Abstract
Thromboembolic events were described in patients with Chagas disease without cardiomyopathy. We aim to confirm if there is a hypercoagulable state in these patients and to determine if there is an early normalization of hemostasis factors after antiparasitic treatment. Ninety-nine individuals from Chagas disease-endemic areas were classified in two groups: G1, with T.cruzi infection (n = 56); G2, healthy individuals (n = 43). Twenty-four hemostasis factors were measured at baseline. G1 patients treated with benznidazole were followed for 36 months, recording clinical parameters and performance of conventional serology, chemiluminescent enzyme-linked immunosorbent assay (trypomastigote-derived glycosylphosphatidylinositol-anchored mucins), quantitative polymerase chain reaction, and hemostasis tests every 6-month visits. Prothrombin fragment 1+2 (F1+2) and endogenous thrombin potential (ETP) were abnormally expressed in 77% and 50% of infected patients at baseline but returned to and remained at normal levels shortly after treatment in 76% and 96% of cases, respectively. Plasmin-antiplasmin complexes (PAP) were altered before treatment in 32% of G1 patients but normalized in 94% of cases several months after treatment. None of the patients with normal F1+2 values during follow-up had a positive qRT-PCR result, but 3/24 patients (13%) with normal ETP values did. In a percentage of chronic T. cruzi infected patients treated with benznidazole, altered coagulation markers returned into normal levels. F1+2, ETP and PAP could be useful markers for assessing sustained response to benznidazole.
Author Summary
The manuscript describes the results of a study whose aim was to assess the tendency to coagulate in people suffering from a parasitic infection frequent in Latin America named T. cruzi infection or Chagas disease, by the study of several coagulation factors. According to the state of the art in this topic, specific treatment for Chagas disease is recommended in recent (acute) and late (chronic) stages of the infection. The effectiveness of current available drugs in the chronic stage of infection is still a topic of debate due to inconsistent results across studies and a lack of early measurable parameters of response to specific treatment. Another aim of this study was to determine if the presence of an upregulated procoagulative activity in plasma in people suffering T. cruzi infection could be used as potential marker that indicates therapeutic response in people at chronic stage of the disease. The results of this study suggest that measurements of alterations of procoagulative activity may be useful to indicate specific treatment for T. cruzi chronically infected patients and new data concerning early response to treatment biomarkers.
Introduction
Chagas disease (CD) is one of 17 neglected tropical diseases recognized by the World Health Organization. Caused by the protozoan parasite Trypanosoma cruzi, it mainly affects people with poor socioeconomic status and limited health care access in endemic and nonendemic countries. [1, 2]
Thrombosis is considered as a pathological deviation of haemostasis, and it is characterized by intravascular thrombus formation and vessel occlusion. Perturbation of hemostasis is an important factor in the pathogenesis of thromboembolic events, which can be caused by blood flow dysregulation, endothelial injury, and coagulation system alterations.
Recently, is has been described that under certain circumstances thrombosis is a physiological process that constitutes an intrinsic effector mechanism of innate immunity, and the process has been defined as “immunothrombosis”. [3] It is activated after the recognition of pathogens and damaged cells, and inhibits pathogen dissemination and survival. Immunothrombosis can therefore be regarded as a newly identified, crucial element of intravascular immunity, which is a part of the immune system that encompasses a wide range of host strategies to detect and protect against pathogens in the vasculature. Dysregulation of immunothrombosis is likely to constitute a key event in the development of thrombotic disorders. [3]
Infectious disease can cause a hypercoagulable state through the upregulation of tissue factor in monocytes, the generation of procoagulant microparticles, the activation of the coagulation intrinsic pathway, platelet activation, and NETs (Neutrophil Extracellular Traps) release.[3] Different infectious agents may cause different responses but a final degree of hypercoagulability can be a common trait as one of the biological endpoints. Additionally, patients with chronic inflammation may also present platelet adhesion events, which are considered inflammatory processes and can be observed in patients with chronic T. cruzi infection, even in the asymptomatic stages. [4] Infection itself can cause vasculitis, increasing proinflammatory cytokine levels and perpetuating the risk of thrombotic events. [5] In the case of the Chagas’ disease the effect of hemostasis in the bradikinin formation, through the effect of factor XII activation in the Kallikrein-Kinin system, can modify the type 1 immune response and then modulate the antiparasite immunity as suggested in a mice model of subcutaneous infection by T.cruzi. [6]
Thromboembolic events and dilated cardiomyopathy, ventricular aneurysms, and intracavitary thrombosis are associated with CD. [7, 8] Rheological factors can induce intraluminal thrombus formation with the risk of embolism. [9] Alterations of molecular markers of coagulation system activation have been described in T. cruzi infection individuals with or without clinical thrombosis. [9–12] Other factors, such as injury to vessel walls by parasites or changes in blood viscosity due to host immune response, may influence in the development of thromboembolic events in T. cruzi-infected individuals without Chagas cardiomyopathy or other vascular risk factors. [13]Based on studies performed in humans with chronic T. cruzi infection, there are controversial results regarding the existence of a prothrombotic status in T. cruzi-infected patients. [13,14] There is an study in which a of higher prothrombotic status in the CD group was not found, but the control group were individuals without T. cruzi infection and heart failure. [14] In previous studies performed in murine models, several abnormalities of the heart microcirculation of individuals with chronic CD were pointed out, but they did not find evidence of thrombi and neither thromboembolism. [15, 16] Higher levels of the hypercoagulability markers prothrombin fragment 1+2 (F1+2), thrombin-antithrombin complexes (TAT), fibrinogen/fibrin degradation products, plasminogen activator inhibitor type 1 (PAI-1), and D-dimer have been reported in T. cruzi–infected patients compared with healthy individuals. [10, 11] A pilot study performed by our group showed that endogenous thrombin potential (ETP) and F1+2 levels were outside normal ranges in 73% and 80% of T. cruzi–infected patients without advanced heart disease, respectively. [12] We demonstrated a 100% and 73% decrease in these levels six months after treatment with benznidazole. Thus, if they prove to remain stable in time, hypercoagulability factors could be used as biomarkers of therapeutic response in CD. Besides, although whether or not chronic Chagas disease is an independent vascular risk factor remains to be confirmed. [17,18]
While specific treatment is recommended in both acute and chronic stages of infection [19,20], there are only two drugs (i.e., benznidazole and nifurtimox) available for the treatment of CD. The mechanism of action of benznidazole relates to the nitro-reduction of components of the parasite, the binding of metabolites of the nuclear DNA and k-DNA of T. cruzi and the lipids and proteins of the parasite. [21] In adults, benznidazole has a high rate of adverse effects, which can be classified into three groups: (i) hypersensitivity, including dermatitis with cutaneous eruptions (usually appearing between days 7 and 10), myalgias, arthralgias, and lymphadenopathy; (ii) polyneuropathy, paresthesias, and polineuritis usually during the 4th week of treatment); and (iii) bone marrow disorders, such as thrombopenic purpura and agranulocytosis (usually after the second week of treatment). [22]Furthermore, the effectiveness of these drugs in the chronic stage of infection is still a topic of debate due to inconsistent studies’ results [23–25] and a lack of early biomarkers of response to specific T. cruzi treatment with benznidazole. [26]
Following on from our pilot study [12], here we increased the sample size and extended follow-up to further investigate the value of hypercoagulability factors as biomarkers of treatment response in CD. We also added current treatment response parameters measured by conventional serology, serology for lytic anti-α-galactosyl (anti-α-Gal) antibodies against T.cruzi [27–29], and quantitative reverse transcription polymerase chain reaction (qRT-PCR). [30]
The aims of the study were to investigate alterations of hypercoagulability factors in patients chronically infected with T. cruzi and determine whether there is an early and sustainable improvement of the hypercoagulability factors after antiparasitic treatment.
Methods
Ethics Statement
Written informed consent was obtained from participants before being recruited (all of them were adults). Approval for the protocols and for the informed consent was obtained from the Hospital Clínic of Barcelona Ethics Review Committee.
Design and Setting
This is a descriptive study of 99 individuals (56 with T.cruzi infection and 43 healthy individuals) from Latin American, where CD is endemic. All the individuals were evaluated at the Centre for International Health at Hospital Clínic in Barcelona, Spain.
Recruitment and Participants
Ninety-nine individuals from CD-endemic areas living in Barcelona were invited to participate. Inclusion criteria were an age of over 18 years and provision of signed informed consent. Exclusion criteria were pregnancy, non-Chagasic cardiopathy, late chronic cardiac or digestive forms of CD, other acute or chronic infections, inflammatory or immunological diseases, and chronic systemic diseases (high blood pressure and diabetes).
Procedures
After signing the informed consent form, participants were asked for clinical and epidemiological data, including area of origin and risk factors for the CD transmission. The information recorded included vascular risk factors, toxic habits, and cardiological and/or vascular events.
Conventional serology of T.cruzi infection was established using two ELISA kits: a commercial kit with recombinant antigens (BioELISA Chagas, Biokit S.A.,Barcelona-Spain) and an in-house kit with whole T.cruzi epimastigote antigen, as described. [12, 31]. Diagnosis was confirmed by a positive result on both tests. [19] Following serological tests results, participants were divided into two groups: those with T.cruzi infection (Group 1 [G1]) and those without (Group 2 [G2]). All the participants underwent human immunodeficiency virus testing, basic blood and biochemical tests (including renal and liver function), and specific evaluation of hemostasis factors.
For the hemostasis studies, blood was collected in citrate-containing tubes (Becton Dickinson), samples were centrifuged, and platelet-poor plasma aliquots were frozen at –80°C until assayed. Prothrombin time, activated partial thromboplastin time, coagulation factor VIII, protein C activity, free and total protein S levels, antithrombin and plasminogen activity, F1+2, plasmin-antiplasmin complexes (PAP), factor VIIa, PAI-1, P-selectin, factor V Leiden and prothrombin gene G20210A mutation, lupus anticoagulant and anticardiolipin antibodies were measured as previously described. [12] D-dimer was measured using an automated turbidimetric test (Siemens Healthcare Diagnostics) and ETP was assessed using a continuous chromogenic thrombin generation assay and ETP Curves software (Siemens). The ETP coagulation test was initiated by using human recombinant tissue factor, phospholipids, and calcium ions. ADAMTS-13 was measured using a commercial chromogenic method (American Diagnostica). Factor XIIa was determined by a direct quantitative commercially available immunoassay (Shield Diagnostics) with a highly specific monoclonal antibody that does not recognize its zymogen factor XII.[32] Plasma tissue factor levels were determined using a commercial kit (American Diagnostica) according to the manufacturer’s protocol. Plasma levels of von Willebrand factor antigen were determined by enzyme-linked immunosorbent assay (ELISA) (Corgenix). Procoagulant activity of microparticles was measured using a functional assay with the addition of factors Xa, Va, and prothrombin after microparticle capture in the solid phase using annexin V (Hyphen Biomed). Soluble CD40L was measured by ELISA (R&D Systems).
qRT-PCR [30] and a chemiluminescent ELISA assay based on a highly purified, trypomastigote-derived glycosylphosphatidylinositol-anchored mucin (tGPI-mucin) antigen for the serological detection of lytic anti-α-Gal antibodies against T.cruzi (AT CL-ELISA) [27–29, 33–36], were performed in G1 at month 0 (baseline), and 6, 12, 18, 24, 30, and 36 months post-treatment. For AT CL-ELISA, a serum sample was considered positive when the titer was ≥1.0 and negative when it was ≤0.9. Inconclusive or equivocal results were determined by a titer between 0.9 and 1.0. [27, 35]All sera were tested in duplicate and the results were expressed as the mean of two simultaneous determinations.
G1 patients were studied using a protocol that included a 12-lead electrocardiogram, chest X-ray, and echocardiogram. They were followed up every 6 months for at least 36 months. At each visit, clinical data were collected and the following tests were performed: ELISA, AT CL-ELISA, qRT-PCR, and hemostasis tests. Other tests were performed according to individual symptoms. Specific treatment with benznidazole (5 mg/kg/day for 60 days) was offered to all T.cruzi–infected patients, and those treated were monitored fortnightly for clinical and analytical assessment. Treatment was considered complete when at least 80% of the total dose was reached.
A hypercoagulable state is defined as the presence, in certain individuals, of thrombotic potentialities that activate the endothelium and the formative elements of the blood (mainly, platelets) that favors plasma kinetics that lead to the formation of thrombin, which disturbs fibrinolytic activity and produces hemorheological changes with turbulence phenomena that predispose to thrombogenesis. [18]
Statistical Analysis
Quantitative variables were presented as medians and interquartile range (IQR) and were compared between groups using the Wilcoxon rank sum test. Qualitative variables were reported using absolute frequencies and percentages and between-group comparisons were made using Fisher’s exact test. Hypercoagulability biomarker variation over time was assessed using a mixed-effect linear regression model with a random intercept structure. Hypercoagulability factors were used as dependent variables and follow-up time as the explanatory variable, with one category for each time point: baseline, month 6 (reference for comparisons), and months 12, 18, 24, and 36. This type of model allows for the inclusion of random effects in addition to the overall error term. Random intercept regression was also used to assess whether antibody levels measured by ELISA and AT CL-ELISA approached the negative threshold during follow-up. The response variable was the distance from this threshold (i.e., the difference between each ELISA or AT CL-ELISA value and the negative cutoff) and the explanatory variable was the follow-up time from month six (reference) to month 36. The regression coefficients express the effect estimate of follow-up on the outcome variable.
The pattern of the relationships between hypercoagulability biomarkers was assessed by multiple correspondence analysis (MCA) using the Burt matrix approach. [37, 38] The MCA represents a method for analyzing multi-way contingency table containing measure of correspondence between row (subjects) and columns (levels of variables). The interpretation is based upon proximities between levels of variables (or points) in a low-dimensional map. The firsts dimensions (usually one or two) account for meaningful amounts of variance and are those retained for the map definition and interpretation. The first dimension accounts for a maximal amount of total variance in the observed variables. Under typical conditions, this means that the first component will be correlated with at least some of the observed variables. The second dimension has two important characteristics: it accounts for a maximal amount of variance in the data set that is not accounted for by the first dimension, thus it is correlated with some of the observed variables that not display strong correlations with dimension 1; and it is uncorrelated with dimension 1. Looking at the map, the proximity between levels of different variables means that these levels tend to appear together in the observations. Since the levels of the same variable cannot occur together, the proximity between levels of the same variable means that the groups of observations associated with these levels are themselves similar. A level far away from the origin (of the dimensions) means that is well-represented in the map, thus that level is meaningful for the interpretation of the dimension(s). All levels that are not useful for the solution are near the origin. Supplementary (passive) variables are those not used for the solution but mapped in the graph in order to help in the interpretation.
The biomarkers were classified into three categories: normalization of values throughout follow-up, non-sustained normalization during follow-up and normal values at baseline. Two additional variables were considered: qRT-PCR results during follow-up (categories: always negative and sometime positive) and level of adherence (categories: 80% and 100%). All the tests were 2-tailed and the confidence level was set at 95%. The analyses were performed using Stata 13 (Stata Corporation, College Station, TX, USA).
Results and Discussion
Ninety-nine individuals (76 women) were studied. Fifty-six of these (43 women) were T.cruzi–positive (G1) and 43 (33 women) were T.cruzi–negative. The mean ages were 34 (SD, 9) years for the overall group (range 17–56, median 33), 37 (SD, 9) years for G1, and 32 (SD, 7) years for G2. Fifty G1 patients were treated with benznidazole (six were lost to follow-up before starting treatment due to unexpected work-related changes in the migratory process). Forty-five (90%) completed treatment. Eighty-six participants (87%) (51 [91%] in G1 and 35 [81%] in G2) were from Bolivia. None of the participants traveled to their countries or other CD-endemic areas during follow-up. The clinical and demographic data are summarized in Table 1. The epidemiological and baseline clinical data were similar in both groups, making them statistically comparable.
Table 1. Epidemiological data, vascular risk factors, and cardiovascular events in healthy and T. cruzi-infected individuals.
| Group 2: Healthy Individuals n (%) | Group 1: T. cruzi-Infected Patients (Baseline) n (%) | ||
|---|---|---|---|
| Country of origin | Bolivia | 35 (81) | 51(91) |
| Argentina | 1 (2) | 2(4) | |
| Brazil | 1 (2) | 1 (2) | |
| Colombia | 3 (7) | 0 | |
| Ecuador | 2 (5) | 1 (2) | |
| Paraguay | 0 | 1 (2) | |
| Peru | 1 (2) | 0 | |
| Toxic habits | Smoking | 1 (2) | 1 (2) |
| Alcohol intake | 5 (12) | 1 (2) | |
| Vascular risk factors | High blood pressure | 0 | 0 |
| Hyperlipidemia | 4 (9) | 5 (9) | |
| Diabetes mellitus | 0 | 0 | |
| Cardiovascular events | Atrial fibrillation | 0 | 0 |
| Valvulopathy* | 0 | 4 (8) | |
| Cardiac failure | 0 | 0 | |
| Myocardial ischemia | 0 | 0 | |
| Stroke | 0 | 0 | |
Comparison of the 24 hypercoagulability biomarkers at baseline between (untreated) G1 and G2 individuals showed statistically significant differences for D-dimer (P = .0262); F1+2 (abnormal values in 43/56 G1 patients [77%], P < .0001), PAP (abnormal values in 17/56 G1 patients [30%], P = .0111), P-selectin (abnormal values in 7/56 G1 patients [13%] P = .0177), and ETP (abnormal values in 28/56 G1 patients [50%], P < .0013), and circulating microparticles (P = .0112) (Table 2). D-dimer levels were normal in all the individuals in G1 and G2, and microparticles were within the normal range in a high percentage of patients (86% in G1 and 93% in G2, P = .3402). Our findings showed that a high percentage of patients with chronic T.cruzi infection have a hypercoagulable state regardless the clinical stage of disease, thus confirming the observations of previous studies. [11–13]
Table 2. Descriptive analysis and comparisons of hemostasis parameters between pretreatment Group 1 (N = 56) and Group 2 (N = 43).
| VARIABLE | Group 1 Median (IQR) [n] | Group 2 Median (IQR) [n] | P Value a | Normal range (units) | |
|---|---|---|---|---|---|
| D-dimer | 228.5 (119.0) [56] | 201.0 (125.0) [43] | 0.0262 | 50–400 (μg/L) | |
| Prothrombin fragment 1+2 | 1.8 (1.3) [56] | 0.8 (0.4) [43] | < 0.0001 | 0.40–1.1 (nM) | |
| PAI-1 | 24.6 (14.5) [56] | 21.3 (14.5) [43] | 0.0680 | 4.0–43.0 (ng/mL) | |
| Factor VIIa | 3.5 (1.4) [56] | 2.9 (1.8) [43] | 0.4312 | 1.5–4.1 (ng/mL) | |
| PAP complexes | 360.9 (275.8) [56] | 258.1 (225.8) [43] | 0.0006 | 80–470 (μg/L) | |
| P-selectin | 41.8 (40.0) [56] | 32.1 (21.7) [43] | 0.0200 | 3–90 (μg/mL) | |
| ETP | 475.2 (99.2) [56] | 412.4 (75.8) [43] | <0.0001 | 351–473 (mEq) | |
| Prothrombin time | 98.5 (5.0) [56] | 98.0 (5.0) [43] | 0.4701 | 0.85–1.15 (ratio)/ 80–100 (%) | |
| Attp | 30.0 (3.0) [56] | 30.0 (4.0) [43] | 0.4849 | 25–35 (sec) | |
| Fibrinogen | 3.5 (0.8) [56] | 3.4 (0.8) [43] | 0.8540 | 1.5–4.5 (g/L) | |
| Antithrombin | 104.5 (16.5) [56] | 101.0 (18.0) [43] | 0.4518 | 60–140 (%) | |
| Plasminogen | 108.5 (17.5) [56] | 107.0 (16.0) [43] | 0.1467 | 60–140 (%) | |
| Protein C | 103.0 (30.5) [56] | 104.0 (27.0) [43] | 0.7082 | 60–140 (%) | |
| Total protein S | 87.0 (14.5) [56] | 88.0 (12.0) [43] | 0.8156 | 60–140 (%) | |
| Free protein S | 85.5 (11.5) [56] | 88.0 (13.0) [43] | 0.1125 | 60–140 (%) | |
| FVIII | 112.5 (51.0) [56] | 103.0 (36.0) [43] | 0.1391 | 60–140 (%) | |
| FvWAg | 136.0 (52.0) [56] | 116.0 (46.0) [43] | 0.0758 | 65–150 (U/dL) | |
| Microparticles | 21.1 (11.0) [56] | 17.7 (13.5) [43] | 0.0112 | 8–30 (nM) | |
| CD40L | 98.2 (39.3) [56] | 89.0 (42.1) [43] | 0.6952 | 30–145 (pg/mL) | |
| Tissue factor | 116.5 (50.1) [56] | 124.2 (57.3) [43] | 0.3737 | 80–280 (ng/mL) | |
| ADAMT13 | 103.8 (41.9) [56] | 97.9 (60.1) [43] | 0.8905 | 50–120 (ng/mL) | |
| Factor XIIa | 3.7 (3.9) [56] | 3.1 (4.6) [43] | 0.3178 | 1.0–4.4 (ng/mL) | |
| Factor V Leiden b | No mutation | 55 (98%) | 42 (98%) | 1.0000 c | Mutations/no mutations |
| Heterocygote | 1 (2%) | 1 (2%) | |||
| G20210Ab | No mutation | 56 (100%) | 43 (100%) | Mutations/no mutations | |
a Wilcoxon rank sum test P value
b Absolute frequency (column percentage)
c Fisher’s exact test
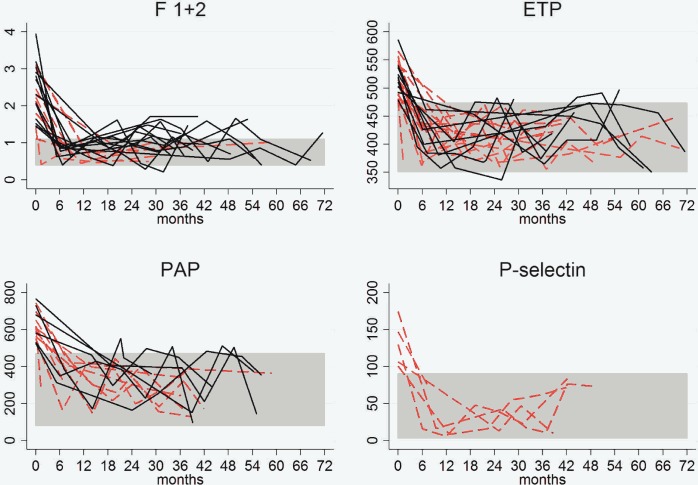
Thirty-three (76%) of the 43 patients with abnormal baseline F1+2 values achieved normal levels after a median follow-up of 9 month (IQR, 8). All but one of the 28 patients with abnormal ETP values before treatment showed normal values at 6 months (IQR, 3). These values were maintained throughout follow-up (30 months; IQR, 28) in 15 patients (60%). Fifteen of the 17 patients with abnormal baseline PAP values showed normal values 7 months (IQR, 7) after treatment and nine of these (60%) maintained these values throughout follow-up (28 months; IQR,11). However, PAP values at 12 and 48 months seemed to be higher than those at 6 months, but the confidence interval indicates a lack of precision for both time point effect estimates (Table 3). Thus, once normalized, F1+2 and ETP levels did not increase again significantly after treatment. Fig 1 shows a graphic representation of these results.
Table 3. Variations in hemostasis F1+2, ETP, and PAP during the follow-up of 50 treated patients.
| F1+2b | ETPb | PAPb | |||||
|---|---|---|---|---|---|---|---|
| Variable | Effect Estimate (95% CI) | P Value | Effect Estimate (95% CI) | P Value | Effect Estimate (95% CI) | P Value | |
| TIME | Baseline c | 0.88 (0.66; 1.10) | 52.58 (33.96; 71.21) | 101.26 (48.39; 154.14) | |||
| 6 mo | 0 | 0 | 0 | ||||
| 12 mo | -0.08 (-0.36; 0.21) | -8.54 (-31.96; 14.88) | 65.30 (-1.58; 132.18) | ||||
| 18 mo | -0.01 (-0.27; 0.25) | < 0.0001 | -6.06 (-27.87; 15.75) | < 0.0001 | 29.03 (-33.24; 91.29) | < 0.0001 | |
| 24 mo | 0.03 (-0.25; 0.30) | 2.88 (-19.65; 25.41) | -3.97 (-68.14; 60.20) | ||||
| 30 mo | -0.10 (-0.39; 0.19) | 13.19 (-10.58; 36.95) | 49.83 (-17.91; 117.57) | ||||
| 36 mo | 0.10 (-0.18; 0.37) | 0.92 (-21.88; 23.73) | -27.76 (-92.97; 37.45) | ||||
b F 1+2, prothrombin fragment 1+2; ETP, endogenous thrombin potential; PAP, plasmin-antiplasmin
c Baseline value compared to value at 6 months of follow-up.
Fig 1. Hemostasis factor levels (baseline and follow-up) in patients with altered levels before treatment who achieved levels within normal ranges during follow-up.
*The discontinuous red lines indicate patients who maintained normal values throughout follow-up. The continuous black lines indicate patients who experienced a return to abnormal values at some time during the follow-up. Abbreviations: ETP, endogenous thrombin potential; F 1+2, prothrombin fragment 1+2; PAP, plasmin-antiplasmin.
F1+2 values are an indirect measure of the amount of thrombin generated in vivo (mainly due to endothelial injury, even in subclinical states) [39], and ETP levels indicate the potential amount of thrombin that can be formed when blood coagulation is activated through the addition of tissue factor. PAP complexes are markers of fibrinolysis. Upon activation, plasmin, which is primarily responsible for a controlled and regulated dissolution of the fibrin polymers into soluble fragments, is immediately inactivated by antiplasmin, forming PAP complexes. [40] Therefore, it is conceivable that the increase formation of PAP complexes stems from excessive formation of fibrin in the blood stream of untreated T. cruzi infected patients. Soluble P-selectin is considered a biomarker of in vivo platelet activation. P-selectin is contained in the α-granules of platelets; following platelet activation, the soluble form is expressed on the platelet surface and then shed by cleavage. P-selectin has been shown to act as a link between thrombosis and inflammation. [41] Additionally, the four biomarkers-F1+2, ETP, PAP complexes, and P-selectin-reflect are highly stable over time.
A hypercoagulable state is a term that pretends to denominate a condition in which there is an increased tendency toward blood clotting. There is not a universally accepted definition for this state based in biomarkers values, but an increase in several of them suggests the possibility of an increase in the person's chances of developing blood clots. The increases in F1+2, PAP and ETP are congruent with this idea: F1+2 and PAP indicate the actual amount of thrombin and plasmin formed, as markers in procoagulant and fibrinolysis pathways, respectively; and ETP indicates the potential amount of thrombin that can be formed considering globally all the activators, inhibitors and substrates of the hemostasis present in the plasma. The increase observed in these biomarkers is good enough to be an argument to point out a hypercoagulable state in patients with Chagas’ disease.
Sixteen (33%) of the 56 G1 patients had a positive qRT-PCR result at baseline, but only four of these had a positive result after treatment (treatment failure rate of 25% in this subgroup). Five of the 34 patients with a negative baseline qRT-PCR result showed a positive result during follow-up. None of the patients with normal F1+2 values during follow-up had a positive qRT-PCR result, but 3(13%) of the 24 patients with normal ETP values during follow-up did. Of the patients with altered levels of F1+2, ETP, or PAP complexes at baseline, a positive qRT-PCR result during follow-up was not significantly associated with changes observed in lytic anti-α-Gal antibodies, F1+2, ETP, and/or PAP levels.
A positive qRT-PCR result after treatment in patients who achieved normalization of F1+2, ETP, and/or PAP could mean that a decrease in parasite load is sufficient to modify the hypercoagulable state or that benznidazole, which acts on the redox system, could modify these biomarkers without eliminating the parasites. This would limit the use of these factors as biomarkers for parasite elimination, although they could be valuable indicators of treatment response and add support to the theory that, by reverting the hypercoagulable state, benznidazole may also prevent clinical thrombotic events.
Conventional ELISA results were positive in all the patients in G1. Although, as expected, antibodies remained positive throughout follow-up, a slight decrease was detected by the commercial and in-house methods during this period. A statistically significant relevant decrease, was only observed with the in-house test from month 18 onwards (P = .0006).
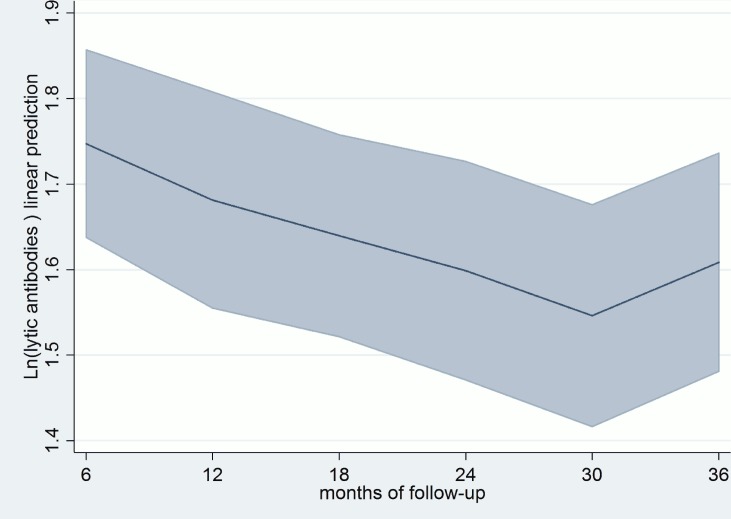
Lytic anti-α-Gal antibodies were positive in 52 (96%) of the 54 patients tested before treatment, and in all patients AT CL-ELISA remained within positive levels to the end of the follow-up (Fig 2). Besides, there was no correlation between lytic anti-α-Gal antibody assay and the hemostasis factors evaluated. In relation to previous studies’ results, early decreases in lytic anti-α-Gal antibodies were expected to be observed. On the contrary, a decrease in levels was evident at month 12 and this was significant since month 18 and forward (P = .0052). [28, 34]
Fig 2. Variations in AT CL-ELISA levels.
Months of follow-up predictive margins with 95% CI. Cutoff AT CL ELISA = 0.
Adherence to treatment was high, with only five patients not achieving 80% of the total dose. All five patients showed abnormal F1+2 values throughout follow-up and 3 (60%) had abnormal ETP and PAP values. One of the five patients had a positive qRT-PCR result during follow-up, and all five maintained the same positive ELISA and AT CL-ELISA results throughout follow-up. A large cohort of adolescents with T cruzi infection treated with benznidazole showed seronegativity in lytic anti-α-Gal antibodies, as measured by AT CL-ELISA, in 58% and 85% of the patients 36 and 72 months after treatment, respectively. [28, 34] The differences between those studies and ours may be due to the nature of the cohorts (adolescents vs. adults) and the stage of the disease. Nevertheless, both studies showed a similar trend towards a reduction in lytic anti-α-Gal antibodies following treatment with benznidazole.
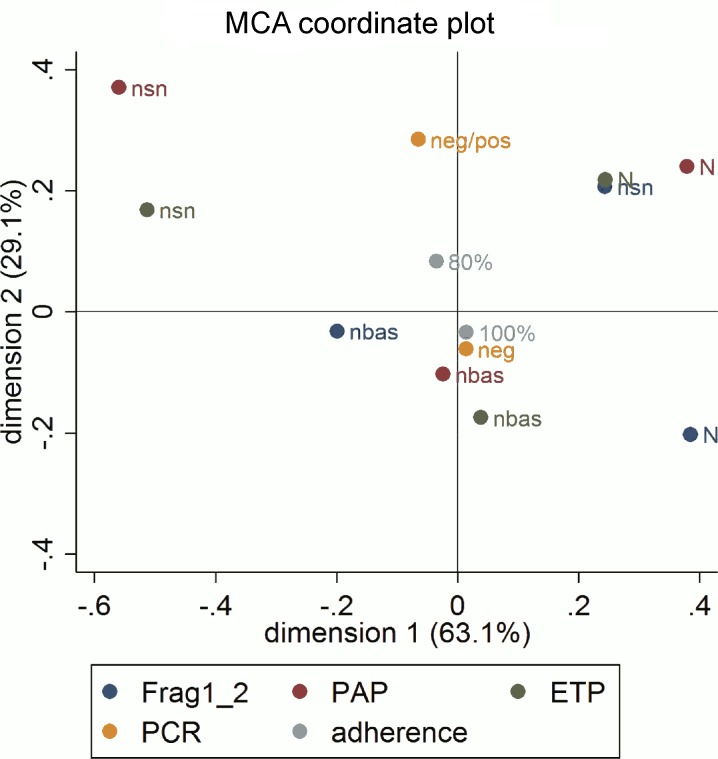
We studied the relationship between normalization of hypercoagulability markers F1+2, PAP, and ETP and qRT-PCR results by multiple correspondence analyses (MCA). Due to the low rate of positive qRT-PCR results, this variable was used as a supplementary variable jointly with treatment adherence. The MCA results (Fig 3) showed an association between complete normalization of PAP and ETP levels and non-sustained and marginally abnormal values in F1+2. These factors had the highest contribution and correlation in the positive part of the second dimension, while normal baseline ETP and PAP values had the highest contribution and correlation in the negative part. F1+2 normalization clearly characterized the positive part of the first dimension, while non-sustained normalization of PAP and ETP values clearly characterized the negative part. In other words, the sustained normalization observed post-treatment in PAP and ETP, could, despite the non-sustained normalization of F1+2 values, reflect response to antiparasitic treatment due to the strong correlation between these three variables.
Fig 3. Multiple correspondence analysis coordinate plot.
Biomarkers: N, sustained normalization of values throughout follow-up; nsn: non-sustained normalization throughout follow-up; nbas, normal value at baseline. Supplementary variables: qRT-PCR during follow-up: neg (negative); pos (positive); Level of adherence: 80%; 100%. Abbreviations: ETP, endogenous thrombin potential; F1+2, prothrombin fragment 1+2; PCR, polymerase chain reaction.
The projection of qRT-PCR results and adherence to treatment in the solution space provided little additional information. Consistently negative qRT-PCR results throughout follow-up appear to be related to 100% treatment adherence.
In a recent study, the authors found that the serum samples of 37 individuals with chronic Chagas disease showed an upregulation of specific fragments of apolipoprotein A-1 (Apo A1) and one fibronectin fragment, that returned to normal levels in 43% of them three years after a treatment with nifurtimox. [38] Apo A1 and fibronectin fragment were altered in all the 37 patients with T.cruzi infection before treatment, but the number of patients treated with that normalized levels was lower than in our series (60% and 96% of patients who normalized F1+2 and ETP values).
This study has some limitations. Although the sample size was calculated to obtain sufficient statistical power to answer the hypothesis, a larger sample may have detected differences that would be expected to appear earlier (e.g., before 12 months). The lost to follow-up samples also affected the estimates. Even within Spain, it is difficult to follow individuals with high migratory mobility for long periods. In addition, the fact that only 30% of patients had a positive baseline qRT-PCR result was a constraint for assessing the effect of treatment.
In conclusion, patients with chronic T.cruzi infection have a potential hypercoagulable state, regardless of cardiological and/or digestive involvement. The hypercoagulability markers F1+2 and ETP were abnormally expressed in a high percentage of patients with chronic T.cruzi infection before treatment (77% and 50%, respectively) but returned to and remained at normal levels shortly after treatment in 76% and 96% of patients, respectively. Baseline PAP values were altered in just 30% of patients before treatment, but normalized several months after treatment in 88% of these. These three hypercoagulability biomarkers could be useful for assessing short-term response to treatment. However, the fact that normal values were seen in some infected patients, including some with positive post-treatment qRT-PCR results, reduces their usefulness as universal biomarkers. The decrease in hypercoagulability factor levels could be explained by a decrease in parasitemia or by other benznidazole effect.
Data Availability
Consent forms informed participants that their data would be anonymized. Release of data would compromise patient privacy and would be a breach of protocol approved by the IRB committee. However, qualified researchers will be able to access the data by requesting it from Sergi Sanz (sergi.sanz@isglobal.org), the person in charge of research data at the Global Health Institute.
Funding Statement
This work was supported by Fundación Mundo Sano. ISGlobal Research group receives funds from the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) [grant number 2009SGR385], and from the Tropical Disease Cooperative Research Network (RICET) [grant number RD12/0018/0010]. ICdA was supported by NIH grants R01AI070655-A5, R01AI070655-A5S1, and 5G12MD007592; AFM was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 2010; 115(1–2): 14–21. 10.1016/j.actatropica.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 2010; 115(1–2): 22–27. 10.1016/j.actatropica.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 3.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013;13(1): 34–45. 10.1038/nri3345 [DOI] [PubMed] [Google Scholar]
- 4.Schuetz P, Christ-Crain M, Morgenthaler NG, Struck J, Bergman A, Müller B. Circulating precursor levels of endothelin-1 and adrenomedullin, two endothelium-derived, counteracting substances, in sepsis. Endothelium 2007; 14(6): 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreutz RP, Tantry US, Bliden KP, Gurbel PA. Inflammatory changes during the 'common cold' are associated with platelet activation and increased reactivity of platelets to agonists. Blood Coagul Fibrinolysis 2007; 18(8): 713–718. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro AC, Schmitz V, Svensjo E, Gazzinelli RT, Almeida IC, Todorov A, et al. Cooperative activation of TLR2 and bradykinin B2 receptor is required for induction of type 1 immunity in a mouse model of subcutaneous infection by Trypanosoma cruzi. J Immunol. 2006; 177(9):6325–6335. [DOI] [PubMed] [Google Scholar]
- 7.Alejandro Salomone O. Chagas cardiomyopathy and thrombosis: the beginning and the end of a dangerous affair. Rev Esp Cardiol 2003; 56(4): 333–334. [DOI] [PubMed] [Google Scholar]
- 8.Carod-Artal FJ, Vargas AP, Horan TA, Nunes LG. Chagasic cardiomyopathy is independently associated with ischemic stroke in Chagas disease. Stroke 2005; 36(5): 965–970. [DOI] [PubMed] [Google Scholar]
- 9.Berra HH, Piaggio E, Revelli SS, Luquita A. Blood viscosity changes in experimentally Trypanosoma cruzi-infected rats. Clin Hemorheol Microcirc 2005; 32(3): 175–182. [PubMed] [Google Scholar]
- 10.Herrera RN, Diaz de Amaya EI, Perez Aguilar RC, Joo Turoni C, Marañón R, Berman SG, et al. Inflammatory and prothrombotic activation with conserved endothelial function in patients with chronic, asymptomatic Chagas disease. Clin Appl Thromb Hemost 2011; 17(5): 502–507. 10.1177/1076029610375814 [DOI] [PubMed] [Google Scholar]
- 11.Herrera RN, Diaz E, Perez Aguilar R, Bianchi J, Berman S, Luciardi HL. Prothrombotic state in early stages of chronic Chagas' disease. Its association with thrombotic risk factors. Arch Cardiol Mex 2005; 75(3): 38–48. [PubMed] [Google Scholar]
- 12.Pinazo MJ, Tassies D, Muñoz J, Fisa R, Posada E de J, Monteagudo J, et al. Hypercoagulability biomarkers in Trypanosoma cruzi -infected patients. Thromb Haemost 2011; 106(4): 617–623. 10.1160/TH11-04-0251 [DOI] [PubMed] [Google Scholar]
- 13.Carod-Artal FJ, Vargas AP, Falcao T. Stroke in asymptomatic Trypanosoma cruzi-infected patients. Cerebrovasc Dis 2011; 31(1): 24–28. 10.1159/000320248 [DOI] [PubMed] [Google Scholar]
- 14.Melo LM, Souza GE, Valim LR, Moreira LF, Damico EA, Rocha TR, et al. Study of pro-thrombotic and pro-inflammatory factors in Chagas cardiomyopathy. Arq Bras Cardiol 2010; 95(5):655–662. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi MdeL, De Morais CF, Sambiase NV, Pereira-Barretto AC, Bellotti G, Pileggi F. Histopathological criteria of myocarditis—a study based on normal heart, chagasic heart and dilated cardiomyopathy. Jpn Circ J 1990;54(4):391–400. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi ML, Fukasawa S, De Brito T, Parzianello LC, Bellotti G, Ramires JA. Different microcirculatory and interstitial matrix patterns in idiopathic dilated cardiomyopathy andChagas' disease: a three dimensional confocal microscopy study.Heart. 1999. September;82(3):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carod-Artal FJ, FdST, Vilela Nunes S et al. Thrombophilia is not a predictive factor of stroke in Chagas Disease. Neurology 2004; 62: A240–241. [Google Scholar]
- 18.Herrera RN, Díaz E, Pérez R, Chaín S, Sant-Yacumo R, Rodríguez E, et al. The prothrombotic state in early stages of chronic Chagas' disease. Rev Esp Cardiol 2003; 56: 377–382. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). Control of Chagas’ disease; 2002. [PubMed]
- 20.Bern C, Montgomery SP, Herwaldt BL, Rassi A Jr, Marin-Neto JA, Dantas RO, et al. Evaluation and treatment of chagas disease in the United States: a systematic review. Jama 2007; 298(18): 2171–2181. [DOI] [PubMed] [Google Scholar]
- 21.Urbina JA. Specific treatment of Chagas disease: current status and new developments. Curr Opin Infect Dis 2001;14(6):733–41. [DOI] [PubMed] [Google Scholar]
- 22.Sosa Estani S., Armenti A., Araujo G., Viotti R., Lococo B., Ruiz Vera B., et al. Treatment of Chagas’disease with benznidazole and thioctic acid. Medicina (B. Aires) 2004; 64:1–6. [PubMed] [Google Scholar]
- 23.Marin-Neto JA, Rassi A Jr., Morillo CA, Avezum A, Connolly SJ, Sosa-Estani S, et al. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas' cardiomyopathy: the BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT). Am Heart J 2008; 156(1): 37–43. 10.1016/j.ahj.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 24.Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Mem Inst Oswaldo Cruz 2002; 97(1): 3–24. [DOI] [PubMed] [Google Scholar]
- 25.Viotti R, Vigliano C, Lococo B, Bertochi G, Petti M, Álvarez MG, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med 2006; 144(10): 724–734. [DOI] [PubMed] [Google Scholar]
- 26.Requena-Mendez A, Lopez MC, Angheben A, Izquierdo L, Ribeiro I, Pinazo MJ, et al. Evaluating Chagas disease progression and cure through blood-derived biomarkers: a systematic review. Expert Rev Anti Infect Ther 2013; 11(9): 957–976. 10.1586/14787210.2013.824718 [DOI] [PubMed] [Google Scholar]
- 27.Almeida IC, Covas DT, Soussumi LM, Travassos LR. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion 1997; 37(8): 850–857. [DOI] [PubMed] [Google Scholar]
- 28.de Andrade AL, Zicker F, de Oliveira RM, Almeida Silva S, Luquetti A, Travassos LR. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 1996; 348(9039): 1407–1413. [DOI] [PubMed] [Google Scholar]
- 29.Krettli AU. The utility of anti-trypomastigote lytic antibodies for determining cure of Trypanosoma cruzi infections in treated patients: an overview and perspectives. Mem Inst Oswaldo Cruz 2009; 104 (1): 142–151. [DOI] [PubMed] [Google Scholar]
- 30.Piron M, Fisa R, Casamitjana N, López-Chejade P, Puig L, Vergés M, et al. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop 2007; 103(3): 195–200. [DOI] [PubMed] [Google Scholar]
- 31.Riera C, Verges M, López-Chejade P, et al. Desarrollo y evaluación de una técnica ELISA con antígeno crudo de Trypanosoma cruzi para el diagnóstico de la enfermedad de Chagas. Enfermedades emergentes 2009; 11: 19–22. [Google Scholar]
- 32.Ford RP, Esnouf MP, Burgess AI, Sarphie AF. An enzyme linked immunosorbent assay (ELISA) for the measurement of activated factor XII (Hageman factor) in human plasma. J Immunoassay 1996;17:119–131. [DOI] [PubMed] [Google Scholar]
- 33.Almeida IC, Ferguson MA, Schenkman S, Travassos LR. Lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem J 1994; 304 (3): 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrade AL, Martelli CM, Oliveira RM, Silva SA, Aires AI, Soussumi LM, et al. Short report: benznidazole efficacy among Trypanosoma cruzi-infected adolescents after a six-year follow-up. Am J Trop Med Hyg 2004; 71(5): 594–597. [PubMed] [Google Scholar]
- 35.Izquierdo L, Marques AF, Gállego M, Sanz S, Tebar S, Riera C, et al. Evaluation of a chemiluminescent enzyme-linked immunosorbent assay for the diagnosis of Trypanosoma cruzi infection in a nonendemic setting. Mem Inst Oswaldo Cruz 2013; 108(7):928–931. 10.1590/0074-0276130112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almeida IC, Krautz GM, Krettli AU, Travassos LR. Glycoconjugates of Trypanosoma cruzi: a 74 kD antigen of trypomastigotes specifically reacts with lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas disease. J Clin Lab Anal 1993; 7(6): 307–316. [DOI] [PubMed] [Google Scholar]
- 37.Greenacre MJ. Theory and Applications of Correspondence Analysis. London: Academic Press; 1984. [Google Scholar]
- 38.Greenacre MJ, Blasius J. From simple to multiple correspondence analysis In Multiple Correspondence Analysis and Related Methods, ed. Raton Boca, 2006. FL: Chapman & Hall/CRC. [Google Scholar]
- 39.Manucci PM. Mechanisms, markers and management of coagulation activation. Thrombosis 1993; 44: 851–870. [DOI] [PubMed] [Google Scholar]
- 40.Schaller J, Gerber SS. The plasmin-antiplasmin system: structural and functional aspects. Cell Mol Life Sci 2011; 68(5): 785–801. 10.1007/s00018-010-0566-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferroni P, Martini F, Riondino S, La Farina F, Magnapera A, Ciatti F, et al. Soluble P-selectin as a marker of in vivo platelet activation. Clin Chim Acta 2009; 399(1–2): 88–91. 10.1016/j.cca.2008.09.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Consent forms informed participants that their data would be anonymized. Release of data would compromise patient privacy and would be a breach of protocol approved by the IRB committee. However, qualified researchers will be able to access the data by requesting it from Sergi Sanz (sergi.sanz@isglobal.org), the person in charge of research data at the Global Health Institute.