Abstract
Background: Evidence is growing that the equilibrium between reactive oxygen species and antioxidants plays a vital role in women’s reproductive health.
Objective: The objective of this study was to evaluate variations in serum antioxidant concentrations across the menstrual cycle and associations between antioxidants and reproductive hormones and anovulation among healthy women.
Methods: The BioCycle Study, a prospective cohort, followed 259 women aged 18–44 y for up to 2 menstrual cycles. Serum fat-soluble vitamin and micronutrient (α-tocopherol, γ-tocopherol, retinol, lutein, lycopene, and β-carotene), ascorbic acid, and reproductive hormone concentrations were measured 5–8 times/cycle. We used weighted linear mixed models to assess associations between antioxidants and hormone concentrations, after adjustment for age, race, body mass index, parity, sleep, pain medication use, total energy intake, concurrent hormones, serum cholesterol, F2-isoprostanes, and other antioxidants. Generalized linear models were used to identify associations with anovulation.
Results: Serum antioxidant concentrations varied across the menstrual cycle. Retinol and α-tocopherol were associated with higher estradiol [RR: 1.00 pg/mL (95% CI: 0.67, 1.34 pg/mL); RR: 0.02 pg/mL (95% CI: 0.003, 0.03 pg/mL), respectively] and testosterone [RR: 0.61 ng/dL (95% CI: 0.44, 0.78 ng/dL); RR: 0.01 ng/dL (95% CI: 0.001, 0.01 ng/dL), respectively]. Ascorbic acid was associated with higher progesterone (RR: 0.15 ng/mL; 95% CI: 0.05, 0.25 ng/mL) and with lower follicle-stimulating hormone (RR: −0.06 mIU/mL; 95% CI: −0.09, −0.03 mIU/mL). The ratio of α- to γ-tocopherol was associated with an increased risk of anovulation (RR: 1.03; 95% CI: 1.01, 1.06).
Conclusions: These findings shed new light on the intricate associations between serum antioxidants and endogenous hormones in healthy premenopausal women and support the hypothesis that concentrations of serum vitamins affect steroidogenesis even after adjustment for oxidative stress.
Keywords: antioxidants, estrogen, ovulation, progesterone, vitamins
Introduction
The equilibrium between reactive oxygen species (ROS)10 and antioxidants plays an important role in maintaining women’s reproductive health (1–8). Indeed, accumulation of ROS, resulting in oxidative stress, is linked to defective oocyte maturation, impaired fertilization, and development of endometriosis (9–13).
Antioxidants reduce excess ROS and may help support normal reproduction in women. For example, ascorbic acid (1 form of vitamin C) can stimulate human placental/trophoblastic steroidogenesis (14), and supplementation with ascorbic acid may increase serum progesterone concentrations in women with a luteal phase defect (15). Moreover, lower concentrations of ascorbic acid and α-tocopherol (the form of vitamin E that is preferentially absorbed by humans) were observed among women with recurrent spontaneous abortions linked to a luteal phase defect than among women with better reproductive outcomes (16).
Although these previous studies show the importance of antioxidants in relation to reproductive complications, less is known about antioxidant concentrations and their associations with menstrual function in healthy women of reproductive age. In addition, assessment of antioxidants with the use of serum biomarkers improves research on the basis of self-reported intakes, given known individual differences in metabolism (17). A previous report from the BioCycle Study found that among healthy, regularly menstruating, premenopausal women, oxidative stress was positively associated with endogenous estradiol (E2) and inversely associated with follicle-stimulating hormone (FSH) and sex hormone-binding globulin (SHBG) (18), but the associations between serum antioxidants and endogenous hormones have yet to be determined.
Therefore, the primary objective of this study was to evaluate variation in serum antioxidant concentrations across the menstrual cycle and the relations between serum antioxidant concentrations and endogenous reproductive hormone concentrations and sporadic anovulation in regularly menstruating young women.
Methods
Participants and study design.
The BioCycle Study (2005–2007) was a prospective cohort study of 259 regularly menstruating, healthy female volunteers, aged 18–44 y, recruited from western New York. The primary objective of the BioCycle Study was to determine the association of oxidative stress concentrations with endogenous reproductive hormone concentrations and antioxidants, including vitamin concentrations, across the menstrual cycle in a prospective cohort of premenopausal women (18). Further details of the study design are described elsewhere (19). Exclusion criteria included use of vitamin/mineral or herbal antioxidant supplements over the study period; current use of oral contraceptives (or use during the previous 3 mo); pregnancy or breastfeeding in the previous 6 mo; and diagnosis of certain chronic conditions, including history of menstrual and ovulation disorders and uterine abnormalities, such as uterine fibroids. Women with a self-reported BMI (in kg/m2) of <18 or >35 at screening were also excluded.
The University at Buffalo Health Sciences Institutional Review Board approved the study and served as the institutional review board, designated by the NIH for this study under a reliance agreement. All participants provided written informed consent. Participants were followed for 1 (n = 9 women) or 2 (n = 250 women) menstrual cycles. Fasting blood samples were collected for serum antioxidant and hormone assessment during the following 8 phases: second day of menstruation (visit M1); mid-follicular phase (visit F1); 3 visits around expected ovulation (visits O1–O3); and early, mid-, and late luteal phases (visits L1–L3). Fertility monitors [Clearblue Easy Fertility Monitor; Alere (previously Inverness Medical)] indicated low, high, and peak fertility and were used to time mid-cycle visits and biospecimen collection (20). Other visits were scheduled according to an algorithm that took each woman’s reported cycle length into consideration (21). The participants were highly compliant to the study protocol; 94% completed at least 7 clinic visits per cycle, and all completed at least 5 clinic visits per cycle.
Laboratory assays.
Total ascorbic acid was determined by the dinitrophenylhydrazine method (22). Samples for ascorbate analysis were stabilized immediately after phlebotomy and centrifugation by adding 0.5 mL heparin plasma to 2.0 mL of 6% meta-phosphoric acid and centrifuging at 3000 × g for 10 min. Clear supernatant fluid was decanted and frozen at −80°C for analysis. The absorbance of each dinitrophenylhydrazine derivatized sample was determined at 520 nm on a Shimadzu 160U spectrophotometer (Shimadzu Scientific Instruments, Inc.). Across the study period, the CV for this test reported by the laboratory was 10%.
Fat-soluble vitamins and carotenoid micronutrients (retinol, α- and γ-tocopherols, lutein, lycopene, and β-carotene) were measured simultaneously in serum with the use of HPLC with photodiode array detection (23). Lutein was quantified as a single co-eluting peak with zeaxanthin. δ-Tocopherol was detected but was below the lower limit of quantification for our assay; thus, results are not presented in this analysis. Across the study period the CV for these tests reported by the laboratory were <6% for retinol, <2% for α- and γ-tocopherols, and <8% for the carotenoids. Continuous monitoring of standard reference material 968c from the National Institute of Standards and Technology, and participation in the National Institute of Standards and Technology Micronutrients Measurement Quality Assurance Program provided external checks on analytical accuracy. Because ascorbic acid participates in the recycling of the α-tocopherol radical (24–28), we examined the ratio of α-tocopherol to ascorbate. Similarly, although γ-tocopherol seems to be the more powerful antioxidant (29, 30), α-tocopherol is incorporated into lipoproteins preferentially to γ-tocopherol (31). We therefore also examined the α- to γ-tocopherol ratio.
Serum cholesterol was measured with the use of a LX20 automated chemistry analyzer (Beckman; CV: <5%). Plasma free F2-isoprostanes were measured with a gas chromatography-mass spectrometry–based method by the Molecular Epidemiology and Biomarker Research Laboratory (University of Minnesota; CV: 9%).
Reproductive hormones were measured in fasting serum samples collected at each cycle visit (5–8 visits per cycle for up to 2 cycles) at the Kaleida Health Center for Laboratory Medicine (Buffalo, New York). E2, FSH, luteinizing hormone (LH), progesterone, and SHBG were measured with the use of solid-phase competitive chemiluminescent enzymatic immunoassays by Specialty Laboratories, Inc. on the DPC Immulite 2000 analyzer (Siemens Medical Solutions Diagnostics). The albumin assay was tested with the Beckman LX20 autoanalyzer with the use of bromocresol purple methods. Calculation of free E2 (i.e., bioavailable E2) was performed via the equation proposed by Södergård et al. (32) with the use of total E2, SHBG, and albumin concentrations. Total testosterone concentration (ng/dL) was determined by liquid chromatography/tandem mass spectrometry with the use of a Shimadzu Prominence Liquid Chromatogram (Shimadzu Scientific Instruments, Inc.) with an ABSceix 5500 tandem mass spectrometer (AB SCIEX). Increased sensitivity was obtained with the use of Mobile Phase B (100% acetonitrile) with a low standard of 4 ng/dL added to the standard curve. Across the study period the CV for these tests reported by the laboratory were <10% for E2 and SHBG, <5% for LH and FSH, <14% for progesterone, and <7% for testosterone. Cycles were defined as anovulatory if progesterone concentrations were ≤5 ng/mL across the cycle and no serum LH peak was observed during the mid- or late luteal phase visit [42 of 509 cycles (8.3%)] (33).
Covariates.
At study enrollment, height and weight were obtained with the use of standardized protocols by trained study staff and used to calculate BMI. Participants also completed questionnaires at baseline for demographic characteristics, lifestyle, reproductive history, health history (19), and physical activity with the use of the International Physical Activity Questionnaire (34). High, moderate, and low physical activity categories were formed on the basis of standard International Physical Activity Questionnaire cutoffs (34). Numbers of hours of sleep and pain medication use for the day before biospecimen collection were assessed via self-report on a daily diary. Cycle length was defined as the number of days from the first day of bleeding (menstruating by 1600) until the day before the next onset of bleeding. Energy intake was assessed with 24-h dietary recalls conducted 4 times per cycle (corresponding to menstruation, mid-follicular phase, ovulation, and mid-luteal phase) and analyzed with the use of the Nutrition Data System for Research software version 2005 (Nutrition Coordinating Center, University of Minnesota). All covariates assessed had at least a 95% response rate.
Statistical analysis.
Descriptive statistics were calculated for demographic and lifestyle characteristics and for mean concentrations of serum antioxidants, cholesterol, and F2-isoprostanes and were compared between tertiles of average retinol, ascorbic acid, and α-tocopherol across the study. Fisher’s exact tests and ANOVA were used to test for differences across tertiles. In addition, variation in average antioxidant concentrations across the menstrual cycle was assessed with the use of linear mixed models to account for repeated cycles per woman and repeated visits per cycle. Pairwise comparisons were made between cycle phases with the use of the Tukey’s method to account for multiple comparisons.
Weighted linear mixed models were used to estimate the associations between antioxidant concentrations and reproductive hormones, adjusting for age, race, BMI, parity, hours of sleep, pain medication use, total energy intake, concurrent reproductive hormones, serum cholesterol concentrations, F2-isoprostanes, and other antioxidants. Because E2, progesterone, LH, and FSH concentrations change over the cycle in response to complex feedback mechanisms with other hormones, cholesterol, and antioxidants, traditional regression adjustment for other hormone concentrations and antioxidants across the cycle may be inadequate (35). Therefore, we used marginal structural models to appropriately adjust for this time-varying confounding affected by prior exposure with the use of inverse probability weights (35, 36). Hormone concentrations were log-transformed for normality. Lag models were additionally evaluated in which we assessed associations between antioxidant concentrations on 1 visit and reproductive hormones during the next cycle visit. To assess the association between cycle average antioxidant concentrations and sporadic anovulation, RRs were estimated with the use of generalized linear models, adjusted for age, race, BMI, total energy intake, and cycle-average serum cholesterol, with results compared with models that additionally adjusted for F2-isoprostanes and other antioxidants. Models for anovulation were additionally run with the use of the average antioxidant concentrations up to the time of expected ovulation to preserve temporality. P < 0.05 was considered statistically significant. SAS version 9.4 (SAS Institute) was used for all statistical analyses.
Results
Overall, women in the BioCycle Study were relatively young (mean age: 27.3 y), of healthy weight (mean BMI: 24.1), physically active (moderate-to-high physical activity: 90%), and nonsmokers (96%) (Table 1). Age was significantly associated with α-tocopherol concentrations, with the average age between the lower and upper tertiles differing by 7.6 y (23.7 and 31.3 y, respectively; P < 0.01). Accordingly, women in the highest tertile of α-tocopherol were also more likely to be married, parous, and have a history of past oral contraceptive use than were women in the first and second tertiles. Race was also significantly associated with α-tocopherol (40% compared with 73% white for the lower and upper tertiles, respectively) and for retinol tertile (43% compared with 72% white for the lower and upper tertiles, respectively). Women in the highest ascorbic acid tertile had lower BMI than women in the lowest tertile; no differences were observed in BMI between tertiles of retinol (P = 0.42) or tertiles of α-tocopherol (P = 0.60). Women in the highest tertile of serum retinol had higher mean α-tocopherol and γ-tocopherol concentrations than women in the middle or lower tertile. Women with the highest tertile of ascorbic acid similarly had the higher mean α-tocopherol concentration and higher mean total cholesterol, lutein, lycopene, and β-carotene concentrations than women in the lowest tertile of ascorbic acid concentration. However, women in the highest tertile of ascorbic acid had the lowest mean F2-isoprostane concentrations relative to the other tertiles. Finally, women in the highest tertile of α-tocopherol had higher mean serum concentrations of total cholesterol, retinol, ascorbic acid, lutein, lycopene, and β-carotene than women in the lowest tertile.
TABLE 1.
Characteristics of healthy, premenopausal women participating in the BioCycle Study by tertile of mean serum concentrations of retinol, ascorbic acid, and α-tocopherol across the study1
| Tertiles of mean retinol, μg/dL |
Tertiles of mean ascorbic acid, mg/dL |
Tertiles of mean α-tocopherol, μg/dL |
|||||||||||
| Total cohort | Low(0.22–0.34) | Medium (>0.34 to 0.40) | High(>0.40 to 0.65) | P2 | Low(0.70–1.54) | Medium (>1.54 to 1.85) | High(>1.85 to 4.22) | P2 | Low(3.68–7.33) | Medium (>7.33 to 8.75) | High(>8.75 to 28.77) | P2 | |
| Women, n | 254 | 84 | 85 | 85 | 86 | 83 | 85 | 84 | 85 | 85 | |||
| Age, y | 27.3 ± 8.2 | 27.5 ± 8.4 | 26.7 ± 7.6 | 27.7 ± 8.7 | 0.69 | 27.5 ± 8.1 | 26.1 ± 7.7 | 28.4 ± 8.7 | 0.19 | 23.7 ± 6.3c | 26.9 ± 7.7b | 31.3 ± 8.7a | <0.001 |
| BMI, kg/m2 | 24.1 ± 3.9 | 24.1 ± 4.1 | 23.7 ± 3.4 | 24.5 ± 4.0 | 0.42 | 25.4 ± 4.1a | 24.0 ± 3.8b | 22.8 ± 3.2b | <0.001 | 24.0 ± 3.9 | 23.8 ± 3.8 | 24.5 ± 3.9 | 0.60 |
| Energy intake, kcal/d | 1610 ± 37 | 1520 ± 376b | 1650 ± 373a,b | 1660 ± 370a | 0.02 | 1590 ± 401 | 1630 ± 342 | 1620 ± 388 | 0.77 | 1560 ± 332 | 1600 ± 361 | 1670 ± 410 | 0.19 |
| Race | <0.001 | 0.16 | <0.001 | ||||||||||
| White | 152 (60) | 36 (43) | 55 (65) | 61 (72) | 52 (61) | 53 (64) | 47 (55) | 34 (40) | 56 (66) | 62 (73) | |||
| Black | 50 (20) | 30 (36) | 10 (12) | 10 (12) | 20 (23) | 17 (20) | 13 (15) | 26 (31) | 12 (14) | 12 (14) | |||
| Other | 52 (20) | 18 (21) | 20 (23) | 14 (16) | 14 (16) | 13 (16) | 25 (29) | 24 (29) | 17 (20) | 11 (13) | |||
| ≤High school education | 33 (13) | 12 (14) | 8 (9) | 13 (15) | 0.47 | 11 (13) | 15 (18) | 7 (8) | 0.17 | 11 (13) | 12 (14) | 10 (12) | 0.92 |
| Married | 65 (26) | 16 (19) | 21 (25) | 28 (33) | 0.12 | 17 (20) | 21 (25) | 27 (32) | 0.21 | 5 (6) | 27 (32) | 33 (39) | <0.001 |
| Nulliparous3 | 183 (72) | 61 (73) | 64 (75) | 58 (68) | 0.79 | 62 (72) | 62 (75) | 59 (69) | 0.70 | 73 (87) | 61 (72) | 49 (58) | <0.001 |
| Current smoker | 10 (4) | 1 (1) | 5 (6) | 4 (5) | 0.36 | 4 (5) | 4 (5) | 2 (2) | 0.72 | 4 (5) | 0 (0) | 6 (7) | 0.03 |
| Physical activity | 0.40 | 0.47 | 0.80 | ||||||||||
| Low | 25 (10) | 10 (12) | 9 (11) | 6 (7) | 6 (7) | 9 (11) | 10 (12) | 10 (12) | 8 (9) | 7 (8) | |||
| Moderate | 91 (36) | 30 (36) | 35 (41) | 26 (31) | 30 (35) | 26 (31) | 35 (41) | 27 (32) | 34 (40) | 30 (35) | |||
| High | 138 (54) | 44 (52) | 41 (48) | 53 (62) | 50 (58) | 48 (58) | 40 (47) | 47 (56) | 43 (51) | 48 (56) | |||
| Past OC use3 | 138 (54) | 44 (52) | 49 (58) | 45 (53) | 0.90 | 53 (62) | 41 (49) | 44 (52) | 0.49 | 34 (40) | 49 (58) | 55 (65) | 0.01 |
| Serum biomarkers | |||||||||||||
| Total cholesterol, mg/dL | 165 ± 27.3 | 163 ± 28.8 | 161 ± 25.1 | 169 ± 27.6 | 0.15 | 158 ± 25.1b | 167 ± 27.6a,b | 169 ± 28.2a | 0.02 | 145 ± 17.4c | 165 ± 19.6b | 183 ± 29.0a | <0.001 |
| F2-isoprostane, pg/mL | 52.9 ± 22.2 | 51.1 ± 20.0 | 52.6 ± 24.4 | 54.8 ± 22.2 | 0.55 | 59.6 ± 26.0a | 53.4 ± 22.9a | 45.5 ± 13.8b | <0.001 | 57.5 ± 28.6 | 50.0 ± 16.3 | 51.2 ± 19.6 | 0.06 |
| Retinol, μg/mL | 0.38 ± 0.08 | 0.30 ± 0.03c | 0.37 ± 0.02b | 0.46 ± 0.06a | <0.001 | 0.37 ± 0.08 | 0.38 ± 0.08 | 0.38 ± 0.08 | 0.25 | 0.35 ± 0.06c | 0.38 ± 0.06b | 0.41 ± 0.09a | <0.001 |
| Ascorbic acid, mg/dL | 1.77 ± 0.48 | 1.70 ± 0.46 | 1.77 ± 0.45 | 1.84 ± 0.51 | 0.18 | 1.33 ± 0.17c | 1.71 ± 0.08b | 2.28 ± 0.43a | <0.001 | 1.59 ± 0.37b | 1.81 ± 0.46a | 1.91 ± 0.54a | <0.001 |
| α-Tocopherol, μg/mL | 8.25 ± 2.19 | 7.80 ± 1.70b | 7.95 ± 1.74b | 8.98 ± 2.78a | <0.001 | 7.79 ± 2.76b | 8.27 ± 1.67a,b | 8.69 ± 1.89a | 0.03 | 6.35 ± 0.72c | 8.00 ± 0.41b | 10.4 ± 2.35a | <0.001 |
| γ-Tocopherol, μg/mL | 1.80 ± 0.57 | 1.75 ± 0.53b | 1.70 ± 0.51b | 1.95 ± 0.64a | 0.01 | 1.84 ± 0.54 | 1.83 ± 0.53 | 1.73 ± 0.64 | 0.41 | 1.64 ± 0.48b | 1.82 ± 0.54a,b | 1.94 ± 0.65a | 0.01 |
| Lutein, μg/mL | 0.12 ± 0.05 | 0.12 ± 0.04 | 0.12 ± 0.04 | 0.13 ± 0.06 | 0.17 | 0.10 ± 0.04c | 0.12 ± 0.03b | 0.15 ± 0.05a | <0.001 | 0.11 ± 0.04b | 0.12 ± 0.04a,b | 0.14 ± 0.06a | <0.001 |
| Lycopene, μg/mL | 0.46 ± 0.16 | 0.47 ± 0.17 | 0.45 ± 0.15 | 0.48 ± 0.16 | 0.42 | 0.40 ± 0.13b | 0.47 ± 0.14b | 0.52 ± 0.18a | <0.001 | 0.42 ± 0.17b | 0.46 ± 0.13b | 0.52 ± 0.16a | <0.001 |
| β-carotene, μg/mL | 0.20 ± 0.12 | 0.19 ± 0.13 | 0.21 ± 0.13 | 0.19 ± 0.11 | 0.24 | 0.13 ± 0.07c | 0.17 ± 0.07b | 0.30 ± 0.15a | <0.001 | 0.15 ± 0.09b | 0.21 ± 0.13a | 0.23 ± 0.13a | <0.001 |
Values are means ± SDs or n (%) as indicated. Labeled means without a common letter differ, P < 0.05 (adjusted for multiple comparisons). OC, oral contraceptives.
Two-sided P values for continuous variables were calculated with ANOVA and for categorical variables with Fisher’s exact test.
Five women were missing alpha serum Vitamin E concentrations on all visits, 6 women were missing information on parity, and 4 were missing information on past OC use.
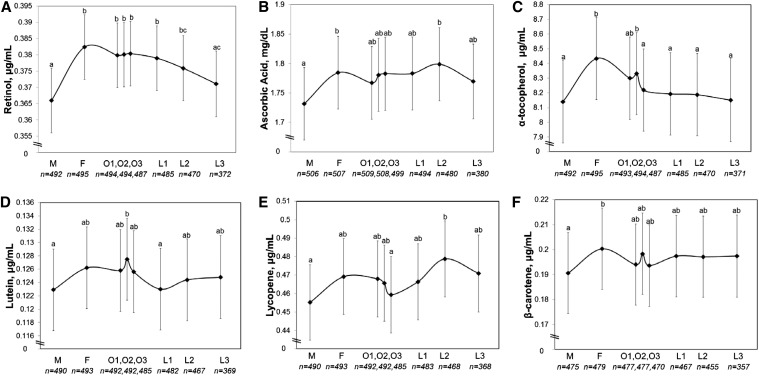
Antioxidant concentrations were observed to vary significantly across phases of the menstrual cycle for retinol, ascorbic acid, α-tocopherol, lutein, lycopene, and β-carotene (Figure 1). Specifically, the mean concentrations of these fat-soluble vitamins and macronutrients and ascorbic acid were lower during menses: retinol [compared with the early follicular (F1), periovulatory (O1–O3), and early and mid-luteal (L1, L2) phases], ascorbic acid [compared with the early follicular (F1) and mid-luteal (L2) phases], α-tocopherol [compared with the early follicular (F1), and periovulatory (O1, O2) phases], lutein [compared with the periovulatory phase (O2)], lycopene [compared with the mid-luteal phase (L2)], and β-carotene [compared with the early follicular phase (F1)]. No differences were observed for γ-tocopherol or the α-tocopherol:γ-tocopherol ratio across phases of the menstrual cycle (P = 0.29 and P = 0.11, respectively) (Supplemental Figure 1).
FIGURE 1.
Serum concentrations of retinol (A), ascorbic acid (B), α-tocopherol (C), lutein (D), lycopene (E), and β-carotene (F) in 259 healthy premenopausal women in the BioCycle Study across phases of the menstrual cycle for up to 2 cycles (509 cycles). Values are means and 95% CIs. Means without a common letter differ, P < 0.05 (adjusted for multiple comparisons). Sample sizes represent number of cycles (up to 2 per woman) for each cycle phase (all models take multiple visits per cycle and multiple cycles per woman into account). F, follicular phase; L1–L3, luteal phase; M, menses; O1–O3, ovulatory phase.
We consistently observed positive associations between multiple antioxidants and both E2 and testosterone (Table 2). Specifically, we observed positive associations between retinol, α-tocopherol, and lutein and between E2, free E2, and testosterone. Associations between retinol and α-tocopherol and total and free E2 were observed in models in which antioxidants and hormones were measured on the same day, although only retinol was also associated with these hormones in lag models. Ascorbic acid and lutein were associated with total and free E2 only in models with antioxidants and hormones measured on the same day. In general, ascorbic acid and lutein were associated with E2 and other hormones only in same-day models, suggesting potentially acute effects. However, we also observed that several antioxidants were positively associated with testosterone in lag models (retinol, γ-tocopherol, lycopene, β-carotene), suggesting potential longer term effects. We also observed that retinol was positively associated with LH (in both same-day and lag models), whereas α-tocopherol (in same-day and lag models) and ascorbic acid (in the same-day model) were negatively associated with FSH. Sporadic associations were observed between some of the antioxidants measured and other reproductive hormones.
TABLE 2.
Associations between the serum antioxidant concentrations that vary during the menstrual cycle and serum reproductive hormones among healthy premenopausal women in the BioCycle Study1
| Estradiol, pg/mL | Free estradiol, pg/mL | SHBG, nmol/L | Luteal progesterone,2 ng/mL | Testosterone, ng/dL | FSH, mIU/mL | LH, ng/mL | |
| Retinol, μg/mL | |||||||
| Model 13 | 1.00 (0.67, 1.34)* | 1.03 (0.67, 1.40)* | −0.09 (−0.20, 0.02) | 1.94 (0.99, 2.89)* | 0.61 (0.44, 0.78)* | −0.04 (−0.30, 0.23) | 0.51 (0.14, 0.88)* |
| Model 24 | 0.48 (0.11, 0.84)* | 0.49 (0.14, 0.84)* | −0.06 (−0.15, 0.04) | −0.25 (−1.13, 0.63) | 0.18 (0.03, 0.33)* | −0.04 (−0.26, 0.19) | 0.33 (0.01, 0.66)* |
| Ascorbic acid, mg/dL | |||||||
| Model 1 | 0.09 (0.05, 0.13)* | 0.07 (0.03, 0.11)* | 0.04 (0.02, 0.05)* | 0.09 (0.0003, 0.18)* | 0.002 (−0.01, 0.02) | −0.06 (−0.09, −0.03)* | −0.01 (−0.05, 0.04) |
| Model 2 | 0.0007 (−0.04, 0.04) | −0.01 (−0.05, 0.03) | 0.02 (0.004, 0.03)* | −0.01 (−0.13, 0.11) | −0.04 (−0.06, −0.03)* | −0.005 (−0.03, 0.02) | −0.01 (−0.05, 0.03) |
| α-Tocopherol, μg/mL | |||||||
| Model 1 | 0.02 (0.003, 0.03)* | 0.01 (−0.001, 0.02) | −0.003 (−0.01, 0.003) | 0.01 (−0.02, 0.04) | 0.01 (0.001, 0.01)* | −0.02 (−0.03, −0.01)* | 0.004 (−0.01, 0.02) |
| Model 2 | 0.002 (−0.01, 0.02) | 0.0005 (−0.01, 0.01) | −0.004 (−0.01, 0.001) | −0.05 (−0.09, −0.01)* | 0.004 (−0.02, 0.01) | −0.01 (−0.02, −0.0002)* | −0.01 (−0.02, 0.01) |
| γ-Tocopherol, μg/mL | |||||||
| Model 1 | 0.05 (0.01, 0.08)* | 0.05 (0.01, 0.09)* | −0.0006 (−0.01, 0.01) | 0.04 (−0.07, 0.16) | −0.002 (−0.01, 0.01) | 0.05 (0.02, 0.08)* | 0.07 (0.03, 0.10)* |
| Model 2 | 0.05 (0.02, 0.09)* | 0.05 (0.01, 0.08)* | −0.003 (−0.01, 0.01) | −0.05 (−0.15, 0.06) | 0.02 (0.01, 0.03)* | 0.04 (0.01, 0.06)* | 0.05 (0.01, 0.09)* |
| Ratio of α- to γ-tocopherol | |||||||
| Model 1 | 0.002 (−0.01, 0.01) | 0.0008 (−0.008, 0.01) | −0.002 (−0.005, 0.001) | −0.01 (−0.04, 0.01) | 0.01 (0.003, 0.01)* | −0.003 (−0.01, 0.004) | 0.004 (−0.01, 0.01) |
| Model 2 | −0.001 (−0.01, 0.01) | −0.003 (−0.01, 0.01) | 0.001 (−0.001, 0.004) | 0.003 (−0.02, 0.02) | −0.001 (−0.004, 0.002) | −0.004 (−0.01, 0.002) | 0.001 (−0.01, 0.01) |
| Ratio of α-tocopherol to ascorbic acid | |||||||
| Model 1 | −0.0001 (−0.02, 0.02) | 0.004 (−0.01, 0.02) | −0.004, (−0.01, 0.003) | −0.002 (−0.05, 0.05) | −0.01 (−0.01, 0.002) | −0.0001 (−0.01, 0.01) | −0.01 (−0.02, 0.02) |
| Model 2 | −0.01 (−0.02, 0.01) | −0.004 (−0.02, 0.01) | −0.01 (−0.01, −0.0003)* | −0.02 (−0.07, 0.03) | 0.003 (−0.004, 0.01) | −0.004 (−0.02, 0.01) | −0.01 (−0.02, 0.01) |
| Lutein, μg/mL | |||||||
| Model 1 | 1.10 (0.40, 1.80)* | 0.71 (0.05, 1.38)* | 0.25 (0.02, 0.48)* | 0.77 (−0.94, 2.49) | 0.43 (0.12, 0.75)* | 0.17 (−0.31, 0.64) | 0.46 (−0.18, 1.10) |
| Model 2 | 0.10 (−0.58, 0.78) | −0.19 (−0.84, 0.47) | −0.20 (−0.44, 0.04) | −1.41 (−2.96, 0.14) | 0.01 (−0.28, 0.31) | −0.31 (−0.72, 0.11) | −0.18 (−0.77, 0.41) |
| Lycopene, μg/mL | |||||||
| Model 1 | 0.06 (−0.10, 0.21) | 0.02 (−0.12, 0.17) | 0.03 (−0.02, 0.08) | −0.14 (−0.53, 0.24) | −0.02 (−0.08, 0.03) | 0.09 (−0.02, 0.21) | 0.01 (−0.15, 0.17) |
| Model 2 | −0.09 (−0.24, 0.06) | −0.11 (−0.26, 0.03) | 0.02 (−0.02, 0.07) | −0.17 (−0.56, 0.23) | −0.06 (−0.11, −0.001)* | −0.12 (−0.22, −0.01)* | −0.09 (−0.24, 0.06) |
| β-Carotene, μg/mL | |||||||
| Model 1 | 0.08 (−0.15, 0.31) | −0.09 (−0.32, 0.13) | 0.12 (0.04, 0.21)* | 1.06 (0.47, 1.64)* | 0.01 (−0.10, 0.13) | −0.12 (−0.30, 0.05) | −0.08 (−0.32, 0.16) |
| Model 2 | 0.03 (−0.19, 0.25) | −0.09 (−0.31, 0.12) | 0.13 (0.05, 0.21)* | 0.59 (−0.05, 1.24) | −0.13 (−0.23, −0.03)* | −0.29 (−0.43, −0.15)* | −0.18 (−0.39, 0.02) |
Values are RRs (95% CIs). *P < 0.05. FSH, follicle-stimulating hormone; LH, luteinizing hormone; SHBG, sex hormone-binding globulin.
Restricted to the 3 visits during the luteal phase.
Model 1: Linear mixed model analyses for association between antioxidant concentrations and log transformed reproductive hormones during the same cycle visit, adjusting for age, race, BMI, parity, time-varying sleep, pain medication use, total energy intake, serum cholesterol, F2-isoprostanes, other antioxidants, and concurrent hormones with the use of inverse probability weights.
Model 2: Lag linear mixed model analyses for association between antioxidant concentrations on 1 visit and log transformed reproductive hormones during the next cycle visit, adjusting for age, race, BMI, parity, time-varying sleep, pain medication use, total energy intake, serum cholesterol, F2-isoprostanes, other antioxidants, and concurrent hormones with the use of inverse probability weights.
An increased risk of anovulation associated with increasing serum γ-tocopherol and the α-tocopherol:γ-tocopherol ratio was found after adjusting for age, race, BMI, cholesterol, and total energy intake (Table 3). However, after additional adjustment for F2-isoprostanes and other antioxidants, only the α-:γ-tocopherol was significantly associated with an increased risk of anovulation (RR: 1.03; 95% CI: 1.01, 1.05). We observed no other associations between antioxidants and the risk of anovulation. Similar results were observed when evaluating the association between average antioxidant concentrations up to the time of expected ovulation and sporadic anovulation.
TABLE 3.
Associations between average serum antioxidant concentrations per cycle and risk of anovulation among healthy premenopausal women in the BioCycle Study1
| Cycle-average antioxidant concentrations |
Average antioxidant concentrations before expected ovulation |
|||
| Model 12 | Model 23 | Model 12 | Model 23 | |
| Retinol, μg/dL | 1.29 (0.77, 2.16) | 1.17 (0.68, 2.02) | 1.34 (0.82, 2.18) | 1.25 (0.75, 2.08) |
| Ascorbic acid, μmol/L | 0.99 (0.98, 1.01) | 1.00 (0.98, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.98, 1.01) |
| α-Tocopherol, μg/dL | 1.01 (0.99, 1.03) | 1.01 (0.99, 1.02) | 1.01 (1.00, 1.03) | 1.01 (0.99, 1.02) |
| γ-Tocopherol, μg/dL | 1.06 (1.00, 1.12)* | 1.05 (0.99, 1.12) | 1.06 (1.00, 1.12)* | 1.05 (0.99, 1.12) |
| Ratio of α- to γ-tocopherol | 1.03 (1.01, 1.05)* | 1.03 (1.01, 1.06)* | 1.03 (1.01, 1.05)* | 1.03 (1.01, 1.06)* |
| Ratio of α-tocopherol to ascorbic acid | 1.12 (0.96, 1.30) | 1.10 (0.94, 1.28) | 1.10 (0.94, 1.29) | 1.08 (0.93, 1.26) |
| Lutein, μg/dL | 1.42 (0.64, 3.16) | 1.44 (0.56, 3.67) | 1.38 (0.62, 3.04) | 1.32 (0.49, 3.51) |
| Lycopene, μg/dL | 1.10 (0.89, 1.36) | 1.13 (0.88, 1.44) | 1.15 (0.94, 1.40) | 1.18 (0.93, 1.50) |
| β-Carotene, μg/dL | 0.86 (0.50, 1.49) | 0.78 (0.37, 1.65) | 0.88 (0.50, 1.52) | 0.80 (0.37, 1.74) |
Values are RRs (95% CIs). *P < 0.05.
Adjusted for age, race, BMI, serum cholesterol, and total energy intake.
Adjusted for age, race, BMI, serum cholesterol, F2-isoprostanes, total energy intake, and the other antioxidants.
Discussion
Serum concentrations of fat-soluble antioxidant vitamins tended to be lowest during menses and were positively associated with E2 and testosterone concentrations across the menstrual cycle in a cohort of healthy regularly menstruating women. Ascorbic acid was also associated with increased E2, progesterone and decreased FSH concentrations. The α-tocopherol:γ-tocopherol ratio was significantly associated with an increased risk of anovulation. These results support the hypothesis that serum concentrations of antioxidants are associated with steroidogenesis and further elucidate the potential role of antioxidants in women’s reproductive health.
Biomarkers of both antioxidants and oxidative stress exist in normally cycling human ovaries (37, 38), and ROS and antioxidants are shown to surge and ebb across a normal menstrual cycle (8). Our finding of lower antioxidant concentrations during menses is consistent with this previous work and with a few small studies that have directly evaluated fluctuations across the cycle (39–42). In particular, a study of 12 premenopausal women observed serum β-carotene, lutein, and lycopene concentrations to be lowest during menses during controlled feeding cycles and that retinol concentrations were lowest during menses in both controlled feeding and free-living cycles (39). Among these women, serum α-tocopherol was lowest during menses (41), and similar cyclic fluctuations of carotenoids in lipoprotein fractions were observed (40). In addition, Michos et al. (42) reported elevated antioxidant status (n = 13) and an increase in ascorbic acid and total antioxidant plasma status between menstruation and ovulation and positive correlations between antioxidant concentrations and E2. In contrast to our study, α-tocopherol concentrations were reported to be lower in the follicular than in the luteal phase, but these results were based on a small study (n = 10) with the use of single nonfasting measurements during the follicular and luteal phases, with sample collection based on cycle day rather than timed to cycle phase by other methods (43). Collectively, and particularly given that we observed no differences in reported dietary intakes of these antioxidants across the menstrual cycle in this study (data not shown), these findings highlight potential differences in metabolism of antioxidants across the cycle, perhaps because of interactions with reproductive hormones.
Previous investigations have proposed biological mechanisms that may explain our findings of increased E2 and testosterone concentrations associated with α-tocopherol, retinol, and lutein. Some researchers hypothesize that vitamin E affects steroidogenesis by altering prostaglandin concentrations (44, 45) or through effects on cholesterol homeostasis (46, 47). E2 can influence the expression of retinol-binding protein and hepatic uptake of retinol-binding protein, which may also influence serum concentrations (48, 49). Moreover, we previously reported a significant positive relation between E2 and F2-isoprostanes, a global marker of oxidative stress, in the BioCycle cohort (18). Together, these studies may indicate greater antioxidant capacity in association with increased estrogen. This may suggest an adaptive response to estrogen-related oxidative stress, thereby resulting in an observed association also with antioxidant concentrations, or that estrogen may support antioxidant accumulation in the face of high ROS phases of the menstrual cycle.
The association between α-tocopherol:γ-tocopherol ratio and an increased risk of anovulation seems to conflict with past research, although no previous studies have evaluated these associations directly. Specifically, vitamin E supplementation, in the form of α-tocopherol, was associated with increased mid-luteal progesterone among women who had tried unsuccessfully to conceive (50); however, the supplement used had components in addition to vitamin E. Furthermore, lower ascorbic acid and α-tocopherol concentrations were reported among women with luteal phase defects who experienced recurrent spontaneous abortions compared with healthy women (16). Although γ-tocopherol is the main form of vitamin E in the diet, hepatic processing of vitamin E preferentially incorporates α-tocopherol into LDL cholesterol and HDL cholesterol, leading to increased γ-tocopherol excretion into bile. Our findings of a higher serum α-tocopherol:γ-tocopherol ratio being associated with increased risk of anovulation indicates that γ-tocopherol may be the more effective form of vitamin E relative to reproductive outcomes. Indeed, we observed more consistent associations between γ-tocopherol and reproductive hormones in both same-day and lag models. A clue to this complex relation may lie in observations in breast cancer in which γ- and δ-tocopherols, but not α-tocopherol, antagonized estrogen action (51). Given the complexities of hepatic processing and the contribution of diet and lipoprotein metabolism, additional research is needed to further explore the potential relations and mechanisms between particular vitamin E concentrations and reproductive outcomes.
The positive association we observed between serum ascorbic acid and both E2 and progesterone are in line with a prior study that reported increased progesterone concentrations and pregnancy rates in 122 women with luteal phase defect who were administered ascorbic acid supplementation (15). Indeed, in vitro studies with the use of human choriocarcinoma cell lines indicate that ascorbic acid may stimulate hormone synthesis through a vitamin C transporter (14). However, a study among 620 women aged <40 y undergoing in vitro fertilization did not show increases in clinical pregnancy rates or implantation from ascorbic acid supplementation (52). This could suggest a minimal steroidogenic effect or one that is overshadowed by subfertility or exogenous hormone treatments used in assisted reproductive technologies.
Our finding of an association between ascorbic acid and decreased FSH is novel. A decrease in FSH may be expected because we also observed increases in E2, and these 2 hormones have a known negative feedback under control of the hypothalamic-pituitary-ovarian axis in women with normal reproductive function. It is important to note that, although we could not evaluate supplementation by ascorbic acid in this study and women were eligible for the study only if they were willing to stop regular intake of vitamin or mineral supplements during the study, the concentrations of ascorbic acid were considerably higher than among women of reproductive age in the United States on the basis of responses from the NHANES [median: 96.0 μmol/L (95% CI: 61.3, 157.3 μmol/L) in BioCycle compared with 55.3 μmol/L (95% CI: 9.9, 96.1 μmol/L) in NHANES] (53).
Intensive monitoring of a large number of young, ethnically diverse women throughout 2 menstrual cycles, with multiple clinic visits timed with fertility monitors, are unique strengths of this study. Multiple measurements of both hormones and antioxidants enabled us to more precisely model these associations. The biomarker approach to target antioxidant nutrients is a more precise method of assessing these exposures, given individual differences in metabolism compared with self-reported dietary assessment (17). We were also able to adjust for important covariates that affect antioxidant concentrations, such as serum cholesterol and F2-isoprostane concentrations. We attempted to further explain the complex relations between antioxidants and hormones by using marginal structural models and inverse probability weights to account for potential feedback mechanisms and to adjust for potential confounding by reproductive hormone concentrations. Moreover, the use of lag models and anovulation models to evaluate concentrations of antioxidants before the time of expected ovulation preserves temporality and indicates both potential acute and longer term effects of antioxidants on hormone concentrations. Collectively, these unique aspects of the study design allowed us to improve our knowledge of antioxidants and their relations with reproductive hormones and ovulation.
The study faced several limitations, including the absence of a daily transvaginal ultrasound scan to aid in detecting ovulation. In addition, the power of the findings was limited by the small number of anovulatory cycles (n = 42) and because women were only followed for up to 2 menstrual cycles. The median concentrations of retinol, α- and γ-tocopherols, and lutein were lower in our population than the median concentrations reported in NHANES [retinol: 37 μg/dL (BioCycle), 49 μg/dL (NHANES); α-tocopherol: 796 μg/dL (BioCycle), 997 μg/dL (NHANES); γ-tocopherol: 173 μg/dL (BioCycle), 196 μg/dL (NHANES); lutein: 11.4 μg/dL (BioCycle), 13.8 μg/dL (NHANES)], although median concentrations of lycopene and ascorbic acid were higher [lycopene: 44 μg/dL (BioCycle), 43 μg/dL (NHANES); ascorbic acid: 96 μmol/L (BioCycle), 55 μmol/L (NHANES)] (53). Thus, although some differences in the concentrations were observed, the concentrations reported are in large part in line with those reported among women of reproductive age in the United States and are generalizable to similar populations.
In conclusion, we observed that serum concentrations of antioxidants were lower during menses and were positively associated with E2 and testosterone concentrations across the menstrual cycle in healthy premenopausal women. Ascorbic acid was also associated with increased progesterone and decreased FSH concentrations. This study sheds new light on the intricate associations between antioxidant and hormone concentrations among healthy women of reproductive age and builds on our previous work that identified positive associations between markers of oxidative stress and E2. Studies are now needed to elucidate the biologic pathways interconnecting ROS production, antioxidant agents, and reproductive hormones to better understand the potential role dietary and supplemental antioxidants may play in women’s reproductive health and fertility.
Acknowledgments
SLM, JW-W, and EFS conceived the study concept and design; SLM conceived the project and developed the overall research plan; SLM, KCS, and JS performed the statistical analyses; SLM, RWB, KCS, and JS wrote the paper; SLM, RWB, KCS, JS, TCP, KAM, LAS, SMZ, NJP, LCM, RGR, JW-W, and EFS interpreted the data and critically revised the manuscript for important intellectual content; SLM had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; ROS, reactive oxygen species; SHBG, sex hormone-binding globulin.
References
- 1.Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal 2008;10:1375–403. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - a clinician’s perspective. Reprod Biomed Online 2005;11:641–50. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril 2006;86:503–12. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstet Gynecol Surv 2007;62:335–47. [DOI] [PubMed] [Google Scholar]
- 5.Lord KC, Shenouda SK, McIlwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovasc Res 2010;87:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update 2008;14:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol 2009;21:219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costello MF, Shrestha B, Eden J, Johnson NP, Sjoblom P. Metformin versus oral contraceptive pill in polycystic ovary syndrome: a Cochrane review. Hum Reprod 2007;22:1200–9. [DOI] [PubMed] [Google Scholar]
- 10.Jackson LW, Schisterman EF, Dey-Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Hum Reprod 2005;20:2014–20. [DOI] [PubMed] [Google Scholar]
- 11.Mier-Cabrera J, Jimenez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernandez-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG 2011;118:6–16. [DOI] [PubMed] [Google Scholar]
- 12.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, et al. . Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008;44:280–7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig 2006;13:451–8. [DOI] [PubMed] [Google Scholar]
- 14.Wu X, Iguchi T, Itoh N, Okamoto K, Takagi T, Tanaka K, Nakanishi T. Ascorbic acid transported by sodium-dependent vitamin C transporter 2 stimulates steroidogenesis in human choriocarcinoma cells. Endocrinology 2008;149:73–83. [DOI] [PubMed] [Google Scholar]
- 15.Henmi H, Endo T, Kitajima Y, Manase K, Hata H, Kudo R. Effects of ascorbic acid supplementation on serum progesterone levels in patients with a luteal phase defect. Fertil Steril 2003;80:459–61. [DOI] [PubMed] [Google Scholar]
- 16.Vural P, Akgul C, Yildirim A, Canbaz M. Antioxidant defence in recurrent abortion. Clin Chim Acta 2000;295:169–77. [DOI] [PubMed] [Google Scholar]
- 17.Mayne ST, Wright ME, Cartmel B. Assessment of antioxidant nutrient intake and status for epidemiologic research. J Nutr 2004;134:3199S–200S. [DOI] [PubMed] [Google Scholar]
- 18.Schisterman EF, Gaskins AJ, Mumford SL, Browne RW, Yeung E, Trevisan M, Hediger M, Zhang C, Perkins NJ, Hovey K, et al. . Influence of endogenous reproductive hormones on F2-isoprostane levels in premenopausal women: the BioCycle Study. Am J Epidemiol 2010;172:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol 2009;23:171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol 2009;169:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mumford SL, Schisterman EF, Gaskins AJ, Pollack AZ, Perkins NJ, Whitcomb BW, Ye A, Wactawski-Wende J. Realignment and multiple imputation of longitudinal data: an application to menstrual cycle data. Paediatr Perinat Epidemiol 2011;25:448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalmers AH, McWhinney BC. Two spectrophotometric methods compared for measuring low concentrations of ascorbate in plasma and urine. Clin Chem 1986;32:1412–3. [PubMed] [Google Scholar]
- 23.Browne RW, Armstrong D. Simultaneous determination of serum retinol, tocopherols, and carotenoids by HPLC. Methods Mol Biol 1998;108:269–75. [DOI] [PubMed] [Google Scholar]
- 24.Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol 1993;71:725–31. [DOI] [PubMed] [Google Scholar]
- 25.Kagan VE, Serbinova EA, Forte T, Scita G, Packer L. Recycling of vitamin E in human low density lipoproteins. J Lipid Res 1992;33:385–97. [PubMed] [Google Scholar]
- 26.May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys 1998;349:281–9. [DOI] [PubMed] [Google Scholar]
- 27.Niki E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic Biol Med 2014;66:3–12. [DOI] [PubMed] [Google Scholar]
- 28.Scarpa M, Rigo A, Maiorino M, Ursini F, Gregolin C. Formation of alpha-tocopherol radical and recycling of alpha-tocopherol by ascorbate during peroxidation of phosphatidylcholine liposomes. An electron paramagnetic resonance study. Biochim Biophys Acta 1984;801:215–9. [DOI] [PubMed] [Google Scholar]
- 29.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci USA 1993;90:1771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saldeen T, Li D, Mehta JL. Differential effects of alpha- and gamma-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis. J Am Coll Cardiol 1999;34:1208–15. [DOI] [PubMed] [Google Scholar]
- 31.Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am J Clin Nutr 1989;49:517–26. [DOI] [PubMed] [Google Scholar]
- 32.Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–10. [DOI] [PubMed] [Google Scholar]
- 33.Lynch KE, Mumford SL, Schliep KC, Whitcomb BW, Zarek SM, Pollack AZ, Bertone-Johnson ER, Danaher M, Wactawski-Wende J, Gaskins AJ, et al. . Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms. Fertil Steril 2014;102:511–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 35.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 36.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiotani M, Noda Y, Narimoto K, Imai K, Mori T, Fujimoto K, Ogawa K. Immunohistochemical localization of superoxide dismutase in the human ovary. Hum Reprod 1991;6:1349–53. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki T, Sugino N, Fukaya T, Sugiyama S, Uda T, Takaya R, Yajima A, Sasano H. 1999 Superoxide dismutase in normal cycling human ovaries: immunohistochemical localization and characterization. Fertil Steril 1999;72:720–6. [DOI] [PubMed] [Google Scholar]
- 39.Forman MR, Beecher GR, Muesing R, Lanza E, Olson B, Campbell WS, McAdam P, Raymond E, Schulman JD, Graubard BI. The fluctuation of plasma carotenoid concentrations by phase of the menstrual cycle: a controlled diet study. Am J Clin Nutr 1996;64:559–65. [DOI] [PubMed] [Google Scholar]
- 40.Forman MR, Johnson EJ, Lanza E, Graubard BI, Beecher GR, Muesing R. Effect of menstrual cycle phase on the concentration of individual carotenoids in lipoproteins of premenopausal women: a controlled dietary study. Am J Clin Nutr 1998;67:81–7. [DOI] [PubMed] [Google Scholar]
- 41.Lanza E, Forman MR, Johnson EJ, Muesing RA, Graubard BI, Beecher GR. alpha-Tocopherol concentrations in plasma but not in lipoproteins fluctuate during the menstrual cycle in healthy premenopausal women. J Nutr 1998;128:1150–5. [DOI] [PubMed] [Google Scholar]
- 42.Michos C, Kiortsis DN, Evangelou A, Karkabounas S. Antioxidant protection during the menstrual cycle: the effects of estradiol on ascorbic-dehydroascorbic acid plasma levels and total antioxidant plasma status in eumenorrhoic women during the menstrual cycle. Acta Obstet Gynecol Scand 2006;85:960–5. [DOI] [PubMed] [Google Scholar]
- 43.Palan PR, Magneson AT, Castillo M, Dunne J, Mikhail MS. Effects of menstrual cycle and oral contraceptive use on serum levels of lipid-soluble antioxidants. Am J Obstet Gynecol 2006;194:e35–8. [DOI] [PubMed] [Google Scholar]
- 44.Hartman TJ, Dorgan JF, Woodson K, Virtamo J, Tangrea JA, Heinonen OP, Taylor PR, Barrett MJ, Albanes D. Effects of long-term alpha-tocopherol supplementation on serum hormones in older men. Prostate 2001;46:33–8. [DOI] [PubMed] [Google Scholar]
- 45.Mondul AM, Rohrmann S, Menke A, Feinleib M, Nelson WG, Platz EA, Albanes D. Association of serum alpha-tocopherol with sex steroid hormones and interactions with smoking: implications for prostate cancer risk. Cancer Causes Control 2011;22:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barella L, Muller PY, Schlachter M, Hunziker W, Stöcklin E, Spitzer V, Meier N, de Pascual-Teresa S, Minihane AM, Rimbach G. Identification of hepatic molecular mechanisms of action of alpha-tocopherol using global gene expression profile analysis in rats. Biochim Biophys Acta 2004;1689:66–74. [DOI] [PubMed] [Google Scholar]
- 47.Barella L, Rota C, Stocklin E, Rimbach G. Alpha-tocopherol affects androgen metabolism in male rat. Ann N Y Acad Sci 2004;1031:334–6. [DOI] [PubMed] [Google Scholar]
- 48.Jung US, Jeong KJ, Kang JK, Yi K, Shin JH, Seo HS, Kim T, Kim SH, Hur JY. Effects of estrogen receptor alpha and beta on the expression of visfatin and retinol-binding protein 4 in 3T3–L1 adipocytes. Int J Mol Med 2013;32:723–8. [DOI] [PubMed] [Google Scholar]
- 49.Pirani T, Chen J, Vieira A. Effects of estradiol on the endocytic transport of vitamin D carrier protein in hepatocytes. Biochim Biophys Acta 2013;1830:3421–6. [DOI] [PubMed] [Google Scholar]
- 50.Westphal LM, Polan ML, Trant AS, Mooney SB. A nutritional supplement for improving fertility in women: a pilot study. J Reprod Med 2004;49:289–93. [PubMed] [Google Scholar]
- 51.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, Lee MJ, Yang CS, Newmark HL, Suh N. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin Cancer Res 2009;15:4242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griesinger G, Franke K, Kinast C, Kutzelnigg A, Riedinger S, Kulin S, Kaali SG, Feichtinger W. Ascorbic acid supplement during luteal phase in IVF. J Assist Reprod Genet 2002;19:164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.CDC [Internet]. Atlanta: The Organization [updated 2012 Mar 28; cited 2015 Nov 2] Second National Report. 2012. Available from: http://www.cdc.gov/nutritionreport/.