Abstract
A systematic review was conducted to identify evidence-based interventions (EBIs) for increasing HIV medication adherence behavior or decreasing HIV viral load among persons living with HIV (PLWH). We conducted automated searches of electronic databases (i.e., MEDLINE, EMBASE, PsycINFO, CINAHL) and manual searches of journals, reference lists, and listservs. Interventions were eligible for the review if they were U.S.-based, published between 1996 and 2011, intended to improve HIV medication adherence behaviors of PLWH, evaluated the intervention using a comparison group, and reported outcome data on adherence behaviors or HIV viral load. Each intervention was evaluated on the quality of study design, implementation, analysis, and strength of findings. Of the 65 eligible interventions, 10 are EBIs. The remaining 55 interventions failed to meet the efficacy criteria primarily due to null findings, small sample sizes, or low retention rates. Research gaps and future directions for development of adherence EBIs are discussed.
Keywords: HIV/AIDS, Intervention, Medication adherence, Evidence-based, Antiretroviral therapy
Introduction
Due to the availability and advancement of highly active antiretroviral therapy (HAART) as well as an increasing number of persons living with HIV (PLWH), there has been an increased focus on both health promotion and HIV prevention for PLWH [1]. The individual health benefits of antiretroviral treatment (ART) for PLWH are clear [2–6]; however, the success of ART is related to the patient’s level of medication adherence. High adherence rates have consistently been associated with decreased viral load, less risk of progression to AIDS, and a decreased risk of developing drug-resistant strains of HIV [7–10], whereas poor adherence is associated with treatment failure, lower CD4 cell counts, and increased mortality [10–19]. Recently, the HIV Prevention Trials Network 052 study comparing early versus delayed ART for HIV patients with CD4 cell counts between 350 and 550 cells/mm3 found a 96 % reduction in the number of linked HIV transmissions for those with early ART initiation. This finding suggests that, in addition to individual health benefits, ART has significant prevention benefits in that successful viral suppression can lead to a reduction in HIV transmission risk [20].
However, the most recent surveillance data from the Centers for Disease Control and Prevention (CDC) showed that among 1.15 million PLWH in the United States (U.S.) in 2009, 33 % were prescribed ART and only 25 % were estimated to have the suppressed viral load needed to maximally prolong health and prevent transmission [21].
Optimal adherence to ART is critical to fully achieve both the clinical and preventive benefits of ART. However, a recent meta-analysis suggests that adherence levels remain suboptimal. In 84 studies across 20 countries, an average of 62 % of participants reported ≥90 % adherence to HAART [22]. Maintaining high levels of adherence to medications for a chronic condition is extremely difficult and often requires additional support. Some barriers to HIV medication adherence are identified, including lack of knowledge and competence regarding how to maintain good adherence [11, 23–26]; patient-provider relationship [8, 11, 27, 28]; and psychosocial factors such as depression, anxiety, fatigue, and stress, as well as lack of social support and negative attitudes about the HIV disease [8, 11, 23–32].
The scientific literature focusing on developing and testing behavioral interventions to address identified barriers and help PLWH adhere to their medications continues to expand, particularly as PLWH are living longer and as medication regimens are evolving over time. Overall, the positive effects of these interventions on adherence behaviors and viral load has been highlighted through several quantitative and qualitative systematic reviews [25, 33–38]. These reviews are useful for understanding the overall potential for interventions to improve medication adherence; however, they typically do not critically evaluate the study design, implementation, analysis, and strength of findings of individual interventions. Doing so may help identify model programs, with rigorous methods and strong findings, which could be used by prevention providers within their own clinics or communities. Therefore, there remains a need to supplement these reviews by identifying individual interventions with evidence of efficacy.
CDC’s HIV/AIDS Prevention Research Synthesis (PRS) Project
In order to identify evidence-based interventions (EBIs) for the HIV prevention field, the CDC established the HIV/AIDS PRS project in 1996 (http://www.cdc.gov/hiv/dhap/prb/prs/index.html) [39]. The aim of the PRS project is to review and synthesize the cumulative body of evidence of HIV prevention interventions from the scientific research literature to help inform policy decisions and programmatic efforts within the U.S. and to guide future research. Since 1996, the PRS team has been conducting meta-analyses and systematic efficacy reviews focused on interventions to change sex and drug behaviors related to HIV acquisition and transmission. In late 2008, the PRS team expanded the scope to include medication adherence interventions and began a new systematic review to identify EBIs for improving HIV medication adherence among PLWH (referred to as “adherence EBIs”).
This article focuses on the findings from the PRS systematic efficacy review process for identifying adherence EBIs. First, we briefly describe methods for developing the efficacy criteria and the final criteria for evaluating adherence interventions. Second, we provide a summary of the adherence EBIs identified through our systematic review process and compare the EBIs to interventions that did not meet our efficacy review criteria. Finally, we provide recommendations for future programmatic and research activities.
Methods
PRS Efficacy Criteria for HIV Medication Adherence Interventions
Between 2008 and 2010, the PRS team conducted a series of activities to develop the efficacy criteria to evaluate the evidence from published HIV medication adherence intervention studies. These included repeated consultations with CDC scientists, key federal partners including the National Institute of Mental Health (NIMH), the National Institute of Drug Abuse (NIDA), and the Health Resources and Services Administration (HRSA), and non-federal researchers with substantial expertise in HIV medication adherence issues. The existing PRS efficacy criteria for HIV-related sex and drug risk reduction interventions were used as the initial framework and were adapted to address issues relevant for adherence intervention studies.
To ensure a reasonable level of confidence that observed changes could be attributed to the intervention under evaluation, these criteria focus heavily on elements related to internal validity and assess risk of bias in individual studies (e.g., potential bias resulted from allocation method, reassignment, baseline group equivalence, attrition, measurement and confounding factors). The criteria assess factors across four domains: the quality of study design, quality of study implementation, quality of study analysis, and strength of evidence. Based on the overall set of criteria, adherence EBIs are classified as either good-evidence or best-evidence. Good-evidence interventions are considered to have been evaluated using scientifically sound methods and provide sufficient evidence of efficacy and must meet each element in the efficacy criteria (Table 1). Best-evidence interventions are considered to have been rigorously evaluated and provide the strongest evidence of efficacy and must meet additional elements within the efficacy criteria (Table 2). In Tables 1 and 2, we list the PRS efficacy criteria and indicate whether the criteria were supported by other systematic review or evidence-based groups, based on empirical evidence, or recommended by our consultants. Appendix A in supplementary material provides more detailed explanation on the complex elements of our efficacy criteria.
Table 1.
PRS criteria for good-evidence medication adherence behavioral interventions
| Intervention description |
| Clear description of key aspects of the interventiona |
| Quality of study design |
| At least a quasi-prospective study designa |
| Appropriate comparison arma |
| At least a non-concurrent comparison arm that was implemented within 12 months of the start of the intervention and was similar with respect to population characteristics and settingc |
| At least non-random allocation with minimal or moderate selection bias unrelated to the intervention or adherence behaviora |
| Quality of study implementation |
| At least a 1-month post-intervention follow-up assessment for each study arm (with recall not referring to pre-intervention period) for interventions that are clearly discrete or at least a 3-months post-initiation follow-up assessment for each study arm for all other types of interventionsc |
| At least a 60 % retention rate (or medical chart recovery) at a single required assessment time point for each study armb |
| Quality of study analysis |
| Analysis contrasting intervention arm and an appropriate comparison arma |
| Intent-to-treat analysis |
| Analysis of participants in study arms as originally allocateda |
| Analysis of participants regardless of the level of intervention exposurea |
| Comparability of measures |
| Measures must be identical, including recall, for any repeated measures or change score analysesa |
| Baseline measures do not have to be identical, but must be of the same construct as outcome measures, if used as a covariate in analyses (i.e., adjusted for BL)a |
| Analysis based on a 2-sided test and an α = .05 (or more stringent)a |
| Analytic sample of at least 40 participants in each study arma |
| Non-randomized controlled trials (non-RCTs) must either demonstrate baseline equivalence or control for baseline differences in outcome variables. Non-RCTs with moderate bias or non-concurrent comparison must also demonstrate baseline equivalence or control for baseline differences in demographics and other critical variablesa |
| Strength of evidence—significant positive intervention effects |
| Positive and statistically significant (p ≤ .05) intervention effect for at least 1 relevant behavioral outcome measure or 1 relevant biologic outcome measure(defined as greater improvement in, or better level of, medication adherence behavioral or biologic outcome in the intervention arm relative to the comparison arm)a |
| A relevant behavioral outcome measure may include electronic data monitoring (e.g., MEMs caps), pill count, pharmacy refill, or self-reported adherence. |
| A relevant biologic outcome measure may include a lab test or medical chart recovery of HIV viral load levelsc |
| Effect at the follow-up and based on the analyses that meet study design, implementation and analysis criteriac |
| Strength of evidence—significant negative intervention effects |
| No negative and statistically significant (p ≤ .05) intervention effect for any relevant outcomea |
| A negative intervention effect is defined as a statistically significant greater improvement in, or better level of, HIV-related behavioral or biologic outcomes in the comparison arm relative to the intervention arm. |
| No other statistically significant harmful intervention effect on other outcomesa |
| For intervention with a replication evaluation, no significant negative intervention effectsa |
| Additional limitations to evaluate |
| The totality of the limitations (as described below) cannot introduce considerable bias that substantially reduces the confidence placed on the findings |
| Examples of limitations to check |
| Intervention and comparison arms did not receive similar medication regimensc |
| Findings based on too many post hoc analysesa |
| Inconsistent evidence between effectsa |
| Inconsistent evidence across intervention comparisons within the studya |
| Effects only found within a potentially biased subgroup analysisa |
| Substantial (>40 %) overall missing data (due to attrition and non-attrition such as missing responses)c |
| Substantial differential attrition in rates (>10 %) or participant characteristics across study armsa |
| Differences in characteristics between those lost-to-follow up and those retained in the studya |
| Any other notable bias threatening internal or external validitya |
Supported by other systematic review or evidence-based groups such as HHS-Office of the Assistant Secretary for Health (http://www.hhs.gov/ash/oah), Community Guide (http://www.thecommunityguide.org/index.html), Department of Education—Institute of Education Science (http://ies.ed.gov/ncee/wwc/), HHS—Administration for Children and Family (http://homvee.acf.hhs.gov/Default.aspx), Office of Justice Programs (http://www.crimesolutions.gov/), Promising Practices Network (http://www.promisingpractices.net/), or Coalition for Evidence-Based Policy (http://toptierevidence.org/; http://evidencebasedprograms.org/)
Based on empirical evidence
Recommended by consultants only
Table 2.
Additional elements for PRS best-evidence medication adherence behavioral interventions
| Intervention description |
| (No additional elements for best-evidence) |
| Quality of study design |
| Prospective study designa |
| Concurrent comparison arma |
| Random allocation of participants to study armsa |
| Quality of study implementation |
| At least a 3-month post-intervention follow-up assessment for each study arm (with recall referring to post-intervention period only) for interventions that are clearly discrete or at least a 6-months post-initiation follow-up assessment for each study arm for all other types of interventionsc |
| At least a 70 % retention rate (or medical chart recovery) at a single required assessment time point for each study armb |
| Quality of study analysis |
| Intent-to-treat analysis |
| Analysis using appropriate imputations to account for missing data due to attrition or other reasonsc |
| Use of appropriate cluster-level analyses if allocated to study arms by clustera |
| Analytic sample of at least 50 participants in each study arma |
| Strength of evidence—significant positive intervention effects |
| Positive and statistically significant (p ≤ .05) intervention effect for at least 1 relevant behavioral outcome measure and 1 relevant biologic outcome measure |
Supported by other systematic review or evidence-based groups such as HHS-Office of the Assistant Secretary for Health (http://www.hhs.gov/ash/oah), Community Guide (http://www.thecommunityguide.org/index.html), Department of Education—Institute of Education Science (http://ies.ed.gov/ncee/wwc/), HHS—Administration for Children and Family (http://homvee.acf.hhs.gov/Default.aspx), Office of Justice Programs (http://www.crimesolutions.gov/), Promising Practices Network (http://www.promisingpractices.net/), or Coalition for Evidence-Based Policy (http://toptierevidence.org/; http://evidencebasedprograms.org/)
Based on empirical evidence
Recommended by consultants only
Systematic Search Strategy
Two librarians with expertise in systematic searches developed and conducted a comprehensive and systematic search strategy, including both annual automated and quarterly manual searches, to identify all relevant HIV medication adherence intervention reports for the PRS cumulative database. The annual automated search component focused on literature published between 1996 and 2011 using the following electronic databases and platforms: CINAHL (EBSCOhost platform), EMBASE (OVID), MEDLINE (OVID), and PsycINFO (OVID). We selected 1996 as the start date for our search to be consistent with the year that HAART was made more available to HIV positive persons in the U.S. The automated search component used indexing and keyword terms, cross-referenced using Boolean logic, in four areas: (a) HIV/AIDS; (b) intervention and prevention evaluation; (c) HAART, anti-retroviral therapy or treatment; and (d) adherence. Indexing terms for the electronic searches were varied according to each database, but keywords remained constant across all databases and searches. The search was not restricted by country or language. The last automated search for this efficacy review was conducted in March 2012. As required by the PRISMA checklist, the full search strategy of the MEDLINE database is provided in Appendix B in supplementary material. The searches of the other databases are available from the corresponding author.
The quarterly manual search component involved reviewing all articles published in the previous 3 months of 20 journals to identify potentially relevant articles not yet indexed in electronic databases (see Appendix C in supplementary material). The last quarterly manual search for this review was conducted in January 2012. To supplement our routine automated and manual searches, PRS also examined the reference lists of relevant published articles, HIV/AIDS Internet listserves (e.g., www.RobertMalow. org), various research databases (i.e., ISI Web of Knowledge, RePORTER, Cochrane), and unpublished manuscripts submitted by study authors. Further details of the supplemental searches can be obtained from the corresponding author.
Study Selection
We searched the CDC’s PRS database for eligible studies. Studies were included for the efficacy review if they (1) were conducted in the U.S. or a U.S. territory; (2) were published or accepted for publication between 1996 and 2011; (3) reported on an intervention that focused on improving HIV medication adherence among PLWH by including either an educational or behavioral component (i.e., excluding studies exclusively comparing drug regimens), treatment delivery method (e.g., directly administered antiretroviral therapy [DAART]), or monitoring device to facilitate adherence (e.g., pager); (4) compared an intervention group to a comparison group; and (5) reported data on at least one behavioral adherence outcome (i.e., as measured by medication event monitoring system [MEMS caps], electronic data monitoring [EDM], pill count, self-report, or pharmacy refill) or laboratory-based HIV viral load outcome (i.e., not self-report). Our review allowed for behavioral interventions delivered to individuals, small groups or communities, but excluded interventions that were exclusively changes in policy or structure. Linked citations, defined as publications providing additional information on the same study, were included in this efficacy review if they provided relevant intervention evaluation information.
Qualitative Data Coding
Pairs of trained coders independently evaluated each eligible intervention against the newly established efficacy criteria on study design, implementation, analysis, and strength of findings. The reliability between coders on the efficacy coding was not calculated. All the coders go through standardized and stringent coding training and, on average, the overall percentage agreement among the trained coders is 96 % with a kappa rate of 80 % on our regular citation-level coding, indicating a high inter-rater reliability. All discrepancies were reconciled between paired coders. The first author of individual studies was contacted to provide missing data or clarification as needed. Of the 15 authors (out of 57 studies) we contacted for additional information, the response rate was 87 %. Final efficacy determination for each study was reached by PRS group consensus.
Results
PRS evaluated 65 interventions from the 57 unique studies eligible for this efficacy review (Fig. 1). Of these, we identified 10 interventions from 9 unique studies that met the good-evidence efficacy criteria and are considered evidence-based [40–48]. Fifteen percent of eligible medication adherence interventions (i.e., 10/65) met the PRS efficacy criteria. Below, we describe the characteristics of the 10 EBIs.
Fig. 1.
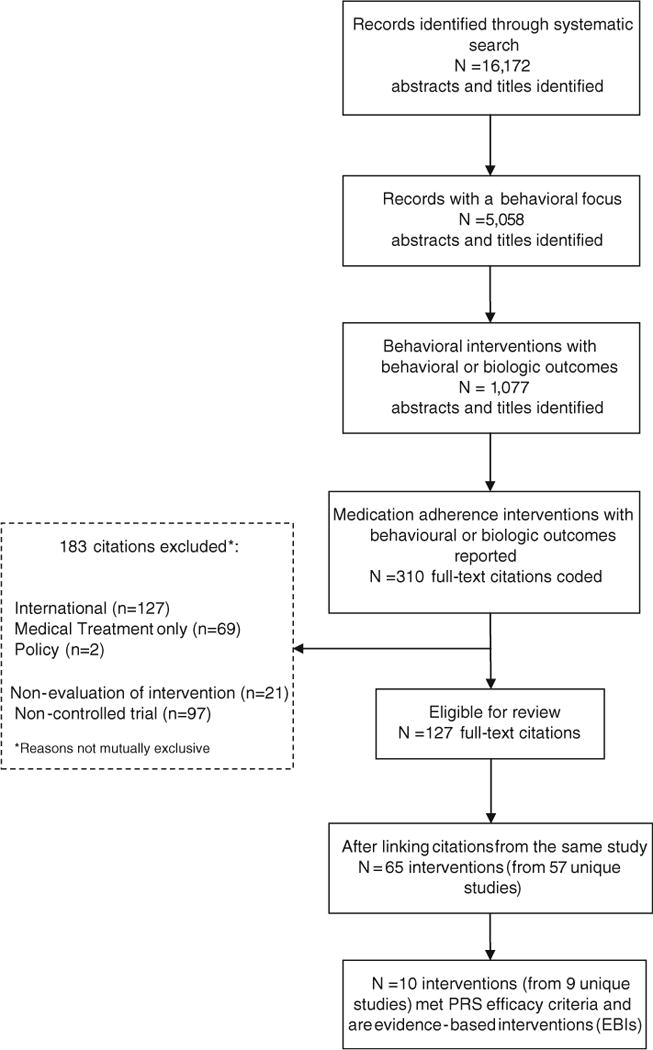
Medication adherence systematic review flow chart (1996–2011)
Population Characteristics of EBIs
All of the EBIs targeted adults. As shown in Table 3, eight interventions targeted clinic patients [40–43, 45, 47, 48] and two targeted drug users [40, 44]. None of the EBIs specifically targeted men who have sex with men (MSM), although one targeting discordant couples included gay male couples [46] and one included a majority of MSM participants [45]. One intervention targeted treatment-naïve individuals initiating therapy [43], four targeted treatment-experienced individuals [41, 45, 46, 48], and five included both treatment-experienced and -naïve individuals [40, 42, 44, 47].
Table 3.
Study population characteristics of good-evidence interventions (n = 10): 1996–2011
| Source | Intervention name | Target population | Target group | No. | Gender, % male/female | Race (%) |
Mean age (range) | Baseline Adherence |
Baseline Viral Load |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American | Hispanic | White | Other | |||||||||
| Altice et al. [40] | DAARTa for drug users | Drug-using clinic patients | Treatment-experienced or naïve | 141 | 69/31 | 58 | 19 | 22 | 1 | 44 | 25 % with >75 % self-reported adherence | 36 % with undetectable viral load (≤400 copies/mL) |
| Johnson et al. [41] | Healthy Living Project | Clinic patients and CBO attendees | Treatment-experienced | 204 | 78/22 | 56 | 7 | 31 | 6 | 40 | 61 % with <85 % self-reported adherence | 20 % with undetectable viral load (<50 copies/mL) |
| Kalichman et al. [42] | Integrated HIV risk reduction and adherence intervention | Clinic patients and CBO attendees | Treatment-experienced or naïve | 436 | 71/29 | 91 | 2 | 6 | 2 | 44 | 37 % with ≤90 % self-reported adherence | 64 % with undetectable viral load (level not defined) |
| Koenig et al. [43] | Project HEARTb | Clinic patients | Treatment-naïve | 226 | 64/36 | 83 | 2 | 12 | 3 | 37 (31–13)g | NA—treatment-naïve | 100 % with detectable viral load (>400 copies/mL) |
| Lucas et al. [44] | DAARTa in a methadone clinic | Injection drug users in treatment | Treatment-experienced or naïve | 891 | 65/35 | 79 | 43 (38–19)g | NA—No adherence behavior measure | 100 % with detectable viral load (>500 copies/mL) | |||
| Milam et al. [45] | Partnership for Health | Clinic patients | Treatment-experienced | 437 | 88/12 | 15 | 39 | 40 | 6 | 39 | 75 % with ≥ 95 % self-reported medication adherence | 59 % with undetectable viral load (<500 copies/mL) |
| Remien et al. [46] | SMARTc couples | HIV-serodiscordant couples | Poor medication adherence in HIV-positive partner | 215f | 54/46 | 62 | 24 | 42 | 100 % missed >80 % prescribed doses in past 2 weeks measured by MEMS | 41 % with undetectable viral load (level not defined) | ||
| Simoni et al.e [47] | Pager messaging | Clinic patients | Treatment-experienced or naïve | 226 | 76/24 | 30 | 11 | 47 | 12 | 40 (19–60) | NR | NR |
| Simoni et al.e [47] | Peer support | Clinic patients | Treatment-experienced or naïve | 226 | 76/24 | 30 | 11 | 47 | 12 | 40 (19–60) | NR | NR |
| Williams et al. [48] | ATHENAd | Clinic patients | Treatment-experienced | 171 | 52/48 | 35 | 19 | 42 | 4 | NR | 70 % with >90 % self-reported adherence; 41 % with >90 % adherence (MEMS) | 53 % with undetectable viral load (<400 copies/mL) |
Additional information about the efficacy review and the interventions identified can be found at http://www.cdc.gov/hiv/dhap/prb/prs/index.html
NR not reported, NA not applicable
Directly Administered Antiretroviral Therapy (DAART)
Helping Enhance Adherence to antiRetroviral Therapy (Project HEART)
Sharing Medical Adherence Responsibilities Together (SMART Couples)
Adherence through Home Education and Nursing Assessment (ATHENA)
One Study; two interventions
Couples
Median (interquartile range)
All 10 EBIs had greater than 50 % minority participants (range 53–94 %), six of which included a majority of African Americans [40–44, 46]. In addition, all 10 interventions had greater than 50 % male participants (range 52–88 %), and participants ranged in age from 19 to 67 years.
Intervention Characteristics of EBIs
Overall, there were three discrete interventions, defined as those in which participants had to receive all intervention sessions [41, 42, 46], two repetitive dosing interventions, defined as those in which sessions were implemented repeatedly without an explicit end point (both DAART; [40, 44]), and 5 EBIs with both discrete and repetitive dosing components [43, 45, 47, 48] (see Appendix A in supplementary material for detailed description of length of follow-up criteria). All EBIs, except for the two delivering DAART, relied on at least 1 behavioral change theory or model such as Social Cognitive Theory [49], Social Support Theory [50], Self-determination Theory [51], the Social Problem Solving Model [52], Paolo Freire’s Educational Model [53], and Social Action Theory [54].
As shown in Table 4, six EBIs were delivered in public or private outpatient clinics (one of which was also implemented in community-based organizations [41, 42]). Additional intervention settings included a mobile community health care van [40], anywhere the patient had access to a pager [47], and residential and community settings [48]. The interventions were delivered by a health care provider such as a nurse (n = 5), peer (n = 3), community/outreach worker (n = 2), or facilitator (n = 3). All EBIs included components delivered to individuals, except SMART Couples [46] which was group-based. Three interventions included both individual and group components: Project HEART [43], the Integrated HIV Risk Reduction and Adherence Intervention [42], and Peer Support [47].
Table 4.
Intervention characteristics of good-evidence interventions (n = 10): 1996–2011
| Source | Intervention name | Type of setting | Unit of delivery | Deliverer | No. of sessions and duration | Session duration | Intervention effects |
|---|---|---|---|---|---|---|---|
| Altice et al. [40] | DAARTa for drug users | Mobile community health care van | Individual | DAART specialist, who is an outreach worker trained to supervise DAART | Every week day over 6 months | NR | Reduced viral load |
| Johnson et al. [41] | Healthy Living Project | Private community based organizations and clinics | Individuaul | Ethnically diverse, gender-matched facilitators | Fifteen sessions grouped into three modules of five sessions each | 90 min each | Achieved medication adherence
(self-report) Decrease in HIV risk behaviors |
| Kalichman et al. [42] | Integrated HIV risk reduction and adherence intervention | Community-based AIDS service provider | Individual and group | Trained male-female facilitator pairs | Seven sessions over 5 weeks | One 45-min session, five 2-h sessions, and one 1-h session | Achieved medication adherence (unannounced
pill counts and pharmacy prescription records) Decrease in HIV risk behaviors |
| Koenig et al. [43] | Project HEARTb | Public HIV primary care outpatient clinic | Individual and group | A nurse interventionist, group discussion facilitator, and access to a peer advocate | Five sessions, with 5 support phone calls between sessions and a booster session at 6 months | Two 2–3 h sessions; three 1.5 h sessions, 1.5 h booster | Achieved medication adherence (MEMs caps) |
| Lucas et al. [44] | DAARTa in a methadone clinic | Methadone clinic | Individual | Nurse or medical assistant | Every morning of methadone clinic visit, over at least one year | NR | Reduced viral load Achieved undetectable viral load |
| Milam et al. [45] | Partnership for Health | HIV primary care outpatient clinics | Individual | Primary care provider (e.g., physician, physician assistant, nurse practitioner) | A session at each clinic visit over 10 to 11 months | 3- to 5-min | Maintained medication adherence
(self-report) Achieved undetectable viral load |
| Remien et al. [46] | SMARTc couples | Public & private outpatient clinics | Group | Nurse practitioner | Four sessions over 5 weeks | 45–60 min | Increased medication adherence (MEMS caps) |
| Simoni et al.e [47] | Pager messaging | Anywhere patient has pager access | Individual | 2-way pager | Daily customized pager messages over 3 months | NA | Reduced viral load |
| Simoni et al.e [47] | Peer support | Public HIV primary care outpatient clinic | Individual and group | Peer and research staff | Six twice-monthly group meetings and weekly phone calls over 3 months | 1-h group meeting | Increased medication adherence (self-report) |
| Williams et al. [48] | ATHENAd | Residence and community settings | Individual | Nurse and community/peer worker pair | Twenty-four home visits on a schedule of declining frequency over 12 months (weekly for 3 months, biweekly for 3 months, and monthly for 6 months) | NR | Increased medication adherence (MEMS caps) |
Additional information about the efficacy review and the interventions identified can be found at http://www.cdc.gov/hiv/dhap/prb/prs/index.html
NR not reported, NA not applicable
Directly Administered Antiretroviral Therapy (DAART)
Helping Enhance Adherence to antiretroviral Therapy (Project HEART)
Sharing Medical Adherence Responsibilities Together (SMART Couples)
Adherence through Home Education and Nursing Assessment (ATHENA)
One Study; two interventions
Although the content of the EBIs differed substantially, the majority of the interventions included a cognitive-behavioral component (e.g., addressing barriers to adherence and problem-solving). Three interventions focused on skill-building: technical (e.g., practice medication adherence with candies), personal (e.g., practice ways to overcome barriers), and interpersonal (e.g., couple communication exercises). In addition to medication adherence, one EBI focused on patient-provider relationships in clinic settings [45], and three focused on both medication adherence and safer sex [41, 42, 46]. Social support was also incorporated as an important component in three EBIs [43, 46, 47].
Outcomes Measures of EBIs
Among the ten EBIs, one measured viral load only [44], two measured adherence behavior only [41, 42], and the remaining seven measured both viral load and adherence behavior. Among the nine interventions that measured adherence behavior, three relied on MEMS caps only [43, 46, 48], two relied on EDM data and self-report ([47] Peer Support and Pager Messaging), one relied on unannounced pill counts and pharmacy prescription records [42] and three relied on self-report only [40, 41, 45].
Among the seven EBIs that assessed viral load and adherence behavior, three observed a significant intervention effect on viral load only and three on adherence behavior only. Only one found a significant intervention effect on both outcomes [45]. Significant intervention effects were observed over a range of follow-up times, from 3 to 18 months post-initiation of the seven interventions with repetitive-dosing components and 1–13 months post-completion of the three discrete interventions [41, 42, 46].
Reasons for Not Meeting Best-Evidence Criteria
The ten good-evidence interventions did not meet the best-evidence efficacy criteria for the following reasons (not mutually exclusive): did not find a significant positive intervention effect on both behavioral and biologic measures of adherence (n = 9; three of these did not measure both outcomes); did not meet the requirement for retention (n = 2) or follow-up time point (n = 3), did not impute missing data (n = 4) or adjust for clusters (n = 2). One additional study was identified as a non-RCT with moderate allocation bias. Most of these limitations are a result of the design of the study and/or analysis of data.
Comparison Between EBIs and Non-EBIs
Although 10 interventions were identified as EBIs, 55 interventions from 48 unique studies [55–102] did not meet the minimal criteria for good-evidence. The most common reasons (not mutually exclusive) were: small sample size (n = 31; 56 %), null/non-significant findings (n = 18; 33 %), no appropriate follow-up (n = 12; 22 %), poor retention (n = 7; 13 %). Several studies had other design or analytic issues that did not meet criteria (n = 9; 22 %; e.g., biased allocation to study arms, harmful negative effects, substantial missing data). The comparisons between the 10 EBIs and the 55 non-EBIs on key population and intervention characteristics are shown in Table 5. Both groups are similar on several population and intervention characteristics; however, there are a couple notable differences. All of the EBIs had at least one positive significant outcome (100 %) whereas only two-third (64 %) of the non-EBIs did. More EBIs than non-EBIs targeted both treatment-experienced and -naïve patients combined (50 vs. 7 %). Additionally, more non-EBIs than EBIs focused on specific populations (e.g., women only, men only, high risk youth only); whereas, more EBIs include a majority of African American participants than non-EBIs (60 vs. 49 %).
Table 5.
Comparison of characteristics of EBIs and non-EBIs included in the PRS efficacy review (N = 65)
| Characteristic | 10 EBIs n (%) | 55 non-EBIs n (%) |
|---|---|---|
| Target population | ||
| MSM | 0 (0) | 1 (2) |
| Drug users/IDU only | 2 (20) | 9 (16) |
| High risk youth only | 0 (0) | 3 (5) |
| Women only | 0 (0) | 9 (16) |
| Men only | 0 (0) | 2 (4) |
| Race/ethnicity (not mutually exclusive) | ||
| Majority AA | 6 (60) | 27 (49) |
| Majority people of color (including AA, Hispanic, API, other) | 5 (50) | 26 (47) |
| Majority white | 0 (0) | 5 (9) |
| Target groupa | ||
| Treatment-experienced | 4 (40) | 27 (49) |
| Treatment-naïve | 1 (10) | 18 (33) |
| Both | 5 (50) | 4 (7) |
| Type of setting (not mutually exclusive) | ||
| Clinic | 6 (60) | 34 (62) |
| Community | 4 (40) | 1 (2) |
| Other | 2 (20) | 34 (62) |
| Unit of delivery | ||
| Individual only | 6 (60) | 41 (75) |
| Group only | 1 (10) | 6 (11) |
| Individual and group | 3 (30) | 8 (15) |
| Community | 0 (0) | 0 (0) |
| Deliverer (not mutually exclusive) | ||
| Clinic staff | 6 (60) | 24 (44) |
| Facilitator/other | 7 (70) | 41 (75) |
| Intervention sessions | ||
| Single-session discrete | 0 (0) | 2 (4) |
| Multi-session discrete | 3 (30) | 25 (45) |
| Repetitive dosing or combinationb | 7 (70) | 28 (51) |
| Outcomes measured | ||
| Adherence only | 2 (20) | 19 (35) |
| Viral load only | 1 (10) | 5 (9) |
| Both | 7 (70) | 31 (56) |
| At least one statistically significant positive intervention effectc | ||
| Yes | 10 (100) | 35 (64) |
| No | 0 (0) | 20 (36) |
| Sample size at baseline | ||
| Median | 226 | 77 |
| Follow-up time | ||
| Median time for first follow-up (in month) | 3 | 2 |
| Median time for last follow-up (in month) | 9 | 6 |
| Median retention | ||
| At first “good-evidence” follow-upd | 85 % | 81 % |
n = 6 non-EBIs did not target treatment naïve or experienced
Repetitive dosing or combination = includes interventions that had repetitive dosing and one or more discrete sessions
At least one statistically significant positive intervention effect on viral load or medication adherence outcomes
1-month post completion of intervention or 3-month post implementation of intervention
Discussion
Given the importance of adherence for both prevention and treatment efficacy, it is very encouraging to have identified 10 EBIs for promoting adherence among PLWH. These interventions can serve as model programs for providers and other prevention planners looking to implement EBIs best suited for their community’s needs.
The 10 EBIs represent 15 % of eligible interventions (i.e., 10/65) for this first efficacy review of HIV medication adherence interventions. In comparison, the first published PRS sex and drug risk reduction efficacy review, which included a review of the scientific literature, published from 2000 to 2004, identified 18 % of eligible interventions as meeting the original PRS efficacy criteria [103]. Over the years, the scientific field has evolved, with advancements in research and improvements in study quality. Cumulatively, through 2011, roughly 20–22 % of the eligible risk reduction behavioral interventions have met the risk reduction efficacy criteria (personal correspondence with PRS team, 2011). The results of this medication adherence efficacy review are comparable to those initial findings for risk reduction interventions. Similarly, we anticipate an increase in medication adherence EBIs over time as the field matures. To remain a valuable source to HIV-care and prevention providers, the PRS team plans to continually update this review and post new adherence EBIs on the PRS website (http://www.cdc.gov/hiv/prevention/research/compendium/ma/index.html) as they are identified.
The 55 interventions that did not meet the efficacy criteria reported similar population and intervention characteristics but failed to meet evidence-based criteria, primarily due to small sample sizes, null findings or low retention rates. Among these 55 interventions, 35 (64 %) found at least 1 significant positive intervention effect. These interventions could be considered for re-testing, in particular with more rigorous evaluation methods.
Although the study samples across the 10 EBIs consist of greater than 50 % minority participants, we do not know specifically the percentage of MSM of color or minority women across these studies. Non-EBIs more often targeted specific populations (e.g., women, high-risk youth, MSM, men) whereas the EBIs more often targeted general clinic populations. Given that the EBIs tended to target the general HIV clinic population, this suggests that these effects may be robust and can be generalized to a wide variety of HIV care clinics. One exception may be HIV-positive injection drug users (IDUs) since their lifestyle and active substance use may create a barrier to adherence that others may not experience. The two EBIs targeting drug users were DAART interventions, which may not be easily implementable or sustainable in typical HIV clinics. Systematic reviews of DAART interventions have shown them to be efficacious during implementation but not so after DAART services end. Future research should evaluate the extent to which current and newly developed interventions are effective for IDUs, drug users, and other groups with unique structural barriers (such as homeless persons) to adherence.
Those involved in developing and implementing HIV medication adherence interventions also have the opportunity to engage PLWH at the onset of treatment and to help them establish a high level of adherence from the beginning. Of the ten EBIs, only one focuses exclusively on treatment-naïve participants [43]. There is opportunity here for providers to identify participants as soon as they are linked to care and assist them in developing and maintaining good adherence behaviors.
A few limitations of this review and the literature warrant comment. First, our criteria primarily focused on internal validity and did not focus on evidence from replication studies, external validity, scalability, cost and population-level impact which should be incorporated in the criteria as the medication adherence field advances. Second, our criteria are designed to evaluate risk of bias in individual studies; however, there is a potential risk of bias across studies in our review as we only evaluated published reports. Third, there remains considerable variability regarding a “gold standard” for adherence measurement in research and practice. As recently recommended by Williams and her colleagues, the prevention field is encouraged to adopt quality standards for measuring and reporting on adherence measures so that adherence behaviors are reported consistently and reliably [104].
Despite these limitations, there are a few implications from our review findings for further research. For improving the quality of study design, implementation, and analyses, researchers should aim to assess both behavioral and biologic measures of adherence, develop strategies to retain participants over longer periods of time (particularly among prioritized populations and those known to have poor retention in care), and use robust analytic methods for dealing with complex data that result from missing data and design elements (e.g., allocating clusters of individuals). We also encourage researchers to use the PRS efficacy criteria to evaluate their own interventions as they are being developed. The current fiscal environment requires a deliberate effort to identify and support the interventions most likely to have a large impact on the HIV epidemic. It is imperative that EBIs are also evaluated to determine which ones are most easily scalable and cost-effective. Researchers, therefore, are further encouraged to report cost data related to intervention implementation.
Translating EBIs into Practice
Similar to recommendations in other public health sectors, [105, 106] the National HIV/AIDS Strategy [1] calls for greater focus on evidence-based HIV prevention by drawing upon interventions and strategies with proven efficacy. Thus, once EBIs are identified, they need to be made available and accessible for wide-scale use in practice to achieve a larger public health impact. Many of the HIV risk reduction interventions previously identified by PRS as EBIs (http://www.cdc.gov/hiv/prevention/research/compendium/index.html) have been translated into easy-to-use intervention materials and are being disseminated to prevention providers across the nation (https://www.effectiveinterventions.org) [107]. Recently, CDC has developed web-based and e-learning training and implementation materials for five of these medication adherence EBIs for national dissemination and wide-spread practice (https://www.effectiveinterventions.org) [107]. These interventions were designed for healthcare and/or non-healthcare providers. For clinic settings, there is a need for brief intervention tools that are feasible to be implemented within the short period of time that providers have with their patients during routine HIV care. The more intensive EBIs can be more realistically implemented in non-healthcare settings to provide additional support to improve PLWH’s ART adherence behavior. Efforts in both healthcare and non-healthcare settings are important to fully support PLWH in achieving optimal adherence to ART and viral load suppression.
Conclusions
This efficacy review contributes to the research translation of HIV medication adherence interventions; that is, translating proven scientific research into routine practice. Our systematic review identified several EBIs that can serve as model programs for providers and other prevention planners who are looking to implement evidence-based HIV medication adherence interventions best suited for their community’s needs. The medication adherence field can be further improved if identified research opportunities are explored. Scalable, cost-effective, evidence-based adherence interventions are imperative for improving the health outcomes and reducing HIV transmission risk among PLWH.
Supplementary Material
Acknowledgments
The authors would like to thank the following external consultants for providing invaluable insight and feedback on the medication adherence criteria development process: Rivet Amico, Deborah L. Jones, Robert H. Remien, Steven Safren, Jane Simoni, Ann Williams, and Ira Wilson. The authors would also like to thank all principal investigators of the original research who facilitated our review process by providing the necessary additional information or analyses as requested by PRS. Other members of the HIV/AIDS Prevention Research Synthesis Team who contributed to this review (listed alphabetically): Adebukola Adegbite (ICF International), Brittney Baack (CDC), Terrika Barham (ICF International), Mary M. Mullins (CDC), and Maria Luisa Tungol (CDC). This work was supported by the Prevention Research Branch, Division of HIV/AIDS Prevention, U.S. Centers for Disease Control and Prevention and was not funded by any other organization.
Footnotes
Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Electronic supplementary material The online version of this article (doi:10.1007/s10461-013-0594-x) contains supplementary material, which is available to authorized users.
Contributor Information
Mahnaz R. Charania, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA
Khiya J. Marshall, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA
Cynthia M. Lyles, Email: clyles@cdc.gov, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA.
Nicole Crepaz, Email: ncrepaz@cdc.gov, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA.
Linda S. Kay, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA
Linda J. Koenig, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA
Paul J. Weidle, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA
David W. Purcell, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA
HIV/AIDS Prevention Research Synthesis (PRS) Team, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention, 1600 Clifton Rd., Mailstop E-37, Atlanta, GA 30333, USA.
References
- 1.Office of National AIDS Policy. National HIV/AIDS strategy for the United States. Washington, DC: Office of National AIDS Policy; http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf. Accessed 10 June 2013. [Google Scholar]
- 2.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa F, Lodwick RK, Smith CJ, et al. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS. 2011;26(3):335–43. doi: 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- 4.van Sighem AI, Gras LA, Reiss P, Brinkman K, De Wolf F, Athena National Observational Cohort Study Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24(10):1527–35. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- 5.CDC. High-Impact HIV Prevention: CDC’s approach to reducing HIV infections in the United States. Atlanta, GA: Centers for Disease Control and Prevention; http://www.cdc.gov/hiv/strategy/hihp/report/index.htm. Accessed 10 June 2013. [Google Scholar]
- 6.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care Panel. Ann Intern Med. 2012;156(11):817–33. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett JA. Addressing the challenges of adherence. J Acquir Immune Defic Syndr. 2002;29(Suppl 1):S2–10. doi: 10.1097/00126334-200202011-00002. [DOI] [PubMed] [Google Scholar]
- 8.Chesney MA. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl 2):S171–6. doi: 10.1086/313849. [DOI] [PubMed] [Google Scholar]
- 9.Gardner EM, Maravi ME, Rietmeijer C, Davidson AJ, Burman WJ. The association of adherence to antiretroviral therapy with healthcare utilization and costs for medical care. Appl Health Econ Health Policy. 2008;6(2–3):145–55. doi: 10.1007/bf03256129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson MJ, Petrozzino JJ. An evidence-based review of treatment-related determinants of patients’ nonadherence to HIV medications. AIDS Patient Care STDS. 2009;23(11):903–14. doi: 10.1089/apc.2009.0024. [DOI] [PubMed] [Google Scholar]
- 12.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–66. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 13.Bangsberg DR. Less than 95 % adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–41. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 14.Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16(16):2175–82. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 15.Lima VD, Geller J, Bangsberg DR, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21(9):1175–83. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 16.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34(8):1115–21. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 17.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan PS, Campsmith ML, Nakamura GV, Begley EB, Schulden J, Nakashima AK. Patient and regimen characteristics associated with self-reported nonadherence to antiretroviral therapy. PLoS ONE. 2007;2(6):e552. doi: 10.1371/journal.pone.0000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaragoza-Macias E, Cosco D, Nguyen ML, Del Rio C, Lennox J. Predictors of success with highly active antiretroviral therapy in an antiretroviral-naive urban population. AIDS Res Hum Retrovir. 2010;26(2):133–8. doi: 10.1089/aid.2009.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall HI, Frazier EL, Rhodes P, et al. Continuum of care: differences in care and treatment by sex and race/ethnicity in the United States. Proceedings of the XIX International AIDS Conference; 2012 July 22–27; Washington, DC. [Abstract FRLBX05] [Google Scholar]
- 22.Ortego C, Huedo-Medina TB, Llorca J, et al. Adherence to highly active antiretroviral therapy (HAART): a meta-analysis. AIDS Behav. 2011;15(7):1381–96. doi: 10.1007/s10461-011-9942-x. [DOI] [PubMed] [Google Scholar]
- 23.Ammassari A, Trotta MP, Murri R, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S123–7. doi: 10.1097/00126334-200212153-00007. [DOI] [PubMed] [Google Scholar]
- 24.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reisner SL, Mimiaga MJ, Skeer M, Perkovich B, Johnson CV, Safren SA. A review of HIV antiretroviral adherence and intervention studies among HIV-infected youth. Top HIV Med. 2009;17(1):14–25. [PMC free article] [PubMed] [Google Scholar]
- 26.Simoni JM, Montgomery A, Martin E, New M, Demas PA, Rana S. Adherence to antiretroviral therapy for pediatric HIV infection: a qualitative systematic review with recommendations for research and clinical management. Pediatrics. 2007;119(6):e1371–83. doi: 10.1542/peds.2006-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns. 2002;46(2):93–108. doi: 10.1016/s0738-3991(01)00219-1. [DOI] [PubMed] [Google Scholar]
- 28.Sandelowski M, Voils CI, Chang Y, Lee EJ. A systematic review comparing antiretroviral adherence descriptive and intervention studies conducted in the USA. AIDS Care. 2009;21(8):953–66. doi: 10.1080/09540120802626212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae JW, Guyer W, Grimm K, Altice FL. Medication persistence in the treatment of HIV infection: a review of the literature and implications for future clinical care and research. AIDS. 2011;25(3):279–90. doi: 10.1097/QAD.0b013e328340feb0. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS Behav. 2010;14(4):731–47. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- 32.Wutoh AK, Elekwachi O, Clarke-Tasker V, Daftary M, Powell NJ, Campusano G. Assessment and predictors of antiretroviral adherence in older HIV-infected patients. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S106–14. doi: 10.1097/00126334-200306012-00007. [DOI] [PubMed] [Google Scholar]
- 33.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–97. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 34.Heyer A, Ogunbanjo GA. Adherence to HIV antiretroviral therapy. Part I: a review of factors that influence adherence. S Afr Fam Pract. 2006;48(8):5–9. [Google Scholar]
- 35.Leeman J, Chang YK, Lee EJ, Voils CI, Crandell J, Sandelowski M. Implementation of antiretroviral therapy adherence interventions: a realist synthesis of evidence. J Adv Nurs. 2010;66(9):1915–30. doi: 10.1111/j.1365-2648.2010.05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malta M, Petersen ML, Clair S, Freitas F, Bastos FI. Adherence to antiretroviral therapy: a qualitative study with physicians from Rio de Janeiro, Brazil. Cad Saude Publica. 2005;21(5):1424–32. doi: 10.1590/s0102-311x2005000500015. [DOI] [PubMed] [Google Scholar]
- 37.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyles CM, Crepaz N, Herbst JH, Kay LS. Evidence-based HIV behavioral prevention from the perspective of the CDC’s HIV/AIDS Prevention Research Synthesis Team. AIDS Educ Prev. 2006;18(4 Suppl A):21–31. doi: 10.1521/aeap.2006.18.supp.21. [DOI] [PubMed] [Google Scholar]
- 40.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45(6):770–8. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007;46(5):574–80. doi: 10.1097/qai.0b013e318158a474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalichman SC, Cherry C, Kalichman MO, et al. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. Am J Public Health. 2011;101(3):531–8. doi: 10.2105/AJPH.2010.197608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenig LJ, Pals SL, Bush T, Pratt PM, Stratford D, Ellerbrock TV. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27(2):159–69. doi: 10.1037/0278-6133.27.2.159. [DOI] [PubMed] [Google Scholar]
- 44.Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42(11):1628–35. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- 45.Milam J, Richardson JL, McCutchan A, et al. Effect of a brief antiretroviral adherence intervention delivered by HIV care providers. J Acquir Immune Defic Syndr. 2005;40(3):356–63. doi: 10.1097/01.qai.0000159710.98960.81. [DOI] [PubMed] [Google Scholar]
- 46.Remien RH, Stirratt MJ, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19(8):807–14. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 47.Simoni JM, Huh D, Frick PA, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52(4):465–73. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams AB, Fennie KP, Bova CA, Burgess JD, Danvers KA, Dieckhaus KD. Home visits to improve adherence to highly active antiretroviral therapy: a randomized controlled trial. J Acquir Immune Defic Syndr. 2006;42(3):314–21. doi: 10.1097/01.qai.0000221681.60187.88. [DOI] [PubMed] [Google Scholar]
- 49.Bandura A. Social cognitive theory and excercise of control over HIV infection. In: DiClemente RJ, Peterson JL, editors. Preventing AIDS: theories and methods of behavioral preventions. New York: Plenum Press; 1994. pp. 25–59. [Google Scholar]
- 50.Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychol. 2006;25(1):74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deci E. Intrinsic motivation and self-determination in human behavior. New York: Plenum Press; 1985. [Google Scholar]
- 52.D’Zurilla TJ, Nezu A. Social problem solving in adults. In: Kendall P, editor. Advances in cognitive-behavioral research and therapy. Vol. 1. New York: Academic Press; 1982. pp. 202–74. [Google Scholar]
- 53.Freire P. Pedagogy of the oppressed. New York: Continuum Press; 1986. [Google Scholar]
- 54.Ewart CK. Social action theory for a public health psychology. Am Psychol. 1991;46(9):931–46. doi: 10.1037//0003-066x.46.9.931. [DOI] [PubMed] [Google Scholar]
- 55.Margolin A, Avants SK, Warburton LA, Hawkins KA, Shi J. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psychol. 2003;22(2):223–8. [PubMed] [Google Scholar]
- 56.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151–62. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 57.Smith SR, Rublein JC, Marcus C, Brock TP, Chesney MA. A medication self-management program to improve adherence to HIV therapy regimens. Patient Educ Couns. 2003;50(2):187–99. doi: 10.1016/s0738-3991(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 58.McPherson-Baker S, Malow RM, Penedo F, Jones DL, Schneiderman N, Klimas NG. Enhancing adherence to combination antiretroviral therapy in non-adherent HIV-positive men. AIDS Care. 2000;12(4):399–404. doi: 10.1080/09540120050123792. [DOI] [PubMed] [Google Scholar]
- 59.Jones DL, Ishii M, LaPerriere A, et al. Influencing medication adherence among women with AIDS. AIDS Care. 2003;15(4):463–74. doi: 10.1080/0954012031000134700. [DOI] [PubMed] [Google Scholar]
- 60.Rawlings MK, Thompson MA, Farthing CF, et al. Impact of an educational program on efficacy and adherence with a twice-daily Lamivudine/Zidovudine/Abacavir regimen in underrepresented HIV-infected patients. J Acquir Immune Defic Syndr. 2003;34(2):174–83. doi: 10.1097/00126334-200310010-00007. [DOI] [PubMed] [Google Scholar]
- 61.Safren SA, Hendriksen ES, Desousa N, Boswell SL, Mayer KH. Use of an on-line pager system to increase adherence to antiretroviral medications. AIDS Care. 2003;15(6):787–93. doi: 10.1080/09540120310001618630. [DOI] [PubMed] [Google Scholar]
- 62.Rigsby MO, Rosen MI, Beauvais JE, et al. Cue-dose training with monetary reinforcement: pilot study of an antiretroviral adherence intervention. J Gen Intern Med. 2000;15(12):841–7. doi: 10.1046/j.1525-1497.2000.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rotheram-Borus MJ, Swendeman D, Comulada WS, Weiss RE, Lee M, Lightfoot M. Prevention for substance-using HIV-positive young people: telephone and in-person delivery. J Acquir Immune Defic Syndr. 2004;37(Suppl. 2):S68–77. doi: 10.1097/01.qai.0000140604.57478.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wyatt GE, Longshore D, Chin D, et al. The efficacy of an integrated risk reduction intervention for HIV-positive women with child sexual abuse histories. AIDS Behav. 2004;8(4):453–62. doi: 10.1007/s10461-004-7329-y. [DOI] [PubMed] [Google Scholar]
- 65.Berrien VM, Salazar JC, Reynolds E, McKay K, HIV Medication Adherence Intervention Group Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care STDS. 2004;18(6):355–63. doi: 10.1089/1087291041444078. [DOI] [PubMed] [Google Scholar]
- 66.Murphy DA, Lu MC, Martin D, Hoffman D, Marelich WD. Results of a pilot intervention trial to improve antiretroviral adherence among HIV-positive patients. J Assoc Nurses AIDS Care. 2002;13(6):57–69. doi: 10.1177/1055329002238026. [DOI] [PubMed] [Google Scholar]
- 67.Rathbun RC, Farmer KC, Stephens JR, Lockhart SM. Impact of an adherence clinic on behavioral outcomes and virologic response in treatment of HIV infection: a prospective, randomized, controlled pilot study. Clin Ther. 2005;7(2):199–209. doi: 10.1016/j.clinthera.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 68.DiIorio C, Resnicow K, McDonnell M, Soet J, McCarty F, Yeager K. Using motivational interviewing to promote adherence to antiretroviral medications: a pilot study. J Assoc Nurses AIDS Care. 2003;14(2):52–62. doi: 10.1177/1055329002250996. [DOI] [PubMed] [Google Scholar]
- 69.Samet JH, Horton NJ, Meli S, et al. A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antivir Ther. 2005;10(1):83–93. doi: 10.1177/135965350501000106. [DOI] [PubMed] [Google Scholar]
- 70.Antoni MH, Carrico AW, Duran RE, et al. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosom Med. 2006;68(1):143–51. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- 71.Golin CE, Earp J, Tien HC, Stewart P, Porter C, Howie L. A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. J Acquir Immune Defic Syndr. 2006;42(1):42–51. doi: 10.1097/01.qai.0000219771.97303.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner GJ, Kanouse DE, Golinelli D, et al. Cognitive-behavioral intervention to enhance adherence to antiretroviral therapy: a randomized controlled trial (CCTG 578) AIDS. 2006;20(9):1295–302. doi: 10.1097/01.aids.0000232238.28415.d2. [DOI] [PubMed] [Google Scholar]
- 73.Javanbakht M, Prosser P, Grimes T, Weinstein M, Farthing C. Efficacy of an individualized adherence support program with contingent reinforcement among nonadherent HIV-positive patients: results from a randomized trial. J Int Assoc Physicians AIDS Care. 2006;5(4):143–50. doi: 10.1177/1545109706291706. [DOI] [PubMed] [Google Scholar]
- 74.Rosen MI, Dieckhaus K, McMahon TJ, et al. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21(1):30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- 75.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007;26(4):488–95. doi: 10.1037/0278-6133.26.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones DL, McPherson-Baker S, Lydston D, et al. Efficacy of a group medication adherence intervention among HIV positive women: the SMART/EST Women’s Project. AIDS Behav. 2007;11(1):79–86. doi: 10.1007/s10461-006-9165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Purcell DW, Latka MH, Metsch LR, et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46(Suppl. 2):S35–47. doi: 10.1097/QAI.0b013e31815767c4. [DOI] [PubMed] [Google Scholar]
- 78.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–50. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naar-King S, Lam P, Wang B, Wright K, Parsons JT, Frey MA. Brief report: maintenance of effects of motivational enhancement therapy to improve risk behaviors and HIV-related health in a randomized controlled trial of youth living with HIV. J Pediatr Psychol. 2008;33(4):441–5. doi: 10.1093/jpepsy/jsm087. [DOI] [PubMed] [Google Scholar]
- 80.Wohl AR, Garland WH, Valencia R, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006;42(11):1619–27. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- 81.Andrade AS, McGruder HF, Wu AW, et al. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infect Dis. 2005;41(6):875–82. doi: 10.1086/432877. [DOI] [PubMed] [Google Scholar]
- 82.DiIorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20(3):273–83. doi: 10.1080/09540120701593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Westling E, Garcia K, Mann T. Discovery of meaning and adherence to medications in HIV-infected women. J Health Psychol. 2007;12(4):627–35. doi: 10.1177/1359105307078169. [DOI] [PubMed] [Google Scholar]
- 84.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21(11):1473–7. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 85.Van Servellen G, Nyamathi A, Carpio F, et al. Effects of a treatment adherence enhancement program on health literacy, patient-provider relationships, and adherence to HAART among low-income HIV-positive Spanish-speaking Latinos. AIDS Patient Care STDS. 2005;19(11):745–59. doi: 10.1089/apc.2005.19.745. [DOI] [PubMed] [Google Scholar]
- 86.Reynolds NR, Testa MA, Su M, et al. Telephone support to improve antiretroviral medication adherence: a multisite, randomized controlled trial. J Acquir Immune Defic Syndr. 2008;47(1):62–8. doi: 10.1097/QAI.0b013e3181582d54. [DOI] [PubMed] [Google Scholar]
- 87.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT–AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Enriquez M, Cheng AL, McKinsey DS, Stanford J. Development and efficacy of an intervention to enhance readiness for adherence among adults who had previously failed HIV treatment. AIDS Patient Care STDS. 2009;23(3):177–84. doi: 10.1089/apc.2008.0170. [DOI] [PubMed] [Google Scholar]
- 89.Visnegarwala F, Rodriguez-Barradass MC, Graviss EA, Caprio M, Nykyforchyn M, Laufman L. Community outreach with weekly delivery of anti-retroviral drugs compared to cognitive-behavioural health care team-based approach to improve adherence among indigent women newly starting HAART. AIDS Care. 2006;18(4):332–8. doi: 10.1080/09540120500162155. [DOI] [PubMed] [Google Scholar]
- 90.Mannheimer SB, Morse E, Matts JP, et al. Sustained benefit from a long-term antiretroviral adherence intervention: results of a large randomized clinical trial. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S41–7. doi: 10.1097/01.qai.0000245887.58886.ac. [DOI] [PubMed] [Google Scholar]
- 91.Levin TR, Klibanov OM, Axelrod P, et al. A randomized trial of educational materials, pillboxes, and mailings to improve adherence with antiretroviral therapy in an Inner City HIV clinic. J Clin Outcomes Manag. 2006;13(4):217–21. [Google Scholar]
- 92.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88(1):54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murphy DA, Marelich WD, Rappaport NB, Hoffman D, Farthing C. Results of an antiretroviral adherence intervention: STAR (Staying Healthy: Taking Antiretrovirals Regularly) J Int Assoc Provid AIDS Care. 2007;6(2):113–24. doi: 10.1177/1545109707301243. [DOI] [PubMed] [Google Scholar]
- 94.Webel AR. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22(9):1029–40. doi: 10.1080/09540120903214389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 2011;113(2–3):192–9. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson MO, Dilworth SE, Taylor JM, Neilands TB. Improving coping skills for self-management of treatment side effects can reduce antiretroviral medication nonadherence among people living with HIV. Ann Behav Med. 2011;41(1):83–91. doi: 10.1007/s12160-010-9230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fisher JD, Amico KR, Fisher WA, et al. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows Project. AIDS Behav. 2011;15(8):1635–46. doi: 10.1007/s10461-011-9926-x. [DOI] [PubMed] [Google Scholar]
- 98.Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug Alcohol Depend. 2011;116(1–3):177–87. doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hardy H, Kumar V, Doros G, et al. Randomized controlled trial of a personalized cellular phone reminder system to enhance adherence to antiretroviral therapy. AIDS Patient Care STDS. 2011;25(3):153–61. doi: 10.1089/apc.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalichman SC, Kalichman MO, Cherry C, et al. Brief behavioral self-regulation counseling for HIV treatment adherence delivered by cell phone: an initial test of concept trial. AIDS Patient Care STDS. 2011;25(5):303–10. doi: 10.1089/apc.2010.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holstad MM, DiIorio C, Kelley ME, Resnicow K, Sharma S. Group motivational interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav. 2011;15(5):885–96. doi: 10.1007/s10461-010-9865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duncan LG, Moskowitz JT, Neilands TB, Dilworth SE, Hecht FM, Johnson MO. Mindfulness-based stress reduction for HIV treatment side effects: a randomized, wait-list controlled trial. J Pain Symptom Manage. 2012;43(2):161–71. doi: 10.1016/j.jpainsymman.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lyles CM, Kay LS, Crepaz N, et al. Best-evidence interventions: findings from a systematic review of HIV behavioral interventions for US populations at high risk, 2000–2004. Am J Public Health. 2007;97(1):133–43. doi: 10.2105/AJPH.2005.076182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams AB, Amico KR, Bova C, Womack JA. A proposal for quality standards for measuring medication adherence in research. AIDS Behav. 2012;17(1):284–97. doi: 10.1007/s10461-012-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Committee on HIV Prevention in the United States, Division of Health Promotion and Disease Prevention. No time to lose: getting more from HIV prevention. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 106.Committee on Assuring the Health of the Public in the 21st Century. The future of the public’s health in the 21st century. Washington, DC: National Academies Press; 2002. [Google Scholar]
- 107.Collins C, Harshbarger C, Sawyer R, Hamdallah M. The diffusion of effective behavioral interventions project: development, implementation, and lessons learned. AIDS Educ Prev. 2006;18(4 Suppl A):5–20. doi: 10.1521/aeap.2006.18.supp.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


