Abstract
All animals are ecosystems, home to diverse microbial populations.
Animal-associated microbes play important roles in the normal development and physiology of their hosts, but can also be agents of infectious disease. Traditionally, mice have been used to study pathogenic and beneficial associations between microbes and vertebrate animals. The zebrafish is emerging as a valuable new model system for host-microbe interaction studies, affording researchers with the opportunity to survey large populations of hosts and to visualize microbe-host associations at a cellular level in living animals. This chapter provides detailed protocols for the analysis of zebrafish-associated microbial communities, the derivation and husbandry of germ-free zebrafish, and the modeling of infectious disease in different stages of zebrafish development via different routes of inoculation. These protocols offer a starting point for researchers to address a multitude of questions about animals’ coexistence with microorganisms.
I. Introduction
No animal is ever truly alone, but instead each lives in constant association with single-celled microorganisms. Bacteria, Archaea, fungi, single-celled eukaryotes, and viruses are present both in and on the body from birth to death. These organisms have a spectrum of interactions with their hosts, ranging from beneficial contributions to host development and physiology to harmful infections. For example, beneficial microbes contribute unique enzymatic activities required to break down ingested food and make the caloric content accessible to the host. Additionally, mutualistic microbes promote the development of the digestive tract and the immune system. On the other hand, pathogenic microbes cause harm to the body, sometimes through the active release of toxins or through invasion and expansion in host tissue from which they are normally excluded. It is often tricky to define a host-associated microbe as a strict mutualist or a pathogen because the outcome of any host-microbe interaction can depend on the context of the association, including such factors as the microbial ecology and the immune status of the host.
As a framework to begin to define the functional consequences of a particular microbial interaction with a host, and in particular to ascribe the disease-causing capacity of a potential pathogen, the eminent founding father of microbiology, Robert Koch, defined a set of postulates in 1890 to determine whether a microorganism is the cause of a disease. These rules stipulated that:
The microorganism must be found in abundance in all organisms suffering from the disease, but should not be found in healthy organisms.
The microorganism must be isolated from a diseased organism and grown in pure culture.
The cultured microorganism should cause disease when introduced into a healthy organism.
The microorganism must be reisolated from the inoculated, diseased experimental host and identified as being identical to the original specific causative agent.
Over a century later, these postulates still provide a useful experimental approach for infectious disease researchers to establish causation between infection with a microbe and symptoms in a host, and have been extended to “molecular Koch’s postulates” to establish causation between specific microbial factors and host responses (Falkow, 1988). Such an experimental framework is relevant because infectious diseases continue to play a major role in the human condition worldwide. Since the discovery of microorganisms as the cause of infectious diseases, our ability to control and treat these diseases has advanced enormously with the development of sterile practices, vaccines, antibiotic drugs, and intervention strategies. However, microbial adaptation and environmental changes continue to trigger the emergence of new pathogens, fueling the need for alternative methods for studying infectious diseases.
A prerequisite for fulfilling Koch’s postulates is the existence of an animal model that can be infected with a microorganism. Traditionally mice have been the workhorse model for infectious disease researchers. An emerging model system in this field is the zebrafish, Danio rerio. The zebrafish model system offers numerous advantages, including external fertilization, large clutches, optical clarity during development, and the rapid development of organ primordia (within 5 days postfertilization [dpf]). These advantages first made zebrafish attractive to embryolo-gists and developmental geneticists, but the model has since been adopted by other fields, including toxicology, immunology, and infectious diseases. The model continues to evolve as an effective tool within the field of biomedicine as researchers learn to exploit its unique advantages to address their specific questions. Today large collections of transgenic zebrafish lines and molecularly defined mutants are available; molecular, forward, and reverse genetics techniques have been developed; the zebrafish genome project is nearly complete; and compelling human disease models have been created. In particular, the zebrafish model is now being used to answer questions about infectious disease and immunity (reviewed in Traver et al., 2003; Trede et al., 2004). Zebrafish rely solely on the innate immune response for approximately the first 30 days of development to protect against pathogen infection (Lam et al., 2004). This temporal segregation of innate versus adaptive immune response renders the zebrafish an excellent model for the study of infectious diseases. Table I, adapted from Kanther and Rawls (2010), provides a summary of the infection models that have been established to date in the zebrafish.
Table I.
Bacterial and viral infection models in zebrafish
| Pathogen | Infection method | Embryos/adults | Reference(s) |
|---|---|---|---|
| Bacteria | |||
| Aeromonas hydrophila | Injection, static immersion | Larvae, adult | Rawls et al. (2004, 2006), Rodriguez et al. (2008) |
| Aeromonas salmonicida | Static immersion | Adult | Lin et al. (2007) |
| Aeromonas veronii | Static immersion | Larvae | Bates et al. (2006) |
| Bacillus subtilus | Injection | Embryo | Herbomel et al. (1999) |
| Burkholderia cenocepacia | Injection | Embryo, adult | Deng et al. (2009), Vergunst et al. (2010) |
| Edwardsiella ictaluri | Injection | Adult | Petrie-Hanson et al. (2007) |
| Edwardsiella tarda | Injection, static immersion | Embryo, larvae, adult |
Nayak et al. (2007), Phelan et al. (2005a), Pressley et al. (2005) |
| Escherichia coli MG1655 | Static immersion | Larvae | Rawls et al. (2006, 2007) |
| E. coli O157:H7 | Static immersion | Larvae | Szabady et al. (2009) |
| Flavobacterium columnare | Injection, static immersion | Adult | Moyer and Hunnicutt (2007) |
| Flavobacterium johnsoniae | Injection, static immersion | Adult | Moyer and Hunnicutt (2007) |
| Francisella spp. | Injection | Adult | Vojtech et al. (2009) |
| Leptospira interrogansa | Injection | Embryo |
Davis and Ramakrishnan (2009), PMID 19547748 |
| Listeria monocytogenes | Injection | Embryo, adult | Levraud et al. (2009), Menudier et al. (1996) |
| Listeria spp. | Injection | Adult | Menudier et al. (1996) |
| Listonella anguillaruma | Injection, static immersion | Adult | Rojo et al. (2007) |
| Mycobacterium haemophiluma | Injection | Adult | Whipps et al. (2007) |
| Mycobacterium marinum | Injection, static immersion | Embryo, larvae, adult |
Adams et al. (2011), Carvalho et al. (2011), Clay et al. (2007, 2008), Davis et al. (2002), Davis et al. (2009), Gao et al. (2006), Harriff et al. (2007), Meijer et al. (2005), Stoop et al. (2011), Volkman et al. (2010) |
| Mycobacterium peregrinum | Static immersion | Adult | Harriff et al. (2007) |
| Pseudomonas aeruginosa | Injection, static immersion | Embryo, larvae |
Brannon et al. (2009), Clatworthy et al. (2009), Llamas et al. (2009), Phennicie et al. (2010), Rawls et al. (2004, 2006, 2007), Singer et al. (2010), Vasil et al. (2009) |
| Pseudomonas fluorescens | Static immersion | Larvae | Bates et al. (2006) |
| Salmonella arizonae | Injection | Embryo | Davis et al. (2002) |
| Salmonella typhimurium | Injection | Embryo, larvae |
Ordas et al. (2010), Stockhammer et al. (2009), van der Sar et al. (2003, 2006) |
| Staphylococcus aureus | Injection | Embryo, adult | Lin et al. (2007), Prajsnar et al. (2008) |
| Streptococcus iniae | Injection | Adult |
Lowe et al. (2007), Miller and Neely (2005), Neely et al. (2002) |
| Streptococcus pyogenes | Injection | Adult |
Bates et al. (2005), Cho and Caparon (2005), Kizy and Neely (2009), Montanez et al. (2005), Neely et al. (2002) |
| Vibrio anguillarum | Static immersion | Larvae | O’Toole et al. (2004) |
| Virus | |||
| Herpes simplex virus type 1 (HSV-1) |
Injection | Adult | Burgos et al. (2008) |
| Infectious hematopoietic necrosis virus (IHNV) |
Injection | Adult |
LaPatra et al. (2000), Ludwig et al. (2011), Wang et al. (2006) |
| Infectious pancreatic necrosis virus (IPNV) |
Injection | Adult | Garner et al. (2003), LaPatra et al. (2000) |
| Infectious spleen and kidney necrosis virus (ISKNV) |
Injection | Embryo, adult |
Wang et al. (2008), Xiong et al. (2011), Xu et al. (2008) |
| Nervous necrosis virus (NNV) | Injection | Larvae, adult | Lu et al. (2008) |
| Snakehead rhabdovirus (SHRV) | Injection, static immersion | Embryo, larvae, adult |
Alonso et al. (2004), Altmann et al. (2003, 2004), Hermann and Kim (2005), Nayak et al. (2007), Phelan et al. (2005a, 2005b) |
| Spring viremia of carp virus (SVCV) |
Injection, static immersion | Adult | Sanders et al. (2003), Wang et al. (2006) |
| Viral hemorrhagic septicemia virus (VHSV) |
Injection | Adult | Encinas et al. (2010), Novoa et al. (2006) |
A. Host–Pathogen Interactions
A number of factors must be taken into account when considering the zebrafish as an infection model. The zebrafish supports the growth and replication of a number of fish and human pathogens (Table I), but no animal model is suitable for propagation of all animal pathogens. When developing an infection model in the zebrafish, a researcher should consider questions such as: Can the pathogen be transmitted through the water or must it be injected? Is mimicking the natural route of infection essential for the infection model? What is the optimal temperature for the pathogen? Does the replication temperature of the pathogen match the maintenance temperature of the zebrafish? The maintenance temperature of the zebrafish may be varied to accommodate infection by a pathogen with a growth temperature range that is either higher or lower than 28 °C. If infection studies are initiated to study immune function of the host, the effects of varying the maintenance temperature outside of the host’s normal range should be carefully considered. Altering the temperature beyond the optimal range for the host may permit infection with a pathogen, but will not necessarily reflect the typical homeostasis between host and pathogen. A researcher should consider whether the temperature will change the host’s ability to resist or be susceptible to infection and whether the immune response to this infection will reflect a normal outcome.
Infection protocols presented here were developed to investigate host–pathogen interactions via a number of infection routes. With some pathogens, infections can be achieved by static emersion of embryos, larvae, or adults, but for many nonindigenous pathogens passive exposure does not result in acceptable rates of infection. Systemic infections with bacteria and virus can be established by injection of the pathogen into the duct of Cuvier or tail artery (Fig. 1b and c). Such models are useful for studying global responses to infection, such as cytokine profiles and activation of immune pathways as assayed by quantitative RT-PCR, luciferase assays, and respiratory burst assays (Hermann et al., 2004; Nayak et al., 2007; Sullivan et al., 2007, 2009). Pathogen injection into the hindbrain ventricle (Fig. 1a) typically leads to a contained infection. Localized infection models are useful for tracking the movement of neutrophils or macrophages to a site of infection (Davis et al., 2002; Phennicie et al.; Prajsnar et al., 2008).
Fig. 1.

Microinjection sites of zebrafish embryos. Three methods are commonly used for microinjection of pathogens into zebrafish embryos. The figure demonstrates microinjection into the (a) hindbrain ventricle (HBV), (b) duct of Cuvier (DC), and (c) tail artery of a 48 hpf zebrafish embryo.
Several genetic tools are available to aid in the study of the immune reaction to infections in zebrafish. A number of transgenic zebrafish reporter lines are useful for in vivo studies of infections, including those with fluorescent protein–labeled macrophages and neutrophils (Ellett et al., 2011; Gray et al., 2011; Lawson and Weinstein, 2002; Mathias et al., 2006; Renshaw et al., 2006). Zebrafish lines with immune deficiencies also exist, including loss-of-function alleles of csf1ra and rag1 (Parichy et al., 2000; Wienholds et al., 2002), which lack macrophage and mature lymphocytes, respectively. Antisense morpholinos are frequently used to knock down specific immune-related genes transiently, to discern their relevance and significance during infection. For example, spic-morpholinos inhibit myeloid development for the first 5 dpf and prevent macrophage and neutrophil development (Rhodes et al., 2005; Su et al., 2007). Injection of morpholinos that target myd88 significantly impairs clearance of Salmonella enterica (van der Sar et al., 2006). Several IFN-related genes such as crfb1–8, crfb12–17, ifnph1, ifng1, and infg2 can be targeted for knockdown and observed during viral and bacterial infections (Aggad et al., 2009, 2010). By taking advantage of the optical clarity of the embryo, these transgenic or mutant lines can be used in conjunction with fluorescent protein–labeled pathogens to assist in the characterization of host–pathogen interactions.
Several of the protocols outlined in this chapter were developed for infection of zebrafish with specific pathogens, including snakehead rhabdovirus, Edwardsiella tarda, or Pseudomonas aeruginosa. Adaptation of these protocols to other pathogens will be governed by the specific organism under investigation.
B. Host–Microbiota Interactions
As noted previously, Koch’s postulates are useful for establishing causation between a putative pathogen or virulence determinant and a disease, but the same logic can be applied to investigations into mutualisms between microbes and animals. There are several beautiful examples of animal mutualisms in which the presence of a single microbe profoundly shapes the development or physiology of an animal host, such as the gut endosymbionts of insects and the bioluminescent symbionts of squid (Fraune and Bosch, 2010). Vertebrates, however, are typically associated with complex microbial communities (microbiota) that are difficult to characterize and often recalcitrant to culture in the lab. With a loosening of the requirement for the growth of the microorganism in pure culture, Koch’s postulates can be applied to understanding the effects of these complex microbial communities on their hosts. The collective effect of the microbial community can be evaluated by the comparison of developmental, physiological, and immune markers between conventionally colonized and “germ-free” animals (which lack the microbial community). Alternatively, the effects of individual or subsets of culturable microbes can be evaluated in monoassociated animals, in which a single microbe is introduced into an otherwise germ-free animal, or animals with simple, defined microbial communities. Finally the microbiota’s collective effects can be approximated by transplantation of microbial communities harvested from one donor host into a germ-free recipient host. All together, these experiments can provide powerful evidence for the roles of microbial associations in normal animal development and physiology.
The mouse has been the traditional animal used in the field of gnotobiology (Greek for “known life”), in which the microbial associates of animals are entirely defined. Recently, the zebrafish has emerged as a powerful new gnotobiotic model. The ex utero development of the zebrafish allows for easy surface sterilization of the embryo’s chorion, facilitating the derivation of thousands of germ-free animals at a time. Although we have not yet established methods to rear germ-free zebrafish to adulthood, as is possible for germ-free mice, recent husbandry advances have resulted in maintenance of germ-free fish through a month of age, potentially to the onset of adaptive immunity. Studies of germ-free mice and zebrafish have revealed a number of common differences from their conventionally reared counterparts, indicating a conserved vertebrate program of responses to their microbiota. These responses are listed in Table II.
Table II.
Germ-free traits shared between zebrafish and rodents
| Germ-free trait | Zebrafish | Rodents |
|---|---|---|
| Reduced cell proliferation (as measured by incorporation of nucleotide analogues) |
Cheesman et al. (2011), Rawls et al. (2004, 2006) |
Savage et al. (1981) |
| Reduced numbers of goblet cells | Bates et al. (2006) | Kandori et al. (1996) |
| Altered expression of genes involved in metabolism (e.g., fasting-induced adipose factor) |
Kanther et al. (2011), Rawls et al. (2004, 2006) |
Hooper et al. (2001) |
| Reduced expression of genes involved in innate immunity (e.g., serum amyloid A1) |
Kanther et al. (2011), Rawls et al. (2004, 2006) |
Hooper et al. (2001) |
| Reduced numbers of intestinal associated immune cells |
Bates et al. (2007) (fewer intestinal neutrophils) |
Bouskra et al. (2008), Cebra et al. (1998) (fewer lamina propria cells and lymphoid follicles) |
| Differences in glycan expression | Bates et al. (2006) | Bry et al. (1996) |
| Altered gut motility |
Bates et al. (2006) (increased in germ-free) |
Husebye et al. (1994) (decreased in germ-free) |
This chapter provides the tools for the zebrafish researcher to fulfill Koch’s postulates to establish a functional connection between the presence of a microorganism and an effect on the host. We will begin by addressing the first postulate of characterizing the microbial associates of the zebrafish. We provide protocols for culture-dependent and -independent enumeration of associated bacteria from the intestine, but these can be extended to other anatomical sites and classes of microorganisms. We then provide protocols for the derivation and rearing of germ-free zebrafish, and methods for the generation of zebrafish with defined microbial associates. Finally we provide a series of protocols for infecting zebrafish at different ages and via different routes with different classes of infectious agents. These protocols are designed to provide researchers with the starting point for a diversity of experiments. We leave the final analysis of the experiments – the particular methodologies of 16S rRNA gene sequence analysis and the endpoint analyses of gnotobiotic and infection experiments, including microbiological measurements, pathological assessments, and molecular measures of host responses – to the experimenter.
II. Laboratory Protocols
A. Characterization of Microbial Communities in the Gut
Bacterial communities that reside in the vertebrate guts are not homogenous, but include a wide taxonomic diversity of organisms. Not all of the organisms are cultivable outside of the zebrafish gut, but a good representation of the bacteria present in the gut can be inferred from culturing. The choice of media used to culture the bacteria will depend on what organisms are targeted for culturing. For a broad idea of what is present, aerobic growth of intestinal material on tryptic soy agar (TSA) is sufficient. For isolation of anaerobic bacteria, anaerobe plates made with Oxoid Wilkins-Chalgren anaerobe agar and enriched with equine blood should be incubated in anaerobic chambers. However, some bacteria are present in low numbers, are outcompeted by other organisms due to slow growth rates, or selectively grow on media higher in some nutrients. For example, to isolate Fusobacteria, intestinal material should be plated on Fusobacteria-selective agar, which contains low levels of drugs that will inhibit growth of most Gram-positive and Gram-negative anaerobes, and incubated in an anaerobic chamber to enrich for those bacteria. (See Buller (2004) for lists of different media used to isolate specific strains.)
1. Gut Dissections
Materials
Insect pins and holders (Fine Science Tools, cat. no. 26002-15, 26018-17)
3-5% methylcellulose (viscosity is a matter of preference)
Glass slides
0.4% tricaine in embryonic medium
- 0.4% tricaine (AKATricaine-S; tricaine methanesulfonate; MS-222; 3-aminobenzoic acid ethyl ester (Western Chemical, Inc.)):
- 400 mg tricaine
- 97.9 mL H2O
- 2.1 mL Tris 1 M (pH 9.0)
Adjust to pH 7.0; filter sterilize with 0.22 μm filter
This can be made ahead of time and stored for long periods at 20° C or for up to 2 weeks at 4°C
Sterile embryonic medium (EM, sterilized through 0.22 μm filter; Westerfield, 2000)
200 μL aliquots of sterile EM in 1.5-mL tubes (one per sample)
- Motor and pestle (Pellet Pestle® Motor, Kontes; Pellet Pestle®, Kimble Chase Kontes)
-
(1)Immediately before dissections, anesthetize fish with 2.1 mL 0.4% tricaine.
-
(2)Briefly rinse fish by transferring to a clean Petri dish with sterile EM containing tricaine.
-
(3)On a clean slide, spread a thin layer of 3–5% methylcellulose using a sterile glass pipette or sterile wooden stick.
-
(4)Transfer fish from the rinse medium onto the methylcellulose, minimizing the amount of EM carried over. Remove remaining EM from the methylcellulose.
-
(5)Gently press the fish into the methylcellulose to immobilize it during dissection.
-
(6)Dissections:
-
(a)Insert one pin into the head of the fish and another through the mouth, as shown in Fig. 2a. Begin by pulling the lower jaw away from the head.
-
(b)Repeat the process further posterior. Insert one pin into the medial trunk of the animal, and the second pin immediately dorsal to the intestine. Pull the intestine ventrally with the second pin. At this point, the rest of the intestine will often begin to slide out of the animal’s body cavity fully intact.
-
(c)As needed, make additional cuts with the pins to separate the intestine from the rest of the body without puncturing the intestine. Pull the rest of the intestine out, taking care not to stretch it to the point of breaking.
-
(d)Depending on downstream application, remove liver from the anterior intestine, and remove mouth and esophagus anterior to intestinal junction (Fig. 2b).
-
(e)Use an insect pin to transfer intestines into a 1.5 mL tube containing 200 μL sterile EM, PBS, or other solution depending on downstream application.
-
(a)
-
(7)Homogenize intestines with motorized pestle and continue with sample preparation depending on experiment. This homogenate can be used to prepare genomic DNA for 16S rRNA gene sequencing, or can be plated to phenotypically characterize bacterial colonies or determine the colony-forming units (CFU) of known bacteria in the gut.
-
(1)
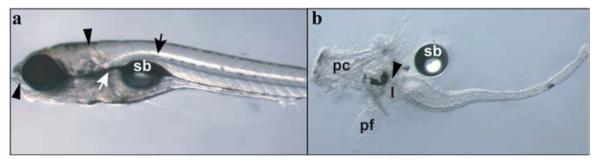
Fig. 2.
Dissection of zebrafish larval intestine. (a) Lateral view of 7 dpf larvae indicating initial (arrowheads) and subsequent (arrows) placement of pins for intestine dissection. (b) Seven dpf dissected intestine illustrating anatomical structures that are often pulled out with the intestine and are generally removed prior to analysis. The arrowhead indicates the esophageal–intestinal junction; sb, swim bladder; pc, pharyngeal cartilage; pf, pectoral fin; l, liver. (For color version of this figure, the reader is referred to the web version of this book.)
2. Plating Gut Microbiota
Materials
Insect pins and holders (Fine Science Tools, cat. no. 26002-15, 26018-17)
3-5% methylcellulose (viscosity is a matter of preference)
Glass slides
0.4% tricaine
200 μL aliquots of sterile EM in 1.5-mL tubes (one per sample)
Mortar and pestle (Pellet Pestle® Motor, Kontes; Pellet Pestle®, Kimble Chase Kontes)
Filter-sterilized EM
Sterilize 1.5 mL snap top tubes
TSA plates
- Beads
-
(1)Dissect guts and homogenize with pestles as described above in 200 μL of filter-sterilized EM in 1.5 mL snap top tube.
-
(2)Bring volume in tube up to 1 mL with filter-sterilized EM. Vortex to distribute bacterial cells evenly.
-
(3)Dilute homogenized, vortexed guts in filter-sterilized EM in sterile 1.5 mL snap top tubes. Most bacteria can be detected on plates diluted between 10−1 and 10−4.
-
(4)Plate bacteria diluted between 10−1 and 10−4 on medium of choice.
-
(5)Incubate plates at 28 °C between 24 and 48 h aerobically or anaerobically before counting.
-
(1)
3. Bacterial gDNA Extractions from Fish Intestines for 16S rRNA Gene Sequencing
The methods described here are for larval zebrafish intestines. The same techniques can be employed for adult intestines, although the elution volumes should be increased. The genomic DNA isolated using this protocol can be used for a variety of downstream applications including sequencing of 16S rRNA gene amplicons or the whole metagenome. Indeed, similar methods have already been successfully used to define the membership and structure of bacterial communities in the zebrafish intestine using 16S rRNA gene sequencing (Bates et al., 2006; Brugman et al., 2009; Rawls et al., 2004, 2006; Roeselers et al., 2011).
Column-based DNA isolation methods (such as Qiagen’s DNeasy columns) are often sufficient for isolating bacterial DNA from fish intestines and generally yield the highest quality DNA for downstream enzymatic reactions, including PCR. However, these methods rely on enzymatic digestion to lyse bacterial cells and may bias the final DNA composition toward certain species with membranes and/or cell walls that are more susceptible to digestion by these enzymes (Morgan et al., 2010). DNA extraction that includes physical disruption of bacterial cells by bead beating appears to be less biased, but tends to yield more variable downstream results. The purification method should be determined based on the purpose of the experiment. The Qiagen DNeasy blood and tissue kits, with the accompanying modification for Gram-positive bacteria, yield consistent 16S rRNA gene PCR amplicons from single 7 dpf zebrafish intestines. This is particularly useful if the intestines harbor a known community of bacteria. If the aim of the study is to describe an unknown community, the bead-beating protocol below may be a more suitable choice.
Materials
0.1 mm sterile zirconia/silica beads (Biospec Products, cat. no. 11079105z)
120 mM sodium phosphate buffer, pH 8.0, filter sterilized
SDS lysis buffer (10% SDS, 0.5 M Tris–Cl, pH 8.0, 0.1 M NaCl), filter sterilized
100 mg/mL lysozyme in sterile PBS. Make fresh each time
7.5 M ammonium acetate
95% EtOH
2 mL sterile screw cap tubes
Bead beater (Biospec Products, Inc.)
TE (pH 8.0) or Qiagen buffer EB, filter sterilized
- 1.5 ml sterile tube Isopropanol
-
(1)Combine in 2-mL screw top tubes:
-
(a)Fish intestines
-
(b)0.5 mL beads
-
(c)0.4 mL 120 mM sodium phosphate buffer
-
(d)0.2 mL SDS lysis buffer
-
(a)
-
(2)Bead beat on high 2 min for adult or 1 min for larval intestines.
-
(3)Remove supernatant to fresh tube and add lysozyme to 10 mg/mL final concentration.
-
(4)Incubate tubes for 45 min at 37 °C.
-
(5)Add 2 volumes 7.5 M ammonium acetate to 5 volumes lysis/DNA solution.
-
(6)Centrifuge at 13,000 × g for 5 min to precipitate protein.
-
(7)Transfer supernatant to new 1.5-mL tube.
-
(8)Add 0.7 volumes room temperature isopropanol and mix.
-
(9)Centrifuge at 13,000 × g for 30 min at 4 °C.
-
(10)Remove supernatant and wash pellet with ice-cold 95% EtOH. (Note: Single larval intestines do not generally yield enough DNA to generate a visible pellet.)
-
(11)Centrifuge at 13,000 × g for 5 min.
-
(12)Carefully remove supernatant from pellet and air dry pellet.
-
(13)Resuspend DNA pellet in TE (pH 8.0) or Qiagen buffer EB (10 mM Tris–Cl, pH 8.5).
-
(1)
4. Fluorescent in situ Hybridization for the Detection of Bacteria
Fluorescent in situ hybridization (FISH) with short, fluorophore-conjugated oligonucleotide probes targeting unique sequences in the bacterial 16S rRNA gene is commonly used to examine localization and abundance of known bacteria in a mixed community, such as the intestinal microbiota. Several databases exist that curate known probes (probeBase.org) and facilitate the design of novel probes (Greengenes, the Ribosomal Database Project, and SILVA). New probes must be optimized for specificity and hybridization conditions (particularly the percent of formamide). Here we describe a basic FISH protocol that works well on zebrafish paraffin sections (Bates et al., 2006).
Materials
4% Paraformaldehyde (PFA)
- Hybridization buffer:
- 20 mM Tris-HCl, pH 7.4
- 0.9 M NaCl
- 0.1% SDS
- 35% (v/v) formamide
Probe (short, fluorophore-conjugated oligo-nucleotides resuspended in nuclease free water)
70°C heat block or water bath
Hybridization chambers (Corning #2551)
48°C water bath
PBS, filter sterilized
PBSt (0.05% Triton X-100 in filter-sterilized PBS)
- Vectashield (Vector labs) or another mounting medium
-
(1)Fix 7 dpf larval fish in 4% PFA overnight with shaking. Adult fish should be gavaged with fixative, the head removed behind the gills and the tail removed behind the vent, incisions made on both flanks of the fish above the intestine but below and parallel to the spine, and fixed overnight in 4% PFA with shaking. Section the fish and paraffin fix the sections to slides.
-
(2)Deparaffin slides and rehydrate through decreasing EtOH concentrations into sterile ddH2O.
-
(3)Prewarm 200 μL hybridization buffer per slide at 70 °C.
-
(4)Dilute probes (final concentration is dependent on type of probe) in hybridization buffer and heat to 70 °C for 5 min.
-
(5)To equilibrate slide, add 200 μL prewarmed hybridization buffer without probes to each slide; let sit for 1 min, and then wick off excess with paper towel.
-
(6)Add 150 μL prewarmed, diluted probes to each slide.
-
(7)Add glass coverslips and place in hybridization chambers with 10 μL hybridization buffer in wells.
-
(8)Incubate at 48 °C overnight in the dark.
-
(9)Heat sterile PBS in 48 °C water bath.
-
(10)Remove slides from hybridization chambers and soak in Coplin jars with PBS at 48 °C for 10 min, or until coverslips fall off when slides are lifted out of Coplin jars.
-
(11)Rinse twice more, 10 min each, with PBS prewarmed to 48 °C; allow second rinse to come to room temperature.
-
(12)Rinse briefly with PBSt and mount with glycerol-based mounting media such as Vectashield.
-
(1)
B. Rearing Germ-Free or Gnotobiotic Zebrafish to 30 dpf
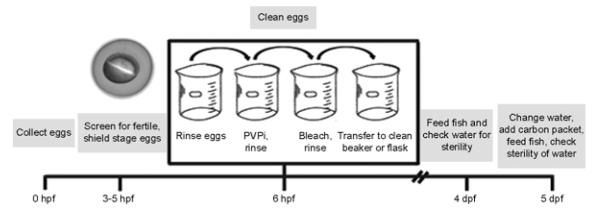
There are several different ways to fertilize eggs for the derivation of germ-free fish, each with advantages and disadvantages. Fertilized eggs may be obtained by surgical removal of gametes from adults, squeezing the eggs and sperm from the fish, and fertilizing the eggs in a sterile Petri dish, or by natural breeding. High success in generating bacteria-free zebrafish is achieved using the in vitro fertilization method. However, surgical removal of the gametes is not ideal as the adults must be euthanized, and the process of squeezing the gametes from live adult results in a shortened reproductive life and can only be performed every 2 weeks due to the stress on the fish. On the other hand, if a limited number of females are available for generating eggs, a conventional cross is sufficient and can be performed weekly, but usually results in higher contamination rates. Further discussion of these different fertilization methods can be found in Pham et al. (2008). Following the fertilization of the eggs, the sorting and cleaning of the eggs is the same for both methods, as outlined in Fig. 3. The protocol has been optimized to generate the cleanest eggs with the highest survival rate and lowest rate of abnormal development.
Fig. 3.
Timeline for generation and maintenance of germ-free zebrafish.
Zebrafish may be reared for up to 30 days in a sterile environment (Rawls et al., 2006). The choice of housing for the fish will depend on the length of the experiment, number of fish involved, and equipment available. For experiments with fewer than 300 fish and lasting less than 2 weeks, fish may be reared in sterile flasks with changes of EM and feeding occurring in a tissue culture hood. For larger experiments (we typically start with up to 1200 fish) and longer time commitments (up to 30 dpf), fish should be reared in a gnotobiotic isolator. The gnotobiotic isolator requires special equipment and more time to prepare for the experiment, but once the equipment is set up, the EM changes and feeding will be less time consuming. There is also a reduced risk of contamination in a gnotobiotic isolator, because the reagents and fish are maintained in a sterile environment from the start to the end of the experiment. Further discussion of gnotobiotic isolator husbandry and associated protocols can be found in Pham et al. (2008).
Sterility of the EM and zebrafish can be monitored in many ways. For daily monitoring, EM can be spotted onto TSA plates and incubated aerobically overnight at 28 ° C. Many microbes cannot be cultured on TSA or may require anaerobic conditions for growth resulting in false-negative results; however, we have found that for daily monitoring of sterility, this method is sufficient because many common contaminants are aerobic. Alternatively, 16S rRNA genes may be amplified from the EM by PCR and run on an agarose gel for visualization. PCR may result in false-positives due to amplification of DNA from dead organisms or contaminating DNA. For final analysis of the sterility of the water and fish at the end of an experiment, a combination of culturing the water in several liquid media – including TSA, BHI, or nutrient media – 16S rRNA gene PCR amplification, and in situ hybridization with 16S rRNA gene-specific probes is sufficient for determining the sterility of the environment.
If experiments are to be carried out to 8 dpf or earlier, zebrafish do not need to be fed. However, zebrafish housed for more than 8 dpf will require a food source because the yolk will be depleted by then. Zebrafish can be fed dry powder diets sterilized either (i) by combining with water to create a slurry that can be sterilized by autoclaving or (ii) by γ-irradiation. However, in our experience, sterile powder diets are not sufficient to support growth and maturation of germ-free zebrafish through metamorphosis (Pham et al., 2008). We are therefore currently exploring the possibility of culturing live germ-free paramecia and brine shrimp to facilitate growth of germ-free zebrafish through metamorphosis toward reproductive maturity.
1. Derivation of Germ-Free Zebrafish
Materials
Flasks (75 cm2, 200 mL, sterile, with vented screw tops; for smaller, shorter experiments; 50 zebrafish per flask) or beakers (200–400 mL, autoclaved with foil covers; for use in the gnotobiotic isolator; 50 zebrafish per 50 mL EM in 200-mL beakers or 100 zebrafish per 100 mL EM in 400-mL beakers)
40 to 50-mL beakers (bleached, rinsed, and autoclaved with foil covers)
Sterile, individually wrapped pediatric gradient 3.0-mL pipettes
25 or 50-mL graduated pipettes, sterile
Large beaker (1–2 L) to collect waste
70% ethanol in spray bottle
6% bleach (sodium hyperchlorite solution, Fisher S5290-1, stored at 4 °C)
10% PVP-I stock (polyvinylpyrrolidone–iodine 0.01% free iodine; Western Chemical, Inc.)
Filter-sterilized EM
Clidox-S activator and base (if using a gnotobiotic isolator; Pharmacal Research Laboratories, cat. no. 95120F and 96120F)
- Drug stocks (all stored at −20 °C):
- 100 mg/mL ampicillin
- 50 mg/mL kanamycin
- 8 mg/mL amphotericin B
Additional items required for feeding and changing water for sterile fish:
Sterile food in 1- to 4-mL aliquots
Filter-sterilized EM (enough to change 70% of water per day)
10 μL sterile loops
TSA plates for plating water
Beads to spread bacteria on TSA plates, or p20 pipette to plate 10 μL of water
Ammo-Carb (Aquarium Pharmaceuticals, Inc., PA), rinsed, dried, placed in mesh packets or cotton muslin tea bags, and autoclaved
Solutions (Make the Same Day as Use)
Antibiotic EM:
150 μL ampicillin 100 mg/mL (100 μg/mL final)
25 μL kanamycin 50 mg/mL (5 μg/mL final)
7.8 μL amphotericin B 8 mg/mL (250 ng/mL final)
250 μL EM
Filter sterilize with 0.22 μm filter
0.003% bleach solution:
125 μL 6.0% bleach solution
250 mL EM
Filter sterilize with 0.22 μm filter
0.1% PVP-I solution (polyvinylpyrrolidone–iodine [0.01% free iodine] Western Chemical, Inc.):
2.5 mL 10% PVP-I stock (5 g PVP-I in 50 mL nanopure water)
247.5 mL EM
Filter sterilize with 0.22 μm filter
0.4% tricaine (3-aminobenzoic acid ethyl ester):
400 mg tricaine
97.9 mL H2O
2.1 mL Tris 1 M (pH 9.0)
Adjust to pH 7.0; filter sterilize with 0.22 μm filter
This can be made ahead of time and stored for long periods at −20 ° C or for up to 2 weeks at 4 °C
-
(1)
Collect embryos in antibiotic EM.
-
(2)
Incubate embryos at 28–30 °C between 4 and 6 h.
-
(3)Collect viable embryos in 50 mL antibiotic EM in beakers.
-
(a)For embryos grown at 30 °C, collect embryos at about 5 hours postferti-lization (hpf); for embryos kept at 28 °C, wait until 6–7 hpf.
-
(b)Wear gloves.
-
(c)Transfer up to 300 embryos in 50% epiboly, germ ring or shield stage into sterile, autoclaved 50-mL beaker containing ~40 mL filter-sterilized EM.
-
(a)
-
(4)Clean the following with 70% ethanol; transfer into the hood and UV treat for up to 10 min (per 300 embryos cleaned):
-
(a)3 sterile 50-mL beakers with foil tops
-
(b)Individually wrapped, sterile transfer pipettes
-
(c)1 L filter-sterilized EM
-
(d)0.003% bleach
-
(e)0.1% PVP-I
-
(f)Sterile flasks – 75 cm2 with vented screw topped caps (in hood, add 48 mL sterile EM to each flask prior to adding embryos)
-
(g)15 or 50 mL pipettes, sterile, individually wrapped
-
(h)Pipette aid
-
(i)Large 1- to 2-L beaker for collecting waste
-
(a)
-
(5)Transfer embryos to clean beaker.
-
(a)Pour off all but ~ 10 mL EM into waste container.
-
(b)Transfer remaining 10 mL EM with eggs into clean 50-mL beaker.
-
(c)Add ~20 mL sterile EM to first beaker to get remaining eggs.
-
(d)Transfer remaining eggs to beaker.
-
(e)Bring volume in second beaker up to 50 mL with sterile EM.
-
(a)
-
(6)
Rinse embryos 3 × with 50 mL filter-sterilized EM.
-
(7)
Immerse embryos in ~50 mL 0.1% PVP-I solution for exactly 2 min.
-
(8)
Rinse embryos with sterile EM 1 ×.
-
(9)
Transfer embryos to fresh beaker as in step 5.
-
(10)
Rinse embryos in sterile EM an additional 2×.
-
(11)
Immerse embryos in 0.003% bleach for 10 min. Do not bleach more than 30 min as this will result in embryo mortality.
-
(12)
Rinse embryos in sterile EM 1 ×.
-
(13)
Transfer embryos to fresh beaker as in step 5.
-
(14)
Rinse embryos additional 2× in sterile EM (if using gnotobiotic isolator, transfer embryos to sterile 15-mL conical tube and clean outside of tube with Clidox for at least 20 min).
-
(15)
Transfer 40–50 embryos to flasks containing sterile EM using sterile, individually wrapped pipettes (gnotobiotic isolator: transfer embryos to beakers containing sterile EM).
-
(16)
Incubate embryos at 28.5 °C.
-
(17)
Feed fish if maintained longer than 8 dpf, starting at 4 dpf.
-
(18)Feeding: spray the following with 70% EtOH and place into a sterile hood (gnotobiotic isolator should already contain these items):
-
(a)Large flask for liquid waste
-
(b)10 to 25- and 1- to 50-mL pipettes, sterile and individually wrapped
-
(c)1 L filter-sterilized EM
-
(d)Sterile 10-μL loops
-
(e)Aliquot of sterile food
-
(a)
-
(19)
Use a 25-mL pipette to remove 70% of the EM and most of the debris from the flask, including any dead zebrafish, undeveloped eggs, and empty chorions.
-
(20)
Plate 10–100 μL of flask EM on TSA; incubate plate at 28.5 °C to screen for contaminants.
-
(21)
Replace the EM with an equal volume of filter-sterilized EM.
-
(22)
Place a loop full of food into the flask.
-
(23)
Return flasks to 28.5 °C.
-
(24)At 5 dpf, add rinsed, autoclaved ammo-carb packets or cotton muslin tea bags to flasks or beakers during water change and feeding:
-
(a)Rinse ammo-carb with distilled water until water is clear.
-
(b)Dry rinse ammo-carb in fume hood overnight.
-
(c)Place about 5 g ammo-carb into mesh packets (should fit through neck of 75-cm2 flask; for beakers use cotton muslin tea bags) and seal.
-
(d)Autoclave ammo-carb packets in aluminum foil.
-
(e)Store at room temperature.
-
(f)When changing water and feeding on 5 dpf, remove sterile ammo-carb packet from foil and gently place into flask.
-
(a)
2. Bacterial Association of Germ-Free Zebrafish
At 3 dpf, the anterior alimentary tract is patent and the intestine may be colonized by bacteria from the medium. The bacterial inoculums should be determined empirically for each species used. For many bacteria, 104 CFU/mL EM suffices for full colonization of the intestine and minimal toxicity to the fish. Prior to inoculation of the EM with bacteria, dead larvae in the flask should be removed as this will often result in increased bacterial growth, which can ultimately be fatal to the remaining larvae. The method below is used for several Proteobacteria strains in our laboratory. Please note that any experiments involving exposure of zebrafish to commensal microorganisms must be approved in advance by the appropriate Institutional Animal Care and Use Committee. Materials:
Spectrophotometer, 600 nm
75cm2 flask
Tryptic Soy Agar (TSA) plates
-
(1)
Incubate bacteria for 16 h in 50 mL of growth medium in 250-mL flask at 28–30 °C, 200 rpm.
-
(2)
Measure the optical density at 600 nm (OD600) of the culture.
-
(3)
Calculate the original culture density, assuming 1 OD600 = 109 CFU/mL.
-
(4)
Dilute culture to predetermined concentration as needed in filter-sterilized EM and inoculate flask in tissue culture hood.
-
(5)
Plate dilutions of the original bacterial culture in order to verify the CFU/mL of the inoculum.
C. Infection of Zebrafish with Pathogens
1. Infections of Embryos and Larvae by Static Immersion with Bacterial Pathogens
The factors discussed above should be taken into account when designing the parameters for infection of zebrafish with a bacterial pathogen. For example, as we developed the E. tarda infection model, we considered the fact that E. tarda is a fish pathogen and likely could be used in a static immersion infection. Interestingly, E. tarda and the related pathogen Edwardsiella ictaluri were recently found to be members of the normal intestinal microbiota in wild yellow catfish and wild-caught zebrafish, respectively (Roeselers et al., 2011). The following section outlines the protocols for infecting zebrafish embryos by immersion using E. tarda as an example (Pressley et al., 2005). Please note that any experiments involving exposure or infection of zebrafish to pathogenic microorganisms must be approved in advance by the appropriate Institutional Animal Care and Use Committee.
Materials
Spectrophotometer, 600nm
1.5 ml tubes
Cuvettes for 600nm readings
Dumont watchmaker forceps #5
35 × 10 mm disposable polystyrene Petri dishes (USA Scientific, no. 5662-7161)
Extra deep 100 × 20 mm polystyrene Petri dishes (Fisher Scientific, no. 08-757-11Z)
Transfer pipettes
0.5% methylene blue
Spectrophotometer
15 ml conical tubes
Cuvettes
Dumont Watchmaker’s forceps no. 5
Solutions
- Egg water:
- 60 mg Instant Ocean® sea salts
- Bring to 1 L with dH2O
- Autoclave
- Prewarm to 28 °C
- E. ictaluri medium (EIM, described by Shotts and Waltman, 1990):
- 40 g/L TSA
- 10 g/L yeast extract
- 0.03 g/L Bromthymol Blue
- 1.0 g/L bile salts and 3.5 g/L mannitol
- 1.0 mg/L colistin B
- Bring to 1 L with ddH2O; autoclave to sterilize
- Luria broth:
- 10 g/L casein peptone
- 5 g/L yeast extract
- 5 g/L NaCl
- Bring to 1 L with ddH2O; autoclave to sterilize
-
(1)Prepare bacterial culture:
-
(a)Streak E. tarda from a freezer stock on an EIM plate. Incubate at 28 °C overnight.
-
(b)Twelve hours prior to infection, prepare two cultures of E. tarda in Luria broth by aseptically picking a colony from an EIM agar plate and inoculating 4 mL LB in a 15-mL conical tube.
-
(c)Shake cultures overnight (12 h) at 250 rpm in a 28 °C incubator.
-
(a)
-
(2)Prepare and quantify the bacterial culture for infection:
-
(a)Subculture E. tarda by adding 1 mL of the overnight culture into 3 mL of fresh media.
-
(b)Measure the OD600 of the subculture after 1–1.5 h by diluting the culture 1:10 (100 μL into 0.9 mL PBS), transferring the dilution to a cuvette and measuring the OD600 in a spectrophotometer.
-
(c)Dilute the bacterial cultures with sterile egg water in a final volume of 2 mL.
-
(a)
-
(3)Collect embryos:
-
(a)Separate embryos into no more than 100 per dish.
-
(b)Remove most of the water from the dish using a transfer pipette.
-
(c)Add 60 mL of egg water to each dish and supplement with 20 μL of 0.5% methylene blue on the first day only, as methylene blue may inhibit infection with pathogen. Add 60 mL of egg water every day following.
-
(a)
-
(4)Prepare embryos for infection:
-
(a)Remove most of the water and dead embryos with a transfer pipette and add 60 mL of fresh egg water (with no methylene blue) to each dish.
-
(b)Manually dechorionate all embryos with Dumont Watchmaker’s forceps no. 5 in each dish and move the embryos into new dishes, approximately 100 per dish, with 60 mL of fresh egg water.
-
(a)
-
(5)Infect 24 hpf embryos by immersion:
-
(a)Transfer the manually dechorionated 24 hpf embryos to a 35-mm Petri dish and remove as much of the egg water as possible.
-
(b)Add the egg water containing the bacterial culture at the appropriate concentration to the dish of embryos.
-
(c)Incubate the embryos in the bacterial suspension for 5 h at 28 °C.
-
(d)After 5 h, carefully transfer the embryos to a wash dish containing 60 mL of sterile egg water.
-
(e)Carefully transfer all the embryos from this dish into a new dish of fresh egg water and hold at 28 °C.
-
(f)Dishes of embryos should be monitored daily, dead embryos removed, and fresh egg water added.
-
(a)
2. Infections of Embryos and Larva by Injection with Bacterial or Viral Pathogens
In contrast to the natural fish pathogen E. tarda, P. aeruginosa, a ubiquitous microbe that is isolated in fresh water environments, is not likely to cause infection in an aquatic species through immersion unless the host is immune-compromised. We found that it was necessary to inject P. aeruginosa to establish infection. The following protocol outlines the methods for infecting zebrafish embryos by microinjection using P. aeruginosa as an example (Phennicie et al., 2010). A similar injection technique is used when infecting embryos or larva with virus. Preparation of virus should be carried out as outlined in sectino 3.
Materials
Extra deep 100 × 20 mm polystyrene Petri dishes (Fisher Scientific, no. 08-757-11Z)
100 × 15 mm disposable polystyrene Petri dishes (Fisher Scientific, no. 08-757-13) that have been filled half with 3% agarose in deionized water
1 × 0.01 mm stage micrometer for droplet calibration (Fisher, cat. no. 12-561-SM1)
Halocarbon oil, series 27 (Sigma, cat. no. H8773)
MPPI-2 pressure injection system (Applied Scientific Instrumentation, Eugene, OR) mounted on an Olympus SZ61 stereo microscope
Microinjection needles drawn from 1.2 mm OD, 0.69 mm ID borosilicate glass micropipette tubes (Sutter, no. BF120-69-10) using a P97 Flaming/Brown micropipette puller (Sutter, no. P97) or similar equipment
Dumont Watchmaker’s forcep no.5
0.5% methylene blue
PBS
Solutions
- Egg water:
- 60 mg Instant Ocean® sea salts
- Bring to 1 L with dH2O
- Autoclave
- Prewarm to 28 °C
- Luria broth:
- 10 g/L casein peptone
- 5 g/L yeast extract
- 5 g/L NaCl
- Bring to 1 L with ddH2O; autoclave to sterilize
Tricaine (Western Chemical, Inc.) at 200 μg/mL in sterile egg water. Note: Extended periods of time in the tricaine solution should be avoided. A stock solution of 4 mg/mL tricaine in Tris-buffered egg water can be prepared in advance and stored at −20 °C for about a month
Cetrimide agar (Neogen, no. 7222A)
-
(1)Embryo collection:
-
(a)Separate embryos into no more than 100 per dish.
-
(b)Remove most of the water from the dish using a transfer pipette.
-
(c)Add 60 mL of egg water to each dish and supplement with 20 μL of 0.5% methylene blue.
-
(d)Remove dead embryos from each dish and place viable embryos in 60 mL of fresh egg water every 24 h.
-
(a)
-
(2)Prepare bacterial culture:
-
(a)Prepare an isolation streak of P. aeruginosa from a freezer stock on a cetrimide agar plate. Place the plate in a 37 °C incubator overnight.
-
(b)Twelve hours prior to infection, prepare two cultures of P. aeruginosa in Luria broth by aseptically picking a colony from a cetrimide agar plate and inoculate 5 mL of Luria broth in a 20-mL test tube. Shake overnight at 250 rpm in a 37 °C incubator.
-
(c)Wash the overnight culture twice by centrifuging at 4000 rpm for 15 min at 4 °C and resuspend in 5 mL of PBS.
-
(d)Determine the relationship between OD600 and viable cell density in the growing culture. Use OD600 to determine the bacterial cell density.
-
(e)Dilute washed culture in PBS to the desired inoculum size.
-
(a)
-
(3)Prepare zebrafish embryos for injection:
-
(a)Manually dechorionate all fish using Dumont Watchmaker’s forceps no. 5.
-
(b)Remove dead fish and most of the egg water with transfer pipette and add 60 mL of fresh egg water to each dish.
-
(c)Thaw a frozen 2-mL aliquot of the 4 mg/mL tricaine stock solution.
-
(a)
-
(4)Infect 48 hpf zebrafish embryos by microinjection:
-
(a)Load the culture dilution into the needle and clip the end of the microinjection needle so that the droplet is equal to 5 nL.
- Clean the calibration micrometer ruler slide with ethanol and on it place a single drop of halocarbon oil.
- Inject a single droplet of liquid into the oil and measure the diameter of the spherical droplet.
- Adjust the injection volume accordingly.
-
(b)Prepare 45 mL of egg water with 2 mL of tricaine to a final concentration of 200 μg/mL and move the embryos for injection into this dish for no more than 5 min.
-
(c)Transfer the embryos from the tricaine to the surface of a Petri dish that has been filled half with 3% agarose (this provides a semisolid substrate to align the sedated embryos), leaving as little water on the surface of the agarose as possible and aligning the fish in the same direction on the plate.
-
(d)Inject 5 nL of the bacterial suspension into the duct of Cuvier of each embryo as shown in Fig. 1.
-
(e)Carefully rinse and transfer the embryos from the injection plate into a new dish containing 60 mL of fresh egg water and maintain the embryos at 28 °C.
-
(f)Dishes of embryos should be monitored daily, dead embryos removed, and fresh egg water added.
-
(a)
3. Infections of Embryos and Larva by Static Immersion with Viral Pathogens
The following section outlines the protocols for infecting zebrafish embryos by immersion, using snakehead rhabdovirus as an example (Phelan et al., 2005b). The titer of the virus should be determined and a range of doses tested. Factors to consider include the infectious dose that will cause 50% mortality and whether or not infection is dose dependent.
Materials
100 × 20 and 35 × 10 mm Petri dishes
Dumont Watchmaker’s forceps no. 5
Transfer pipettes
- Egg water:
- 60 mg Instant Ocean® sea salts
- Bring to 1 L with dH2O
- Autoclave and prewarm to 28 ° C
- Perosan (1.5 mL/L)
PBS
Perosan (1.5 mL/L).
Methods
-
(1)Embryo collection:
-
(a)Separate embryos into no more than 100 per 100 × 20 mm Petri dish.
-
(b)Remove most of the water from the dish using a transfer pipette.
-
(c)Add 60 mL of egg water to each dish.
-
(d)At 4–6 hpf, disinfect embryos by rinsing in Perosan (1.5 mL/L) for 1 min.
-
(a)
-
(2)Prepare embryos for infection:
-
(a)Remove most of the water and dead embryos with a transfer pipette and add 60 mL of fresh egg water to each dish.
-
(b)At 3 dpf, manually dechorionate all embryos using Dumont Watchmaker’s forceps no. 5 and transfer the embryos into new dishes, approximately 100 per dish, with 60 mL of fresh egg water.
-
(a)
-
(3)Infect 3 dpf embryos by static immersion:
-
(a)Transfer the manually dechorionated 3 dpf embryos to a 35 × 10 mm Petri dish and remove as much of the egg water as possible.
-
(b)Expose the embryos to 106 TCID50 of SHRV/mL in 3 mL of egg water. Control embryos should be exposed to an equal volume of PBS in 3 mL of egg water.
-
(c)Immerse the embryos for 5 h at 28 °C.
-
(d)After 5 h, carefully transfer the embryos to a wash dish containing 60 mL of sterile egg water.
-
(e)Carefully transfer all of the embryos from this dish into a new dish of fresh egg water and hold at 28 °C.
-
(f)Dishes of embryos should be monitored daily, dead embryos removed, and fresh egg water added.
-
(a)
4. Infections of Adults by Intraperitoneal Injection with Viral or Bacterial Pathogens
The following section outlines the protocols for infecting zebrafish adults by intraperitoneal injection using snakehead rhabdovirus as an example (Phelan et al., 2005b). The injection technique is similar when infecting adults with bacteria that have been prepared as outlined above.
An alternative method for infecting adult zebrafish involves abrading the skin of the fish and infecting by static immersion (Nayak et al., 2007; Neely et al., 2002; Phelan et al., 2005b; Pressley et al., 2005). In nature, fish are often rendered more susceptible to infection under crowded conditions, for instance, during spawning, when fish rub and bump into one another repeatedly. Minor injuries and surface abrasion provide entryways for pathogens to penetrate and infect the host. In the laboratory, a hypodermic needle can be used to scrape and remove a small area of scales and skin from the zebrafish. The host can then be exposed to pathogen by static immersion as described above.
Materials
Repeating pipetter
0.5-mL syringe tip (VWR, no. 80085-980)
30-gauge needles
Small strips of Parafilm to attach needle to syringe tip
Nets
500 mL beaker/tub for anesthetization
PBS
Tricaine (0.4 mg/100 mL)
Methods
-
(1)Prepare adults for infection:
-
(a)All adult fish (>3 months old) used in infection experiments should be placed in an isolated flow-through system, maintained at 28 °C with a flow rate of 150 L/min, and acclimated for several days before infection.
-
(b)Prepare anesthetization solution by adding 20 mL of Tricaine to 480 mL of system water. Anesthetize as many fish as can be injected in 5 min. Ensure the fish have stopped gill movements before injection to ensure adequate anesthesia.
-
(a)
-
(2)Infect adult zebrafish by intraperitoneal injection:
-
(a)Virus or bacterial dilutions should be prepared in PBS. Control uninfected fish should be injected with PBS alone, and should be injected before preparing the virus to prevent any virus contamination.
-
(b)Install a 0.5-mL syringe tip onto the repeating pipettor. Place a 30-gauge needle on the tip of the 0.5-mL syringe tip and hold in place by wrapping Parafilm around the base of the needle and end of the syringe tip.
-
(c)Place the fish in a supine position. With the needle parallel to the spine, insert it into the midline of the abdomen just posterior to the pectoral fins pointing to the anterior section of the fish. The needle should penetrate only 1–2 mm.
-
(d)Inject 10 μL into each fish. Place injected fish into a tank of system water and allow them to recuperate before placing back into the flow-through system.
-
(e)Fish should be monitored and fed daily.
-
(a)
References
- Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggad D, Mazel M, Boudinot P, Mogensen KE, Hamming OJ, Hartmann R, Kotenko S, Herbomel P, Lutfalla G, Levraud JP. The two groups of zebrafish virus-induced interferons signal via distinct receptors with specific and shared chains. J. Immunol. 2009;183:3924–3931. doi: 10.4049/jimmunol.0901495. [DOI] [PubMed] [Google Scholar]
- Aggad D, Stein C, Sieger D, Mazel M, Boudinot P, Herbomel P, Levraud JP, Lutfalla G, Leptin M. In vivo analysis of Ifn-gamma1 and Ifn-gamma2 signaling in zebrafish. J. Immunol. 2010;185:6774–6782. doi: 10.4049/jimmunol.1000549. [DOI] [PubMed] [Google Scholar]
- Alonso M, Kim CH, Johnson MC, Pressley M, Leong JA. The NV gene of snakehead rhabdovirus (SHRV) is not required for pathogenesis, and a heterologous glycoprotein can be incorporated into the SHRV envelope. J. Virol. 2004;78:5875–5882. doi: 10.1128/JVI.78.11.5875-5882.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann SM, Mellon MT, Distel DL, Kim CH. Molecular and functional analysis of an interferon gene from the zebrafish. Danio rerio. J. Virol. 2003;77:1992–2002. doi: 10.1128/JVI.77.3.1992-2002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann SM, Mellon MT, Johnson MC, Paw BH, Trede NS, Zon LI, Kim CH. Cloning and characterization of an Mx gene and its corresponding promoter from the zebrafish. Danio rerio. Dev. Comp. Immunol. 2004;28:295–306. doi: 10.1016/j.dci.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev. Biol. 2006;297:374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Bates CS, Toukoki C, Neely MN, Eichenbaum Z. Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect. Immun. 2005;73:5743–5753. doi: 10.1128/IAI.73.9.5743-5753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Brannon MK, Davis JM, Mathias JR, Hall CJ, Emerson JC, Crosier PS, Huttenlocher A, Ramakrishnan L, Moskowitz SM. Pseudomonas aeruginosa type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell. Microbiol. 2009;11:755–768. doi: 10.1111/j.1462-5822.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S, Liu KY, Lindenbergh-Kortleve D, Samsom JN, Furuta GT, Renshaw SA, Willemsen R, Nieuwenhuis EE. Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology. 2009;137:1757–17670. doi: 10.1053/j.gastro.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Bry L, Falk PG, Midtvedt T, Gordon JI. A model of hostx–microbial interactions in an open mammalian ecosystem. Science. 1996;273:1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- Buller NB. Bacteria from Fish and Other Aquatic Animals: A Practical Identification Manual. CABI Publishing; Wallingford, UK: 2004. [Google Scholar]
- Burgos JS, Ripoll-Gomez J, Alfaro JM, Sastre I, Valdivieso F. Zebrafish as a new model for herpes simplex virus type 1 infection. Zebrafish. 2008;5:323–333. doi: 10.1089/zeb.2008.0552. [DOI] [PubMed] [Google Scholar]
- Carvalho R, de Sonneville J, Stockhammer OW, Savage ND, Veneman WJ, Ottenhoff TH, Dirks RP, Meijer AH, Spaink HP. A high-throughput screen for tuberculosis progression. PLoS One. 2011;6:e16779. doi: 10.1371/journal.pone.0016779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra JJ, Periwal SB, Lee G, Lee F, Shroff KE. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev. Immunol. 1998;6:13–18. doi: 10.1155/1998/68382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheesman SE, Neal JT, Mittge E, Seredick BM, Guillemin K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc. Natl. Acad. Sci. U. S. A. 2011;108(suppl. 1):4570–4577. doi: 10.1073/pnas.1000072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, Caparon MG. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol. Microbiol. 2005;57:1545–1556. doi: 10.1111/j.1365-2958.2005.04786.x. [DOI] [PubMed] [Google Scholar]
- Clatworthy AE, Lee JS, Leibman M, Kostun Z, Davidson AJ, Hung DT. Pseudomonas aeruginosa infection of zebrafish involves both host and pathogen determinants. Infect. Immun. 2009;77:1293–1303. doi: 10.1128/IAI.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe. 2007;2:29–39. doi: 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- Davis JM, Haake DA, Ramakrishnan L. Leptospira interrogans stably infects zebrafish embryos, altering phagocyte behavior and homing to specific tissues. PLoS Negl. Trop. Dis. 2009;3:e463. doi: 10.1371/journal.pntd.0000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Boon C, Eberl L, Zhang LH. Differential modulation of Burkholderia cenocepacia virulence and energy metabolism by the quorum-sensing signal BDSF and its synthase. J Bacteriol. 2009;191:7270–7278. doi: 10.1128/JB.00681-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 2011;117:e49–e56. doi: 10.1182/blood-2010-10-314120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas P, Rodriguez-Milla MA, Novoa B, Estepa A, Figueras A, Coll J. Zebrafish fin immune responses during high mortality infections with viral haemorrhagic septicemia rhabdovirus. A proteomic and transcriptomic approach. BMC Genomics. 2010;11:518. doi: 10.1186/1471-2164-11-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Molecular Koch’s postulates applied to microbial pathogenicity. Rev. Infect. Dis. 1988;10(suppl. 2):S274–S276. doi: 10.1093/cid/10.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- Fraune S, Bosch TC. Why bacteria matter in animal development and evolution. Bioessays. 2010;32:571–580. doi: 10.1002/bies.200900192. [DOI] [PubMed] [Google Scholar]
- Gao LY, Pak M, Kish R, Kajihara K, Brown EJ. A mycobacterial operon essential for virulence in vivo and invasion and intracellular persistence in macrophages. Infect. Immun. 2006;74:1757–1767. doi: 10.1128/IAI.74.3.1757-1767.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JN, Joshi B, Jagus R. Characterization of rainbow trout and zebrafish eukaryotic initiation factor 2alpha and its response to endoplasmic reticulum stress and IPNV infection. Dev. Comp. Immunol. 2003;27:217–231. doi: 10.1016/s0145-305x(02)00096-4. [DOI] [PubMed] [Google Scholar]
- Gray C, Loynes CA, Whyte MK, Crossman DC, Renshaw SA, Chico TJ. Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb. Haemost. 2011;105:811–819. doi: 10.1160/TH10-08-0525. [DOI] [PubMed] [Google Scholar]
- Harriff MJ, Bermudez LE, Kent ML. Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: a potential model for environmental mycobacterial infection. J. Fish Dis. 2007;30:587–600. doi: 10.1111/j.1365-2761.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- Hermann AC, Kim CH. Effects of arsenic on zebrafish innate immune system. Mar. Biotechnol. (NY) 2005;7:494–505. doi: 10.1007/s10126-004-4109-7. [DOI] [PubMed] [Google Scholar]
- Hermann AC, Millard PJ, Blake SL, Kim CH. Development of a respiratory burst assay using zebrafish kidneys and embryos. J. Immunol. Methods. 2004;292:119–129. doi: 10.1016/j.jim.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host–microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Husebye E, Hellstrom PM, Midtvedt T. Intestinal microflora stimulates myoelectric activity of rat small intestine by promoting cyclic initiation and aboral propagation of migrating myoelectric complex. Dig. Dis. Sci. 1994;39:946–956. doi: 10.1007/BF02087542. [DOI] [PubMed] [Google Scholar]
- Kandori H, Hirayama K, Takeda M, Doi K. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germffree and conventional mice. Exp. Anim. 1996;45:155–160. doi: 10.1538/expanim.45.155. [DOI] [PubMed] [Google Scholar]
- Kanther M, Rawls JF. Host–microbe interactions in the developing zebrafish. Curr. Opin. Immunol. 2010;22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanther M, Sun X, Muhlbauer M, Mackey LC, Flynn EJ, 3rd, Bagnat M, Jobin C, Rawls JF. Microbial colonization induces dynamic temporal and spatial patterns of NF-kappaB activation in the zebrafish digestive tract. Gastroenterology. 2011;146:197–207. doi: 10.1053/j.gastro.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizy AE, Neely MN. First Streptococcus pyogenes signature-tagged mutagenesis screen identifies novel virulence determinants. Infect. Immun. 2009;77:1854–1865. doi: 10.1128/IAI.01306-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- LaPatra SE, Barone L, Jones GR, Zon LI. Effects of infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus infection on hematopoietic precursors of the zebrafish. Blood Cells Mol. Dis. 2000;26:445–452. doi: 10.1006/bcmd.2000.0320. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Levraud JP, Disson O, Kissa K, Bonne I, Cossart P, Herbomel P, Lecuit M. Real-time observation of Listeria monocytogenes-phagocyte interactions in living zebrafish larvae. Infect. Immun. 2009;77:3651–3660. doi: 10.1128/IAI.00408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Chen S, Cao Z, Lin Y, Mo D, Zhang H, Gu J, Dong M, Liu Z, Xu A. Acute phase response in zebrafish upon Aeromonas salmonicida and Staphylococcus aureus infection: striking similarities and obvious differences with mammals. Mol. Immunol. 2007;44:295–301. doi: 10.1016/j.molimm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Llamas MA, van der Sar A, Chu BC, Sparrius M, Vogel HJ, Bitter W. A novel extracytoplasmic function (ECF) sigma factor regulates virulence in Pseudomonas aeruginosa. PLoS Pathog. 2009;5:e1000572. doi: 10.1371/journal.ppat.1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe BA, Miller JD, Neely MN. Analysis of the polysaccharide capsule of the systemic pathogen Streptococcus iniae and its implications in virulence. Infect. Immun. 2007;75:1255–1264. doi: 10.1128/IAI.01484-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MW, Chao YM, Guo TC, Santi N, Evensen O, Kasani SK, Hong JR, Wu JL. The interferon response is involved in nervous necrosis virus acute and persistent infection in zebrafish infection model. Mol. Immunol. 2008;45:1146–1152. doi: 10.1016/j.molimm.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Palha N, Torhy C, Briolat V, Colucci-Guyon E, Bremont M, Herbomel P, Boudinot P, Levraud JP. Whole-body analysis of a viral infection: vascular endothelium is a primary target of infectious hematopoietic necrosis virus in zebrafish larvae. PLoS Pathog. 2011;7:e1001269. doi: 10.1371/journal.ppat.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- Meijer AH, Verbeek FJ, Salas-Vidal E, Corredor-Adamez M, Bussman J, van der Sar AM, Otto GW, Geisler R, Spaink HP. Transcriptome profiling of adult zebrafish at the late stage of chronic tuberculosis due to Mycobacterium marinum infection. Mol. Immunol. 2005;42:1185–1203. doi: 10.1016/j.molimm.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Menudier A, Rougier FP, Bosgiraud C. Comparative virulence between different strains of Listeria in zebrafish (Brachydanio rerio) and mice. Pathol. Biol. (Paris) 1996;44:783–789. [PubMed] [Google Scholar]
- Miller JD, Neely MN. Large-scale screen highlights the importance of capsule for virulence in the zoonotic pathogen Streptococcus iniae. Infect. Immun. 2005;73:921–934. doi: 10.1128/IAI.73.2.921-934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez GE, Neely MN, Eichenbaum Z. The streptococcal iron uptake (Siu) transporter is required for iron uptake and virulence in a zebrafish infection model. Microbiology. 2005;151:3749–3757. doi: 10.1099/mic.0.28075-0. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Darling AE, Eisen JA. Metagenomic sequencing of an in vitro-simulated microbial community. PLoS One. 2010;5:e10209. doi: 10.1371/journal.pone.0010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer TR, Hunnicutt DW. Susceptibility of zebra fish Danio rerio to infection by Flavobacterium columnare and F. johnsoniae. Dis. Aquat. Organ. 2007;76:39–44. doi: 10.3354/dao076039. [DOI] [PubMed] [Google Scholar]
- Nayak AS, Lage CR, Kim CH. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio) Toxicol. Sci. 2007;98:118–124. doi: 10.1093/toxsci/kfm072. [DOI] [PubMed] [Google Scholar]
- Neely MN, Pfeifer JD, Caparon M. Streptococcus-zebrafish model of bacterial pathogenesis. Infect. Immun. 2002;70:3904–3914. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa B, Romero A, Mulero V, Rodriguez I, Fernandez I, Figueras A. Zebrafish (Danio rerio) as a model for the study of vaccination against viral haemorrhagic septicemia virus (VHSV) Vaccine. 2006;24:5806–5816. doi: 10.1016/j.vaccine.2006.05.015. [DOI] [PubMed] [Google Scholar]
- O’Toole R, Von Hofsten J, Rosqvist R, Olsson PE, Wolf-Watz H. Visualisation of zebrafish infection by GFP-labelled Vibrio anguillarum. Microb. Pathog. 2004;37:41–46. doi: 10.1016/j.micpath.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Ordas A, Hegedus Z, Henkel CV, Stockhammer OW, Butler D, Jansen HJ, Racz P, Mink M, Spaink HP, Meijer AH. Deep sequencing of the innate immune transcriptomic response of zebrafish embryos to Salmonella infection. Fish Shellfish Immunol. 2010 doi: 10.1016/j.fsi.2010.08.022. (Epub ahead of print) 2010 Sep 8. PMID: 20816807. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Ransom DG, Paw B, Zon LI, Johnson SL. An orthologue of the kit-related gene fms is required for development ofneural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development. 2000;127:3031–3044. doi: 10.1242/dev.127.14.3031. [DOI] [PubMed] [Google Scholar]
- Petrie-Hanson L, Romano CL, Mackey RB, Khosravi P, Hohn CM, Boyle CR. Evaluation of zebrafish Danio rerio as a model for enteric septicemia of catfish (ESC) J. Aquat. Anim. Health. 2007;19:151–158. doi: 10.1577/H06-026.1. [DOI] [PubMed] [Google Scholar]
- Pham LN, Kanther M, Semova I, Rawls JF. Methods for generating and colonizing gnotobiotic zebrafish. Nat. Protoc. 2008;3:1862–1875. doi: 10.1038/nprot.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan PE, Mellon MT, Kim CH. Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio) Mol. Immunol. 2005a;42:1057–1071. doi: 10.1016/j.molimm.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Phelan PE, Pressley ME, Witten PE, Mellon MT, Blake S, Kim CH. Characterization ofsnakehead rhabdovirus infection in zebrafish (Danio rerio) J. Virol. 2005b;79:1842–1852. doi: 10.1128/JVI.79.3.1842-1852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phennicie RT, Sullivan MJ, Singer JT, Yoder JA, Kim CH. Specific resistance to Pseudomonas aeruginosa infection in zebrafish is mediated by the cystic fibrosis transmembrane conductance regulator. Infect. Immun. 2010;78:4542–4550. doi: 10.1128/IAI.00302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajsnar TK, Cunliffe VT, Foster SJ, Renshaw SA. A novel vertebrate model of Staphylococcus aureus infection reveals phagocyte-dependent resistance of zebrafish to non-host specialized pathogens. Cell. Microbiol. 2008;10:2312–2325. doi: 10.1111/j.1462-5822.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- Pressley ME, Phelan PE, 3rd, Witten PE, Mellon MT, Kim CH. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev. Comp. Immunol. 2005;29:501–513. doi: 10.1016/j.dci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Mahowald MA, Goodman AL, Trent CM, Gordon JI. In vivo imaging and genetic analysis link bacterial motility and symbiosis in the zebrafish gut. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7622–7627. doi: 10.1073/pnas.0702386104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- Rhodes J, Hagen A, Hsu K, Deng M, Liu TX, Look AT, Kanki JP. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev. Cell. 2005;8:97–108. doi: 10.1016/j.devcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Novoa B, Figueras A. Immune response of zebrafish (Danio rerio) against a newly isolated bacterial pathogen Aeromonas hydrophila. Fish Shellfish Immunol. 2008;25:239–249. doi: 10.1016/j.fsi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Roeselers G, Mittge E, Parichy D, Cavanaugh CM, Guillemin K, Rawls JF. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011 doi: 10.1038/ismej.2011.38. (Epub ahead of print) 2011 Apr 7. PMID: 21472014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo I, de Ilarduya OM, Estonba A, Pardo MA. Innate immune gene expression in individual zebrafish after Listonella anguillarum inoculation. Fish Shellfish Immunol. 2007;23:1285–1293. doi: 10.1016/j.fsi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Sanders GE, Batts WN, Winton JR. Susceptibility of zebrafish (Danio rerio) to a model pathogen, spring viremia of carp virus. Comp. Med. 2003;53:514–521. [PubMed] [Google Scholar]
- Savage DC, Siegel JE, Snellen JE, Whitt DD. Transit time of epithelial cells in the small intestines of germfree mice and ex-germfree mice associated with indigenous microorganisms. Appl. Environ. Microbiol. 1981;42:996–1001. doi: 10.1128/aem.42.6.996-1001.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotts EB, Waltman 2nd WD. A medium for the selective isolation of Edwardsiella ictaluri. J. Wildl. Dis. 1990;26:214–218. doi: 10.7589/0090-3558-26.2.214. [DOI] [PubMed] [Google Scholar]
- Singer JT, Phennicie RT, Sullivan MJ, Porter LA, Shaffer VJ, Kim CH. Broad-host-range plasmids for red fluorescent protein labeling of gram-negative bacteria for use in the zebrafish model system. Appl. Environ. Microbiol. 2010;76:3467–3474. doi: 10.1128/AEM.01679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhammer OW, Zakrzewska A, Hegedus Z, Spaink HP, Meijer AH. Transcriptome profiling and functional analyses of the zebrafish embryonic innate immune response to Salmonella infection. J. Immunol. 2009;182:5641–5653. doi: 10.4049/jimmunol.0900082. [DOI] [PubMed] [Google Scholar]
- Stoop EJ, Schipper T, Rosendahl Huber SK, Nezhinsky AE, Verbeek FJ, Gurcha SS, Besra GS, Vandenbroucke-Grauls CM, Bitter W, van der Sar AM. Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis. Model Mech. 2011;4:526–536. doi: 10.1242/dmm.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Juarez MA, Cooke CL, Lapointe L, Shavit JA, Yamaoka JS, Lyons SE. Differential regulation of primitive myelopoiesis in the zebrafish by Spi-1/Pu.1 and C/ebp1. Zebrafish. 2007;4:187–199. doi: 10.1089/zeb.2007.0505. [DOI] [PubMed] [Google Scholar]
- Sullivan C, Charette J, Catchen J, Lage CR, Giasson G, Postlethwait JH, Millard PJ, Kim CH. The gene history of zebrafish tlr4a and tlr4b is predictive of their divergent functions. J. Immunol. 2009;183:5896–5908. doi: 10.4049/jimmunol.0803285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan C, Postlethwait JH, Lage CR, Millard PJ, Kim CH. Evidence for evolving Toll-IL-1 receptor-containing adaptor molecule function in vertebrates. J. Immunol. 2007;178:4517–4527. doi: 10.4049/jimmunol.178.7.4517. [DOI] [PubMed] [Google Scholar]
- Szabady RL, Lokuta MA, Walters KB, Huttenlocher A, Welch RA. Modulation of neutrophil function by a secreted mucinase of Escherichia coli O157:H7. PLoS Pathog. 2009;5:e1000320. doi: 10.1371/journal.ppat.1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D, Herbomel P, Patton EE, Murphey RD, Yoder JA, Litman GW, Catic A, Amemiya CT, Zon LI, Trede NS. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 2003;81:253–330. [PubMed] [Google Scholar]
- Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20:367–379. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- van der Sar AM, Musters RJ, van Eeden FJ, Appelmelk BJ, Vandenbroucke-Grauls CM, Bitter W. Zebrafish embryos as a model host for the real time analysis of Salmonella typhimurium infections. Cell. Microbiol. 2003;5:601–611. doi: 10.1046/j.1462-5822.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- van der Sar AM, Stockhammer OW, van derLaan C, Spaink HP, Bitter W, Meijer AH. MyD88 innate immune function in a zebrafish embryo infection model. Infect. Immun. 2006;74:2436–2441. doi: 10.1128/IAI.74.4.2436-2441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil ML, Stonehouse MJ, Vasil AI, Wadsworth SJ, Goldfine H, Bolcome RE, 3rd, Chan J. A complex extracellular sphingomyelinase of Pseudomonas aeruginosa inhibits angiogenesis by selective cytotoxicity to endothelial cells. PLoS Pathog. 2009;5:e1000420. doi: 10.1371/journal.ppat.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergunst AC, Meijer AH, Renshaw SA, O’Callaghan D. Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection. Infect. Immun. 2010;78:1495–1508. doi: 10.1128/IAI.00743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtech LN, Sanders GE, Conway C, Ostland V, Hansen JD. Host immune response and acute disease in a zebrafish model of Francisella pathogenesis. Infect. Immun. 2009;77:914–925. doi: 10.1128/IAI.01201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZL, Xu XP, He BL, Weng SP, Xiao J, Wang L, Lin T, Liu X, Wang Q, Yu XQ, et al. Infectious spleen and kidney necrosis virus ORF48R functions as a new viral vascular endothelial growth factor. J. Virol. 2008;82:4371–4383. doi: 10.1128/JVI.02027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang HX, Zhang JH, Chen WH, Ruan XF, Xia C. In vitro effects of recombinant zebrafish IFN on spring viremia of carp virus and infectious hematopoietic necrosis virus. J. Interferon Cytokine Res. 2006;26:256–259. doi: 10.1089/jir.2006.26.256. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene, OR: 2000. [Google Scholar]
- Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiol. Lett. 2007;270:21–26. doi: 10.1111/j.1574-6968.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Wienholds E, Schulte-Merker S, Walderich B, Plasterk RH. Target-selected inactivation of the zebrafish rag1 gene. Science. 2002;297:99–102. doi: 10.1126/science.1071762. [DOI] [PubMed] [Google Scholar]
- Xiong XP, Dong CF, Xu X, Weng SP, Liu ZY, He JG. Proteomic analysis of zebrafish (Danio rerio) infected with infectious spleen and kidney necrosis virus. Dev. Comp. Immunol. 2011;35:431–440. doi: 10.1016/j.dci.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang L, Weng S, Huang Z, Lu J, Lan D, Zhong X, Yu X, Xu A, He J. A zebrafish (Danio rerio) model of infectious spleen and kidney necrosis virus (ISKNV) infection. Virology. 2008;376:1–12. doi: 10.1016/j.virol.2007.12.026. [DOI] [PubMed] [Google Scholar]