Abstract
While global amphibian declines are associated with the spread of Batrachochytrium dendrobatidis (Bd), undetected concurrent co-infection by other pathogens may be little recognized threats to amphibians. Emerging viruses in the genus Ranavirus (Rv) also cause die-offs of amphibians and other ectotherms, but the extent of their distribution globally, or how co-infections with Bd impact amphibians are poorly understood. We provide the first report of Bd and Rv co-infection in South America, and the first report of Rv infections in the amphibian biodiversity hotspot of the Peruvian Andes, where Bd is associated with extinctions. Using these data, we tested the hypothesis that Bd or Rv parasites facilitate co-infection, as assessed by parasite abundance or infection intensity within individual adult frogs. Co-infection occurred in 30% of stream-dwelling frogs; 65% were infected by Bd and 40% by Rv. Among terrestrial, direct-developing Pristimantis frogs 40% were infected by Bd, 35% by Rv, and 20% co-infected. In Telmatobius frogs harvested for the live-trade 49% were co-infected, 92% were infected by Bd, and 53% by Rv. Median Bd and Rv loads were similar in both wild (Bd = 101.2 Ze, Rv = 102.3 viral copies) and harvested frogs (Bd = 103.1 Ze, Rv = 102.7 viral copies). While neither parasite abundance nor infection intensity were associated with co-infection patterns in adults, these data did not include the most susceptible larval and metamorphic life stages. These findings suggest Rv distribution is global and that co-infection among these parasites may be common. These results raise conservation concerns, but greater testing is necessary to determine if parasite interactions increase amphibian vulnerability to secondary infections across differing life stages, and constitute a previously undetected threat to declining populations. Greater surveillance of parasite interactions may increase our capacity to contain and mitigate the impacts of these and other wildlife diseases.
Introduction
Emerging infectious diseases are threatening biodiversity [1]. In particular, the recent emergence of the chytrid fungus Batrachochytrium dendrobatidis (Bd) is linked to extirpations and even extinctions of amphibians globally; especially in Central America [2, 3], the tropical Andes [4, 5], the western US [6] and Australia [7]. In addition, emerging viral pathogens in the genus Ranavirus (Rv) have caused massive die-offs of amphibians and other ectothermic vertebrates in North America and Europe [8–10], with fewer reports in other regions [11–14]. More recently, Bd and Rv have been reported to co-occur in varied habitats [15, 16], and to co-infect individual hosts [13]. Beyond these reports, however, little is known about the prevalence of ranaviruses in other regions of high amphibian biodiversity, the extent to which Bd and Rv co-infect amphibians, modes of disease spread, or the threat that any such co-infections present for amphibians.
The tropical Andes in South America are among the most species rich regions for amphibians on Earth. Chytridiomycosis has been associated with population declines of frogs throughout the Andes [4, 17], and Bd has been affecting frogs in Peru since at least 1999 [5]. Bd is widely distributed across elevation gradients and ecosystems in the Andes despite variation in precipitation and thermal regimes that can influence the growth of Bd.
The live-trade of comestible amphibians in the Andes of Peru and Bolivia may be a critical vector for the spread of Bd. In particular, we recently found that threatened frogs Telmatobius marmoratus that are harvested for human consumption [18–20] were heavily infected by Bd [21]. Because these wild-caught animals are kept in high-density conditions that increase Bd transmission, and they are transported across regional markets, the live-trade is a probable route for disease spillover and spread to wild amphibian populations. If Rv exhibits similar prevalence patterns to Bd or if it is similarly associated with the live-trade in Peru is unknown. However, disease spillover from confined market to wild amphibian populations is a probable route for the invasion of ranaviruses in the United States [22].
Ranavirus outbreaks are thought to occur globally and can be highly virulent, resulting in mass-mortality events that affect multiple species of ectothermic vertebrates [23–25]. In addition, ranaviruses are not host-specific, so a single strain can infect fish, reptiles and amphibians [23, 26–29]. Therefore, Rv can threaten entire wetland, stream and riparian communities, as was reported for a recent outbreak in Spain [10]. Rv disease outbreaks are likely influenced by variation in susceptibility among species [30] and life stages [31, 32]. Environmental stressors like altered habitats, pollution and climate change are also thought to contribute to Rv outbreaks [9, 33]. In addition, another but little explored factor that may also contribute to the emergence and spread of diseases such as Rv is prior or concurrent infection by other parasites like Bd.
Interactions among one or more co-infecting parasites can be antagonistic or facilitative, by which they may influence disease related morbidity and mortality in their hosts [34]. Antagonistic interactions may occur through resource competition or induction of cross-effective immune responses within the host [35, 36]. Facilitative interactions in contrast, may increase infection and disease in a host via immunosuppression and resource depletion, thereby increasing infectious spread of one or both parasites [34–37]. Indeed, co-infection by multiple parasites is likely common for most wild animals [38–40] and has been associated with increased susceptibility to subsequent infections and increases in disease-related mortality rates in mammals [35, 37, 41]. There are good reasons to suspect that both Rv and Bd could also facilitate co-infections among one another, as well as other parasites in amphibians [13, 15]. Amphibian immunity to Rv and Bd include both innate and adaptive effectors. For example, while T-cell and antibody mediated immunity are critical to fighting Rv infection [42, 43], Bd can suppress these immune factors because it inhibits lymphocyte production and induces apoptosis in these adaptive immune cells [44]. Conversely, innate immunity that includes phagocytic cells, lysozymes, and antimicrobial peptides are central to amphibian immunity against chytridiomycosis. However, Rv evades the amphibian immune system by targeting and infecting macrophages in addition to system wide disruption of amphibian tissues [45]. These direct impacts on immune function suggest that both Bd and Rv could facilitate co-infection via immunosuppression, as well as through general host tissue disruption and resource depletion. To shed light on these little explored dynamics, we examined patterns of co-infection between Bd and Rv in wild frogs and in frogs harvested for the live trade in Peru. Using these data, we tested the hypothesis that Bd or Rv parasite abundance within individuals contributes to the likelihood of co-infection among adult frogs. We also tested for the potential of co-infection to increase the infection intensity of both parasites, which could suggest facilitation.
Methods
Animal sampling
Our sampling design encompassed two distinct study systems: wild-harvested live frogs from the San Pedro city market in Cusco [21], and wild frogs in montane forests of the eastern slopes of the Andes at the Kosñipata Valley near Manu National Park [17]. The frogs used in this study were previously obtained as part of broader efforts to document Bd susceptibility patterns in wild and captive trade amphibians; this work thus maximizes the use of these specimens. All wild and captive frog collections and sampling were conducted under the approval of the Southern Illinois University Animal Care and Use Committee (IACUC) and the Peruvian Ministry of Agriculture (permit #292-2014-MINAGRI-DGFFS/DGEFFS). All frogs obtained from the city market were individuals of Telmatobius marmoratus (n = 87), a Vulnerable species according to the IUCN Red List [46]. These T. marmoratus frogs were obtained alive from 22 June 2012 to 23 July 2013, and then euthanized by a 20% benzocaine overdose following guidelines of the Herpetological Animal Care and Use Committee [47]. Although the exact origin of these frogs is unknown, it is likely to be within the region of Cusco, because the species is common throughout this region and inhabits streams surrounding the city. The species naturally occurs in creeks, streams, ponds and wetlands, predominantly in high-elevation grasslands and other open areas.
The second study region is located on the eastern slopes of the Cordillera de Paucartambo, Cusco in the drainage basin of the Río Kosñipata, southern Peru [17]. The study sites are located in the Kosñipata valley and range from the submontane forest in the Amazonian foothill of the Andes at 945 m to the cloud forest at 2410 m [17]. We hand-captured 94 frogs from 12 June to 31 July 2013 during nocturnal surveys conducted along the Paucartambo-Shintuya road (S1 Fig). The road lies at the southern border of Manu National Park and of its buffer zone. Manu NP covers 17,163 km2 of Amazonian lowland, montane and high-elevation Andean habitats between 300 m and 4020 m elevation, and is the protected area harboring the largest number of amphibians on Earth [48]. All frogs were first swabbed for Bd following standard methods (see below) and then euthanized by a 20% benzocaine overdose following guidelines of the Herpetological Animal Care and Use Committee [47] for tissue collection needed for Rv analysis (see below).
Bd qPCR
We collected skin swabs [49] by stroking a dry synthetic cotton swab across the skin of each frog; a standard technique used in previous surveys [17]. The swabbing protocol included 5 strokes on each side of the abdominal midline, 5 strokes on the inner thighs of each hind leg, and 5 strokes on the foot webbing of each hind leg for a total of 30 strokes/frog. We extracted DNA from swabs by using Prepman Ultra® (Life Technologies), and analyzed extracts with a real-time PCR (qPCR) assay on a StepOnePlus™ Real-Time PCR System (Life Technologies) to quantify the amount of genomic material [50].The assay uses genetic markers specific for Bd (primers of ITS gene); and compares each sample to a set of standards (four serial dilutions at concentrations from100 to 0.1 zoospore genomic equivalents, each in triplicate) to calculate a genomic equivalent. Each plate also includes four negative controls. To calculate Bd infection intensity, we multiplied the qPCR score by 80 to account for subsampling and dilution that occurred during the DNA extraction, resulting in a zoospore equivalent (Ze) estimate for each frog (Briggs et al., 2010; Vredenburg et al., 2010).
Ranavirus qPCR
qPCR was used to detect and quantify the presence of ranavirus DNA in the livers of frogs [32, 51]. DNA was extracted and purified from the livers, stored in ethanol, by DNeasy® Blood and Tissue mini-spin extraction kits (Qiagen Inc.), following the manufacturers protocol. To standardize DNA used in qPCR analysis, total purified DNA was quantified with a Take3™ Microvolume Plate on an Epoch Spectrophotometer (BioTek Instruments INC) and diluted to 20 ng DNA · μL-1. Samples were assayed on a StepOnePlus in duplicate using TaqMan primers and probes that amplify a 70-bp region within the ranavirus major capsid protein (MCP) sequence. Reactions included 20 ng DNA · μL-1 in 20 μL reactions with TaqMan Universal PCR Master mix (Life Technologies), 300 nmol forward rtMCP primer (5-ACACCACCGCCCAAAA GTAC-3), 900 nmol reverse rtMCP primer (5-CCGTTCATGATGCGGATAATG-3), and 250 nmol of rtMCP- probe (5-FAM-CCTCATCGTTCTGGCCATCAACCAC-TAMRA-3). gBlocks® gene fragments (Integrated DNA Technologies, Inc.) specific to the ranavirus major capsid protein (MCP) sequence [52] were included in each 96-well qPCR plate as standards in log10 increments (100 to 106 copies) for quantification of viral concentrations in our samples.
Statistical analyses
To assess Bd prevalence, swabs were categorized as Bd-positive when Ze>0 and as Bd-negative when Ze = 0. For Rv prevalence, samples were considered Rv-positive with qPCR cycles of C(t) < 34; a standard known to exclude false-positives. Sample sizes varied across analyses because some frogs were successfully assayed for only one of either parasite (sample sizes reported for each analysis). We calculated infection prevalence for Bd and Rv by dividing the number of infected frogs by the total number of assayed frogs. We used the R package binom to compute binomial 95% credible intervals intervals using Bayesian inference using Jeffrey’s non-informative priors. To test if parasite abundance within individuals (zeros included; [53]) is associated with the probability of co-infection, we used logistic regression with parasite abundance as a predictor and either Bd or Rv infection status (i.e. infected or not) as a response variable. We then tested for facilitation among these parasites by correlation analysis of Bd and Rv infection intensity (zeros excluded; [53]). Note that we parsed the samples into three distinct populations for these analyses. First, we analyzed the captive T. marmoratus separately from wild frogs because this population, held in high densities, was unique in their high parasite exposure and co-infection status (χ2 = 20.5, P < 0.001). Second, we compared all wild frogs using logistic regression with frog taxa and parasite abundance as predictors of the probability of co-infection. Last, we tested for elevation effects on parasite prevalence, intensity, and abundance by focusing only on the genus Pristimantis; the most widely distributed taxa for which we had sufficient sample size. Here we used logistic regression with elevation class and parasite abundance as predictors of the probability of co-infection.
Results
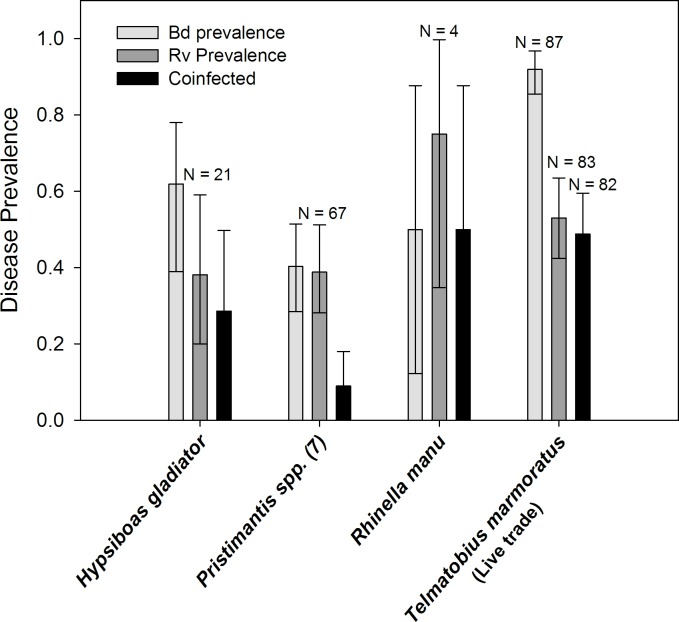
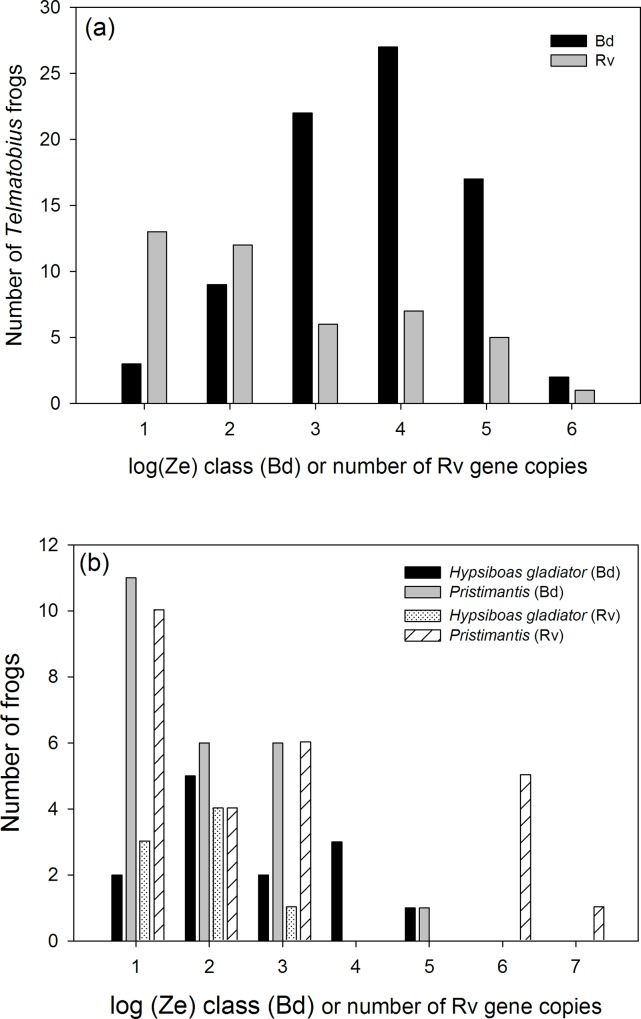
Telmatobius marmoratus sampled from the live trade in Cusco were heavily infected by Bd (Table 1; prevalence = 91.9%, Credible Interval = 85.5–96.8%, n = 87), Rv (prevalence = 53.0%, CI = 42.4–63.5%, n = 83), and co-infected (Fig 1; prevalence = 48.8%, CI = 38.1–59.5%, n = 82). Bd infection intensity ranged from 101 to 106 zoospore equivalents (median = 103.1 Ze, n = 80). 58% of individuals had Bd infection intensity values above 1,000 zoospore equivalents and 24% were above the 10,000 zoospore threshold that is often associated with a high likelihood of mortality [6, 54] (Fig 2A). Rv infection intensity ranged from 101 to 106 virion gene copy equivalents (median = 102.7), and 43% of individuals had Rv viral loads above 103 viral copies (Fig 2A). There was no association between Bd and Rv infection intensity (excluding zeros for uninfected) among co-infected Telmatobius frogs (r = 0.22, P = 0.17, n = 39). The abundance of either parasite within individuals (including zeros for uninfected) was not associated with the probability of infection by the other (Bd:χ2 = 0.39, P = 0.53; Rv:χ2 = 2.0, P = 0.16).
Table 1. Batrachochytrium dendrobatidis and Ranavirus infection prevalence in ten species of frogs from Peru.
Total sampled is the number of frogs examined, but does not always represent the number of samples for each assay (see text for details). 95% Bayesian credible intervals using Jeffrey’s priors.
| Species | Source | Developmental Mode/ Habitat | Bd Infected | Prevalence & CI | Rv Infected | Prevalence & CI | Co-infected | Prevalence & CI | Total Sampled |
|---|---|---|---|---|---|---|---|---|---|
| Hypsiboas gladiator | Wild | Aquatic larvae/ Lotic | 13 | 61.9% (39.0–78.0%) | 8 | 38.0% (20–59%) | 6 | 28.6% (12.9–49.7%) | 21 |
| Pristimantis danae | Wild | Direct/ Terrestrial | 1 | – | 0 | – | 0 | – | 2 |
| Pristimantis fenestratus | Wild | Direct/ Terrestrial | 1 | – | 0 | – | 0 | – | 1 |
| Pristimantis lindae | Wild | Direct/ Terrestrial | 1 | 33.3% (1.0–77.1%) | 1 | 33.3% (1.0–77.1%) | 0 | 0% (0–44.4%) | 3 |
| Pristimantis pharangobates | Wild | Direct/ Terrestrial | 1 | 12.5% (0.1–39.7%) | 3 | 37.5% (10.4–68.6%) | 0 | 0% (0–20.7%) | 8 |
| Pristimantis platydactylus | Wild | Direct/ Terrestrial | 11 | 34.4% (19.3–51.0%) | 13 | 41.9% (25.6–59.0%) | 3 | 9.7% (1.8–21.6%) | 32 |
| Pristimantis reichlei | Wild | Direct/ Terrestrial | 4 | 66.7% (32.0–94.6%) | 0 | 0% (0–26.4%) | 0 | 0% (0–26.4%) | 6 |
| Pristimantis sp. | Wild | Direct/ Terrestrial | 1 | – | 0 | – | 0 | – | 1 |
| Pristimantis toftae | Wild | Direct/ Terrestrial | 7 | 46.7% (23.6–70.3%) | 8 | 53.3% (29.7–76.4%) | 3 | 20.0% (4.3–41.5%) | 15 |
| Rhinella manu | Wild | Presumed direct/ Terrestrial | 2 | 50% (12.2–87.7%) | 3 | 75% (34.7–99.7%) | 2 | 50% (12.2–87.7%) | 4 |
| Telmatobius marmoratus | Live Trade | Aquatic larvae/ Streams and wetlands | 80 | 91.9 (85.5–96.8%) (n = 87) | 44 | 53.0 (42.4–63.5%) (n = 83) | 40 | 48.8 (38.1–59.5%) (n = 82) | 88 |
Fig 1. Prevalence of infection by the emerging pathogens Batrachochtrytium dendrobatidis (Bd) and Ranavirus (Rv) in frogs sampled during 2012 (live trade only) and 2013 (wild and live trade frogs) in Peru.
While Telmatobius were sampled from live trade sources, the other species were wild caught. Error bars are 95% Bayesian credible intervals using Jeffreys prior.
Fig 2. Infection loads in captive Telmatobius for both Bd and Rv (a); and in wild stream breeding Hypsiboas gladiator and in 7 species of terrestrial, direct-developing Pristimantis frogs (b).
Among the wild frogs sampled along the eastern slopes of the Andes, there was no difference in infection prevalence between stream/riparian dwelling Hypsiboas and terrestrial, direct-developing Pristimantis frogs (Fig 1; Bd odds ratio = 0.46, 95% CI = 0.17–1.21, P = 0.14; Rv odds ratio = 1.45, 95% CI = 0.5–4.2, P = 0.61). Ranavirus infected three of the four Rhinella manu sampled, while two of these frogs were infected by Bd (Fig 1). In R. manu, median Bd infection intensity was low 0.5–0.7 Ze, while median Rv infection intensity was 104 viral copies (range = 101.6 to 105). Among the stream-breeding Hypsiboas gladiator (n = 21), 65% were infected by Bd, 40% by Rv, and 30% were co-infected; the difference in Bd and Rv prevalence was not significant (Table 1; odds ratio = 2.1, 95% CI = 0.3–15.35, P = 0.64). Bd infection intensity for H. gladiator ranged broadly from 0.5 to 105 zoospore equivalents (Fig 2B; median = 101.7 Ze, n = 13). Rv infection intensity ranged from 101 to 103.3 virion gene copy equivalents (Fig 2B; median = 102.1, n = 13). There was no association between Bd infection intensity and Rv loads among co-infected wild frogs (r = -0.35, P = 0.22, n = 14). The abundance of Bd within individuals was not associated with the probability of Rv infection (χ2 = 0.01, P = 0.91) or frog taxa (χ2 = 2.0, P = 0.36). Rv abundance within individuals was marginally associated with the probability of infection by Bd (χ2 = 3.8, P = 0.05; log odds estimate for uninfected/infected = 0.25 ± 0.14, P = 0.07) but not frog taxa (χ2 = 2.7, P = 0.25).
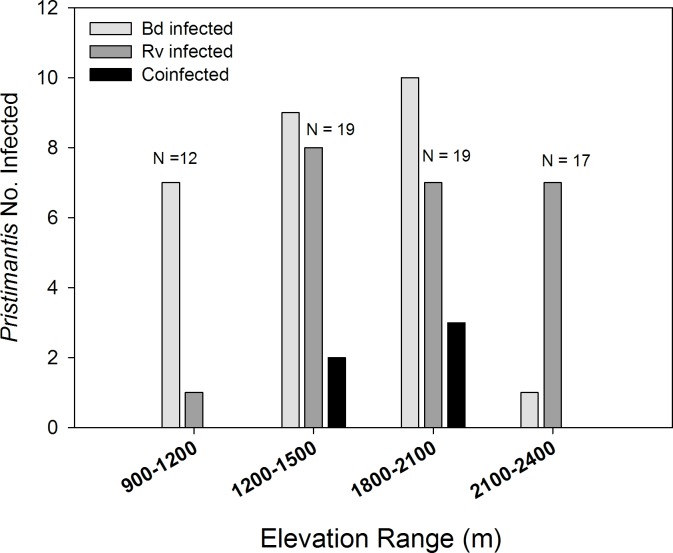
Bd and Rv infection prevalence among seven species of direct-developing Pristimantis also varied across an elevation gradient ranging from sub-montane forests at 900 meters to cloud forests at 2400 meters (Fig 3). Bd prevalence varied across this gradient (χ2 = 13.9, P = 0.003), driven by a sharp decline in the number of infections above 2100 meters. Rv infections were common across all elevations (χ2 = 5.4, P = 0.15), but lowest below 1200 meters. Co-infection was only present in animals in the cloud forest from 1200 to 2100 meters. Among these seven Pristimantis species 39.4% were infected by Bd (Table 1; CI = 28.5–51.4%, n = 68), 38.2% were infected by Rv (CI = 28.1–51.2%, n = 66), but only 9% were co-infected (CI = 4–18%, n = 6; Fig 1); Bd and Rv prevalence was significantly different among Pristimantis spp. (odds ratio = 0.26, 95% CI = 0.08–0.83, P = 0.02). Bd infection intensity for Pristimantis ranged from 0.1 to 104 zoospore equivalents (Fig 2B; median = 1 Ze, n = 68). Rv infection intensity ranged from 101 to 107.2 viral gene copy equivalents (Fig 2B; median = 102.6, n = 68). There was no association of Bd and Rv infection intensity among the few co-infected frogs. Rv abundance within individuals was marginally associated with the probability of infection by Bd (χ2 = 4.8, P = 0.03; log odds estimate for uninfected/infected = 0.45 ± 0.23, P = 0.05), as well as elevation (χ2 = 11.3, P = 0.01). These patterns were driven by the decline in Bd, but not Rv infections above 2100 meters (Fig 3; log odds estimate for uninfected/Bd infected = 2.1 ± 0.82, P = 0.01). The abundance of Bd within individuals was not associated with the probability of Rv infection (χ2 = 0.86, P = 0.35) or elevation (χ2 = 6.6, P = 0.09).
Fig 3. The number of Bd and Rv infections in wild frogs in the genus Pristimantis (7 species) varied across an elevation range on the eastern slopes of the Andes (N = total sample size for each elevation range).
Discussion
This is the first report of co-infection by Bd and Rv in South America, and the first report of ranavirus infections among amphibians in Peru. These findings raise several crucial conservation concerns for amphibians in the Andes, which is among the most species rich regions for amphibians on Earth [55]. While many factors are threatening amphibians both in Peru and globally, emerging diseases such as Bd induced chytridiomycosis are a primary factor contributing to extirpations and even extinctions among amphibians. However, the extent to which interactions among multiple emerging diseases may be contributing to population declines is unknown.
Several studies have reported co-ocurrence of Bd and Rv in a number of aquatic communities in North America [15, 16], and more recently Whitfield and Kerby [13] found co-infection in several species of frogs in Costa Rica. While Bd induced chytridiomycosis is undoubtedly a primary driver of recent extirpations and extinctions of amphibians, especially in Central America, the growing reports of Bd and Rv co-infection raise questions regarding the distribution and prevalence of Rv and these co-infections (in addition to other parasites like Ribeiroia macroparasites), and to what extent concurrent parasite infections contribute to epizootics [15]. We hypothesized that infection by either parasite could facilitate subsequent infection by the other via processes such as immunosuppression, host resource depletion, and/or tissue disruption. However, our analysis of correlations among Bd and Rv infection intensity, as well as the effects of parasite abundance on the likelihood of co-infection did not suggest such facilitation between Bd and Rv in adult frogs. Indeed, the abundance of Rv in wild frogs was associated with a marginally reduced probability of Bd infection, but this was driven by contrasting ecological patterns of Bd and Rv prevalence in Pristimantis frogs at their elevation extremes (Fig 3). We do not believe, however, that our results are a definitive test of the facilitation hypothesis because both parasites primarily infect larval amphibians and induce the greatest disease and mortality in metamorphic and juvenile frogs; our data is all for adult frogs [32]. Given this, we expect any facilitation to occur in larvae during initial exposure to these parasites and primary infections. Experimental infection trials in a factorial design that accounts for developmental stages are required to truly test for facilitation of co-infection.
Our results do suggest that infected adult frogs could serve as potential reservoirs for both Rv and Bd. What is more, adult frogs may be an important source for epizootic outbreaks among larval amphibian communities if during the reproductive season they shed these parasites into breeding ponds and streams [56]. Reproducing frogs may be induced to shed parasites if they are immunosuppressed during the breeding season, and quiescent infections thus become active. Breeding frogs may be immunosuppressed, because the reproduction can induce physiological stress [57]. During such periods of breeding or development when larvae or frogs are potentially immunosuppressed is when facilitation between Bd and Rv is most likely to be apparent. This view of parasite facilitation assumes that (i) such interactions between parasites and/or their apparent effects on their host are temporary or periodic; and (ii) that the state or condition of a host influences community interactions among co-infecting parasites [34, 36, 37, 58]. If these assumptions are true, then the detection of parasite interactions and their effects on hosts are similar to the detection of life history trade-offs, which are only apparent when animals are physiologically stressed [59, 60]. In the context of our stated hypotheses, correlations of infection intensity between co-infecting parasites, and parasite abundance effects on the likelihood of co-infection would only be apparent during life states in animals when they are most vulnerable due to factors like physiological stress or shifts in critical developmental windows [32]. Given this, we suggest that future tests of co-infection in animals take into account the life stage/state and condition of hosts.
Wild amphibians, and most taxa for that matter, are rarely assayed for multiple infections, especially during critical periods of infection or epizootic events. While chytridiomycosis is likely the ultimate causative agent of mortality in recent amphibian declines, it is possible that susceptibility to Bd infection is shaped by prior or concurrent infections by parasites such as Rv. In addition, Bd infections may be contributing to the spread and invasion of other emerging pathogens such as Rv, if these parasites facilitate multiple infections via immunosuppression and host resource depletion [13]. Alternatively, it is possible that Rv is endemic to Peru and does not strongly influence amphibians in this region. However, without greater surveillance and data it is impossible to determine the threat posed by Rv and other potential co-infecting parasites. Therefore, future surveys and amphibian conservation programs focused on Bd should also test for the presence of Rv and monitor for signs of secondary infections. In addition, broad surveys of Rv are warranted to determine if this parasite is a threat to many already endangered amphibian species.
While Rv epizootics can be devastating, causing greater than 90% mortality among larvae in an affected pond, these outbreaks are often rapid and sporadic, which make them difficult to detect. In Peru, however, a recent report of diseased adult T. marmoratus frogs near Cusco described animals exhibiting signs (lesions, edema) consistent with ranavirus infection (A. Ttito, pers. comm.). Ranavirus induced disease in adult amphibians is alarming because this could suggest a highly virulent strain is present in Peru; most Rv induced mortality is in metamorphic larvae [32]. Reports of ranavirus infections and associated epizootics in Central America also could suggest that this disease is widespread and of potential conservation concern [13, 61]. In addition, ranaviruses are not host-specific so a single virulent strain can infect fish, reptiles and amphibians [23, 26–29], which suggests that this pathogen can threaten entire wetland communities; as was recently described in ranavirus epizootics in Spain [10].
While ranavirus outbreaks occur sporadically [62, 63], the factors driving the cryptic pattern of disease outbreaks are likely influenced by variation in susceptibility among species [30] and life stages, with larvae nearing metamorphosis being most susceptible possibly due to the stress associated with metamorphic tissue remodeling [32, 64, 65]. Ranavirus emergence and disease outbreaks may also be linked to environmental change like altered habitats, changes in water chemistry, pollution and climate change [33]; as well as potentially the stress imposed by infection with other diseases like Bd. Clearly, to understand the extent which Rv is distributed in Peru and South America, its prevalence, and any threat it poses to amphibian conservation in conjunction with other parasites, we need more extensive and thorough surveys and assays testing for animal health [66]. Last, genome sequencing of Rv strains present in Peru and other regions of the Americas could shed light on variance in distribution of differing strains, and potentially the means of spread of these emergent diseases [10, 67–69].
Rv and Bd are water-borne parasites that are often associated with infections in the aquatic, larval life-stage of most amphibians, and which impose the highest mortality during the metamorphic and post-metamorphic life-stage of aquatic amphibians. Our study documents for the first time Rv and Bd co-infection in Pristimantis, which are largely terrestrial frogs that lay their eggs in leaf litter and exhibit direct-development (i.e. small froglets hatch directly from eggs with no free-swimming larval stage) and that comprise a sizable component of amphibian diversity in the Andes [70, 71]. Because these frogs and their larvae interact little with ponds or streams, our finding of high infection rates in Pristimantis frogs is rather surprising. We can only speculate about the source(s) and mode of Bd and Rv transmission, but a likely source is environmental. Bd spores and free Rv virions may be present in the moist leaf litter, and spread to these habitats by infected individuals [17, 72]. Another source of exposure could be when these frogs forage in or along riparian habitats that harbor these parasites [17]. Last, shedding of virus from reproducing frogs may also be a source of transmission [56], if infected parental frogs shed Rv or Bd onto the hatchlings, and leaf litter during egg laying and fertilization or if they exhibit parental care. Further research is needed to characterize these potential patterns of Rv and Bd presence in terrestrial habitats and their transmission dynamics.
An important means of potential Rv and Bd spread into these wild communities, with critical conservation implications, is infections in captive Telmatobius frogs, that are wild-caught and harvested for human consumption [18–21]. Captive animals are kept in high densities in communal tubs that favor the transmission of diseases, and dead animals as well as their water are likely discarded without concern for the release of diseases to the environment [21]. Thus, captive animals might become spreaders of pathogens that can spill over to wild populations. Indeed, disease spillover from confined market to wild amphibian populations is a probable route for the spread of ranaviruses in the United States [22, 69]. Clearly, the patterns of Rv and Bd co-infection we have characterized in captive and wild populations in Peru warrant concern and greater testing. To address this likely threat that the live-trade poses for disease spread, outreach and education are greatly needed to inform the sellers and consumers of the risks of live-trade frogs for disease spread to wildlife.
In fact, seventy-four percent of Telmatobius species are threatened, and a quarter of these threatened species are in the category of Critically Endangered in the IUCN Red List [46]. The genus Telmatobius is endemic to the Andes, where it occurs from Ecuador to Argentina and Chile, with the largest center of diversification in Peru and Bolivia. Chytridiomycosis has been associated with population declines of Telmatobius throughout the Andes [5, 17, 20, 73–77]. For example, the three Telmatobius species known from Ecuador were extirpated in the 1990s and are now thought to be extinct [75]. The last individuals of Telmatobius found in Ecuador showed symptoms of chytridiomycosis [75]. Moreover, population declines of T. marmoratus, T. mendelsoni and T. timens in Peru [5, 17, 20, 76] and of three species of Telmatobius in Argentina [73] have been associated with outbreaks of Bd, and high prevalence of Bd infection has been reported from high-elevation populations of T. jelskii in central Peru [48] and T. gigas in Bolivia [77]. While Bd is thus known to threaten these [21] and other vulnerable frogs in Peru [17], the extent to which Rv and co-infection have or are contributing to these declines is unknown. Because amphibians globally are the most threatened group of vertebrates [46], we believe that these co-infection patterns are of great concern and that future research should aim to detect the prevalence of Rv, Bd, and other parasites, as well as test their interacting roles in driving threatened populations to extinction. With concerted efforts, and greatly increased data regarding any such parasite interactions, and the role of humans in spreading these pathogens, we can increase our capacity to contain and mitigate the emergence of these and other wildlife diseases.
Supporting Information
(KML)
Acknowledgments
We thank the anonymous reviewers for their thoughtful comments that helped to improve this paper. We thank Manu NP, Peru for research permits. We also thank the staff of the Amazon Conservation Association for their support.
Data Availability
Data have been deposited to OpenSIUC: http://opensiuc.lib.siu.edu/zool_data/10.
Funding Statement
This research was funded by SIUC Startup grants to RWW and AC, the Amazon Conservation Association to AC, the Disney Worldwide Conservation Fund, the Rufford Small Grants Foundation to AC and VTV, and National Science Foundation, Grant 1120283 to VTV. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, et al. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484(7393):186–94. doi: http://www.nature.com/nature/journal/v484/n7393/abs/nature10947.html#supplementary-information. 10.1038/nature10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford AJ, Lips KR, Bermingham E. Epidemic disease decimates amphibian abundance, species diversity, and evolutionary history in the highlands of central Panama. Proceedings of the National Academy of Sciences. 2010;107(31):13777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lips K, Brem F, Brenes R, Reeve J, Alford R, Voyles J, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci U S A. 2006;103(9):3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La Marca E, Lips KR, Lötters S, Puschendorf R, Ibáñez R, Rueda-Almonacid JV, et al. Catastrophic Population Declines and Extinctions in Neotropical Harlequin Frogs (Bufonidae: Atelopus). Biotropica. 2005;37(2):190–201. 10.1111/j.1744-7429.2005.00026.x [DOI] [Google Scholar]
- 5.Catenazzi A, Lehr E, Vredenburg VT. Thermal Physiology, Disease, and Amphibian Declines on the Eastern Slopes of the Andes. Conservation Biology. 2014;28(2):509–17. 10.1111/cobi.12194 [DOI] [PubMed] [Google Scholar]
- 6.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proceedings of the National Academy of Sciences. 2010;107(21):9689–94. 10.1073/pnas.0914111107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences. 1998;95(15):9031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green D, Converse K, Schrader A. Epizootiology of sixty-four amphibian mortality events in the USA, 1996–2001. Annals of the New York Academy of Science. 2002;969:323–39. [DOI] [PubMed] [Google Scholar]
- 9.Gray M, Miller D. Rise of ranavirus: an emerging pathogen threatens ectothermic vertebrates. Wildlife Professional. 2013;7:51–5. [Google Scholar]
- 10.Price SJ, Garner TW, Nichols RA, Balloux F, Ayres C, de Alba AM-C, et al. Collapse of Amphibian Communities Due to an Introduced Ranavirus. Current Biology. 2014;24:2586–91. 10.1016/j.cub.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 11.Fox SF, Greer AL, Torres-Cervantes R, Collins JP. First case of ranavirus-associated morbidity and mortality in natural populations of the South American frog Atelognathus patagonicus. Diseases of aquatic organisms. 2006;72(1):87 [DOI] [PubMed] [Google Scholar]
- 12.Une Y, Sakuma A, Matsueda H, Nakai K, Murakami M. Ranavirus outbreak in North American bullfrogs (Rana catesbeiana), Japan, 2008. Emerg Infect Dis. 2009;15(7):1146–7. 10.3201/eid1507.081636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitfield SM, Geerdes E, Chacon I, Rodriguez EB, Jimenez RR, Donnelly MA, et al. Infection and co-infection by the amphibian chytrid fungus and ranavirus in wild Costa Rican frogs. Diseases of aquatic organisms. 2013;104(2):173–8. 10.3354/dao02598 [DOI] [PubMed] [Google Scholar]
- 14.Fey SB, Siepielski AM, Nusslé S, Cervantes-Yoshida K, Hwan JL, Huber ER, et al. Recent shifts in the occurrence, cause, and magnitude of animal mass mortality events. Proceedings of the National Academy of Sciences. 2015;112(4):1083–8. 10.1073/pnas.1414894112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoverman J, Mihaljevic J, Richgels KD, Kerby J, Johnson PJ. Widespread Co-occurrence of Virulent Pathogens Within California Amphibian Communities. EcoHealth. 2012;9(3):288–92. 10.1007/s10393-012-0778-2 [DOI] [PubMed] [Google Scholar]
- 16.Souza MJ, Gray MJ, Colclough P, Miller DL. Prevalence of infection by Batrachochytrium dendrobatidis and Ranavirus in eastern hellbenders (Cryptobranchus alleganiensis alleganiensis) in eastern Tennessee. J Wildl Dis. 2012;48(3):560–6. Epub 2012/06/29. 10.7589/0090-3558-48.3.560 . [DOI] [PubMed] [Google Scholar]
- 17.Catenazzi A, Lehr E, Rodriguez LO, Vredenburg VT. Batrachochytrium dendrobatidis and the Collapse of Anuran Species Richness and Abundance in the Upper Manu National Park, Southeastern Peru. Conservation Biology. 2011;25(2):382–91. 10.1111/j.1523-1739.2010.01604.x [DOI] [PubMed] [Google Scholar]
- 18.Angulo A. Conservation Needs of Batrachophrynus and Telmatobius Frogs of the Andes of Peru. Conservation and Society. 2008;6(4):328–33. 10.4103/0972-4923.49196 [DOI] [Google Scholar]
- 19.Lehr E, Lavilla E. The Telmatobius and Batrachophrynus species of Peru. Monogr Herpetol. 2005;7:39–64. [Google Scholar]
- 20.von May R, Catenazzi A, Angulo A, Brown JL, Carrillo J, Chávez G, et al. Current state of conservation knowledge on threatened amphibian species in Peru. Tropical Conservation Science. 2008;1(4):376–96. [Google Scholar]
- 21.Catenazzi A, Vredenburg VT, Lehr E. Batrachochytrium dendrobatidis in the live frog trade of Telmatobius (Anura: Ceratophryidae) in the tropical Andes. Dis Aquat Org. 2010;92:187–91. 10.3354/dao02250 [DOI] [PubMed] [Google Scholar]
- 22.Picco A, Collins J. Amphibian commerce as a likely source of pathogen pollution. Conservation Biology. 2008;22(6):1582–9. 10.1111/j.1523-1739.2008.01025.x [DOI] [PubMed] [Google Scholar]
- 23.Robert J, Gregory Chinchar V. “Ranaviruses: An emerging threat to ectothermic vertebrates” Report of the First International Symposium on Ranaviruses, Minneapolis MN July 8, 2011. Developmental & Comparative Immunology. 2012;36(2):259–61. doi: 10.1016/j.dci.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Hoverman JT, Gray MJ, Miller DL, Haislip NA. Widespread occurrence of ranavirus in pond-breeding amphibian populations. EcoHealth. 2012;9(1):36–48. 10.1007/s10393-011-0731-9 [DOI] [PubMed] [Google Scholar]
- 25.Schock DM, Bollinger TK, Gregory Chinchar V, Jancovich JK, Collins JP. Experimental Evidence that Amphibian Ranaviruses Are Multi-Host Pathogens. Copeia. 2008;2008(1):133–43. 10.1643/CP-06-134 [DOI] [Google Scholar]
- 26.Bandín I, Dopazo CP. Host range, host specificity and hypothesized host shift events among viruses of lower vertebrates. Vet Res. 2011;42(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allender M, Mitchell M, Torres T, Sekowska J, Driskell E. Pathogenicity of Frog Virus 3-like Virus in Red-eared Slider Turtles at Two Environmental Temperatures. Journal of Comparative Pathology. 2013;149:356–67. 10.1016/j.jcpa.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 28.Currylow AF, Johnson AJ, Williams RN. Evidence of Ranavirus Infections among Sympatric Larval Amphibians and Box Turtles. Journal of Herpetology. 2014;48(1):117–21. [Google Scholar]
- 29.Abrams AJ, Cannatella DC, Hillis DM, Sawyer SL. Recent host-shifts in ranaviruses: signatures of positive selection in the viral genome. Journal of General Virology. 2013;94(Pt 9):2082–93. 10.1099/vir.0.052837-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoverman J, Gray M, Haislip N, Miller D. Phylogeny, Life History, and Ecology Contribute to Differences in Amphibian Susceptibility to Ranaviruses. EcoHealth. 2011;8(3):301–19. 10.1007/s10393-011-0717-7 [DOI] [PubMed] [Google Scholar]
- 31.Haislip NA, Gray MJ, Hoverman JT, Miller DL. Development and disease: How susceptibility to an emerging pathogen changes through anuran development. PLoS ONE. 2011;6(7):e22307 10.1371/journal.pone.0022307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warne RW, Crespi EJ, Brunner JL. Escape from the pond: stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct Ecol. 2011;25(1):139–46. 10.1111/j.1365-2435.2010.01793.x [DOI] [Google Scholar]
- 33.Kerby J, Hart A, Storfer A. Combined Effects of Virus, Pesticide, and Predator Cue on the Larval Tiger Salamander (Ambystoma tigrinum). EcoHealth. 2011;8(1):46–54. 10.1007/s10393-011-0682-1 [DOI] [PubMed] [Google Scholar]
- 34.Graham AL. Ecological rules governing helminth–microparasite coinfection. Proceedings of the National Academy of Sciences. 2008;105(2):566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolles AE, Ezenwa VO, Etienne RS, Turner WC, Olff H. Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology. 2008;89(8):2239–50. 10.1890/07-0995.1 [DOI] [PubMed] [Google Scholar]
- 36.Pedersen AB, Fenton A. Emphasizing the ecology in parasite community ecology. Trends Ecol Evol. 2007;22(3):133–9. doi: 10.1016/j.tree.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, et al. Species Interactions in a Parasite Community Drive Infection Risk in a Wildlife Population. Science. 2010;330(6001):243–6. 10.1126/science.1190333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigaud T, Perrot-Minnot M-J, Brown MJF. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proceedings of the Royal Society B. 2010;277:3693–702. 10.1098/rspb.2010.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petney TN, Andrews RH. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. International Journal for Parasitology. 1998;28(3):377–93. doi: 10.1016/S0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- 40.Woolhouse MEJ, Thumbi SM, Jennings A, Chase-Topping M, Callaby R, Kiara H, et al. Co-infections determine patterns of mortality in a population exposed to parasite infection. Science Advances. 2015;1(e140002):1–10. 10.1126/sciadv.1400026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez M, Terrazas LI, Marquez R, Bojalil R. Susceptibility to Trypanosoma cruzi is modified by a previous non‐related infection. Parasite immunology. 1999;21(4):177–85. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Robert J. Antiviral immunity in amphibians. Viruses. 2011;3(11):2065–86. 10.3390/v3112065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales H, Robert J. Characterization of primary and memory CD8 T-cell responses against ranavirus (FV3) in Xenopus laevis. Journal of Virology. 2007;81(5):2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fites JS, Ramsey JP, Holden WM, Collier SP, Sutherland DM, Reinert LK, et al. The invasive chytrid fungus of amphibians paralyzes lymphocyte responses. Science. 2013;342(6156):366–9. 10.1126/science.1243316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grayfer L, Andino FJ, Chen G, Chinchar GV, Robert J. Immune evasion strategies of ranaviruses and innate immune responses to these emerging pathogens. Viruses. 2012;4(7):1075–92. 10.3390/v4071075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.IUCN. IUCN Red List of Threatened Species: www.iucnredlist.org; 2014.
- 47.Beaupre SJ, Jacobson ER, Lillywhite HB, Zamudio K. Guidelines for the use of live amphibians and reptiles in field and laboratory research. Herpetological Animal Care and Use Committee (HACC) of the American Society of Ichthyologists and Herpetologists, 2004 Second Edition.
- 48.Catenazzi A, von May R, Vredenburg VT. High prevalence of infection in tadpoles increases vulnerability to fungal pathogen in high-Andean amphibians. Biological Conservation. 2013;159(0):413–21. doi: 10.1016/j.biocon.2012.11.023. [DOI] [Google Scholar]
- 49.Hyatt A, Boyle D, Olsen V, Boyle D, Berger L, Obendorf D, et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of aquatic organisms. 2007;73:175–92. [DOI] [PubMed] [Google Scholar]
- 50.Boyle D, Boyle D, Olsen V, Morgan J, Hyatt A. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of aquatic organisms. 2004;60:141–8. [DOI] [PubMed] [Google Scholar]
- 51.Greer A, Collins J. Sensitivity of a diagnostic test for amphibian Ranavirus varies with sampling protocol. Journal of Wildlife Diseases. 2007;43(3):525 [DOI] [PubMed] [Google Scholar]
- 52.Mao J, Green D, Fellers G, Chinchar V. Molecular characterization of Iridoviruses isolated from sympatric amphibians and fish. Virus research. 1999;63(1–2):45–52. [DOI] [PubMed] [Google Scholar]
- 53.Rózsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of hosts. Journal of Parasitology. 2000;86(2):228–32. [DOI] [PubMed] [Google Scholar]
- 54.Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ. Seasonal Pattern of Batrachochytrium dendrobatidis Infection and Mortality in Lithobates areolatus: Affirmation of Vredenburg's “10,000 Zoospore Rule”. PLoS ONE. 2011;6(3):e16708 10.1371/journal.pone.0016708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duellman WE. Distribution patterns of amphibians in South America. Patterns of Distribution of Amphibians: A Global Perspective. 1999:255–328. . [Google Scholar]
- 56.Brunner J, Schock D, Davidson E, Collins J. Intraspecific reservoirs: complex life history and the persistence of a lethal ranavirus. Ecology. 2004;85(2):560–6. [Google Scholar]
- 57.Moore IT, Jessop TS. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm Behav. 2003;43(1):39–47. [DOI] [PubMed] [Google Scholar]
- 58.Warne RW. The Micro and Macro of Nutrients across Biological Scales. Integrative and Comparative Biology. 2014;54(5):864–72. 10.1093/icb/icu071 [DOI] [PubMed] [Google Scholar]
- 59.van Noordwijk AJ, de Jong G. Acquisition and allocation of resources: Their influence on variation in life history tactics. Am Nat. 1986;128(1):137–42. [Google Scholar]
- 60.Warne RW, Gilman CA, Garcia DA, Wolf BO. Capital breeding and allocation to life-history demands are highly plastic in lizards. Am Nat. 2012;180(1):130–41. 10.1086/665995 [DOI] [PubMed] [Google Scholar]
- 61.Stark T, Laurijssens C, Weterings M, Spitzen-van der Sluijs A, Martel A, Pasmans F. Death in the clouds: ranavirus associated mortality in assemblage of cloud forest amphibians in Nicaragua. Acta Herpetologica. 2014;9(1):125–7. [Google Scholar]
- 62.Duffus ALJ. Ranavirus ecology in common frogs (Rana Temporaria) from United Kingdom: transmission dynamics, alternate hosts and host-strain interactions 2009. [Google Scholar]
- 63.Gahl MK, Calhoun AJK. Landscape setting and risk of Ranavirus mortality events. Biological Conservation. 2008;141(11):2679–89. doi: 10.1016/j.biocon.2008.08.003. [DOI] [Google Scholar]
- 64.Warne RW, Crespi EJ. Larval growth rate and sex determine resource allocation and stress responsiveness across life stages in juvenile frogs. Journal of Experimental Zoology Part A 2015;323:191–201. [DOI] [PubMed] [Google Scholar]
- 65.Crespi EJ, Warne RW. Environmental Conditions Experienced During the Tadpole Stage Alter Post-metamorphic Glucocorticoid Response to Stress in an Amphibian. Integrative and Comparative Biology. 2013;53(6):989–1001. 10.1093/icb/ict087 [DOI] [PubMed] [Google Scholar]
- 66.Warne RW, Proudfoot GA, Crespi EJ. Biomarkers of animal health: integrating nutritional ecology, endocrine ecophysiology, ecoimmunology, and geospatial ecology. Ecology and Evolution. 2015;5(3):1–10. 10.1002/ece3.1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goldberg TL, Coleman DA, Grant EC, Inendino KR, Philipp DP. Strain Variation in an Emerging Iridovirus of Warm-Water Fishes. Journal of Virology. 2003;77(16):8812–8. 10.1128/jvi.77.16.8812-8818.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mavian C, López-Bueno A, Balseiro A, Casais R, Alcamí A, Alejo A. The genome sequence of the emerging common midwife toad virus identifies an evolutionary intermediate within ranaviruses. Journal of Virology. 2012. 10.1128/jvi.07108-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jancovich J, Davidson E, Parameswaran N, Mao J, Chinchar V, Collins J, et al. Evidence for emergence of an amphibian iridoviral disease because of human enhanced spread. Molecular Ecology. 2005;14(1):213–24. [DOI] [PubMed] [Google Scholar]
- 70.Blair C, Doan TM. Patterns of Community Structure and Microhabitat Usage in Peruvian Pristimantis (Anura: Strabomantidae). Copeia. 2009;2009(2):303–12. 10.1643/CH-08-062 [DOI] [Google Scholar]
- 71.García-R JC, Crawford AJ, Mendoza ÁM, Ospina O, Cardenas H, Castro F. Comparative Phylogeography of Direct-Developing Frogs (Anura: Craugastoridae:Pristimantis) in the Southern Andes of Colombia. PLoS ONE. 2012;7(9):e46077 10.1371/journal.pone.0046077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Puschendorf R, Bolaños F, Chaves G. The amphibian chytrid fungus along an altitudinal transect before the first reported declines in Costa Rica. Biological Conservation. 2006;132(1):136–42. doi: 10.1016/j.biocon.2006.03.010. [DOI] [Google Scholar]
- 73.Barrionuevo JS, Ponssa LM. Decline of three species of the genus Telmatobius (Anura: Leptodactylidae) from Tucumán Province, Argentina. Herpetologica. 2008;64(1):47–62. [Google Scholar]
- 74.Barrionuevo S, Mangione S. Chytridiomycosis in two species of Telmatobius (Anura: Leptodactylidae) from Argentina. Diseases of aquatic organisms. 2006;73(2):171–4. [DOI] [PubMed] [Google Scholar]
- 75.Merino-Viteri A, Coloma L, Almendariz A. Studies on the Andean Frogs of the Genera Telmatobius and Batrachophrynus In: Lavilla E, De la Riva I, editors.2005. p. 9–37.
- 76.Seimon TA, Seimon A, Daszak P, Halloy SRP, Schloegel LM, Aguilar CA, et al. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Global Change Biology. 2007;13(1):288–99. . [Google Scholar]
- 77.De la Riva I, Burrowes PA. Rapid Assessment of the Presence of Batrachochytrium dendrobatidis in Bolivian Andean Frogs. Herpetological Review. 2011;42(3):372–5. . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(KML)
Data Availability Statement
Data have been deposited to OpenSIUC: http://opensiuc.lib.siu.edu/zool_data/10.