Abstract
EEG/fMRI takes advantage of the high temporal resolution of EEG in combination with the high spatial resolution of fMRI. These features make it particularly applicable to the study of epilepsy in which the event duration (e.g., interictal epileptiform discharges) is short, typically less than 200 milliseconds. Interictal or ictal discharges can be identified on EEG and be used for source localization in fMRI analyses. The acquisition of simultaneous EEG/fMRI involves the use of specialized EEG hardware that is safe in the MR environment and comfortable to the participant. Advanced data analysis approaches such as independent component analysis conducted alone or sometimes combined with other, e.g., Granger Causality or “sliding window” analyses are currently thought to be most appropriate for EEG/fMRI data. These approaches make it possible to identify networks of brain regions associated with ictal and/or interictal events allowing examination of the mechanisms critical for generation and propagation through these networks. After initial evaluation in adults, EEG/fMRI has been applied to the examination of the pediatric epilepsy syndromes including Childhood Absence Epilepsy, Benign Epilepsy with Centrotemporal Spikes (BECTS), Dravet Syndrome, and Lennox-Gastaut Syndrome. Results of EEG/fMRI studies suggest that the hemodynamic response measured by fMRI may have a different shape in response to epileptic events compared to the response to external stimuli; this may be especially true in the developing brain. Thus, the main goal of this review is to provide an overview of the pediatric applications of EEG/fMRI and its associated findings up until this point.
1. Introduction
Since its introduction, electroencephalography (EEG) has been used in the study and diagnosis of epilepsy. Its low cost and ease of use make it a ubiquitous staple in the examination of paroxysmal neurological disorders. However, EEG has poor spatial resolution, especially in regions deep in the brain, and the localization of sources (e.g., epileptiform discharges) is dependent on multiple assumptions. Whereas EEG measures the direct electrical activity of a large number of neurons with a high temporal resolution and low spatial resolution, fMRI measures blood oxygen level dependent (BOLD) signal change, which is an indirect measure of neuronal activity at a low temporal resolution, on the order of seconds, and high spatial resolution, on the order of millimeters. The differences in the temporal and spatial resolution of these techniques and in the types of measured neuronal signals make EEG and fMRI complementary techniques enabling examination of dynamic changes associated with paroxysmal neurological disorders such as epilepsy.
The engineering challenges of merging these two technologies were addressed in a paper in 1993 [1]. At that point, EEG could be safely recorded while the participant was in the MRI scanner but the resulting EEG traces were plagued by noise from ballistocardiographic artifacts and echo planar imaging (EPI) gradients that completely consumed any clinically relevant EEG findings. Initially, a technique called “EEG-triggered fMRI” was used – simply put, the fMRI data collection was triggered by an electroencephalographer who was reviewing the real time EEG and who triggered the scanner to collect fMRI data once EEG event was identified (event-related fMRI). In 1998 an algorithm was introduced for removing the ballistocardiographic artifact and later an algorithm for removing the EPI gradient noise [2, 3]. Recently, other methods for ballistocardiographic artifact removal have been developed based on principal component analyses [4, 5]. The ballistocardiographic artifact is believed to be related to the interaction of the magnetic field with the motion of the blood as it accelerates and changes direction in the aorta [1]. Using these noise removal algorithms, EEG can be recorded concurrently to the fMRI with minimal noise and little obstruction to neuronal signals – these advances allow for continuous EEG data collection and, more recently, real-time EPI artifact removal, to assess for the presence of events of interest (e.g., epileptiform discharges).
Performing EEG/fMRI studies in children carries several additional problems that need to be resolved in order for good quality data to be collected. First and foremost is the comfort level of children as young as few months of age who, in the case of those with epilepsy, frequently have cognitive impairments. This is, in many cases, resolved by the use of natural sleep and/or sedating agents that do not affect EEG signals by themselves, e.g., chloral hydrate. Although MRI, including repeated scans, is considered to be safe in children and adolescents [7, 8], the benefits of the research study need to be weighed against the potential risks of the procedure including the use of sedating pharmaceuticals. Finally, to examine group results in a research context, another obstacle that needs to be overcome is splicing data of children of various ages because of developmental differences. Several pediatric brain MRI templates have been developed in order to address developmental changes in anatomy [9, 10].
The majority of previous EEG-fMRI studies have been completed in adults but the childhood epilepsies are unique with respect to their etiologies, semiology, pathogenesis, EEG findings, and long-term prognosis [11]. The developing brain has more frequent epileptiform discharges and they are usually poorly localized when compared to adults. It is likely that the immature brain has neuronal networks leading to interictal discharges that are influenced by the process of development. In addition, many epilepsy syndromes are specific to the pediatric population and can only be studied within children. Consequently, the use of EEG/fMRI in children has the potential to provide unique insights into the pathophysiological mechanisms of epilepsies, which can then be applied across the age span.
2. Requirements for EEG/fMRI data acquisition
MRI-compatible EEG equipment is required for EEG/fMRI data acquisition; standard clinical EEG hardware cannot be used in the MRI scanner without significant risk of damage to the hardware and physical injury to participants. Currently, commercial EEG hardware is available from several vendors that is safe for use in MRI and provides software that allows synchronization of the EEG to the fMRI data.
Typically, in order to remove the ballistocardiographic artifacts from the EEG recordings EKG and respiratory signals are recorded along with the EEG. These artifacts are then modeled and removed from the EEG recording using various freeware or commercially software packages that have implemented various methods of artifact removal including the previously mentioned Allen method [2, 3].
In MRI studies, participant compliance is an important issue that deserves considerable thought in the study design, especially in the pediatric population [12]. At our center, we have successfully performed fMRI in awake participants as young as 2.5 years [13] and EEG/fMRI scans on awake participants as young as 5 years old. While it is possible to perform EEG/fMRI on children who are sedated, the interpretation of signals obtained with the use of sedation may be difficult as sedative pharmaceuticals may alter the BOLD response [14–16]; in addition, use of sedation precludes functional or cognitive testing during the fMRI experiment. It is important when using EEG/fMRI in children to make sure that they have a good understanding of all the steps involved, that they are comfortable with all steps, and that there are no surprises that could cause discomfort leading to movement and resulting in failure of the experiment. The participants should understand that while some steps may be slightly uncomfortable, such as slight abrasion of the scalp during placement of EEG electrodes, nothing will be painful; this helps eliminate much of the anxiety the participant might otherwise have.
An important step to increase the success rate of the fMRI scans is to ensure patient comfort while in the scanner. Common complaints during scanning are a discomfort in the back of the head where the participant is resting on the EEG electrodes. Using toroid shaped foam pads around each posterior electrode is one way of increasing patient comfort, which may extend the time a participant is able to tolerate the cap, but a slight discomfort of the cap remains since pressure is still being exerted on a relatively small area. The best solution is a gel pad that spreads the pressure out across the entire back of the head and absorbs the impression of the electrodes. In our experience, switching to a gel pad has eliminated complaints relating to comfort in the scanner. Using this approach, our current study of patients with Benign Epilepsy with Centrotemporal Spikes (BECTS) has successfully collected EEG/fMRI data in 27 of 35 scans of BECTS patients (23 patients total ages 5–12 years, several patients with two scans). In 17 of these 27 successful scans, centrotemporal spikes were recorded during a resting state acquisition (a 15 minute sequence). In the remaining 8 scans, 2 children (both 6 year olds) refused to wear the EEG cap, 2 children (both 5 year olds) exited the scanner before data could be collected, and 4 scans were unusable due to excessive motion.
3. Analysis Methods
3.1 EEG Preprocessing
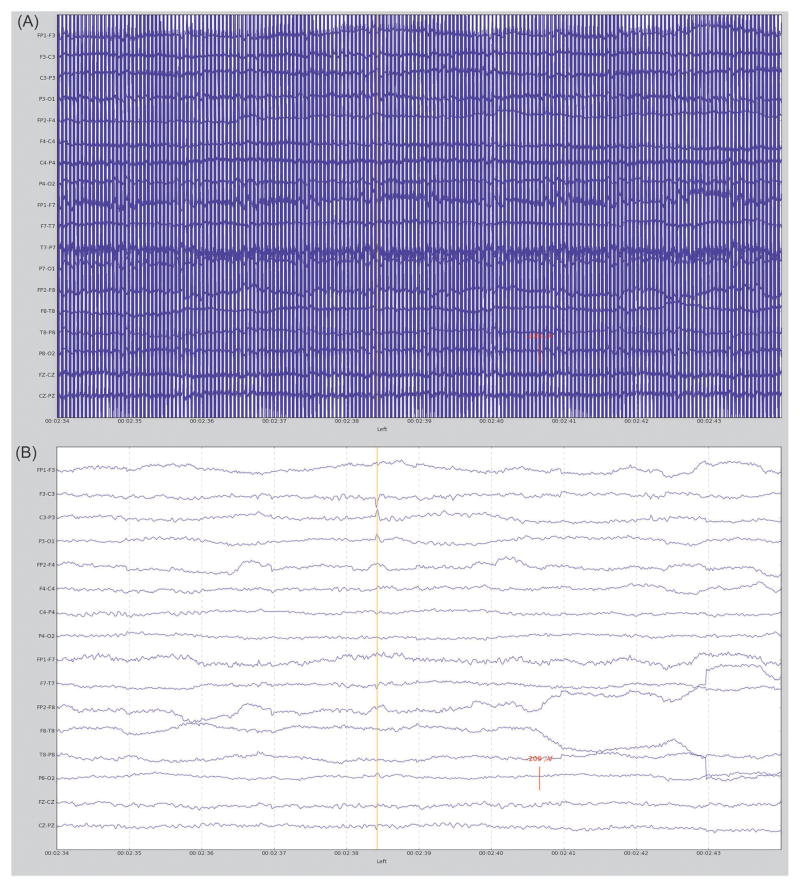
As previously mentioned, the fMRI environment creates significant noise that obfuscates neurologically generated electrical signals in the EEG data. It is possible to perform an analysis using EEG without the gradient artifacts removed, this is accomplished using an interleaved scanner acquisition [17] or by triggering EPI volumes off of an EEG event detection [18]. The downside to these methods is that it is impossible to account for any EEG events that may occur while the functional volume is being acquired and decisions regarding EEG events need to be made in a split second in order for the peak of the BOLD response to be collected. A more effective method, as mentioned above, is to use a gradient noise removal algorithm such as the one initially developed by Allen et al. [2] to completely remove the gradient noise allowing the use of a continuous EPI acquisition. Other algorithms have been used successfully as well including e.g., linear spatial filtering method [19, 20]. After the first processing step, the EPI artifact removal, ballistocardiographic artifacts are removed followed by censoring out EEG samples with excessive motion artifact. Once the EEG artifacts are removed, EEG signals can be evaluated for the presence of expected abnormalities (Figure 1).
Fig. 1.
EEG data collected from a BECTS patient during fMRI scan showing A) EEG prior to EPI gradient artifact and ballistocardiographic artifact removal and B) EEG at same location after artifact removal with a left sided centrotemporal spike identified.
3.2 Functional MRI Processing
The simultaneous EEG acquisition has little effect (occasional signal loss in the cerebellar region due to interference from the EEG cables) on the fMRI and the preprocessing of the fMRI data proceeds in the typical fashion [21]. These steps include motion correction of the functional scan volumes, coregistration of the functional scan to the anatomical, normalization of functional and anatomical scans to a normalized space and spatial and temporal smoothing. All of these steps are incorporated into freeware packages such as FSL or SPM [22].
3.3 General Linear Model
One of the most common methods to analyze EEG/fMRI data is the general linear model (GLM) [23]. One approach is to convolve the timing of the events of interest from EEG data with a hemodynamic response function (HRF) and use the resulting time series as a covariate in the GLM. It is common to model the interictal epileptic discharges (IED) as a gamma function [24] but other methods have been used as well such as counting the number of IED’s before an fMRI volume is acquired [17] or, in the case of generalized spike and wave discharges (GSWDs), using the entire length of the GSWD convolved with the HRF [25].
Most commonly, canonical HRF implemented in software packages such as SPM and FSL has been used [20, 26]. However, multiple studies report that the hemodynamic response arising from EEG events cannot be adequately modeled by the canonical HRF and HRFs in different areas of the brain may vary [27]. One method of evaluating BOLD signal responses to EEG events that occur outside the standard HRF window is to run several GLM analyses with a phase shifted HRF [25, 26, 28]. It is also possible to run a GLM analysis without restricting the shape of the hemodynamic response by using a basis set such as the finite impulse response (FIR), sine or gamma basis set [29].
3.4 Blood Oxygenation-Level Dependent Signal Change Estimation
In order to examine how the BOLD signal changes in relation to an EEG event, one can analyze the BOLD percent signal change in specific regions of interest. First, regions of interest must be identified either by statistical analysis (e.g. GLM), or hypothesis-driven, based on literature or relationship to seizure semiology. For each region of interest, BOLD intensity is averaged over all voxels for each functional volume, giving an average time course for that region. The percent change in BOLD for each region can then be calculated as (I − I0)/I0 × 100 where I is the average BOLD signal at each time point and I0 is the average over the time course. See [30] and [31] for examples of this type of analysis. Examining the percent change in BOLD provides a way of examining the shape of the hemodynamic response independent of any assumed HRF model; a limitation of this approach is that the areas must be defined a-priori.
3.5 Independent Component Analysis
EEG/fMRI GLM analysis has shown that there is a wide network involved in the initiation and maintenance of epileptiform discharges in many epilepsies that occur in childhood and adolescence (e.g., childhood absence epilepsy or juvenile myoclonic epilepsy) with BOLD signal changes occurring before and after the EEG events [26, 28, 30, 32, 33]. To better evaluate these changes, a method called event-related independent component analysis (eICA) has been developed that allows for a model free way of finding network components involved in a defined event with the assumption that the network components are temporally and spatially consistent [34]. The first step is to apply a GLM event-related analysis using a finite impulse response basis set, which is followed by feeding the beta estimates from the GLM analysis into an ICA, which identifies functional networks along with their time courses. The eICA can be implemented at both the individual and group level.
4. EEG/fMRI findings in pediatric epilepsy
4.1 Localization of sources in focal epilepsy syndromes
Benign Epilepsy with Centrotemporal Spikes (BECTS)
BECTS is an electroclinical genetic syndrome of childhood, which is characterized by nocturnal seizures with focal onset and secondary generalization and diurnal focal seizures with EEG onset localized to the Rolandic region [35–37]. Seizures begin during childhood with an age range of 3 to 13 years with a peak between 7 and 8 years. The EEG has characteristic interictal abnormalities consisting of high amplitude spikes or sharp waves which occur with maximum over the central (rolandic; C3, C4) and mid-temporal (T3, T4) head regions. Individual spikes may be unilateral or bilateral, however, when unilateral, there is usually an equal representation over the right and left hemispheres. The spikes are usually activated with sleep with up to 30% of children having spikes only during sleep [38–41]. Seizures are typically infrequent and treatment may not be not necessary [42]. The prognosis is excellent with the epilepsy remitting completely by mid-adolescence.
One study compared EEG multiple source analysis (MSA) with EEG/fMRI BOLD activity to localize the irritative zone in 11 BECTS patients [17]. MSA located the first dipole in all participants in the Rolandic region as expected from previous studies [43–45]. For the EEG/fMRI analysis, good EEG/fMRI data with interictal epileptiform discharges (IEDs) were acquired in only 4 patients. These authors did not use EEG artifact removal but instead used the sparse fMRI acquisition technique and convolved the number of IEDs occurring prior to each fMRI volume scan with the canonical HRF; final analysis was performed with GLM. EEG/fMRI analyses showed activation in all 4 patients in unilateral perisylvian central regions.
Another study used a finite impulse response (FIR) basis set to look at the hemodynamic response from CTS in 8 patients with BECTS [46]. BOLD activation presented in the ipsilateral sensorimotor region in response to IEDs for all patients from which a time course was extracted from the most statistically significant voxel in that region. The time courses were averaged to obtain the shape of the average group response that peaked 2 seconds earlier than the canonical HRF and that had a much greater undershoot. Using the group average response shape in GLM analysis, statistically significant activation in the ipsilateral postcentral gyrus was detected in 8 out 9 patients, compared with only 4 when using the canonical HRF. Using GLM convolved with canonical HRF, one of their patients who exhibited bilateral independent spikes showed significant activation outside of the Rolandic areas including the occipital regions that disappeared when HRF defined by the hemodynamics of the Rolandic region was used in the analysis. This may be an indication of a wider network involved in the generation or maintenance of CTS that has a different timing and/or HRF than the Rolandic region. The results from the FIR analysis were not shown, only the location of the representative voxel in each patient’s Rolandic region from which the time course was taken. These authors also hypothesized that the larger BOLD undershoot following the CTS might represent a reduction in neuronal activity. For an example of the difference between a GLM analysis with a standard Gamma HRF and a GLM analysis with FIR basis see figure 2. Based on their data, we hypothesize that individual differences in the extent of the decreasing BOLD signal may help explain the subtle cognitive deficits that have been reported in patients with BECTS [13, 47–49].
Fig. 2.
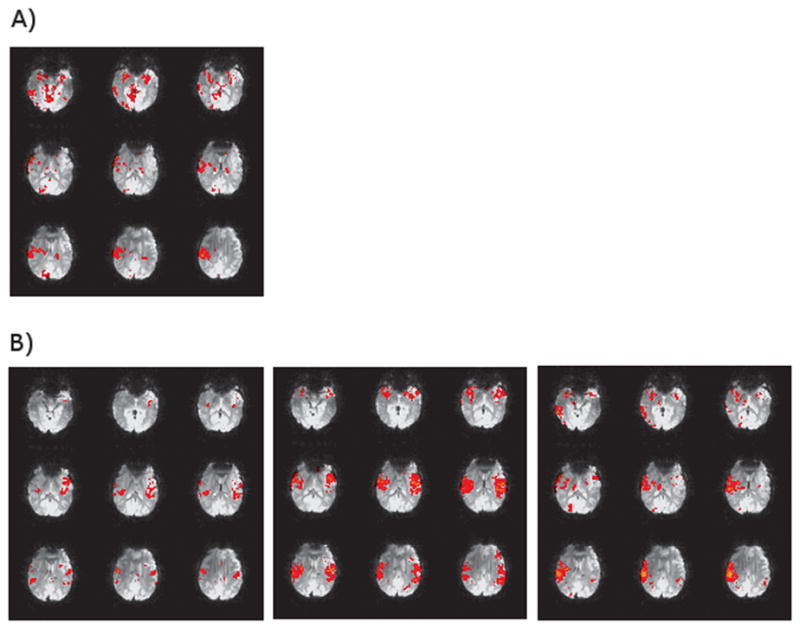
EEG/fMRI analysis implemented in FSL’s FEAT with standard preprocessing steps. Subject was diagnosed with BECTS and had 56 spikes during a resting EEG/fMRI acquisition (26 Right, 23 Left, 7 Bilateral). Images show positive BOLD activation for right spikes with spike times convolved with A) FSL’s standard Gamma HRF (6 second lag, 3 second standard deviation) and B) a FIR basis set starting at the time of the spike with a two second window (i.e. each image is 2 seconds apart showing the progression from time of the spike to 6 seconds after).
Subsequently, the same group investigated the location and timing of network components involved in the generation of the patients’ IEDs in 14 patients with BECTS using eICA [34]. They examined these networks at both the individual and group level; the data from patients with right sided CTS were normalized to one side in the left-right direction for the group analysis. The group eICA analysis detected a single component with activation in the postcentral gyrus ipsilateral to the CTS with a smaller activation on the contralateral side. The time course of the component did not follow the canonical HRF pattern but had an earlier peak time and a larger BOLD decrease as well as an increase in BOLD prior to the CTS. A group GLM analysis with canonical HRF of the same patients produced no significant activation most likely due to the fact the individual GLM analyses showed a mix of CTS related activation and deactivation in the Rolandic region. In their eICA analysis of individual patients, BOLD activity in the ipsilateral Rolandic region was found in 11 of the 15 analyses (14 patients, one of which had independent left and right sided CTS which were analyzed separately) compared with only 8 of the 15 when a GLM analysis with canonical HRF was used. Aside from activation in the Rolandic regions, eICA analysis also identified components with activation in the occipital and peri-Rolandic regions in a few patients. They hypothesize that additional components from the individual analysis did not show up in the group analysis because those regions may be secondary responses or precursors to the IED with individual variability.
Focal Cortical Dysplasia
Several studies in adults documented variable BOLD signal responses to IEDs including local and remote activations and deactivations [50, 51]. Overall, the results of studies in children are similar. E.g., one group used EEG/fMRI to compare BOLD activation associated with focal cortical dysplasia IEDs to seizure onset zone (SOZ) as detected on intracranial electroencephalography (icEEG) [52]. They recorded IEDs in 12 patients during the EEG/fMRI scan, 11 of which had significant BOLD changes. For their analysis they convolved the marked timing of the IED with the canonical HRF to be used as a regressor in a GLM. The fMRI results showed BOLD activation within 2 cm of the SOZ, as detected with icEEG, in 9 out of 11 patients, 5 of which had all BOLD clusters localized with the SOZ at the lobar level. In 5 of 6 cases where surgical outcome was poor, some or all of BOLD clusters of activation were located in lobes outside of the lobe where the SOZ was localized. They concluded that EEG/fMRI is currently unable to replace icEEG, the gold-standard of SOZ localization, but may be able to be used as a factor when deciding whether or not to undergo icEEG, as extensive BOLD activation showed a trend with poor post-surgical outcome. Using a model free approach or other types of hemodynamic response may provide better localization but this remains to be shown.
Tuberous Sclerosis Complex
One group studied 5 patients, mean age 5.2 years, with tuberous sclerosis complex, a genetic syndrome typically associated with multiple cortical abnormalities and epilepsy [6]. The patients in this study had various seizure types including complex partial seizures (CPS), atypical absences, hypermotor seizures, infantile spasms and myoclonic jerks. All but one of the patients were treated with antiepileptic drugs (AEDs) with one of the medicated patients seizure-free at the time of the study. All patients were sedated prior to EEG/fMRI. IEDs and spike-wave-bursts were marked and differentiated according to spatial distribution and morphology. Four GLM analyses were performed with IEDs timing convolved with a Gamma function with peak at 3, 5, 7 or 9 seconds after the IEDs onset. Each IEDs type was included in each analysis but each remained differentiated from the others, with one study per patient per IEDs type this resulted in 13 total studies. Positive BOLD response was identified in 10 studies and negative in 2 with only 1 study not showing any BOLD response. All patients showed BOLD response in multiple areas where a tuber was present as well as areas where the tissue appeared to be normal. No specific patterns in regard to the sign of the BOLD response were identified across the group. This study suggests that there is a wider brain network involved in IEDs with involvement of multiple tubers and healthy tissue
4.2 Epileptic networks in generalized epilepsy syndromes
Childhood Absence Epilepsy
Childhood Absence Epilepsy (CAE) Absence epilepsy is the most common pediatric epilepsy syndrome; absence seizures are part of many forms of pediatric and adult epilepsy syndromes. Typical absence seizures (AS) are characterized as “generalized” and consist of multiple, brief (up to 20 seconds) impairments of consciousness that have an abrupt onset and offset [53]. Absences are unique due to their pharmacologic treatments and characteristic bilaterally synchronous 3Hz generalized spike wave discharges (GSWDs) on EEG. A typical AS is manifested behaviorally as a “staring spell” and can be accompanied by atonic postures such as drooping of the head and/or automatisms such as lip smacking. The majority of children affected by AS will become seizure-free as they enter adulthood [54–56].
One study evaluated 10 drug-naïve children with CAE using EEG/fMRI and captured GSWDs in 6 of them [57]. Focusing on pre-defined ROIs in the thalamus, the precuneus, left and right parietal cortex, and caudate nucleus, they used a canonical HRF to model the BOLD signal in each of these ROIs. They found that a group analysis across the 6 patients showed a BOLD signal increase in the thalamus. BOLD signal decreases were observed in the caudate nucleus and the left parietal cortex. Then, extracting the average BOLD signal time course from each ROI, they found consistent patterns of BOLD signal change associated with GSWDs. Specifically, BOLD signal increase in the thalamus began to rise at the onset of the GSWD and peaked at approximately 6 seconds regardless of the length of the seizure. In the precuneus and caudate nucleus, the decrease in BOLD response began at the onset of the GSWD and reached a maximum decrease several seconds later. These “deactivations” may correspond to previously defined default mode networks and could be involved in the loss of awareness which is the hallmark of absence seizures [57, 58]. Additionally, positive and negative BOLD changes in areas other than the thalamus were variable and may indicate phenotypic variation of the epilepsy syndrome or individual differences in the hemodynamic response. They also noted that in these regions, no changes in BOLD responses were detected prior to the onset of the GSWD. However, subsequent studies by other groups have demonstrated BOLD signal changes occurring up to several seconds prior to absence [25, 30, 59] thus the lack of pre-IEDs BOLD signal changes may be related e.g., to the fact that the participants were drug naïve.
Another study analyzed EEG/fMRI data from 9 CAE patients, mean age 11.9 years, with absence seizures [30]. While scanning they used a continuous performance task (CPT), repetitive tapping task (RTT) and a visual fixation task (VFT) to measure impairment of consciousness. They used a GLM analysis with the GSWD modeled as a boxcar function and convolved with the canonical HRF. This resulted in BOLD increases in bilateral thalamus, occipital cortex, midline cerebellum, anterior and lateral temporal lobes, insula and an area near the lateral ventricles and BOLD decreases in lateral parietal cortex, precuneus, cingulate gyrus and basal ganglia. They also examined the fMRI percent signal change on a voxel-by-voxel basis by averaging the time course of single voxels across patients and averaged the resulting voxel time courses in seven anatomical regions in order to look at the hemodynamic response. These results revealed changes that began 8–14 seconds prior to seizure and continued after the seizure had ended. Prior to seizure onset positive BOLD changes occurred in the medial orbital frontal, fronto-polar, cingulate, lateral parietal, precuneus and lateral occipital cortex with additional increases in the lateral frontal and temporal cortex at seizure onset which is similar to adult studies (for detailed review see [60]. The period after AS was marked by mostly BOLD decreases in medial/orbital frontal, cingulate, medial and lateral parietal cortex 10 seconds after seizure onset followed by additional decreases in frontal, temporal, and occipital cortex and basal ganglia with changes lasting for at least 20 seconds after the seizure. Examining the time course in many of these regions they concluded that they did not follow a canonical HRF response to seizure onset with the exception of a region covering the thalamus. Despite BOLD effects lasting for more than 20 seconds after seizure they report that all behavioral impairments during CPT and RTT tasks ended with the seizure.
A third study examined a heterogeneous group of patients with AS and found that they could separate their cohort into two groups based on BOLD signal changes in the frontal cortex [31]. Their GLM group analysis using the canonical HRF showed positive BOLD activation in the thalamus and frontal white matter tracts and negative activation in the posterior parietal, bilateral mesial frontal cortex, caudate and pontine reticular regions. Using ROI’s defined from previous studies they examined the percent signal change in those regions from 32 seconds before to 32 seconds after AS. The percent signal change in the dorsolateral prefrontal cortex (DLPFC) showed either a mostly positive (7 subjects) or mostly negative (6 patients) BOLD response. Only those patients who showed a positive response in the DLPFC had ongoing AS seizures (3 patients) and had seizures other than AS (2 patients). They also found subtle differences in the BOLD response between the two groups in the AS network. The authors predicted that there may be biological significance to their classification although confounding factors such as differences in medication and neuropsychological performance require further investigation.
Similar to findings in other epilepsy syndromes [30], the hemodynamic response in brain networks involved in absence GSWD may not be captured by a canonical HRF. To avoid the limitations with the canonical HRF, one study used eICA to examine the various networks involved in GSWD and to evaluate their timings [61]. Eight absence patients with GSWD during their EEG/fMRI scan were used in both, a GLM analysis with canonical HRF, and a group eICA analysis. Using the GLM analysis they found activations in the thalamus and anterior cingulate cortex and deactivation in the bilateral frontal, parietal and occipital lobes and head of the caudate nucleus. Their eICA analysis revealed 6 networks that showed a consistent event related response across the group. These 6 networks included the same areas that showed up in the GLM analysis but with additional areas of BOLD activity and with information on the timing of (de-)activation. These findings support the hypothesis that there are focal brain regions that lead to activation of a more widespread network resulting in the generalized EEG findings [60]. Additionally, several of these components had different timing of their BOLD response. Prior to any EEG changes there was activity in several regions, which may indicate a widespread pre-ictal network. During the seizure a distinct activation in the mesial frontal cortical could represent not only the mechanism leading to the attentional impairment seen during seizures but also indicate focal onset of what appears to be a generalized epilepsy on the EEG.
Other Genetic Generalized Epilepsies
Other Genetic Generalized Epilepsies (GGEs) are heterogeneous in clinical presentations and genetic etiologies. The EEG typically shows 2–5Hz generalized spike wave discharges (GSWDs) on a normal background and patients can experience absence seizures, generalized tonic clonic seizures, and/or myoclonic seizures [62]. Early EEG/fMRI studies of patients with GGEs have shown thalamic BOLD signal increases, and increases and decreases within other cortical regions during GSWDs [57, 58]. These results are similar to those which have been reported for BOLD signal changes during absence seizures [57]. Other EEG/fMRI studies have shown heterogeneity of BOLD signal changes associated with GSWDs with most patients having thalamic activation (80%) and variable areas of cortical activation including diffuse regions (33%), only anterior head regions (40%), and predominantly posterior cortical regions (20%) [63]. Overall, these findings support the role of deep brain structures in the maintenance of diffuse, bilaterally symmetric EEG discharges. The reason for the variable nature of the cortical BOLD responses is unclear but may be due to heterogeneous patient populations, medication effects on BOLD signals [64, 65], or slight differences in the analysis methods.
More recently, timing of BOLD signal changes during GSWDs has been studied in more detail. In adolescents and adults with GGE, one study found BOLD increases in the thalamus, cerebellum, and anterior cingulate gyrus at GSWD onset [25]. However, they also found that varying the phase of the standard HRF from 18 seconds before to 18 seconds after the GSWD in 3 second intervals showed significant BOLD increases in the typical default mode network and medial/dorsolateral prefrontal cortex up to 1 seconds prior to the GSWD and BOLD decreases in the parietal lobes, medial temporal cortex lateral and medial frontal cortex, associative visual cortex and bilaterally in the cerebellum. They believe that the individual variability in the BOLD signal that was found could reflect genetic and phenotypic heterogeneity [25].
EEG/fMRI has also been used to understand not only the neuronal networks underlying GSWDs but to test hypotheses that variations in responses to clinical treatments may be related to differing networks. When patients with GGE were analyzed based on their response to treatment, those with refractory GGE initially had more widespread frontal and parietal activity during absence seizures, which was followed by thalamic activation [26]. It was hypothesized that patients with refractory GGE may have a cortical initiation of seizures which then propagate to the thalamus (for detailed discussion see [60]. A follow up to the above study contrasted patients GGEs responsive and not responsive to valproic acid, a prototypical AED used for the treatment of GGEs to show differences between these two groups including several cortical but not thalamic regions indicating that responders and non-responders may have different origins of GSWDs [28]. Finally, connectivity analysis of the thalamic and paracingulate areas revealed differences between responders and non responders [66]. These and other studies argue for the cortical or cortico-thalamic rather than thalamic seizure onset in patients with GGEs [30].
Lennox-Gastaut Syndrome
One study used EEG/fMRI to investigate the functional networks in Lennox-Gastaut syndrome (LGS), a type of epilepsy in which patients have multiple seizure types and slow spike wave complexes on EEG [67]. LGS patients also have developmental delay and poor response to anti-epileptic medication. The participants in the above study were 11 children with LGS (mean 6.45 years) and 9 children with focal epilepsy (mean 10.88 years) as controls, all 20 participants had global developmental delay. All participants were sedated prior to scanning. Functional MRI data were analyzed using GLM with interictal discharges convolved with the canonical HRF. In the LGS group, IEDs were separated into left-sided and right-sided spike-slow wave complexes and runs of polyspikes (paroxysmal fast activity), and activation in the brainstem was the most consistent finding at the single-subject level with no consistent findings in the control group. In the group analysis, the IED types were combined and only the LGS showed significant areas of BOLD activation which were in the brainstem and thalamus, activity in both is supported by previous studies.
A more recent study used EEG/fMRI in 13 patients with LGS and attempted to separate the activation patterns associated with spike-slow wave complexes and paroxysmal fast activity [68]. These patients were studied as adults (ages 25–52), but all had childhood onset of LGS. They also used a GLM approach with a canonical HRF to analyze events of paroxysmal fast activity and spike-slow waves at the single-subject level. Paroxysmal fast activity, observed in 6 patients was associated with increased BOLD signal in the brainstem, basal ganglia, thalamus, and widespread cortical regions at the exclusion of primary sensory motor cortices. In contrast, spike-slow wave activity corresponded with decreases in fMRI signal in a number of cortical regions including primary visual and auditory cortices. They concluded that two distinct diffuse patterns of activation underlie the two types of IEDs observed in LGS. These findings are somewhat difficult to interpret given the small sample size, heterogeous structural brain abnormalities, and the presence of prior corpus callosotomy in some patients.
Myoclonic Astatic Epilepsy
A recent study examined patients with myoclonic astatic epilepsy (MAE), a condition characterized clinically by myoclonic, astatic drop attacks, generalized tonic-clonic seizure and absences and GSWD on the EEG [69]. The objective of this study was to investigate the networks involved with GSWD and compare them with the networks found for patients with GGE. Their participants consisted of 11 patients diagnosed with MAE ages 3–13 years, all of whom were sedated prior to the MRI scan. BOLD activation was analyzed using a GLM with GSWD marked and convolved with the canonical HRF. In their group analysis they found activation in the right putamen, right premotor cortex, left temporal lobe and left cerebellum with deactivation in the right occipital cortex and precuneus at an uncorrected threshold of p < 0.001. When they reduced the threshold to p = 0.005 uncorrected they found additional activation in the left putamen, thalamus, left premotor cortex, supplementary motor cortex and right cerebellum with deactivation in the left occipital cortex, and parietal cortex. These authors concluded that the networks associated with MAE show similarity with the networks found in GGE but with activation in the putamen and premotor cortex specific to MAE. They hypothesized that the involvement of premotor cortex, putamen, and SMA leads to manifestation as myoclonic jerks in patients with MAE.
5. Conclusions
EEG/fMRI is a valuable technique for investigating the network activity in the epileptic brain in pediatric subjects. At this point it is clear that the epileptic activity on the scalp EEG does not tell the entire story. The shape and timing of the hemodynamic response to the epileptic events shown on EEG needs to be investigated in detail as it is unlikely to follow a standard canonical response [25, 30, 34, 61]. It could be that the hemodynamic response to spikes and seizures is canonical in its shape but to activity that is undetectable on scalp EEG [30, 34] or the underlying vasculature or metabolic function of epileptic activity may not follow the same response as evoked activity [30, 34].
A major limitation in the design of pediatric epilepsy studies is the variability in the patient population. At this point it is difficult to determine if the variations in BOLD activity at the subject level are due to variations in the patient population, e.g. age, medication, severity, time since diagnosis etc… or whether epilepsy taxonomy can be further subdivided based on the etiology of the brain networks involved. More research into the effects that activity in different epileptic brain networks have on factors such as response to medication or behavioral measures is needed.
Acknowledgments
Work included in this manuscript was supported by NIH/NINDS R01NS065840 (Vannest, PI)
References
- 1.Ives JR, Warach S, Schmitt F, Edelman RR, Schomer DL. Monitoring the patient’s EEG during echo planar MRI. Electroencephalography and clinical neurophysiology. 1993;87(6):417–20. doi: 10.1016/0013-4694(93)90156-p. [DOI] [PubMed] [Google Scholar]
- 2.Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuro Image. 2000;12(2):230–9. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- 3.Allen PJ, Polizzi G, Krakow K, Fish DR, Lemieux L. Identification of EEG events in the MR scanner: the problem of pulse artifact and a method for its subtraction. Neuro Image. 1998;8(3):229–39. doi: 10.1006/nimg.1998.0361. [DOI] [PubMed] [Google Scholar]
- 4.Benar C, Aghakhani Y, Wang Y, Izenberg A, Al-Asmi A, Dubeau F, et al. Quality of EEG in simultaneous EEG-fMRI for epilepsy. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2003;114(3):569–80. doi: 10.1016/s1388-2457(02)00383-8. [DOI] [PubMed] [Google Scholar]
- 5.Vanderperren K, De Vos M, Ramautar JR, Novitskiy N, Mennes M, Assecondi S, et al. Removal of BCG artifacts from EEG recordings inside the MR scanner: a comparison of methodological and validation-related aspects. Neuro Image. 2010;50(3):920–34. doi: 10.1016/j.neuroimage.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs J, Rohr A, Moeller F, Boor R, Kobayashi E, LeVan Meng P, et al. Evaluation of epileptogenic networks in children with tuberous sclerosis complex using EEG-fMRI. Epilepsia. 2008;49(5):816–25. doi: 10.1111/j.1528-1167.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- 7.Holland SK, Byars AW, Plante E, Szaflarski JP, Dietrich K, Altaye M. Studies support probable long-term safety of MRI. Science. 2010;329(5991):512–3. doi: 10.1126/science.329.5991.512-e. [DOI] [PubMed] [Google Scholar]
- 8.Holland SK, Altaye M, Robertson S, Byars AW, Plante E, Szaflarski JP. Data on the safety of repeated MRI in healthy children. Neuro Image: Clinical. 2014;4(0):526–30. doi: 10.1016/j.nicl.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altaye M, Holland SK, Wilke M, Gaser C. Infant brain probability templates for MRI segmentation and normalization. Neuro Image. 2008;43(4):721–30. doi: 10.1016/j.neuroimage.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuro Image. 2008;41(3):903–13. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Ari Y. Basic developmental rules and their implications for epilepsy in the immature brain. Epileptic disorders: international epilepsy journal with videotape. 2006;8(2):91–102. [PubMed] [Google Scholar]
- 12.Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, Schmithorst VJ, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. Journal of child neurology. 2002;17(12):885–90. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vannest J, Szaflarski JP, Eaton KP, Henkel DM, Morita D, Glauser TA, et al. Functional magnetic resonance imaging reveals changes in language localization in children with benign childhood epilepsy with centrotemporal spikes. Journal of child neurology. 2013;28(4):435–45. doi: 10.1177/0883073812447682. [DOI] [PubMed] [Google Scholar]
- 14.Masamoto K, Kanno I. Anesthesia and the quantitative evaluation of neurovascular coupling. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(7):1233–47. doi: 10.1038/jcbfm.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel AM, Cahill LD, Ret J, Schmithorst V, Choo D, Holland S. FUnctional magnetic resonance imaging of hearing-impaired children under sedation before cochlear implantation. Archives of Otolaryngology–Head & Neck Surgery. 2007;133(7):677–83. doi: 10.1001/archotol.133.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson S, Karunananayaka P, Arjmand E, Greinwald J, Care M, Egelhoff J, et al., editors. fMRI of Infants for Cochlear Implant Staging is Influenced by Sedation. Poster presented at: Soc for Ear, Nose and Throat Advances in Children; 2010 Dec 3–5; Cincinnati, OH. [Google Scholar]
- 17.Boor R, Jacobs J, Hinzmann A, Bauermann T, Scherg M, Boor S, et al. Combined spike-related functional MRI and multiple source analysis in the non-invasive spike localization of benign rolandic epilepsy. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2007;118(4):901–9. doi: 10.1016/j.clinph.2006.11.272. [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Blum A, Pearlman JD, Yousuf N, Ives JR, Saeteng S, et al. Echo-planar functional MR imaging of epilepsy with concurrent EEG monitoring. AJNR American journal of neuroradiology. 1999;20(10):1916–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Lagerlund TD, Sharbrough FW, Busacker NE. Spatial filtering of multichannel electroencephalographic recordings through principal component analysis by singular value decomposition. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society. 1997;14(1):73–82. doi: 10.1097/00004691-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Kay BP, DiFrancesco MW, Privitera MD, Gotman J, Holland SK, Szaflarski JP. Reduced default mode network connectivity in treatment-resistant idiopathic generalized epilepsy. Epilepsia. 2013;54(3):461–70. doi: 10.1111/epi.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stern JM. Simultaneous electroencephalography and functional magnetic resonance imaging applied to epilepsy. Epilepsy & behavior: E&B. 2006;8(4):683–92. doi: 10.1016/j.yebeh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuro Image. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 23.Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuro Image. 1995;2(2):157–65. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- 24.Hawco CS, Bagshaw AP, Lu Y, Dubeau F, Gotman J. BOLD changes occur prior to epileptic spikes seen on scalp EEG. Neuro Image. 2007;35(4):1450–8. doi: 10.1016/j.neuroimage.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Benuzzi F, Mirandola L, Pugnaghi M, Farinelli V, Tassinari CA, Capovilla G, et al. Increased cortical BOLD signal anticipates generalized spike and wave discharges in adolescents and adults with idiopathic generalized epilepsies. Epilepsia. 2012;53(4):622–30. doi: 10.1111/j.1528-1167.2011.03385.x. [DOI] [PubMed] [Google Scholar]
- 26.Szaflarski JP, DiFrancesco M, Hirschauer T, Banks C, Privitera MD, Gotman J, et al. Cortical and subcortical contributions to absence seizure onset examined with EEG/fMRI. Epilepsy & behavior: E&B. 2010;18(4):404–13. doi: 10.1016/j.yebeh.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Munck JC, Goncalves SI, Huijboom L, Kuijer JP, Pouwels PJ, Heethaar RM, et al. The hemodynamic response of the alpha rhythm: an EEG/fMRI study. Neuro Image. 2007;35(3):1142–51. doi: 10.1016/j.neuroimage.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Szaflarski JP, Kay B, Gotman J, Privitera MD, Holland SK. The relationship between the localization of the generalized spike and wave discharge generators and the response to valproate. Epilepsia. 2013;54(3):471–80. doi: 10.1111/epi.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henson RNA. Analysis of fMRI time series. In: Ashburner J, Penny WD, RSJFaKJFaCFaRDaKJFaCJPaSZa, editors. Human Brain Function. Academic Press; 2003. [Google Scholar]
- 30.Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, et al. Dynamic time course of typical childhood absence seizures: EEG, behavior, and functional magnetic resonance imaging. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(17):5884–93. doi: 10.1523/JNEUROSCI.5101-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carney PW, Masterton RA, Flanagan D, Berkovic SF, Jackson GD. The frontal lobe in absence epilepsy: EEG-fMRI findings. Neurology. 2012;78(15):1157–65. doi: 10.1212/WNL.0b013e31824f801d. [DOI] [PubMed] [Google Scholar]
- 32.Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, et al. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuro Image. 2008;39(4):1839–49. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 33.Moeller F, Siebner HR, Ahlgrimm N, Wolff S, Muhle H, Granert O, et al. fMRI activation during spike and wave discharges evoked by photic stimulation. Neuro Image. 2009;48(4):682–95. doi: 10.1016/j.neuroimage.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 34.Masterton RA, Jackson GD, Abbott DF. Mapping brain activity using event-related independent components analysis (eICA): specific advantages for EEG-fMRI. Neuro Image. 2013;70:164–74. doi: 10.1016/j.neuroimage.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Holmes GL. Rolandic epilepsy: clinical and electroencephalographic features. Epilepsy research Supplement. 1992;6:29–43. [PubMed] [Google Scholar]
- 36.Lerman P, Kivity S. Benign focal epilepsy of childhood. A follow-up study of 100 recovered patients. Archives of neurology. 1975;32(4):261–4. doi: 10.1001/archneur.1975.00490460077010. [DOI] [PubMed] [Google Scholar]
- 37.Ma CK, Chan KY. Benign childhood epilepsy with centrotemporal spikes: a study of 50 Chinese children. Brain & development. 2003;25(6):390–5. doi: 10.1016/s0387-7604(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 38.Clemens B, Majoros E. Sleep studies in benign epilepsy of childhood with rolandic spikes. II. Analysis of discharge frequency and its relation to sleep dynamics. Epilepsia. 1987;28(1):24–7. doi: 10.1111/j.1528-1157.1987.tb03617.x. [DOI] [PubMed] [Google Scholar]
- 39.Clemens B, Olah R. Sleep studies in benign epilepsy of childhood with rolandic spikes. I. Sleep pathology. Epilepsia. 1987;28(1):20–3. doi: 10.1111/j.1528-1157.1987.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 40.Blom S, Brorson LO. Central spikes or sharp waves (Rolandic spikes) in children’s EEG and their clinical significance. Acta paediatrica Scandinavica. 1966;55(4):385–93. doi: 10.1111/j.1651-2227.1966.tb08809.x. [DOI] [PubMed] [Google Scholar]
- 41.Blom S, Heijbel J. Benign epilepsy of children with centro-temporal EEG foci. Discharge rate during sleep. Epilepsia. 1975;16(1):133–40. doi: 10.1111/j.1528-1157.1975.tb04730.x. [DOI] [PubMed] [Google Scholar]
- 42.Loiseau P, Beaussart M. The seizures of benign childhood epilepsy with Rolandic paroxysmal discharges. Epilepsia. 1973;14(4):381–9. doi: 10.1111/j.1528-1157.1973.tb03977.x. [DOI] [PubMed] [Google Scholar]
- 43.Baumgartner C, Graf M, Doppelbauer A, Serles W, Lindinger G, Olbrich A, et al. The functional organization of the interictal spike complex in benign rolandic epilepsy. Epilepsia. 1996;37(12):1164–74. doi: 10.1111/j.1528-1157.1996.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 44.Kamada K, Moller M, Saguer M, Kassubek J, Kaltenhauser M, Kober H, et al. Localization analysis of neuronal activities in benign rolandic epilepsy using magnetoencephalography. Journal of the neurological sciences. 1998;154(2):164–72. doi: 10.1016/s0022-510x(97)00226-8. [DOI] [PubMed] [Google Scholar]
- 45.van der Meij W, Wieneke GH, van Huffelen AC. Dipole source analysis of rolandic spikes in benign rolandic epilepsy and other clinical syndromes. Brain topography. 1993;5(3):203–13. doi: 10.1007/BF01128988. [DOI] [PubMed] [Google Scholar]
- 46.Masterton RA, Harvey AS, Archer JS, Lillywhite LM, Abbott DF, Scheffer IE, et al. Focal epileptiform spikes do not show a canonical BOLD response in patients with benign rolandic epilepsy (BECTS) Neuro Image. 2010;51(1):252–60. doi: 10.1016/j.neuroimage.2010.01.109. [DOI] [PubMed] [Google Scholar]
- 47.Lillywhite LM, Saling MM, Simon Harvey A, Abbott DF, Archer JS, Vears DF, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- 48.Northcott E, Connolly AM, Berroya A, Sabaz M, McIntyre J, Christie J, et al. The neuropsychological and language profile of children with benign rolandic epilepsy. Epilepsia. 2005;46(6):924–30. doi: 10.1111/j.1528-1167.2005.62304.x. [DOI] [PubMed] [Google Scholar]
- 49.Overvliet GM, Aldenkamp AP, Klinkenberg S, Nicolai J, Vles JS, Besseling RM, et al. Correlation between language impairment and problems in motor development in children with rolandic epilepsy. Epilepsy and Behavior. 2011;22(3):527–31. doi: 10.1016/j.yebeh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Tyvaert L, Hawco C, Kobayashi E, LeVan P, Dubeau F, Gotman J. Different structures involved during ictal and interictal epileptic activity in malformations of cortical development: an EEG-fMRI study. Brain: a journal of neurology. 2008;131(Pt 8):2042–60. doi: 10.1093/brain/awn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Federico P, Archer JS, Abbott DF, Jackson GD. Cortical/subcortical BOLD changes associated with epileptic discharges: an EEG-fMRI study at 3 T. Neurology. 2005;64(7):1125–30. doi: 10.1212/01.WNL.0000156358.72670.AD. [DOI] [PubMed] [Google Scholar]
- 52.Thornton R, Vulliemoz S, Rodionov R, Carmichael DW, Chaudhary UJ, Diehl B, et al. Epileptic networks in focal cortical dysplasia revealed using electroencephalography-functional magnetic resonance imaging. Annals of neurology. 2011;70(5):822–37. doi: 10.1002/ana.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penry JK, Dreifuss FE. Automatisms associated with the absence of petit mal epilepsy. Archives of neurology. 1969;21(2):142–9. doi: 10.1001/archneur.1969.00480140042004. [DOI] [PubMed] [Google Scholar]
- 54.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B, et al. Two-year remission and subsequent relapse in children with newly diagnosed epilepsy. Epilepsia. 2001;42(12):1553–62. doi: 10.1046/j.1528-1157.2001.21101.x. [DOI] [PubMed] [Google Scholar]
- 55.Fois A, Malandrini F, Mostardini R. Clinical experiences of petit mal. Brain & development. 1987;9(1):54–9. doi: 10.1016/s0387-7604(87)80011-6. [DOI] [PubMed] [Google Scholar]
- 56.Trinka E, Baumgartner S, Unterberger I, Unterrainer J, Luef G, Haberlandt E, et al. Long-term prognosis for childhood and juvenile absence epilepsy. Journal of neurology. 2004;251(10):1235–41. doi: 10.1007/s00415-004-0521-1. [DOI] [PubMed] [Google Scholar]
- 57.Moeller F, Siebner HR, Wolff S, Muhle H, Granert O, Jansen O, et al. Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia. 2008;49(9):1510–9. doi: 10.1111/j.1528-1167.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- 58.Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(42):15236–40. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carney PW, Masterton RA, Harvey AS, Scheffer IE, Berkovic SF, Jackson GD. The core network in absence epilepsy. Differences in cortical and thalamic BOLD response. Neurology. 2010;75(10):904–11. doi: 10.1212/WNL.0b013e3181f11c06. [DOI] [PubMed] [Google Scholar]
- 60.Kay B, Szaflarski JP. EEG/fMRI contributions to our understanding of genetic generalized epilepsies. Epilepsy & behavior: E&B. 2014;34:129–35. doi: 10.1016/j.yebeh.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masterton RA, Carney PW, Abbott DF, Jackson GD. Absence epilepsy subnetworks revealed by event-related independent components analysis of functional magnetic resonance imaging. Epilepsia. 2013;54(5):801–8. doi: 10.1111/epi.12163. [DOI] [PubMed] [Google Scholar]
- 62.Szaflarski JP, Lindsell CJ, Zakaria T, Banks C, Privitera MD. Seizure control in patients with idiopathic generalized epilepsies: EEG determinants of medication response. Epilepsy & behavior: E&B. 2010;17(4):525–30. doi: 10.1016/j.yebeh.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, et al. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain: a journal of neurology. 2004;127(Pt 5):1127–44. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- 64.Szaflarski JP, Allendorfer JB. Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy & behavior: E&B. 2012;24(1):74–80. doi: 10.1016/j.yebeh.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasuda CL, Centeno M, Vollmar C, Stretton J, Symms M, Cendes F, et al. The effect of topiramate on cognitive fMRI. Epilepsy research. 2013;105(1–2):250–5. doi: 10.1016/j.eplepsyres.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kay BP, Holland SK, Privitera MD, Szaflarski JP. Differences in paracingulate connectivity associated with epileptiform discharges and uncontrolled seizures in genetic generalized epilepsy. Epilepsia. 2014;55(2):256–63. doi: 10.1111/epi.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siniatchkin M, Coropceanu D, Moeller F, Boor R, Stephani U. EEG-fMRI reveals activation of brainstem and thalamus in patients with Lennox-Gastaut syndrome. Epilepsia. 2011;52(4):766–74. doi: 10.1111/j.1528-1167.2010.02948.x. [DOI] [PubMed] [Google Scholar]
- 68.Pillay N, Archer JS, Badawy RA, Flanagan DF, Berkovic SF, Jackson G. Networks underlying paroxysmal fast activity and slow spike and wave in Lennox-Gastaut syndrome. Neurology. 2013;81(7):665–73. doi: 10.1212/WNL.0b013e3182a08f6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moeller F, Groening K, Moehring J, Muhle H, Wolff S, Jansen O, et al. EEG-fMRI in myoclonic astatic epilepsy (Doose syndrome) Neurology. 2014;82(17):1508–13. doi: 10.1212/WNL.0000000000000359. [DOI] [PubMed] [Google Scholar]