Abstract
Background
Esophageal adenocarcinoma is a lethal malignancy whose incidence is rapidly growing in recent years. Previous reports suggested that Barrett’s esophagus (BE), which is represented by metaplasia-dysplasia-carcinoma transition, is regarded as the premalignant lesion of esophageal neoplasm. However, our knowledge about the development of esophageal adenocarcinoma is still very limited.
Material/Methods
In order to acquire better understanding about the pathological mechanisms in this field, we obtained gene profiling data on BE, esophageal adenocarcinoma patients, and normal controls from the Gene Expression Omnibus (GEO) database. Bioinformatics analyses, including Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, were conducted.
Results
Our results revealed that several pathways, such as the wound healing, complement, and coagulation pathways, were closely correlated with cancer development and progression. The mitogen-activated protein kinase (MAPK) pathway was discovered to be responsible for the predisposition stage of cancer; while response to stress, cytokine-cytokine receptor interaction, nod-like receptor signaling pathway, and ECM-receptor interaction were chief contributors of cancer progression. More importantly, we discovered in this study that LYN was a critical gene. It was found to be the key nodule of several significant biological networks, which suggests its close correlation with cancer initiation and progression.
Conclusions
These results provided more information on the mechanisms of esophageal adenocarcinoma, which enlightened our way to the clinical discovery of novel therapeutic makers for conquering esophageal cancer. Keywords: esophageal adenocarcinoma; LYN; Go analysis; KEGG pathway.
MeSH Keywords: Esophageal Neoplasms; Genes, abl; Genetic Linkage; Signal Transduction
Background
Esophageal cancer is the eighth most prevalent cancers worldwide [1,2]. The incidence of esophageal adenocarcinoma has increased tremendously over the past several decades, much more than any other malignancies [3].
The understanding of the pathogenesis of esophageal cancer at the molecular level has not led to the development of clinical drugs that have potent anticancer effects in treatment of this disease. Specifically, researchers have shown great interest in the use of agents targeting cell surface receptors, such as epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF). Well-known gene mutations that generally occur in carcinoma, such as P53, P16, E-cadherin, and Cyclin D, were also found in esophageal adenocarcinoma, which could also be regarded as potential medication targets [3]. Although there have been great improvements in surgical techniques, reduced preoperative mortality, and the introduction of multimodal therapies, this malignancy remains difficult to cure [4]. Better prognosis of esophageal cancer can be only achieved by diagnosis in the very early stage [4]; therefore, more and more attention has been focused on finding potential diagnostic and therapeutic markers of esophageal cancer. However, much work remains in defining the molecular mechanisms underlying the development and progression of esophageal neoplasm.
Barrett’s esophagus (BE) is characterized by the replacement of normal stratified squamous epithelium lining of the upper part of esophagus by simple columnar epithelium with goblet cells [5,6]. Barrett’s esophagus can be potential risk factor of esophageal adenocarcinoma, which is the established predisposition to esophageal adenocarcinoma that progresses through a metaplasia-dysplasia-carcinoma transition. The close association with esophageal adenocarcinoma gives its diagnosis great importance in cancer prevention [7]. The molecular mechanism underlying the formation of Barrett’s esophagus has been partially elucidated. Gene polymorphisms that play important roles in the inflammatory response, DNA repair, and chemical detoxification have been associated with progression of the lesion [8]. Multiple biomarkers have been identified that are involved in extensive biological pathways, such as cell cycle control, DNA abnormality, genomic instability, and cell proliferation regulation [9]. However, validation of these markers is still necessary and will benefit the estimation of risk of malignant transformation, early diagnosis of disease, and prognosis prediction [9].
Previous studies in this field have been relatively small-scale, with only a few genes examined. Due to the complexity of cancer evolution, multiple genes and pathways deviated from normal condition should have been involved. An overall study would be more helpful to understand the pathogenesis and progression of esophageal adenocarcinoma. Therefore, in this study we compared the global expression profile of the samples from Barrett’s esophagus-associated cancer patients, and found that LYN gene could play an important role in mediating the pathogenesis and progression of esophageal cancer.
Material and Methods
Data extraction
Microarray expression data (accession number GSE1420) submitted by Kimchi et al. [10], which was deposited in the Gene Expression Omnibus (GEO) database, were downloaded and analyzed. Samples for gene profile analysis were acquired from 8 patients with Barrett’s-associated adenocarcinoma after esophagectomy. The dataset included samples representative of the normal esophageal epithelium (normal), Barrett’s esophagus (BE), and esophageal adenocarcinomas from every patient. All microarray data were obtained by use of the Affymetrix Human Genome U133A Array GeneChip.
Identification and analysis of DEGs
We set up 2 comparisons to identify differentially expressed genes (DEGs). Comparison 1 was between the Barrett’s esophagus and the normal esophageal epithelium samples. Comparison 2 was between the esophageal adenocarcinoma and the normal esophageal epithelium samples. We first normalized the microarray expression data with the robust multi-array average (RMA) algorithm [11] method with background correction, quantile normalization, and log transformation. Then we identified DEGs by R Limma package (v.2.13.0) [12] for each comparison. Results with |log Fold Change| (|log FC|) >2 and P<0.05 were considered significant. To acquire more profound information on these DEGs, we performed hierarchical clustering and statistical analysis [13]. The annotation of DEGs was conducted by online DAVID [14,15] and ingenuity pathway analysis (IPA) [16].
GO and KEGG pathway enrichment analysis for DEGs
To evaluate the pathways of the DEGs between each comparison, we performed Gene Ontology analysis (GO) analysis. We also conducted pathway enrichment analysis using the KEGG database (Kyoto Encyclopedia of Genes and Genomes, http://www.genome.jp/kegg/, Last updated: Dec 1, 2014) and online DAVID tools [14,15] for data Annotation, Visualization, and Integrated Discovery [17].
Results
Identification of DEGs
We used R Limma package software to analyze which gene sets were deregulated in both comparisons with the threshold of |log FC| >2 and P<0.05. There were 55 genes up-regulated and 6 genes down-regulated in Comparison 1; accordingly, the numbers in Comparison 2 were 233 and 244. Altogether, Comparisons 1 and 2 share a DEGs pool including 49 up-regulated genes and 5 down-regulated genes (Figure 1, Tables 1, 2).
Figure 1.
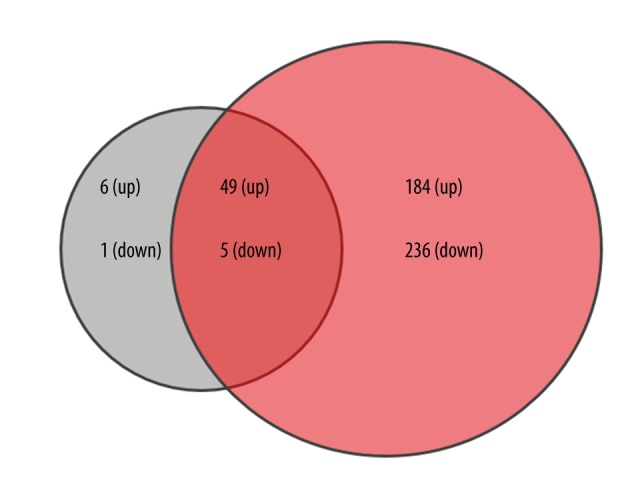
Venn diagram of different expressed genes of Comparison 1 and Comparison 2. Green color represents DEGs of Comparison 1 and red represents Comparison 2.
Table 1.
Up-regulated genes among commonly changed DEGs of both comparison (log2(FC) >2 and adjusted p<0.01).
| Gene symbol | Comparison 1 | Comparison 2 | ||||
|---|---|---|---|---|---|---|
| Log2 (FC) | P value | Adjusted P value | Log2 (FC) | P value | Adjusted P value | |
| AGMAT | 4.17 | 7.45E-05 | 9.40E-03 | 5.26 | 3.25E-06 | 4.90E-04 |
| AGR2 | 7.28 | 7.78E-07 | 5.93E-04 | 7.86 | 2.27E-07 | 1.68E-04 |
| ANXA10 | 6.49 | 2.05E-05 | 4.40E-03 | 5.15 | 3.25E-04 | 4.94E-03 |
| ARPC1B | 2.19 | 6.36E-06 | 2.08E-03 | 3.07 | 2.99E-08 | 8.03E-05 |
| ATP2A3 | 3.97 | 3.93E-07 | 4.46E-04 | 2.25 | 1.06E-04 | 2.67E-03 |
| C1orf115 | 3.30 | 1.27E-05 | 3.14E-03 | 3.00 | 4.59E-05 | 1.76E-03 |
| CASP7 | 2.66 | 3.47E-05 | 6.12E-03 | 2.24 | 2.68E-04 | 4.43E-03 |
| CD97 | 3.12 | 4.57E-06 | 1.70E-03 | 3.46 | 9.78E-07 | 2.94E-04 |
| CFB | 3.13 | 1.23E-05 | 3.14E-03 | 3.88 | 5.03E-07 | 2.33E-04 |
| CREB3L1 | 4.50 | 3.27E-06 | 1.53E-03 | 5.24 | 3.12E-07 | 1.91E-04 |
| CTSE | 7.34 | 6.51E-07 | 5.56E-04 | 7.84 | 2.23E-07 | 1.68E-04 |
| EFNB2 | 2.20 | 1.10E-05 | 2.90E-03 | 2.10 | 2.12E-05 | 1.21E-03 |
| EPCAM | 4.78 | 4.95E-05 | 7.53E-03 | 5.80 | 3.60E-06 | 5.02E-04 |
| FAM46A | 3.05 | 1.57E-05 | 3.62E-03 | 3.10 | 1.26E-05 | 9.94E-04 |
| FAT1 | 2.56 | 3.58E-07 | 4.46E-04 | 2.24 | 2.85E-06 | 4.72E-04 |
| GALNT6 | 5.40 | 2.21E-08 | 2.45E-04 | 4.10 | 2.06E-06 | 4.15E-04 |
| GLRX | 3.26 | 4.71E-05 | 7.41E-03 | 2.45 | 1.94E-05 | 1.16E-03 |
| GOLM1 | 5.70 | 1.60E-05 | 3.62E-03 | 6.57 | 2.13E-06 | 4.19E-04 |
| GPRC5A | 4.06 | 1.51E-06 | 8.09E-04 | 4.77 | 1.15E-07 | 1.28E-04 |
| HOXB7 | 3.11 | 2.61E-05 | 5.18E-03 | 4.06 | 1.43E-07 | 1.44E-04 |
| ITPR3 | 2.54 | 4.02E-05 | 6.66E-03 | 2.74 | 1.52E-05 | 1.02E-03 |
| KDELR3 | 3.92 | 1.28E-06 | 7.49E-04 | 4.28 | 3.16E-07 | 1.91E-04 |
| KIF3B | 2.16 | 6.62E-05 | 8.65E-03 | 3.10 | 3.94E-07 | 1.91E-04 |
| KRT8 | 3.94 | 5.27E-05 | 7.80E-03 | 4.68 | 5.24E-06 | 6.33E-04 |
| LAMC2 | 2.10 | 5.08E-05 | 7.62E-03 | 2.89 | 5.58E-07 | 2.39E-04 |
| LGALS4 | 7.97 | 1.10E-07 | 4.46E-04 | 8.37 | 4.77E-08 | 8.03E-05 |
| LYN | 3.99 | 3.48E-06 | 1.53E-03 | 3.72 | 9.22E-06 | 8.47E-04 |
| LYZ | 4.11 | 2.06E-05 | 4.40E-03 | 4.49 | 6.26E-06 | 6.66E-04 |
| MARCKSL1 | 3.95 | 4.90E-07 | 4.94E-04 | 4.63 | 3.41E-08 | 8.03E-05 |
| MET | 2.68 | 5.97E-05 | 8.19E-03 | 3.72 | 5.80E-07 | 2.39E-04 |
| MISP | 2.97 | 6.25E-05 | 8.37E-03 | 3.33 | 1.40E-05 | 1.02E-03 |
| MLPH | 4.31 | 4.80E-06 | 1.72E-03 | 4.61 | 1.79E-06 | 3.97E-04 |
| ODC1 | 3.53 | 4.73E-05 | 7.41E-03 | 2.69 | 9.17E-04 | 8.83E-03 |
| PIP5K1B | 3.73 | 8.02E-05 | 9.90E-03 | 4.22 | 1.63E-05 | 1.04E-03 |
| PLAUR | 4.22 | 8.55E-07 | 5.93E-04 | 4.47 | 3.42E-07 | 1.91E-04 |
| PLVAP | 2.68 | 5.56E-05 | 8.13E-03 | 2.59 | 8.38E-05 | 2.39E-03 |
| SEL1L3 | 4.32 | 2.79E-07 | 4.46E-04 | 4.87 | 3.76E-08 | 8.03E-05 |
| SERPINA1 | 6.28 | 1.53E-06 | 8.09E-04 | 6.30 | 1.46E-06 | 3.51E-04 |
| SLC20A1 | 2.28 | 5.83E-06 | 2.02E-03 | 2.43 | 2.26E-06 | 4.26E-04 |
| SLC39A14 | 4.60 | 3.32E-06 | 1.53E-03 | 5.29 | 3.74E-07 | 1.91E-04 |
| SLC44A4 | 4.99 | 3.00E-05 | 5.55E-03 | 6.20 | 1.41E-06 | 3.47E-04 |
| SPINK1 | 6.86 | 2.84E-05 | 5.35E-03 | 8.57 | 1.20E-06 | 3.22E-04 |
| SULT1A1 | 2.69 | 4.83E-05 | 7.45E-03 | 2.63 | 6.47E-05 | 2.10E-03 |
| TFF1 | 8.35 | 2.75E-07 | 4.46E-04 | 7.76 | 8.84E-07 | 2.80E-04 |
| TFF2 | 6.63 | 1.50E-05 | 3.54E-03 | 5.84 | 7.66E-05 | 2.25E-03 |
| TMEM2 | 3.51 | 1.48E-07 | 4.46E-04 | 3.58 | 1.04E-07 | 1.28E-04 |
| TNFRSF10B | 2.19 | 8.38E-07 | 5.93E-04 | 2.70 | 2.55E-08 | 8.03E-05 |
| TSPAN1 | 4.77 | 1.43E-05 | 3.45E-03 | 4.01 | 1.28E-04 | 2.99E-03 |
| TSPAN8 | 8.28 | 1.96E-07 | 4.46E-04 | 9.02 | 4.59E-08 | 8.03E-05 |
| TSPAN8 | 8.28 | 1.96E-07 | 4.46E-04 | 9.02 | 4.59E-08 | 8.03E-05 |
Table 2.
Down regulated genes in commonly changed DEGs of both comparisons (log2(FC) <−2 and adjusted p<0.01).
| Gene symbol | Comparison 1 | Comparison 2 | ||||
|---|---|---|---|---|---|---|
| Log2 (FC) | P value | Adjusted P value | Log2 (FC) | P value | Adjusted P value | |
| AGFG2 | −3.39 | 2.83E-05 | 5.35E-03 | −3.25 | 4.72E-05 | 1.78E-03 |
| CD207 | −3.78 | 3.83E-07 | 4.46E-04 | −3.58 | 9.14E-07 | 2.82E-04 |
| HLA-DQB2 | −3.11 | 3.79E-06 | 1.56E-03 | −2.64 | 3.53E-05 | 1.58E-03 |
| TP53AIP1 | −2.70 | 1.08E-06 | 6.65E-04 | −2.55 | 2.62E-06 | 4.42E-04 |
| WNT4 | −2.75 | 3.59E-05 | 6.14E-03 | −2.53 | 1.01E-04 | 2.60E-03 |
Gene ontology analysis for DEGs and biological processes
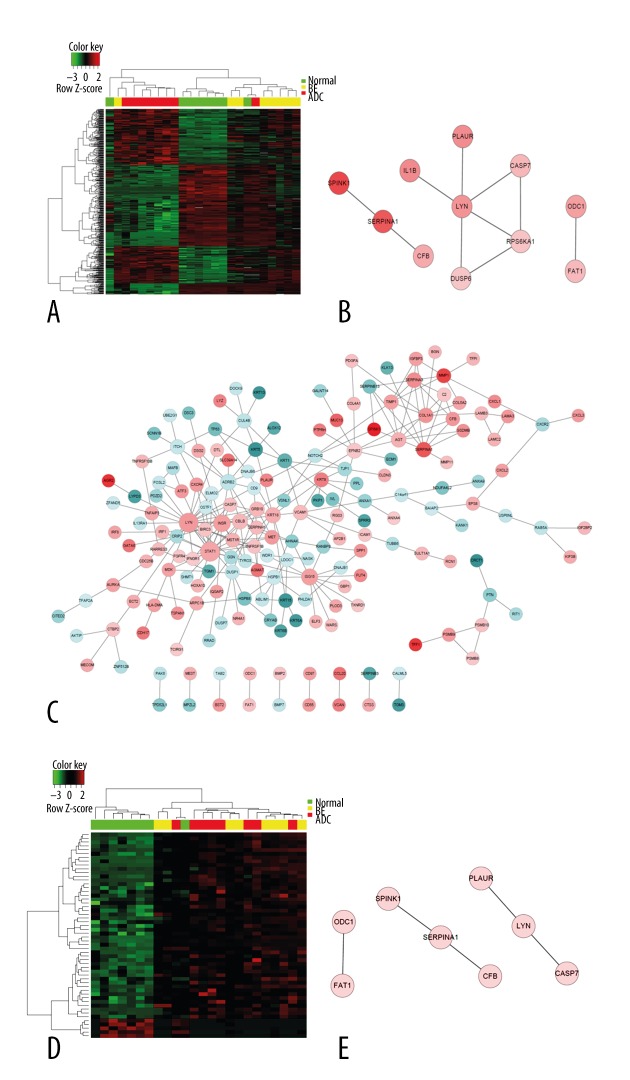
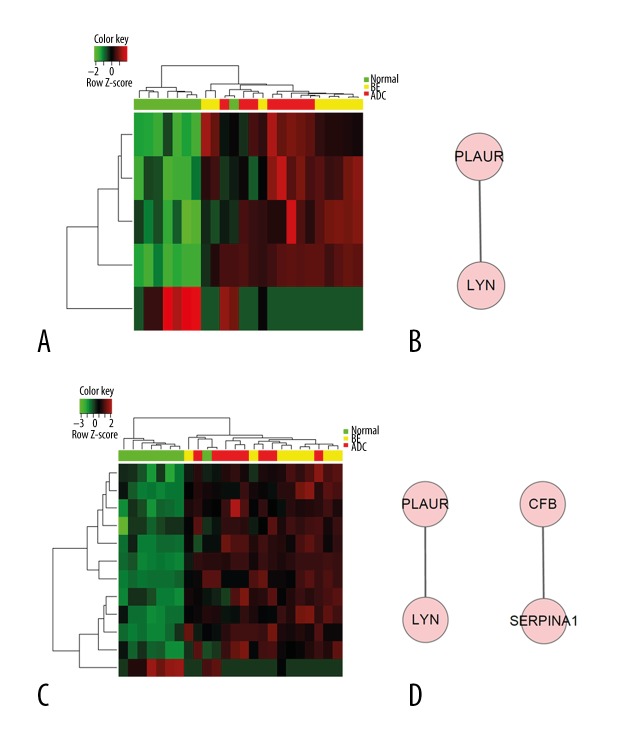
We performed Gene Ontology analysis for DEGs (Figures 2, 3, Table 3). Gene expression profiles of all DEGs is shown in Figure 2A, showing some genes were shared among groups, while some showed different expression patterns between groups. Commonly-changed DEGs expression profile is shown in Figure 2D. The protein network of Comparison 2 (esophageal adenocarcinoma versus normal) (Figure 2C) showed more complexity and information compared with Comparison 1 (BE versus normal) (Figure 2B). As a result, protein network of shared and single comparisons (Figures 2, 3) all identified LYN as the key module. Additionally, this gene was over-expressed in both BE and cancer samples (Table 2). These observations indicate its important function in esophageal adenocarcinoma pathogenesis and progression. Biological processes involved in both and each single comparison are shown in Table 3, in which wound healing is shown to take part in pathological transitions from normal to BE and normal to esophageal carcinoma (Figure 3, Table 3). The mitogen-activated protein kinases (MAPK) pathway was found to be specifically associated with Comparison 1 (Supplementary Table 1), which indicated that the activation of MAPK might contribute to the formation of BE. Cell migration, proliferation, differentiation, and classical cancer-related biological events were related with the pathogenesis of esophageal adenocarcinoma in this analysis (Supplementary Table 2).
Figure 2.
LYN gene was identified as the key network module in both comparisons. Red color indicates relatively high expression, and green indicates relatively low expression. (A) Heat map of all DEGs in both comparisons. (B) Biological network of Comparison 1. (C) Biological network of Comparison 2. (D) Heat map of common DEGs in both comparisons. (E) Biological network of commonly-changed DEGs.
Figure 3.
Regulation of wound healing process and corresponding biological network is significantly enriched in both comparisons. Red color indicates high expression and green color indicates low expression. (A) Heat map of hierarchical clustering of DEGs in “regulation of wound healing”. (B) Biological network of “wound healing”. (C) Heat map of hierarchical clustering of DEGs in “response to wounding”. (D) Biological network of “response to wound healing”.
Table 3.
Significantly changed GO biological processes of DEGs in both comparisons.
| GO-BP-ID | P value | Count | Term |
|---|---|---|---|
| GO: 0061041 | 2.26E-05 | 5 | Regulation of wound healing |
| GO: 0009611 | 1.44E-04 | 11 | Response to wounding |
KEGG pathway enrichment for DEGs
To better clarify the pathological mechanisms, we did KEGG enrichment analysis for the DEGs. As shown in Figure 4 and Table 4, complement and coagulation pathways showed significant association with both comparisons, indicating their possible role in driving pre-malignant transformation and cancer progression. We analyzed the corresponding pathways in each single comparison. In Comparison 1, MAPK pathway showed close significance, which is consistent with GO analysis (Figure 4A, Supplementary Table 3). Significantly changed pathways in Comparison 2 converged on immunology-related pathways as cytokine-cytokine receptor interaction, nod-like receptor signaling pathway, and ECM-receptor interaction (Supplementary Table 4).
Figure 4.
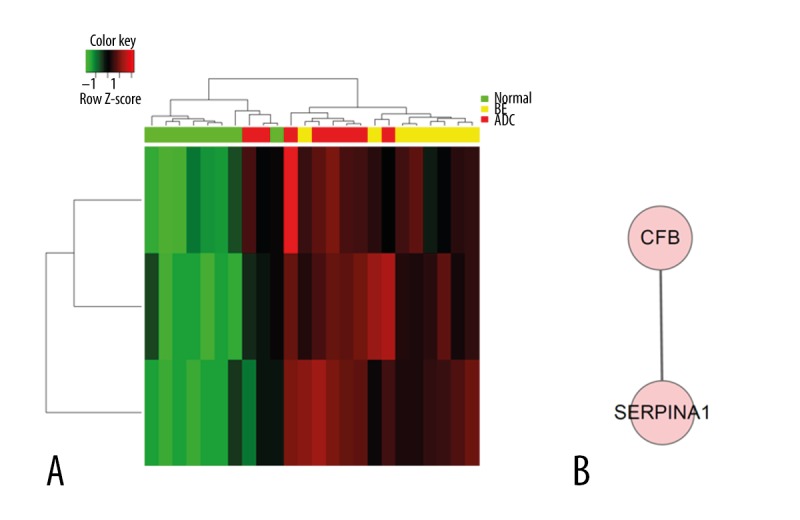
Complement and coagulation cascades pathway and corresponding biological network significantly enriched in both comparisons. Red indicates high expression, and green indicates low expression. (A) Heat map of hierarchical clustering of DEGs in “complement and coagulation cascades”. (B) Biological network of “complement and coagulation cascades”.
Discussion
Esophageal adenocarcinoma has the most rapidly increasing incidence in the Western world, especially in the USA. The overall dismal 5-year survival is below 20%. During the past 4 decades, the incidence of esophageal adenocarcinoma increased at an annual rate of around 1~8% [18]. Compelling evidence demonstrated that Barrett esophagus is a precursor lesion for the development of esophageal adenocarcinoma, whose progression involves the transition from BE [18]. However, our understanding of the progression from BE to cancer is very limited. In order to fill in this gap, we performed an overall investigation by using bioinformatics analysis of BE and esophageal adenocarcinoma patients compared with normal controls, trying to clarify possible mechanisms. We discovered that multiple pathways, especially 1-gene pathways, are closely related with the pathogenesis and progression of esophageal adenocarcinoma.
GO analysis revealed that wound healing may be crucial in both cancer initiation and progression. Wound healing in humans functions as an important tissue repair system, involving multiple processes, such as inflammation component recruitment, fibroblast proliferation, and the formation of new connective tissue [19]. Wounds and carcinomas have striking phenotypic similarities in terms of cellular behavior, signaling molecules, and gene expression. These common features were not only found in epithelial cell behaviors, but also in the stroma of wound healing and carcinomas [20]. Therefore, some researchers described cancer as a wound that would not heal [21], indicating the huge influence of wound healing on cancer.
The complement and coagulation pathways are part of the innate immune system, which serves an important purpose in maintaining physical homeostasis [22,23]. In most pathological situations, the activation of both the complement and coagulation cascades occur simultaneously [22], and cooperate during inflammation to stabilize homeostasis of the human body [24]. As important participants in the immune system, the complement and coagulation pathways sometimes could suppress tumor initiation and progression [25].
Each pathway has its own key player. The most striking finding of this study is that over-expression of the LYN gene was shared in both comparisons, suggesting its important function in both early and late stages of esophageal adenocarcinoma pathogenesis. Being identified as the key nodule of majority of the biological networks involved in cancer enhances this hypothesis. LYN is a kinase belonging to the Src family, and has been reported to be an important mediator in several signal transduction pathways in various malignancies of different cell types [26]. As a Src family kinase, LYN performs its function by regulating downstream signals, such as growth factors, cytokines, and antigen stimulation factors [26]. LYN was also found to regulate epithelial-mesenchymal transition in breast cancer [27], and mediate tumor cell motility and growth in EGFR VIII-expressing head and neck cancer [28]. Additionally, LYN can promote the proliferation of lung adenocarcinoma cells through the activation of EGFRs [29], and regulate androgen receptor expression and activity in castrate-resistant prostate cancer [30]. In our study, LYN gene was also found to be the key nodule in all of the GO networks, such as wound healing. This further strengthened the biological importance of this gene in the development and progression of esophageal cancer, which is possibly accomplished through regulating some essential biological pathways, including wound healing.
Apart from the common features of these comparisons, we also identified some specific pathways that may be associated with each individual esophageal disorder we focused on. Both GO and KEGG analysis found that MAPKs pathway was highly associated with BE. The MAPK family includes multiple extracellular signal-regulated kinases, whose activation regulates a variety of cellular activities, such as proliferation, differentiation, and cell death [31]. The MAPK signaling pathway has been implicated in the development of many human diseases [23,31]. This finding suggests that this pathway may contribute greatly to the malignant transition from BE, the precursor disorder, into esophagus cancer.
On the other hand, response to stress, cytokine-cytokine receptor interaction, nod-like receptor (NLRs) signaling pathway, and ECM-receptor interaction were found to be possible predictors of esophageal adenocarcinoma in our study. By referring to the background of these pathways, we can then explicitly understand their importance in regulating cancer progression. Human cells are constantly exposed to various stresses, such as radiation, carcinogens, oxidative stress, and oncogene activation [32], which can cause damage to the cell, and thus lead to malignant transformation [33]. Growing evidence shows that tumors can progress in a microenvironment not necessarily filled with transformed cells, which can be attributed to the crosstalk accomplished by secreted cytokines between normal and malignant cells [34]. NLRs have been implicated in various diseases caused by its involvement in promoting inflammation and immunity, and its ability to inhibit anti-tumor immunosurveillance. Therefore, nod-like receptors have been believed to contribute to the development of cancer [35]. The extracellular matrix (ECM) is a very essential intracellular component that regulates tissue development and maintains homeostasis. Its deregulation contributes to tumor progression [32]. By interacting with specific receptors, such as integrins, ECM can also regulate the availability of growth factors and cytokines, which also broaden its function in regulating tumor cell progression [36].
Conclusions
Our study shows that the over-expression of LYN gene is highly correlated with the development and progression of esophageal adenocarcinoma by being the key nodule of all GO networks. Although its function needs to be further confirmed, this result strongly indicates that LYN gene might be a novel and potential diagnostic and therapeutic target in clinical esophageal adenocarcinoma treatment.
Supplementary materials
Supplementary Table 1.
Significantly changed GO biological processes for DEGs only in comparison 1.
| GO-BP-ID | P Value | Count | Term |
|---|---|---|---|
| GO: 0051403 | 9.37E-05 | 3 | Stress-activated MAPK cascade |
Supplementary Table 2.
Significantly changed GO biological processes for DEGs only in comparison 2.
| GO-BP-ID | P Value | Count | Term |
|---|---|---|---|
| GO: 0043588 | 9.95E-14 | 36 | Skin development |
| GO: 0006950 | 8.51E-11 | 135 | Response to stress |
| GO: 0060429 | 7.09E-09 | 56 | Epithelium development |
| GO: 0030154 | 1.09E-08 | 126 | Cell differentiation |
| GO: 0016477 | 2.25E-07 | 52 | Cell migration |
| GO: 0008283 | 2.54E-07 | 76 | Cell proliferation |
Supplementary Table 3.
Significantly changed KEGG pathways for DEGs changed only in comparison 1.
| KEGGID | P Value | Count | Term |
|---|---|---|---|
| 04010 | 3.64E-04 | 3 | MAPK signaling pathway |
Supplementary Table 4.
Significantly changed KEGG pathways for DEGs changed only in comparison 2.
| KEGGID | P Value | Count | Term |
|---|---|---|---|
| 04060 | 1.50E-02 | 14 | Cytokine-cytokine receptor interaction |
| 04621 | 2.18E-02 | 5 | Nod-like receptor signaling pathway |
| 04512 | 3.02E-02 | 6 | ECM-receptor interaction |
Footnotes
Source of support: Departmental sources
References
- 1.Wheeler JB, Reed CE. Epidemiology of esophageal cancer. Surg Clin North Am. 2012;92(5):1077–87. doi: 10.1016/j.suc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–87. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 3.Denlinger CE, Thompson RK. Molecular basis of esophageal cancer development and progression. Surg Clin North Am. 2012;92(5):1089–103. doi: 10.1016/j.suc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg. 2013;61(6):330–35. doi: 10.1007/s11748-013-0246-0. [DOI] [PubMed] [Google Scholar]
- 5.Streppel MM, Montgomery EA, Maitra A. New advances in the pathogenesis and progression of barrett’s esophagus. Curr Mol Med. 2014;14(1):58–68. doi: 10.2174/15665240113139990068. [DOI] [PubMed] [Google Scholar]
- 6.Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med. 2014;371(9):836–45. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 7.Hagen CE, Lauwers GY, Mino-Kenudson M. Barrett esophagus: diagnostic challenges. Semin Diagn Pathol. 2014;31(2):100–13. doi: 10.1053/j.semdp.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373(9666):850–61. doi: 10.1016/S0140-6736(09)60487-6. [DOI] [PubMed] [Google Scholar]
- 9.Fouad YM, Mostafa I, Yehia R, El-Khayat H. Biomarkers of Barrett’s esophagus. World J Gastrointest Pathophysiol. 2014;5(4):450–56. doi: 10.4291/wjgp.v5.i4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimchi ET, Posner MC, Park JO, et al. Progression of Barrett’s metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Res. 2005;65(8):3146–54. doi: 10.1158/0008-5472.CAN-04-2490. [DOI] [PubMed] [Google Scholar]
- 11.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 12.Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21(9):2067–75. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- 13.Sharov AA, Dudekula DB, Ko MS. A web-based tool for principal component and significance analysis of microarray data. Bioinformatics. 2005;21(10):2548–49. doi: 10.1093/bioinformatics/bti343. [DOI] [PubMed] [Google Scholar]
- 14.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 15.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao XY, Li W, Lv Z, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461(7260):86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 17.Dennis G, Jr, Sherman BT, Hosack DA, et al. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 18.Anaparthy R, Sharma P. Progression of Barrett oesophagus: role of endoscopic and histological predictors. Nat Rev Gastroenterol Hepatol. 2014;11(9):525–34. doi: 10.1038/nrgastro.2014.69. [DOI] [PubMed] [Google Scholar]
- 19.Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12(3):170–80. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- 20.Antsiferova M, Werner S. The bright and the dark sides of activin in wound healing and cancer. J Cell Sci. 2012;125(Pt 17):3929–37. doi: 10.1242/jcs.094789. [DOI] [PubMed] [Google Scholar]
- 21.Riss J, Khanna C, Koo S, et al. Cancers as wounds that do not heal: differences and similarities between renal regeneration/repair and renal cell carcinoma. Cancer Res. 2006;66(14):7216–24. doi: 10.1158/0008-5472.CAN-06-0040. [DOI] [PubMed] [Google Scholar]
- 22.Markiewski MM, Nilsson B, Ekdahl KN, et al. Complement and coagulation: strangers or partners in crime? Trends Immunol. 2007;28(4):184–92. doi: 10.1016/j.it.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Yu SH, Bordeaux JS, Baron ED. The immune system and skin cancer. Adv Exp Med Biol. 2014;810:182–91. doi: 10.1007/978-1-4939-0437-2_10. [DOI] [PubMed] [Google Scholar]
- 24.Krarup A, Wallis R, Presanis JS, et al. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2(7):e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messina NL, Banks KM, Vidacs E, et al. Modulation of antitumour immune responses by intratumoural Stat1 expression. Immunol Cell Biol. 2013;91(9):556–67. doi: 10.1038/icb.2013.41. [DOI] [PubMed] [Google Scholar]
- 26.Iqbal MS, Tsuyama N, Obata M, Ishikawa H. A novel signaling pathway associated with Lyn, PI 3-kinase and Akt supports the proliferation of myeloma cells. Biochem Biophys Res Commun. 2010;392(3):415–20. doi: 10.1016/j.bbrc.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 27.Choi YL, Bocanegra M, Kwon MJ, et al. LYN is a mediator of epithelial-mesenchymal transition and a target of dasatinib in breast cancer. Cancer Res. 2010;70(6):2296–306. doi: 10.1158/0008-5472.CAN-09-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wheeler SE, Morariu EM, Bednash JS, et al. Lyn kinase mediates cell motility and tumor growth in EGFRvIII-expressing head and neck cancer. Clin Cancer Res. 2012;18(10):2850–60. doi: 10.1158/1078-0432.CCR-11-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutton P, Borgia JA, Bonomi P, Plate JM. Lyn, a Src family kinase, regulates activation of epidermal growth factor receptors in lung adenocarcinoma cells. Mol Cancer. 2013;12:76. doi: 10.1186/1476-4598-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zardan A, Nip KM, Thaper D, et al. Lyn tyrosine kinase regulates androgen receptor expression and activity in castrate-resistant prostate cancer. Oncogenesis. 2014;3:e115. doi: 10.1038/oncsis.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Pflaum J, Schlosser S, Muller M. p53 family and cellular stress responses in cancer. Front Oncol. 2014;4:285. doi: 10.3389/fonc.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haigis KM, Sweet-Cordero A. New insights into oncogenic stress. Nat Genet. 2011;43(3):177–78. doi: 10.1038/ng0311-177. [DOI] [PubMed] [Google Scholar]
- 34.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4(1):11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 35.Kent A, Blander JM. Nod-like receptors: key molecular switches in the conundrum of cancer. Front Immunol. 2014;5:185. doi: 10.3389/fimmu.2014.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15(12):1243–53. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Significantly changed GO biological processes for DEGs only in comparison 1.
| GO-BP-ID | P Value | Count | Term |
|---|---|---|---|
| GO: 0051403 | 9.37E-05 | 3 | Stress-activated MAPK cascade |
Supplementary Table 2.
Significantly changed GO biological processes for DEGs only in comparison 2.
| GO-BP-ID | P Value | Count | Term |
|---|---|---|---|
| GO: 0043588 | 9.95E-14 | 36 | Skin development |
| GO: 0006950 | 8.51E-11 | 135 | Response to stress |
| GO: 0060429 | 7.09E-09 | 56 | Epithelium development |
| GO: 0030154 | 1.09E-08 | 126 | Cell differentiation |
| GO: 0016477 | 2.25E-07 | 52 | Cell migration |
| GO: 0008283 | 2.54E-07 | 76 | Cell proliferation |
Supplementary Table 3.
Significantly changed KEGG pathways for DEGs changed only in comparison 1.
| KEGGID | P Value | Count | Term |
|---|---|---|---|
| 04010 | 3.64E-04 | 3 | MAPK signaling pathway |
Supplementary Table 4.
Significantly changed KEGG pathways for DEGs changed only in comparison 2.
| KEGGID | P Value | Count | Term |
|---|---|---|---|
| 04060 | 1.50E-02 | 14 | Cytokine-cytokine receptor interaction |
| 04621 | 2.18E-02 | 5 | Nod-like receptor signaling pathway |
| 04512 | 3.02E-02 | 6 | ECM-receptor interaction |