Abstract
Porphyromonads play an important role in human periodontal disease and recently have been shown to be highly prevalent in canine mouths. Porphyromonas cangingivalis is the most prevalent canine oral bacterial species in both plaque from healthy gingiva and plaque from dogs with early periodontitis. The ability of P. cangingivalis to flourish in the different environmental conditions characterized by these two states suggests a degree of metabolic flexibility. To characterize the genes responsible for this, the genomes of 32 isolates (including 18 newly sequenced and assembled) from 18 Porphyromonad species from dogs, humans, and other mammals were compared. Phylogenetic trees inferred using core genes largely matched previous findings; however, comparative genomic analysis identified several genes and pathways relating to heme synthesis that were present in P. cangingivalis but not in other Porphyromonads. Porphyromonas cangingivalis has a complete protoporphyrin IX synthesis pathway potentially allowing it to synthesize its own heme unlike pathogenic Porphyromonads such as Porphyromonas gingivalis that acquire heme predominantly from blood. Other pathway differences such as the ability to synthesize siroheme and vitamin B12 point to enhanced metabolic flexibility for P. cangingivalis, which may underlie its prevalence in the canine oral cavity.
Keywords: periodontal disease, comparative genomics, Porphyromonas, canine, plaque
Introduction
The genus Porphyromonas is considered one of the most important in human periodontitis and a large amount of research has been undertaken into the role of Porphyromonas gingivalis in particular (Lamont et al. 1995; Griffen et al. 1998; Holt et al. 1999; Holt and Ebersole 2005). The central role of P. gingivalis in the progression of human periodontitis has led to its classification as a “keystone pathogen” (Darveau et al. 2012) which influences the composition of the oral microbiome even when present at low levels. Recent surveys of the canine oral microbiota (Dewhirst et al. 2012; Davis et al. 2013) have hinted at a role for Porphyromonads in canine oral health and periodontitis. For instance, Porphyromonas gulae which is closely related to P. gingivalis (∼98% sequence identity) based on 16S rDNA sequencing data (Fournier et al. 2001) is the second most common Porphyromonad measured by 16S rDNA sequencing of plaque from dogs with early periodontitis (Davis et al. 2013). In addition, Porphyromonas cangingivalis showed the highest relative abundance of 16S rDNA sequences of any canine oral species, with an average of 10.5% in plaque from healthy gums and 4.6% in plaque from periodontitis samples and reached as high as 25% relative abundance in both health and disease samples (Davis IJ, unpublished data). The ability of P. cangingivalis to predominate in both health and disease environments suggests that it is both metabolically flexible enough to colonize in health and also able to compete against other Porphyromonas spp. in a disease environment (Davis et al. 2013). A comparison of the genes present in the P. cangingivalis genome compared with those found in other Porphyromonads therefore has the potential to identify genes that support that metabolic flexibility. The genomes of various Porphyromonads from dog, human, and other mammals were compared to look at their relatedness and for differences in gene content. The ultimate aim is to better understand the role of the Porphyromonads found in canine plaque and generate information to assist the future development of treatments to reduce periodontitis in dogs.
Materials and Methods
Genomes Used
This study used 32 Porphyromonas genomes including representatives from all publicly available Porphyromonas species (table 1). Of the 32 genomes, 18 were of canine oral isolates previously sequenced and assembled by the authors (Coil et al. 2015). The remaining 14 were complete or partially assembled genomes downloaded from National Center for Biotechnology Information (NCBI) (Sayers et al. 2009). Species characteristics (assembly statistics, host species, host sites, and disease associations) (table 1) for the canine isolates were acquired from previous studies (Sayers et al. 2009; Davis et al. 2013), for noncanine strains from NCBI or individual genome release papers (Hirasawa and Takada 1994; Summanen et al. 2005, 2009; Sakamoto and Ohkuma 2013; Sakamoto et al. 2013). In addition to the Porphyromonas genomes, four further Bacteroidales genomes were used as outgroups in the phylogenetic analyses. These were Bacteroides fragilis, Paludibacter propionicigenes, Tannerella forsythia, and the canine isolate Porphyromonadaceae [G-1] sp. COT-184.
Table 1.
Summary of Porphyromonas Genomes
| Isolate | Accession | Number of Contigs | Size (Mb) | GC% | Proteins | Host | Site | Health Status of Mouth at Point of Isolation | Disease Association of Species (Health/Disease) |
|---|---|---|---|---|---|---|---|---|---|
| Porphyromonas asaccharolytica DSM 20707 uid66603 | NC_015501 | 1 | 2.19 | 52.5 | 1,699 | Human | Nonoral—puss | — | — |
| Porphyromonas asaccharolytica PR426713P I uid61039 | AENO00000000 | 58 | 2.20 | 52.3 | 1,655 | Human | Nonoral | — | — |
| Porphyromonas bennonis DSM 23058 | AQWR00000000 | 87 | 2.02 | 56.3 | 1,407 | Human | Nonoral—skin | — | — |
| Porphyromonas catoniae F0037 uid183770 | AMEQ00000000 | 44 | 2.11 | 51.0 | 1,855 | Human | Oral | — | — |
| Porphyromonas endodontalis ATCC 35406 uid55449 | ACNN00000000 | 37 | 2.06 | 47.5 | 1,965 | Human | Oral | — | — |
| Porphyromonas gingivalis ATCC 33277 uid58879 | NC 010729 | 1 | 2.35 | 48.4 | 2,089 | Human | Oral | — | D |
| Porphyromonas gingivalis TDC60 uid67407 | NC 015571 | 1 | 2.34 | 48.3 | 2,217 | Human | Oral | — | D |
| Porphyromonas gingivalis W50 uid180461 | AJZS00000000 | 104 | 2.24 | 48.3 | 2,016 | Human | Oral | — | D |
| Porphyromonas gingivalis W83 uid57641 | NC 002950.2 | 1 | 2.34 | 48.3 | 1,909 | Human | Oral | — | D |
| Porphyromonas oral taxon 279 F0450 uid174237 | ALKJ00000000 | 51 | 2.27 | 55.5 | 1,729 | Human | Oral | — | — |
| Porphyromonas somerae DSM 23386 | AQVC00000000 | 95 | 2.36 | 47.0 | 1,922 | Human | Nonoral—leg ulcer | — | — |
| Porphyromonas uenonis 60 3 uid55869 | ACLR00000000 | 250 | 2.24 | 52.5 | 1,977 | Human | Nonoral—vagina | — | — |
| Porphyromonas cangingivalis COT-109 OH1379 | JQJF00000000 | 21 | 2.36 | 47.7 | 1,708 | Dog | Oral | Gingivitis | H |
| Porphyromonas cangingivalis COT-109 OH1386 | JQJD00000000 | 65 | 2.44 | 47.6 | 1,771 | Dog | Oral | Gingivitis | H |
| Porphyromonas canoris COT-108 OH1224 | JQZX00000000 | 21 | 2.31 | 44.6 | 1,708 | Dog | Oral | PD1 | — |
| Porphyromonas canoris COT-108 OH1349 | JRAH00000000 | 43 | 2.33 | 44.6 | 2,153 | Dog | Oral | Gingivitis | — |
| Porphyromonas canoris COT-108 OH2762 | JQZV00000000 | 14 | 2.20 | 44.7 | 1,612 | Dog | Oral | Gingivitis | — |
| Porphyromonas canoris COT-108 OH2963 | JRAP00000000 | 21 | 2.18 | 44.8 | 1,955 | Dog | Oral | PD1 | — |
| Porphyromonas cansulci JCM 13913 | BAOV00000000 | 89 | 2.11 | 45.4 | 2,180 | Dog | Oral | — | D |
| Porphyromonas crevioricanis COT-253 OH1447 | JQJC00000000 | 30 | 2.16 | 45.4 | 1,607 | Dog | Oral | PD1 | D |
| Porphyromonas crevioricanis COT-253 OH2125 | JQJB00000000 | 14 | 2.11 | 45.4 | 1,583 | Dog | Oral | Gingivitis | D |
| Porphyromonas gingivicanis COT-022 OH1391 | JQZW00000000 | 19 | 1.98 | 42.7 | 1,433 | Dog | Oral | Gingivitis | — |
| Porphyromonas gulae I COT-052 OH1355 | JRAG01000000 | 40 | 2.34 | 48.6 | 1,847 | Dog | Oral | Gingivitis | D |
| Porphyromonas gulae I COT-052 OH3471 | JRAQ01000000 | 44 | 2.37 | 48.6 | 1,877 | Dog | Oral | Health | D |
| Porphyromonas gulae I COT-052 OH4946 | JQZY00000000 | 34 | 2.38 | 48.5 | 2,127 | Dog | Oral | PD1 | D |
| Porphyromonas gulae II COT-052 OH2857 | JRFD01000000 | 53 | 2.33 | 48.7 | 1,874 | Dog | Oral | Gingivitis | D |
| Porphyromonas gulae II COT-052 OH3856 | JRAT00000000 | 31 | 2.39 | 48.5 | 1,904 | Dog | Oral | Health | D |
| Porphyromonas macacae COT-192 OH2859 | JRFA00000000 | 32 | 2.36 | 43.2 | 1,843 | Dog | Oral | Gingivitis | D |
| Porphyromonas sp. COT-239 OH1446 | JRAO00000000 | 37 | 1.96 | 53.9 | 1,412 | Dog | Oral | PD1 | D |
| Porphyromonas sp. COT-290 OH0860 | JRAR01000000 | 82 | 2.34 | 49.7 | 1,619 | Dog | Oral | Gingivitis | H |
| Porphyromonas sp. COT-290 OH3588 | JRFC01000000 | 48 | 2.29 | 49.8 | 1,582 | Dog | Oral | — | H |
| Porphyromonas levii ATCC 29147 | ARBX00000000 | 125 | 2.51 | 45.7 | 2,150 | Cow | Nonoral—rumen | — | — |
| Porphyromonadaceae COT-184 OH4590 | JRAN01000000 | 79 | 2.39 | 37.6 | 1,859 | Dog | Oral | — | — |
| Bacteroides fragilis | NC_016776 | 1 | 5.37 | 43.4 | 4,290 | Human | Nonoral—gut | — | — |
| Tannerella forsythia | NC_016610 | 1 | 3.41 | 47.0 | 3,001 | Human | Oral | — | D |
| Paludibacter propionicigenes | NC_014734 | 1 | 3.69 | 38.9 | 3,020 | Environmental | Nonoral—soil | — | — |
Functional Analysis of Genomes
The genomes for all strains were uploaded to RAST (v2.0) (Aziz et al. 2008; Overbeek et al. 2014) and annotated with the ClassicRAST scheme with automatic error fixing and backfilling of gaps. Annotations were then manually curated to reduce the impact of partial/misassemblies or erroneous gene annotations; this was done using a combination of visual inspection, homology searches, and multiple sequence alignments. The subsequent amino acid sequences and genome annotations were used for downstream analyses.
Identification of Homologous Clusters
Homologous clusters used in the subsequent analyses of phylogeny and gene content were identified by using a Markov clustering approach. Predicted protein sequences from RAST were first filtered to remove those shorter than 50 amino acids in length. A reciprocal Basic Local Alignment Search Tool (BLAST) was performed, BLASTall (v2.2.25) (-b 10000 -v 10000 -p blastp -e 1e-5) (Altschul et al. 1990). The BLAST results were filtered to remove spurious hits (percentage identity ≥ 25, alignment length ≥ 40, and bit score ≥ 45), then further filtered so that only queries with reciprocal matches were retained. Markov cluster algorithms were applied to group the sequences, MCL (v12.135) ((mcxload –stream-mirror –stream-neg-log10 -stream-tf ‘ceil(200)') and (mcl -I 1.4)) (Li et al. 2003). The resultant clusters were then converted into counts and binary matrices using in house Perl scripts (supplementary tables S1 and S2, Supplementary Material online) and annotated using the consensus annotation within the cluster. Sequences that failed to meet the above criteria or failed to form clusters were excluded from downstream analyses.
Phylogenetic Analysis
To investigate the phylogenetic relationship between Porphyromonas species, core sequence and gene content methods were applied. For the supermatrix method, single copy core sequences were extracted from the set of homologous clusters. The sequences from each cluster were aligned using “muscle” (v3.8.31) (default settings) (Edgar 2004) and then concatenated for each strain. The consequent concatenated alignment was filtered for low-quality sites using Gblocks (v.0.91b) (maximum contiguous nonconserved positions 8, minimum block length 10) (Castresana 2000). The filtered alignments were used to construct a maximum-likelihood (ML) tree using FastTree (v2.1.7) (using a Whelan and Goldman matrix and optimized using Gamma20 likelihood for 1,000 bootstraps) (Price et al. 2010).
Gene Content Clustering
The binary presence/absence matrix of homologous clusters computed by MCL was used to calculate the Jaccard distance between each strain, using the “dist.binary” function from the R (v3.0.2) package “ade4” (v1.6-2) (Dray and Dufour 2007). The resultant distance matrix was used to create a hierarchical clustering of the strains. The trees from both the supermatrix and gene content methods were plotted using FigTree (v1.4.1) (unpublished) and annotated using Inkscape (v0.48.4) (unpublished). The gene content of strains is also represented as a heatmap calculated from the binary matrix using the binary distance function from R base which was subsequently converted into similarities.
Average Nucleotide Identity
The average nucleotide identity (ANI) analysis used the assembled whole-genome nucleotide sequence data and followed the method and 95% species criteria as previously reported (Goris et al. 2007; Chan et al. 2012; Kim et al. 2014). For all 32 genomes and for each reciprocal pair, one genome was chosen as a query and the other as a reference. The query was split into consecutive fragments of 500 bp, smaller fragments were discarded. The query fragments were then used to interrogate the reference genome using BLASTn (v2.2.28) (–xdrop_gap_final 150 –penalty -1 –reward 1 –gapopen 3 –gapextend 2 –dust no) (Altschul et al. 1990). For each query if the alignment exhibited at least 70% identity for at least 70% of the query length, the hit with the best bit-score was retained for further evaluation. The ANI score was calculated as the average alignment identity of the retained hits for each reciprocal pair. The ANI scores were used to create a distance matrix to represent the ANI divergence (100% − ANI), this matrix was used to compute a hierarchical clustering using “hclust” function in R (v3.0.2) (method=“complete”) the hierarchical clustering was plotted using “ggplot2” (Wickham 2009) (supplementary fig. S1, Supplementary Material online).
Results
Phylogenetic Analysis
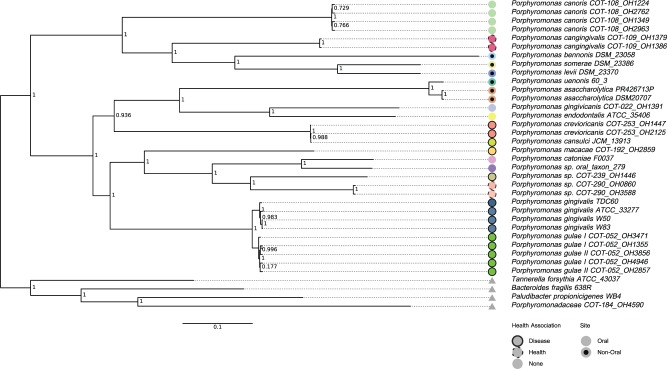
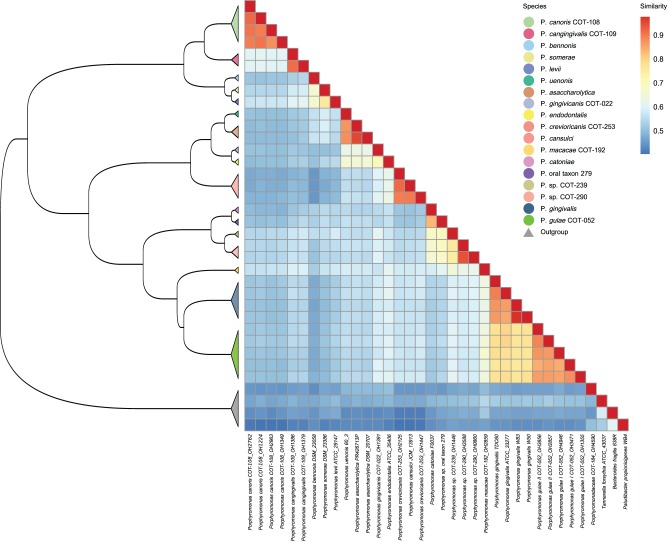
To understand the relatedness of the Porphyromonad strains, two complementary techniques were applied. First, a phylogenetic tree was inferred based on sequences common to all of the strains (core genome). The core genome was constructed for the 32 Porphyromonas strains (ingroup) and also for the 36 genome set including the four outgroup species. The ingroup core genome consisted of 657 (18%) core clusters from a pan-genome of 3,751 clusters, 492 of 657 core clusters were identified as single copy. After including the outgroup 560 (13%) of 4,460 were core clusters and of these 363 were single copy. The clusters were aligned separately and then concatenated to form core genomes. The ingroup alignment consisted of 199,261 amino acid positions which reduced to 131,810 (66% average global identity) positions after filtering (supplementary file S1, Supplementary Material online). The outgroup alignment contained 146,429 positions which reduced to 91,552 (66% average global identity) after filtering (supplementary file S2, Supplementary Material online). These alignments were then used to infer a phylogenetic tree (fig. 1). The second method clustered strains based on their shared gene content using the gene cluster matrix. This method did not have the scope to reconstruct evolutionary distance but was able to group strains based on which genes were present or absent using a Jaccard distance matrix (supplementary file S3, Supplementary Material online). The Jaccard distance is the size of the intersection divided by the size of the union subtracted from one; therefore, identical samples would have a pairwise value of zero whereas unrelated samples would have a value of 1. The two most similar pairwise comparisons were between P. gingivalis W50 and P. gingivalis W83 (0.16) and between Porphyromonas asaccharolytica DSM20707 and P. asaccharolytica PR426713P (0.23). The two least similar strains were between Pa. propionicigenes WB4 and the two Porphyromonas canoris strains; OH1554 and OH1349 (0.77 and 0.77). A hierarchical clustering of the pairwise Jaccard distances along with similarity heatmap is presented in figure 2. For both the ML and binary gene content methods, isolates of the same named species were grouped together at the nodes creating monophyletic groups. Canine and human oral strains did not form separate clusters neither did strains classed as health or disease associated. There were divides seen between the ingroup and outgroup strains which separated at the earliest point forming the root, the nonoral strains also separated but formed two disparate groups either side of the root.
Fig. 1.—
Core sequence ML tree. Supermatrix ML tree inferred from the concatenation of 492 single copy core cluster sequences showing node bootstrap values. Circles represent species groups, colored by species. Triangles are used to represent the four Porphyromonadaceae family strains (outgroup). Circles with black insertion were from stains isolated from nonoral sites. Circles with full outlines have known oral disease associations; staggered outlines have associations with oral health; and no outline have no known association.
Fig. 2.—
Binary gene content dendrogram and heatmap. The dendrogram was inferred by hierarchical clustering based of dissimilarities of gene content using Jaccard distances. Isolates of the same species are represented by triangles, nodes are colored by species. *Porphyromonas crevioricanis and Porphyromonas cansulci have been co-colored as thought to be isoforms of the same species. Heatmap constructed using binary distance (converted to similarity) between strains.
Comparison of Species Relatedness
Given the small distance between P. gulae and P. gingivalis isolates in the core genome phylogenetic tree (fig. 1), it was of interest to determine whether P. gulae and P. gingivalis were distinct species or could be reclassified as related subspecies. ANI values were calculated, pairwise ANI comparisons between P. gingivalis isolates gave values between 98.5% and 100%. Between P. gulae isolates the pairwise ANI values were between 98.0% and 98.3% and when P. gingivalis isolates were compared with P. gulae isolates the pairwise ANI values were between 92.7% and 92.9% (supplementary table S3, Supplementary Material online).
Porphyromonas crevioricanis and Porphyromonas cansulci were also closely related (fig. 1) and have previously been reported to be very similar if not the same species (Sakamoto and Ohkuma 2013; Sakamoto et al. 2013). Pairwise ANI calculations between these strains were performed and the ANI between the two P. crevioricanis strains and one P. cansulci strain was 99.5% (supplementary table S3, Supplementary Material online).
Metabolic Processes that Differ between P. cangingivalis and Other Porphyromonads
Iron
The role of iron and its regulation and acquisition in P. gingivalis has been studied extensively. Based on previous research 21 iron-related genes were identified in the P. gingivalis strains and then their presence or absence in the 16 other Porphyromonad species was determined (table 2). The genes fell into two main groups, the first group comprised genes conserved in all or almost all of the species examined. These included a tonB homolog putatively involved in ferric siderophore transport, ferritin-like protein (iron storage), ferrochetalase, and a ferrous iron transport protein (table 2). The other main group was present largely in only P. gingivalis and P. gulae. These included various hemagglutins, two hemolysins, two arginine-specific proteases (RGPA and RGPB) and a hemophore-like protein HusA. Outside of these two groups, a ferric uptake regulator (Fur) was found in P gingivalis, P. gulae and some of the oral human isolates but in none of the canine isolates. In addition, two iron compound ATP-binding cassette (ABC) transporter proteins were only found in P. gingivalis, P. gulae, and P. cangingivalis. Finally, lysine protease KGP was only found in P. gingivalis as were most of the hemagglutinins.
Table 2.
Genes Involved in Iron Acquisition, Regulation, or Utilization
| HagA ref: YP_004508794 | HagB ref: YP_004508981 | HagC ref: YP_004508984 | HagD gbk: AAB49691 | HagE gbk: AAQ66991 | Hemagglutinin gbk: WP_012458040 | Hemagglutinin gbk: WP_005873402 | Hemolysin ref: WP_023847110 | Hemolysin ref: WP_005873396 | Cell Envelope Biogenesis Protein TonB ref: WP_013815139 | TonB-Dependent Receptor ref: WP_013816293 | Ferritin ref: WP_004585574 | Ferrochelatase ref:WP_013815385 | Iron Transport FeoB ref:WP_013816689 | Iron ABC Transporter ref:WP_013816382 | Iron ABC Transporter Substrate-Binding Protein ref:WP_013816383 | Fur Family Transcriptional Regulator ref:WP_004584994 | Arginine-Specific Protease RGPA | Arginine-Specific Protease RGPB | Lysine-Specific Protease KGP | HusA ref:WP_005874769 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hemagglutanins | Hemolysins | Iron Transport | Gingipains | ||||||||||||||||||
| Porphyromonas canoris | + | + | + | + | |||||||||||||||||
| Porphyromonas cangingivalis | + | + | + | + | + | + | + | ||||||||||||||
| Porphyromonas bennonis | + | + | + | ||||||||||||||||||
| Porphyromonas somerae | + | + | + | + | |||||||||||||||||
| Porphyromonas levii | + | + | + | + | |||||||||||||||||
| Porphyromonas uenonis | + | + | + | + | + | + | |||||||||||||||
| Porphyromonas asaccharolytica | + | + | + | + | + | + | |||||||||||||||
| Porphyromonas gingivicanis | + | + | + | + | + | + | |||||||||||||||
| Porphyromonas endodontalis | + | + | + | + | + | + | |||||||||||||||
| Porphyromonas crevioricanis | + | + | + | + | + | + | |||||||||||||||
| Porphyromonas catoniae | + | + | + | + | + | + | |||||||||||||||
| Porphyromonas sp. oral taxon 279 | + | + | + | + | + | ||||||||||||||||
| Porphyromonas sp. COT-239 | + | + | + | + | + | + | |||||||||||||||
| Porphyromonas sp. COT-290 | + | + | + | + | + | ||||||||||||||||
| Porphyromonas macacae | + | + | + | + | + | ||||||||||||||||
| Porphyromonas gingivalis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Porphyromonas gulae | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
Protoporphyrin IX
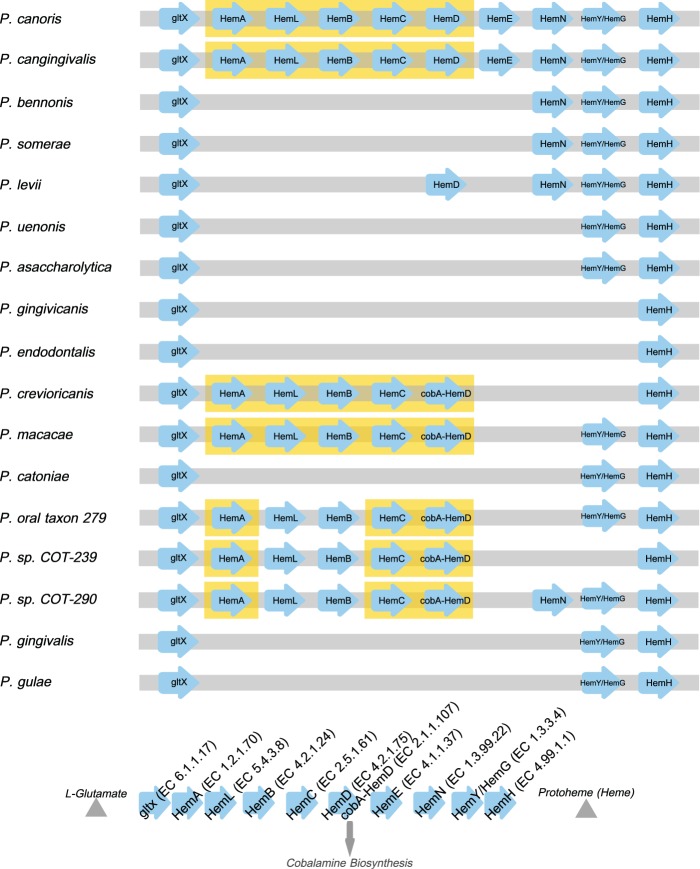
To identify genes that might confer metabolic flexibility, metabolic pathways that contained genes which differed between P. cangingivalis and the other Porphyromonads were analyzed. Given the importance of heme and its likely limited availability in healthy gingiva, the genes required for protoporphyrin IX synthesis were promising candidates and therefore investigated along with other related pathways.
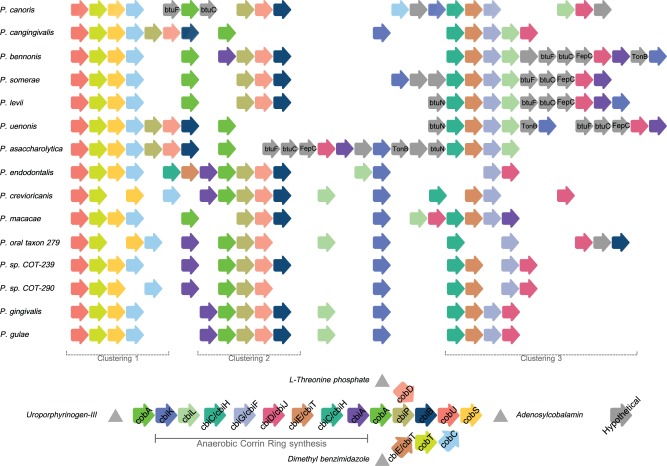
The metabolic pathway for protoporphyrin IX synthesis consists of ten enzymes that catalyze the conversion of L-glutamate to protoheme (heme) (fig. 3). Figure 3 is a schematic of the presence of these enzymes within the genomes of the Porphyromonas species. The first (gltx) and last (HemH) enzymes of the pathway were annotated in all 17 species, the penultimate enzyme of the pathway HemY/HemG was present in 13 species whereas the remaining seven enzymes were absent from the majority of the species. The only species to have all ten enzymes annotated within their genomes were P. cangingivalis and P. canoris. There were five species that contained a partially complete pathway these were P. sp. COT-290, P. sp. COT-239, P. oral taxon 279, Porphyromonas macacae, and P. crevioricanis, this group contained all of the enzymes required to convert L-glutamate to uroporphyrinogen III.
Fig. 3.—
The protoporphyrin IX synthesis pathway. Arrow icons indicate individual enzymes ordered by the pathway order, missing icons indicate missing enzymes from the genomes and yellow boxing highlights enzymes forming clusters. Schematic represents pathway order.
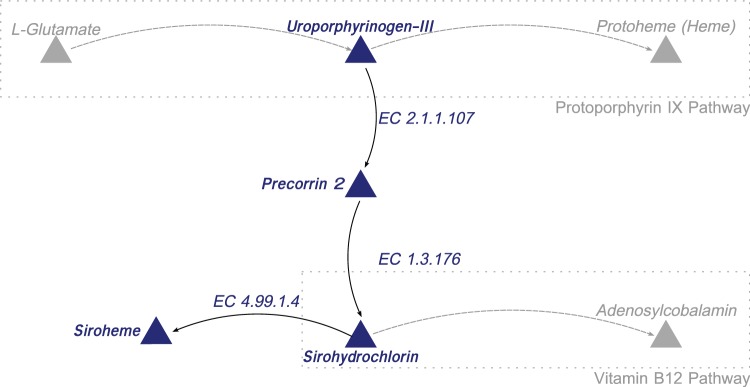
The pathway for protoporphyrin IX branches at uroporphyrinogen III leading to the synthesis of two other critical enzymatic cofactors siroheme and vitamin B12 (fig. 4). Investigating these pathways revealed further differences between the Porphyromonad species. Out of all of the 32 genomes examined, only P. cangingivalis possessed siroheme synthase and was therefore the only Porphyromonad that had the capacity to synthesize siroheme from uroporphyrinogen III (fig. 4). The pathway for synthesizing vitamin B12 (cobalamin) is complex and branches from the siroheme pathway with which it shares cobA (figs. 4 and 5). Of the 17 Porphyromonads analyzed, the genomes of 13 species contained all of the genes required to synthesize cobalamin from uroporphyrinogen III regardless of whether they had the genes to synthesize uroporphyrinogen III or not. The only exceptions to this were P. cangingivalis and P. canoris, which were missing cbiA (cobyrinic acid a,c-diamide) (fig. 5) and Porphyromonas gingivicanis and Porphyromonas catoniae, which lacked the entire pathway.
Fig. 4.—
Links between the protoporphyrin IX, siroheme and vitamin B12 synthesis pathways. The figure shows the connection between the protoporphyrin IX pathway and vitamin B12 synthesis pathway. Staggered lines represent a summary of the pathway, full lines represent specific reactions, arrows indicate pathway direction, and compounds are represented by triangles.
Fig. 5.—
The anaerobic vitamin B12 synthesis pathway. Colored arrow icons indicate individual enzymes grouped by clustering, hypothetical or nonpathway enzymes are gray and named where possible. Missing icons indicate enzymes not found within the genomes. Schematic represents pathway order.
As the starting molecule for protoporphyrin IX synthesis is glutamate, it was feasible that P. cangingivalis might have an increased glutamate requirement and therefore the genes relating to glutamate synthesis were examined. Analysis of genes relating to glutamate synthesis identified several differences between P. cangingivalis strains and other Porphyromonads. Porphyromonas cangingivalis strains contained three genes involved in converting nitrite to ammonia (a precursor of glutamate) that were absent in P. gulae (table 3). In addition the genome of P. cangingivalis contained an NADP(H)-dependent glutamate dehydrogenase that could synthesize glutamate from ammonia. In contrast, P. gulae had an NAD(H)-dependent glutamate dehydrogenase that could catabolize glutamate to ammonia (table 3). Porphyromonas cangingivalis also lacked aspartate amino transferase (AAT) which either synthesizes glutamate from aspartate and α-ketoglutarate or in the reverse direction generates aspartate from glutamate and oxaloacetate. Finally, three genes were identified that had the potential to supply the extra NADPH required by glutamate dehydrogenase in P. cangingivalis. These genes make up the oxidative phase of the pentose phosphate pathway that generates two NADPH molecules and were present in P. cangingivalis strains but not in P. gulae strains (table 4).
Table 3.
Glutamate Enzymes
| Species | Cytochrome c Biogenesis Protein CcsA | Cytochrome c Nitrite Reductase, Small Subunit NrfH | Cytochrome c552 Precursor NrfA (EC 1.7.2.2) | Aspartate Aminotransferase (EC 2.6.1.1) | NAD-Specific Glutamate Dehydrogenase | NADP-Specific Glutamate Dehydrogenase |
|---|---|---|---|---|---|---|
| (EC 1.4.1.2) | (EC 1.4.1.4) | |||||
| Porphyromonas canoris | + | + | + | + | + | |
| Porphyromonas cangingivalis | + | + | + | + | ||
| Porphyromonas bennonis | + | + | + | |||
| Porphyromonas somerae | + | |||||
| Porphyromonas levii | + | |||||
| Porphyromonas uenonis | + | |||||
| Porphyromonas asaccharolytica | + | |||||
| Porphyromonas gingivicanis | + | |||||
| Porphyromonas endodontalis | + | |||||
| Porphyromonas crevioricanis | + | + | ||||
| Porphyromonas catoniae | + | |||||
| Porphyromonas sp. oral taxon 279 | + | + | + | |||
| Porphyromonas sp. COT-239 | + | |||||
| Porphyromonas sp. COT-290 | + | + | + | + | ||
| Porphyromonas macacae | + | + | + | + | + | |
| Porphyromonas gingivalis | + | + | + | + | + | |
| Porphyromonas gulae | + | + |
Table 4.
Pentose Phosphate Enzymes
| Glucose-6-Phosphate1-Dehydrogenase(EC 1.1.1.49) | 6-Phosphogluconate Dehydrogenase, Decarboxylating(EC 1.1.1.44) | 6-Phosphogluco-Nolactonase(EC 3.1.1.31) | |
|---|---|---|---|
| G6PD | PGD | PGLS | |
| Porphyromonas canoris | + | + | + |
| Porphyromonas cangingivalis | + | + | + |
| Porphyromonas bennonis | + | ||
| Porphyromonas somerae | + | ||
| Porphyromonas levii | |||
| Porphyromonas uenonis | + | ||
| Porphyromonas asaccharolytica | + | ||
| Porphyromonas gingivicanis | + | ||
| Porphyromonas endodontalis | + | ||
| Porphyromonas crevioricanis | |||
| Porphyromonas catoniae | + | + | + |
| Porphyromonas sp. oral taxon 279 | + | + | + |
| Porphyromonas sp. COT-239 | + | + | + |
| Porphyromonas sp. COT-290 | + | + | + |
| Porphyromonas macacae | + | + | + |
| Porphyromonas gingivalis | |||
| Porphyromonas gulae |
Discussion
Phylogenetic Analysis
The core genome by definition is a pool of genes common to all members of a group and considered integral to fundamental aspects of biology and phenotypic traits (Medini et al. 2005). The genes within the core are therefore involved in functions, such as regulation, metabolism, and cell division. This is seen in the core set identified within the Porphyromonas genomes, which includes genes such as MutL, RpoN, and RnfB. It has been argued that core genes are recalcitrant to transfer (Eisen 2000) and stable over time as recipient taxa would likely already contain orthologs of similar function. However, there is some evidence to suggest that certain genes defined as being of core function are liable to core gene transfer (Everitt et al. 2014), which would have the potential to obscure the results of a core gene phylogeny.
The ML tree inferred using the core gene set roots at the midpoint, separating P. canoris, P. cangingivalis, P. bennonis, Porphyromonas somerae, and Porphyromonas levii from the other Porphyromonas species (fig. 1). This separation has also been demonstrated previously using analysis of 16S rDNA gene sequences (Fournier et al. 2001; Summanen et al. 2009, 2005; Gronow et al. 2011). The nonoral isolates (isolated from a skin abscess, leg ulcer, and bovine rumen) have been described as opportunistic pathogens, with their presence reported in clinical infections in a number of studies (Finegold et al. 2004; Summanen et al. 2005; Sweeney et al. 2009). In contrast, P. cangingivalis and P. canoris are among the most abundant Porphyromonas species in the canine oral environment (quantified by 16S rDNA sequences) (Davis et al. 2013), with P. cangingivalis being the most abundant of all canine oral species, with a high prevalence in plaque from healthy gingiva as well as plaque during periodontitis (Davis et al. 2013). This topology is repeated in the gene content clustering (fig. 2), suggesting that these species not only had an early split from the remaining species inferred from the ML tree but have core differences in gene content and therefore protein-coding capacity.
The remaining nonoral strains P. asaccharolytica and Porphyromonas uenonis form a clade, confirming previous observations using 16S rDNA genes (Finegold et al. 2004; Mikkelsen et al. 2008). Porphyromonas asaccharolytica particularly has been associated with pathogenesis at sites in addition to the oral cavity (Sharma and Rouphael 2009; Caiano Gil et al. 2013; Takeda et al. 2013). Porphyromonas asaccharolytica and P. uenonis have been shown to be biochemically similar (Finegold et al. 2004) to the oral associate Porphyromonas endodontalis (Cao et al. 2012; Lombardo Bedran et al. 2012), which is a sister clade to P. asaccharolytica and P. uenonis. Bifolious to P. endodontalis is P. gingivicanis a novel canine strain (Hirasawa and Takada 1994). Next to this clade are P. crevioricanis and P. cansulci which were confirmed by pairwise ANI comparisons (99.5%) to be the same species (supplementary table S3, Supplementary Material online). Strains of the human periodontitis-related species P. gingivalis (Pihlstrom et al. 2005) form a monophyletic group that is similar but distinct from the putative canine periodontitis P. gulae strains (Nomura et al. 2012; Yamasaki et al. 2012).
The ML tree is mainly in agreement with previously published studies looking at 16S rDNA genes in isolation, and this topology is recapitulated in the gene content of the strains as seen in the gene content clustering. The tree topology however is not entirely concordant with some phenotypic differences observed within the genus. For example, it has been previously reported from biochemical tests (Fournier et al. 2001; Mikkelsen et al. 2008) that approximately half of the Porphyromonad species test positive for catalase (Mikkelsen et al. 2008), an enzyme commonly found in aerobic bacteria that detoxifies molecular oxygen. This observation was confirmed here in silico by the identification of the catalase (EC 1.11.1.6) enzyme. However, the distribution of species containing catalase does not fall where one would expect for a single point of origin. For example, it is absent from P. gingivalis, P. somerae and P. levii but present in P. gulae, P. cangingivalis and P. canoris. This would suggest that through parsimony catalase has been acquired or lost in multiple events; the acquisition of catalase was supported by looking at the neighboring genes which were different for each species. A phylogeny inferred using the catalase genes found within the canine species did not resemble the phylogeny shown by the core gene set (supplementary fig. S2, Supplementary Material online). Furthermore, catalase is absent from all of the human oral isolates but present in all (with the exception of P. crevioricanis) of the dog species. This would advocate that catalase provides an advantage to bacteria living within canine mouths. It is therefore plausible that canine Porphyromonads have acquired catalase from other bacteria within the mouth, this is consistent with previous reports where similar results were attributed to multiple migrations of catalase (Fast et al. 2003; Passardi et al. 2007). The value of catalase may be enhanced by its high turnover rate (Chidrawi et al. 2008), with its high level of enzyme activity magnifying the advantage of incorporation. The presence of catalase may help canine Porphyromonads to tolerate higher levels of oxygen in the environment potentially allowing them to become more prevalent in healthy plaque than are their human cousins.
The close relationship between P. gingivalis and P. gulae isolates was investigated further to determine whether the two are from different species. Porphyromonas gulae was first described as a separate species largely as a result of DNA–DNA hybridization results (Fournier et al. 2001). However, the 16S rDNA sequence data in the same study were less clear cut. In this study, ANI values (Goris et al. 2007) were calculated. When the P. gulae and P. gingivalis isolates were compared (supplementary table S3, Supplementary Material online) the pairwise ANI values were in the range 92.7–92.9%. Based on a previously determined threshold of 95% or above for isolates being from the same species (Chan et al. 2012; Kim et al. 2014) this confirms that despite their high level of similarity P. gulae and P. gingivalis are sufficiently different to be considered separate species.
Adaptations of P. cangingivalis for Proliferating in Health
Porphyromonas cangingivalis is the most prevalent canine oral bacterium found in 16S rDNA studies. It therefore has properties that make it able to persist in the canine oral cavity, particularly in healthy plaque where it averages over 10% of the bacteria present (Davis et al. 2013). Differences between P. cangingivalis and other Porphyromonads were identified that are consistent with what we already know about the likely conditions present in healthy plaque compared to during periodontal disease; a number of linked metabolic pathways were analyzed that all center on the production of heme.
Heme
One of the key differences for bacteria seeking to proliferate in plaque from healthy gingiva compared with the plaque associated with periodontal disease is the availability of various nutrients such as sources of energy and cofactors like heme. In Porphyromonads, heme is thought to be acquired predominantly from the breakdown of hemoglobin from blood (Amano 1995; Nhien et al. 2010). Many other bacteria do however contain the machinery to synthesize their own heme (Hansson et al. 1991; McNicholas et al. 1997; Hashimoto et al. 1997; Guégan et al. 2003; Xiong et al. 2007), which offers an alternative strategy to heme acquisition particularly when this is limited in an environment. The relationship between sourcing heme endogenously or exogenously is not fully understood, but may center on the energy expense of synthesizing rather than foraging (Anzaldi and Skaar 2010). Heme is not only important for growth but also has important roles in defense and virulence (Olczak et al. 2005), Porphyromonads in general are black pigmented because they store heme at the cell surfaces in vast quantities. This is thought to provide some level of protection from peroxidases generated as part of the host’s immune response (Smalley et al. 2000).
Porphyromonas gingivalis has an array of genes which are important for acquiring and utilizing iron (Olczak et al. 2008). The presence or absence of many of these heme- and iron-related proteins was analyzed across the various Porphyromonad genomes studied here. First a key difference between P. gulae (and other Porphyromonads) and its human counterpart P. gingivalis was observed, despite most of the iron-related genes being the same between the two strains, a copy of the lysine gingipains gene (KGP) was not identified in any P. gulae strain. In contrast, both of the arginine gingipains genes (RGPA and RGPB) were present. The gingipains genes have been heavily studied and shown to be important for P. gingivalis virulence. The genes encode for outer membrane cysteine proteases that are required for host colonization, inactivation of host defenses, tissue destruction, and modulation of the host immune system (Holt et al. 1999). Gene knockout experiments have shown that a lysine gingipains mutant of P. gingivalis had reduced heme agglutination and increased sensitivity to complement compared with its wild-type parent (Grenier et al. 2003), which results from an increased sensitivity to damage by H2O2. Given that none of the canine Porphyromonads have a lysine gingipains gene and only P. gulae has arginine gingipains genes, it might be supposed that the canine Porphyromonads have an increased sensitivity to H2O2. However as already mentioned, all the canine Porphyromonads (except P. crevioricanis) have a catalase gene that may protect against H2O2. Therefore it is feasible that P. gulae has lost the lysine gingipains gene because it has become redundant, with its main function (protection against H2O2) now being provided by catalase.
Comparing the iron-related genes from P. cangingivalis with those from other Porphyromonads showed that many are retained in P. cangingivalis. Genes for iron transport and iron storage (ferritin) are all present (table 2). In contrast to this, the genes for hemagglutination, the arginine gingipains genes, and the ferric uptake regulator gene are missing. Porphyromonas cangingivalis may therefore still have the ability to uptake heme, potentially as a source of iron, but the absence of hemagglutinating genes in P. cangingivalis compared with P. gulae may relate to a divergence in lifestyle. Assuming the latter retains the pathogenic properties of P. gingivalis, an important part of its mechanism of action may be to attach to and invade canine gingival tissue cells. If P. cangingivalis has evolved to a commensal modality, there may be a disadvantage to attacking canine cells that might elicit an inflammatory response. Under those circumstances the loss of the ability to hemagglutinate red blood cells may be a strategy to prevent attachment to canine gingival cells and the concomitant initiation of inflammation. This may be particularly important in a healthy plaque environment where stimulating the inflammatory response will lead to the generation of altered physiological conditions that other species (particularly other Porphyromonads) may be better adapted to utilize. Other gene differences between P. cangingivalis and P. gulae also appear to relate to their likely exposure to iron. Porphyromonas cangingivalis lacks HusA which is a hemophore-like protein that has been previously described to have a role in the uptake of heme from gingipains in P. gingivalis (Gao et al. 2010). HusA is expressed when heme levels are very low as it has a high affinity for heme and can effectively scavenge it from the environment. In contrast, P. cangingivalis may not need to scavenge heme under limiting conditions as it may be able to synthesize its own from protoporphyrin IX and iron.
The ferric uptake regulator homolog (Har) in P. gingivalis (Butler et al. 2014) regulates a number of genes in response to heme and has a role in biofilm formation in a heme responsive manner. Within the canine Porphyromonads Har is only present in P. gulae (table 4). Similarly the loss of ferric uptake regulator (Fur) has been reported in the genus Rickettsia and attributed to obligatory intracellular milieu (Panek and O’Brian 2002). It may therefore be possible that health associated Porphyomonad species have dispensed with gene regulation of heme uptake because they are likely to be operating in conditions where heme levels are low or alternatively are using a currently unidentified novel ortholog.
In a healthy mouth where blood is not present, iron will be complexed to the salivary proteins lactoferrin and transferrin. These are host scavenging systems that seek to reduce the iron available to oral bacteria as an antimicrobial defense. Some bacteria have been shown to subvert the host defense by being able to import transferrin and its associated iron (Banerjee et al. 2012). Examples of bacteria that are able to make use of iron bound within transferrin and lactoferrin include Neisseriaceae and Pasteurellaceae (Ratledge and Dover 2000), within these families of bacteria are other canine oral health-associated species such as Neisseria shayeganii and Pasteurella dagmatis (Davis et al. 2013; Holcombe et al. 2014). Studies have shown that the genes required to uptake host expressed siderophores are generally expressed in operons of genes which include; a linked outer membrane receptor, a periplasmic-binding protein (PBP), and an inner membrane ABC transporter (Krewulak and Vogel 2008). Within the iron-related genes analyzed in table 2, a PBP protein and an ABC transporter were found to be both present in only P. cangingivalis, P. gulae, and P. gingivalis. This may be part of a siderophore scavenging system used by P. cangingivalis in healthy plaque, although that does not explain the presence of the same genes found in P. gingivalis and P. gulae. It is however feasible that genes related to iron uptake that are unique to P. cangingivalis may remain undetected through lack of annotation. Assuming that P. cangingivalis is able to acquire iron, it also requires a source of protoporphyrin IX to be able to synthesize heme. A complete protoporphyrin IX synthesis pathway is present in P. cangingivalis and the same pathway is also present to varying degrees across the other Porphyromonads (fig. 3), which is suggestive that those species without a complete pathway are able to synthesize protoporphyrin IX by foraging precursors liberated by other species with more intact pathways.
The presence of the heme synthesis pathways is well documented across the tree of life, likewise is its loss (Panek and O’Brian 2002; Kořený et al. 2013). The loss of the pathway may arise due to degeneration of genes that are no longer under selective pressure, for example, when heme is readily available within a host environment. The loss of the pathway may also be more directly advantageous to bacteria by reducing the potential of cell damage from reactive oxygen species produced by the accumulation of heme (Anzaldi and Skaar 2010). Along with examples of the heme pathway being lost there are also examples of the pathway being regained as new niches are explored, for example, the group Kinetoplastea (Korený et al. 2010; Alves et al. 2011) which have regained partial or full pathways despite a complete absence within their ancestors. Whether the Porphyromonads have lost, gained, or lost and regained the heme pathway is unclear, but the completeness of the heme pathway may be reflective of the evolutionary strategy employed by the bacteria. Bacteria such as P. cangingivalis have maintained a complete (or near complete) pathway and therefore remained independent of the host, whereas more pathogenic bacteria such as P. gingivalis may have discarded their machinery in favor of exogenous heme acquisition, which requires less energy and therefore enables rapid growth when environmental conditions are favorable.
Other Pathways Linked to Protoporphyrin IX
Branching off the protoporphyrin IX pathway are genes for the synthesis of two other key cofactors, siroheme and vitamin B12 (cobalamin). Of all the Porphyromonads studied, only P. cangingivalis has the siroheme synthase gene. Siroheme has been found to be associated with two different classes of enzymes—nitrite and sulfite reductases (Krewulak and Vogel 2008). This raises the possibility that the nitrite reductase identified as potentially helping to supply glutamate in P. cangingivalis requires siroheme.
Vitamin B12 is required by all bacteria and the majority of the Porphyromonads has the second half of the pathway from precorrin 2 onwards (fig. 4). The exceptions are P. gingivicanis and P. catoniae that lack the entire pathway (and presumably scavenge vitamin B12) and P. cangingivalis and P. canoris that appear to lack CbiA. Given that these species have all the other genes (19 in total) required for synthesizing vitamin B12 from glutamate, it is possible that CbiA function in these strains is performed by another gene. Of particular interest is that P. cangingivalis uniquely among porphyromonads appears to have the genes required to make the CobN cobalt chelatase. This enzyme is normally part of the aerobic route to vitamin B12 synthesis and encodes the step in the pathway immediately before the anaerobic and aerobic pathways converge (Rodionov et al. 2003). One possibility is that P. cangingivalis has acquired this gene complex to allow it to scavenge intermediates from its aerobic neighbors which could be an advantage in the more oxygen rich environment of healthy gingiva.
Generating a Supply of Glutamate
To ensure an adequate amount of protoporphyrin IX can be synthesized, the starting precursor molecule(s) need to be present in sufficient amounts. The protoporphyrin IX pathway requires glutamate as a precursor (fig. 3). Porphyromonas cangingivalis therefore possesses a number of related genes that supply the building blocks and the energy required to provide a ready supply of glutamate. Three genes were identified which are homologs of three components of a cytochrome C nitrite reductase (NrfA, NrfH, and Cytochrome C biogenesis protein CcsA; Kranz et al. 2009; Einsle 2011). The combined action of these enzymes converts nitrite to ammonia by combining it with formate. Nitrite is readily available in saliva through the action of various bacteria converting nitrate into nitrite (Duncan et al. 1995). Reservoirs of nitrate reducing bacteria have been found on the human tongue (Duncan et al. 1995). To make use of the ammonia generated by nitrite reductase, it must be converted to glutamate. In P. cangingivalis, this may be achieved by the action of NADPH-dependent glutamate dehydrogenase. A related enzyme is also present in P. gulae. However, the P. gulae gene is an NADH-dependant glutamine dehydrogenase that catalyzes the opposite reaction, that is, the degradation of glutamate to ammonia. This gene correlates well with canine oral pathogenesis, being present in all of the disease-associated Porphyromonads (except COT-239) and none of the health-associated ones (table 3). This potentially reflects the differing requirements in the two health states, with amino acids freely available during periodontitis (from proteolytic degradation of host proteins and tissues) but in shorter supply in healthy gingiva with an increased requirement for glutamate to feed protoporphyrin IX synthesis.
The use of an NADPH requiring glutamine dehydrogenase places an increased demand on NADPH levels as an energy source. Perhaps to solve this problem P. cangingivalis has three extra genes in the pentose phosphate pathway (table 4). These genes form the oxidative phase of the pathway that generates two extra NADPH molecules for every glucose 6-phosphate molecule processed. In addition to further bolster the glutamate pool P. cangingivalis has lost the gene for AAT, which converts aspartate to glutamate and back. In an environment where glutamate levels are high, AAT will favor the glutamate catabolic route, so deletion of this gene possibly allows the generation of a higher concentration of glutamate. Of course, protoporphyrin IX is not the only destination for glutamate in the bacterial cell. Glutamate is a precursor for the synthesis of several amino acids. Therefore, the genes listed above that drive increased glutamate levels may also have a role in supporting the synthesis of amino acids which again is consistent with an environment where sources of amino acids are in short supply.
Conclusions and Summary
In an attempt to understand the metabolic flexibility of P. cangingivalis that might explain its prevalence in the canine mouth both in plaque from healthy gingiva and plaque associated with the development of periodontal disease, genes relating to the generation or acquisition of heme have been analyzed. Even within this limited field of study many genes have been identified which could give P. cangingivalis an advantage within the canine oral environment. These differences are summarized in table 5. Along with P. canoris, P. cangingivalis is the only Porphyromonad to have all the genes required to synthesize protoporphyrin IX, which is potentially required in the absence of blood as a source of heme. Linked to protoporphyrin IX a number of genes were identified that point to the need to generate elevated levels of glutamate which could be used as a precursor for protoporphyrin IX. Porphyromonas cangingivalis is the only species with the gene to synthesize siroheme and the only species potentially able to scavenge from the aerobic pathway for vitamin B12 synthesis in other bacteria.
Table 5.
Summary of Adaptations of Porphyromonas cangingivalis for Proliferating in Health
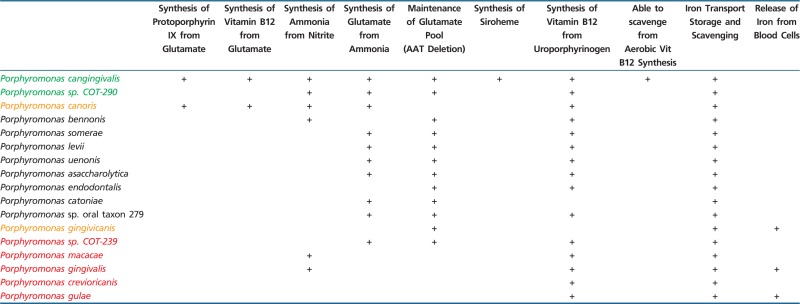 |
Note.—Red: periodontitis associated in dog; yellow: no association in dog; green: health associated in dog; black: noncanine species. Associations are taken from Davis et al. (2013).
Perhaps just as important are the genes that P. cangingivalis lacks. The gingipains genes and most of the hemagglutinin genes are unique to P. gulae and P. gingivalis and are not found in any other Porphyromonads. These have been shown in P. gingivalis to be key virulence factors linked to survival, degradation of host tissues and invasion of host cells. This suggests that the other Porphyromonads may not have a similar role and whether they are disease associated, such as P. macacae and P. crevioricanis, or just prevalent in disease such as P. cangingivalis, they may be responding to pathogenic events rather than initiating them. It might be considered somewhat ironic that P. cangingivalis, which has dispensed with many of the reported Porphyromonad virulence genes (e.g., hemolysins, hemagglutinins and gingipains) allowing a more commensal lifestyle, is actually as prevalent in early periodontitis as its cousin P. gulae which has these virulence genes. This may relate to the sequential nature of disease development with disease biofilms developing from healthy ones. We therefore speculate that P. cangingivalis dominates in healthy biofilms thanks to various metabolic adaptations that allow it to prosper such as the ability to synthesize its own heme. Establishing a strong foothold in a biofilm may be a good way to ensure continued success even when the environmental conditions change as periodontitis develops.
Supplementary Material
Supplementary files S1–S3, figures S1 and S2, and tables S1–S3 are available at Genome Biology and Evolution online (http://www.gbe.oxfordjournals.org/).
Acknowledgment
This work was funded by WALTHAM Centre for Pet Nutrition.
Literature Cited
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol. 215:403–410. [DOI] [PubMed] [Google Scholar]
- Alves JMP, et al. 2011. Identification and phylogenetic analysis of heme synthesis genes in trypanosomatids and their bacterial endosymbionts. PLoS One 6:e23518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano A. 1995. Binding of hemoglobin by Porphyromonas gingivalis. FEMS Microbiol Lett. 134:63–67. [DOI] [PubMed] [Google Scholar]
- Anzaldi LL, Skaar EP. 2010. Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect Immun. 78:4977–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz RK, et al. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, et al. 2012. Evidence of Fe3+ interaction with the plug domain of the outer membrane transferrin receptor protein of Neisseria gonorrhoeae: implications for Fe transport. Metallomics 4:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CA, et al. 2014. The Porphyromonas gingivalis ferric uptake regulator orthologue binds hemin and regulates hemin-responsive biofilm development. PLoS One 9:e111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiano Gil J, Calisto R, Amado J, Barreto V. 2013. Eikenella corrodens and Porphyromonas asaccharolytica pleural empyema in a diabetic patient with obstructive sleep apnea syndrome on noninvasive ventilation. Rev Port Pneumol. 19:76–79. [DOI] [PubMed] [Google Scholar]
- Cao H, et al. 2012. Detection of Porphyromonas endodontalis, Porphyromonas gingivalis and Prevotella intermedia in primary endodontic infections in a Chinese population. Int Endod J. 45:773–781. [DOI] [PubMed] [Google Scholar]
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 17:540–552. [DOI] [PubMed] [Google Scholar]
- Chan JZ-M, Halachev MR, Loman NJ, Constantinidou C, Pallen MJ. 2012. Defining bacterial species in the genomic era: insights from the genus Acinetobacter. BMC Microbiol. 12:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidrawi G, Robson M, Hollis S. 2008. Biology in focus: HSC course. Sydney: (Australia): McGraw-Hill Sydney. Available from: http://books.google.co.uk/books/about/Biology_in_Focus.html?id=fW6HSQAACAAJ&pgis=1 (accessed November 13, 2014). [Google Scholar]
- Coil DA, et al. 2015. Draft genome sequences of 26 Porphyromonas strains isolated from the canine oral microbiome. Genome Announc. 3:e00187–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP, Hajishengallis G, Curtis MA. 2012. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 91:816–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IJ, et al. 2013. A cross-sectional survey of bacterial species in plaque from client owned dogs with healthy gingiva, gingivitis or mild periodontitis. PLoS One 8:e83158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, et al. 2012. The canine oral microbiome. PLoS One 7:e36067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray S, Dufour A. 2007. The ade4 package: implementing the duality diagram for ecologists. J Stat Softw. 22(4):1–20. [Google Scholar]
- Duncan C, et al. 1995. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1:546–551. [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsle O. 2011. Structure and function of formate-dependent cytochrome c nitrite reductase, NrfA. Methods Enzymol. 496:399–422. [DOI] [PubMed] [Google Scholar]
- Eisen JA. 2000. Horizontal gene transfer among microbial genomes: new insights from complete genome analysis. Curr Opin Genet Dev. 10:606–611. [DOI] [PubMed] [Google Scholar]
- Everitt RG, et al. 2014. Mobile elements drive recombination hotspots in the core genome of Staphylococcus aureus. Nat Commun. 5:3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast NM, Law JS, Williams BAP, Keeling PJ. 2003. Bacterial catalase in the microsporidian Nosema locustae: implications for microsporidian metabolism and genome evolution. Eukaryot Cell. 2:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, et al. 2004. Porphyromonas uenonis sp. nov., a pathogen for humans distinct from P. asaccharolytica and P. endodontalis. J Clin Microbiol. 42:5298–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier D, et al. 2001. Porphyromonas gulae sp. nov., an anaerobic, Gram-negative coccobacillus from the gingival sulcus of various animal hosts. Int J Syst Evol Microbiol. 51:1179–1189. [DOI] [PubMed] [Google Scholar]
- Gao J-L, Nguyen K-A, Hunter N. 2010. Characterization of a hemophore-like protein from Porphyromonas gingivalis. J Biol Chem. 285:40028–40038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J, et al. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 57:81–91. [DOI] [PubMed] [Google Scholar]
- Grenier D, et al. 2003. Effect of inactivation of the Arg- and/or Lys-gingipain gene on selected virulence and physiological properties of Porphyromonas gingivalis. Infect Immun. 71:4742–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen AL, Becker MR, Lyons SR, Moeschberger ML, Leys EJ. 1998. Prevalence of Porphyromonas gingivalis and periodontal health status. J Clin Microbiol. 36:3239–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronow S, et al. 2011. Complete genome sequence of Paludibacter propionicigenes type strain (WB4). Stand Genomic Sci. 4:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guégan R, Camadro J-M, Saint Girons I, Picardeau M. 2003. Leptospira spp. possess a complete haem biosynthetic pathway and are able to use exogenous haem sources. Mol Microbiol. 49:745–754. [DOI] [PubMed] [Google Scholar]
- Hansson M, Rutberg L, Schröder I, Hederstedt L. 1991. The Bacillus subtilis hemAXCDBL gene cluster, which encodes enzymes of the biosynthetic pathway from glutamate to uroporphyrinogen III. J Bacteriol. 173:2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Yamashita M, Murooka Y. 1997. The Propionibacterium freudenreichii hemYHBXRL gene cluster, which encodes enzymes and a regulator involved in the biosynthetic pathway from glutamate to protoheme. Appl Microbiol Biotechnol. 47:385–392. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Takada K. 1994. Porphyromonas gingivicanis sp. nov. and Porphyromonas crevioricanis sp. nov., isolated from beagles. Int J Syst Bacteriol. 44:637–640. [DOI] [PubMed] [Google Scholar]
- Holcombe LJ, et al. 2014. Early canine plaque biofilms: characterization of key bacterial interactions involved in initial colonization of enamel. PLoS One 9:e113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. 2005. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex,” a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000. 38:72–122. [DOI] [PubMed] [Google Scholar]
- Holt SC, Kesavalu L, Walker S, Genco CA. 1999. Virulence factors of Porphyromonas gingivalis. Periodontology 2000. 20:168–238. [DOI] [PubMed] [Google Scholar]
- Kim M, Oh H-S, Park S-C, Chun J. 2014. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 64:346–351. [DOI] [PubMed] [Google Scholar]
- Korený L, Lukes J, Oborník M. 2010. Evolution of the haem synthetic pathway in kinetoplastid flagellates: an essential pathway that is not essential after all? Int J Parasitol.. 40:149–156. [DOI] [PubMed] [Google Scholar]
- Kořený L, Oborník M, Lukeš J. 2013. Make it, take it, or leave it: heme metabolism of parasites. PLoS Pathog. 9:e1003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz RG, Richard-Fogal C, Taylor J-S, Frawley ER. 2009. Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol Mol Biol Rev. 73:510–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewulak KD, Vogel HJ. 2008. Structural biology of bacterial iron uptake. Biochim Biophys Acta. 1778:1781–1804. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, et al. 1995. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 63:3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stoeckert CJ, Roos DS. 2003. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13:2178–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo Bedran TB, et al. 2012. Porphyromonas endodontalis in chronic periodontitis: a clinical and microbiological cross-sectional study. J Oral Microbiol. 4:10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas PM, Javor G, Darie S, Gunsalus RP. 1997. Expression of the heme biosynthetic pathway genes hemCD, hemH, hemM, and hemA of Escherichia coli. FEMS Microbiol Lett. 146:143–148. [DOI] [PubMed] [Google Scholar]
- Medini D, Donati C, Tettelin H, Masignani V, Rappuoli R. 2005. The microbial pan-genome. Curr Opin Genet Dev. 15:589–594. [DOI] [PubMed] [Google Scholar]
- Mikkelsen D, et al. 2008. Phylogenetic analysis of Porphyromonas species isolated from the oral cavity of Australian marsupials. Environ Microbiol. 10:2425–2432. [DOI] [PubMed] [Google Scholar]
- Nhien NTT, et al. 2010. Neutralization of toxic haem by Porphyromonas gingivalis haemoglobin receptor. J Biochem. 147:317–325. [DOI] [PubMed] [Google Scholar]
- Nomura R, et al. 2012. Diversity of fimbrillin among Porphyromonas gulae clinical isolates from Japanese dogs. J Vet Med Sci. 74:885–891. [DOI] [PubMed] [Google Scholar]
- Olczak T, Simpson W, Liu X, Genco CA. 2005. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol Rev. 29:119–144. [DOI] [PubMed] [Google Scholar]
- Olczak T, Sroka A, Potempa J, Olczak M. 2008. Porphyromonas gingivalis HmuY and HmuR: further characterization of a novel mechanism of heme utilization. Arch Microbiol. 189:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek R, et al. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42:D206–D214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panek H, O’Brian MR. 2002. A whole genome view of prokaryotic haem biosynthesis. Microbiology 148:2273–2282. [DOI] [PubMed] [Google Scholar]
- Passardi F, et al. 2007. Phylogenetic distribution of catalase-peroxidases: are there patches of order in chaos? Gene 397:101–113. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. 2005. Periodontal diseases. Lancet 366:1809–1820. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. 2010. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C, Dover LG. 2000. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 54:881–941. [DOI] [PubMed] [Google Scholar]
- Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. 2003. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J Biol Chem. 278:41148–41159. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Ohkuma M. 2013. Porphyromonas crevioricanis is an earlier heterotypic synonym of Porphyromonas cansulci and has priority. Int J Syst Evol Microbiol. 63:454–457. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Tanaka N, Shiwa Y, Yoshikawa H, Ohkuma M. 2013. Draft genome sequences of Porphyromonas crevioricanis JCM 15906T and Porphyromonas cansulci JCM 13913T isolated from a canine oral cavity. Genome Announc. 1(4):e00483–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers EW, et al. 2009. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 37:D5–D15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Rouphael N. 2009. Case report: atypical Lemierre’s disease secondary to Porphyromonas asaccharolytica. Curr Opin Pediatr. 21:397–400. [DOI] [PubMed] [Google Scholar]
- Smalley JW, Birss AJ, Silver J. 2000. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the mu-oxo bishaem of iron protoporphyrin IX to protect against hydrogen peroxide. FEMS Microbiol Lett. 183:159–164. [DOI] [PubMed] [Google Scholar]
- Summanen PH, et al. 2005. Porphyromonas somerae sp. nov., a pathogen isolated from humans and distinct from Porphyromonas levii. J Clin Microbiol. 43:4455–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summanen PH, Lawson PA, Finegold SM. 2009. Porphyromonas bennonis sp. nov., isolated from human clinical specimens. Int J Syst Evol Microbiol. 59:1727–1732. [DOI] [PubMed] [Google Scholar]
- Sweeney M, et al. 2009. Identification of Porphyromonas levii isolated from clinical cases of bovine interdigital necrobacillosis by 16S rRNA sequencing. Vet Ther. 10:E1–E10. [PubMed] [Google Scholar]
- Takeda K, Kenzaka T, Morita Y, Kuroki S, Kajii E. 2013. A rare case of Lemierre`s syndrome caused by Porphyromonas asaccharolytica. Infection 41:889–892. [DOI] [PubMed] [Google Scholar]
- Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York: Springer. [Google Scholar]
- Xiong J, Bauer CE, Pancholy A. 2007. Insight into the haem d1 biosynthesis pathway in heliobacteria through bioinformatics analysis. Microbiology 153:3548–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y, et al. 2012. Distribution and molecular characterization of Porphyromonas gulae carrying a new fimA genotype. Vet Microbiol. 161:196–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.