Abstract
Background
The endogenous protein annexin A1 (ANXA1) is an anti-inflammatory mediator in the brain that is thought to contribute to the progression of many neurological conditions. However, its exact role in temporal lobe epilepsy (TLE) remains unclear. We hypothesized that ANXA1 exerts negative actions on TLE by alleviating inflammatory damage in neurons. To identify the potential mechanism of TLE by assessing ANXA1 expression in TLE rats.
Material/Methods
TLE was induced in rats (n=70) via an intraperitoneal injection of lithium chloride (LiCl) and pilocarpine (PILO). The control group (n=10) received an injection of the equivalent amount of saline. ANXA1 expression was detected via immunohistochemistry and Western blotting.
Results
Successful establishment of the TLE model in rats resulted in epileptic seizures. ANXA1 was immunohistochemically detected as brownish yellow particles in the dentate gyrus and the CA1 region of the door zone; this expression was predominantly localized to the cytoplasm of glia rather than neurons. ANXA1 expression was stronger in TLE rats compared with the control group. ANXA1 expression in TLE was also assessed via Western blotting, and compared between groups at various time points. ANXA1 expression was significantly increased in the acute (the first 24 h) and chronic (after 1 month) phases (P<0.001) but significantly decreased during the recovery phase (72 h, 1 week, and 2 weeks) (P<0.001). These findings suggest that ANXA1 expression is correlated with TLE activity.
Conclusions
Our data suggest that ANXA1 plays an important role in TLE by alleviating inflammatory damage and protecting neurons.
MeSH Keywords: Annexin A1; Epilepsy, Temporal Lobe; Glial Cells
Background
Temporal lobe epilepsy (TLE) is a type of focal and refractory epilepsy [1]. Approximately 1.2 million individuals in China with TLE suffer chronic seizures that cannot be controlled by antiepileptic drugs, which consequently leads to cognitive deficits and chronic health problems [2]. Currently, the mechanism of antiepileptic drugs (AEDs) is mainly aimed at enhancing the function of nervous system inhibition, keeping the membrane of neurons stable, and preventing release of the excitatory neurotransmitters. However, it is urgent to investigate the mechanism of TLE to develop new efficacious treatments.
Epilepsy is a brain disease, and its pathogenesis remains unclear. Although various hypotheses have been proposed, the exact mechanism responsible for disease development is unknown. The inflammatory response in the brain following a seizure is one of the main causes of pathological damage [3]. Inflammatory injury, microglial activation, and cell proliferation typically occur after epileptic seizures, and these processes also contribute to the detrimental effects of epileptic seizures [4]. Thus, it has been suggested that the frequency of epileptic seizures should be decreased to alleviate the inflammatory reaction in epilepsy via therapeutic methods.
The endogenous protein annexin A1 (ANXA1) is an anti-inflammatory mediator in the brain [5]. Previous studies have addressed the role of ANXA1 in humans, and the most important function involves the phagocytosis of apoptotic leukocytes during peripheral inflammatory resolution [6]. Additionally, evidence suggests that ANXA1 is up-regulated in many nervous system diseases, such as multiple sclerosis [7] and Alzheimer’s disease [8]. This phenomenon cannot be explained by an accepted theory, although researchers have suggested that ANXA1 released from microglia exerts neuroprotective effects directly at the neuronal cell membrane [9]. Furthermore, ANXA1 was shown to suppress the excessive production of cytotoxic pro-inflammatory mediators secreted by activated microglial cells in an autocrine and/or paracrine fashion [10]. Thus, ANXA1 is thought to contribute to the progression of many neurological conditions, and it appears to be associated with epilepsy via the inflammatory response.
Previous evidence has indicated that the ANXA1 gene is up-regulated more than 2-fold in hippocampal tissues after lithium chloride (LiCl) and pilocarpine (PILO)-induced TLE; however, the mechanism responsible for this effect remains unclear [11]. We hypothesize that ANXA1 inhibits inflammation in TLE to protect neurons from injury and promote recovery. Thus, this study aimed to identify the relationship between ANXA1 and TLE.
Material and Methods
Animals
All animal experimental protocols were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, which were approved by the Institutional Animal Care and Use Committee of Renmin Hospital of Wuhan University. All animal experiments were conducted in a humane manner. Eighty male Sprague-Dawley rats that weighed 180–220 g were obtained from the Experimental Animal Center of Wuhan University, China. The animals were housed at 24–25ºC and 50–60% humidity on a 12-h light/dark cycle and allowed free access to food and water.
Methods
Establishment of the TLE animal model
The rats were treated with LiCl (Sigma-Aldrich, Santa Clara, CA, USA; 127 mg/kg) via an intraperitoneal (i.p.) injection. After 18 h, the animals were injected with PILO (15 mg/kg, i.p.) (Sigma-Aldrich) diluted in physiological saline. Atropine sulfate (1 mg/kg, i.p.) (Harvest, Shanghai, China) was injected approximately 30 min prior to PILO administration. The seizure severity was graded according to the Racine standard as follows: stage 1, mouth and face movements; stage 2, head nods; stage 3, clonus of the unilateral forelimb; stage 4, clonus and rears of the bilateral forelimb; and stage 5, falling episodes after a stage 4 seizure [12]. PILO was administered every 30 min if the seizure attacks or seizure activities were above stage 4 on Racine’s scale. The rats graded at stages 4 or 5 were used in the present study. The maximum dose for PILO was 60 mg/kg. Status epilepticus that lasted for 1 h was terminated via diazepam (10 mg/kg, i.p.) (Jinyao, Tianjin, China). The rats with status epilepticus that entered the latency period at 24 h after an acute attack were selected and reared in single cages.
Behavioral observation
Fifteen days after status epilepticus onset, monitor the occurrences of spontaneous recurrent seizures for 4 h. Behavioral parameters, such as the latency period (the period between the acute seizure and spontaneous recurrent seizures), seizure frequency, duration of the chronic phase, and mortality during all periods, were also recorded.
Immunohistochemistry
Immunohistochemistry was performed using antibodies against ANXA1 in the hippocampus, followed by horseradish peroxidase labeling. The sections were examined microscopically after staining.
Western blot analysis
Proteins were extracted from rat brain tissues using a protein extraction kit. Anti-β-actin labeling was used as a control. ANXA1 expression was determined for each group at different time points. We used 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for separating protein. For Western blot analysis, protein bands on the PAGE were transferred in wet condition into the polyvinylidene difluoride (PVDF) membrane, and protein was detected by anti-His(C-term)-HRP antibody. The yield of expressed protein was determined by AlphaEase FC software after image acquisition.
Statistical analysis
All data are presented as the mean ± SEM. The Mann-Whitney test was used to analyze the differences between 2 groups, and comparisons of >2 groups were conducted via a Kruskal-Wallis analysis (nonparametric test, SPSS 17.0). The statistical significance was set at P<0.05.
Results
Behavioral changes with TLE
Eighty rats were randomly divided into the control group (10 rats) and the model group (70 rats), which was further subdivided into 5 groups based on the time of epileptic seizure (24 h, 72 h, 1 week, 2 weeks, and 1 month). Seventy rats suffered an epileptic fit, and all rats were characterized as Racine stage 4 or higher; 6 rats died due to severe status epilepticus. Several minutes after LiCl and PILO administration, the rats in the model group exhibited a series of peripheral cholinergic reactions, such as oral and masticatory movements, hypokinesia, head nodding, and wet-dog shakes. During the 72 h to 2 weeks after model induction, the balance continued to swing away from status epilepticus to behaviors of listlessness, lack of activity, and dullness of the mind. Two weeks later, the rats suffered spontaneous recurrent epileptic seizures that lasted 30 s to 40 s every half an hour. The entire process of the epileptic state following the incubation period represents the typical characteristics of TLE in rats.
ANXA1 expression in TLE
ANXA1 expression was measured via Western blot. ANXA1 has a molecular weight of 37 KD. The ANXA1 expression was maintained at a low level in each group; however, the expression in the model groups was higher than that in the control group. Furthermore, the ANXA1 expression fluctuated with disease period; the expression increased within 24 h, decreased to the lowest level between 72 h to 2 weeks, recovered during the chronic stage (1 month) and was then maintained at a high level. These findings suggest that ANXA1 is expressed in response to epilepsy; however, the functional significance of ANXA1 expression is unknown (Table 1, Figure 1).
Table 1.
The ratio of OD described ANXA1/β-actin in each group.
| Control | 24 h | 72 h | 1 week | 2 weeks | 1 month | |
|---|---|---|---|---|---|---|
| ANXA1/β-actin | 0.434±0.092 | 1.102±0.103* | 1.005±0.098* | 0.847±0.132* | 0.793±0.118* | 1.214±0.102* |
Compared with the control group, there was a significant difference in each model group.
P<0.001.
Figure 1.
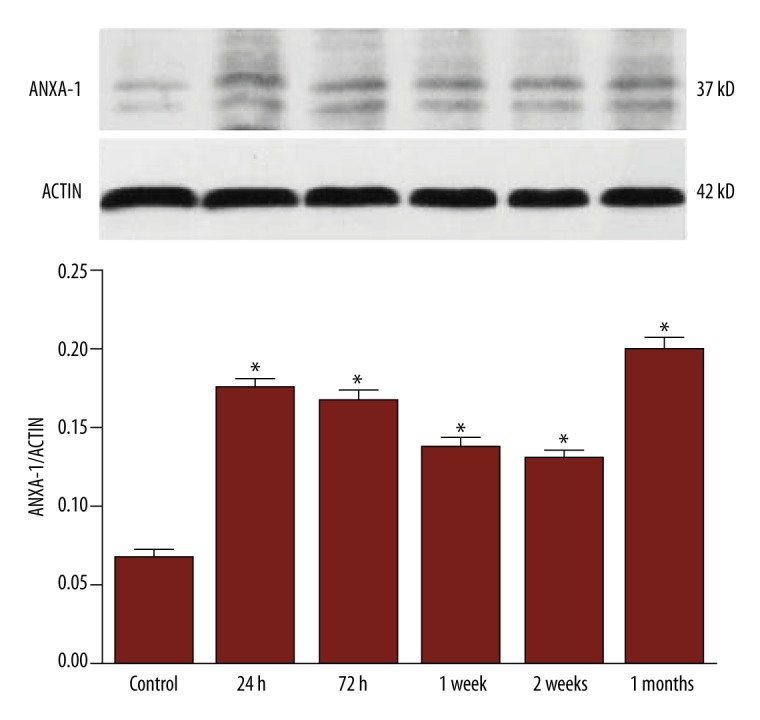
Dynamic changes in ANXA1 expression in TLE. ANXA1 expression was dynamically altered in rats with TLE; this expression visibly increased during the acute phase (24 h) and gradually decreased during the incubation period (72 h to 2 weeks). There was no significant difference between the acute phase and the initial part of the latent period (72 h); however, during the second week, there was a significant difference between groups (* P<0.001). A significant increase was also observed from the incubation period (2 weeks) to the chronic stage (1 month), after which ANXA1 expression was maintained at a high level (* P<0.001).
Localization of ANXA1 expression in TLE
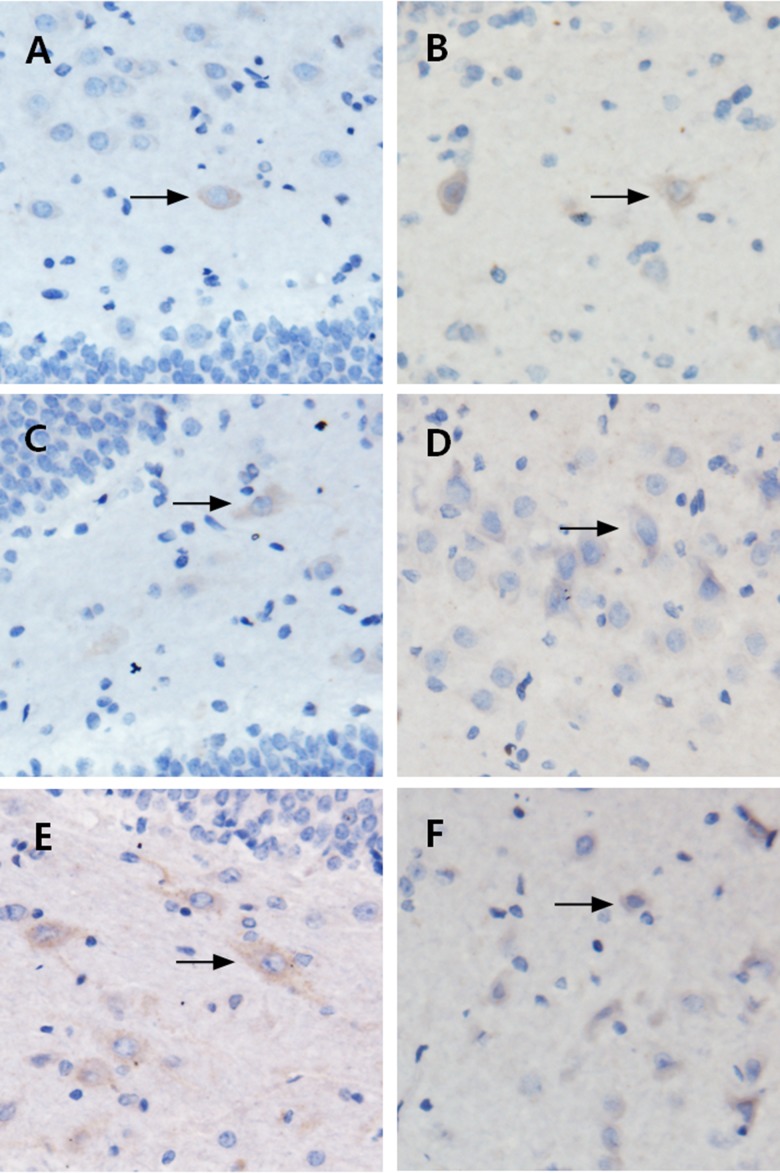
The dentate gyrus of the hippocampus was shaped like a “V” or “U” and it mainly contained granule cells and polymorphic cells. Glial cells were scattered inside the dentate gyrus and showed pleomorphism. ANXA1 expression was visualized as brownish yellow particles by immunohistochemical staining. These findings confirmed that ANXA1 was mainly distributed in the cytoplasm of glial cells in the hippocampus, and the staining in the model groups was stronger than that in the control group (Figure 2).
Figure 2.
Localization of ANXA1 via immunohistochemistry. A (control), B (24 h), C (72 h), D (1 week), E (2 weeks), and F (1 month). Immunohistochemically, the glial cells in the hippocampus stained positive for ANXA1, as demonstrated by brownish yellow-stained particles distributed in the cytoplasm. (A) The control group, showed brownish yellow particles with weakly positive staining; (B–F) The model groups showed brownish yellow particles with deep staining, especially in B and F. The ANXA1 expression was more pronounced in the model groups compared to the control group.
Discussion
During epileptic seizures, neurons exhibit varying degrees of damage due to hypoxia, which can lead to abnormal physiological neuron metabolism and the inhibition of brain development. Furthermore, hypoxic conditions also aggravate epilepsy and causes recurrent seizures.
ANXAl is involved in phagocytosis, inflammation, cell signaling, cell differentiation, and apoptosis [13,14], as well as other related regulatory functions. A study found that over-expression of ANXA1 could enhance NO production and iNOS expression by a post-transcriptional mechanism [15]. The mechanism by which ANXA1 may modulate inflammation is by affecting inflammatory protein translation within the cell. Moreover, cyclooxygenase-2 (COX-2) is an enzyme involved in the conversion of arachidonic acid to prostaglandins. Glucocorticoids are highly effective inhibitors of COX-2 expression. The steroid-regulated protein ANXA1, which is implicated in mediating some of the activities of glucocorticoids, may also be involved in the inflammatory network linked to COX-2 [16,17]. Some studies suggest that ANXA1 in the central nervous system plays a role in anti-inflammation and the maintenance of stability in the brain [18]. Therefore, we propose that ANXA1 contributes to the regulation of inflammation and cell apoptosis in the central nervous system, which may protect neurons and reduce damage through increased ANXA1 expression. In this study, we evaluated changes in ANXA1 expression following epileptic seizures to explore the potential mechanism of its function.
In particular, we established the TLE model via intraperitoneal injection of LiCl-PILO, and the evaluation criteria indicated that the model was successfully induced based on the behavior of the rats. According to the Racine classification criteria, the success of the model was based on a seizure level at Racine grades 4–5. The TLE rats in our study all achieved these requirements, which indicated the model was reliable.
The hippocampus is the most sensitive brain tissue to hypoxic injury; thus, our study investigated ANXA1 expression in hippocampal tissue. Our results demonstrated that ANXA1 expression increased during the acute phase (the first 24 h) after seizure. It has been reported that the regulation of ANXA1 expression represents a mechanism by which glucocorticoids respond to inflammation [19,20]; thus, glucocorticoids may stimulate immune cells to produce ANXA1 for anti-inflammatory effects. Therefore, we suspect that ANXA1 is induced via inflammatory cytokines and glucocorticoids as a result of epileptic seizure and the stress reaction during the acute phase of injury. Up-regulated ANXA1 may also inhibit inflammatory factors to reduce inflammation and protect neurons from inflammatory injury, thereby promoting recovery. During the incubation period (72 h, 1 week, and 2 weeks), ANXA1 expression decreased over time. We suspect that high levels of ANXA1 precede the anti-inflammatory and cell-repair status in the brain. Furthermore, the stress reaction is terminated when the glucocorticoid levels decrease, at which point ANXA1 expression was also reduced. However, injury to the central nervous system persists during the incubation period, and other variables in addition to ANXA1 are involved in the anti-inflammatory response; thus, the amount of ANXA1 remained higher than that in the control group. During the chronic phase (after 1 month), ANXA1 again increased and reached its highest level of expression. We suspect that recurrent seizures during the chronic phase led to continuous neuronal damage and persistent impairment of the central nervous system. The maintenance of a high level of ANXA1 may be required to perform anti-inflammatory and cell-repair functions and promote apoptosis. At this time, the balance between injury to the central nervous system caused by epilepsy and ANXA1 expression reaches equilibrium.
These findings indicate that ANXA1 expression is altered at various times following epileptic seizures, which may be related to the extent of subsequent brain damage. We suggest that ANXA1 plays a protective role in epilepsy; however, additional research is required to explore the mechanism responsible for this function.
Conclusions
In summary, our data suggest that TLE is associated with increased and dynamic ANXA1 expression, which appears to have anti-inflammatory effects involving glial cells during epileptic seizure injury. However, the specific mechanism and the pathway responsible for this role of ANXA1 remain unclear. Thus, additional research is needed to explore the reaction process, as well as to determine whether ANXA1 reduces epileptic seizures in humans.
Acknowledgements
The authors appreciate the support received from members of the Renmin Hospital of WuHan University.
Footnotes
Source of support: Departmental support
Statements and declarations regarding conflicts of interest
There were no direct or indirect relationships, such as equity, consulting, research support and funding, or corporate patents, that dealt with the material or subject matter of this study.
References
- 1.Beleza P. Refractory epilepsy: a clinically oriented review. Eur Neurol. 2009;62:65–71. doi: 10.1159/000222775. [DOI] [PubMed] [Google Scholar]
- 2.Quan Z, Xianrui Y, Zhidong S. The epidemiological and sociological analysis, current situation and our strategies of the epilepsy. J Shanxi Med U. 2005;7:217–20. [Google Scholar]
- 3.Ravizza T, Vezzani A. Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neurosci. 2006;137(1):301–8. doi: 10.1016/j.neuroscience.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 4.Qian X, Chunling Z. Advances in research of pathogenesis of epilrpsy. Modern Med Health. 2009;9:1373–75. [Google Scholar]
- 5.Solito E, McArthur S, Christian H, et al. Annexin A1 in the brain – undiscovered roles? Trends Pharmacol Sci. 2008;29:135–42. doi: 10.1016/j.tips.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 6.McArthur S, Cristante E, Paterno M, et al. Annexin A1: a central player in the anti-inflammatory and neuroprotective role of microglia. J Immunol. 2010;185:6317–28. doi: 10.4049/jimmunol.1001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Probst-Cousin S, Kowolik D, Kuchelmeister K, Kayser C, et al. Expression of annexin-1 in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 2002;28:292–300. doi: 10.1046/j.1365-2990.2002.00396.x. [DOI] [PubMed] [Google Scholar]
- 8.Savchenko VL, McKanna JA, Nikonenko IR, Skibo GG. Microglia and astrocytes in the adult rat brain: comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neurosci. 2000;96:195–203. doi: 10.1016/s0306-4522(99)00538-2. [DOI] [PubMed] [Google Scholar]
- 9.Young KA, Hirst WD, Solito E, Wilkin GP. De novo expression of lipocortin-1 in reactive microglia and astrocytes in kainic acid lesioned rat Cerebellum. Glia. 1999;26:333–43. doi: 10.1002/(sici)1098-1136(199906)26:4<333::aid-glia7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–39. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- 11.Jin Z, Meiping D, Zhao L, et al. [Gene and protein alterations in the hippocampus of rats with temporal lobe epilepsy]. J Pathophysiol. 2006;09:1779–83. in Chinese. [Google Scholar]
- 12.Racine R, Okujava V, Chipashvili S. Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr Clin Neurophysiol. 1972;32:295–99. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- 13.Patel DM, Ahmad SF, Weiss DG, et al. Annexin A1 is a new functional linker between actin filaments and phagosomes during phagocytosis. J Cell Sci. 2011;124:578–88. doi: 10.1242/jcs.076208. [DOI] [PubMed] [Google Scholar]
- 14.Horlacher T, Noti C, de Paz JL, et al. Characterization of annexin A1 glycan binding reveals binding to highly sulfated glycans with preference for highly sulfated heparan sulfate and heparin. Biochemistry. 2011;50:2650–59. doi: 10.1021/bi101121a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smyth T, Harris HJ, Brown A, et al. Differential modulatory effects of Annexin 1 on nitric oxide synthase induction by lipopolysaccharide in macrophages. Immunology. 2006;117(3):340–49. doi: 10.1111/j.1365-2567.2005.02307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bo-wei L, Jin-min Z, Guo-qian Y, et al. Progress on the physiological function of ANXA1. Journal of SNAKE. 2012;24(2):160–62. [Google Scholar]
- 17.Gastardelo TS, Cunha BR, Raposo LS, et al. Inflammation and cancer: Role of Annexin A1 and FPR2/ALX in proliferation and metastasis in human laryngeal squamous cell carcinoma. PLoS One. 2014;9(12):e111317. doi: 10.1371/journal.pone.0111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim LH, Pervaiz S. Annexin 1: The new face of an old molecule. FASEB J. 2007;21:968–75. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- 19.Luthra R, Singh RR, Luthra MG, et al. MicroRNA-196a targets annexin A1: a microRNA-mediated mechanism of annexin A1 downregulation in cancers. Oncogene. 2008;27:6667–78. doi: 10.1038/onc.2008.256. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Yoshihara E, Son A, et al. Differential roles of Annexin A1 (ANXA1/lipocortin-1/lipomodulin) and thioredoxin binding protein-2(TBP-2/VDUP1/TXNIP) in glucocorticoid signaling of HTLV-I-transformed T cells. Immunol Lett. 2010;131:11–18. doi: 10.1016/j.imlet.2010.04.003. [DOI] [PubMed] [Google Scholar]