Abstract
Cocaine abuse in HIV patients accelerates the progression and severity of neuropathology, motor impairment and cognitive dysfunction compared to non-drug using HIV patients. Cocaine and HIV interact with the dopamine transporter (DAT); however, the effect of their interaction on DAT binding remains understudied. The present study compared the dose-response functions for intravenous self-administration of cocaine and heroin between male HIV-1 transgenic (HIV-1 Tg) and Fischer 344 rats. The cocaine and heroin dose-response functions exhibit an inverted U-shape for both HIV-1 Tg and F344 rats. For cocaine, the number of infusions for each dose on the ascending limb was greater for HIV-1 Tg versus F344 rats. No significant changes in the heroin dose-response function were observed in HIV-1 Tg animals. Following the conclusion of self-administration experiments, DAT binding was assessed in striatal membranes. Saturation binding of the cocaine analog [125I] 3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester ([125I]RTI-55) in rat striatal membranes resulted in binding curves that were best fit to a two-site binding model, allowing for calculation of dissociation constant (Kd) and binding density (Bmax) values that correspond to high- and low-affinity DAT binding sites. Control HIV-1 Tg rats exhibited a significantly greater affinity (i.e., decrease in Kd value) in the low-affinity DAT binding site compared to control F344 rats. Furthermore, cocaine self-administration in HIV-1 Tg rats increased low-affinity Kd (i.e., decreased affinity) compared to levels observed in control F344 rats. Cocaine also increased low-affinity Bmax in HIV-1 Tg rats as compared to controls, indicating an increase in the number of low-affinity DAT binding sites. F344 rats did not exhibit any change in high- or low-affinity Kd or Bmax values following cocaine or heroin self-administration. The increase in DAT affinity in cocaine HIV-1 Tg rats is consistent with the leftward shift of the ascending limb of the cocaine dose-response curve observed in HIV-1 Tg vs. F344 rats, and has major implications for the function of cocaine binding to DAT in HIV patients. The absence of HIV-related changes in heroin intake are likely due to less dopaminergic involvement in the mediation of heroin reward, further emphasizing the preferential influence of HIV on dopamine-related behaviors.
Keywords: HIV, reinforcement, addiction, stimulant, opiate, self-administration, receptor binding
INTRODUCTION
Abuse of drugs such as cocaine and heroin is more common in HIV-infected individuals than the general population and is a major contributing factor for poor outcomes for HIV and substance abuse treatment (Cook et al. 2008). Epidemiological studies on drug abusers with AIDS link cocaine abuse, more than other drugs, to increased incidence of HIV seroprevalence and progression to AIDS (Anthony et al. 1991; Baldwin et al. 1998; Chaisson et al. 1989; Doherty et al. 2000). Cocaine abuse increases the incidence and severity of HIV neuropathology and associated cognitive deficits by enhancing viral replication (Burdo et al. 2006; Dhillon et al. 2007a; Ferris et al. 2008; Nath et al. 2002). Furthermore, cocaine abuse is a known risk factor for susceptibility to and progression of neurocognitive decline in HIV patients (Buch et al. 2011; Fiala et al. 1998; Larrat and Zierler 1993; Norman et al. 2009; Webber et al. 1999). HIV patients with cocaine dependence show greater neurocognitive impairment than their non-dependent HIV counterparts (Meade et al. 2011). Such cognitive impairments likely undermine the abuser’s ability to abstain from subsequent drug intake and may serve as a biological impediment to treatment, as well as hinder treatment adherence and increase mortality in HIV cocaine abusers.
A substantial literature exists on the role of dopamine in cocaine abuse and similarly on the role of dopamine in HIV-1 neuropathology and HIV associated neurocognitive disorders (HAND). The dopamine transporter (DAT), the primary mechanism for regulating dopaminergic tone in the synapse, is directly affected by cocaine and HIV-1 infection. The reinforcing and behavioral effects of cocaine are associated with the drug’s affinity for binding directly to the DAT. Chronic cocaine administration alters dopamine release and DAT uptake dynamics, dopamine receptor function and related intracellular signaling. The HIV viral proteins Tat and gp120 interact directly and indirectly, respectively, with DAT (Midde et al. 2013) to decrease DAT surface expression (Midde et al. 2012) and correspondingly decrease uptake rate and function (Aksenova et al. 2006; Ferris et al. 2009; Gaskill et al. 2009; Hu et al. 2009; Wallace et al. 2006; Zhu et al. 2009). Because DAT expression and function are reduced by these HIV viral proteins, cocaine administration enhances HIV-induced neuropathology by further elevating synaptic dopamine concentrations via DAT inhibition. This in turn exacerbates viral replication and Tat release, leading to compromised dopamine neuronal function and the manifestation of HAND (Burdo et al. 2006; Davies et al. 1997; Dhillon et al. 2007b; Ellis et al. 2003; Ferris et al. 2008; Nath et al. 2002; Roth et al. 2002; Shapshak et al. 1996).
Previous in vitro and in vivo studies have provided critical information of the mechanisms by which cocaine exacerbates the effects of HIV proteins on dopamine signaling (Bennett et al. 1995; Ferris et al. 2008; Nath et al. 2002; Zauli et al. 2000). Given the prevalence and severity of HIV-associated neuropathologies and neurocognitive disorders in drug-abusing HIV patients, there is a critical need to utilize models that recapitulate compulsive drug intake to more fully comprehend the impact of cocaine/HIV interactions in the brain. Intravenous self-administration, an experimental method in which an animal subject presses a lever to receive an infusion of a drug, has a high degree of face validity and is widely accepted as the most appropriate model of human compulsive drug intake (Lynch and Hemby 2011; Vanderschuren and Ahmed 2013). In addition, several studies have demonstrated significant differences in neurochemical and neurobiological responses to drugs of abuse depending on whether the drug is self-administered by the subject or administered by the experimenter in a non-contingent manner (Hemby et al. 1997a; Hemby et al. 1995a; LaLumiere and Kalivas 2008; Miguens et al. 2008; Wydra et al. 2013). Therefore, intravenous self-administration is highly relevant, replicable and applicable for studying the neurobiological consequences of HIV/cocaine interactions.
The current study was undertaken to examine the effects of HIV-1 infection on DAT binding in the striatum, a subcortical area of the forebrain that coordinates motivation and movement, with and without intravenous cocaine or heroin self-administration. Comparisons were made between the HIV-1 transgenic rat (HIV-1 Tg) and Fischer 344 rat strains. The HIV transgenic rat is a model of persistent systemic HIV-1 infection and recapitulates several of the key features of human AIDS, including HIV-associated neurological abnormalities (Basselin et al. 2011; Kass et al. 2010; Reid et al. 2001). Fischer 344 rats were selected as comparators because the HIV-1 Tg line was developed from an F344/NHsd stock, the Fischer 344 inbred strain. HIV-1 Tg and F344 rats were trained to self-administer cocaine or heroin intravenously. Given the different relative dopaminergic influence between cocaine and heroin, comparisons were made between these two drugs to assess the influence of HIV on dopamine-related behaviors. Cocaine and heroin dose-response curves were generated and compared between the strains. Since the reinforcing properties of cocaine are primarily mediated by inhibition of uptake through DAT, we examined whether changes in cocaine self-administration could be correlated with changes in DAT radioligand binding in striatal membranes using the cocaine analog [125I] 3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester ([125I]RTI-55).
METHODS
Subjects
Male HIV-1 Tg (100–130 days; Harlan Laboratories, Frederick, MD) and male Fischer 344 rats (100–130 days; Charles River, Wilmington, MA) were housed in a temperature-controlled vivarium on a 12-hour reversed light/dark cycle (lights on at 6:00 PM). Rats were group-housed two per cage before surgery and housed individually after catheterization. Water was available ad libitum while food access was restricted to maintain consistent body weight during the experiment. Experimental sessions were conducted during the dark phase of the light/dark cycle. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996.
Drugs
Cocaine hydrochloride and heroin hydrochloride were obtained from the Drug Supply Program of the National Institute on Drug Abuse (Bethesda, MD). Pentobarbital and sodium thiopental were purchased from the pharmacy at Wake Forest Baptist Hospital (Winston-Salem, NC). Sodium heparin was purchased from Elkin-Sinn (Cherry Hill, NJ), methyl atropine nitrate was from Sigma-Aldrich (St Louis, MO) and penicillin G was purchased from Webster Vet Supply (Devens, MA). Iodinated 3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester ([125I]RTI-55; 2200 Ci/mmol) was purchased from PerkinElmer (Waltham, MA). Unlabeled RTI-55 was supplied by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). WF-23 [2β-propanoyl-3β-(2-naphthyl) tropane] was previously synthesized and dissolved in phosphate-buffered saline (Davies et al. 1994).
Surgery
Animals were pretreated with administered atropine nitrate (10 mg/kg; i.p.) and anesthesia was induced by pentobarbital (40 mg/kg; i.p.). Under anesthesia, rats were implanted with chronic indwelling venous catheters as described previously (Hemby et al. 1999; Hemby et al. 1997a; Hemby et al. 1995b; Hemby et al. 1996c).(Hemby et al. 1999; Hemby et al. 1997a; Hemby et al. 1995b; Hemby et al. 1996c). Briefly, catheters were inserted into the right jugular vein and anchored to muscle near the point of entry into the vein. The distal end of the catheter was guided subcutaneously to exit above the scapulae through a Teflon shoulder harness. The harness provided a point of attachment for a spring leash connected to a single-channel swivel at the opposing end. The catheter was threaded through the leash for attachment to the swivel. The fixed end of the swivel was connected to a syringe (for saline and drug delivery) by polyethylene tubing. Infusions of sodium thiopental (150 μl; 15 mg/kg; i.v.) were manually administered as needed to assess catheter patency. Rats were administered penicillin G procaine (75,000 units in 0.25 ml, i.m.) at the termination of catheter implantation. Health of the rats was monitored daily by the experimenters and weekly by institutional veterinarians according to the guidelines issued by the Wake Forest University Animal Care and Use Committee and the National Institute of Health.
Self-Administration
Self-administration chambers have been described previously (Pattison et al. 2012; Pattison et al. 2014). Rats from each strain were assigned randomly into groups to self-administer cocaine or heroin. Responding was engendered under a FR 1: timeout (TO) 20-s schedule of three 1-hour components (Hemby et al. 1999; Hemby et al. 1996b). The ratio was increased to FR2. Before each component, a 10-min blackout was followed by a priming infusion of the dose to be administered in the succeeding component. After an additional 10-min blackout period, the levers were extended and the cue lights above the levers were illuminated. Upon completion of the response requirement, a drug infusion was delivered, the lever was retracted, the lever lights extinguished, a tone was generated, and the house light illuminated.
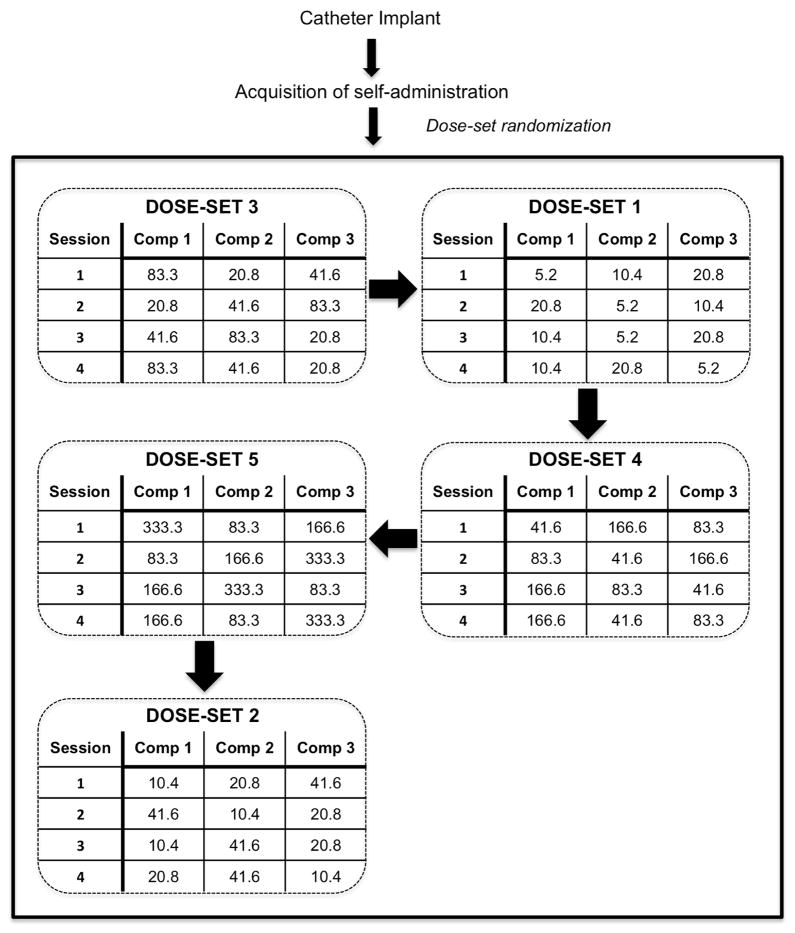
Once responding stabilized (less than 20% variation between the three 1-hour components), dose-response functions were generated for all subjects. The following doses were used: 5.2, 10.4, 20.8, 41.6, 83.3, 166.5 and 333 μg/infusion for cocaine; 0.28, 0.56, 1.12, 2.25, 4.5, 9.0, and 18 μg/infusion for heroin. Sessions were comprised of three one hr components with one dose available per component. The three doses available during a session constitute a dose-set. Table 1 shows the dose-sets for cocaine and heroin with the corresponding volume and rate of infusion for each dose with in the dose-set. The order in which the dose-sets were presented was randomly determined for each subject. Furthermore, the order of dose presentation for each dose-set was randomized across sessions (Figure 1). Doses were varied by changing the time of operation of the infusion pump, hence the volume of drug delivered from the syringe. Note that the volume of infusion is proportional to the rate of infusion (100 μl:2.8 sec; 200 μl:5.6 sec; 400 μl:11.2 sec). Body weights varied between 260 and 285 and there was no significant difference in weight between the rat strains during the time of self-administration. Eighteen hours following the last self-administration session rats were sacrificed and each brain was removed from the skull and immediately immersed in isopentane (200-ml beaker packed in dry ice). Brains were then stored at −80°C.
Table 1. Dose-sets for cocaine and heroin self-administration sessions.
Each dose-set consisted of three doses, with each dose available for a hour component of the self-administration session. The order in which the dose-sets were presented was randomized for each subject. The order of dose presentation for each dose-set was randomized across sessions.
| Dose Sets for Cocaine | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||||||
|
| ||||||||||||||
| Dose (μg/inf) | Vol (μl) | Rate (sec) | Dose (μg/inf) | Vol (μl) | Rate (sec) | Dose (μg/inf) | Vol (μl) | Rate (sec) | Dose (μg/inf) | Vol (μl) | Rate (sec) | Dose (μg/inf) | Vol (μl) | Rate (sec) |
|
| ||||||||||||||
| 5.2 | 100 | 2.8 | ||||||||||||
| 10.4 | 200 | 5.6 | 10.4 | 100 | 2.8 | |||||||||
| 20.8 | 400 | 11.2 | 20.8 | 200 | 5.6 | 20.8 | 100 | 2.8 | ||||||
| 41.6 | 400 | 11.2 | 41.6 | 200 | 5.6 | 41.6 | 100 | 2.8 | ||||||
| 83.3 | 400 | 11.2 | 83.3 | 200 | 5.6 | 83.3 | 100 | 2.8 | ||||||
| 166.6 | 400 | 11.2 | 166.6 | 200 | 5.6 | |||||||||
| 333.3 | 400 | 11.2 | ||||||||||||
| Dose Sets for Heroin | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||||||||
|
| ||||||||||||||
| Dose (μg/inf) | Vol (μl) | Rate (sec) | Dose (μg/inf) | Vol (μl) | Rate (sec) | Dose (μg/inf) | Vol (μl) | Rate (sec) | Dose (μg/inf) | Vol (μl) | Rate (sec) | Dose (μg/inf) | Vol (μl) | Rate (sec) |
|
| ||||||||||||||
| 0.28 | 100 | 2.8 | ||||||||||||
| 0.56 | 200 | 5.6 | 0.56 | 100 | 2.8 | |||||||||
| 1.12 | 400 | 11.2 | 1.12 | 200 | 5.6 | 1.12 | 100 | 2.8 | ||||||
| 2.25 | 400 | 11.2 | 2.25 | 200 | 5.6 | 2.25 | 100 | 2.8 | ||||||
| 4.5 | 400 | 11.2 | 4.5 | 200 | 5.6 | 4.5 | 100 | 2.8 | ||||||
| 9.0 | 400 | 11.2 | 9.0 | 200 | 5.6 | |||||||||
| 18.0 | 400 | 11.2 | ||||||||||||
Figure 1. Schematic of experimental design.
Example of dose-set and dose presentation. After catheter implantation and recovery, rats within each strain were randomly assigned to self-administer either cocaine or heroin. Following acquisition, the sequence of dose-set presentation was randomly determined. Within each dose-set, the order of dose presentation was randomized across sessions.
DAT radioligand binding in membranes
The temperature of the brains was increased to −20°C and each striatum was dissected under a 10X microscope. A scalpel blade and forceps were used to remove the olfactory tubercles and the cerebellum. The striatum was dissected according the following anatomical boundaries: rostral/caudal: genu of corpus callosum to joining of anterior commissure at midline; dorsal and lateral: genu of the corpus callosum and adjacent external capsule; medial: lateral ventricle and septum; ventral: piriform cortex and ventral pallidum. Striatal tissue was thawed on ice and homogenized in 10 ml cold assay buffer (10 mM sodium phosphate buffer, pH 7.4, with 0.32 M sucrose). Membranes were prepared by high-speed centrifugation (10 min at 48,000 × g) and re-suspended in 50 ml buffer at 4°C. Protein concentrations were determined using colorimetric bicinchoninic acid (BCA) protein assay reagents (Thermo Scientific) by absorbance at 562 nm.
To determine dissociation constant (Kd) and binding density (Bmax) values of [125I]RTI-55 binding to DAT, saturation binding of [125I]RTI-55 (Boja et al. 1991; Rothman et al. 1994) was accomplished using a constant concentration (0.1 nM) of [125I]RTI-55 with a range of concentrations of unlabeled RTI-55 (0.001–30 nM) in rat striatal membranes Fluoxetine (30 nM) was added to each tube to block any potential binding of [125I]RTI-55 to 5-HT uptake sites (Boja et al, 1991). Each striatal membrane sample was assayed in triplicate (2 ml of assay buffer at 4°C), incubated at 25°C for 50 min and reactions were terminated by rapid filtration with 3 × 5 ml of 50 mM Tris-HCl buffer (4°C, pH 7.4), through Whatman GF/B glass fiber filters pre-soaked in Tris buffer containing 0.1% bovine serum albumin. Nonspecific binding was determined using 1 μM WF-23 (Davies et al. 1994). Radioactivity was determined by liquid scintillation spectrometry in filters eluted for 8 hours in 5 ml of Ecolite scintillation fluid. Binding curves were fit to two-site models by iterative non-linear curve fitting, where the contribution of the high-affinity Bmax values has been subtracted from those of low-affinity sites.
Data Analysis
Comparison of the number of days to stable self-administration between the strains for both cocaine and heroin was conducted using a two-way ANOVA with Strain and Drug as the main factors. Comparison of the number of days to stable self-administration of cocaine and heroin within each strain was conducted using a one way ANOVA with Drug as the main factor. The number of infusions between the groups was analyzed using a two-way ANOVA with Strain and Dose as the main factors. Bonferroni test was used for post hoc analysis (P<0.05). Total [125I]RTI-55 binding was compared using a two way ANOVA with Group and Strain as the main effect. For post hoc analysis, Bonferroni test was used (P<0.05). Saturation binding data were analyzed using JMP Stats software for nonlinear regression curve fitting to determine the Kd and Bmax from data fit to one- and two-site binding hyperbolae. In all cases, the fits to two-site models were significantly better than analyses fit to one-site models (P<0.05). Comparison of strain differences in drug naïve rats was conducted using t-test. For each strain, the effects of drug self-administration on each [125I]RTI-55 binding parameter was analyzed using a one way ANOVA with Group as the main effect. Bonferroni test for selected group comparisons was used as the post hoc analysis (P<0.05).
RESULTS
Self-administration
HIV-1 Tg and F344 rats acquired intravenous self-administration of cocaine and heroin. For F344 rats, responding was engendered using 166.6 mg/infusion of cocaine or 9.0 mg of heroin. In contrast, these doses were not effective for engendering responding in HIV-1 Tg rats so the doses were decreased to 83.25 mg/infusion for cocaine and 4.5 mg/infusion for heroin. While there was a significant difference in the amount of time to reach stable self-administration between the strains [F(1,21)=29.288, P<0.001], the strain x drug interaction was not statistically significant [F(1,21)=1.394, P=0.253]. Interestingly, there was no significant difference between cocaine and heroin in the number of days to stable self-administration for HIV-1 Tg rats [F(1,9)=2.939, P=0.125]. Similarly, there was no significant difference in the number of days to stable self-administration for F344 rats [F(1,11)=0.0108, P=0.919]. These results suggest that while there is a preferential influence of HIV-1 on the acquisition of drug self-administration, the effect is not specific to cocaine or heroin.
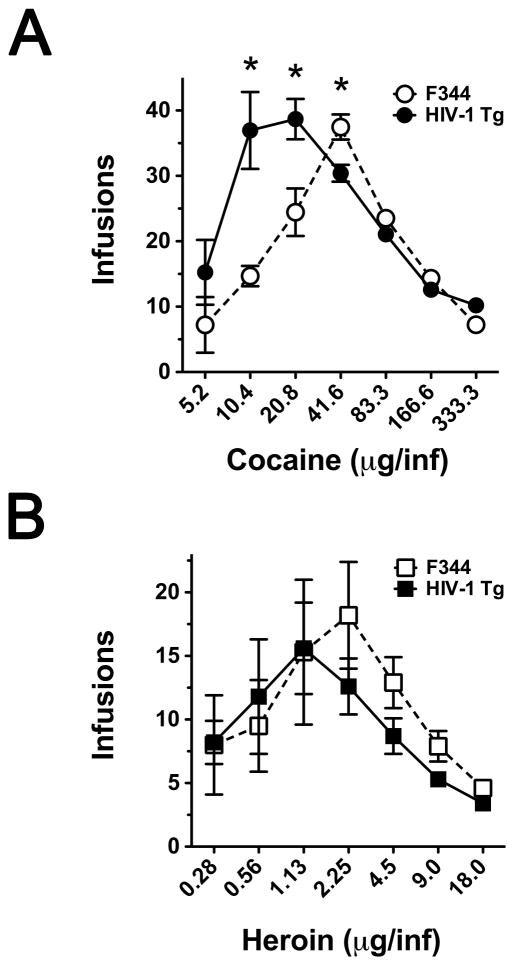
Following stable self-administration under an FR2 schedule of reinforcement, dose-response curves were generated as described in the Methods. Cocaine and heroin self-administration are characterized by inverted U-shape dose-response curves in both Fischer 344 and HIV-1 Tg rats (Figure 2). There was no significant difference in the maximal number of infusions obtained with cocaine self-administration; however, the cocaine dose that maintained the maximal number of infusions was significantly different (41.6 μg/inf and 20.8 μg/inf for F344 and HIV-1 Tg rats, respectively). The ascending limb of the dose-response curve was significantly different between the strains [F(3,32)=15.83, P=0.003]; specifically, the ascending limb was shifted leftward in HIV-1 Tg rats with more infusions/dose. Post hoc analysis revealed the 10.4 and 20.8 μg/inf doses significantly increased the number of infusions delivered in HIV-1 Tg versus F344 rats (P<0.05). There was a significant difference between the two strains for the descending limb as well [F(3,32)=0.0003], with post hoc analysis indicating a significant reduction in the number of infusions for 41.6 μg/inf for HIV-1 Tg compared to F344 rats.
Figure 2. Dose-effect curves for cocaine and heroin self-administration in HIV-1 Tg and Fischer 344 male rats.
Dose-effect curves were obtained using a within-session dose intake procedure described in Methods. * P<0.05
For heroin self-administration, there was a trend towards a significant difference in the maximal number of infusions obtained between F344 and HIV-1 Tg rats (P=0.054). Furthermore, the doses that maintained the maximal number of infusions for heroin self-administration were 1.12 μg/inf and 2.25 μg/inf for HIV-1 Tg and F344 rats, respectively (Figure 2). There was no significant main effect of Strain [F(1,32) = 0.19, P=0.66] or Interaction (Strain x Dose) [F(3,32) = 0.82, P=0.31] for the ascending limb of the heroin dose-effect function. For the descending limb of the dose-response curve, there was a significant effect of Strain [F(1,32) = 6.04, P=0.02] but no significant Interaction [F(3,32) = 0.48, P=0.70]. There was no significant difference in total drug intake between HIV-1 Tg and Fischer 344 subjects for either cocaine or heroin.
In order to determine if the rate (or volume) of infusion affected drug intake, we compared the number of infusions obtained for a dose at each rate/volume 2.8 sec/100 μl, 5.6 sec/ 200 μl and 11.2 sec/400 μl) in which they were delivered (see Table 1), using t-test or one-way ANOVA, as appropriate. Analysis revealed no statistically significant effect of rate/volume on the number of infusions obtained for 10.4, 20.8, 41.6, 83.3 or 166.6 μg/infusion of cocaine in either the Fischer F344 or HIV-1 Tg rats (P>0.05). Similarly, we found no statistically significant effect of rate/volume on the number of infusions obtained for 0.56, 1.12, 2.25, 4.5 or 9.0 μg/infusion of heroin in either the Fischer F344 or HIV-1 Tg rats (P>0.05).
DAT binding
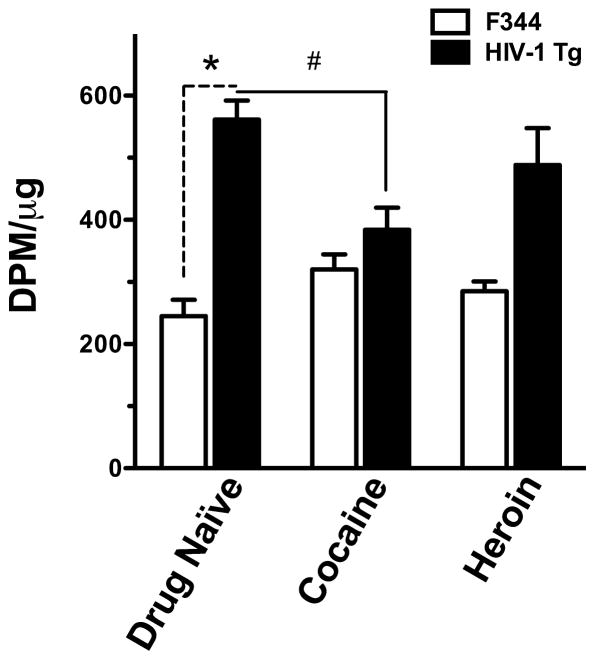
There was no statistically significant difference in total lifetime intake of cocaine (t=1.055, df=11, P=0.388) or heroin (t=0.713, df=11, P=0.491) between the strains. To compare the effects of cocaine and heroin self-administration behavior between HIV-1 Tg and F344 rats on dopamine uptake sites in striatum, preliminary radioligand binding to DAT was performed in striatal membranes from Fischer 344 and HIV-1 Tg rats using a single concentration of the cocaine analog radioligand [125I]RTI-55 (Figure 3). Fluoxetine was added to the assays to selectively block any contribution from serotonin transporter reuptake sites. Comparison of drug-naïve F344 and HIV-1 Tg rats revealed a significant difference in [125I]RTI-55 binding (t=7.918, df=12, P<0.0001), with striatal membranes from HIV-1 Tg displaying over two-fold higher binding compared to membranes from F344 rats. In HIV-1 Tg rats, drug self-administration significantly altered [125I]RTI-55 striatal binding [F(2,15)=4.559, P=0.032]. Post hoc analysis revealed significantly lower levels of binding following cocaine self-administration compared to drug naïve rats. In contrast, neither cocaine nor heroin self-administration significantly altered [125I]RTI-55 binding in the striatum of F344 rats [F(2,23)=2.807, P=0.083].
Figure 3. Total [125I]RTI-55 binding in rat striatal membranes from Fischer 344 and HIV-1 Tg male rats in drug naïve rats and following cocaine or heroin self-administration.
Bars represent mean +/− S.E.M. *. P<0.05 HIV-1 Tg drug naïve vs. F344 drug naïve; #, P<0.05 HIV-1 Tg drug naïve vs. HIV-1 Tg cocaine. DPM/μg = disintegrations per minute per μg of striatal membrane tissue.
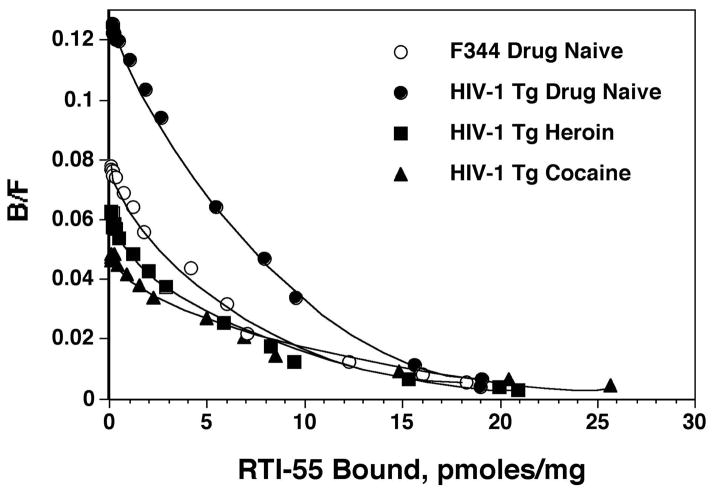
To distinguish whether these changes in radioligand binding were caused by changes in the number of transporters (Bmax) or in the affinity (Kd) of the transporter for [125I]RTI-55, detailed saturation binding isotherms in striatal membranes were performed. It has been well established that cocaine analogs display biphasic binding to DAT, with binding to both high- and low-affinity sites (Madras et al. 1989a; Madras et al. 1989b; Pattison et al. 2014; Staley et al. 1994a; Staley et al. 1994b). In the present study, this phenomenon was observed in striatal membranes from both F344 and HIV-1 Tg rats (Figure 4). Statistical analyses of iterative non-linear fits of binding data revealed significantly better fits to two-site compared to one-site models (P<0.05). The difference between high- and low-affinity binding for [125I]RTI-55 can clearly be seen in Figure 4, which shows mean values of binding data from all animals in the F344 drug-naive group, as well as from the HIV-1 Tg drug-naive, cocaine and heroin self-administration groups.
Figure 4. Scatchard plots of saturation [125I]RTI-55 binding to striatal membrane preparations.
Membranes were incubated with 0.1 nM [125I]RTI-55 along with 15 concentrations of unlabeled RTI-55 (0.001–30 nM), in triplicate. Binding data were analyzed using a nonlinear curve fit and a two-site-specific binding model. Each point represents mean values from binding data pooled from individual binding data, N=6–8 rats per group. Lines are best fits of high- and low-affinity binding sites predicted from two-site models. For the sake of clarity, data from drug self-administration in F344 are not shown, since those results are essentially identical to those in drug-naive F344 rats. B/F = Bound/Free ligand.
Comparison of drug-naïve F344 and HIV-1 Tg rats revealed a trend towards a significant difference in Kd values (Kd1: t=2.04, df=12, P=0.064) and no significant difference in Bmax values (Bmax1: t=0.79, df=12, P=0.442) at the high-affinity DAT binding site. However, Kd values at the low-affinity DAT binding site were significantly decreased in HIV-1 Tg rats versus F344 rats (Kd2, t=2.28, df=11, P=0.043), indicating a significant increase in DAT affinity for this cocaine analog at the low-affinity binding sites; however, there was no difference in the abundance of low-affinity DAT sites (Bmax2, t=1.62, df=12, P=0.132) (Table 2).
Table 2.
Comparison of [125I]RTI-55 binding in striatal membranes at high- and low-affinity DAT binding sites in Fischer 344 and HIV-1 Tg rats following cocaine or heroin intravenous self-administration.
| Kd1 | Bmax1 | Kd2 | Bmax2 | |
|---|---|---|---|---|
| F344 | ||||
| Control | 3.16 ± 0.64 | 6.08 ± 1.09 | 72.9 ± 20.5 | 27.4 ± 6.6 |
| Cocaine | 2.54 ± 0.37 | 6.56 ± 1.14 | 55.7 ± 17.0 | 21.8 ± 2.2 |
| Heroin | 2.07 ± 0.73 | 5.03 ± 1.80 | 38.3 ± 6.7 | 31.2 ± 4.5 |
| HIV-1 Tg | ||||
| Control | 1.60 ± 0.22 | 7.31 ± 1.05 | 21.3 ± 4.1 # | 14.8 ± 0.7 |
| Cocaine | 0.96 ± 0.36 | 6.09 ± 1.34 | 77.5 ± 24.2 * | 30.5 ± 4.8 * |
| Heroin | 1.61 ± 0.35 | 6.39 ± 1.56 | 39.8 ± 9.8 | 21.0 ± 3.3 |
P<0.05, compared to F344 control
P<0.05, compared to HIV-1 Tg control
For HIV-1 Tg rats, both Kd and Bmax for the low-affinity binding site were altered following drug self-administration [Kd2: F(2,15)=4.03, P=0.044; Bmax2: F(2,14)=6.957, P<0.01]. Post hoc analysis revealed cocaine self-administration in HIV-1 Tg rats significantly increased Kd2 (decreased affinity) and Bmax2 values compared to drug-naive controls (P<0.05; Table 2). No significant differences were observed following heroin self-administration. For HIV-1 Tg rats, there were no significant differences in Kd or Bmax values at the high-affinity binding site following drug self-administration [Kd1: F(2,15)=0.451, P=0.647; Bmax1: F(2,23)=0.253, P=0.780]. Drug self-administration did not significantly alter Kd or Bmax at the high-affinity [Kd1: F(2,23)=0.849, P=0.442; Bmax1: F(2,23)=0.318, P=0.731] or low-affinity DAT binding site [Kd2: F(2,21)=1.12, P=0.348; Bmax2: F(2,22)=0.922, P=0.414] in F344 rats (Table 2).
DISCUSSION
Given the high incidence and prevalence of drug abuse in HIV seropositive individuals and the consequential increased severity and accelerated progression of HIV-related neuropathology and neurocognitive decline, there is a critical need to utilize animal models that recapitulate critical aspects of HIV infection and compulsive drug intake in humans. The present study is the first published report assessing the effects of HIV-1 on the intravenous self-administration of cocaine and heroin in a rodent model and the first to determine the effect of HIV-1, cocaine and heroin self-administration, and the combination of HIV-1 and these drugs of abuse on DAT binding parameters. Specifically, we found that: 1) HIV increased striatal DAT binding via increased affinity at the low-affinity DAT site (Kd2), 2) HIV-1 Tg rats exhibit an increased sensitivity to the reinforcing effects of cocaine as evidenced by a leftward shift in the cocaine self-administration dose-response curve, and 3) chronic cocaine, but not heroin, self-administration decreased affinity and increased the abundance of the low-affinity DAT site in HIV-1 Tg rats. These novel findings expand the current knowledge in the field by demonstrating the impact of the synergistic relationship between HIV and drug abuse on biochemical and behavioral measures.
In order to determine the impact of HIV-1 on DAT binding parameters, binding of the cocaine analog [125I]RTI-55 was examined in striatal membrane preparations from drug-naïve HIV-1 Tg and Fischer 344 rats. [125I]RTI-55 binding was significantly greater in drug-naïve HIV-1 Tg versus drug-naïve F344 rats. These results are parsimonious with a previous study demonstrating elevated DAT mRNA and DAT immunostaining in the striatum of individuals with HIV encephalitis (HIVE) from the autopsy cohort of the National NeuroAIDS Tissue Consortium (Gelman et al. 2006). As changes in binding may be attributable to changes in abundance or affinity, saturation binding analyses were conducted and revealed the presence of high- and low-affinity binding sites in agreement with human (Staley et al. 1994a; Staley et al. 1994b), non-human primate (Madras et al. 1989a; Madras et al. 1989b) and rat (Pattison et al. 2014) striatal tissue, with low-affinity binding comprising 79.8 +/− 3.7% of the sites in F344 rats and 67.7 +/−4.2% in HIV-1 Tg rats. While no differences in the abundance of the DAT binding sites were observed between strains, HIV-1 Tg rats exhibited a significant increase in affinity for the cocaine analog RTI-55 at the low-affinity DAT binding sites, compared to Fischer 344 controls. A previous study has shown that PKC-induced phosphorylation of the Ser7 residue modulates the conformational equilibrium of DAT between high- and low-affinity binding states (Moritz et al. 2013). Whether the increased affinity of the low-affinity site in HIV-1 Tg rats is conferred by increased phosphorylation of the Ser7 residue or other post-translational modifications remains to be determined. Nonetheless, these results indicate that persistent HIV-1 infection results in significant functional alterations of DAT in the striatum.
Previous studies have demonstrated that the high-affinity binding site exerts a low-capacity dopamine uptake process that maintains extracellular dopamine ([DA]e) at relatively low concentrations (Graef and Bonisch 1988; Horn 1990), whereas the low-affinity site is sodium-independent and contributes to a high-capacity uptake process (Kimelberg 1986; Mireylees et al. 1986; Stamford et al. 1986). Given that the low-affinity site operates most efficiently at high synaptic dopamine concentrations, increased affinity at the low-affinity DAT site in drug-naïve HIV-1 Tg rats likely reflects a compensatory response to elevated basal [DA]e in the striatum, as shown previously (Ferris et al. 2009). These findings support Gelman and colleagues’ postulate of striatal hyperdopaminergic tone in patients with HIVE (Gelman et al. 2006), but are in apparent contrast to previous results showing decreased striatal DAT in patients diagnosed with HIV associated dementia (HAD) (Wang et al. 2004). The apparent discrepancy between results of in vivo neuroimaging and in vitro protein analysis is partly explained by the influence of [DA]e levels on DAT binding (Gatley et al. 1995; Gatley et al. 1997). Dopamine competes with ligands for DAT binding sites such that high dopamine levels would decrease binding of the PET ligand leading to the conclusion of reduced DAT binding sites. Therefore, the reduced DAT binding reported may be due in part to elevated [DA]e levels in the striatum of these patients. The in vitro binding procedure used in the present study minimizes the influence of dopamine by removing the neurotransmitter via thorough rinsing of the tissue preparations and by suspending the membrane preparations in excess amounts of buffer. Elevated dopamine levels are involved in the pathogenesis of HIV by enhancing viral replication, which results in the release of inflammatory mediators (e.g. chemokines and TNF-α) (Gaskill et al. 2009) and HIV proteins (e.g. Tat and gp120) directly and indirectly affecting dopaminergic function (Purohit et al. 2011). Results of the present study are an important first step towards understanding the impact of HIV-1 infection on dopaminergic synaptic function in the intact brain. A more complete characterization of HIV-induced dysregulation of dopamine signaling in the HIV-1 Tg model requires analysis of dopamine receptor binding and function, as well as the determination of basal [DA]e in the striatum and other terminal projection areas of midbrain dopamine systems.
There are several potential biobehavioral implications of HIV-induced differences in DAT uptake binding measures in the striatum. Previous studies in subjects diagnosed with HIV found that decreased striatal DAT binding is correlated with increased cognitive impairment (Wang et al. 2004), irrespective of cocaine abuse (Chang et al. 2008). While we are not aware of any published studies in humans or animal models that have assessed the relationship between HIV-associated changes in DAT binding and the reinforcing effects of cocaine, previous studies have found that injection of the HIV protein Tat into the striatum increases the acute locomotor response to cocaine but attenuates the sensitized response following repeated injections (Ferris et al. 2010; Harrod et al. 2008). Based on the assumption that the transition from recreational to compulsive drug intake is associated with behavioral sensitization (Kalivas 2004; Kalivas and Volkow 2005; Pierce and Kalivas 1997; Robinson and Berridge 1993) along with the attenuated sensitized response in Tat treated rats, it is reasonable to hypothesize that HIV would reduce the abuse liability of cocaine and heroin. In contrast, we found that cocaine and heroin were self-administered by HIV-1 Tg rats across a range of doses resulting in an inverted “U” shaped dose-response function similar to F344 rats. Interestingly, the ascending limb of the cocaine dose-response curve was shifted to the left for HIV-1 Tg rats, indicative of an increase in the reinforcing effects of cocaine (Howell and Wilcox 2001). The change in sensitivity reflects a change in affinity of the binding site of the drug, in this case cocaine, at the low-affinity DAT site (Swift and Lewis 2008), which we also observed as a significant increase in the value of Kd2 in HIV-1 Tg animals following cocaine self-administration. In contrast, there was no significant difference in the dose-effect functions for heroin self-administration between HIV-1 Tg and F344 rats. The observed effect of cocaine but not heroin self-administration in the HIV-1 Tg rats emphasizes the preferential impact of HIV on dopamine-related behaviors. Several lines of evidence indicate that the mesolimbic dopamine system influences the reinforcing effects of cocaine to a greater extent than for heroin. Cocaine, but not heroin, self-administration is altered by selective destruction of dopamine neurons or terminal regions within this pathway (Pettit et al. 1984; Roberts and Koob 1982; Roberts et al. 1980) and peripheral and central administration of dopamine receptor antagonists (Ettenberg et al. 1982; Hemby et al. 1997b; Hemby et al. 1996a). Furthermore, extracellular dopamine concentrations in the nucleus accumbens are increased to a significantly greater extent during cocaine compared to heroin self-administration (Hemby et al. 1999; Hemby et al. 1997a; Hemby et al. 1995a) and cocaine, but not heroin, self-administration significantly alters striatal DAT binding (Pattison et al. 2014). Therefore, we surmise that the absence of HIV-related changes in heroin intake are likely due to negligible dopaminergic involvement in the mediation of heroin reward, further emphasizing the preferential influence of HIV on dopamine-related behaviors.
Chronic cocaine self-administration has been shown to increase DAT binding in humans (Little et al. 1998a; Little et al. 1998b; Little et al. 1999), monkeys (Beveridge et al. 2008; Letchworth et al. 2001) and rats (Wilson and Kish 1996; Wilson et al. 1994), presumably as compensation for chronically elevated levels of [DA]e due to prolonged cocaine exposure. In contrast, the binding potential (Bmax/Kd), a measure used in PET imaging analyses, for striatal DAT is decreased in HIV-associated dementia patients and is associated with decreased cognitive performance (Wang et al. 2004). Likewise, Chang and colleagues found decreased striatal DAT binding potential is associated with cognitive impairment in HIV subjects, with a “trend for further minimal decreases” in DAT binding in HIV patients with cocaine histories following one month of abstinence (Chang et al. 2008). In the present study, DAT binding in HIV-1 Tg rats was compared to drug-naïve controls and showed a trend towards increased DAT binding in F344 rats following cocaine self-administration. In the HIV-1 Tg rats, the decrease in binding is likely attributable to increased Kd (decreased affinity) at the low-affinity DAT binding site. Furthermore, decreased affinity of the low-affinity DAT binding site in the HIV-1 Tg rats following cocaine self-administration may be due in part to reduced [DA]e following chronic cocaine self-administration. As noted previously, the low-affinity DAT site operates most efficiently at high synaptic dopamine concentrations, such that decreased affinity may reflect a compensatory response to a reduction in cocaine-stimulated dopamine levels. The absence of significant reductions in the number of DAT binding sites, Bmax, suggests that the number of dopamine terminals was not reduced during the period in which the study was conducted.
Results from our study are in agreement with previous studies showing little or no effect of infusion rate on acquisition or maintenance of self-administration in rats (Crombag et al. 2008; Liu et al. 2005); however, others have reported that the rate of drug delivery influences the reinforcing effects of cocaine in rats (Schindler et al. 2011; Schindler et al. 2009; Wakabayashi et al. 2010). Drawing direct comparisons with the present study is complicated by several factors including the range of infusion rates, doses and/or the duration of self-administration sessions and history. For example, Wakabayashi et al., 2010 study compared the effects of infusion rate (5, 45 and 90 sec) between short access (one hr) and long access (6 hr) sessions. While there was no significant effect of infusion rate on cocaine intake during short access sessions, there was a significant effect of infusion rate on number of infusions during long access sessions. Similarly, Schindler and colleagues found a significant difference between short infusion rates (1.7 and 10 sec) compared with the longer duration of 100 sec (Schindler et al. 2009). The present study design does not allow an analysis of the separate contributions of dose, volume or infusion rate on the reinforcing effects of cocaine. Given that volume is directly related to rate of infusion in the present study, the infusion rate varied between 2.8 and 11.2 which is within the range
The generalizability of the present findings in HIV-1 Tg rats to humans is complicated by several factors. First, the HIV-1 Tg rat model expresses an engineered, partial HIV-1 transgene incorporated into the genome that is expressed in cells of all tissues, including brain (Peng et al. 2010; Sultana et al. 2010), and is not recognized as immunologically foreign. A recent study demonstrated increased systemic and central nervous system inflammatory responses, along with the presence of HIV proteins in the brains of HIV-1 Tg rats (Royal et al. 2012). Secondly, whereas the model exhibits several characteristics that mimic those observed in HIV infected in humans (Peng et al. 2010; Reid et al. 2001; Royal et al. 2007; Sultana et al. 2010), other pathologies, such as congenital cataracts, observed in this model are not generally indicative of, or associated with, the disease in humans. The presence of these pathologies in HIV-1 Tg but not F344 rats may contribute to differences between in the strains observed in the present study. For example, congenital cataracts present in the HIV-1 Tg rats may could reduce the ability to visually discriminate the stimulus light and lever leading to a longer period of time to reach stable self-administration. Secondly, the HIV-1 Tg rat model can not address the impact or effects of drug use that occur prior to HIV infection, for example drug-induced neuroadaptations, occurring in the brain prior to HIV infection. In addition, several factors may contribute to apparent differences between the results of the present study and the results of PET imaging studies in humans. One potential difference is the influence of [DA]e on measures of binding availability. In vivo, extracellular dopamine competes with the ligand limiting the availability of DAT binding sites (Gatley et al. 1995; Gatley et al. 1997) leading to an apparent decrease in the number of DAT binding sites. For saturation binding analysis, the influence of endogenous extracellular dopamine is minimized by thorough washing of the membrane preparations prior to incubation with the ligand. Secondly, PET imaging studies use binding potential (Bmax/Kd) as the dependent measure of DAT binding in the striatum whereas in the present study Bmax and Kd were obtained, enabling separate measures of DAT abundance and affinity, respectively, at the two binding sites on the transporter. Finally, the PET studies used the radioligand [11C]cocaine, a relatively non-specific monoamine transporter ligand. In the present study, dopamine transporter binding was determined using the relatively selective DAT ligand [125I]RTI-55 and the potential influence of serotonin transporter binding was negated by the addition of fluoxetine to the binding assays. The paucity of available information regarding the biochemical effects of HIV/cocaine abuse comorbidity highlights the need for additional investigation, especially in light of the growing percentage of the HIV population who abuse drugs and the deleterious effects on disease progression and severity in this population.
Acknowledgments
The study was supported in part by the National Institute of Drug Abuse through grant R01DA012498 (SEH).
Footnotes
ETHICAL STANDARDS
The experiments contained herein were approved by the Wake Forest School of Medicine Animal Care and Use Committee and were conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Vlahov D, Nelson KE, Cohn S, Astemborski J, Solomon L. New evidence on intravenous cocaine use and the risk of infection with human immunodeficiency virus type 1. Am J Epidemiol. 1991;134:1175–1189. doi: 10.1093/oxfordjournals.aje.a116021. [DOI] [PubMed] [Google Scholar]
- Baldwin GC, Roth MD, Tashkin DP. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J Neuroimmunol. 1998;83:133–138. doi: 10.1016/s0165-5728(97)00229-4. [DOI] [PubMed] [Google Scholar]
- Basselin M, et al. Imaging upregulated brain arachidonic acid metabolism in HIV-1 transgenic rats. J Cereb Blood Flow Metab. 2011;31:486–493. doi: 10.1038/jcbfm.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bennett BA, Rusyniak DE, Hollingsworth CK. HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res. 1995;705:168–176. doi: 10.1016/0006-8993(95)01166-8. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from Chronic Cocaine Self-Administration Alters Striatal Dopamine Systems in Rhesus Monkeys. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boja JW, et al. [125I]RTI-55: a potent ligand for dopamine transporters. Eur J Pharmacol. 1991;194:133–134. doi: 10.1016/0014-2999(91)90137-f. [DOI] [PubMed] [Google Scholar]
- Buch S, Yao H, Guo M, Mori T, Su TP, Wang J. Cocaine and HIV-1 interplay: molecular mechanisms of action and addiction. J Neuroimmune Pharmacol. 2011;6:503–515. doi: 10.1007/s11481-011-9297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Katner SN, Taffe MA, Fox HS. Neuroimmunity, drugs of abuse, and neuroAIDS. J Neuroimmune Pharmacol. 2006;1:41–49. doi: 10.1007/s11481-005-9001-3. [DOI] [PubMed] [Google Scholar]
- Chaisson RE, Bacchetti P, Osmond D, Brodie B, Sande MA, Moss AR. Cocaine use and HIV infection in intravenous drug users in San Francisco. J Am Med Assoc. 1989;261:561–565. [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Ferrario CR, Robinson TE. The rate of intravenous cocaine or amphetamine delivery does not influence drug-taking and drug-seeking behavior in rats. Pharmacol Biochem Behav. 2008;90:797–804. doi: 10.1016/j.pbb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HM, Saikali E, Huby NJ, Gilliatt VJ, Matasi JJ, Sexton T, Childers SR. Synthesis of 2 beta-acyl-3 beta-aryl-8-azabicyclo[3.2.1]octanes and their binding affinities at dopamine and serotonin transport sites in rat striatum and frontal cortex. J Med Chem. 1994;37:1262–1268. doi: 10.1021/jm00035a005. [DOI] [PubMed] [Google Scholar]
- Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL. HIV-associated brain pathology in the United Kingdom: an epidemiological study. Aids. 1997;11:1145–1150. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Dhillon NK, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007a;13:483–495. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- Dhillon NK, et al. Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007b;13:483–495. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- Doherty MC, Garfein RS, Monterroso E, Brown D, Vlahov D. Correlates of HIV infection among young adult short-term injection drug users. AIDS. 2000;14:717–726. doi: 10.1097/00002030-200004140-00011. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, Tat1-86, impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: a nonet-flux microdialysis study. Neuroscience. 2009;159:1292–1299. doi: 10.1016/j.neuroscience.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. Hyperdopaminergic tone in HIV-1 protein treated rats and cocaine sensitization. J Neurochem. 2010;115:885–896. doi: 10.1111/j.1471-4159.2010.06968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, et al. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine’s connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol. 2009;175:1148–1159. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Fowler JS, Dewey SL, Logan J. Sensitivity of striatal [11C]cocaine binding to decreases in synaptic dopamine. Synapse. 1995;20:137–144. doi: 10.1002/syn.890200207. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Gifford AN, Ding YS, Logan J, Wang GJ. Model for estimating dopamine transporter occupancy and subsequent increases in synaptic dopamine using positron emission tomography and carbon-11-labeled cocaine. Biochem Pharmacol. 1997;53:43–52. doi: 10.1016/s0006-2952(96)00655-7. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Spencer JA, Holzer CE, 3rd, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2006;1:410–420. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- Graef KH, Bonisch H. The transport of amines across the axonal membranes of noradrenergic and dopaminergic neurones. In: Trendelenburg U, Weiner N, editors. Handbook of Experimental Pharmacology. Springer; Berlin: 1988. pp. 193–245. [Google Scholar]
- Harrod SB, Mactutus CF, Fitting S, Hasselrot U, Booze RM. Intra-accumbal Tat1-72 alters acute and sensitized responses to cocaine. Pharmacol Biochem Behav. 2008;90:723–729. doi: 10.1016/j.pbb.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999;288:274–280. [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997a;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Johnson BA, Dworkin SI. Neurobiological basis of drug reinforcement. In: Johnson BA, Roache JD, editors. Drug Addiction and Its Treatment: Nexus of Neuroscience and Behavior. Lippincott-Raven Publishers; Philadelphia: 1997b. pp. 137–169. [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. Journal of Pharmacology & Experimental Therapeutics. 1995a;273:591–598. [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J Pharmacol Exp Ther. 1995b;273:591–598. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. The Journal of Pharmacology and Experimental Therapeutics. 1996a;277:1247–1258. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. Journal of Pharmacology & Experimental Therapeutics. 1996b;277:1247–1258. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996c;277:1247–1258. [PubMed] [Google Scholar]
- Horn AS. Dopamine uptake: a review of progress in the last decade. Prog Neurobiol. 1990;34:387–400. doi: 10.1016/0301-0082(90)90033-d. [DOI] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. Intravenous drug self-administration in nonhuman primates. In: JJB, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; Boca Raton, FL: 2001. pp. 91–110. [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK, Rock RB. Preferential sensitivity of human dopaminergic neurons to gp120-induced oxidative damage. J Neurovirol. 2009;15:401–410. doi: 10.3109/13550280903296346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Recent understanding in the mechanisms of addiction. Current psychiatry reports. 2004;6:347–351. doi: 10.1007/s11920-004-0021-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kass MD, Liu X, Vigorito M, Chang L, Chang SL. Methamphetamine-induced behavioral and physiological effects in adolescent and adult HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2010;5:566–573. doi: 10.1007/s11481-010-9221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. Occurrence and functional significance of serotonin and catecholamine uptake by astrocytes. Biochem Pharmacol. 1986;35:2273–2281. doi: 10.1016/0006-2952(86)90451-x. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrat EP, Zierler S. Entangled epidemics: cocaine use and HIV disease. J Psychoactive Drugs. 1993;25:207–221. doi: 10.1080/02791072.1993.10472272. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Carroll FI, Butts JD. Striatal [125I]RTI-55 binding sites in cocaine-abusing humans. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1998a;22:455–466. doi: 10.1016/s0278-5846(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Little KY, et al. Brain dopamine transporter messenger RNA and binding sites in cocaine users: a postmortem study. Archives of General Psychiatry. 1998b;55:793–799. doi: 10.1001/archpsyc.55.9.793. [DOI] [PubMed] [Google Scholar]
- Little KY, Zhang L, Desmond T, Frey KA, Dalack GW, Cassin BJ. Striatal dopaminergic abnormalities in human cocaine users. The American journal of psychiatry. 1999;156:238–245. doi: 10.1176/ajp.156.2.238. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Hemby SE. Drug Reinforcement in Animals. In: Johnson BA, editor. Addiction Medicine: Science and Practice. Vol. 1. Springer; New York: 2011. pp. 117–128. [Google Scholar]
- Madras BK, Fahey MA, Bergman J, Canfield DR, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. I. [3H]cocaine binding sites in caudate-putamen. The Journal of Pharmacology and Experimental Therapeutics. 1989a;251:131–141. [PubMed] [Google Scholar]
- Madras BK, Spealman RD, Fahey MA, Neumeyer JL, Saha JK, Milius RA. Cocaine receptors labeled by [3H]2 beta-carbomethoxy-3 beta-(4-fluorophenyl)tropane. Mol Pharmacol. 1989b;36:518–524. [PubMed] [Google Scholar]
- Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. J Behav Med. 2011;34:128–138. doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Zhu J. HIV-1 Tat protein decreases dopamine transporter cell surface expression and vesicular monoamine transporter-2 function in rat striatal synaptosomes. J Neuroimmune Pharmacol. 2012;7:629–639. doi: 10.1007/s11481-012-9369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J. Mutation of Tyrosine 470 of Human Dopamine Transporter is Critical for HIV-1 Tat-Induced Inhibition of Dopamine Transport and Transporter Conformational Transitions. J Neuroimmune Pharmacol. 2013 doi: 10.1007/s11481-013-9464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- Mireylees SE, Brammer NT, Buckley GA. A kinetic study of the in vitro uptake of [3H]dopamine over a wide range of concentrations by rat striatal preparations. Biochem Pharmacol. 1986;35:4065–4071. doi: 10.1016/0006-2952(86)90029-8. [DOI] [PubMed] [Google Scholar]
- Moritz AE, et al. Phosphorylation of dopamine transporter serine 7 modulates cocaine analog binding. J Biol Chem. 2013;288:20–32. doi: 10.1074/jbc.M112.407874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Norman LR, Basso M, Kumar A, Malow R. Neuropsychological consequences of HIV and substance abuse: a literature review and implications for treatment and future research. Current drug abuse reviews. 2009;2:143–156. doi: 10.2174/1874473710902020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison LP, McIntosh S, Budygin EA, Hemby SE. Differential regulation of accumbal dopamine transmission in rats following cocaine, heroin and speedball self-administration. J Neurochem. 2012;122:138–146. doi: 10.1111/j.1471-4159.2012.07738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison LP, McIntosh S, Sexton T, Childers SR, Hemby SE. Changes in dopamine transporter binding in nucleus accumbens following chronic self-administration cocaine: Heroin combinations. Synapse. 2014 doi: 10.1002/syn.21755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–173. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Research Reviews. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Mol Neurobiol. 2011;44:102–110. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Reid W, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roth MD, Tashkin DP, Choi R, Jamieson BD, Zack JA, Baldwin GC. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis. 2002;185:701–705. doi: 10.1086/339012. [DOI] [PubMed] [Google Scholar]
- Rothman RB, et al. Studies of the biogenic amine transporters. IV. Demonstration of a multiplicity of binding sites in rat caudate membranes for the cocaine analog [125I]RTI-55. J Pharmacol Exp Ther. 1994;270:296–309. [PubMed] [Google Scholar]
- Royal W, 3rd, Wang H, Jones O, Tran H, Bryant JL. A vitamin A deficient diet enhances proinflammatory cytokine, Mu opioid receptor, and HIV-1 expression in the HIV-1 transgenic rat. J Neuroimmunol. 2007;185:29–36. doi: 10.1016/j.jneuroim.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, 3rd, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Cogan ES, Thorndike EB, Panlilio LV. Rapid delivery of cocaine facilitates acquisition of self-administration in rats: an effect masked by paired stimuli. Pharmacol Biochem Behav. 2011;99:301–306. doi: 10.1016/j.pbb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Thorndike EB. Effect of rate of delivery of intravenous cocaine on self-administration in rats. Pharmacol Biochem Behav. 2009;93:375–381. doi: 10.1016/j.pbb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapshak P, et al. HIV-1 neuropathogenesis and abused drugs: current reviews, problems, and solutions. Adv Exp Med Biol. 1996;402:171–186. doi: 10.1007/978-1-4613-0407-4_23. [DOI] [PubMed] [Google Scholar]
- Staley JK, Basile M, Flynn DD, Mash DC. Visualizing dopamine and serotonin transporters in the human brain with the potent cocaine analogue [125I]RTI-55: in vitro binding and autoradiographic characterization. J Neurochem. 1994a;62:549–556. doi: 10.1046/j.1471-4159.1994.62020549.x. [DOI] [PubMed] [Google Scholar]
- Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC. High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. The Journal of Pharmacology and Experimental Therapeutics. 1994b;271:1678–1685. [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. In vivo voltammetric characterization of low affinity striatal dopamine uptake: drug inhibition profile and relation to dopaminergic innervation density. Brain Res. 1986;373:85–91. doi: 10.1016/0006-8993(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Sultana S, Li H, Puche A, Jones O, Bryant JL, Royal W. Quantitation of parvalbumin+ neurons and human immunodeficiency virus type 1 (HIV-1) regulatory gene expression in the HIV-1 transgenic rat: effects of vitamin A deficiency and morphine. J Neurovirol. 2010;16:33–40. doi: 10.3109/13550280903555712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift RM, Lewis DC. Principles of Nervous System Pharmacology. In: Golan DE, Tashjian AHJ, Armstrong EJ, Armstrong AW, editors. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 2. Lippincott Williams & Wilkins; 2008. pp. 283–304. [Google Scholar]
- Vanderschuren LJ, Ahmed SH. Animal studies of addictive behavior. Cold Spring Harb Perspect Med. 2013;3:a011932. doi: 10.1101/cshperspect.a011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Weiss MJ, Pickup KN, Robinson TE. Rats markedly escalate their intake and show a persistent susceptibility to reinstatement only when cocaine is injected rapidly. J Neurosci. 2010;30:11346–11355. doi: 10.1523/JNEUROSCI.2524-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain: a journal of neurology. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kish SJ. The vesicular monoamine transporter, in contrast to the dopamine transporter, is not altered by chronic cocaine self-administration in the rat. J Neurosci. 1996;16:3507–3510. doi: 10.1523/JNEUROSCI.16-10-03507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, et al. Heterogeneous subregional binding patterns of 3H-WIN 35,428 and 3H-GBR 12,935 are differentially regulated by chronic cocaine self-administration. J Neurosci. 1994;14:2966–2979. doi: 10.1523/JNEUROSCI.14-05-02966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydra K, Golembiowska K, Zaniewska M, Kaminska K, Ferraro L, Fuxe K, Filip M. Accumbal and pallidal dopamine, glutamate and GABA overflow during cocaine self-administration and its extinction in rats. Addict Biol. 2013;18:307–324. doi: 10.1111/adb.12031. [DOI] [PubMed] [Google Scholar]
- Zauli G, et al. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem. 2000;275:4159–4165. doi: 10.1074/jbc.275.6.4159. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [3H]dopamine uptake: dissociation of [3H]dopamine uptake and [3H]2beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. The Journal of Pharmacology and Experimental Therapeutics. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]