Abstract
Background: Green tea consumption has been associated with favorable changes in body weight and obesity-related hormones, although it is not known whether these changes result from green tea polyphenols or caffeine.
Objective: We examined the impact of decaffeinated green tea extract (GTE) containing 843 mg of (−)-epigallocatechin-3-gallate on anthropometric variables, obesity-associated hormones, and glucose homeostasis.
Methods: The Minnesota Green Tea Trial was a 12-mo randomized, double-blind, placebo-controlled clinical trial of 937 healthy postmenopausal women assigned to either decaffeinated GTE (1315 mg total catechins/d) or a placebo, stratified by catechol-O-methyltransferase (COMT) genotype. This study was conducted in a subset of 237 overweight and obese participants [body mass index (BMI) ≥25 kg/m2].
Results: No changes in energy intake, body weight, BMI, or waist circumference (WC) were observed over 12 mo in women taking GTE (n = 117) or placebo (n = 120). No differences were seen in circulating leptin, ghrelin, adiponectin, or glucose concentrations at month 12. Participants randomly assigned to GTE with baseline insulin ≥10 μIU/mL (n = 23) had a decrease in fasting serum insulin from baseline to month 12 (−1.43 ± 0.59 μIU/mL), whereas those randomly assigned to placebo with baseline insulin ≥10 μIU/mL (n = 19) had an increase in insulin over 12 mo (0.55 ± 0.64 μIU/mL, P < 0.01). Participants with the homozygous high-activity (G/G) form of COMT had significantly lower adiponectin (5.97 ± 0.50 compared with 7.58 ± 0.53 μg/mL, P = 0.03) and greater insulin concentrations (7.63 ± 0.53 compared with 6.18 ± 0.36 μIU/mL, P = 0.02) at month 12 compared with those with the low-activity (A/A) genotype, regardless of treatment group.
Conclusions: Decaffeinated GTE was not associated with reductions in body weight, BMI, or WC and did not alter energy intake or mean hormone concentrations in healthy postmenopausal women over 12 mo. GTE decreased fasting insulin concentrations in those with elevated baseline fasting concentrations. The high-activity form of the COMT enzyme may be associated with elevations in insulin and a reduction in adiponectin concentrations over time. This trial was registered at http://www.clinicaltrials.gov as NCT00917735.
Keywords: green tea extract, obesity, postmenopausal women, insulin, adiponectin
Introduction
Excess adiposity, especially visceral adiposity, has been accepted as a risk factor for both postmenopausal breast carcinogenesis and insulin resistance, a condition associated with the development of metabolic syndrome and type 2 diabetes mellitus (1). Numerous studies have indicated that green tea consumption may have beneficial effects on weight loss and weight maintenance as well as breast cancer risk (2–5). Catechins, a class of flavan-3-ol polyphenols that includes epicatechin, epicatechin gallate, epigallocatechin, and (−)-epigallocatechin-3-gallate (EGCG)8, are major constituents of green tea (6). EGCG is the most abundant of these catechins and is thought to be a major contributor to many of the beneficial effects of green tea, including its antiobesity and antidiabetic properties (7, 8). Green tea also contains caffeine (9), and it remains unclear whether its health benefits result from its catechin content, caffeine content, or synergism between the 2 compounds.
One of the major metabolic pathways of green tea catechin degradation is O-methylation, which is catalyzed by catechol-O-methyltransferase (COMT). At the same time, flavanolic compounds such as catechins have been shown to reduce the activity of COMT in vitro, although this has not been conclusively demonstrated in vivo. This enzyme also metabolizes several other compounds, including catecholamines. One of the proposed mechanisms by which green tea consumption may influence body weight is through the inhibition of COMT and the resulting increased and/or prolonged effects of norepinephrine, including increased energy expenditure and fat oxidation (10). Genetic variability may also have an impact on this relation because the COMT enzyme is polymorphic—a single-nucleotide polymorphism at codon 108/158 (rs4680) results in a guanine (G) to adenine (A) transition that leads to a 66–75% decrease in enzymatic activity (11, 12). Individuals with the low-activity COMT genotype may metabolize tea catechins slower than those with the high-activity genotype, allowing the bioactive components to be retained longer and resulting in greater benefits from green tea intake.
Although there has been substantial focus on green tea’s regulation of energy expenditure (13) and fat oxidation (14, 15), less research has been directed toward its potential effects on glucose homeostasis and adiposity-associated hormones such as leptin, ghrelin, and adiponectin in postmenopausal women. The objective of this study was to clarify the effects of 12 mo of caffeine-free green tea extract (GTE) supplementation on obesity-related hormones, anthropometrics, and glucose homeostasis in postmenopausal women in the absence of other lifestyle-related changes (diet and physical activity). We hypothesized that GTE supplementation would reduce body weight, BMI, waist circumference (WC), and waist-to-hip ratio (WHR) and parallel changes in obesity-related hormones and glucose homeostasis. We also hypothesized that participants with the low-activity COMT genotype would experience greater effects than those with the high-activity genotype.
Methods
Design.
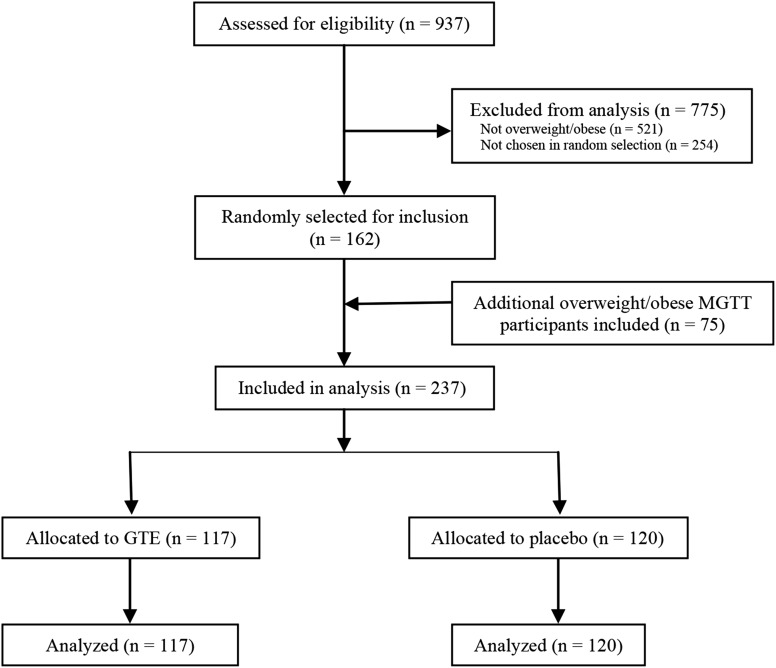
The Minnesota Green Tea Trial (MGTT) (NCT00917735) was a 12-mo randomized, double-blinded, placebo-controlled study designed to examine the effect of high-dose GTE on breast cancer risk factors. Details of the study design, eligibility criteria, randomization, blinding, study conduct, and patient flow through the trial have been previously published (16). Briefly, healthy postmenopausal women aged 50–70 y and classified as having high mammographic density (a breast cancer risk factor) were recruited from 2009 to 2013 at clinical centers in the Minneapolis–St. Paul metropolitan area. Of 1075 randomly assigned women, 538 were assigned to receive 4 oral GTE capsules containing 1315 mg ± 116 total catechins per day (843 ± 44 mg as EGCG), and 537 were randomly assigned to receive placebo. In total, 937 women (87.2%) completed the study. Of these, 162 overweight (BMI = 25.0–29.9 kg/m2) and obese (BMI = 30.0–40.0 kg/m2) women were randomly selected for obesity-associated hormone analysis through a computer-generated random number sequence; an additional 75 overweight and obese participants enrolled in another MGTT ancillary study of body composition variables were included in this analysis. The final sample sizes were 117 (GTE) and 120 (placebo). Of these, 5 participants from the GTE and 5 from the placebo groups discontinued the intervention at some point during the study period, although they remained in the study in accordance with the intention-to-treat analytic model (16). Figure 1 details the participant flow through the trial.
FIGURE 1.
Flow of participants through the Minnesota Green Tea Trial. GTE, green tea extract; MGTT, Minnesota Green Tea Trial.
Baseline health status was assessed by a standardized questionnaire and blood samples were taken at the initial screening clinic visit. Exclusionary criteria included any history of breast cancer, proliferative breast disease, or ovarian cancer; any cancer diagnosis within 5 y; regular consumption of >7 alcoholic drinks per week [1 drink = 12 fl oz (355 mL) regular beer, 5 fl oz (148 mL) wine, or 1.5 fl oz (44 mL) spirits]; regular consumption of green tea (>240 mL/wk); BMI <25.0 or >40 kg/m2; weight change >4.5 kg during the previous year; current or recent use (within 6 mo) of menopausal hormone therapy or chemopreventive agents; current use of methotrexate or Enbrel (etanercept) (anti-inflammatory agents); current smoking; history of breast augmentation; positive serology for hepatitis B or C antibodies; or alanine aminotransferase >1.5 times the upper limit of normal (defined as 60 U/L).
Randomization, blinding, and ethics.
Participants were randomly assigned to receive oral GTE or a placebo for 12 mo. Randomization was performed by the Investigational Drug Services pharmacy at the University of Minnesota Medical Center–Fairview using a computer-generated permuted block randomization scheme with blocks of 8 stratified by COMT genotype: low (A/A or A/G) or high (G/G). Participants and study staff were blind to treatment allocation throughout the trial. Institutional Review Board approval was obtained at each clinical center, and all participants provided written informed consent.
COMT genotyping.
COMT genotype was determined by the University of Minnesota Genomics Center. DNA was extracted from buffy coat samples by the Qiagen DNAeasy Blood and Tissue Kit method. A TaqMan assay was developed for defining the COMT H/L polymorphism using a TaqMan PCR Core Reagents Kit (Applied Biosystems). Coriell cell lines with a known COMT genotype were used as quality controls with each PCR run.
Study supplement.
Decaffeinated green tea extract catechin complex (GTE) and placebo capsules were supplied by Corban Laboratories (Eniva Nutraceutics) and dispensed by the Investigative Drug Service pharmacy in 3-mo increments. Mean total catechin content of each GTE capsule was 328 ± 30 mg, including 211 ± 11 mg of EGCG (1315 ± 116 mg of total catechins per day, 843 ± 44 mg as EGCG), which is equivalent to 5 8-oz (240 mL) cups of brewed green tea per day (17). Placebo capsules were identical in appearance to the GTE capsule and contained 816 mg of maltodextrin, 808 mg of cellulose, and 8 mg of magnesium stearate as a flow agent. Each GTE capsule contained <4 mg of caffeine. Participants were instructed to consume 2 capsules twice daily with morning and evening meals (4 capsules per day).
Anthropometry and body composition.
Body weight was recorded at screening, baseline, and months 3, 6, 9, and 12 using a calibrated digital stand-on scale. Standing height was assessed by a wall-mounted stadiometer to the nearest 0.1 cm at the screening clinic visit and month 12. BMI was calculated by dividing the weight in kilograms by the height in meters squared (kg/m2). Waist and hip circumference were recorded to the nearest 0.1 cm in duplicate using a flexible body tape at baseline and month 12. WC was measured at the uppermost lateral border of the iliac crest at the narrowest point of the torso. Hip circumference was measured at the widest part of the buttocks. WHR was calculated by dividing WC by hip circumference.
Dietary assessment.
Participants completed an FFQ at the beginning and end of the study to capture dietary patterns from the previous 12 mo. The Diet History Questionnaire (18) is an FFQ that consists of 124 food items and includes both portion size and dietary supplement questions. The food list and nutrient database used with the Diet History Questionnaire are based on national dietary data (19). FFQ data were analyzed using Diet*Calc software developed at the National Cancer Institute.
Physical activity.
Recreational physical activity was assessed at baseline and month 12 by a health history questionnaire, in which participants were asked about the frequency and duration of several types of physical activity. Metabolic equivalent (MET) hours, defined as the ratio of work metabolic rate to a standard resting metabolic rate, were computed as the product of average hours per week of each activity multiplied by its MET-hour equivalent. All recorded activities were summed to obtain the total MET-hours of activity performed per week for a given participant.
Obesity-associated hormone and glucose homeostasis marker analysis.
Blood samples for the obesity-associated hormone and glucose homeostasis marker assessment were collected at baseline and month 12 by a research nurse or licensed phlebotomist after an overnight fast of >10 h. Whole blood samples were separated into plasma and serum, measured into 1.5-mL aliquots, and stored at −80°C until the sample analysis was performed. Plasma leptin, ghrelin, and adiponectin were measured using EMD Millipore radioimmunoassay kits [interassay CV: leptin = 7.1%, ghrelin = 7.8%, and adiponectin = 8.9%; intra-assay CV: leptin = 6.6%, ghrelin = 5.5%, and adiponectin = 7.4%]. Serum insulin was measured via the Access Ultrasensitive Insulin simultaneous 1-step immunoenzymatic chemiluminescent assay (Quest Diagnostics; intra-assay CV = 3–5.0%; inter-assay CV = 3.9%). Serum glucose concentrations were measured using a hexokinase enzymatic reference method (Quest Diagnostics). HOMA-IR was used to evaluate insulin resistance [fasting insulin (μIU/mL) × fasting glucose (mg/dL)/405].
Adverse event reporting.
Information on adverse events (AEs) that occurred during the study was obtained at clinic visits or self-reported via phone or e-mail. Hepatic function was measured monthly during the first 6 months of the study and at months 9 and 12 because of GTE’s association with hepatotoxicity (20, 21). AE information was coded and graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.03 (22). The clinical course of each event was tracked in an ongoing AE record in the study clinic, and each event was followed until resolution.
Statistical analyses.
Differences in baseline demographic and anthropometric characteristics between groups and baseline, month 12, and changes from baseline in dietary intake and anthropometrics were assessed by nonpaired Student’s t tests. Change from baseline was calculated by subtracting the baseline from the month 12 values. A repeated measures linear regression was used to assess the within-treatment group and within-COMT genotype differences in anthropometric variables between baseline and month 12. Obesity-associated hormones, glucose, and HOMA-IR were natural log-transformed, and a repeated measures 2-factor ANOVA was used to compare geometric means between and within groups at baseline and month 12. The pairwise differences between groups were used to compute the P values. A similar analysis was performed to compare geometric means between and within COMT genotype groups at baseline and month 12.
Mean change in obesity-associated hormones, glucose, and HOMA-IR at 12 mo was analyzed using linear regression and calculated by subtracting the baseline value from the value at 12 mo. The explanatory variables included treatment, baseline value of the variable, baseline glucose concentration, change in BMI, COMT genotype, and all 2-factor interactions. Main effects were kept in the model. Model reduction was considered using backward elimination for 2-factor interactions that had P values >0.05. All-randomized (intention-to-treat) and per-protocol analyses were performed, and data are reported for the all-randomized dataset unless otherwise noted. To examine the difference in baseline characteristics and AEs by treatment group and AE status, the chi-square test or Fisher’s exact test was used for categorical variables, and Student’s t test was used for continuous variables. Sample sizes were calculated using power analysis based on previous obesity study reports. Defining α as equal to 0.05, the present study had >80% power to detect a 0.5-kg difference in body weight and 0.5 kg/m2 difference in BMI between treatment groups and sufficient power to detect an adiponectin increase of 0.62 μg/mL in participants randomly assigned to GTE compared with placebo. There was also sufficient power to detect an interaction effect between GTE and the COMT genotype, with >81 participants per group. All analyses were performed using SAS version 9.3 (IBM) mixed procedure. Values in tables are presented as arithmetic mean ± SEM or geometric mean (95% CI) for continuous variables and as count (%) for categorical variables.
Results
Participant characteristics.
Baseline characteristics and demographics were similar for both treatment groups (Table 1). The mean age of the study sample was 60.8 y, and most subjects (94.0%) were non-Hispanic whites. Mean BMI did not differ between treatment groups, although the GTE group had a greater proportion of obese participants at baseline compared with the placebo group (P = 0.02). The mean total energy intake and daily intake of carbohydrates, protein, fat, caffeine, and micronutrients were similar at baseline and were not significantly different between or within treatment or COMT genotype groups at month 12. Characteristics of the study population did not differ by COMT genotype group at baseline or month 12.
TABLE 1.
Baseline characteristics of overweight or obese postmenopausal MGTT participants given GTE or placebo for 12 mo1
| Characteristic | GTE (n = 117) | Placebo (n = 120) | P value |
| Age at baseline, y | 60.9 ± 0.45 | 60.6 ± 0.47 | 0.64 |
| Race/ethnicity, n (%) | 0.52 | ||
| Non-Hispanic white | 111 (46.8) | 112 (47.2) | |
| African American | 2 (0.8) | 5 (2.1) | |
| Hispanic | 1 (0.4) | 0 (0) | |
| Other | 3 (1.3) | 3 (1.3) | |
| COMT genotype, n (%) | 0.65 | ||
| Low (A/A) | 38 (16.0) | 37 (15.6) | |
| Intermediate (A/G) | 51 (21.6) | 59 (24.9) | |
| High (G/G) | 28 (11.8) | 24 (10.1) | |
| Weight, kg | 75.6 ± 0.87 | 74.3 ± 0.86 | 0.27 |
| Height, cm | 163 ± 0.59 | 163 ± 0.53 | 0.94 |
| BMI, kg/m2 | 28.5 ± 0.28 | 27.9 ± 0.25 | 0.13 |
| BMI, n (%) | 0.02 | ||
| 25.0–29.9 kg/m2 | 79 (67.6) | 97 (80.8) | |
| 30.0–40.0 kg/m2 | 38 (32.4) | 23 (19.2) | |
| Waist circumference, cm | 91.9 ± 0.8 | 90.5 ± 0.8 | 0.23 |
| Waist:hip ratio | 0.86 ± 0.01 | 0.86 ± 0.01 | 0.43 |
| Age at menopause, y | 47.2 (46.0, 48.4) | 48.3 (47.1, 49.6) | 0.20 |
| Physical activity, MET-h/wk | 26.1 (21.6, 31.6) | 28.3 (23.5, 34.1) | 0.55 |
| Alcohol consumption, n (%) | 0.79 | ||
| Yes | 96 (40.5) | 100 (42.2) | |
| No | 21 (8.9) | 20 (8.4) | |
| Alcohol intake,2 drinks/wk | 2.0 (1.6, 2.4) | 1.8 (1.5, 2.2) | 0.53 |
| Energy intake, kcal/d | 1390 (1300, 1480) | 1400 (1310, 1490) | 0.87 |
| Carbohydrate, g/d | 182 ± 67.8 | 190 ± 79.8 | 0.43 |
| Protein, g/d | 59.7 ± 23.7 | 61.2 ± 26.0 | 0.65 |
| Total Fat, g/d | 54.3 ± 25.8 | 55.4 ± 28.1 | 0.76 |
| Caffeine, mg/d | 152 (112, 207) | 202 (149, 273) | 0.20 |
Baseline age, weight, height, BMI, waist circumference, and waist-hip ratio are presented as arithmetic means ± SEMs; age at menopause, physical activity, alcohol intake, energy intake, and caffeine intake are presented as geometric means (95% CIs). COMT, catechol-O-methyltransferase; GTE, green tea extract; MET, metabolic equivalent; MGTT, Minnesota Green Tea Trial.
Drinkers only; 1 alcoholic drink was quantified as 12 fl oz (355 mL) regular beer, 5 fl oz (148 mL) wine, or 1.5 fl oz (44 mL) liquor.
Change in dietary intake and anthropometric variables.
A comparison of dietary intake and anthropometric changes from baseline to month 12 is shown in Table 2. Total energy intake decreased in both treatment groups from baseline to month 12, although the between-group comparison was not significant. Caffeine intake did not appreciably change from baseline to month 12 in either group (P = 0.78). Weight change from baseline to month 12 was not significantly different when GTE and placebo groups were compared, although weight change from baseline was marginally greater in GTE participants (P = 0.13). Similarly, a nonsignificant decrease in BMI was seen in GTE participants compared with placebo (P = 0.14). We did not observe any differences in WC or WHR changes between GTE and placebo groups. No differences in within-group comparisons of baseline and month 12 values were observed for any anthropometric variable. Results did not differ when analyzed by COMT genotype. These anthropometric outcomes for this subset of participants were consistent with those seen for the full study population (n = 937; data not shown).
TABLE 2.
Energy intake and anthropometric variables in overweight or obese postmenopausal MGTT participants given GTE or placebo for 12 mo1
| Variable | GTE (n = 117) | Placebo (n = 120) | P value |
| Total energy intake, kcal/d | |||
| Baseline | 1480 ± 52.3 | 1500 ± 51.7 | 0.77 |
| Month 12 | 1360 ± 52.3 | 1390 ± 51.9 | 0.69 |
| Change from baseline | −115 ± 505 | −108 ± 416 | 1.0 |
| Body weight, kg | |||
| Baseline | 75.6 ± 0.9 | 74.3 ± 0.9 | 0.27 |
| Month 12 | 75.4 ± 0.9 | 74.3 ± 0.9 | 0.39 |
| Change from baseline | −0.28 ± 2.2 | −0.14 ± 3.2 | 0.13 |
| BMI, kg/m2 | |||
| Baseline | 28.5 ± 0.3 | 27.9 ± 0.3 | 0.13 |
| Month 12 | 28.5 ± 0.3 | 27.9 ± 0.3 | 0.14 |
| Change from baseline | −0.10 ± 0.8 | −0.05 ± 1.2 | 0.14 |
| Waist circumference, cm | |||
| Baseline | 91.9 ± 0.8 | 90.5 ± 0.8 | 0.24 |
| Month 12 | 93.2 ± 0.8 | 91.6 ± 0.8 | 0.17 |
| Change from baseline | 1.45 ± 0.5 | 1.29 ± 0.5 | 0.82 |
| Waist-to-hip ratio | |||
| Baseline | 0.86 ± 0.01 | 0.86 ± 0.01 | 0.41 |
| Month 12 | 0.86 ± 0.01 | 0.86 ± 0.01 | 0.34 |
| Change from baseline | −0.001 ± 0.005 | −0.001 ± 0.005 | 0.98 |
Data are presented as arithmetic means ± SEMs. GTE, green tea extract; MGTT, Minnesota Green Tea Trial.
Comparison of obesity-associated hormones and glucose homeostasis factors.
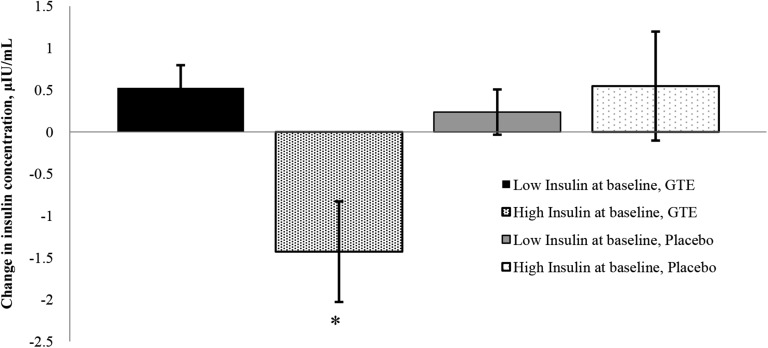
No differences were observed in measurements of leptin, ghrelin, adiponectin, insulin, glucose, or HOMA-IR between treatment groups at baseline or month 12 (Table 3). There were also no significant differences in change from baseline to month 12 for any of the hormones or glucose homeostasis variables within treatment groups. Changes in obesity-associated hormones, glucose, and HOMA-IR after 12 mo were not associated with treatment, change in BMI, or baseline values. An interaction was observed between treatment groups and insulin concentration at baseline in GTE participants only (Figure 2). When analyzed categorically at month 12, participants randomly assigned to the GTE group with a baseline fasting serum insulin ≥10 μIU/mL (n = 23) had a decrease in fasting serum insulin over time (−1.43 ± 0.59 μIU/mL), whereas those randomly assigned to the placebo group with a baseline fasting serum insulin ≥10 μIU/mL (n = 19) had an increase in insulin over the same 12-mo period (0.55 ± 0.64 μIU/mL). Similarly, participants with a baseline fasting insulin <10 μIU/mL in either study group (GTE, n = 97; placebo, n = 101) increased fasting insulin concentrations from baseline to month 12 [0.63 ± 0.30 μg/mL and 0.36 ± 0.29 μIU/mL in GTE and placebo, respectively; P < 0.01 for comparing GTE (high insulin) to all other categories)]. Compared with participants with insulin <10 μIU/mL, those with baseline insulin concentrations ≥10 μIU/mL had significantly higher body weight, BMI, fasting glucose concentrations, and HOMA-IR at baseline (Supplemental Table 1).
TABLE 3.
Concentrations of plasma obesity-associated hormones and serum insulin and glucose in overweight or obese postmenopausal MGTT participants given GTE or placebo for 12 mo1
| Variable | GTE (n = 117) | Placebo (n = 120) | P value |
| Insulin, μIU/mL | |||
| Baseline | 6.65 (6.05, 7.31) | 6.24 (5.68, 6.85) | 0.33 |
| Month 12 | 6.96 (6.33, 7.65) | 6.63 (6.03, 7.28) | 0.46 |
| Change from baseline | 0.31 (−0.17, 0.78) | 0.29 (−0.18, 0.77) | 0.97 |
| Glucose, mg/dL | |||
| Baseline | 97.6 (95.3, 100.0) | 97.3 (95.0, 99.7) | 0.84 |
| Month 12 | 95.3 (93.0, 97.6) | 94.7 (92.5, 97.0) | 0.74 |
| Change from baseline | −1.60 (−3.2, 0.007) | −1.97 (−3.58, −0.36) | 0.74 |
| HOMA-IR | |||
| Baseline | 1.60 (1.45, 1.78) | 1.50 (1.35, 1.66) | 0.35 |
| Month 12 | 1.64 (1.48, 1.82) | 1.55 (1.40, 1.72) | 0.45 |
| Change from baseline | 0.07 (−0.06, 0.19) | 0.05 (−0.08, 0.18) | 0.85 |
| Adiponectin, μg/mL | |||
| Baseline | 6.37 (5.68, 7.15) | 6.80 (6.06, 7.63) | 0.42 |
| Month 12 | 6.62 (5.90, 7.43) | 6.62 (5.90, 7.44) | 0.99 |
| Change from baseline | 0.72 (0.14, 1.29) | 0.11 (−0.48, 0.69) | 0.14 |
| Ghrelin, pg/mL | |||
| Baseline | 1130 (1020, 1250) | 1100 (991, 1210) | 0.65 |
| Month 12 | 1130 (1020, 1250) | 1150 (1040, 1270) | 0.82 |
| Change from baseline | 13.4 (−50.9, 77.6) | 57.9 (−6.98, 122.7) | 0.32 |
| Leptin, μg/L | |||
| Baseline | 31.6 (28.2, 35.5) | 29.9 (26.6, 33.6) | 0.49 |
| Month 12 | 30.7 (27.4, 34.5) | 30.0 (26.4, 33.2) | 0.64 |
| Change from baseline | 0.65 (−1.79, 3.10) | −1.14 (−3.63, 1.34) | 0.30 |
Baseline and month 12 data are presented as geometric means (95% CIs). Changes from baseline are presented as arithmetic means (95% CIs), adjusted for changes in BMI, baseline value, and COMT genotype. COMT, catechol-O-methyltransferase; GTE, green tea extract; MGTT, Minnesota Green Tea Trial.
FIGURE 2.
Effect of GTE on mean changes in fasting serum insulin in overweight or obese postmenopausal MGTT participants given GTE or placebo for 12 mo by baseline insulin concentration. High insulin at baseline defined as ≥10.0 μIU/mL; low insulin defined as <10.0 μIU/mL. Values are mean changes ± SEMs, n = 97 (low insulin, GTE), 101 (low insulin, placebo), 23 (high insulin, GTE), or 19 (high insulin, placebo). *Different from all other groups, P < 0.01. For comparisons between all other groups, P > 0.2. GTE, green tea extract; MGTT, Minnesota Green Tea Trial.
No treatment by genotype interactions was observed. To examine the effect of COMT genotype independent of GTE supplementation, an exploratory analysis was conducted in which participants were stratified into 3 groups based on their COMT genotype (high-, intermediate-, or low-activity). When analyzed by the COMT genotype irrespective of treatment group, participants with the homozygous high-activity (G/G) form of the enzyme had lower adiponectin concentrations at month 12 compared with participants with the low (A/A) genotype (5.99 ± 0.50 μg/mL compared with 7.66 ± 0.53 μg/mL; P = 0.03) (Table 4). Adiponectin concentrations at month 12 were significantly lower in those with the intermediate (G/A) genotype compared with the A/A group (7.66 ± 0.53 μg/mL compared with 6.33 ± 0.37 μg/mL; P = 0.05). Mean insulin concentrations were significantly higher in participants with the high-activity COMT genotype compared with those with the low-activity genotype in the all-randomized model (7.55 ± 0.53 μIU/mL compared with 6.33 ± 0.36 μIU/mL; P = 0.02); this comparison was only marginally significant in the per-protocol analysis (GTE, n = 107; placebo, n = 110) (7.56 ± 0.53 μIU/mL compared with 6.32 ± 0.38 μIU/mL; P = 0.06). HOMA-IR was significantly higher at month 12 in those with the G/G genotype compared with the G/A genotype (1.82 ± 0.14 μg/mL compared with 1.52 ± 0.08 μg/mL; P = 0.05) and the A/A genotype (1.80 ± 0.14 μg/mL compared with 1.48 ± 0.09 μg/mL; P = 0.02). Mean baseline glucose concentrations were significantly higher in participants with the high-activity COMT genotype compared with those with the low-activity COMT genotype (P = 0.03), but this was no longer significant at month 12. No significant differences were observed at month 12 for fasting leptin, ghrelin, or glucose concentrations among COMT genotypes.
TABLE 4.
Concentrations of plasma obesity-associated hormones and serum insulin and glucose in overweight or obese postmenopausal MGTT participants by COMT genotype1
| Variable | High (G/G) (n = 52) | Intermediate (G/A) (n = 110) | Low (A/A) (n = 75) |
| Insulin, μIU/mL | |||
| Baseline | 6.50 (5.76, 7.49) | 6.38 (5.81, 7.02) | 6.33 (5.62, 7.24) |
| Month 12 | 7.55a (6.58, 8.68) | 6.56a,b (5.97, 8.68) | 6.33b (5.62, 7.12) |
| Change from baseline | 0.85 (0.14, 1.56) | 0.14 (−0.34, 0.62) | −0.09 (−0.67, 0.5) |
| Glucose, mg/dL | |||
| Baseline | 100.1a (96.6, 103.7) | 95.1a,b (92.8, 97.4) | 94.4b (91.6, 100.3) |
| Month 12 | 96.5 (93.1, 100.0) | 94.1 (91.9, 100.0) | 94.4 (91.6, 97.3) |
| Change from baseline | −2.17 (−4.57, 0.22) | −1.77 (−3.4, −0.13) | −1.42 (−3.42, 0.57) |
| HOMA-IR | |||
| Baseline | 1.61 (1.38, 1.87) | 1.50 (1.35, 1.65) | 1.48 (1.30, 1.76) |
| Month 12 | 1.80a (1.54, 2.09) | 1.52b (1.38, 2.09) | 1.48b (1.30, 1.68) |
| Change from baseline | 0.15 (−0.04, 0.33) | 0.02 (−0.10, 0.15) | 0.009 (−0.15, 0.16) |
| Adiponectin, μg/mL | |||
| Baseline | 6.70 (5.66, 7.94) | 6.16 (5.49, 6.92) | 7.66 (6.63, 7.97) |
| Month 12 | 5.99b (5.05, 7.09) | 6.33b (5.64, 7.09) | 7.66a (6.60, 8.85) |
| Change from baseline | −0.41 (−1.27, 0.45) | 0.51 (−0.08, 1.1) | 1.14 (0.42, 1.85) |
| Ghrelin, pg/mL | |||
| Baseline | 1060 (913, 1223) | 1100 (979, 1200) | 1260 (1110, 1370) |
| Month 12 | 1090 (936, 1260) | 1080 (972, 1260) | 1260 (1110, 1430) |
| Change from baseline | 11.6 (−83.4, 106.7) | 22.5 (−43.5, 88.6) | 72.6 (−6.67, 151.9) |
| Leptin, μg/L | |||
| Baseline | 31.6 (28.2, 30.7) | 29.9 (26.6, 33.6) | 31.6 (28.2, 35.5) |
| Month 12 | 30.7 (27.4, 34.5) | 29.6 (27.1, 33.2) | 30.7 (27.4, 34.5) |
| Change from baseline | 0.07 (−3.55, 3.70) | −0.94 (3.44, 1.56) | 0.13 (−2.91, 3.18) |
Data are presented as unadjusted geometric means (95% CIs). Labeled means in a row without a common letter differ, P < 0.05. COMT, catechol-O-methyltransferase; MGTT, Minnesota Green Tea Trial.
Tolerability/AEs.
AEs in MGTT participants have been previously reported (23). In this subset of participants, no difference was seen in the numbers of participants who experienced any AE (22 compared with 25 in GTE and placebo, respectively; P = 0.70) or in the distribution of AE grade (severity) between treatment groups (P = 0.74; data not shown).
Discussion
Our results indicate that oral supplementation of decaffeinated GTE containing 1315 mg of total catechins (843 mg as EGCG) for 1 y does not alter total energy intake, anthropometric variables, or obesity-associated hormones in overweight and obese, free-living, postmenopausal women, although GTE was shown to reduce fasting insulin concentrations in participants with higher insulin at baseline. Women with the high-activity form of the COMT enzyme showed reductions in fasting plasma adiponectin and increased insulin over 12 mo compared with participants with an intermediate- or low-activity form of the enzyme regardless of treatment group.
Green tea and GTE have recently gained popularity as dietary aids for weight reduction. Several randomized trials have examined the association between GTE and body weight, and the results have been largely inconclusive—likely as a result of differences in study design, short durations of intervention, variable study populations, and differences in green tea preparations and caffeine content. The GTE used in this study contained a high amount of catechins and <16 mg of caffeine per day to test the independent effects of green tea catechins. Results indicated that decaffeinated GTE did not significantly reduce energy intake, body weight, WC, or WHR compared with placebo. These results are comparable to those of a recent Cochrane review of the effects of green tea on weight loss in overweight and obese adults (24) that concluded that green tea was associated with small, nonsignificant decreases in body weight. Because a weight loss of 5–10% of body weight is considered to be beneficial for reducing several disease risk factors associated with overweight and obesity (25), small losses resulting from green tea preparations are not likely to be clinically meaningful.
There was a significant association between high baseline insulin concentrations and reduced insulin in GTE participants, suggesting that GTE is most effective at lowering insulin in those with baseline fasting insulin ≥10 μIU/L. GTE supplementation has been associated with reductions in fasting insulin in several randomized trials (26–28), although to our knowledge this study is the largest, with the longest duration of intervention that has demonstrated an association specifically in overweight or obese postmenopausal women. Animal studies have suggested that green tea catechins may prevent hyperglycemia by enhancing insulin activity (29, 30), and epidemiologic studies have noted a lower incidence of type 2 diabetes in individuals who have consumed green tea for a long time and in women who consume >3 cups (720 mL) of tea per day (31, 32). Given that women with baseline insulin >10 μIU/mL tended to have higher fasting glucose concentrations, increased body weight, and increased baseline BMI compared with those with baseline insulin <10 μIU/mL, they may be at risk for developing metabolic syndrome. The proportion of participants in this study with fasting insulin >10 μIU/mL was small [n = 23 (19.7%) and n = 19 (15.8%) in GTE and placebo, respectively)]; therefore, further research in participants with insulin resistance or those with obesity-associated metabolic abnormalities is needed to confirm this effect.
Immunohistochemical staining has indicated that the COMT enzyme is present in the β cells of the pancreas (33), therefore demonstrating the potential for crosstalk between its enzymatic activity and glucose homeostasis. In an animal model of diabetes, COMT activity was markedly decreased in the liver, and correcting hyperglycemia restored enzyme activity (34). Adiponectin has known insulin-sensitizing effects, and circulating concentrations of this hormone have been found to predict the development of type 2 diabetes (35). Circulating adiponectin and adiponectin gene expression in adipose tissue are reduced in patients with type 2 diabetes and in obese populations (36–38). Aside from this study, there is, to our knowledge, a lack of research—in both epidemiologic and randomized controlled trials—regarding the role of the COMT genotype in insulin sensitivity in women. No treatment by genotype interactions were observed. Fasting insulin increased and fasting plasma adiponectin decreased in women who were homozygous for the high-activity (G) allele of the COMT enzyme compared with women with the intermediate- (G/A) and low-activity (A/A) forms of the enzyme, a direction consistent with insulin resistance development and an increased risk for type 2 diabetes (39, 40). These results are similar to the findings of Kring et al. (41), who noted that the frequency of the COMT G/G genotype was 11.6% higher in insulin-resistant and type 2 diabetics compared with that of the COMT A/A and G/A genotypes. To our knowledge, this is the first study that has correlated the COMT genotype with a change in insulin and adiponectin concentrations over time. Because this adipose-derived hormone has notable insulin-sensitizing actions (42, 43), it is possible that the observed relation of the COMT genotype with insulin sensitivity could be mediated through the enzyme’s interaction with adiponectin. However, because this analysis was done irrespective of the intervention, it represents a cross-sectional reporting of differences between COMT genotypes. It is unclear whether the changes we observed from baseline to month 12 were already on this trajectory before the study and whether they would continue in the same direction after the intervention concluded.
The MGTT is to our knowledge the first long-term randomized trial to evaluate the effects of green tea catechins, independent of caffeine, on obesity-associated hormones, glucose homeostasis markers, and anthropometric variables. In addition to having the longest study duration to date (12 mo), this intervention provided the highest catechin dose and randomized the largest number of subjects compared with previous trials. Because study participants were in free-living conditions and encouraged to maintain typical dietary and physical activity habits, the results likely represent translatable effects for a wider population of postmenopausal white women. Given that this group is one of the largest users of dietary supplements (44), this research is of particular importance. This study is also to our knowledge one of the first to suggest a relation between GTE supplementation and possible insulin-reductive effects for those with higher fasting insulin concentrations and of different COMT genotypes.
Several limitations of this study should be addressed. Aside from being categorized as overweight or obese, participants were generally healthy white women free of diagnosed diabetes or other metabolic diseases, and average circulating hormones and glucose concentrations were within normal ranges. Therefore, the extrapolation of results to diabetic populations or to those with metabolic syndrome cannot be confirmed, although several studies in diabetic populations have noted similar results (45, 46). It was beyond the scope of this study to compare blood concentrations of green tea catechins and their metabolites between COMT genotype groups. These analyses are currently being conducted in a subset of ∼13% of the MGTT study population, and the results are forthcoming.
In conclusion, these results show that decaffeinated GTE is not associated with reductions in WC or WHR and did not alter energy intake or obesity-associated hormone concentrations. GTE may cause small changes in body weight and BMI that are unlikely to be of clinical importance. However, these data suggest that GTE supplementation may be particularly beneficial for individuals with elevated insulin concentrations and for those who possess the high-activity COMT genotype. Additional research is needed to confirm these results and to identify the feasibility of incorporating GTE supplementation into treatment and prevention strategies for breast cancer and obesity-related metabolic disorders.
Acknowledgments
We thank Sarah Bedell, Jane Mobeck-Wilson, Kate Ringsak, Amy Brehm, and Ed Smith for their help in carrying out the study coordination, clinical data collection, and laboratory analysis aspects of our study. AMD, HS, NRS-H, and MSK contributed to the conception, design, and implementation of the project; AMD and AYA contributed to data collection and analytical procedures; LE and AYA conducted the statistical analysis; AMD, LE, AYA, and MSK interpreted data; and AMD and MSK wrote the manuscript and had primary responsibility for final content. All authors have read and approved the final version of the manuscript.
Footnotes
Abbreviations used: AE, adverse event; COMT, catechol-O-methyltransferase; EGCG, (−)-epigallocatechin-3-gallate; GTE, green tea extract; MET, metabolic equivalent; MGTT, Minnesota Green Tea Trial; WC, waist circumference; WHR, waist-to-hip ratio.
References
- 1.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev 2007;8:395–408. [DOI] [PubMed] [Google Scholar]
- 2.Shrubsole MJ, Lu W, Chen Z, Shu XO, Zheng Y, Dai Q, Cai Q, Gu K, Ruan ZX, Gao YT, et al. Drinking green tea modestly reduces breast cancer risk. J Nutr 2009;139:310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res 2003;63:7526–9. [PubMed] [Google Scholar]
- 4.Zhang M, Holman CD, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis 2007;28:1074–8. [DOI] [PubMed] [Google Scholar]
- 5.Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A, Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta-analysis. Am J Clin Nutr 2010;91:73–81. [DOI] [PubMed] [Google Scholar]
- 6.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000;141:980–7. [DOI] [PubMed] [Google Scholar]
- 7.Zamora-Ros R, Forouhi NG, Sharp SJ, Gonzalez CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr 2014;144:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr 2010;29:31–40. [DOI] [PubMed] [Google Scholar]
- 9.Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res 2011;64:87–99. [DOI] [PubMed] [Google Scholar]
- 10.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res 2006;50:176–87. [DOI] [PubMed] [Google Scholar]
- 11.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res 2001;61:6716–22. [PubMed] [Google Scholar]
- 12.Syvänen AC, Tilgmann C, Rinne J, Ulmanen I. Genetic polymorphism of catechol-O-methyltransferase (COMT): correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and parkinsonian patients in Finland. Pharmacogenetics 1997;7:65–71. [DOI] [PubMed] [Google Scholar]
- 13.Hursel R, Westerterp-Plantenga MS. Catechin- and caffeine-rich teas for control of body weight in humans. Am J Clin Nutr 2013; 98(6, Suppl)1682S–93S. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson AB, Randell RK, Jeukendrup AE. The effect of green tea extract on fat oxidation at rest and during exercise: evidence of efficacy and proposed mechanisms. Adv Nutr 2013;4:129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem 2011;22:1–7. [DOI] [PubMed] [Google Scholar]
- 16.Samavat H, Dostal AM, Wang R, Bedell S, Emory TH, Ursin G, Torkelson CJ, Gross MD, Le CT, Yu MC, et al. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: study rationale, design, methods, and participant characteristics. Cancer Causes Control 2015;26:1405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhagwat S, Haytowitz DB, Holden JM. USDA database for the flavonoid content of selected foods: release 3.1. Beltsville (MD): USDA; 2014. [Google Scholar]
- 18.NIH. Diet history questionnaire, version 1.0. Bethesda (MD): Applied Research Program, National Cancer Institute; 2007. [Google Scholar]
- 19.USDA, Human Nutrition Research Center.Continuing survey of food intakes by individuals 1994-96, 1998; 2000.
- 20.Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, Marles RJ, Pellicore LS, Giancaspro GI, Low Dog T. Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf 2008;31:469–84. [DOI] [PubMed] [Google Scholar]
- 21.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol 2009;65:331–41. [DOI] [PubMed] [Google Scholar]
- 22.NIH. Common terminology criteria for adverse events v4.0. Bethesda (MD): National Cancer Institute; 2009. [Google Scholar]
- 23.Dostal AM, Samavat H, Bedell S, Torkelson C, Wang R, Swenson K, Le C, Wu AH, Ursin G, Yuan JM, et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem Toxicol 2015;83:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurgens TM, Whelan AM, Killian L, Doucette S, Kirk S, Foy E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst Rev 2012;12:CD008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res (Phila) 2012;5:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res 2012;32:421–7. [DOI] [PubMed] [Google Scholar]
- 28.Narotzki B, Reznick AZ, Navot-Mintzer D, Dagan B, Levy Y. Green tea and vitamin E enhance exercise-induced benefits in body composition, glucose homeostasis, and antioxidant status in elderly men and women. J Am Coll Nutr 2013;32:31–40. [DOI] [PubMed] [Google Scholar]
- 29.Ortsater H, Grankvist N, Wolfram S, Kuehn N, Sjoholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr Metab (Lond) 2012;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson RA, Polansky MM. Tea enhances insulin activity. J Agric Food Chem 2002;50:7182–6. [DOI] [PubMed] [Google Scholar]
- 31.Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med 2006;144:554–62. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Mao QX, Xu HX, Ma X, Zeng CY. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open 2014;4:e005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karhunen T, Tilgmann C, Ulmanen I, Julkunen I, Panula P. Distribution of catechol-O-methyltransferase enzyme in rat tissues. J Histochem Cytochem 1994;42:1079–90. [DOI] [PubMed] [Google Scholar]
- 34.Wang JP, Liu IM, Tzeng TF, Cheng JT. Decrease in catechol-O-methyltransferase activity in the liver of streptozotocin-induced diabetic rats. Clin Exp Pharmacol Physiol 2002;29:419–22. [DOI] [PubMed] [Google Scholar]
- 35.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord 2008;6:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y, Funahashi T. Adiponectin as a biomarker of the metabolic syndrome. Circ J 2004;68:975–81. [DOI] [PubMed] [Google Scholar]
- 37.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003;361:226–8. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Retnakaran R, Hanley A, Tungtrongchitr R, Shaw C, Sweeney G. Total and high molecular weight but not trimeric or hexameric forms of adiponectin correlate with markers of the metabolic syndrome and liver injury in Thai subjects. J Clin Endocrinol Metab 2007;92:4313–8. [DOI] [PubMed] [Google Scholar]
- 39.Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens) 2012;11:8–20. [DOI] [PubMed] [Google Scholar]
- 40.Mather KJ, Goldberg RB. Clinical use of adiponectin as a marker of metabolic dysregulation. Best Pract Res Clin Endocrinol Metab 2014;28:107–17. [DOI] [PubMed] [Google Scholar]
- 41.Kring SI, Werge T, Holst C, Toubro S, Astrup A, Hansen T, Pedersen O, Sorensen TI. Polymorphisms of serotonin receptor 2A and 2C genes and COMT in relation to obesity and type 2 diabetes. PLoS One 2009;4:e6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wannamethee SG, Tchernova J, Whincup P, Lowe GD, Rumley A, Brown K, Cherry L, Sattar N. Associations of adiponectin with metabolic and vascular risk parameters in the British Regional Heart Study reveal stronger links to insulin resistance-related than to coronory heart disease risk-related parameters. Int J Obes (Lond) 2007;31:1089–98. [DOI] [PubMed] [Google Scholar]
- 43.Gao H, Fall T, van Dam RM, Flyvbjerg A, Zethelius B, Ingelsson E, Hagg S. Evidence of a causal relationship between adiponectin levels and insulin sensitivity: a Mendelian randomization study. Diabetes 2013;62:1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States. Natl Health Stat Report 2007;12:1–23. [PubMed] [Google Scholar]
- 45.Liu K, Zhou R, Wang B, Chen K, Shi LY, Zhu JD, Mi MT. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr 2013;98:340–8. [DOI] [PubMed] [Google Scholar]
- 46.Hsu CH, Liao YL, Lin SC, Tsai TH, Huang CJ, Chou P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern Med Rev 2011;16:157–63. [PubMed] [Google Scholar]