Summary
The outcome of chronic viral infections, which affect millions of people worldwide, are greatly dependent on CD4+ T cells. Here we showed that T cell specific ablation of the common interleukin-6 (IL-6) family receptor, gp130, profoundly compromised virus-specific CD4+ T cell survival, T follicular helper responses and IL-21 production at late stages of a chronic murine viral infection. These effects were cell-intrinsic for CD4+ T cells and were accompanied by a reduction of CD8+ T cells, antibodies and a severe failure in viral control. We identified IL-27 as a gp130 cytokine that promoted antiviral CD4+ T cell survival in vivo and that rapidly induced IL-21 ex vivo. Furthermore, IL27R was critical for control of lymphocytic choriomeningitis virus (LCMV) in vivo. These results reveal that gp130 cytokines (particularly IL-27) are key regulators of CD4+ T cell responses during an established chronic viral infection, empowering both humoral and cytotoxic immunity.
Keywords: LCMV - chronic infection, CD4 T cell survival- IL-21, T follicular helper cells, gp130 - IL-6 cytokine family, IL-27, WSX-1
Introduction
Chronic viral infections such as human immunodeficiency virus-type 1 (HIV-1), hepatitis C virus (HCV) and hepatitis B virus (HBV) affect hundreds of millions of people worldwide (Virgin et al., 2009). Viral persistence is associated with decreased numbers of both virus specific CD4+ and CD8+ T cells along with a progressive loss of their ability to kill infected targets and produce important effector molecules such as interferon-γ (IFN-γ), tumor necrosis factor–α (TNF-α) and interleukin-2 (IL-2) (Letvin and Walker, 2003; Rehermann and Nascimbeni, 2005). Indeed, upregulation of multiple inhibitory surface molecules including PD-1, LAG-3 & Tim3 (Barber et al., 2006; Blackburn et al., 2009; Wherry et al., 2007), along with interleukin-10 (IL-10) (Brooks et al., 2006; Ejrnaes et al., 2006), during chronic infection contributes to the hierarchical loss of function seen in virus specific T cells. In addition, transforming growth factor-β (TGF-β) signaling limits the numbers of virus specific T cells (Boettler et al., 2012; Garidou et al., 2012; Tinoco et al., 2009). These features appear common among actively replicating chronic viruses in humans and other animals (Clerici et al., 1994; Cumont et al., 2007; Day et al., 2006; Le Clerc et al., 2009; Rigopoulou et al., 2005; Urbani et al., 2006) and this highly immunosuppressive environment prevents viral eradication and makes the host extremely susceptible to secondary infections and cancers.
Individuals control persistent viruses to differing extents, ranging from elite controllers of HIV-1 and acutely resolving HCV patients, to fast HIV and HCV progressors (Letvin and Walker, 2003; Rehermann and Nascimbeni, 2005). This varied degree of viral control associates with different quality and quantity of CD4+ and CD8+ T cell responses (Virgin et al., 2009). In particular, CD4+ T cells are central for several aspects of anti-viral responses. The outcome of HIV infection depends critically on the rate and the extent of virus-specific CD4+ T cell expansion with decreased CD4+ T cell numbers tightly correlating with progression into AIDS (Porichis and Kaufmann, 2011; Virgin and Walker, 2010). Similarly, spontaneous recovery from HCV and HBV infection associates with vigorous CD4+ and CD8+ T-cell responses while CD4+ T cell depleted chimpanzees fail to clear HBV or HCV and develop severe liver disease (Rehermann and Nascimbeni, 2005). CD4+ T cells are also essential to control the persistent variant of lymphocytic choriomeningitis virus, LCMV Clone 13 (Cl13), in a mouse model of chronic viral infection (Battegay et al., 1994; Matloubian et al., 1994a). Recently this model has been used to uncover CD4+ derived IL-21 as vital for CD8+ (but not CD4+) T cell maintenance during late chronic infection (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2009). IL-21 has also been found to positively correlate with lower viral loads in HIV and HCV infected patients (Chevalier et al., 2011; Iannello et al., 2010; Yue et al., 2010). In addition LCMV Cl13 infection results in the enhanced accumulation of virus specific T follicular helper (Tfh) cells (Fahey et al., 2011; Harker et al., 2011), a process subsequently observed during HBV, HCV, simian immunodeficiency virus (SIV) and HIV infection (Feng et al., 2012; Feng et al., 2011; Lindqvist et al., 2012; Petrovas et al., 2012). This is dependent on IL-6 signaling on CD4+ T cells during the later stages of chronic infection and results in enhanced virus specific antibody responses and viral control in LCMV (Harker et al., 2011; Petrovas et al., 2012).
IL-6 is the archetypal member of the IL-6 cytokine family which includes IL-11, IL 27, Leukaemia inhibitory factor (LIF), cardiotrophin-1, ciliary neurotrophic factor and oncostatin M (OSM) (Silver and Hunter, 2010). The members of the IL-6 family can exert both unique and overlapping functions through their common use of the constitutively expressed trans-membrane receptor gp130, which dimerizes with cytokine specific receptors on cytokine binding. Given this conserved signaling pathway, we speculated that along with IL-6, other members of the IL-6 family of cytokines may have potent effects on T cell function during chronic infection. Supporting this, IL-6 deficiency does not affect in vivo IL-21 during chronic infection, however ex vivo IL-6 stimulation of CD4+ T cells from LCMV Cl 13 infected mice causes rapid expression of Il21 (Harker et al., 2011) suggesting the presence of functional redundancies for IL-21 induction.
To bypass such functional redundancies we examined the effect of genetic ablation of Il6st (which encodes gp130) in T cells during persistent LCMV Cl 13 infection in mice. In the absence of gp130 on T cells mice were incapable of controlling infection, had a profound reduction in the numbers of virus-specific CD8+ and CD4+ T cells and compromised antibody responses. In contrast to CD8+ T cells, which appeared functionally unaltered by gp130 deficiency, Il6st−/− CD4+ T cells also had greatly reduced IL-21 production. While the CD8+ T cell defects were in most part extrinsic, direct gp130 signaling on CD4+ T cells was essential for their survival and function at late stages of chronic LCMV infection. We identified IL-27 as the gp130 signaling cytokine promoting CD4+ T cell survival and that (similarly to IL-6) was capable of rapidly inducing IL-21 ex-vivo but was redundant for its in vivo production. Our data indicate that gp130 signaling cytokines play a vital role during late stages of chronic viral infection including regulation of CD4+ T cell survival and IL-21 production to orchestrate antiviral responses.
Results
Gp130 signaling on T cells was essential for control of chronic viral infection
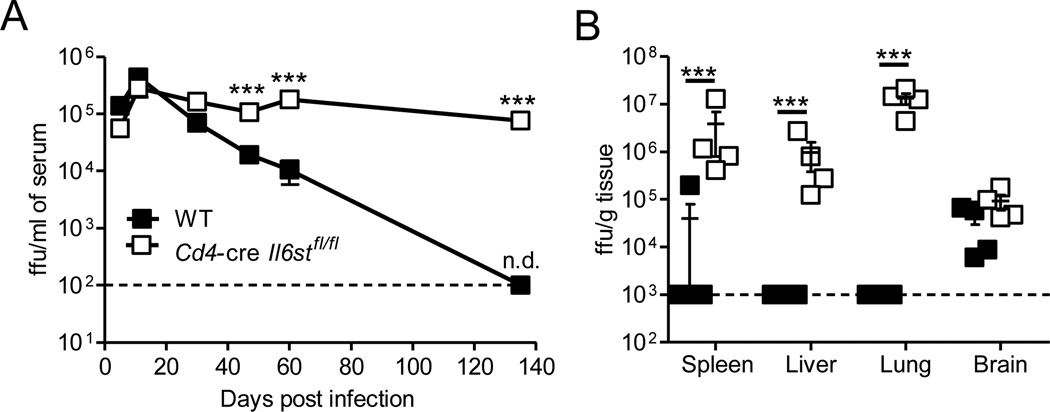
To investigate the role of T cell specific gp130 signaling on control of a chronic viral infection we infected Cd4-cre Il6stfl/fl (where Il6st is deleted in CD4+ and CD8+ T cells) or wildtype (WT) mice with LCMV Cl13. Loss of gp130 signaling did not adversely affect the proportion of regulatory T (Treg) cells, CD4+ or CD8+ T cells, or their capacity to produce TNF-α or IFN-γ, in the spleen prior to infection (Figure S1). Initial and peak viremia were identical, however mice lacking T cell gp130 showed a complete failure to control viremia while WT mice had significantly reduced viral loads from day 45 post infection (p.i.) onward (Figure 1A). By day 130 p.i. virus was readily detectable across multiple tissues in Il6st−/− mice while it was only detected in the brain of WT animals (Figure 1B). Gp130 deficiency did not affect the clearance of acute LCMV Armstrong infection (data not shown). In contrast to the decline in viremia we previously observed in WT and Il6−/− mice between days 30 and 45 p.i., mice deficient in T cell gp130 showed little or no decline in viremia between these two timepoints (Figure 1A and (Harker et al., 2011)). These results demonstrated that gp130 signaling on T cells played a critical role in controlling chronic (but not acute) LCMV infection in vivo. In addition, the fact that viremia was higher in mice lacking gp130 compared with Il6−/− mice suggested that gp130 signaling cytokines (other than IL-6) significantly contributed to viral containment during chronic LCMV infection.
Figure 1. IL-6 independent gp130 signaling is crucial for control of chronic viral infection.
Wildtype (C57B/6 or Il6stfl/fl) and Cd4-cre Il6stfl/fl mice were infected with 2 × 106 pfu of LCMV Cl13 i.v. (A–B) Viral load was monitored in the serum throughout infection (A) and the indicated tissues at day 135 p.i. by immunofocus assay (B). Data is representative of 2 experimental repeats n ≥ 4 mice per group with mean ± S.E.M. depicted. This figure is supported by supplementary figure 1.
T cell gp130 signaling promotes CD8+ and CD4+ T cell numbers at late stages of chronic infection
IL-6 deficiency does not affect the total numbers of virus specific CD8+ or CD4+ T cells throughout chronic LCMV infection (Harker et al., 2011). In the next series of experiments we aimed to identify the immune defects that resulted in the more severe failure of Cd4-cre Il6stfl/fl mice compared to Il6−/− mice to control LCMV Cl13. For that, we first monitored the expansion of virus specific CD8+ and CD4+ T cells after infection.
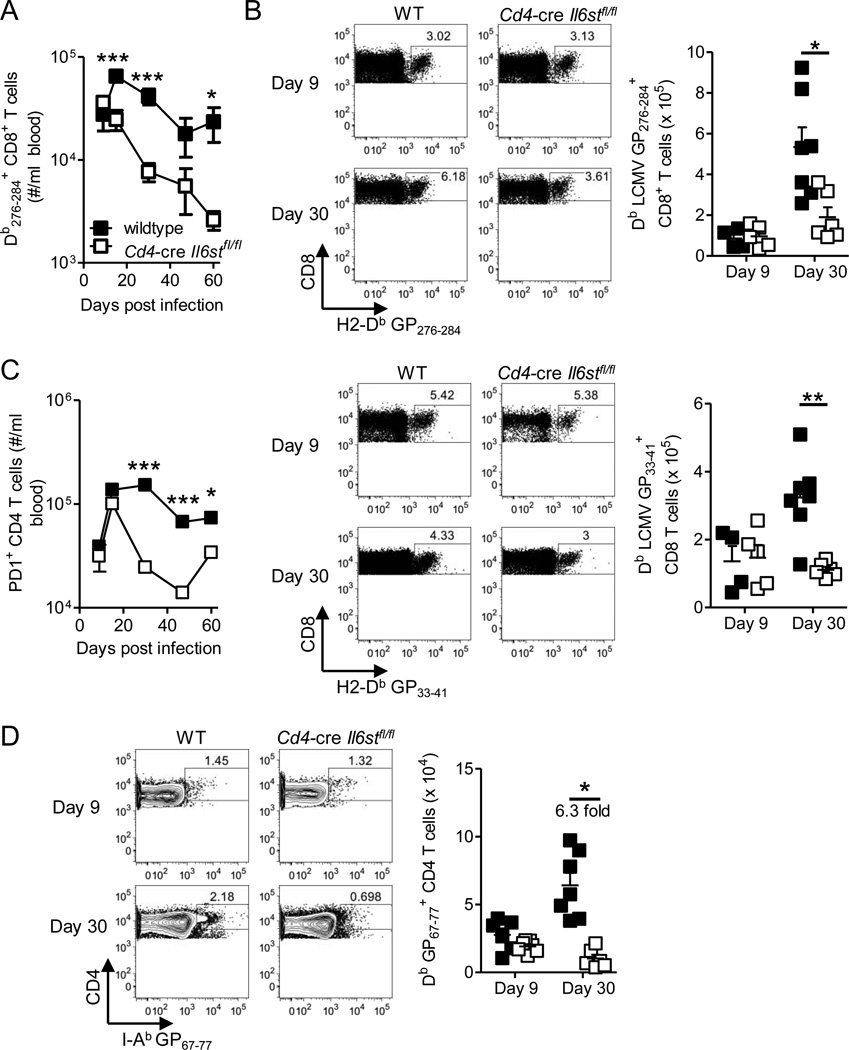
We observed similar numbers of H2-Db LCMV GP276–284 and H2-Db LCMV GP33–41 specific CD8+ T cells in blood and spleens of WT and Cd4-cre Il6stfl/fl animals at day 9 p.i. (Figure 2A and B). By day 15 p.i., however, there were significantly fewer virus specific CD8+ T cells in the blood of Cd4-cre Il6stfl/fl animals, a trend that continued until day 60 p.i., the last time point analyzed (Figure 2A). These findings were confirmed in the spleen where Cd4-cre Il6stfl/fl mice had significantly fewer H2-Db LCMV GP33–41 and GP276–284 specific CD8+ T cells compared to infection matched controls at day 30 (but not day 9) p.i. (Figure 2B).
Figure 2. T cell specific gp130 signaling is required for accumulation of virus specific T cell responses and viral control during chronic infection.
Wildtype (C57B/6 or Il6stfl/fl) and Cd4-cre Il6stfl/fl mice were infected with 2 × 106 pfu of LCMV Cl13 i.v. (A) PBMCs were analyzed to determine the number of GP276–284 CD8+ T cells. (B) At days 9 and 30 p.i. splenocytes were analyzed by flow cytometry to determine the number of H2-Db GP276–284 and GP33–41 CD8+ T cells. (C) As in (A) PBMC were analyzed to determine the number of PD-1+ CD4+ T cells. (D) As in (B) I-Ab GP67–77 + CD4+ T cells numbers were determined in the spleen, the fold increase from CD4-cre Il6fl/fl to WT cells at day 30 p.i. is indicated. Data is representative of 3 experimental repeats n ≥ 4 mice per group with mean ± S.E.M. depicted. This figure is supported by supplementary figure 2.
“Antigen experienced” PD-1+ CD4+ T cells in the blood also showed normal development in Cd4-cre Il6stfl/fl mice on days 9 and 15 p.i., but a significantly reduced number was seen from day 30 onward compared to WT mice (Figure 2C). The number of H2-Ab LCMV GP67–77 specific CD4+ T cells was also dramatically reduced in the absence of gp130 signaling, with a profound reduction of virus specific cells observable at day 30 p.i. (16% of WT numbers), but unaltered numbers at day 9 p.i. (Figure 2D). Combined these results show that gp130 cytokines, while not required for priming and initial expansion of virus specific T cell responses, are essential for their accumulation at late stages of chronic LCMV infection. Given that neither CD4+ nor CD8+ T cell numbers are affected by the absence of IL-6 alone (Harker et al., 2011), the aforementioned results highlight a critical role for IL-6 independent gp130 signaling in the maintenance of virus specific CD8+ and CD4+ T cells at later stages of chronic infection.
Gp130 deletion in T cells alters CD4+ T cell functions at late stages of chronic infection
We next investigated the consequences of ablated gp130 signaling on the function of CD8+ and CD4+ T cells. Chronic infection not only leads to deletion of virus specific CD8+ T cells but also a hierarchical (IL-2>TNF-α>IFN-γ) loss of function of those cells that remain (Wherry et al., 2003). The loss of gp130 signaling on T cells did not, however, alter the level of functional exhaustion in virus specific CD8+ T cells (Figure S2).
During chronic LCMV infection there is a progressive increase in the proportion of virus specific CD4+ Tfh cells (Fahey et al., 2011; Harker et al., 2011). In this context, IL-6 signaling is required for maximal up-regulation of Bcl6 and Tfh cell numbers, but is not required for these cells to produce IL-21 (Harker et al., 2011), a cytokine vital to the maintenance of virus specific CD8+ T cells (Elsaesser et al., 2009; Frohlich et al., 2009; Yi et al., 2009). As expected, loss of gp130 signaling resulted in significant loss of Tfh cell differentiation and Bcl6 expression in both virus-specific and total CD4+ T cells at day 30 p.i., although the expression of Bcl6 within the remaining CXCR5+ virus specific CD4+ T cells was not significantly affected (Figure S3A–C). In contrast to the reduced number of Tfh cells, the proportion of FoxP3+ T regulatory cells (Tregs) was increased at day 30, but not day 9, p.i. in the absence of gp130 signaling (Figure S3D). However the total number of Treg cells was unaltered in gp130 deficient animals at both time points studied. Stimulation of CD4+ T cells from both WT and Cd4-cre Il6stfl/fl mice at day 9 p.i. with LCMV GP67–77 peptide resulted in moderate IFN-γ production and low IL- 21 secretion as previously reported (Fig. 3, A and (Elsaesser et al., 2009)). At day 30 p.i., we observed fewer peptide responsive IFN-γ+ CD4+ T cells, consistent with the reduced number Db GP67–77 + CD4+ T cells in Cd4-cre Il6stfl/fl mice. Cd4-cre Il6stfl/fl IFN-γ+ CD4+ T cells also produced significantly less IL-21 than WT CD4+ T cells after GP67–77 ex-vivo peptide stimulation. Similar observations were seen in response polyclonal PMA and Ionomycin stimulation (Figure 3B). Consistently, Il21 transcripts were significantly reduced in purified Db GP67–77 + CD4+ T cells from CD Cd4-cre Il6stfl/fl mice compared to WT controls (Figure 3C). These results indicated that gp130 signaling is not only essential for CD4+ T cell maintenance and differentiation, but it is also necessary for them to produce IL-21 at late stages of chronic LCMV infection. The defective IL-21 production in the absence of T cell gp130 signaling contrasted with unchanged amounts observed in Il6−/− mice (Harker et al., 2011). This implied the presence of gp130 cytokines (other than IL-6) that regulated this fundamental CD4+ T cell function during an established chronic viral infection.
Figure 3. T cell specific gp130 signaling is required for IL-21 production during chronic LCMV infection.
Wildtype (C57B/6 or Il6stfl/fl) and Cd4-cre Il6stfl/fl mice were infected with 2 × 106 pfu of LCMV Cl13 i.v. At days 9 and 30 p.i. splenocytes were stimulated with (A) LCMV GP67–77 peptide or (B) PMA and ionomycin and the number of IFN-γ secreting cells (GP67–77 specific only), and the proportion of IFN-γ+ cells that were also secreting IL-21, were determined by flow cytometry. (C) At day 30 p.i. CD4+ and I-Ab LCMV GP67–77 + cells were isolated by flow cytomtery and Il21 expression, relative to Gapdh, was determined by qPCR. n.d = not detectable, data is representative of 3 experimental repeats n ≥ 4 mice per group with mean ± S.E.M. depicted. This figure is supported by supplementary figure 3.
Consistent with defective CD4+ T cell accumulation and function we observed dramatic effects on humoral immunity in Cd4-cre Il6stfl/fl compared to WT mice. This included lower numbers of germinal center B cells, reduced concentrations of LCMV specific Ig, IgG, IgG1 and IgG2a antibodies and weakened avidity of LCMV specific IgG (Figure S4). These results further demonstrate the need for gp130 signaling in CD4 T cells for optimal antibody responses.
Cell-intrinsic gp130 is essential for virus specific CD4+ T cells numbers and IL-21 production
The loss of T cell specific gp130 signaling, while not affecting T cell development or the initial expansion of either virus specific CD4+ or CD8+ T cell responses during LCMV infection, had significant impact on the number of virus specific T cells at later stages of infection (day 30 p.i.) and prevented viral control. To determine the role of intrinsic gp130 signaling on either CD8+ or CD4+ T cells we generated mixed WT:Cd4-cre Il6stfl/fl bone marrow (BM) chimeric mice. Injection of an equal ratio of WT and Cd4-cre Il6stfl/fl BM cells favored development of WT T cells, with an approximately 70:30 (WT:Il6st−/−) ratio of CD8+ and CD4+ T cells at 8 weeks post reconstitution (Figure S5A). The bias to WT cells seen was similar to that described in mixed chimeras reconstituted with equal proportions of WT and Il6ra−/− deficient BM cells (Harker et al., 2011) suggesting that IL-6 played a role in the development of T cells under competitive conditions.
At day 30 post LCMV Cl13 infection the numbers of H2-Db LCMV GP33–41 CD8+ T cells were reduced in the gp130 deficient compartment versus WT in some, but not all, mixed chimeras, reaching a p value equal to 0.0649 (Figure 4A). In contrast, as in Cd4-cre Il6stfl/fl mice, the number of Db GP67–77 + CD4+ T cells were significantly and consistently reduced in the Il6st−/− compared to WT cells, and the total number of gp130 deficient virus specific cells was only 3.5% of WT cells in this setting (Figure 4B). Tfh cell differentiation in the remaining virus specific CD4+ T cells, and total CD4+ T cells, was also dramatically reduced (Figure S5B). Consistently, there was low or undetectable expression of Bcl6 in virus specific CD4+ T cells in the absence of gp130, while Prdm1 (Blimp-1) expression was lower but detectable (Figure S5C).
Figure 4. CD4+ intrinsic gp130 signaling is required for CD4+ T cell numbers, accumulation and IL-21 production.
Congenic wildtype (CD45.1) mice were lethally irradiated and reconstituted with a 50:50 mix of CD45.1 and Cd4-cre Il6stfl/fl (CD45.2) bone marrow cells. 8 weeks later chimeras were infected with 2 × 106 pfu of LCMV Cl13. At day 30 p.i. the percentages of splenic CD8+ T cells that were H2-Db GP33–41 + (A) and the % and number of splenic CD4+ T cells that were I-Ab GP67–77 + (B) in CD45.1+ or CD45.2+ compartments was determined by flow cytometry, the fold increase between WT and Il6st−/− is depicted. (C) Splenocytes were stimulated ex vivo with GP67–77 peptide and the proportion of IFN-γ producing CD4+ T cells that were also producing IL-21 was determined by flow cytometry and (D) Il21 expression determined in I-Ab GP67–77 + CD4+ T cells by qPCR. N.d. = not detectable. Data are representative of 4 independent repeats from pooled samples of n ≥ 4 mice, with mean ± S.E.M. depicted. This figure is supported by supplementary figure 4.
Deletion of T cell specific Il6st during intestinal nematode infection, in otherwise highly susceptible IL-10 deficient animals, resulted in enhanced Th2 CD4+ T cell, and reduced Th1 and Th17 CD4+ T cell, mediated immunity and accelerated parasite expulsion and protection (Fasnacht et al., 2009). In this study we saw slight reductions in the expression of both Tbx21 (T-bet) (Th1) and Gata3 (Th2) in virus specific CD4+ T cells in the absence of Il6st, but no change in the balance of the immune response (Figure S5E–F) while we saw little to no Rorc expression indicating few or no Th17 cells were present (data not shown). This difference is most likely due to the different infectious systems used, as this is the first report of the overall role of T cell specific gp130 signaling during a viral infection.
The proportion of IFN-γ producing cells that were also producing IL-21 was significantly reduced in the absence of gp130, indicating that cell intrinsic gp130 signaling is required for IL-21 production after ex vivo peptide stimulation (Figure 4C). Accordingly amounts of Il21 transcript measured directly ex vivo were undetectable in Il6st−/− I-Ab GP67–77 CD4+ T cells from mixed chimeras, while they were readily detectable in their WT counterparts (Figure 4D). These results indicate that while CD8+ T cells might be modestly influenced by intrinsic gp130 signaling, CD4+ T cells are greatly dependent on direct gp130 signaling to both accumulate and acquire key helper properties during late chronic LCMV infection.
Direct gp130 signaling promotes CD4+ T cell survival during chronic viral infection
To discern whether the reduced numbers of virus specific CD4+ T cells resulted from increased cell death, reduced proliferation or both, we analyzed apoptosis and cell cycle entry in WT versus Cd4-cre Il6stfl/fl mice 30 days post LCMV Cl13 infection. Annexin V staining revealed that a higher proportion of virus specific CD4+ T cells were undergoing apoptosis in Cd4-cre Il6stfl/fl compared to WT mice; and this difference was further accentuated in the competitive environment generated in WT:Cd4-cre Il6stfl/fl mixed chimeras (Figure 5A and 5B). These results were validated by the presence of increased cleaved caspase-3 in Il6st−/− PD1+ CD4+ T cells compared to WT cells, another indicator of increased cell-intrinsic apoptosis (Figure 5C).
Figure 5. Virus specific CD4 T cells exhibits an intrinsic survival defect in the absence of gp130 signaling.
WT, Cd4-cre Il6stfl/fl (A&D) or WT:Cd4-cre Il6stfl/fl mixed chimeric (B,C,E&F) mice were infected with 2 × 106 pfu of LCMV Cl13 i.v. and splenocytes analyzed by flow cytometry at day 30 p.i. A&B) The % Annexin V binding in I-Ab GP67–77 + CD4+ T cells was determined. C) The % of PD1+ CD4+ T cells expressing cleaved Caspase-3+ was determined. D&E) The proportion of I-Ab GP67–77 + CD4+ T cells that had incorporated BrdU incorporation was quantified. F) The proportion of Ki67+ was analyzed within PD1+ CD4+ T cells. Data are representative of 2 or 3 independent experiments, of n ≤ 4 mice per group, with mean ± S.E.M. depicted. This figure is supported by supplementary figure 5.
In contrast, analysis of BrdU incorporation revealed that the proliferation of virus specific CD4 T cells was enhanced (likely due to a compensatory effect) in Cd4-cre Il6stfl/fl mice, but similar in WT and Il6st−/− cells in the mixed chimeric setting (Figure 5D and E). There was a very low frequency of BrdU+ virus specific CD4+ T cells at day 30 p.i. in the mixed chimera, however in the same system WT PD1+ CD4+ T cells and Il6st−/− PD1+ CD4+ T cells showed detectable, and similar proportions of, Ki67+ cells supporting the absence of an intrinsic proliferation defect when gp130 signaling is not present (Figure 5F). Overall these data indicate that cell-intrinsic gp130 signaling was fundamental in promoting CD4+ T cell survival at late stages of chronic LCMV infection.
IL-27 drives accumulation of virus specific CD4+ T cells, and promotes IL-21 production ex vivo
To determine which of the cytokines that use gp130 was involved in promoting CD4+ T cell survival and IL-21 production we examined their individual capacity to phosphorylate the downstream molecules STAT-1 and STAT-3. In CD4+ T cells taken from naïve animals IL-6, IL-27, OSM and LIF were all capable of STAT-3 phosphorylation, while IL-6 and IL-27 also caused phosphorylation of STAT-1 (Figure 6A). As we have shown previously ex vivo IL-6 stimulation of transgenic LCMV GP61–80 specific CD4+ T (Smarta) cells taken from WT mice 18 days post LCMV Cl13 infection also caused rapid phosphorylation of STAT-3 (Figure 6A and (Harker et al., 2011)). However IL-6 stimulation of Smarta cells did not promote STAT-1 phosphorylation. In comparison IL-27 stimulation of the same Smarta cells caused slightly weaker phosphorylation of STAT-3, but pronounced phosphorylation of STAT-1. Stimulation with LIF, IL-11 and OSM phosphorylated neither STAT-1 nor STAT-3.
Figure 6. IL-27 signaling on virus specific CD4+ T cells is vital for survival during chronic viral infection.
A) LCMV specific CD45.1+ transgenic CD4+ T cells (Smarta) were transferred i.v. 1 day prior to LCMV Cl13 infection and splenocytes isolated at 18 days p.i.. The amount of pSTAT1 and pSTAT3 were then determined in naïve and Smarta CD4+ T cells 30 minutes after ex vivo stimulation with 50 ng/ml of the indicated recombinant cytokines by flow cytometry. B&C) WT:Il27ra−/− mixed bone marrow chimeras were generated and infected with LCMV Cl13, 30 days p.i. the proportion of splenic CD4+ T cells that were I-Ab Gp67–77 + (B) and CD4+ T cells that were IFN-γ+ IL-21+ after GP67–77 peptide stimulation (C) were determined by flow cytometry. D&E) At day 18 post LCMV Cl13 infection PD1+ CD4+ T cells (D) or Smarta cells (E) prepared as in (A) were isolated by flow cytometry. Expression of Il21 relative to Gapdh was determined 6, 12 and 24 hours (D) or 6 hours (E) after ex vivo stimulation with IL-6, IL-27 or PBS. A is representative of 2 independent experiments with pools of 5 mice per group. B, C and D–E are representative of 5, 3 and 2 independent experiments, respectively with mean ± S.E.M. depicted. This figure is supported by supplementary figure 6.
As IL-27 was the only tested member of the IL-6 family of cytokines, other than IL-6, capable of directly signaling on virus specific CD4+ T cells during LCMV Cl13 infection we evaluated its cell-intrinsic effects on CD4+ T cells in vivo. In WT:Il27ra−/− mixed bone marrow chimeras, unlike Cd4-cre Il6stfl/f and Il6ra−/− mixed bone marrow chimeras, the Il27ra−/− CD4+ T cell compartment was similar in proportion to the WT CD4+ T cell compartment after reconstitution (Figure S6A). There was, however, a slight bias towards WT CD8 T cells and Il27ra−/− B cells during reconstitution, which could indicate a role for IL-27 during lymphocyte development (Figure S6A). After LCMV Cl13 infection direct IL-27 signaling on CD4+ T cells was vital for the accumulation of virus specific CD4+ T cells, indicated by the reduced proportion of I-Db GP67–77 + CD4+ T cells in the Il27ra−/− compared to the WT compartment, as was seen in Cd4-cre Il6stfl/fl mice (Figure 6B). However, IL-21 production in the remaining Il27ra−/− virus specific CD4+ T cells was similar to that seen in WT CD4+ T cells (Figure 6C) as was the proportion of virus-specific CD4 T cells that expressed Bcl6 and differentiated into Tfh cells (Figure S6B and C).
Stimulation of polyclonal virus specific PD-1+ CD4+ T cells taken 18 days post LCMV Cl13 infection with recombinant IL-27 resulted in rapid upregulation of Il21, with the amount of transcript peaking at 6 hours post stimulation, while as we have previously shown IL-6 also caused the upregulation of Il21 transcript, but this peaked at 12 hours post stimulation (Figure 6D). Increased Il21 transcript was also seen 6 hours post IL-27 stimulation in Smarta CD4+ T cells (Figure 6E). Ex vivo IL-27 stimulation also lead to upregulation of Bcl6 expression in PD-1+ and Smarta CD4+ T cells (Figure S6D and E). Together these data show that IL-27 plays an essential role in the accumulation of virus specific CD4+ T cells at late stages during chronic viral infections. Furthermore, both IL-27 and IL-6 were capable of rapidly up-regulating IL-21 in virus specific CD4+ T cells, but were redundant for its production in vivo, where IL-21 production could only be reduced by removal of IL-6 and IL-27 common co-receptor, gp130.
We next quantified the number of virus-specific CD4+ T cells in the spleens of WT versus Il27ra−/− non-chimeric mice at days 9 and 30 post infection (Figure 7A). We observed higher percentage and numbers of virus-specific CD4+ T cells in Il27ra−/− compared to WT mice at day 9 p.i.. Since this early increase in the CD4+ T cell responses was not observed in Cd4-cre Il6stfl/fl mice (Figure 2D) it may result from CD4+ T cell-extrinsic effects due to lack of IL-27R signaling in other cell populations. Il27ra−/− mice showed a significant decline in the proportion and numbers of LCMV specific CD4+ T cells from day 9 to day 30 p.i. while in WT mice the number of LCMV specific CD4+ T cells was maintained or slightly increased. This defect in anti-viral CD4+ T cells maintenance in Il27ra−/− mice was comparable with the one observed in Cd4-cre Il6stfl/fl mice and was also detected in blood samples, where the number of PD1+CD4+ T cells were significantly lower at day 45 p.i (Figure 7B and 7C, respectively). These results were consistent with our conclusions from Cd4-cre Il6stfl/fl mice, WT:Cd4-cre Il6stfl/fl and WT:Il27ra−/− mixed chimeras that cell-intrinsic IL-27R and gp130 signaling promotes the maintenance of virus-specific CD4+ T cells at late stages of chronic viral infection.
Figure 7. IL-27 is critical in controlling viral load at both early and late stages of infection.
WT or Il27ra−/− animals were infected with LCMV Cl13. (A & B) Splenic I-Ab GP67–77+ CD4+ T cells at days 9 and 30 p.i. were enumerated by flow cytometry (A). Example flow plots are shown and the proportion of Db GP67–77 + CD4+ T cells at day 30 p.i. compared to day 9 p.i. was compared to that observed in Cd4-cre Il6stfl/fl animals (B). (C) PD-1+ CD4+ T cells were enumerated in the blood by flow cytometry. (D & E) Viral load was determined in the blood (D) at various timepoints and in tissues (E) at day 130 post infection. Data is representative of n = 2 experimental repeats of n = 5 mice per group, with mean ± S.E.M. depicted.
Finally, we assessed the importance of IL-27R signaling in overall viral control. Il27ra−/− mice exhibited significantly higher viremia than their WT counterparts as early as between days 5 and 9 p.i. (Figure 7D). Given that deletion of Il6st exclusively in T cells did not affect early control of LCMV Cl13 (Figure 1A), it is likely that this initial difference in viral loads is a consequence of deficient IL-27 signaling in non-T cells. Infection of Il27ra−/− mice also resulted in a failure of the long term control of persistent LCMV with virus detectable in the blood and a range of tissues up to 130 days post infection, when the majority of WT animals had cleared virus (Figure 7D and 7E). Altogether, these data indicate that IL-27 signaling plays a critical role in the maintenance of CD4+ T cells and viral control during chronic viral infection in vivo.
Discussion
The presence and function of antigen specific CD4+ T cells is vital for optimal immune responses in many infectious contexts, but especially during chronic viral infections. Here we showed that direct gp130 signaling was vital for the survival of virus specific CD4+ T cells and for their key functional properties such as IL-21 production during chronic viral infection in vivo. Further we demonstrated that different members of the IL-6 family have both unique and redundant roles in this process; IL-27 was essential for CD4+ T cell survival, while we have previously shown that IL-6 plays a dominant role in the differentiation of Tfh cells. On the other hand while both IL-6 and IL-27 were capable of promoting IL-21 production ex vivo they were redundant in vivo, suggesting multiple gp130 signaling cytokines secure optimal IL-21 production.
Higher concentrations of IL-21 are associated with lower viral loads in HIV and HCV infections (Chevalier et al., 2011; Iannello et al., 2010; Yue et al., 2010), and with an increased success rate in vaccination of chronically infected individuals (Pallikkuth et al., 2011). STAT-3 is heavily associated with the transcription of Il21 (Kaplan et al., 2011). Engagement of gp130 with cytokine specific receptors results in rapid phosphorylation of STAT-3 (Ernst and Jenkins, 2004) and our study revealed a vital role for gp130 signaling in IL-21 production in vivo at late stages of chronic LCMV infection. STAT-3 binds not only at the ll21 promoter but also at Batf and Maf loci (Durant et al., 2010), two other transcription factors known to promote IL-21 (Hiramatsu et al., 2010; Schraml et al., 2009), suggesting multiple molecular pathways through which gp130 signaling might trigger, and maintain IL-21 production. Once produced IL-21 itself could act in an autocrine fashion by further phosphorylating STAT-3. Both IL-6 and IL-27, were capable of inducing STAT-3 phosphorylation and Il21 transcription in virus specific CD4+ T cells taken from chronically infected mice but none of the other gp130 cytokines tested (i.e. IL-11, OSM and LIF) were capable of phosphorylating STAT-3 in LCMV specific CD4+ T cells. Given the essential nature of gp130 but non-essential roles for IL-6R or IL-27R our data strongly suggests that IL-6 and IL-27 act redundantly to promote IL-21 production in CD4+ T cells. While our study focused on chronic viral infection, it is conceivable that gp130 signaling may trigger IL-21 production in the context of autoimmune diseases such as diabetes, multiple sclerosis and Sjogren’s synodrome where IL-21 is associated with increased pathology (Jones et al., 2009; McGuire et al., 2011; Spolski et al., 2008).
IL-27-gp130 signaling promoted the survival of virus specific CD4+ T cells at late stages of chronic LCMV infection. However it was dispensable for CD4+ T cell accumulation early after infection, indicating that other signaling pathways must be involved in inducing CD4+ T cell proliferation and survival at these timepoints. Likely candidates are the common γc cytokines such as IL-2, IL-7 and IL-15, which are potent mediators of both naïve and activated T cell survival and proliferation (Rochman et al., 2009). Indeed provision of either IL-2 or IL-7 is sufficient to rescue mice from LCMV Cl13 infection (Blattman et al., 2003; Pellegrini et al., 2011). Many of their effects are mediated by activation of STAT-5, which also blocks the differentiation of Tfh cells (Johnston et al., 2012; Nurieva et al., 2012). Tfh cells only accumulate in LCMV Cl13 infected mice during late stages of infection, at a time when IL-2 production is compromised by T cell exhaustion (Brooks et al., 2005) which may indicate reduced γc-STAT5 signaling, leaving CD4+ T cells reliant on gp130 signaling for survival. In CD8+ T cells, while STAT-3 is not required for initial expansion, it is essential for the formation of long lived memory cells providing evidence that T cells can progressively alter their STAT requirements for accumulation and survival (Cui et al., 2011). Both IL-6 and IL-21 can phosphorylate STAT-3, however, while only IL-27 signaling results in enhanced CD4+ T cell survival in vivo. Meanwhile only IL-27 phosphorylated STAT-1 in virus specific CD4+ T cells, suggesting that STAT-1 could promote CD4+ T cell survival late during chronic infection. Phosphorylation of STAT-1 by IL-27 in this context is most likely due to its use of WSX-1, which contains a box 1 janus kinase (JAK) domain capable of activating the STAT family independently of gp130 (Sprecher et al., 1998). In many contexts STAT-1 is thought to activate an anti-proliferative, pro-apoptotic pathway in T cells (Bromberg et al., 1996; Gil et al., 2006; Tanabe et al., 2005), thus exploration of STAT-1’s functions at late stages of infection is worthy of further investigation. IL-27 is also known to phosphorylate STAT-4 and STAT-5, although less potently than it does STAT-1, both of which have potent effects on CD4+ T cell differentiation, and could have pro-survival effects via these pathways (Kaplan, 2005; Lucas et al., 2003).
The loss of IL-27R signaling across the whole mouse resulted in reduced viral control and a slight increase in the number of virus specific CD4+ T cells at early stages of viral infection. As this was not observed when Il6st was conditionally ablated in T cells, nor in mixed chimeras generated from either Il27ra−/− or Cd4-cre Il6stfl/fl mice, it suggests that IL-27 signaling also has a vital non-T cell role in anti-viral immunity early during LCMV Cl13 infection. Fitting with this, effective IL-27 signaling has also been shown in natural killer cells, NKT cells, B cells and macrophages (Imamichi et al., 2008; Villarino et al., 2005; Yoshimoto et al., 2004), however the effects of IL-27 signaling on cells other than CD4+ T cells during chronic viral infection is yet to be explored. It is possible that the presence of higher viral loads early during LCMV Cl13 infection plays a role in the increased virus specific CD4+ T cell numbers observed in LCMV Cl13 infected Il27ra−/− mice at day 9 p.i. Alternatively this could be a consequence of IL-27’s regulation of cell populations that directly influence CD4+ T cell expansion (e.g. antigen presenting cells). Our data therefore not only shows the importance of IL-27 in the maintenance of virus specific CD4+ T cell responses and viral control during chronic viral infection in vivo, but also highlights the complex and significant role that IL-27 plays in different aspects of immune responses and the need to use models that allow discrimination of cell intrinsic versus extrinsic effects (such as mixed bone marrow chimeras) in the exploration of these processes.
IL-27 signaling has previously been implicated in controlling Tfh cells numbers after vaccination (Batten et al., 2010), while we have previously observed that IL-6 plays an essential role in Tfh cells differentiation during chronic viral infection (Harker et al., 2011). In this context IL-27 appears to be essential for the survival of virus specific CD4+ T cells, the majority of which are Tfh cells, but does not appear to control Tfh cell differentiation or Bcl6 expression. Complete loss of gp130 mediated signaling, however, results in loss of both virus specific CD4+ T cells and Tfh cell differentiation. This leads us to hypothesize that during chronic viral infection IL-6 is vital for Tfh cell differentiation, while IL-27 is required for their ongoing survival.
The number of CD8+ and CD4+ T cells is critical in determining the outcome of chronic viral infections in mice and humans (Rehermann and Nascimbeni, 2005; Virgin and Walker, 2010; Virgin et al., 2009). CD4+ T cells are often not required for the initial generation of virus specific CD8+ T cell responses, but they are vital for the maintenance and quality of these cells and are also fundamental for B cell responses (Matloubian et al., 1994b; Shedlock and Shen, 2003). Notably, the use of vaccines that target both the cytotoxic and humoral arms of the immune system, such as the RV144 prime-boost HIV vaccine trial (Benmira et al., 2010), have demonstrated the efficacy of this approach compared to prior vaccines that aimed to target a single aspect of immunity. Our data places gp130 signaling at the center of CD4+ T cell responses within the highly immunosuppressive environment of an established chronic viral infection and highlights the potential of this pathway as a target for simultaneously empowering both humoral and cellular immunity in this context.
Experimental Procedures
Mice and viral stocks
WT C57BL/6 mice, C57BL/6 CD45.1+ mice and Il27ra−/− mice were purchased from The Jackson laboratory. Cd4-cre Il6stfl/fl mice were kindly provided by Dr. Werner Mueller (University of Manchester, U.K.). Mice were bred and maintained in a closed breeding facility and mouse handling conformed to the requirements of the National Institutes of Health and the Institutional Animal Care and Use Guidelines of UCSD. Unless otherwise stated, 6–8 week old mice were infected intravenously (i.v.) with 2×106 PFU of LCMV ARM or Cl 13. Viruses were grown, identified and quantified as described in (Ahmed et al., 1984; Borrow et al., 1995). For viral quantification by focus forming assay vero cells (ATCC) were seeded in 96 well plates and incubated with serial dilutions of serum or tissue homogenate for 20 hours. Cells were then fixed with 1% paraformaldehyde for 30 minutes, blocked with PBS containing 10% FBS for 1 hour and incubated with supernatant from 113 hybridoma cells (A mouse B cell hybridoma producing monoclonal IgG1 against LCMV NP kindly provided by M.B. Oldstone, TSRI) for 1 hour. Finally the cells were incubated with a Cy3 conjugated goat anti-mouse IgG (Jackson Immunoresearch) for 40 minutes and foci counted by fluorescent microscope.
Generation of BM chimeras
BM chimeras were generated via transfer of a 50:50 ratio of BM cells from Cd4-cre Il6stfl/fl or Il27ra−/− mice and C57BL/6 CD45.1+ mice into lethally irradiated C57BL/6 CD45.1+ as described previously (Harker et al., 2011).
LCMV specific antibody ELISAs
LCMV specific ELISAs and avidity assays were done as we and others have previously described (Hammond et al., 1997; Harker et al., 2011)
Flow cytometry
Flow cytometry was done as previously described (Tinoco et al., 2009). The following fluorochrome labeled antibodies purchased from ebioscience or BD biosciences were used to stain blood or spleen cells: anti-CD8-pacific blue, -CD4- pacific blue, -CD19-PE, -CD38-Alexafluor700, -GL7-FITC, -PD1-PE-Cy7, -ICOS-PE, - SLAM-FITC or PE-Cy7, -CD45.2-APC-Cy7, -CD45.1-PerCy5.5, -CD200-PerCP-eFlour 610, -IFN-γ-APC, -TNF-α-FITC, -IL-2-PE, -CD107a-FITC, -CD107b-FITC, Annexin-VPE and -B220-FITC. CXCR5, FoxP3 and Bcl6 (BD, K112-91) staining were done as previously described (Harker et al., 2011). Cells were acquired using the Digital LSR II flow cytometer (Becton Dickinson, San Jose, CA). To quantify incorporation of BrdU by tetramer+ T cells, mice were injected with 2 mg of BrdU (Sigma-Aldrich) 16 h before analysis and splenocytes stained with BrdU Flow kit (BD Biosciences) following the manufacturer instructions. Staining for fluorescence assisted cell sorting was conducted in an identical fashion and cells were isolated on a FACSAria (Becton Dickinson, San Jose, CA) to >95% purify. Flow cytometric data were analyzed with FlowJo software (TreeStar, CA).
Ex-vivo T cell stimulation
For MHC class-I-restricted GP33–41 peptide (2 µg/ml) or MHC class-II restricted GP67–77 (5 µg/ml) stimulation and staining were carried out as we have previously described (Tinoco et al., 2009). For polyclonal stimulation we used PMA (10 ng/ml) and ionomicyn (0.5 µg/ml) in place of peptide. For intracellular IL-21 staining, cells were permeabilized with saponin and incubated with 1:25 dilution of IL- 21RhFc (R&D Systems) for 30 minutes at 4°C, washed twice and stained with 1:200 anti-human Fc-PE (BD Pharmingen). For phosphorylation of STATs, 1,000 CD45.1+ Smarta CD4+ T cells were transferred i.v. 1 day prior to infection with 2 × 106 pfu of LCMV Cl13. 18 days p.i. splenocytes were isolated, rested for 2 hours at 37°C and stimulated with 50 ng/ml of recombinant human IL-6 (National Cancer Institute) or mouse IL-11, IL-27, LIF (eBioscience) or oncostatin M (Cell Signaling). Cells were fixed after 30 minutes and pSTAT staining was done according to manufacturer’s instructions (BD).
Real-time RT-PCR
Total RNA was extracted from splenocytes using RNeasy kits (Qiagen), and reverse transcribed into cDNA using superscript III RT (Invitrogen). cDNA quantification was performed using SYBR Green PCR kits (Applied Biosystems) and a Real-Time PCR Detection System (ABI). Primers for the genes assessed are described in (Harker et al., 2011). Il21 detection of IL-27 and IL-6 stimulated CD4+ T cells was carried out on 50,000 PD1+ CD4+ T cells and 10,000 Smarta cells per timepoint, resulting in Il21 detection being 18-fold more sensitive in PD1+ CD4+ T cells compared to Smarta based on Gapdh expression.
Statistical analysis
Unpaired student's t-tests or ANOVA tests were performed using the InStat 3.0 software (GraphPad, CA.). For mixed chimeras, paired t-tests were used. * P < 0.05, ** P < 0.01, *** P < 0.001 in all data shown.
Supplementary Material
Acknowledgements
Cd4-cre Il6stfl/fl mice were kindly provided by Dr. Werner Mueller (University of Manchester, U.K.). We would also like to thank Dr Steve Hedrick, University of California San Diego, for critically reading this manuscript. This research was funded by NIH grant A1081923, AI101561 and Leukemia and Lymphoma Society Scholar Award to E.I.Z. J.A.H. was supported by an Irvington Institute Postdoctoral Fellowship from the Cancer Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, Danilenko DM, Caplazi P, Wong M, Fulcher DA, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J Exp Med. 2010;207:2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmira S, Bhattacharya V, Schmid ML. An effective HIV vaccine: A combination of humoral and cellular immunity? Curr HIV Res. 2010;8:441–449. doi: 10.2174/157016210793499286. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- Boettler T, Cheng Y, Ehrhardt K, von Herrath M. TGF-beta blockade does not improve control of an established persistent viral infection. Viral Immunol. 2012;25:232–238. doi: 10.1089/vim.2011.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE., Jr Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci U S A. 1996;93:7673–7678. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768–775. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumont MC, Monceaux V, Viollet L, Lay S, Parker R, Hurtrel B, Estaquier J. TGF-beta in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus. Cell Death Differ. 2007;14:1747–1758. doi: 10.1038/sj.cdd.4402192. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, O'Shea JJ. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203:2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med. 2011;208:987–999. doi: 10.1084/jem.20101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasnacht N, Greweling MC, Bollati-Fogolin M, Schippers A, Muller W. T-cell-specific deletion of gp130 renders the highly susceptible IL-10-deficient mouse resistant to intestinal nematode infection. Eur J Immunol. 2009;39:2173–2183. doi: 10.1002/eji.200838710. [DOI] [PubMed] [Google Scholar]
- Feng J, Hu X, Guo H, Sun X, Wang J, Xu L, Jiang Z, Xu B, Niu J, Jiang Y. Patients with chronic hepatitis C express a high percentage of CD4(+)CXCR5 (+) T follicular helper cells. J Gastroenterol. 2012;47:1048–1056. doi: 10.1007/s00535-012-0568-1. [DOI] [PubMed] [Google Scholar]
- Feng J, Lu L, Hua C, Qin L, Zhao P, Wang J, Wang Y, Li W, Shi X, Jiang Y. High frequency of CD4+ CXCR5+ TFH cells in patients with immune-active chronic hepatitis B. PLoS One. 2011;6:e21698. doi: 10.1371/journal.pone.0021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- Garidou L, Heydari S, Gossa S, McGavern DB. Therapeutic blockade of transforming growth factor beta fails to promote clearance of a persistent viral infection. J Virol. 2012;86:7060–7071. doi: 10.1128/JVI.00164-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil MP, Salomon R, Louten J, Biron CA. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood. 2006;107:987–993. doi: 10.1182/blood-2005-07-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SA, Cook SJ, Lichtenstein DL, Issel CJ, Montelaro RC. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J Virol. 1997;71:3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker JA, Lewis GM, Mack L, Zuniga EI. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu Y, Suto A, Kashiwakuma D, Kanari H, Kagami S, Ikeda K, Hirose K, Watanabe N, Grusby MJ, Iwamoto I, Nakajima H. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J Leukoc Biol. 2010;87:703–712. doi: 10.1189/jlb.0909639. [DOI] [PubMed] [Google Scholar]
- Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, Routy JP, Ahmad A. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- Imamichi T, Yang J, Huang DW, Brann TW, Fullmer BA, Adelsberger JW, Lempicki RA, Baseler MW, Lane HC. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS. 2008;22:39–45. doi: 10.1097/QAD.0b013e3282f3356c. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Phuah CL, Cox AL, Thompson SA, Ban M, Shawcross J, Walton A, Sawcer SJ, Compston A, Coles AJ. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H) J Clin Invest. 2009;119:2052–2061. doi: 10.1172/JCI37878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MH. STAT4: a critical regulator of inflammation in vivo. Immunol Res. 2005;31:231–242. doi: 10.1385/IR:31:3:231. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Glosson NL, Stritesky GL, Yeh N, Kinzfogl J, Rohrabaugh SL, Goswami R, Pham D, Levy DE, Brutkiewicz RR, et al. STAT3-dependent IL-21 production from T helper cells regulates hematopoietic progenitor cell homeostasis. Blood. 2011;117:6198–6201. doi: 10.1182/blood-2011-02-334367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clerc S, Limou S, Coulonges C, Carpentier W, Dina C, Taing L, Delaneau O, Labib T, Sladek R, Deveau C, et al. Genomewide association study of a rapid progression cohort identifies new susceptibility alleles for AIDS (ANRS Genomewide Association Study 03) J Infect Dis. 2009;200:1194–1201. doi: 10.1086/605892. [DOI] [PubMed] [Google Scholar]
- Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9:861–866. doi: 10.1038/nm0703-861. [DOI] [PubMed] [Google Scholar]
- Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994a;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994b;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG, Christ D, Fulcher D, Falcone M, King C. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity. 2011;34:602–615. doi: 10.1016/j.immuni.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, Huang H, Wen R, Wang J, Li HS, et al. STAT5 negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. 2012 doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, Pahwa S. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. J Immunol. 2011;186:6173–6181. doi: 10.4049/jimmunol.1100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porichis F, Kaufmann DE. HIV-specific CD4 T cells and immune control of viral replication. Curr Opin HIV AIDS. 2011;6:174–180. doi: 10.1097/COH.0b013e3283454058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Rigopoulou EI, Abbott WG, Haigh P, Naoumov NV. Blocking of interleukin-10 receptor--a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin Immunol. 2005;117:57–64. doi: 10.1016/j.clim.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraml BU, Hildner K, Ise W, Lee WL, Smith WA, Solomon B, Sahota G, Sim J, Mukasa R, Cemerski S, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88:1145–1156. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A. 2008;105:14028–14033. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher CA, Grant FJ, Baumgartner JW, Presnell SR, Schrader SK, Yamagiwa T, Whitmore TE, O'Hara PJ, Foster DF. Cloning and characterization of a novel class I cytokine receptor. Biochem Biophys Res Commun. 1998;246:82–90. doi: 10.1006/bbrc.1998.8576. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Nishibori T, Su L, Arduini RM, Baker DP, David M. Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-alpha beta responses in T lymphocytes. J Immunol. 2005;174:609–613. doi: 10.4049/jimmunol.174.2.609. [DOI] [PubMed] [Google Scholar]
- Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino AV, Larkin J, 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, de Sauvage FJ, Hunter CA. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- Virgin HW, Walker BD. Immunology and the elusive AIDS vaccine. Nature. 2010;464:224–231. doi: 10.1038/nature08898. [DOI] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T, Okada K, Morishima N, Kamiya S, Owaki T, Asakawa M, Iwakura Y, Fukai F, Mizuguchi J. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173:2479–2485. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, Liu J, Song H, Jones RB, Sheth P, et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol. 2010;185:498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.