Abstract
OBJECTIVE
Osteonecrosis in the growing population of childhood cancer survivors results from disease and treatment. Imagers must be knowledgeable about patient groups at risk for its development, patterns of involvement and potential implications. This review will focus on implications of this potentially life-altering toxicity.
CONCLUSION
Childhood cancer survivors are at increased risk for developing osteonecrosis. Because osteonecrosis is often asymptomatic until late in the process, imaging is critical for its detection and characterization when interventions may be most effective to ameliorate its progression.
Keywords: acute lymphoblastic leukemia, bone mineral density, childhood cancer, osteonecrosis, pediatrics
Osteonecrosis is a significant long-term toxicity that can compromise joint functionality and quality of life in afflicted survivors of childhood cancer. The causes for its development are multifactorial and associated with adverse effects of disease and treatment. However, understanding of this process in pediatrics, particularly pediatric oncology, is limited.
In the United States, osteonecrosis is diagnosed in 10,000–20,000 individuals each year [1]. Osteonecrosis typically occurs in patients 20–50 years old and is rare in the pediatric population. Pediatric patients may develop osteonecrosis from chronic exposure to steroids. Preservation of joint function and control of pain are key factors in managing osteonecrosis. Unique to pediatric patients is concern about lifetime compromise of joint function; maintaining skeletal growth and development; and, when necessary, the potential need for multiple surgeries. The growing number of pediatric oncology patients represents an especially vulnerable population in which toxicities from the primary disease and treatment compound surgical risk and skeletal integrity. This review will focus on implications of this potentially life-altering toxicity as seen in survivors of childhood cancer.
Early Diagnosis
Osteonecrosis often remains asymptomatic or associated with only minimal, nonspecific symptoms until it becomes advanced. Thus, early stage lesions need to be detected when implementation of interventions may ameliorate severity and preserve joint integrity [2–4]. The size of the osteonecrosis lesion inconsistently correlates with the severity of pain. Twenty-one percent of patients with stage III osteonecrosis of the hips classified according to the staging system of the Association Research Circulation Osseous (ARCO) [5] were asymptomatic whereas 28% with ARCO stage I osteonecrosis of the hips were symptomatic [3]. Similar findings have been reported in patients prospectively imaged for detection of osteonecrosis involving the knees [6]. Thus, the growing recognition of treatment-induced osteonecrosis has led to a high index of suspicion for osteonecrosis and prompted prospective monitoring of at-risk patient populations.
Longitudinal Outcomes of Osteonecrosis
The paucity of longitudinal studies and correlative standardized clinical assessment of joint function in pediatric cancer patients hampers understanding of the clinical ramifications of imaging findings. Symptoms may not occur until late in the evolution of osteonecrosis when most of the articular surface has been irreversibly destroyed. At this point, therapeutic options are limited, and patients and usually require surgical intervention. Clinical symptoms are an unreliable indicator of the presence and severity of osteonecrosis [7]. Only lesions reaching and affecting more than 50% of the articular surface are consistently associated with symptoms. Large lesions may also be associated with no or only minor symptoms [3] (Fig. 1). Up to 21% of patients have remained asymptomatic after suffering subchondral collapse of hip osteonecrosis. In a meta-analysis series of adults, more than one half of asymptomatic cases progressed to symptomatic disease or collapse of the femoral head over 2–240 months [7]. Investigations into potential associations between metaphyseal and diaphyseal osteonecrosis, clinical symptoms, and joint function are currently lacking.
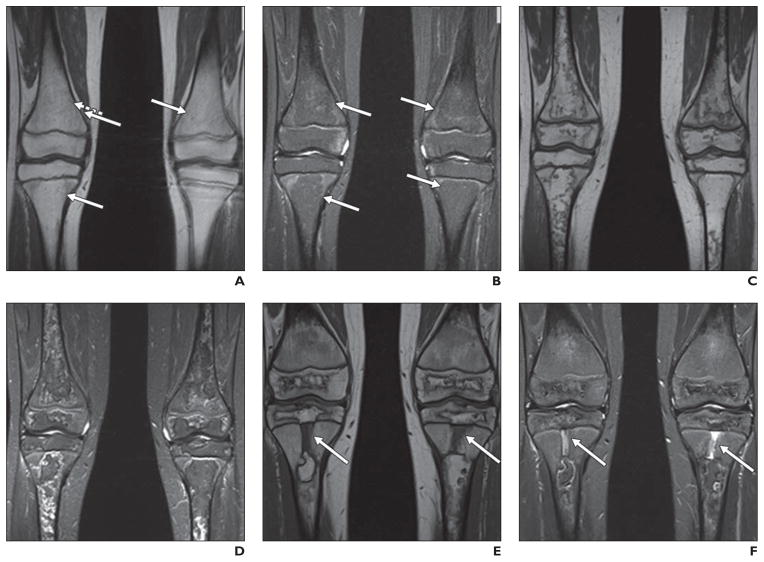
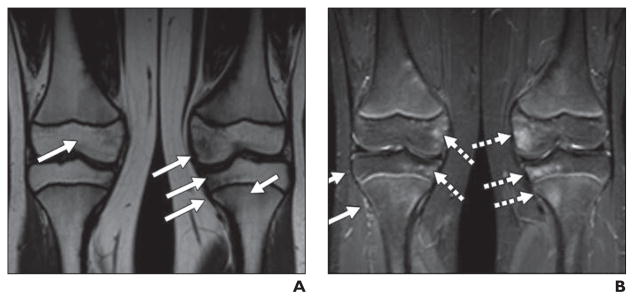
Fig. 1. 9-year-old girl being treated for standard-risk acute lymphoblastic leukemia who underwent routine MR screening for osteonecrosis.
A and B, Coronal unenhanced T1-weighted (A) and STIR (B) images of knees show very subtle geographic signal changes (arrows) in distal femoral and proximal tibial diametaphyses bilaterally. These signal changes are less well defined than those described in literature for osteonecrosis.
C and D, MR images show that over course of 3 months, these changes evolved into typical geographic signal changes of osteonecrosis.
E and F, Annual follow-up examinations (not shown) revealed progressive definition of these extensive lesions, with overall decrease in extent. Note rectangular foci of abnormal centrally located metaphyseal signal in proximal tibias (arrows), dark on T1-weighted image (E) and bright on STIR image (F). Over course of 3 years, these abnormalities narrowed transversely and elongated, coincident with longitudinal growth of patient. These findings represent cartilaginous ingrowths from arrested enchondral ossification.
Few longitudinal studies investigating the outcome of MR findings are available for pediatric cohorts. Similar findings have been reported in adult populations [8, 9]. Osteonecrosis lesions involving at least 30% of the articular surface of the hips or the knees are associated with worse outcomes [3, 8]. Specifically, such lesions in the hips involving the capital femoral epiphysis, are predictive of progression to collapse of the articular surface in 80% of patients within 2 years of presentation, with 50% requiring arthroplasty [10]. Lesions involving < 30% of the articular surface commonly heal without collapse of the articular surface [10]; in some cases, large lesions also heal [10–12] (Fig. 1). As mentioned, osteonecrosis lesions of the hip with a large necrotic angle are also predicted to compromise joint function and ultimately to require arthroplasty [13, 14].
In contrast to skeletally mature adults, the potential for alteration of growth and development of a joint affected by osteonecrosis is a concern in pediatric patients. Merrow and Laor [15] reported a single case of preserved longitudinal bone growth in a patient who developed symptomatic osteonecrosis of the distal tibial epiphysis, metaphysis, and diaphysis during treatment of leukemia. In the reported case, the area of osteonecrosis migrated proximally as unossified physeal cartilage. More recent anecdotal experience supports this case report (Fig. 1), but published longitudinal experience is lacking.
Prevalence of Osteonecrosis in Pediatric Patients Treated for Cancer
The reported incidence of osteonecrosis varies from about 1–72% [16–19] depending on study design, primary diagnosis, symptomatic versus asymptomatic cases, and osteonecrosis definition used. Reports based on symptomatic cases [16–19] and those using radiographic evaluation typically report a lower incidence of osteonecrosis than those prospectively monitoring its development with contemporary MR techniques [19]. In the Childhood Cancer Survivor Study consisting of 9261 patients and a random sample of 2872 siblings, 52 (0.56%) survivors of childhood cancer self-reported osteonecrosis developing in 78 joints; 60% reported multiple joint involvement [18]. Thus, prospective MR monitoring for osteonecrosis is now incorporated into some standard pediatric oncology treatment protocols.
Two populations of childhood cancer survivors at risk for osteonecrosis warrant special mention: acute lymphoblastic leukemia (ALL) and patients who have undergone allogeneic bone marrow transplantation. ALL is the most common pediatric cancer, with survival now exceeding 90% [20]. The reported incidence of symptomatic cases ranges from 1.8% 5-year cumulative incidence [17] to a 3-year life-table incidence of 9.3% [16]. These reported frequencies underestimate the overall prevalence of osteonecrosis. Kawedia et al. [19] reported a cumulative incidence of osteonecrosis involvement in hips or knees in 72% of prospectively monitored patients with ALL while on therapy, irrespective of symptoms.
In patients who have undergone allogeneic bone marrow transplantation, the reported incidence of osteonecrosis of any joint ranges from 3.9% to 44.2% [21–24]. Recently, nearly 22% of prospectively monitored pediatric patients, irrespective of clinical symptoms, were found to have MR-documented osteonecrosis of the hips or knees. Nearly one half of those with osteonecrosis had at least 30% epiphyseal involvement [24].
Risk Factors for the Development of Osteonecrosis in Pediatric Oncology Patients
Development of osteonecrosis is multifactorial. Older patient age at the time of oncologic therapy has been consistently identified as a risk factor for its development [10, 12, 18, 23–28]. Adolescents are at greater risk than younger patients [2, 3, 15–17, 23]. The surge in sex hormones and rapid skeletal maturation during puberty may contribute to the development of osteonecrosis [29, 30]. Increased intraosseous pressures related to growth plate fusion may also contribute to increased risk [16, 31]. Osteonecrosis of the hip in patients older than 16 years treated for hematologic malignancies may be more extensive and progress to end-stage collapse more readily than those lesions in younger patients [3].
Exposure to glucocorticoids (particularly dexamethasone) has consistently been associated with development of osteonecrosis [12, 16, 18]. Glucocorticoid-induced osteonecrosis results from complex interactions of several pathways [32]. One proposed mechanism describes early apoptosis of osteocytes with concurrent increase in intramedullary adipocytosis, contributing to increased intraosseous pressure that subsequently compromises vascular perfusion of the epiphysis and adjacent bone [33]. Glucocorticoid-induced hypoperfusion may result from down-regulation of vascular endothelial growth factor [34]. Investigations into genetic [35–38] and pharmacogenetic factors contributing to osteonecrosis are ongoing [26, 38].
Inconsistent results have been reported from investigations into the association between osteonecrosis and other risk factors, such as patient sex [22, 24, 34], obesity, and body mass index [22, 24, 39].
Imaging of Osteonecrosis
The imaging appearance of osteonecrosis in childhood cancer survivors parallels that seen in the general and other high-risk populations. Many excellent publications are available for review of osteonecrosis imaging characteristics. Thus, the imaging findings discussed here focus on reports targeting pediatric patients receiving or having received therapy for a malignancy.
Radiography
Although readily available and inexpensive, radiographic evaluation for detection of osteonecrosis is insensitive to the early stages of osteonecrosis when intervention may be most effective in preventing progression to collapse of the articular surface [40]. Its use is primarily limited to advanced disease, orthopedic surgical planning, and longitudinal postoperative monitoring. Even in the presence of extensive MR-proven osteonecrosis, involved joints are often radiographically normal [3, 22, 41].
MRI
MRI is the most sensitive and specific method for detection and monitoring of osteonecrosis [41–43]. Minimal MRI comprising coronal unenhanced T1-weighted and STIR sequences exquisitely delineates osteonecrosis lesions (Figs. 1–7). The addition of sagittal or axial sequences may add to volumetric assessment of osteonecrosis involvement. A cartilage-specific sequence, such as FLASH 2D may be useful for assessing secondary changes related to osteonecrosis.
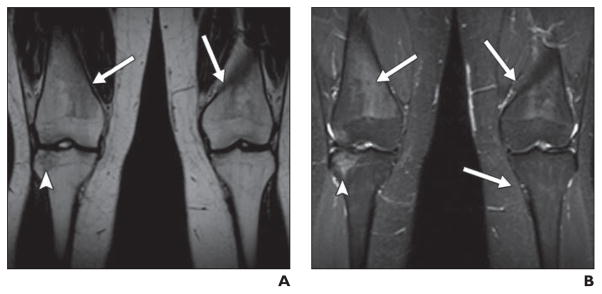
Fig. 7. Healing osteonecrosis of knees in 4-year-old girl undergoing treatment of acute lymphoblastic leukemia who was prospectively monitored annually for osteonecrosis of hips (normal throughout monitoring) and knees.
A and B, Coronal unenhanced T1-weighted (A) and STIR (B) images of knees show small lesion of right distal femoral epiphysis (solid arrows), large lesion of distal right femoral diaphysis (arrowheads), and moderate-sized lesions of proximal tibial diametaphyses bilaterally (dotted arrows).
C and D, Over course of 4 years, osteonecrotic lesions healed. Coronal unenhanced T1-weighted (C) and STIR (D) images of knees show no evidence of osteonecrosis.
The administration of IV contrast material did not contribute to the detection and characterization of osteonecrosis in a single adult series [44]. However, preliminary experience with diffusion- and perfusion-weighted sequences suggests such techniques may identify development of osteonecrosis at an earlier stage and better depict regional vascularity than that shown by unenhanced studies [45–48]. A recent experimental animal study showed that functional perfusion MRI may predict development of steroid-associated osteonecrosis [49]. Whole-body MR techniques show promise for detecting osteonecrosis lesions, but clinical use is currently limited [50, 51].
CT
CT has the distinct advantage of being able to better detect subchondral collapse and even subtle depression of the articular surface [52]. Yeh et al. [52] compared MRI with CT detection of subchondral fractures related to osteonecrosis as interpreted by two blinded readers, a musculoskeletal radiologist and a general radiologist. These investigators found only 58% (16/28) agreement in identifying subchondral collapse by MRI. However, current efforts in pediatric oncology are focusing on early detection of osteonecrosis at stages when intervention may preserve joint integrity. Furthermore, patient exposure to ionizing radiation limits the utility of CT in pediatric patients for assessment of joint integrity.
Functional Imaging
Skeletal scintigraphy has long been advocated as a sensitive diagnostic tool to detect osteonecrosis; it is fairly available and can provide whole-body assessment [53–55]. However, MRI has largely superseded skeletal scintigraphy because of superior anatomic detail, lack of exposure to ionizing radiation, and improved specificity [54].
Metabolic activity in sites of osteonecrosis has been detected during 18F-FDG PET or PET/CT performed for disease staging; these sites of osteonecrosis may mimic metastatic lesions [56]. Preliminary investigations in adults have found FDG PET to be a sensitive imaging method for detecting osteonecrosis [57–59] and in assessing perfusion of the femoral head [57, 58]. However, prospective reports in pediatric cases are lacking.
Coexisting Lesions
The sensitivity of MRI to signal changes in bone marrow can present a challenge when providing a differential diagnosis. Distinguishing osteonecrosis from recurrent leukemia (Fig. 2), metastatic disease, and nonspecific transient signal abnormalities is of utmost importance in providing an appropriate differential diagnosis.
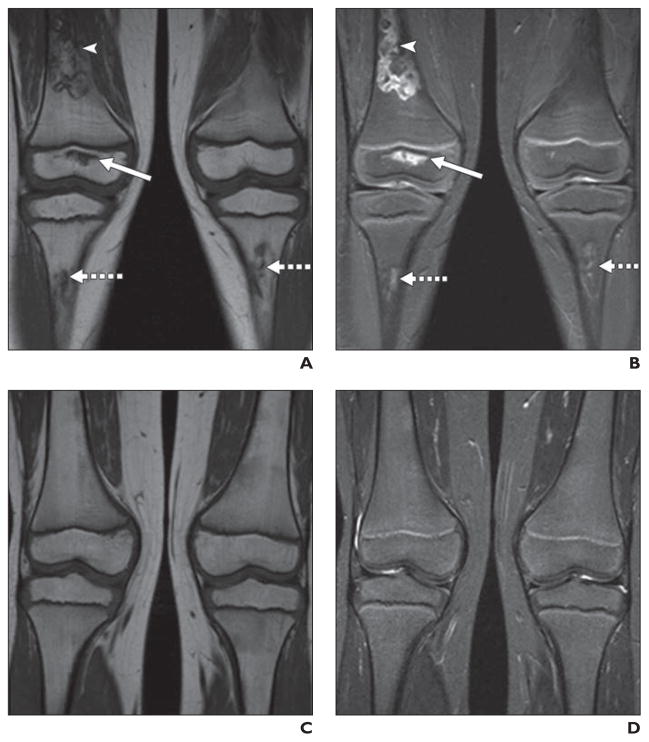
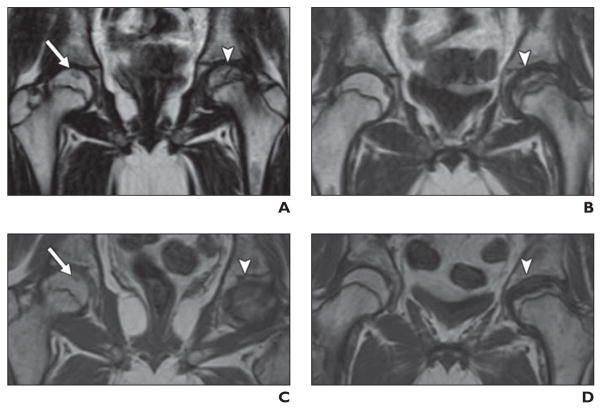
Fig. 2. Relapse of acute lymphoblastic leukemia in 14-year-old boy who underwent stem cell transplantation for acute lymphoblastic leukemia.
A and B, MR images of hips obtained as part of pretransplantation assessment reveal no evidence of osteonecrosis or other abnormality of bones and soft tissues.
C and D, MR images obtained 1 year after bone marrow transplantation reveal numerous foci of decreased signal on T1-weighted and increased signal on STIR sequences indicative of relapsed leukemia.
On MRI, areas of osteonecrosis are typically described as being geographic in distribution and well-marginated by a thin sclerotic line with fat signal emanating from the lesions themselves. These are often irregular in outline. Recurrent leukemia is typically manifested by well-defined smoothly marginated intramedullary foci of decreased signal on T1-weighted sequences and bright signal on STIR [60, 61] (Fig. 2). The sclerotic line has low intensity on all sequences; a high-intensity line lies adjacent to the sclerotic margin on STIR. T2-weighted images reveal a double line sign formed from high-intensity (inner) and low-intensity (outer) lines.
Nonspecific appearance of bone marrow signal has been reported around the knee in children with leukemia [62–64] and is composed of punctuate foci (dotlike abnormalities with 1- to 3-mm diameters with no discernible interior fatlike areas) (Fig. 3), bone marrow edema (Fig. 5), and diffuse marrow heterogeneity [65] (Fig. 4). The distinguishing characteristic of the latter two abnormalities from osteonecrosis or metastatic disease is the lack of sharply defined borders. Diffuse marrow heterogeneity lacks the interior zone of fatlike signal, which is especially evident on STIR. These findings typically resolve over time [65] and may represent red marrow conversion [66]. Varying degrees of edema or edemalike signal changes [66] (Figs. 4 and 5) may be seen in association with osteonecrosis but may also exist transiently and independent of osteonecrosis [64]; possible reported causes include bone marrow contusion, ischemia, and micro-fractures [66]. Areas of edema may be painful whether or not they are associated with developing osteonecrosis [67].
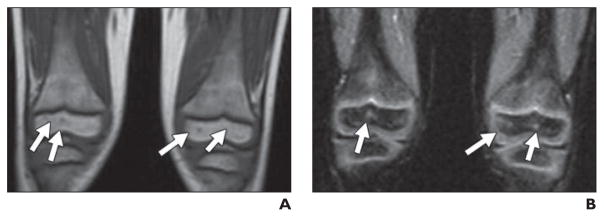
Fig. 3. Nonspecific punctuate lesions that can be confused with tiny areas of osteonecrosis in 4-year-old boy.
A and B, T1-weighted image (A) shows tiny foci of decreased signal (arrows, A) and STIR image (B) shows bright signal (arrows, B). These may be confused with tiny foci of osteonecrosis.
Fig. 5. Nonspecific patchy edema in knees in 13-year-old boy who had completed treatment for leukemia and underwent MRI surveillance for development of osteonecrosis to evaluate knee pain.
A and B, Coronal unenhanced T1-weighted (A) and STIR (B) images of knees show poorly defined decreased signal on T1-weighted and increased signal on STIR images, indicative of edema involving distal femoral and proximal tibial epiphyses and proximal left tibial epiphysis and metaphysis (arrows). These changes resolved at time of follow-up MRI 8 months later.
Fig. 4. Mottled marrow pattern in 18-year-old man previously treated for acute lymphoblastic leukemia who underwent MRI for complaint of chronic knee pain.
A and B, Coronal unenhanced T1-weighted (A) and STIR (B) images of knees show bilateral heterogeneous marrow signal (arrows) in distal femoral and proximal tibial metaphyses, which is mildly dark on T1-weighted and bright on STIR images. These changes became less apparent over course of 2 years (not shown). Also note area of nonspecific edema (arrowheads) in right lateral tibial epiphysis, which completely resolved over 2 years.
Patterns of Osteonecrosis
The joints most often reported to be involved with osteonecrosis vary between reports of symptomatic and asymptomatic cases. Typically, the hips and knees are most often reported although any joint may be involved [18]. In pediatric patients treated for malignancies, osteonecrosis lesions are typically bilateral in distribution [3, 7, 8, 12] unless there has been local radiation therapy, surgery, or other focal insult that would unilaterally predispose a joint to this toxicity. Multijoint involvement is common [3, 18, 24].
Distribution osteonecrosis among the epiphysis, metaphysis, and diaphysis varies by patient as does the extent of such lesions. Several investigators have described the extent of epiphyseal involvement to be predictive of joint destruction [10, 13, 15, 68, 69] (Figs. 1 and 6). Extension of epiphyseal lesions to the articular surface [3, 64] and estimated percentage involvement of the articular surface are important MR findings to describe the extent of osteonecrosis involvement [3, 64, 68]. These aspects are important for predicting the eventual outcome of joint function and risk of arthroplasty [8, 10, 65, 70–72]. Epiphyseal lesions involving at least 900 mm2 [69], large lesions involving more than one third of the femoral condyle on a midcoronal image or more than one of three zones on a midsagittal image [70], and those with a combined epiphyseal necrotic angle of at least 250° [13] are all characteristics reported as predictive of joint destruction and the need for arthroplasty.
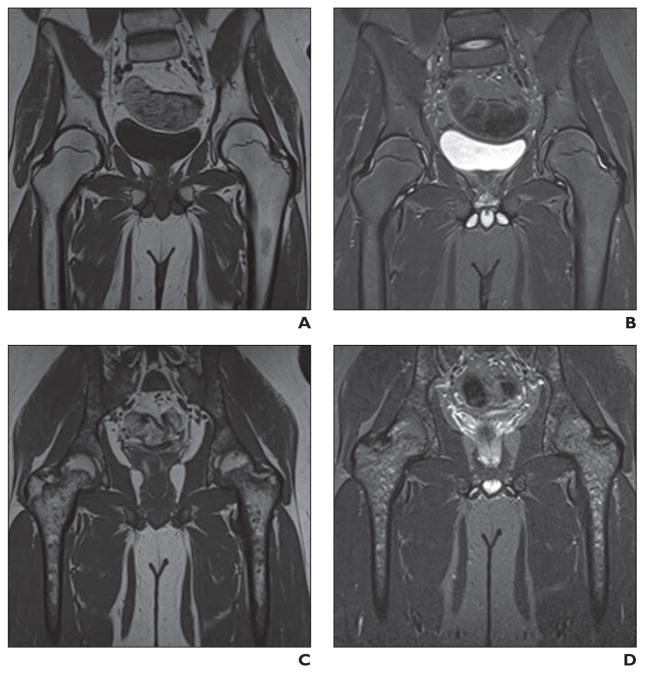
Fig. 6. 15-year-old boy treated for acute lymphoblastic leukemia and asymmetric osteonecrosis of hips.
A and B, Coronal unenhanced T1-weighted MR images of hips show small focus of osteonecrosis in right femoral head (arrow, A) and large (involving greater than 30% of articular surface) focus in left femoral head (arrowhead).
C and D, Over course of 18 months, small lesion remained stable (arrow, C) but eventually healed (images not shown). Lesion of left femoral head (arrowhead) rapidly progressed to collapse.
The correlation between clinical symptoms and functional impairment of one anatomic site over another is limited and currently inconclusive [73]. Because epiphyseal involvement leads to the most significant morbidity, investigative initiatives have been directed toward detection and evolution of these lesions, with little attention paid to the significance and evolution of metaphyseal and diaphyseal lesions.
In the knees, osteonecrosis typically occurs on both the femoral and the tibial sides of the joint. When MR monitoring is prospectively performed, regardless of the presence or absence of clinical symptoms, involvement of the knees is the most common site of osteonecrosis, superseding involvement of the hips [24]. The knees may be the only joints involved. When the hips are involved, the knees are also commonly involved but the reverse less commonly occurs [3, 24].
Monitoring Recommendations for Asymptomatic Patients
To our knowledge, no published recommendations exist regarding monitoring of asymptomatic patients for osteonecrosis in the hips or knees. However, because of the less common development and a lower rate of complications [3, 4, 10] of osteonecrosis in patients younger than 10 or 11 years [3, 20], routine monitoring may not be indicated. For older patients, our current recommendations, supported by preliminary analysis of ongoing unpublished investigations and based on prior publications, seem to indicate a need to monitor asymptomatic patients exposed to glucocorticoids [3, 16] or those who undergo bone marrow transplantation [24]. We have found few instances of new sites of osteonecrosis or progression of existing osteonecrosis beyond 3 years of follow-up [6]. Patients with subtle MR changes in the hips or knees or those who develop symptoms, regardless of the presence or absence of MR changes, warrant an individualized approach to imaging. Further analyses may warrant revision of our current recommendations.
New Challenges
Oncotherapy regimens continue to evolve to improve outcomes and now also to minimize toxicities. Currently incorporated into phase I, II, and III clinical studies are new agents—antiangiogenesis and targeted molecular agents—that have been shown in pre-clinical trials to adversely affect skeletal development. These agents, particularly the antiangiogenesis agents, alter vascularization of chondrocytes, which in turn compromises endochondral ossification of the long bones. Coupled with early apoptosis of chondrocytes, decreased longitudinal growth of the long bones from early physeal fusion has been shown in preclinical models [74–76]. Experience with these agents is preliminary, but similar alteration of skeletogenesis is of concern in growing children.
Conclusion
Children and adolescents treated for malignancies represent a unique and growing population at significant risk for developing osteonecrosis that may limit joint functionality; require surgical intervention at a young age; and, overall, compromise quality of life. Longitudinal outcomes studies are currently limited, and a standardized approach to interventions has not been defined. Imaging plays a key role in identifying and staging osteonecrosis lesions because symptoms are unreliable for identifying affected patients and determining the severity of osteonecrosis and the risk of progression.
Acknowledgments
Supported in part by grant number P30 CA-21765 from the National Institutes of Health, a Center of Excellence grant from the State of Tennessee, and the American Lebanese Syrian Associated Charities (ALSAC).
We thank Sandra Gaither and Erika Thompson for manuscript preparation.
References
- 1.National Institute of Arthritis and Musculoskeletal and Skin Diseases Website. [Accessed December 10, 2010];Osteonecrosis. www.niams.nih.gov/Health_Info/Osteonecrosis/default.asp. Published June 2009.
- 2.Mont MA, Jones LC, Hungerford DS. Non-traumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006;88:1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 3.Karimova EJ, Rai SN, Ingle D, et al. MRI of knee osteonecrosis in children with leukemia and lymphoma. Part 2. Clinical and imaging patterns. AJR. 2006;186:477–482. doi: 10.2214/AJR.04.1597. [DOI] [PubMed] [Google Scholar]
- 4.Niinimaki RA, Harila-Saari AH, Jartti AE, et al. Osteonecrosis in children treated for lymphoma or solid tumors. J Pediatr Hematol Oncol. 2008;30:798–802. doi: 10.1097/MPH.0b013e31818ab29d. [DOI] [PubMed] [Google Scholar]
- 5.Gardeniers JWM. The ARCO perspective for reaching one uniform staging system of osteonecrosis. In: Schoutens A, Arlet J, Gardeniers JWM, et al., editors. Bone circulation and vascularization in normal and pathological conditions. New York, NY: Plenum Press; 1993. pp. 375–380. [Google Scholar]
- 6.Karimova EJ, Wozniak A, Wu J, Neel MD, Kaste SC. How does osteonecrosis about the knee progress in young patients with leukemia? A 2- to 7-year study. Clin Orthop Relat Res. 2010;468:2454–2459. doi: 10.1007/s11999-010-1358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. J Bone Joint Surg Am. 2010;92:2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 8.Hungerford DS, Jones LC. Asymptomatic osteonecrosis: should it be treated? Clin Orthop Relat Res. 2004:124–130. [PubMed] [Google Scholar]
- 9.Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90:477–484. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
- 10.Karimova EJ, Rai SN, Howard SC, et al. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25:1525–1531. doi: 10.1200/JCO.2006.07.9947. [DOI] [PubMed] [Google Scholar]
- 11.Ojala AE, Paakko E, Lanning FP, Lanning M. Osteonecrosis during the treatment of childhood acute lymphoblastic leukemia: a prospective MRI study. Med Pediatr Oncol. 1999;32:11–17. doi: 10.1002/(sici)1096-911x(199901)32:1<11::aid-mpo4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Lackner H, Benesch M, Moser A, et al. Aseptic osteonecrosis in children and adolescents treated for hemato-oncologic diseases: a 13-year longitudinal observational study. J Pediatr Hematol Oncol. 2005;27:259–263. doi: 10.1097/01.mph.0000163215.37147.13. [DOI] [PubMed] [Google Scholar]
- 13.Mont MA, Baumgarten KM, Rifai A, Bluemke DA, Jones LC, Hungerford DS. Atraumatic osteonecrosis of the knee. J Bone Joint Surg Am. 2000;82:1279–1290. doi: 10.2106/00004623-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Sakai T, Sugano N, Nishii T, Hananouchi T, Yoshikawa H. Extent of osteonecrosis on MRI predicts humeral head collapse. Clin Orthop Relat Res. 2008;466:1074–1080. doi: 10.1007/s11999-008-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrow AC, Laor T. Leukemia and treatment: imprint on the growing skeleton. Pediatr Radiol. 2008;38:594. doi: 10.1007/s00247-007-0740-6. [DOI] [PubMed] [Google Scholar]
- 16.Mattano LA, Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 17.Burger B, Beier R, Zimmermann M, Beck JD, Reiter A, Schrappe M. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL)—experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44:220–225. doi: 10.1002/pbc.20244. [DOI] [PubMed] [Google Scholar]
- 18.Kadan-Lottick NS, Dinu I, Wasilewski-Masker K, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:3038–3045. doi: 10.1200/JCO.2007.14.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawedia JD, Kaste SC, Pei D, et al. Pharmacokinetic, pharmacodynamic and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340–2347. doi: 10.1182/blood-2010-10-311969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balduzzi A, Gooley T, Anasetti C, et al. Unrelated donor marrow transplantation in children. Blood. 1995;86:3247–3256. [PubMed] [Google Scholar]
- 22.Kaste SC, Shidler TJ, Tong X, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:435–441. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 23.Faraci M, Calevo MG, Lanino E, et al. Osteonecrosis after allogeneic stem cell transplantation in childhood: a case-control study in Italy. Haematologica. 2006;91:1096–1099. [PubMed] [Google Scholar]
- 24.Sharma S, Yang S, Rochester R, et al. Prevalence of osteonecrosis and associated risk factors in children before allogeneic BMT. Bone Marrow Transplant. doi: 10.1038/bmt.2010.210. Epub 2010 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arico M, Boccalatte MF, Silvestri D, et al. Osteonecrosis: an emerging complication of intensive chemotherapy for childhood acute lymphoblastic leukemia. Haematologica. 2003;88:747–753. [PubMed] [Google Scholar]
- 26.Relling MV, Yang W, Das S, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22:3930–3936. doi: 10.1200/JCO.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Hogler W, Wehl G, van ST, Meister B, Klein-Franke A, Kropshofer G. Incidence of skeletal complications during treatment of childhood acute lymphoblastic leukemia: comparison of fracture risk with the General Practice Research Database. Pediatr Blood Cancer. 2007;48:21–27. doi: 10.1002/pbc.20701. [DOI] [PubMed] [Google Scholar]
- 28.Elmantaser M, Stewart G, Young D, Duncan R, Gibson B, Ahmed SF. Skeletal morbidity in children receiving chemotherapy for acute lymphoblastic leukaemia. Arch Dis Child. 2010;95:805–809. doi: 10.1136/adc.2009.172528. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler MD. Physical changes of puberty. Endocrinol Metab Clin North Am. 1991;20:1–14. [PubMed] [Google Scholar]
- 30.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laroche M. Intraosseous circulation from physiology to disease. Joint Bone Spine. 2002;69:262–269. doi: 10.1016/s1297-319x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- 32.Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol. 2009;114:121–128. doi: 10.1016/j.jsbmb.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong GT, Sklar CA, Hudson MM, Robison LL. Long-term health status among survivors of childhood cancer: does sex matter? J Clin Oncol. 2007;25:4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Zhang CQ, Sun Y, et al. Changes in femoral head blood supply and vascular endothelial growth factor in rabbits with steroid-induced osteonecrosis. J Int Med Res. 2010;38:1060–1069. doi: 10.1177/147323001003800333. [DOI] [PubMed] [Google Scholar]
- 35.Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 36.French D, Hamilton LH, Mattano LA, Jr, et al. A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:4496–4499. doi: 10.1182/blood-2007-11-123885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.te Winkel ML, Appel IM, Pieters R, van den Heuvel-Eibrink MM. Impaired dexamethasone-related increase of anticoagulants is associated with the development of osteonecrosis in childhood acute lymphoblastic leukemia. Haematologica. 2008;93:1570–1574. doi: 10.3324/haematol.12956. [DOI] [PubMed] [Google Scholar]
- 38.Hong JM, Kim TH, Kim HJ, Park EK, Yang EK, Kim SY. Genetic association of angiogenesis and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp Mol Med. 2010;42:376–385. doi: 10.3858/emm.2010.42.5.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niinimaki RA, Harila-Saari AH, Jartti AE, et al. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J Clin Oncol. 2007;25:1498–1504. doi: 10.1200/JCO.2006.06.2539. [DOI] [PubMed] [Google Scholar]
- 40.Mont MA, Marulanda GA, Jones LC, et al. Systematic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(suppl 3):16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 41.Bassounas AE, Karantanas AH, Fotiadis DI, Malizos KN. Femoral head osteonecrosis: volumetric MRI assessment and outcome. Eur J Radiol. 2007;63:10–15. doi: 10.1016/j.ejrad.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Zibis AH, Karantanas AH, Roidis NT, et al. The role of MR imaging in staging femoral head osteonecrosis. Eur J Radiol. 2007;63:3–9. doi: 10.1016/j.ejrad.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Malizos KN, Karantanas AH, Varitimidis SE, Dailiana ZH, Bargiotas K, Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63:16–28. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Khanna AJ, Yoon TR, Mont MA, Hungerford DS, Bluemke DA. Femoral head osteonecrosis: detection and grading by using a rapid MR imaging protocol. Radiology. 2000;217:188–192. doi: 10.1148/radiology.217.1.r00oc26188. [DOI] [PubMed] [Google Scholar]
- 45.Menezes NM, Connolly SA, Shapiro F, et al. Early ischemia in growing piglet skeleton: MR diffusion and perfusion imaging. Radiology. 2007;242:129–136. doi: 10.1148/radiol.2421050680. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Qi J, Xia L, et al. Diffusion MRI in ischemic epiphysis of the femoral head: an experimental study. J Magn Reson Imaging. 2008;28:471–477. doi: 10.1002/jmri.21458. [DOI] [PubMed] [Google Scholar]
- 47.Merlini L, Combescure C, De Rosa V, Anooshiravani M, Hanquinet S. Diffusion-weighted imaging findings in Perthes disease with dynamic gadolinium-enhanced subtracted (DGS) MR correlation: a preliminary study. Pediatr Radiol. 2010;40:318–325. doi: 10.1007/s00247-009-1468-2. [DOI] [PubMed] [Google Scholar]
- 48.Hong N, Du X, Nie Z, Li S. Diffusion-weighted MR study of femoral head avascular necrosis in severe acute respiratory syndrome patients. J Magn Reson Imaging. 2005;22:661–664. doi: 10.1002/jmri.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheng H, Zhang G, Wang YX, et al. Functional perfusion MRI predicts later occurrence of steroid-associated osteonecrosis: an experimental study in rabbits. J Orthop Res. 2009;27:742–747. doi: 10.1002/jor.20765. [DOI] [PubMed] [Google Scholar]
- 50.Darge K, Jaramillo D, Siegel MJ. Whole-body MRI in children: current status and future applications. Eur J Radiol. 2008;68:289–298. doi: 10.1016/j.ejrad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Castro TC, Lederman H, Terreri MT, Kaste SC, Hilario MO. Detection of multifocal osteonecrosis in an adolescent with dermatomyositis using whole-body MRI. Pediatr Radiol. 2010;40:1566–1568. doi: 10.1007/s00247-010-1636-4. [DOI] [PubMed] [Google Scholar]
- 52.Yeh LR, Chen CK, Huang YL, Pan HB, Yang CF. Diagnostic performance of MR imaging in the assessment of subchondral fractures in avascular necrosis of the femoral head. Skeletal Radiol. 2009;38:559–564. doi: 10.1007/s00256-009-0659-0. [DOI] [PubMed] [Google Scholar]
- 53.Sakai T, Sugano N, Nishii T, Haraguchi K, Yoshikawa H, Ohzono K. Bone scintigraphy for osteonecrosis of the knee in patients with non-traumatic osteonecrosis of the femoral head: comparison with magnetic resonance imaging. Ann Rheum Dis. 2001;60:14–20. doi: 10.1136/ard.60.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mont MA, Ulrich SD, Seyler TM, et al. Bone scanning of limited value for diagnosis of symptomatic oligofocal and multifocal osteonecrosis. J Rheumatol. 2008;35:1629–1634. [PubMed] [Google Scholar]
- 55.Warwick BJ, Caristo V, Hartin N, Ihsleish W, Perera C, Van der WH. MRI-negative, bone scintigram-positive in early osteonecrosis of the knees. Clin Nucl Med. 2006;31:750–753. doi: 10.1097/01.rlu.0000246890.17699.2a. [DOI] [PubMed] [Google Scholar]
- 56.Sohn MH, Jeong HJ, Lim ST, Song SH, Yim CY. F-18 FDG uptake in osteonecrosis mimicking bone metastasis on PET/CT images. Clin Nucl Med. 2007;32:496–497. doi: 10.1097/RLU.0b013e318053ed7b. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura F, Fujioka M, Takahashi KA, et al. Evaluation of the hemodynamics of the femoral head compared with the ilium, femoral neck and femoral intertrochanteric region in healthy adults: measurement with positron emission tomography (PET) Ann Nucl Med. 2005;19:549–555. doi: 10.1007/BF02985047. [DOI] [PubMed] [Google Scholar]
- 58.Schiepers C, Broos P, Miserez M, Bormans G, De Roo M. Measurement of skeletal flow with positron emission tomography and 18F-fluoride in femoral head osteonecrosis. Arch Orthop Trauma Surg. 1998;118:131–135. doi: 10.1007/s004020050332. [DOI] [PubMed] [Google Scholar]
- 59.Aratake M, Yoshifumi T, Takahashi A, Takeuchi R, Inoue T, Saito T. Evaluation of lesion in a spontaneous osteonecrosis of the knee using 18F-fluoride positron emission tomography. Knee Surg Sports Traumatol Arthrosc. 2009;17:53–59. doi: 10.1007/s00167-008-0641-8. [DOI] [PubMed] [Google Scholar]
- 60.Porter RP, Kaste SC. Imaging findings of recurrent acute lymphoblastic leukemia in children and young adults, with emphasis on MRI. Pediatr Radiol. 2004;34:400–408. doi: 10.1007/s00247-003-1137-9. [DOI] [PubMed] [Google Scholar]
- 61.Jaramillo D. What is the optimal imaging of osteonecrosis, Perthes, and bone infarcts? Pediatr Radiol. 2009;39(suppl 2):S216–S219. doi: 10.1007/s00247-009-1151-7. [DOI] [PubMed] [Google Scholar]
- 62.Ojala AE, Paakko E, Lanning FP, Harila-Saari AH, Lanning BM. Bone marrow changes on MRI in children with acute lymphoblastic leukaemia 5 years after treatment. Clin Radiol. 1998;53:131–136. doi: 10.1016/s0009-9260(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 63.Bernbeck B, Christaras A, Krauth K, et al. Bone marrow oedema and aseptic osteonecrosis in children and adolescents with acute lymphoblastic leukaemia or non-Hodgkin-lymphoma treated with hyperbaric-oxygen-therapy (HBO): an approach to cure? BME/AON and hyperbaric oxygen therapy as a treatment modality. Klin Padiatr. 2004;216:370–378. doi: 10.1055/s-2004-832341. [DOI] [PubMed] [Google Scholar]
- 64.Karimova EJ, Rai SN, Deng X, et al. MRI of knee osteonecrosis in children with leukemia and lymphoma, Part 1, Observer agreement. AJR. 2006;186:470–476. doi: 10.2214/AJR.04.1598. [DOI] [PubMed] [Google Scholar]
- 65.Karimova EJ, Kaste SC. MR imaging of osteonecrosis of the knee in children with acute lymphocytic leukemia. Pediatr Radiol. 2007;37:1140–1146. doi: 10.1007/s00247-007-0579-x. [DOI] [PubMed] [Google Scholar]
- 66.Roemer FW, Frobell R, Hunter DJ, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage. 2009;17:1115–1131. doi: 10.1016/j.joca.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 67.Koo KH, Ahn IO, Kim R, et al. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: prospective study with serial MR images. Radiology. 1999;213:715–722. doi: 10.1148/radiology.213.3.r99dc06715. [DOI] [PubMed] [Google Scholar]
- 68.Korholz D, Bruder M, Engelbrecht V, Ruther W, Gobel U. Aseptic osteonecrosis in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1998;15:307–315. doi: 10.3109/08880019809014014. [DOI] [PubMed] [Google Scholar]
- 69.Sakai T, Sugano N, Ohzono K, Matsui M, Hiroshima K, Ochi T. MRI evaluation of steroid- or alcohol-related osteonecrosis of the femoral condyle. Acta Orthop Scand. 1998;69:598–602. doi: 10.3109/17453679808999263. [DOI] [PubMed] [Google Scholar]
- 70.Kokubo T, Takatori Y, Ninomiya S, Nakamura T, Kamogawa M. Magnetic resonance imaging and scintigraphy of avascular necrosis of the femoral head: prediction of subsequent segmental collapse. Clin Orthop Relat Res. 1992:54–60. [PubMed] [Google Scholar]
- 71.Takatori Y, Kokubo T, Ninomiya S, Nakamura S, Morimoto S, Kusaba I. Avascular necrosis of the femoral head: natural history and magnetic resonance imaging. J Bone Joint Surg Br. 1993;75:217–221. doi: 10.1302/0301-620X.75B2.8444940. [DOI] [PubMed] [Google Scholar]
- 72.Ha YC, Jung WH, Kim JR, Seong NH, Kim SY, Koo KH. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg Am. 2006;88(suppl 3):35–40. doi: 10.2106/JBJS.F.00535. [DOI] [PubMed] [Google Scholar]
- 73.Marchese VG, Connolly BH, Able C, et al. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescents, and young adults with acute lymphoblastic leukemia. Phys Ther. 2008;88:341–350. doi: 10.2522/ptj.20070108. [DOI] [PubMed] [Google Scholar]
- 74.Smith DW. Is avascular necrosis of the femoral head the result of inhibition of angiogenesis? Med Hypotheses. 1997;49:497–500. doi: 10.1016/s0306-9877(97)90067-0. [DOI] [PubMed] [Google Scholar]
- 75.Roguin A, Levy AP. Angiogenesis: an update. Pediatr Endocrinol Rev. 2005;2:391–398. [PubMed] [Google Scholar]
- 76.Patyna S, Arrigoni C, Terron A, et al. Nonclinical safety evaluation of sunitinib: a potent inhibitor of VEGF, PDGF, KIT, FLT3, and RET receptors. Toxicol Pathol. 2008;36:905–916. doi: 10.1177/0192623308326151. [DOI] [PubMed] [Google Scholar]