS
The study cohort consisted of 73 patients, 32 males (median age at diagnosis, 14.8 years; age range, 8.1–20.1 years) and 41 females (median age at diagnosis, 13.3 years; age range, 6.0–20.7 years). Patients underwent a total of three 201Tl studies with a median dose of 4.4 mCi (162.8 MBq) (range, 2.2–8.4 mCi [81.4–310.8 MBq]) per study. Total median cumulative patient radiation dose for 201Tl studies was 18.6 rem (186 mSv) (range, 8.4–44.2 rem [84–442 mSv]) for males and 21.5 rem (215 mSv) (range, 7.0–43.8 rem [70–438 mSv]) for females. Estimated excess cancer incidence was 6.0 per 100 (male) and 13.0 per 100 (female) if exposed by 5 years of age; 2.0 per 100 (male) and 3.1 per 100 (female) by 15 years of age. Estimated excess cancer mortality was 3.0 per 100 for males and 5.2 per 100 for females at 5 years of age; 1.0 per 100 (male) and 1.4 per 100 (female) exposed at 15 years of age.
CONCLUSION
Further reduction of doses in younger patients is needed to consider 201Tl a viable option for imaging osteosarcoma.
Keywords: 201Tl, osteosarcoma, pediatric cancer risk, thallium-201
Children diagnosed and undergoing therapy for cancer are a growing population at increased risk of long-term sequelae related to treatment toxicities. This cohort is also vulnerable to development of second cancers that may result from inherent genetic predisposition, environmental exposure, or treatment exposure. However, the likelihood of adverse late effects must be weighed against life-saving therapy.
Health care providers for children and adolescents have a responsibility not only to provide specialized medical imaging to these patients but also to understand the implications of such imaging. Abundant literature is available about excess cancer incidence related to radiation exposure that may be induced in healthy children from either medical imaging or radiation therapy as was historically performed for thymic hypertrophy. However, considerably less information is available regarding doses of ionizing radiation from medical imaging in children undergoing therapy for a life-threatening disease such as cancer.
Disease- and cell-specific physiologic imaging using multiple radionuclides provides important information regarding metabolic activity of tumors and other processes that guide clinicians in patient management. It is to the benefit of patients that health care providers understand the implications of such imaging, the risks involved, and the risk–benefit ratios of such imaging in the context of patient treatment. We can expect that most children with cancer would lose their life to disease if left untreated. However, such a cohort may also be at greatest risk for adverse toxicities related to imaging methods. Cancer risk is multifactorial, and the information obtained from an analysis such as the one contained herein is but one piece of estimating cancer risk. We present this article in the spirit of advancing the principles of minimizing patient exposure to ionizing radiation associated with medical imaging—as low as reasonably achievable—as an example of estimating potential risk to patients from medical imaging while considering the advantages of such imaging.
Clinical assessment of the therapeutic response of osteosarcoma, the most common primary bone cancer in children, is unreliable. Pain severity, tumor size, and standard imaging characteristics of the primary tumor have all been used unsuccessfully to assess therapeutic response before surgery. Routine bone scintigraphy using 99mTc-methylene diphosphonate is sensitive to the detection of primary and metastatic osteosarcoma but is nonspecific in differentiating responsive from unresponsive disease, infection from tumor or trauma, and so on. In our therapeutic osteosarcoma protocol (OS-91) [1], we explored the utility of 201Tl bone scintigraphy in assessing tumor response to preoperative chemotherapy and prognosis in pediatric patients with osteosarcoma. The pattern of central tumor photopenia (donut avidity) was found to be a predictor of lower event-free survival [2]. In our subsequent therapeutic osteosarcoma protocol (OS-99) [3], we continued our evaluation of 201Tl imaging. However, this evaluation was halted in December 2003 because of concerns about radiation exposure and potential increased risk for excess lifetime cancer mortality, especially in young children. Our concern prompted us to perform this analysis of patients enrolled in OS-91 and OS-99 who underwent thallium scintigraphy to assess its safety in pediatric patients by calculating the effective radiation dose received by the patients and by estimating the risk for excess lifetime cancer incidence and mortality.
Materials and Methods
We retrospectively reviewed the medical records and 201Tl imaging of pediatric patients treated at our institution between August 1991 and December 2003 for newly diagnosed osteosarcoma who underwent 201Tl imaging as part of our OS-91 and OS-99 protocols. We recorded patient demographics, dates of diagnosis, dates of 201Tl imaging, and 201Tl doses. The HIPAA-compliant study was approved by our institutional office of human protection.
Thallium Technique
The 201Tl doses were adjusted for body surface area and administered IV. Imaging was initiated approximately 30 minutes after injection. Each image (cardiac image; anterior, posterior, and lateral views of the primary tumor; and anterior thigh background) was acquired for 600,000 counts; the time of acquisition was recorded. Images were obtained with a low-energy all-purpose or low-energy high-resolution collimator using a single anterior detector for image acquisition.
Protocol Treatment
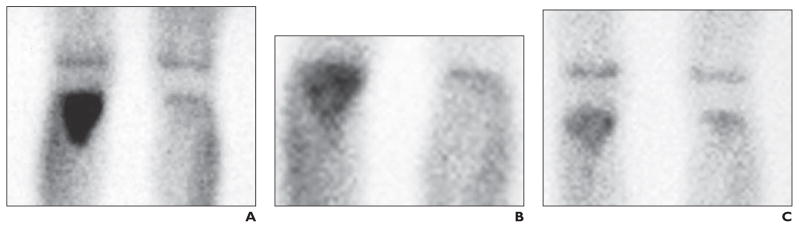
The two protocols (OS-91 and OS-99) incorporated multiagent chemotherapy and aggressive surgery for treatment of newly diagnosed osteosarcoma. OS-91 therapy consisted of 13 cycles of chemotherapy administered every 3 weeks for a total of 38 weeks; surgery for local control was performed after three cycles of neoadjuvant chemotherapy (at week 9) [1]. OS-99 therapy consisted of 12 cycles of chemotherapy administered every 3 weeks for a total of 35 weeks; surgery for local control was performed after four cycles of neoadjuvant chemotherapy (at week 12). In both protocols, thallium scanning was performed at baseline before starting therapy, after two cycles (OS-91) or three cycles (OS-99) of neoadjuvant chemotherapy (to assess changes that could indicate an early response to therapy), and before surgery for local control (to allow optimal correlation between imaging findings and histologic response to therapy (Fig. 1).
Fig. 1. 13-year-old boy with proximal right tibial osteosarcoma. Thallium imaging was performed at diagnosis, after 9 weeks of neoadjuvant chemotherapy, and just before surgical resection for local tumor control. Histologically, tumor responded to neoadjuvant chemotherapy.
A, In 201Tl image obtained at time of diagnosis, intense uptake of 201Tl is present in proximal right tibial diametaphyseal osteosarcoma.
B, After 9 weeks of chemotherapy, 201Tl image shows uptake of 201Tl has diminished.
C, In 201Tl image obtained just before surgical resection, tumor avidity for 201Tl has diminished further and mildly exceeds that of normal bone.
Estimation of Radiation Dose and Cancer Incidence and Mortality
Our osteosarcoma patients received three separate IV doses of 201Tl-chloride during a 3-month period. The radiation dose estimates for children of different ages and for various nuclear medicine procedures have been published [4]. Using the IV-administered 201Tl-chloride data [4], the effective dose (ED) in rem per millicurie was plotted as a function of a patient’s age (Fig. 2). Each patient’s total ED was then calculated by multiplying the age-specific ED/mCi, as noted in Figure 2, by the sum of the three doses administered. The calculation was
Fig. 2.
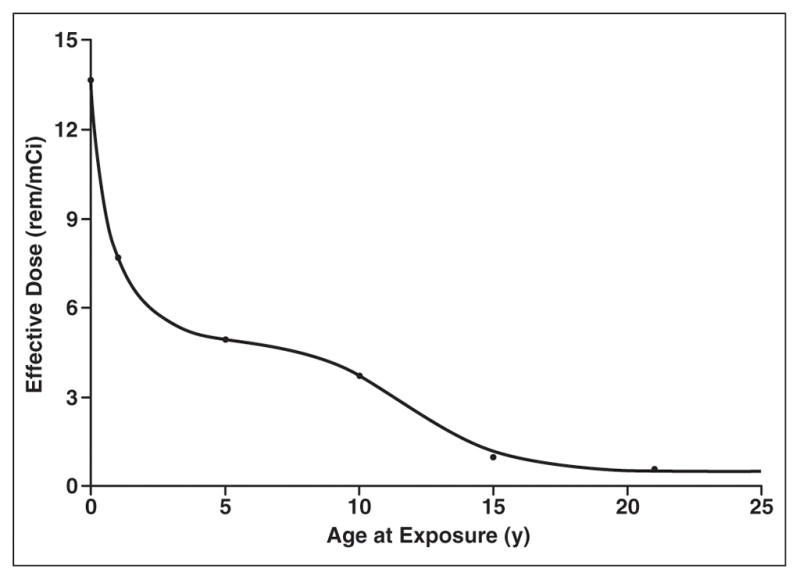
Graph shows estimated radiation dose per mCi of 201Tl-chloride by IV injection according to patient age at exposure.
Updated lifetime attributable risk (LAR) estimates for cancer due to radiation exposure have been recently published [5], with information on sex- and age-specific risk estimates for all cancer, leukemia, all solid cancer, and cancer of several specific sites. This information is presented as the number of excess incidences of cancer or cancer deaths in a population of 100,000 exposed to 0.1 Gy. In terms of ED, the 0.1 Gy would be equivalent to 10 rem (100 mSv). Using the total EDrem for our osteo sarcoma patients and the published LAR estimates for all cancers, we calculated the estimated LAR for all excess cancer incidence and mortality for a population of 100 osteosarcoma patients as follows:
Results
The study cohort was composed of 73 patients, 32 males (median age at diagnosis, 14.8 years; age range, 8.1–20.1 years) and 41 females (median age at diagnosis, 13.3 years; age range, 6.0–20.7 years). Patients underwent a total of three 201Tl studies with a median dose of 4.4 mCi (162.8 MBq) (range, 2.2–8.4 mCi [81.4–310.8 MBq]) per study. The median total cumulative radiation dose per patient for all 201Tl studies identified in this cohort was 18.6 rem (186 mSv) (range, 8.4–44.2 rem [84–442 mSv]) for males and 21.5 rem (215 mSv) (range, 7.0–43.8 rem [70–438 mSv]) for females.
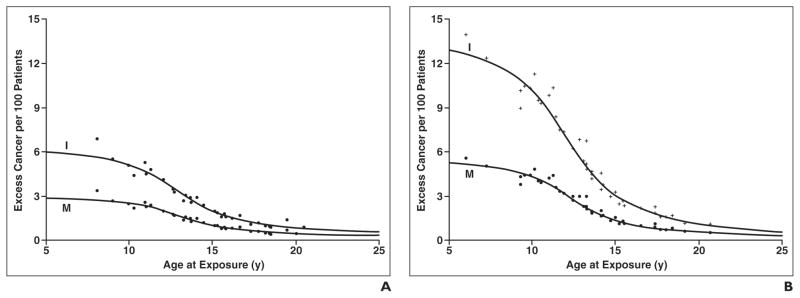
On the basis of the cumulative 201Tl doses, we calculated the estimated excess cancer incidence and mortality by sex and age, as noted in Table 1 and Figure 3. The excess cancer incidence was 6.0 per 100 for males and 13.0 per 100 for females undergoing imaging at 5 years old and decreased to 2.0 per 100 for males and 3.1 per 100 for females undergoing imaging at 15 years old. The excess cancer mortality was estimated to be 3.0 per 100 for males and 5.2 per 100 for females undergoing imaging at 5 years old and decreased to 1.0 for males and 1.4 per 100 for females undergoing imaging at 15 years old.
TABLE 1.
Estimated 201Tl-Related Excess Cancer Incidence and Mortality per 100 Patients by Sex and Age
| Sex | Age at Exposure (y)
|
||||
|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | |
|
| |||||
| Male | |||||
| Incidence | 6.0 | 5.1 | 2.0 | 0.9 | 0.6 |
| Mortality | 3.0 | 2.6 | 1.0 | 0.5 | 0.3 |
| Female | |||||
| Incidence | 13.0 | 10.3 | 3.1 | 1.2 | 1.0 |
| Mortality | 5.2 | 4.4 | 1.4 | 0.5 | 0.3 |
Fig. 3. Graphs of excess incidence and mortality.
A and B, Charts show estimated 201Tl-related excess cancer incidence (I) and mortality (M) per 100 males (A) and females (B).
Discussion
We have shown that there is a considerable potential risk of increased cancer incidence and mortality related to radiation exposure in pediatric patients who undergo thallium scintigraphy for disease assessment during therapy for osteosarcoma. Although the doses administered were adjusted according to the body surface area, young children received the greatest effective radiation dose per mCi (37 MBq) of 201Tl-chloride administered. As shown in Figure 2, the estimated radiation dose ranged widely from about 13.5 rem/mCi (135 mSv/37 MBq) in infants to about 1 rem/mCi (10 mSv/37 MBq) in children 15 years old. In parallel, as shown in Figure 3, the estimated cancer incidence and mortality are greatest not only in young children under 15 years old but also are approximately twice as great in girls compared with boys. These findings are not surprising given that younger age begets considerably more sensitivity to ionizing radiation as does the exquisite sensitivity of female breast tissue [6, 7]. However, this risk is merely one factor in the equation for determining the use of thallium scintigraphy as an imaging technique in children undergoing treatment for osteosarcoma.
With the use of aggressive surgery and multiagent chemotherapy, approximately 70% of patients with localized osteosarcoma and approximately 30% of those with metastatic disease (about 20% of all patients with osteosarcoma) survive [8–12]. The presence or absence of metastasis at diagnosis and histologic response to neoadjuvant chemotherapy are strong predictors of patient outcome [9, 13–16]. However, the latter cannot be assessed until the time of definitive surgery, which is usually performed several weeks after pre-operative chemotherapy. Because responding tumors may not show shrinkage by static imaging techniques, there is a need to identify noninvasive methods to assess tumor response early during treatment to optimize patient management.
Thallium 201 is a potassium analog that has been used extensively for myocardial imaging. Uptake of 201Tl is dependent on a functioning cell membrane sodium–potassium pump system and is therefore an indicator of cell viability [17]. Because there is little concentration of 201Tl in normal bone or bone marrow, increased lesion avidity for the radiopharmaceutical agent reflects increased cellular activity of the tumor. Several investigations of patients with osteosarcoma have shown unchanged thallium avidity of non-responsive tumors and decreased avidity in tumors with histologic evidence of good response to chemotherapy [17–19]. In our experience, thallium scintigraphy has good accuracy for detecting the primary tumor in osteosarcoma, and the pattern of central photopenia correlates with a worse prognosis [2, 20]. Central photopenia is unlikely to reflect central ossification but may be due to central necrosis [20].
Although preliminary experience suggests that 201Tl may be clinically useful, our findings raise concerns about radiation exposure from 201Tl and potential increased risk for excess lifetime cancer mortality in pediatric patients with osteosarcoma. Five-year survivors of childhood and adolescent cancer are known to have a higher mortality rate when compared with age- and sex-specific expected mortality rates in the general population [21–23]. The overall risk of death from the original cancer is 6–7% but is worse for patients with leukemia, brain tumors, or bone tumors, and the risk of treatment-related death, including second cancers, is 2%. A second cancer may result from therapy or may reflect host factors. A 2001 report from the Childhood Cancer Survivor Study showed a 10.8-fold excess in overall all-cause mortality among 5-year survivors, and the risk of death was significantly higher in females (standardized mortality ratio [SMR], 18.2), and individuals diagnosed with cancer before the age of 5 years (SMR, 14.0) [22].
Because SMR calculations are based on the observed and expected numbers of deaths, the higher SMR in females was attributed partly to the overall higher expected numbers of deaths for males compared with females. Although recurrent disease was the leading cause of death, accounting for 67%, there was a significant excess mortality rate due to secondary or subsequent cancer (12.7%), which was related to treatment with radiation, alkylating agents, and epipodophyllotoxins. Another study that assessed the risk of death in patients who survived more than 5 years after diagnosis of childhood cancer in the Nordic countries found an estimated overall mortality rate of 14% at 25 years after diagnosis [23]. The corresponding expected mortality rate was 2%, showing that most of the deaths represented excess mortality relative to the general population. Higher mortality rates were observed among males (mortality at 25 years was 15% in males compared with 12% for females). The overall SMR was 10.8, mainly due to high excess mortality from the primary cancer. The SMR for a second cancer was 4.9 and for noncancer death 3.1. The biggest challenge for pediatric oncologists and radiologists is how to optimize therapy for the primary cancer to improve patient outcome while minimizing the risk of late mortality from treatment-related effects and radiation exposure.
Although the use of thallium imaging in the management of osteosarcoma appears to be promising, the results of our study underscore the need to carefully consider the risk of exposure to ionizing radiation when developing protocols that incorporate imaging studies. The use of 201Tl imaging may be acceptable in mid and older adolescent patients if used with 201Tl doses lower than ours or when other tools to assess tumor response are not available. Other radionuclide imaging agents associated with less radiation exposure should be evaluated. For instance, 18F-FDG PET is a potentially useful technique in the management of osteosarcoma that needs prospective evaluation [24, 25]. MRI has no associated radiation exposure. Preliminary use of dynamic contrast-enhanced MRI is promising in assessing tumor response to therapy, but not all parts of the world have access to this technique and it presents unique challenges related to standardization of image acquisition, processing, and analysis [26].
Although we provide valuable information regarding doses of exposure to ionizing radiation associated with 201Tl imaging and potential excess risk of cancer, our study has limitations. The relatively short follow-up period of patients precludes assessment of the clinical impact of 201Tl bone scintigraphy on patient outcomes. Although the increased risks of second cancers and mortality have been addressed in survivors of childhood cancer, the complexity and interaction of many contributing factors and the role of 201Tl exposure in cancer development remain unclear.
In summary, we have documented the estimated lifetime excess cancer incidence and mortality related to 201Tl imaging in a pediatric cohort under treatment for osteosarcoma. We must be aware of the dose of ionizing radiation associated with medical imaging and incorporate the risks of morbidity and mortality and the potential benefits in the decision process of whether we use this imaging technique or not. From the analysis presented in this article, it is apparent that further reduction of doses in younger patients is needed to consider 201Tl as a viable imaging option in patients with osteosarcoma. Development of new technologies and imaging techniques to effectively image with smaller radionuclide doses would minimize the potential risk of late cancers related to 201Tl.
Acknowledgments
Supported in part by grants P30CA-21765 and P01CA-20180 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and a grant from the American Lebanese Syrian Associated Charities (ALSAC).
The authors thank Sandra Gaither for manuscript preparation and Lisa Mills for collection of 201Tl doses.
References
- 1.Meyer WH, Pratt CB, Poquette CA, et al. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St. Jude Children’s Research Hospital OS-91 trial. J Clin Oncol. 2001;19:171–182. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- 2.Kaste SC, Billips C, Tan M, et al. Thallium bone imaging as an indicator of response and outcome in nonmetastatic primary extremity osteosarcoma. Pediatr Radiol. 2001;31:251–256. doi: 10.1007/s002470000405. [DOI] [PubMed] [Google Scholar]
- 3.Rivera GK, Quintana J, Villarroel M, et al. Transfer of complex frontline anticancer therapy to a developing country: the St. Jude osteosarcoma experience in Chile. Pediatr Blood Cancer. 2008;50:1143–1146. doi: 10.1002/pbc.21444. [DOI] [PubMed] [Google Scholar]
- 4.Stabin MG, Gelfand MJ. Dosimetry of pediatric nuclear medicine procedures. Q J Nucl Med. 1998;42:93–112. [PubMed] [Google Scholar]
- 5.National Research Council of the National Academies Press. Health effects of exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 6.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res. 2005;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMahon B. Epidemiology and the causes of breast cancer. Int J Cancer. 2006;118:2373–2378. doi: 10.1002/ijc.21404. [DOI] [PubMed] [Google Scholar]
- 8.Meyers PA, Heller G, Healey JH, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11:449–453. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- 9.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 10.Bacci G, Briccoli A, Mercuri M, et al. Osteosarcoma of the extremities with synchronous lung metastases: long-term results in 44 patients treated with neoadjuvant chemotherapy. J Chemother. 1998;10:69–76. doi: 10.1179/joc.1998.10.1.69. [DOI] [PubMed] [Google Scholar]
- 11.Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 12.Daw NC, Billups CA, Rodriguez-Galindo C, et al. Metastatic osteosarcoma. Cancer. 2006;106:403–412. doi: 10.1002/cncr.21626. [DOI] [PubMed] [Google Scholar]
- 13.Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (Tl2) protocol. J Clin Oncol. 1998;16:2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 15.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 17.Rosen G, Loren GJ, Brien EW, et al. Serial Tl-201 scintigraphy in osteosarcoma: correlation with tumor necrosis after preoperative chemotherapy. Clin Orthop Relat Res. 1993;293:302–306. [PubMed] [Google Scholar]
- 18.Imbriaco M, Yeh SDJ, Yeung H, et al. Thallium-201 scintigraphy for the evaluation of tumor response to preoperative chemotherapy in patients with osteosarcoma. Cancer. 1997;80:1507–1512. [PubMed] [Google Scholar]
- 19.Kostakoglu L, Panicek DM, Divgi CR, et al. Correlation of the findings of thallium-201 chloride scans with those of other imaging modalities and histology following therapy in patients with bone and soft tissue sarcomas. Eur J Nucl Med. 1995;22:1232–1237. doi: 10.1007/BF00801605. [DOI] [PubMed] [Google Scholar]
- 20.McCarville MB, Barton EH, Cameron JR, et al. The cause and clinical significance of central tumor photopenia on thallium scintigraphy of pediatric osteosarcoma of the extremity. AJR. 2007;188:572–578. doi: 10.2214/AJR.06.0292. [DOI] [PubMed] [Google Scholar]
- 21.Simone JV. Late mortality in childhood cancer: two excellent studies bring good news tempered by room for improvement. J Clin Oncol. 2001;19:3161–3162. doi: 10.1200/JCO.2001.19.13.3161. [DOI] [PubMed] [Google Scholar]
- 22.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 23.Moller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol. 2001;19:3173–3181. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 24.McCarville MB, Christie R, Daw NC, et al. PET/ CT in the evaluation of childhood sarcomas. AJR. 2005;184:1293–1304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- 25.Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. J Nucl Med. 2003;44:930–942. [PubMed] [Google Scholar]
- 26.Reddick WE, Wang SH, Xiong XP, et al. Dynamic magnetic resonance imaging of regional contrast access as an additional prognostic factor in pediatric osteosarcoma. Cancer. 2001;91:2230–2237. [PubMed] [Google Scholar]