Abstract
Myocardial infarction (MI) is the leading cause of death worldwide. Recent advances in stem cell research hold great potential for heart tissue regeneration through stem cell-based therapy. While multiple cell types have been transplanted into MI heart in preclinical studies or clinical trials, reduction of scar tissue and restoration of cardiac function have been modest. Several challenges hamper the development and application of stem cell-based therapy for heart regeneration. Application of cardiac progenitor cells (CPCs) and cardiac tissue engineering for cell therapy has shown great promise to repair damaged heart tissue. This review presents an overview of the current applications of embryonic CPCs and the development of cardiac tissue engineering in regeneration of functional cardiac tissue and reduction of side effects for heart regeneration. We aim to highlight the benefits of the cell therapy by application of CPCs and cardiac tissue engineering during heart regeneration.
Keywords: Heart regeneration, Myocardial infarction, Cell therapy, Cardiac progenitor cells, Cardiac tissue engineering, Biomaterials
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the world. According to WHO 17.3 million people died from CVD in 2008 and the number is estimated to reach 23.3 million by 2030 [1]. In the United States alone, the medical cost of CVD is expected triple from $273 billion in 2008 to $818 billion in 2030, constituting a heavy economic burden [2]. Myocardial infarction (MI) is the most common type of CVD with high morbidity and mortality. Approximately 1 million people suffer from MI annually in the US [3]. MI frequently progresses to heart failure accompanied by ventricle remodeling with the permanent loss of up to 1 billion cardiomyocytes that are replaced by myofibroblasts to form scar tissue [4]. In contrast to amphibians, reptiles, and zebrafish, human cannot sufficiently regenerate the injured heart after MI. The current therapeutic approaches, such as medication, intervention and surgical bypass, can limit the disease developments, but they are ineffective in completely restoring reduced ventricular function and reversing scar formation. While whole heart transplantation is one of the most effective option to treat patients with severe MI, it is limited by the shortage of donor hearts and immune rejection complications [5].
Over the past decade, great breakthroughs in stem cell biology have offered several potential strategies for heart regeneration, such as cell therapy and cell reprogramming [6]. Cell therapy is considered to be a promising option for patients afflicted with heart disease. A variety of candidate cell types, including embryonic stem cells, induced pluripotent stem cells, cardiac progenitor cells (CPCs), cardiomyocytes, mesenchymal stem cells, skeletal myoblasts and others, have been explored to repair the injured hearts in animal models by vasculogenesis, cardiomyogenesis and paracrine effects (Figure 1). Several approaches have moved into clinical trials and applications, providing evidence of the cardiac regenerative possibility by cell therapy. The transplanted cells have been shown to take place of the fibrotic scar tissue, form vascular structure and generate new cardiomyocytes. However, it remains difficult to replace the entire infarcted area with newly generated cardiac tissue by the transplanted cells. Several challenges involving cell survival, cell retention, immune rejection, and vascular blood supply need to be technically and practically overcome before the promise of stem cell therapy is fulfilled.
Figure 1.
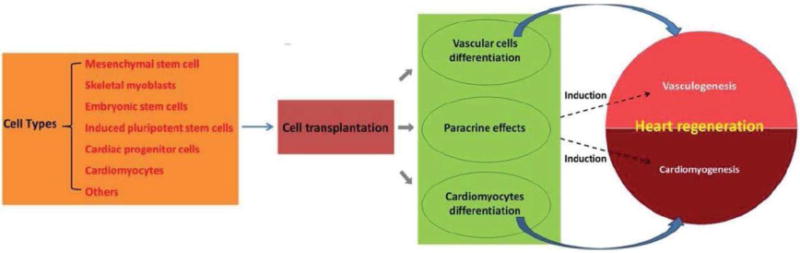
Diagram of cardiac cell therapy. Multiple cell types have been applied to investigate therapeutic potential after transplantation into MI heart. The transplanted cells aim to produce new vascular cells, cardiomyocytes and paracrine effects, leading to vasculogenesis and cardiomyogenesis.
Appropriate cell types and delivery methods are being considered to address these challenges. CPCs, which can give rise to cardiomyocytes, smooth muscle cells and endothelial cells, have been recently reported to significantly improve cardiac functions. Thus, CPCs are believed to be an optimal cell source to address current challenges facing cell therapy.
Cardiac tissue engineering is a vital strategy aimed at improving cell therapy for heart regeneration. It involves application of a series biomaterials designed for facilitating cell delivery and supporting cell functions after transplantation, thus enhancing the regenerative capacity. Moreover, seeding cardiac cells into biomaterials can be used to fabricate engineered vascular and myocardial grafts following transplantation.
In this review, we aim at highlighting the recent advances and major issues in cardiac cell therapy. We especially focus on the advantage and development of CPCs and cardiac tissue engineering during cardiac cell therapy. Furthermore, we attempt to shed light on optimized application of CPCs and biomaterials for heart regeneration after MI.
Current Challenges of Cell Therapy for Heart Re- generation
Since the first application of stem cells in human in 2001, a number of studies have been performed to prove the efficacy and safety of stem cell therapy for MI using multiple cell types. Some studies have advanced from laboratory into clinical trials. However, none of the current cell therapy strategies have been shown to effectively regenerate the injured heart after MI with most clinical trials reporting limited improvement of cardiac function. There are still a number of unsolved problems for cell-based heart regeneration that need to be addressed.
Cell retention and survival
MI typically results in cardiac cell loss, requiring a large number of cells to replace scar tissue and regenerate functional cardiac tissue. However, implanted cells show poor survival and retention after transplantation. Commonly used methods for cell transplantation involve intravenous injection, intracoronary injection and intramyocardial injection. By intravenous and intracoronary injection methods, cells are promptly removed by the circulation with very low numbers homing to the target sites. Intramyocardial injection, which can directly deliver cells into the infarcted region, is more efficient and currently more widely used [7]. But even by intramyocardial injection, more than 90% of injected cells are lost within 2 h [8,9]. Over 75% of retained cells in the first 2 h are lost after 24 h [10]. Possible reasons of the low cell retention and survival after transplantation are shown in Figure 2. -Immediate leakage of injected cells from the puncture holes is one of the major reasons. Blockage of the puncture holes may increase the retained cells by up to 60% 1 h after injection [8,11]. Another route for leakage of cells is through the transepicardial/transendocardial puncture holes and subsequent removal through the venous system. 2 h after injection, up to 30% of lost cells can be found in the lung [12]. Even though a small portion of cells can be retained after injection, numerous cells will also die in the challenging environments of the infarction region characterized by low oxygen, inflammation, cytotoxic cytokines and apoptosis induced by MI. As a result less than 5% of the injected cells can be observed after 4 weeks [7,10].
Figure 2.
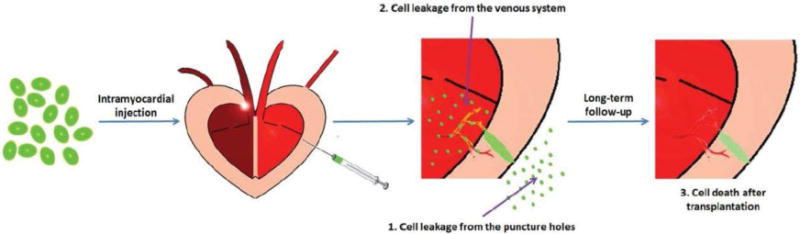
Possible reasons for low cell retention and survival after transplantation. Currently, intramyocardial injection is the most widely used method for cell transplantation. However, most of the transplanted cells are lost for three possible reasons: 1) the cells rapidly leak from the puncture holes after injection; 2) the injected cells leak through the transepicardial/transendocardial puncture holes and then they are washed out from the venous system; and 3) cell death occurs after injection into the infarct region due to the induction of hypoxia, inflammation, cytotoxic cytokines and apoptosis after MI.
Regeneration of the functional cardiac tissue
In order to restore the cardiac function after MI, the well- organized cardiac structure should be constructed to replace the infarcted tissue. An ideal cell therapy should develop new vascular network, provide new contractile muscle tissue and synchronize with the contraction of the host heart. Although cardiomyocytes, smooth muscle cells and endothelial cells can be generated from pluripotent stem cells, no cell transplantation has successfully regenerated a fully organized and functional cardiac tissue due to the failed alignment and integration of transplanted cells with host cells.
After MI, the infarction region is replaced by cardiac fibroblasts. Cardiac fibroblasts excrete collagen to form new extracellular matrix (ECM). Deposition of collagen will create scar fibrosis, leading to a distortion of cardiomyocytes alignment [13–16]. Therefore, the transplanted cells are hampered from aligning properly due to the presence of collagen. Furthermore, the transplanted cells need to be mechanically and electrically integrated with the host myocardium where Connexin43 serves as a key protein for cell-cell coupling and electrical signals transduction [17]. Overexpression of Connexin43 in transplanted cells can improve intercellular mechanical and electrical coupling and reduce post-infarct arrhythmia [18,19], highlighting the importance of reestablishment of gap junction coupling. However, cardiac fibroblasts build up solid gap junction coupling after MI by increased expression of Connexin43 [20], impeding the integration of transplanted cells with host cells.
Side Effects: Tumorigenesis, Arrhythmia, and Immune Rejection
Several safety issues with regard to application of cell therapy for heart regeneration have been raised, including tumorigenesis, immune rejection and arrhythmia. Concern of tumorigenesis and immune rejection exists in all kinds of stem cell therapy and should also be considered in heart regeneration.
Tumorigenesis
Pluripotent stem cells can differentiate into multiple cell types as well as tumor cells. The migration of pluripotent stem cells has been found to result in off-target effects. If the undifferentiated pluripotent stem cells migrate from heart into other organs, it is possible to induce tumor formation [21], although, up to now no tumor formation has been found in animal and human studies using differentiated cells.
Immune rejection
Non-autologous cells are recognized as foreign cells by the immune system of the patients. The activated immune response will eliminate the transplanted cells and reduce the therapeutic efficacy. The transplanted cells may also modulate and influence the patient immune system [22]. Immunosuppressive drugs can inhibit the immune rejection but induce immunodeficiency as an adverse consequence. The risk of immune rejection should be carefully considered in order to maximize the benefits of cell therapy.
Arrhythmia
Ventricular arrhythmia after cell transplantation for heart regeneration has been observed in animal experiments and clinical trials. The first reported occurrence of arrhythmia was reported following transplantation of skeletal myoblasts in a clinical study [23]. Arrhythmia also occurred after injection of human ES cells-derived cardiomyocytes into injured monkey hearts [24]. Although arrhythmia was detected shortly after cell transplantation into heart, the arrhythmia symptoms disappeared after long-term follow-up in both studies [25]. The potential mechanism of arrhythmia is proposed by the different action potential and lack of electrical coupling between transplanted cells and host cells [26,27].
To address these challenges, a variety of cell types and several delivery approaches have been proposed. Among those, CPCs and tissue engineering have shown promising benefits of cell therapy to regenerate the heart tissue.
Embryonic Cardiac Progenitor Cells (Ecpcs) For Heart Regeneration
Endogenous heart regeneration
Heart is able to activate endogenous regenerative mechanism after injury. Zebrafish can fully regenerate the heart after resection of the apex and maintain the regenerative capacity during the whole life [28]. Neonatal mice also exhibit regenerative potential, although the extent of neonatal mice regeneration may need further investigation [29–31]. Recent studies show that proliferation of cardiomyocytes is the major mechanism for endogenous heart regeneration [32,33]. Adult mammalian heart retains the potential of cardiomyocytes proliferation after injury [34]. However, the capacity of endogenous adult cardiomyocytes proliferation is very limited thus providing an inadequate source to fully regenerate the injured heart.
Origin of eCPCs during heart development
CPCs have been identified and characterized during heart development (Figure 3). Cardiac precursors are initiated from lateral plate mesoderm, which arises from the primitive streak during gastrulation. Mesp1, a key transcription factor, is identified as the earliest marker of cardiac precursors. The expression of Mesp1 occurs transiently in the primitive streak stage before being extinguished as the cardiac precursors migrate away from the primitive streak. In addition to cardiac lineages, Mesp1+ cells are also involved in formation of paraxial mesoderm and skeletal muscle of the head and neck [35]. Mesp1+ cells develop into cardiac crescent, when the cardiac precursor cells become CPCs by expression of marker genes, such as Nkx2.5, Gata4 and Isl1 [36]. CPCs irreversibly commit to cardiac lineages, contributing to cardiomyocytes, endothelial cells, and vascular smooth muscle cells [37,38]. The cardiac progenitors are further divided into first heart field (FHF) and second heart field (SHF) [39]. By lineage tracing studies, the FHF, which is marked by the expression of TBX5, gives rise to left ventricle and some part of atrium [40,41]; while SHF, which specifically express Isl1, gives rise to right ventricle, the outflow tract, and some part of atrium[42,43]. With the rapid proliferation and differentiation of CPCs, the heart will undergo heart tube formation, looping and four chambers formation, and finally develop into the functional heart.
Figure 3.
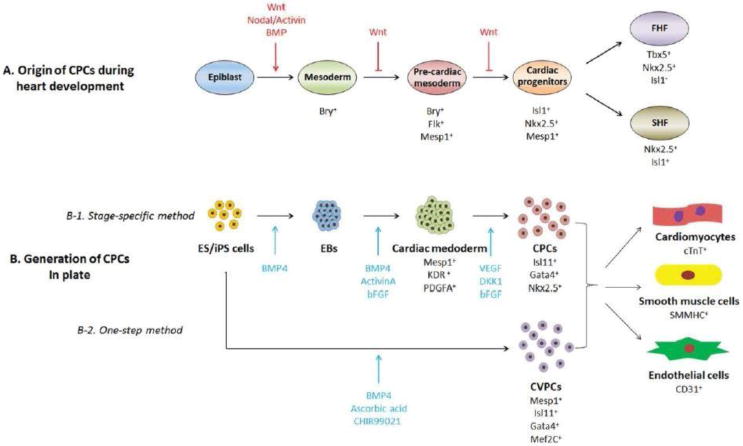
Origin and in vitro generation of eCPCs. (A) Lineage tracing method has been applied to describe specific stages and the origin of CPCs during heart development. Specific marker genes are used to define different stages. In this process, Wnt signaling pathway plays important roles. (B) Stage specific differentiation protocol of CPCs from ES/iPS cells was generated according to the Wnt signaling pathway. An optimized one-step differentiation protocol was developed to generate CPCs in plate. Both methods derived CPCs could further differentiate into cardiomyocytes, smooth muscle cells and endothelial cells.
Embryonic stem (ES) cells/induced pluripotent stem (iPS) cells- derived eCPCs
Generation of ES/iPS cells-derived CPCs
ES cells are pluripotent cells that can give rise to all the cell types found in the adult organism. However, due to the ethical issues, ES cells cannot be directly used for cell therapy. In 2006, adult mice skin fibroblasts were reprogrammed into ESC-like cells by retrovirus- mediated expression of four transcription factors: Oct3/4, Sox2, Klf4, and c-Myc [44]. This technique was soon reproduced in human cells to generate human iPS cells using human fibroblasts [45,46]. Recently, iPS cells were generated by defined chemicals alone without application of virus transfection, providing a more promising method that would not introduce mutation and might be safer for clinical use. iPS cells could be maintained in the ES cell like state in culture medium and have been successfully used to generate various somatic cells for regenerative medicine.
ES/iPS cells can be used to generate cardiac cells for cell therapy (Figure 2). The specification and differentiation of cardiac lineages during in vitro differentiation are modulated by several signaling pathway, including BMP, GSK3, TGF-β/activin/nodal, WNT/β-catenin, and FGF pathway [47,48]. In recent years, multiple differentiation protocols have been developed to direct ES/iPS cells into cardiac lineages and eCPCs can arise during the differentiation process.
The most commonly used method to generate eCPCs was the stage specific differentiation through embryonic body (EB) formation [49] to mimic the embryos development. ES/iPS cells are digested into single cells and suspended in differentiation medium on low-adherence plates to generate EBs, which can differentiate into all 3 germ layers [50]. Then eCPCs are induced by stepwise addition of growth factors in defined medium. By addition of BMP4, ActinvinA and bFGF, EBs are induced into cardiac mesoderm cells marked by Mesp1. Mesp1+ cells specifically differentiate into eCPCs in the presence of VEGF, DKK1 and bFGF. eCPCs can be identified by the expression of surface markers, such as KDR/Flk-1 and PDGFR-α, or by the expression of transcription factors, such as Isl1, Gata4 and Nkx2.5. The derived eCPCs can efficiently differentiate into cardiomyocytes and vascular cells [51,52].
Another method has been reported to directly induce cultured human ES/iPS cells into cardiovascular progenitor cells (CVPCs) by application of BMP4, GSK3 inhibitor CHIR99021 and ascorbic acid [53]. The CVPCs can be characterized by expression of multipotent cardiovascular progenitor cell markers of Mesp1, Isl1, Gata4 and Mef2c. The CVPCs generated by this method can be maintained long-term and expanded by a combination of GSK3 inhibitor, BMP inhibitor and Activin/Nodal inhibitor. The derived CVPCs exhibited expected potential to generate cardiomyocytes, smooth muscle cells and endothelial cells using specific differentiation medium. Further research should be performed to describe whether the CVPCs are subpopulation of eCPCs, which are specifically commited to cardiac lineage and can be used for cell therapy.
Application of human iPS cells-derived eCPCs for heart regeneration
ES/iPS cells-derived eCPCs have shown great advantage for heart regeneration. Heart regeneration requires replacement of injured heart with new cardiac tissue accompanied with myocardiogenesis and angiogenesis. eCPCs have the potential to realize this goal by simultaneously differentiation into three major cardiac lineages. The specific commitment to cardiac lineages results in low potential of teratoma formation after cell transplantation. Furthermore, the autologous cells derived iPS cells could reduce the immune rejection for cell therapy. The therapeutic potential of eCPCs has been well investigated after transplantation into MI heart in multiple animal models, including mouse, rat and monkey.
Ali Nsair [54] reported that the combination of Flt1+/Flt4+ facilitated enrichment for mouse iPS cell-derived Isl1+/Nkx2.5+ CPCs with ability to differentiate into all three cardiac lineages. After transplantation into mouse heart, the transplanted cells were demonstrated to robustly engraft into host tissue and differentiate into mature cardiomyocytes. To verify the therapeutic effects of eCPCs, mouse ES cell-derived CPCs, labeled with EGFP driven by CPC marker gene Nkx2.5, were injected into the mouse heart after MI [55]. Injected CPCs significantly reduced scar area and improved cardiac function within two weeks. The injected cells differentiated into three major cardiac cell types with formation of mechanical and electrical coupling to host cells. No teratomas or arrhythmias were observed after transplantation.
Therapies using human iPS cell-derived eCPCs have been investigated. A modified 6-day generation protocol was established using human iPS cells as a monolayer and the derived CPCs were identified by expression of KDR and PDGFR-α [56]. After transplantation into the MI/reperfusion rats, the CPCs differentiated into cardiomyocytes and smooth muscle cells and persisted for at least 10 weeks, leading to a non-significant trend to protect declined cardiac function after MI. In another study, eCPCs were derived from Rhesus ES cells by treatment of BMP2 and characterized by expression of Oct4, SSEA-1 and Mesp1. eCPCs were purified by cell sorting using an anti–SSEA-1 antibody [57]. After transplantation into the MI Rhesus heart, SSEA-1+ CPCs differentiated into cardiomyocytes and reconstituted 20% of the scar tissue. The SSEA-1+ CPCs did not develop teratomas, whereas the non-purified CPCs, containing SSEA-1- cells could induce teratomas.
Application of eCPCs derivatives for heart regeneration
ES/iPS cell-derived eCPCs can further differentiate into cardiac cells, which have also been used for heart regeneration after MI. The effects of human pluripotent stem cell- derived cardiomyocytes for regenerating injured heart have been extensively investigated. After injection of human ES cell-derived cardiomyocytes into infarcted murine heart, the cells could engraft and electrically couple with host cells, but significant improvement of cardiac remodeling and function was not shown [58–60]. Significantly, a recent study has shown great promise for cell therapy against MI by injection of human ES cell-derived cardiomyocytes into non-human primate heart. After cell transplantation, obvious improvement of infarcted heart was shown. 40% of the infarcted region was replaced by injected cardiomyocytes and the host vessels could extend into the graft to form new vascular network [24]. Besides cardiomyocytes, the therapeutic efficacy of ES cell-derived vascular cells has been investigated. After injection of mouse ES cell-derived endothelial cells into MI mice, the cardiac function was improved by formation of capillaries and venules in the infarct region. Using a single cell type appears inefficient to regenerate the injured heart. To simultaneously regenerate myocardium and vasculature, a recent study was performed where a mixture of human iPS cell- derived cardiomyocytes, endothelial cells, and smooth muscle cells (1:1:1) was transplanted using pig MI model [61]. The survived cardiomyocytes could integrate into the host cells and endothelial and smooth muscle cells involved in vasculature formation, contributing to cardiac function improvement. This study supports potential advantage of application of eCPCs for heart regeneration since eCPCs can automatically differentiate into cardiomyocytes, smooth muscle cells and endothelial cells with optimal efficiency under regulation of in vivo signaling pathways.
Adult CPCs
Identification and isolation of adult CPCs
Adult CPCs have been identified in the adult heart and characterized by the expression of stem cell surface markers, such as c-Kit, Sca-1 and SSEA-1. After MI, adult CPCs can be activated and, they are able to home in on the infarction region [62] but cannot produce sufficient numbers of cardiac cells to efficiently regenerate the injured heart [63]. Nevertheless, adult CPC is considered as an ideal cell source for cell therapy (Figure 2). Adult CPCs have been successfully isolated from cardiac biopsies of mice [64], rat [65], sheep [66], pig [67] and human [68]. Despite small numbers of adult CPCs that can be derived from the biopsies, they can be propagated for long periods of time to obtain sufficient cells for transplantation [65,69]. Notably, this kind of autologous cells may not induce immune rejection. Furthermore, the adult CPCs are committed to the cardiac lineages, indicating that the cells present the possibility to generate new cardiac tissue without tumorigenesis.
Application of adult CPCs for heart regeneration
Several types of adult CPCs, including c-Kit+, Scar-1+, SSEA-1+, “cardiosphere” progenitor cells and side population CPCs, have been applied in vivo studies after MI and have demonstrated the therapeutic potential. Transplantation of c-Kit+ CPCs reduced infarct size and improved cardiac function after MI by vascular system restoration, especially by formation of large coronary arteries [70,71]. Even in a 30-day infarction rat model, intracoronary administration of c-Kit+ CPCs could alleviate the cardiac dysfunction [72]. By 2011, a phase 1 clinical trial was completed by transplantation of c-Kit+ CPCs into MI patients. The results were very encouraging with regard to autologous c-Kit+ CPCs ability to effectively improve LV systolic function and reduce infarct size in MI patients. Similar therapeutic potential was confirmed with Sca-1 and SSEA-1 CPCs. Sca-1+ CPCs have been isolated from adult heart with expression of early cardiac markers of Gata4 and Mef2C, lacking c-Kit [73], and SSEA-1+ CPCs obtained from adult heart have been characterized by expression of SSEA-1 and Oct3/4 without c-Kit and Sca-1[74]. Transplantation of Sca-1+ or SSEA-1+ CPCs showed improvement of cardiac function and attenuation of cardiac remodeling. “Cardiosphere” progenitor cells and side population CPCs have also been isolated from the adult heart and exhibited therapeutic potential for MI. However, they were identified with a mixture of CPCs, vascular progenitor cells and mesenchymal progenitors, which limited their clinical application [75,76].
Although improvement of cardiac function after MI has been described after transplantation of adult CPCs, their utility for clinical application remains controversial due to the origin and differentiation issues. The c-Kit and Sca-1 are the most used surface markers to isolate adult CPCs. They were initially used to isolate hematopoietic stem cells from bone marrow. The mesenchymal markers are found co-expressed in several subpopulations of c-Kit+ and Sca-1+ CPCs [77,78]. It is presumed that the adult CPCs are a different subset of mesenchymal stem cells or a mixture of CPCs and mesenchymal stem cells. Besides of the origin of adult CPCs, the differentiation potential of adult CPCs is also under debated. The CPCs are defined by their ability to differentiate into three types of cardiac cells. Cardiomyocytes are particularly the most important cells to restore the cardiac function. c-Kit+ and Sca-1+ CPCs have been shown to have a minimal potential to differentiate into cardiomyocytes. In vitro, Sca-1+ CPCs were shown to differentiate into cardiomyocytes with very low efficiency, while addition of specific chemicals and growth factors could induce adult CPCs differentiation into cardiomyocytes [73,79,80]. c-Kit+ CPCs were characterized by differentiation into cardiomyocytes in vitro[81], but less than 0.8% of adult c-Kit+ CPCs contributed to cardiomyocytes by lineage tracing studies in vivo, even after injury [82,83]. However, some recent studies showed that after injection of c-Kit+ or Sca-1+ CPCs into infarcted heart, improvement of cardiac function could be observed by formation of vasculature and cardiomyocytes [71,72,84,85], although the efficiency of cardiomyocytes formation of transplanted cells should be further investigated. In addition, a single c-Kit+ progenitor cell isolation and culture method was developed, providing a potential to generate real cardiac specific c-Kit+ CPCs for cell therapy [86].
Cardiac Tissue Engineering
The previous cell transplantation methods for heart regeneration include intravenous injection, intracoronary injection and intramyocardial injection. These methods have severe cell retention and cell survival issues, leading to limited cell therapy potential. The emergence of tissue engineering offers an encouraging strategy to improve cell delivery. Over the past two decades, various engineered tissues were constructed in vitro, such as bone, skin, nerve, liver, blood vessel and myocardium [87]. Cardiac tissue engineering strategies, in which biomaterials play important roles in facilitating cell transplantation, have been developed to repair injured heart.
Currently used biomaterials for heart regeneration
Currently, hydrogel, decellularized ECMs and synthetic matrices are the main reported biomaterials applied for heart regeneration (Table 1) [88]. There are at least several general principles of biomaterials construction for heart regeneration: (1) the materials should be biocompatible and degradable; (2) the materials should not influence transplanted cells’ proliferation and differentiation; (3) the materials should facilitate transplanted cells to establish mechanical and electrical couplings with host cells; and (4) the quality of the materials should be controllable to realize commercialization.
Table 1.
Biomaterials applied for cardiac tissue engineering
| Biomaterials | Hydrogel | Decellularized ECMs | Synthetic matrices |
|---|---|---|---|
| Components or Origin | Collagen Fibrin Matrigel Gelatin Alginate Others |
SIS Pericardium Valves Whole pig heart |
PU PGS PLLA PLGA PCLA Others |
| Advantage | Bioactive Biocompatible Natural environment |
Organized tissue Provide donor heart |
Mechanical strength Consistent qualities Biocompatible Biodegradable |
| Disadvantage | Less mechanical strength Pathogen risks potential Variable qualities |
Pathogen risks potential Variable qualities |
Integration to host Foreign responses |
Hydrogel from natural polymers, such as collagen, fibrin, matrigel, gelatin and alginate, has been extensively investigated for cell-based tissue engineering approaches for heart regeneration. Particularly, the collagen matrix was the pioneered biomaterial to generate engineered cardiac tissue [89]. The natural materials are derived from native tissues thus exhibiting beneficial properties of being bioactive and biocompatible [88]. Additionally, they proivde ECM-like features of natural environment and structure for cell adhesion, proliferation and differentiation. Application of natural biomaterials can form better cell-cell interaction, but it leads to similar mechanical strength to native tissue, which is a very soft tissue, resulting in the difficulties for transplantation. The weakness of mechanical strength can be partly solved by combination of multiple scaffolds [90].
Decellularized ECMs are prepared by removing the cells from tissue, leaving ECMs, cell matrix interactions and blood vessels intact. It provides native ECM as biological scaffolds, following recellularization to fabricate the cardiac tissue. Decellularized ECMs have been generated from blood vessel [91], small intestine submucosa [92,93], pericardium [94,95] and valves [96]. Even the whole heart has been constructed by the decellularized ECMs in mouse and rat [97,98]. The decellularized ECMs cannot be derived from human heart, but pig heart, with many similarities to human heart in histology and physiology, can be used as an alternative to develop decellularized whole heart ECMs, providing a promising candidate for heart transplantation to treat MI [99,100].
Although several advantages have been shown in hydrogel and natural ECMs, the mechanical issues, pathogen risks and variable qualities between batches hamper their wide application [101]. Much effort has been devoted to exploring synthetic matrices due to their the advantages of being biocompatible and biodegradable [102]. Synthetic matrices, which mimic the natural ECMs and in vivo environments, are made with predictable and consistent mechanical, chemical and physical properties [103]. Their properties can be tuned to match specific applications, and they can realize commercialization with precise quality control. Multiple synthetic matrices have been reported for stem cell based heart regeneration, such as polyurethane (PU), polyglicerolsebacate (PGS), poly-L-lactic acid (PLLA), poly-lactic-co-glycolic acid (PLGA) and poly-chitosan-g-lactic acid (PCLA) [104]. Some shortcomings of synthetic materials such as difficulties with the host tissue integration and inability to conduct appropriate signals have been described [105]. These problems can be addressed by combination of regulatory molecules, growth factors and proteins [106,107].
Numerous biomaterials have been used to deliver stem cells and construct cardiac tissue. Some publications have shown the enhanced outcome of cell therapy. According to their application, these biomaterials can be classified into injectable biomaterials and engineered cardiac grafts. Both natural and synthetic materials have been extensively explored as injectable biomaterials and cardiac tissue grafts. The injectable biomaterials aim to directly deliver cells into the injured region with increased cell survival, while the engineered cardiac grafts are designed to generate a functional tissue to replace the infarcted ventricle.
Injectable biomaterials for cardiac cell delivery
Injectable biomaterials, which allow the injected cells to be directly delivered into the target site of the infarct region, are being developed to improve cell retention and survival during cell injection. After direct cell injection, the single cells are rapidly lost due to leakage from the puncture holes. The lack of cell-cell and cell- ECM interactions limit cell survival. The injectable materials offer a temporary ECM as a support structure for cell adherence thereby preventing cell ejection and increasing cell survival after injection [108]. The injectable biomaterials can also be used as a controlled release system to package drugs and growth factors during cell transplantation to further improve cell survival, proliferation, differentiation and cell coupling. In addition, injectable biomaterials can deliver cells with minimal invasive procedure by combination of catheter mediated cell delivery approaches [108,109].
Hydrogel and nanoparticles are the most used injectable biomaterials for heart regeneration. The improved cell retention accompanied by restored cardiac function and reduced scar tissue have been demonstrated by co-injection of injectable materials and various cell types [9,110–113]. Some advanced injectable materials have been designed to improve therapeutic effects. Thermosensitive hydrogel system, which underwent a sol-gel reversible transition upon heating or cooling at ≈32°C, was designed to increase injected cells retention compared with injection of dissociated cells [114]. With this biomaterial, the cardiac function significantly improved in rats and pigs with MI. Thermosensitive biomaterials were also able to suppress oxidative stress damage to cells so that the cell survival could be effectively improved [115]. Magnetic targeting method was combined with nanoparticles to prevent washing out of the injected cells. Magnetic targeting with ferumoxytol nanoparticles delivered cardiosphere-derived stem cells into MI region, leading to augmented cell engraftment and therapeutic benefit without any toxicity [116].
Engineered cardiac grafts
Engineered cardiac grafts are fabricated by seeding cells into biomaterials. Some of them are cultured in a bioreactor for a certain time. An ideal engineered cardiac grafts should exhibit contractility forces, extensive vascularization and electrical transduction. Different types of cell sheets and 3-D scaffolds have been developed by natural or synthetic biomaterials to generate cardiac grafts [117]. Despite some positive therapeutic potential observed from cardiac grafts, constructing a well-organized and functional cardiac tissue remains a significant challenge. Several strategies present promising advantage to improve the engineered cardiac grafts.
Contractility forces formation is a big challenge for generation of cardiac graft in vitro due to the cell alignment and mechanical coupling issues, further leading to limited electrical coupling. Cardiomyocytes are striated for the parallel arrangement of actin and myosin filaments. To address this problem, some biomaterials were designed into parallel channels with certain width so that the seeded cells were aligned in a highly organized parallel manner [118–120]. The laser-patterned array was also reported to provide guidance to direct cells alignment [121,122]. The well-aligned cells revealed higher expression of Connexin43, suggesting improved cell-cell interaction and mechanical coupling. The electrical coupling was also improved in the well-organized engineered grafts. Some natural biomaterials, such as epicardial mimetics and decellularized ECMs, were applied to fabricate recellularized heart tissue with the presence of alignment and electrical coupling [123,124]. Even the acellular biomaterials themselves could attenuate remodeling and improve cardiac function after MI [125]. Mechanical and electrical stimulation approaches were broadly investigated in 2D and 3D engineered cardiac tissue. The stimulation appeared to generate functional cardiac tissue in vitro by mechanical and electrical coupling and improve cardiomyocytes maturation as well [126–130].
Although therapeutic effects of engineered cardiac grafts have been widely reported after MI, the cell survival issue remains unresolved [131–133]. The low survival rate is believed to be due to the low oxygen supply caused by graft thickness and poor revascularization. In a recent study, cardiac tissue slice was used as a model to investigate the maximum thickness of grafts. The results indicated that the cell death was higher at the edge of the grafts and <400 μm was the optimal thickness [134]. The revascularization issue can be addressed by optimization of cellular composition of the grafts. Co-culture of endothelial cells and fibroblasts was shown to revascularize the grafts and improve cardiac function [135,136]. Revascularization of transplanted grafts was also achieved by addition of growth factors [137].
CPCs Based Cardiac Tissue Engineering For Heart Regeneration
There are two potential strategies to regenerate injured heart by application of CPCs and cardiac tissue engineering (Figure 4). One is biomaterial-mediated CPCs injection into infarcted region, allowing CPCs to replace scar tissue with newly formed cardiac tissue; the other is fabrication of cardiac tissue by cultivation of CPCs with biomaterials in vitro, followed by transplantation into ventricle wall of infarct region.
Figure 4.
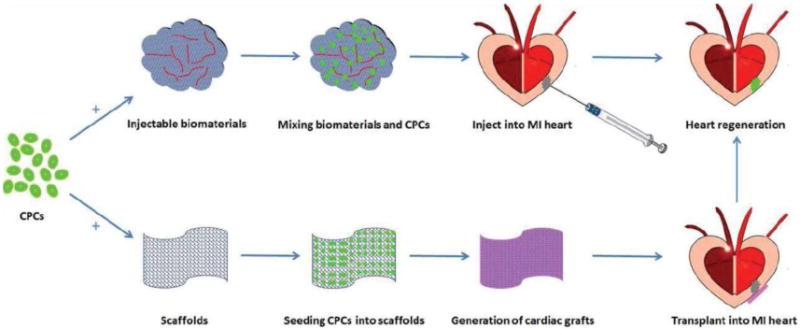
Potential strategies of CPCs based cardiac tissue engineering for heart regeneration. One strategy is to mix CPCs and injectable biomaterials and then directly inject into the infarcted region, allowing CPCs to replace scar tissue with newly formed cardiac tissue; the other involves fabrication of cardiac tissue by cultivation of CPCs with biomaterials in vitro and then transplantation of the tissue engineered cardiac grafts to the surface of the infarct region to achieve heart regeneration.
The application of injectable biomaterials has shown the improvement of retention and survival of CPCs. Matrix-enriched hydrogel capsules were used to deliver human biopsy-derived resident CPCs into mice heart with MI [138]. The cell retention and survival were significantly improved and restored cardiac function was observed. The transplanted CPCs could differentiate into both cardiomyocytes and vascular cells, suggesting the potential to regenerate new cardiac tissue. When naturally derived cardiac ECM was explored to deliver CPCs [139], strong adhesion of CPCs to the cardiac ECM was demonstrated. CPCs exhibited enhanced proliferation and increased tolerance to apoptosis on the cardiac ECMs. Modified biomaterials have been explored to further improve cell survival or for certain other application. For example, an oxygen-releasing system, which consisted of hydrogen peroxide- releasing microspheres, catalase and an injectable, thermosensitive hydrogel, was demonstrated to augment CPCs survival and differentiation under hypoxic condition [140].
Several CPC-based engineered cardiac grafts have been generated in vitro. Gelatin and collagen scaffolds seeded with human adult CPCs were used to construct cardiac grafts [141]. The cells were viable and normally proliferating in the scaffold. CPCs were selectively committed towards cardiomyocytes lineage to generate the myocardium grafts. Printing technology was applied to generate 3-D cardiac grafts by combination of CPCs and alginate scaffold [142]. The CPCs could survive, proliferate and differentiate into cardiac lineages during culture. The printed cells were able to migrate from the matrix to form tubular-like structures. A defined organized cardiac graft was constructed by this method. The engineered heart was successfully constructed in vitro by seeding human iPS cells-derived CPCs into decellularized mouse heart [98]. The existence of natural ECMs directed CPCs into well- organized alignment and generation of functional myocardium. The engineered heart exhibited similar physiological response to normal heart. Repopulation of decellularized heart provided a promising strategy to generate donor heart for transplantation. Since most of the engineered cardiac grafts by application of CPCs and biomaterials are constructed in vitro, their therapeutic effects should be further investigated in the injured heart in vivo.
Conclusions
Collectively, the major challenges for current cell-based heart regeneration include cell retention and survival, functional cardiac tissue formation and side effects. Application of CPCs and cardiac tissue engineering has the potential to address these key issues. The CPCs are committed to cardiac lineages, differentiating into cardiomyocytes, smooth muscle cells and endothelial cells, suggesting the potential to generate a cardiac tissue without tumorigenesis. The autologous derived CPCs can reduce immune rejection after transplantation. Meanwhile, tissue engineering technique has shown advantages in improving cell retention and survival, cell alignment, cell coupling and integration into the host tissue. However, there are still various limitations of application of CPCs and tissue engineering. In order to optimize the application of CPCs, future studies are required to illustrate the mechanism of cardiac remodeling after MI and the mechanisms of CPCs in heart regeneration. Furthermore, novel biomaterials and delivery systems are required to further advance the tissue engineering for heart regeneration.
Acknowledgments
Z.W. was supported by NIH grant (1R01HL109054), CVC Inaugural Grant from University of Michigan and a Pilot Grant from University of Michigan. P.X.M. was supported by NIH grant (1R01HL114038).
References
- 1.Global status report on non-communicable diseases 2010. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47(9):1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi R, Kuruma Y, Sekine H, Dobashi I, Yamato M, Umezu M, et al. In vivo vascularization of cell sheets provided better long-term tissue survival than injection of cell suspension. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1854. [DOI] [PubMed] [Google Scholar]
- 6.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elhami E, Dietz B, Xiang B, Deng J, Wang F, Chi C, et al. Assessment of three techniques for delivering stem cells to the heart using PET and MR imaging. EJNMMI Res. 2013;3(1):72. doi: 10.1186/2191-219X-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyette JP, Fakharzadeh M, Burford EJ, Tao ZW, Pins GD, Rolle MW, et al. A novel suture-based method for efficient transplantation of stem cells. J Biomed Mater Res A. 2013;101(3):809–818. doi: 10.1002/jbm.a.34386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roche ET, Hastings CL, Lewin SA, Shvartsman DE, Brudno Y, Vasilyev NV, et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials. 2014;35(25):6850–6858. doi: 10.1016/j.biomaterials.2014.04.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, et al. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108(3):346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chik TK, Ma XY, Choy TH, Li YY, Diao HJ, Teng WK, et al. Photochemically crosslinked collagen annulus plug: a potential solution solving the leakage problem of cell-based therapies for disc degeneration. Acta Biomater. 2013;9(9):8128–8139. doi: 10.1016/j.actbio.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Lang C, Lehner S, Todica A, Boening G, Zacherl M, Franz WM, et al. In-vivo comparison of the acute retention of stem cell derivatives and fibroblasts after intramyocardial transplantation in the mouse model. Eur J Nucl Med Mol Imaging. 2014;41(12):2325–36. doi: 10.1007/s00259-014-2858-8. [DOI] [PubMed] [Google Scholar]
- 13.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104(4):557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 14.Menon SC, Eidem BW, Dearani JA, Ommen SR, Ackerman MJ, Miller D. Diastolic dysfunction and its histopathological correlation in obstructive hypertrophic cardiomyopathy in children and adolescents. J Am Soc Echocardiogr. 2009;22(12):1327–1334. doi: 10.1016/j.echo.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Deitch S, Gao BZ, Dean D. Effect of matrix on cardiomyocyte viscoelastic properties in 2D culture. Mol Cell Biomech. 2012;9(3):227–249. [PMC free article] [PubMed] [Google Scholar]
- 16.Pichler M, Rainer PP, Schauer S, Hoefler G. Cardiac fibrosis in human transplanted hearts is mainly driven by cells of intracardiac origin. J Am Coll Cardiol. 2012;59(11):1008–1016. doi: 10.1016/j.jacc.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, Kardami E, et al. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15(3):289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450(7171):819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 19.Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97(2):159–167. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Kanter EM, Yamada KA. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc Pathol. 2010;19(6):e233–240. doi: 10.1016/j.carpath.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66(9):4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 22.Nasef A, Ashammakhi N, Fouillard L. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med. 2008;3(4):531–546. doi: 10.2217/17460751.3.4.531. [DOI] [PubMed] [Google Scholar]
- 23.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41(7):1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 24.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42(12):2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Chong JJ, Murry CE. Cardiac regeneration using pluripotent stem cells-Progression to large animal models. Stem Cell Res. 2014;13(3 Pt B):654–65. doi: 10.1016/j.scr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol. 2000;149(3):731–740. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 29.Bryant DM, O’Meara CC, Ho NN, Gannon J, Cai L, Lee RT. A systematic analysis of neonatal mouse heart regeneration after apical resection. J Mol Cell Cardiol. 2015;79:315–8. doi: 10.1016/j.yjmcc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darehzereshki A, Rubin N, Gamba L, Kim J, Fraser J, Huang Y, et al. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev Biol. 2015;399(1):91–9. doi: 10.1016/j.ydbio.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bicknell KA, Coxon CH, Brooks G. Can the cardiomyocyte cell cycle be reprogrammed? J Mol Cell Cardiol. 2007;42(4):706–721. doi: 10.1016/j.yjmcc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 33.O’Meara C, Wamstad JA, Gladstone R, Fomovsky G, Butty V, Shrikumar A, et al. Transcriptional Reversion of Cardiac Myocyte Fate During Mammalian Cardiac Regeneration. Circ Res. 2015;116(5):804–15. doi: 10.1161/CIRCRESAHA.116.304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126(15):3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 36.Devine WP, Wythe JD, George M, Koshiba-Takeuchi K, Bruneau BG. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. Elife. 2014;3 doi: 10.7554/eLife.03848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127(6):1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127(6):1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 39.Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, Shao Y, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326(5951):426–429. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruneau BG, Logan M, Davis N, Levi T, Tabin CJ, Seidman JG, et al. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev Biol. 1999;211(1):100–108. doi: 10.1006/dbio.1999.9298. [DOI] [PubMed] [Google Scholar]
- 41.Liberatore CM, Searcy-Schrick RD, Yutzey KE. Ventricular expression of tbx5 inhibits normal heart chamber development. Dev Biol. 2000;223(1):169–180. doi: 10.1006/dbio.2000.9748. [DOI] [PubMed] [Google Scholar]
- 42.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6(11):826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 48.Moretti A, Laugwitz KL, Dorn T, Sinnecker D, Mummery C. Pluripotent stem cell models of human heart disease. Cold Spring Harb Perspect Med. 2013;3(11) doi: 10.1101/cshperspect.a014027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8(2):228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 51.El-Mounayri O, Mihic A, Shikatani EA, Gagliardi M, Steinbach SK, Dubois N, et al. Serum-free differentiation of functional human coronary-like vascular smooth muscle cells from embryonic stem cells. Cardiovasc Res. 2013;98(1):125–135. doi: 10.1093/cvr/cvs357. [DOI] [PubMed] [Google Scholar]
- 52.Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29(11):1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao N, Liang H, Huang J, Wang J, Chen Y, Chen Z, et al. Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Res. 2013;23(9):1119–1132. doi: 10.1038/cr.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nsair A, Schenke-Layland K, Van Handel B, Evseenko D, Kahn M, Zhao P, et al. Characterization and therapeutic potential of induced pluripotent stem cell-derived cardiovascular progenitor cells. PLoS One. 2012;7(10):e45603. doi: 10.1371/journal.pone.0045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christoforou N, Oskouei BN, Esteso P, Hill CM, Zimmet JM, Bian W, et al. Implantation of mouse embryonic stem cell-derived cardiac progenitor cells preserves function of infarcted murine hearts. PLoS One. 2010;5(7):e11536. doi: 10.1371/journal.pone.0011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carpenter L, Carr C, Yang CT, Stuckey DJ, Clarke K, Watt SM. Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev. 2012;21(6):977–986. doi: 10.1089/scd.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120(4):1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489(7415):322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol. 2010;49(6):941–949. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1(1):9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15(6):750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, et al. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154(4):827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13(8):970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.French KM, Davis ME. Isolation and expansion of c-kit-positive cardiac progenitor cells by magnetic cell sorting. Methods Mol Biol. 2014;1181:39–50. doi: 10.1007/978-1-4939-1047-2_4. [DOI] [PubMed] [Google Scholar]
- 65.Freire AG, Nascimento DS, Forte G, Valente M, Resende TP, Pagliari S, et al. Stable phenotype and function of immortalized Lin-Sca-1+ cardiac progenitor cells in long-term culture: a step closer to standardization. Stem Cells Dev. 2014;23(9):1012–1026. doi: 10.1089/scd.2013.0305. [DOI] [PubMed] [Google Scholar]
- 66.Hou X, Appleby N, Fuentes T, Longo LD, Bailey LL, Hasaniya N, et al. Isolation Characterization, and Spatial Distribution of Cardiac Progenitor Cells in the Sheep Heart. J Clin Exp Cardiolog. 2012:S6. [PMC free article] [PubMed] [Google Scholar]
- 67.Vanelli A, Pennarossa G, Maffei S, Galvez BG, Cossu G, Rahaman M, et al. Isolation, characterization and differentiation potential of cardiac progenitor cells in adult pigs. Stem Cell Rev. 2012;8(3):706–719. doi: 10.1007/s12015-011-9339-2. [DOI] [PubMed] [Google Scholar]
- 68.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, Simpson D, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123(4):364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyamoto S, Kawaguchi N, Ellison GM, Matsuoka R, Shin’oka T, Kurosawa H. Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev. 2010;19(1):105–116. doi: 10.1089/scd.2009.0041. [DOI] [PubMed] [Google Scholar]
- 70.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, et al. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105(5):1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102(10):3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121(2):293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100(21):12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ott HC, Matthiesen TS, Brechtken J, Grindle S, Goh SK, Nelson W, et al. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S27–39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 75.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107(11):1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carr CA, Stuckey DJ, Tan JJ, Tan SC, Gomes RS, Camelliti P, et al. Cardiosphere-derived cells improve function in the infarcted rat heart for at least 16 weeks–an MRI study. PLoS One. 2011;6(10):e25669. doi: 10.1371/journal.pone.0025669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gambini E, Pompilio G, Biondi A, Alamanni F, Capogrossi MC, Agrifoglio M, et al. C-kit+ cardiac progenitors exhibit mesenchymal markers and preferential cardiovascular commitment. Cardiovasc Res. 2011;89(2):362–373. doi: 10.1093/cvr/cvq292. [DOI] [PubMed] [Google Scholar]
- 78.Valente M, Nascimento DS, Cumano A, Pinto-do OP. Sca-1+ cardiac progenitor cells and heart-making: a critical synopsis. Stem Cells Dev. 2014;23(19):2263–2273. doi: 10.1089/scd.2014.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279(12):11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 80.Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, et al. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1(2):138–149. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 81.Smith AJ, Lewis FC, Aquila I, Waring CD, Nocera A, Agosti V, et al. Isolation and characterization of resident endogenous c-Kit+ cardiac stem cells from the adult mouse and rat heart. Nat Protoc. 2014;9(7):1662–1681. doi: 10.1038/nprot.2014.113. [DOI] [PubMed] [Google Scholar]
- 82.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, et al. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci U S A. 2012;109(33):13380–13385. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, et al. The role of the sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24(7):1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 85.Rong XS, Li MQ, Xu Y, Hua XQ, Jiao GQ, Wei LY. Injection of Cardiac Stem Cells Prolongs the Survival of Cardiac Allograft Rats. Am J Med Sci. 2015;349(1):67–71. doi: 10.1097/MAJ.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 86.Choi SH, Jung SY, Suh W, Baek SH, Kwon SM. Establishment of isolation and expansion protocols for human cardiac C-kit-positive progenitor cells for stem cell therapy. Transplant Proc. 2013;45(1):420–426. doi: 10.1016/j.transproceed.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 87.Wang H, Zhou J, Liu Z, Wang C. Injectable cardiac tissue engineering for the treatment of myocardial infarction. J Cell Mol Med. 2010;14(5):1044–1055. doi: 10.1111/j.1582-4934.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lam MT, Wu JC. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev Cardiovasc Ther. 2012;10(8):1039–1049. doi: 10.1586/erc.12.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11(8):683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 90.Pok S, Myers JD, Madihally SV, Jacot JG. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013;9(3):5630–5642. doi: 10.1016/j.actbio.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108(22):9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okada M, Payne TR, Oshima H, Momoi N, Tobita K, Huard J. Differential efficacy of gels derived from small intestinal submucosa as an injectable biomaterial for myocardial infarct repair. Biomaterials. 2010;31(30):7678–7683. doi: 10.1016/j.biomaterials.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 93.Vukadinovic-Nikolic Z, Andree B, Dorfman SE, Pflaum M, Horvath T, Lux M, et al. Generation of bioartificial heart tissue by combining a three-dimensional gel-based cardiac construct with decellularized small intestinal submucosa. Tissue Eng Part A. 2014;20(3–4):799–809. doi: 10.1089/ten.TEA.2013.0184. [DOI] [PubMed] [Google Scholar]
- 94.Ghodsizad A, Bordel V, Wiedensohler H, Elbanayosy A, Koerner MM, Gonzalez Berjon JM, et al. Magnetically guided recellularization of decellularized stented porcine pericardium-derived aortic valve for TAVI. ASAIO J. 2014;60(5):582–586. doi: 10.1097/MAT.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 95.Mirsadraee S, Wilcox HE, Korossis SA, Kearney JN, Watterson KG, Fisher J, et al. Development and characterization of an acellular human pericardial matrix for tissue engineering. Tissue Eng. 2006;12(4):763–773. doi: 10.1089/ten.2006.12.763. [DOI] [PubMed] [Google Scholar]
- 96.Grabow N, Schmohl K, Khosravi A, Philipp M, Scharfschwerdt M, Graf B, et al. Mechanical and structural properties of a novel hybrid heart valve scaffold for tissue engineering. Artif Organs. 2004;28(11):971–979. doi: 10.1111/j.1525-1594.2004.00007.x. [DOI] [PubMed] [Google Scholar]
- 97.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 98.Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat Commun. 2013;4:2307. doi: 10.1038/ncomms3307. [DOI] [PubMed] [Google Scholar]
- 99.Wang B, Borazjani A, Tahai M, Curry AL, Simionescu DT, Guan J, et al. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J Biomed Mater Res A. 2010;94(4):1100–1110. doi: 10.1002/jbm.a.32781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weymann A, Patil NP, Sabashnikov A, Jungebluth P, Korkmaz S, Li S, et al. Bioartificial heart: a human-sized porcine model – the way ahead. PLoS One. 2014;9(11):e111591. doi: 10.1371/journal.pone.0111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Radhakrishnan J, Krishnan UM, Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv. 2014;32(2):449–461. doi: 10.1016/j.biotechadv.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 102.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomater. 2011;7(1):1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 104.Reis LA, Chiu LL, Feric N, Fu L, Radisic M. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 106.Kuppan P, Sethuraman S, Krishnan UM. Tissue engineering interventions for esophageal disorders–promises and challenges. Biotechnol Adv. 2012;30(6):1481–1492. doi: 10.1016/j.biotechadv.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 107.Yamamoto M, Sakakibara Y, Nishimura K, Komeda M, Tabata Y. Improved therapeutic efficacy in cardiomyocyte transplantation for myocardial infarction with release system of basic fibroblast growth factor. Artif Organs. 2003;27(2):181–184. doi: 10.1046/j.1525-1594.2003.06993.x. [DOI] [PubMed] [Google Scholar]
- 108.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, et al. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30(29):5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59(8):751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang H, Shi J, Wang Y, Yin Y, Wang L, Liu J, et al. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials. 2014;35(13):3986–3998. doi: 10.1016/j.biomaterials.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 111.Panda NC, Zuckerman ST, Mesubi OO, Rosenbaum DS, Penn MS, Donahue JK, et al. Improved conduction and increased cell retention in healed MI using mesenchymal stem cells suspended in alginate hydrogel. J Interv Card Electrophysiol. 2014;41(2):117–127. doi: 10.1007/s10840-014-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen CH, Chang MY, Wang SS, Hsieh PC. Injection of autologous bone marrow cells in hyaluronan hydrogel improves cardiac performance after infarction in pigs. Am J Physiol Heart Circ Physiol. 2014;306(7):H1078–1086. doi: 10.1152/ajpheart.00801.2013. [DOI] [PubMed] [Google Scholar]
- 113.Chen J, Guo R, Zhou Q, Wang T. Injection of composite with bone marrow-derived mesenchymal stem cells and a novel synthetic hydrogel after myocardial infarction: a protective role in left ventricle function. Kaohsiung J Med Sci. 2014;30(4):173–180. doi: 10.1016/j.kjms.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang CC, Liao ZX, Chen DY, Hsiao CW, Chang Y, Sung HW. Injectable cell constructs fabricated via culture on a thermoresponsive methylcellulose hydrogel system for the treatment of ischemic diseases. Adv Healthc Mater. 2014;3(8):1133–1148. doi: 10.1002/adhm.201300605. [DOI] [PubMed] [Google Scholar]
- 115.Li J, Shu Y, Hao T, Wang Y, Qian Y, Duan C, et al. A chitosan-glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials. 2013;34(36):9071–9081. doi: 10.1016/j.biomaterials.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 116.Vandergriff AC, Hensley TM, Henry ET, Shen D, Anthony S, Zhang J, et al. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials. 2014;35(30):8528–8539. doi: 10.1016/j.biomaterials.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 117.Ye L, Zimmermann WH, Garry DJ, Zhang J. Patching the heart: cardiac repair from within and outside. Circ Res. 2013;113(7):922–932. doi: 10.1161/CIRCRESAHA.113.300216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J Biomed Mater Res B Appl Biomater. 2011;98(2):379–386. doi: 10.1002/jbm.b.31862. [DOI] [PubMed] [Google Scholar]
- 119.Annabi N, Tsang K, Mithieux SM, Nikkhah M, Ameri A, Khademhosseini A, et al. Highly Elastic Micropatterned Hydrogel for Engineering Functional Cardiac Tissue. Adv Funct Mater. 2013;23(39) doi: 10.1002/adfm.201300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Salick MR, Napiwocki BN, Sha J, Knight GT, Chindhy SA, Kamp TJ, et al. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials. 2014;35(15):4454–4464. doi: 10.1016/j.biomaterials.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kiefer K, Lee J, Haidar A, Martinez Miro M, Kaan Akkan C, Veith M, et al. Alignment of human cardiomyocytes on laser patterned biphasic core/shell nanowire assemblies. Nanotechnology. 2014;25(49):495101. doi: 10.1088/0957-4484/25/49/495101. [DOI] [PubMed] [Google Scholar]
- 122.Pijnappels DA, Schalij MJ, Ramkisoensing AA, van Tuyn J, de Vries AA, van der Laarse A, et al. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ Res. 2008;103(2):167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 123.Bian W, Badie N, Himel HDt, Bursac N. Robust T-tubulation and maturation of cardiomyocytes using tissue-engineered epicardial mimetics. Biomaterials. 2014;35(12):3819–3828. doi: 10.1016/j.biomaterials.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yasui H, Lee JK, Yoshida A, Yokoyama T, Nakanishi H, Miwa K, et al. Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials. 2014;35(27):7839–7850. doi: 10.1016/j.biomaterials.2014.05.080. [DOI] [PubMed] [Google Scholar]
- 125.Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013;34(36):9048–9055. doi: 10.1016/j.biomaterials.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mihic A, Li J, Miyagi Y, Gagliardi M, Li SH, Zu J, et al. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials. 2014;35(9):2798–2808. doi: 10.1016/j.biomaterials.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 127.Cannizzaro C, Tandon N, Figallo E, Park H, Gerecht S, Radisic M, et al. Practical aspects of cardiac tissue engineering with electrical stimulation. Methods Mol Med. 2007;140:291–307. doi: 10.1007/978-1-59745-443-8_16. [DOI] [PubMed] [Google Scholar]
- 128.Gomez JF, Cardona K, Martinez L, Saiz J, Trenor B. Electrophysiological and structural remodeling in heart failure modulate arrhythmogenesis. 2D simulation study. PLoS One. 2014;9(7):e103273. doi: 10.1371/journal.pone.0103273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Bornchen C, Müller C, et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J Mol Cell Cardiol. 2014;74:151–161. doi: 10.1016/j.yjmcc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 130.Hollweck T, Akra B, Haussler S, Uberfuhr P, Schmitz C, Pfeifer S, et al. A novel pulsatile bioreactor for mechanical stimulation of tissue engineered cardiac constructs. J Funct Biomater. 2011;2(3):107–118. doi: 10.3390/jfb2030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang BJ, Kim H, Lee SK, Kim J, Shen Y, Jung S, et al. Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 2014;10(7):3007–3017. doi: 10.1016/j.actbio.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 132.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12(4):459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 133.Cohen JE, Purcell BP, MacArthur JW, Jr, Mu A, Shudo Y, Patel JB, et al. A bioengineered hydrogel system enables targeted and sustained intramyocardial delivery of neuregulin, activating the cardiomyocyte cell cycle and enhancing ventricular function in a murine model of ischemic cardiomyopathy. Circ Heart Fail. 2014;7(4):619–626. doi: 10.1161/CIRCHEARTFAILURE.113.001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Riegler J, Gillich A, Shen Q, Gold JD, Wu JC. Cardiac tissue slice transplantation as a model to assess tissue-engineered graft thickness, survival, and function. Circulation. 2014;130:S77–86. doi: 10.1161/CIRCULATIONAHA.113.007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106(39):16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 137.Chiu LL, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31(2):226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 138.Mayfield AE, Tilokee EL, Latham N, McNeill B, Lam BK, Ruel M, et al. The effect of encapsulation of cardiac stem cells within matrix-enriched hydrogel capsules on cell survival, post-ischemic cell retention and cardiac function. Biomaterials. 2014;35(1):133–142. doi: 10.1016/j.biomaterials.2013.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, et al. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012;8(12):4357–4364. doi: 10.1016/j.actbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Z, Guo X, Guan J. An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials. 2012;33(25):5914–5923. doi: 10.1016/j.biomaterials.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 141.Chimenti I, Rizzitelli G, Gaetani R, Angelini F, Ionta V, Forte E, et al. Human cardiosphere-seeded gelatin and collagen scaffolds as cardiogenic engineered bioconstructs. Biomaterials. 2011;32(35):9271–9281. doi: 10.1016/j.biomaterials.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 142.Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33(6):1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]


