One hundred years ago, in the first paper in the first edition of GENETICS, Calvin Blackman Bridges provided evidence for the chromosome theory of inheritance, laying the groundwork for much of the genetics research that has followed (Bridges 1916). As we discuss a paper that is arguably a cornerstone of modern genetic analysis, it is well worth remembering that this two-part paper was the report of Bridges’s Ph.D. thesis work (indeed, we find it sobering to compare the impact of our own theses to that of Bridges’s). Bridges’s 1916 paper described nondisjunction (improper chromosome segregation), explained how evidence of nondisjunction during meiosis provided proof that chromosomes contained the genetic material and illustrated how sex determination works in Drosophila melanogaster. The scientific insights Bridges made in this seminal paper were instrumental to subsequent experimental studies of meiosis, and his influence is still felt in genetics labs today.
As much as we are able to appreciate the significance of this accomplishment with a century of hindsight, we cannot help but wonder if the importance of Bridges’s paper was equally obvious to the first editorial board of GENETICS, of which Bridges’s thesis advisor Thomas Hunt Morgan was a member. It would be an interesting exercise for the current editorial board to try to identify a paper to be published in the next year in GENETICS that they believe will still be lauded a century later for its continuing legacy and enduring impact. The selection of Bridges’s masterpiece as the first paper published in GENETICS reflects either amazing prescience by the editorial board or an extremely fortuitous choice. Either way, they could not have gotten the journal off to a better start.
Origins of the Chromosome Theory
The chromosome theory of inheritance is a cornerstone of modern genetics. It postulates that chromosomes are the carriers of Mendelian factors (genes) and are the physical basis of heredity. This theory, first proposed in 1903, led to the inseparable union of two previously distinct fields of investigation—cytological observation of chromosomes and genetic analysis of inheritance from breeding experiments. It was vital to subsequent rapid advances in genetics and the eventual understanding of the physical and chemical nature of genes. Our ability to think interchangeably about genes and chromosomes is so integral to genetics today that it is hard to conceive that this theory did not gain immediate widespread acceptance. Nonetheless, prior to Bridges’s 1916 investigation, the chromosome theory remained an unresolved issue.
Although Theodor Boveri and Walter Sutton are generally given equal credit for first expounding the chromosome theory, the classic paper by Sutton (1903), a student of E. B. Wilson, most clearly articulates the parallels between Mendel’s factors in breeding experiments and the properties of chromosomes (particularly during meiosis) observed directly by cytological analysis. Sutton points out that both exist in pairs, that they segregate 1:1, and that the segregation of one pair is independent from the segregation of all other pairs. Sutton concludes his analysis by explicitly stating that chromosomes “may constitute the physical basis of the Mendelian law of heredity.” Recognition of this parallelism was a profound insight, for if genes were not physically associated with chromosomes then it would be necessary to find some other cellular component that behaved exactly as chromosomes did2.
Notable among the skeptics of chromosome theory were William F. Bateson (see Cock 1983), who coined the word “genetics,” and Bridges’s mentor Thomas Hunt Morgan, who was a close colleague of E. B. Wilson (Sutton’s mentor) and who initially rejected Mendelism as well. Benson (2001), citing Morgan, points out that by 1906, Morgan was not only skeptical but had “become antagonistic, arguing that he was going after chromosome theory and that he personally was ‘… not in sympathy with all this modern way of referring everything to the chromosomes and I am continually in hot water, for I live in an atmosphere saturated with chromosomic acid and blue dyes.’”
Morgan’s opposition is ironic since he would go on to win the Nobel Prize in 1933 for his discoveries concerning the role of chromosomes in heredity. He began to waver in 1910 once he started working with Drosophila, stating that the focus on chromosomes was “worth considering” (Morgan 1910a). By the end of that year, he had become a full convert with the discovery of the white-eyed mutation in Drosophila and his realization that, according to Mendelian principles, inheritance of the white-eyed trait could only be explained if the mutant gene producing this phenotype were physically associated with the X chromosome (Morgan 1910b). This conversion was significant. When Morgan encountered genetic linkage, he sought a chromosomal explanation and found one in Frans A. Janssens’ chiasmatype theory (Janssens 1909; Koszul et al. 2012), which postulated that chiasmata represented sites at which paired homologous chromosomes in meiosis I broke and rejoined to exchange corresponding chromosome segments. Morgan correctly recognized that the genetic consequence of this exchange would be recombination between genes on the same chromosome and that the frequency of recombination between any two genes provided information about the relative distance between those genes—an inference of monumental importance. Clearly, Morgan had come a long way in a short time. Morgan’s student, Alfred H. Sturtevant, still an undergraduate at the time, carried this reasoning to its ultimate conclusion using recombination frequencies to construct the world’s first linkage map (Sturtevant 1913).
Chromosome Theory: The Final Proof
In the opening paragraphs of his 1916 paper, Bridges summarizes the evidence supporting the chromosome theory: (1) the parallels between the inferred behavior of Mendelian factors and the observed behavior of chromosomes in meiosis; (2) the discovery of sex chromosomes, with the implication that inheritance of a specific chromosome determines the development of a particular sex, presumably because these chromosomes contain unique sets of genetic instructions; and (3) the discovery of sex linkage, with the demonstration that certain genes showed a mode of inheritance that deviated from the usual Mendelian pattern but is entirely interpretable if those genes are assumed to be physically associated with the X chromosome. He goes on to state that what was still required to make the argument complete was “to demonstrate the identity of distribution between specific genes and specific chromosomes” in such a way that permits no reasonable explanation other than the physical association of the genes and chromosomes and that “the experimental and cytological evidence in the case of nondisjunction furnishes such a demonstration.”
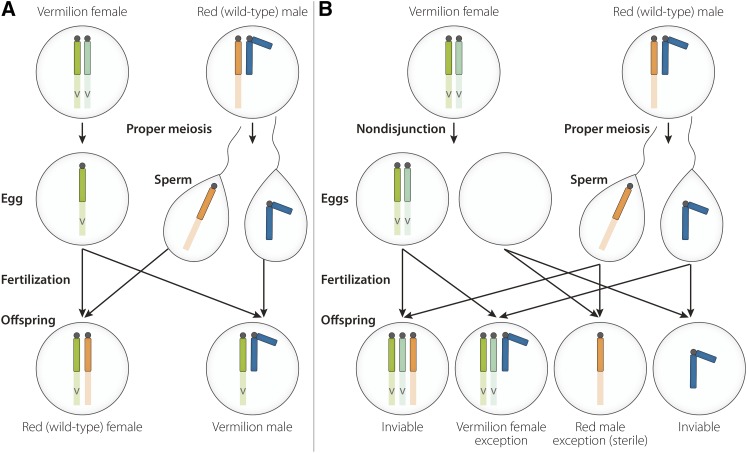
From experiments analyzing the inheritance of sex-linked traits, Bridges discovered the rare occurrence of offspring whose pattern of inheritance deviated from the usual outcome. These exceptional progeny were females that inherited all of their sex-linked traits exclusively from their mothers and males that inherited their sex-linked traits exclusively from their fathers, exactly opposite of the expected pattern of transmission. Purely on the basis of this phenotypic analysis, Bridges proposes a chromosomal mechanism to account for this outcome: the rare occurrence of a meiotic error in the female (nondisjunction) resulting in a failure of the two homologous X chromosomes to properly segregate at the first meiotic division (Figure 1). The outcome of such an error would be the production of an aberrant egg containing either both maternal X chromosomes or neither. This explanation made definite predictions about the chromosome composition of progeny resulting from subsequent crosses using the exceptional females. In particular, half of the regular females and all of the exceptional females should have the XXY chromosome constitution (Figure 2A), which had never been observed previously. In each instance, Bridges fully verified these predictions by cytological analysis. Thus, not only does the pattern of inheritance of sex-linked traits parallel the inheritance of the X chromosome as first ascertained by Morgan, but errors in the transmission of X chromosomes produce corresponding deviations in the inheritance of sex-linked traits. There is only one reasonable interpretation of this outcome: the genes for sex-linked traits are physically part of the misbehaving chromosomes. As Bridges said,
Figure 1.
X chromosome nondisjunction leads to aberrant inheritance of sex-linked traits. (A) Proper chromosome segregation of X chromosomes in females results in wild-type XX females and vermilion-eyed XY males. (B) Nondisjunction of X chromosomes at the first meiotic division produces diplo-X and nullo-X eggs containing two and zero X chromosomes, respectively. Fertilization of these nondisjunctional eggs results in the production of exceptional red-eyed sterile X0 males and exceptional vermilion-eyed XXY females (adapted from Bridges 1916).
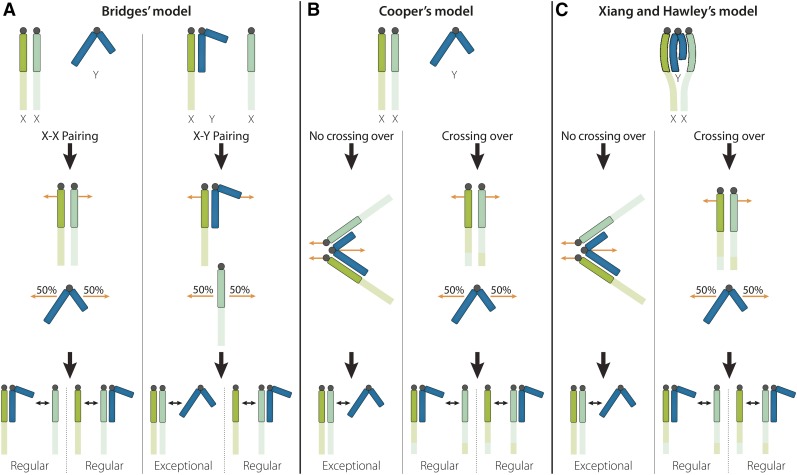
Figure 2.
Models of secondary nondisjunction. (A) In Bridges’s model, an X chromosome has a choice of two pairing partners in meiosis, giving rise to different patterns of segregation: (1) the two X’s could pair and segregate, leaving the unpaired Y to segregate at random, generating X and XY eggs with equal frequency; or (2) an X could pair and segregate from the Y chromosome, leaving the unpaired X to segregate at random and producing X, XY, XX, and Y eggs with equal frequencies. (B) To explain secondary nondisjunction frequencies greater than 50%, Cooper proposed an alternative model wherein X chromosomes undergo exchange without regard for the presence of the Y chromosome. However, nonexchange X chromosomes will engage in trivalent association with the Y chromosome, which segregates to one pole and preferentially directs the two nonexchange X chromosomes to the other pole. (C) Xiang and Hawley confirmed the formation of a trivalent, but found that it precedes and does not inhibit recombination between the X’s. X chromosomes that do recombine disengage from the Y and segregate normally, leaving the unpaired Y to segregate at random. X chromosomes that fail to recombine remain associated with the Y and preferentially segregate away from it.
“The genetic and cytological evidence in the case of non-disjunction leaves no escape from the conclusion that the X chromosomes are the carriers of the genes for the sex-linked characters. The distribution of sex-linked genes (as tested by experimental breeding methods) has been demonstrated to be identical, through all the details of a unique process, with the distribution of the X chromosomes (as tested by direct cytological examination).”
Sex Determination
In the late 1800s and early 1900s, cytologists including Henking and McClung discovered departures from the usual observation that chromosomes were present in homologous pairs. Instead, one chromosome in the diploid set was an extra or “accessory” chromosome that lacked a morphologically similar partner. Because of the unknown nature of this accessory chromosome it became known as the X chromosome. Further studies, primarily in insects, revealed that the X chromosome was present in one copy in males and two copies in females. McClung proposed as early as 1901 that the X chromosome was in some way associated with sex determination. However, various complications and errors in cytology and interpretation led to a period of confusion until E. B. Wilson and one of his students, Nettie Stevens, clarified the situation by examining several species of insects (Stevens 1905; Wilson 1905). These studies revealed the existence of two different types of sex chromosome composition: the X0-XX type, in which the single X chromosome in males lacks a homolog entirely; and the XY-XX type, in which males possess an additional chromosome (the Y chromosome) not present in females. The Y chromosome behaves as a homologous pairing partner with the X chromosome in male meiosis but differs from it in morphological appearance. Drosophila was found to be of the XY-XX type.
The production of two qualitatively different types of sperm (either X and 0 or X and Y, depending on species) that upon fertilization of X-bearing eggs resulted in the production of two qualitatively different types of progeny (male and female) in equal frequency was ultimately taken as evidence that the X and Y chromosomes were not just associated with sex but were directly involved in determining sex. However, the basis of the sex-determining mechanism remained unclear. For example, in the case of an XY male, is maleness due to the presence of a single X, the presence of the Y, or perhaps some combination of the two? Cytological examination alone could not provide further insight into these mechanistic questions.
Bridges realized that individuals containing aberrant sex chromosome constitutions could provide the answer, and inferred that the exceptional males resulting from primary nondisjunction in XX female flies had an X0 sex chromosome constitution. These individuals were normal males in all respects except that they were sterile. Moreover, Bridges confirmed the occurrence of XXY females that were fully normal in morphological appearance. Thus, Bridges was able to conclude that the Y chromosome did not play a role in sex determination in Drosophila and apparently contained no genes other than those required for male fertility, which was itself of significance:
“The fact that X0 males are totally sterile is of unusual interest since it is the first indication that the Y chromosome is something more than a gear wheel in the mechanism of synapsis and reduction. The evidence of this paper proves that the Y has no effect upon the sex or the sex-linked characters of either the male or female, but that the Y does play some positive role is proved by the fact that XY males are fertile and X0 males are sterile.”
The mechanism of sex determination and the role of the Y chromosome were found to differ between flies and mammals, but key insights about mammalian sex determination some 40 years later were based on observations comparable to those of Bridges. Again, cytological analysis of individuals containing abnormal sex chromosome constitutions resulting from nondisjunction provided crucial information. For example, Ford et al. (1959) described an X0 human female with Turner syndrome, while Welshons and Russell (1959) reported the discovery of X0 female mice that were fertile. Furthermore, Jacobs and Strong (1959) described an XXY patient with gonadal dysgenesis who was overall male in appearance (Klinefelter’s syndrome), providing evidence for the male-determining role of the Y chromosome.
Although Bridges did not delve further into the mechanism of sex determination in Drosophila in the 1916 paper, his interest was clearly sparked and he continued to investigate this problem in ensuing years. The model he eventually proposed was based on phenotypic and cytological analysis of flies containing varying numbers of X chromosomes and sets of autosomes (Bridges 1921, 1925). He recovered triploid flies (3X:3A, where A represents a haploid set of autosomes) from the fertilization of an egg that had undergone meiotic nondisjunction for all the chromosomes in a diploid female and found that they were normal females in appearance. These females were also fertile and in crosses with diploid males (1X:2A) produced some flies that had two X chromosomes and three sets of autosomes (2X:3A). Those flies were sterile intersexes composed of a mixture of both male and female structures. From these and similar observations, Bridges proposed that the key determinant for sexual development in Drosophila was the ratio of X chromosomes to haploid sets of autosomes (Bridges 1925). A ratio of 1.00, as in 3X:3A and 2X:2A flies, produced normal females; a ratio of 0.5, as in 1X:2A flies, produced normal males; and an intermediate ratio of 0.66, as in 2X:3A, produced sterile intersexes. Flies with X:A ratios of greater than 1.00 (e.g., 3X:2A) or less than 0.5 (e.g., 1X:3A) were abnormal and generally did not survive. In Bridges’s genic balance model to explain these results, he imagined that many genes were involved in determining sexual phenotype, some of which promoted male development and some of which promoted female development. These genes acted in opposition to one another such that sex determination depended on the relative proportion of female-determining genes (which he imagined were enriched on the X chromosomes) to male-determining genes (enriched on the autosomes) present in each cell.
Although this model stood for decades and is still frequently taught in textbooks, more recent studies, primarily by Baker, Cline, and Schedl and colleagues (Baker 1989; Bell et al. 1991; Cline 2005), have led to an updated model that is at once both more complicated and more fascinating. A detailed description of this model is beyond the scope of the present article, but in essence, it now appears that sex determination in Drosophila is more a mechanism for counting X chromosomes than measuring the X:A ratio (Salz and Erickson 2010)3. Although the details of Bridges’s model have been revised, he was the first to use genetics to investigate mechanisms of sex determination, a field of study that remains highly vigorous.
Meiotic Nondisjunction
Meiosis reduces chromosome number precisely in half to form haploid eggs and sperm and ensures that each gamete receives exactly one member of each pair of homologs. However, meiosis is not an error-free mechanism, and the failure to correctly execute a number of steps along the way can cause homologs to segregate improperly. In some organisms—notably human females—these errors are astoundingly common. Recent estimates suggest that segregation errors in meiosis cause the spontaneous miscarriage of more than 30% of all human conceptions, often before the pregnancy is even recognized (Nagaoka et al. 2012). This number rises dramatically with increasing maternal age, particularly around the age of 40–45. Thus, nondisjunction resulting primarily from errors in the first meiotic division in the mother is the most prevalent cause of pregnancy loss and congenital disorders such as Down syndrome in humans (Hassold and Jacobs 1984). As such, major research efforts are now devoted to understanding the basis of these errors and their relationship to maternal age.
It is unlikely that Bridges anticipated any of this when, in the course of carrying out routine breeding experiments in D. melanogaster (but known then as D. ampelophila), he observed exceptional progeny (approximately 1 of 1600) displaying aberrant inheritance of sex-linked markers. It is a testimony to Bridges’s genius that rather than merely discarding these flies as some rare, meaningless fluke or the result of careless husbandry, he made them the focus of his investigation4. In an imaginative leap, Bridges recognized that the exceptional progeny could be the result of improper segregation of the X chromosomes in the female, resulting in the production of diplo-X and nullo-X eggs (Figure 1). He coined the term primary nondisjunction to refer to these meiotic errors. Bridges published short preliminary accounts of his studies on nondisjunction (Bridges 1913, 1914), but the full details of his exhaustive analysis awaited his 1916 masterpiece in GENETICS. The rare spontaneous occurrence of primary nondisjunction in Drosophila females made the process difficult to investigate, so Bridges (1916) could only suggest a possible mechanical mechanism for their generation—one in which homologs failed to separate because they were somehow entangled.
Bridges went on to demonstrate by progeny tests that the diplo-X eggs contained two nonrecombinant homologous X chromosomes5. He then discovered that in contrast with the rare occurrence of primary nondisjunction, exceptional females produced both male and female exceptional offspring at a much higher frequency (4–5%) than normal females.
Bridges called this subsequent X chromosome missegregation “secondary nondisjunction” (not to be confused with nondisjunction at the second meiotic division). The bulk of Bridges’s 1916 paper focuses on his analysis of secondary nondisjunction and his efforts to determine the underlying mechanism.
Bridges’s first suggested that perhaps an X-linked mutation caused meiotic misbehavior of chromosomes that the primary exceptional females inherited from their mother. However, he eventually ruled out this explanation because he was unable to map any gene associated with the occurrence of nondisjunction. Although the idea proved incorrect, one must admire Bridges’s insight in conceiving it. Bridges thought of this possibility only 16 years after the rediscovery of Mendel, at a time when virtually all known mutations in Drosophila had visible phenotypes affecting eyes, wings, bristles, or body color. The notion that meiosis itself could be under genetic control and that mutations might exist whose phenotype would be aberrant chromosome segregation was nothing short of brilliant. Although the first bona fide meiotic mutant in Drosophila [c(3)G] was recovered in 1922 (Gowen and Gowen 1922), it would be another 50 years before there were systematic efforts to find such mutations in Drosophila (e.g., Sandler et al. 1968; Baker and Carpenter 1972).
Bridges did eventually hit upon the correct interpretation: the inappropriate presence of the Y chromosome in XXY primary exceptional females caused the meiotic misbehavior of the two X chromosomes at high frequency6. To explain this effect of the Y chromosome, Bridges proposed the competitive pairing model (Figure 2A) based on the shared heterochromatic homology between the X and Y and the possible competition for pairing partners when three sex chromosomes were present.
Subsequent work by Sturtevant and Beadle (1936), Cooper (1948), Grell (1962b), and others uncovered details of secondary nondisjunction that were inconsistent with Bridges’s model, necessitating a succession of new models (Figure 2, B and C), each of which attempted to accommodate the latest observations and each of which revealed some new facet of meiosis (see Box 1 for details). From these studies, it emerged that X-Y association in XXY females is not in competition with X-X pairing, but rather all three chromosomes can associate in a trivalent as first proposed by Cooper (1948). A trivalent model based on homologous heterochromatic associations was ultimately confirmed and refined by Xiang and Hawley (2006) once direct cytological observation of meiosis in Drosophila females became possible. Nonetheless, 100 years after Bridges, there are still aspects of secondary nondisjunction that remain unresolved and under active study (c.f., Gilliland et al. 2015).
The Legacy of Bridges
With his discovery of primary nondisjunction, Bridges revealed for the first time that meiosis is an imperfect process subject to spontaneous, unprovoked errors. His key insight about secondary nondisjunction—that some type of X-Y pairing interfered with the proper segregation of nonexchange X chromosomes—is fundamentally correct, even if his model ultimately proved inaccurate. Moreover, Bridges’s work provided the impetus for all subsequent efforts to explain secondary nondisjunction, and those efforts became a driving force from which we learned many of the essential features of normal meiosis. Among these we may include the following: the importance of crossing over to ensure proper chromosome segregation, the important role heterochromatin plays in homologous chromosome pairing, and the recognition of distinct mechanisms for segregating nonexchange chromosomes. These conclusions hold true in yeast, worms, and likely, humans. Perhaps most important of all, Bridges’ realization that he could infer important aspects of meiosis by examining the ability of a Y chromosome to disrupt the normal segregation of the X chromosomes provided an experimental paradigm for subsequent studies of meiosis in Drosophila: inferring the normal meiotic mechanism by examining the outcome when it operates in an abnormal chromosome environment. Thus, the studies of Sturtevant and Beadle, Cooper, Grell, Novitski, Sandler, Lindsley, Hawley, and many others after Bridges depended on generating flies containing a variety of different chromosome constitutions or unusual chromosomes (inversions, free duplications, compound chromosomes, etc.) to determine their effect on normal segregation.
It must be emphasized that cytological analysis of meiotic chromosome segregation in Drosophila females was notoriously difficult, and until quite recently (Theurkauf and Hawley 1992; Xiang and Hawley 2006), insights about meiosis by previous investigators were made without the benefit of examining chromosome behavior directly. (Earlier, Carpenter 1975a,b pioneered ultrastructural analysis of synapsis and crossing over during meiosis in Drosophila females). One can only admire and respect the creative imagination of these investigators in their ability to construct often elaborate (albeit sometimes incorrect) models about meiotic chromosome behavior based exclusively on the phenotypes and frequencies of offspring they recovered from genetic crosses. In the most general sense, Bridges’s analysis of secondary nondisjunction demonstrated how it was possible to take an experimental genetic approach to dissect meiosis, and one can still find clear intellectual links to Bridges’s work in virtually any recent study of meiosis in Drosophila.
Bridges’s studies described here represent only a small fraction of his extensive contributions to genetics. In many ways, he was the father of modern genomics. He led the effort to use the giant salivary chromosomes to correlate the map position of genes (as defined by recombination mapping) with their physical position on the chromosomes, providing the first physical map (at almost single-gene resolution) of the Drosophila genome decades before anyone even dreamed of determining its DNA sequence. Bridges correctly identified the polytene nature of these chromosomes and produced detailed cytological maps of each chromosome. These maps were of incalculable value to Drosophila biologists in the ensuing decades. Bridges’s meticulous drawings of each chromosome and the nomenclature he introduced to name each polytene band are still standard. Bridges’s recognition of repeat segments in the polytene chromosomes stimulated Edward B. Lewis to propose the hypothesis of gene evolution by tandem duplication and led ultimately to the discovery of the bithorax and antennapedia HOX complexes in Drosophila (Lewis 1951, 1998). Similarly, Bridges’s studies of the bobbed locus, which defines the rDNA gene cluster, initiated analysis of repetitive genes. There are more such examples than we have space to describe. Equally important to his intellectual contributions, Bridges largely shaped Drosophila into the facile experimental system it is today. Most of the standard tools and techniques still used by Drosophilists were either invented by Bridges or are subsequent variants of his inventions: he introduced the use of dissecting microscopes and anesthesia (ether) for carefully examining flies; invented the standard cornmeal, molasses, agar synthetic medium for raising flies; and designed etherizers, incubators, and culture bottles that made fly husbandry convenient and consistent.
At the personal level, all who interacted with Bridges described him as unpretentious, enthusiastic, generous, and unfailingly helpful and friendly. Bridges’ creative spirit was not bound by conventional thinking, and he was equally unconstrained in his private life, which would be considered flamboyant even by today’s standards. Although his lifestyle never impeded his scientific accomplishments, it did contribute to an unfortunately early death at age 49. (For more information about Bridges and the history of Drosophila genetics see Kohler 1994 and the online exhibit http://library.cshl.edu/exhibits/bridges/).
The totality of Bridges’s work surely constitutes one of the most significant scientific legacies of the 20th century. In closing, we cannot help but note again, with satisfaction, that this legacy began in this journal. Perhaps it is not too much of an overstatement to suggest that Bridges’s 1916 masterpiece not only serves as the cornerstone of this journal, but also as a cornerstone of 20th century genetics.
BOX 1: Three models of secondary nondisjunction
In Bridges’ competitive pairing model (Figure 2A), the two X chromosomes pair and segregate from each other, leaving the unpaired Y to segregate at random and generating X and XY eggs with equal frequency (both of these gametes are considered “regular” because the X chromosomes have segregated properly even though the XY egg has an unusual chromosome constitution). Alternatively, an X can pair with and segregate from the Y chromosome (as they would do normally in male meiosis) leaving the unpaired X to segregate at random. This segregation pattern produces four types of eggs with equal frequencies: 50% of the time it would produce equal numbers of regular X and XY gametes, and 50% of the time it would produce equal numbers of exceptional XX and Y gametes. Bridges assumed that X-X pairings would normally be more common than X-Y pairings because of the more complete homology between the X’s.
This model made specific predictions that Bridges was able to test. For example, since the exceptional gametes arose when there was X-Y pairing and the other X remained unpaired, the two X’s would not have been able to engage in crossing over. Thus, the exceptional gametes should contain nonrecombinant X chromosomes7. Among the regular female progeny, half should be XX and half should be XXY. Phenotypically these two classes of regular females would appear identical, but they could be distinguished by progeny tests because secondary nondisjunction would occur only in the XXY females. Moreover, direct cytological examination of chromosomes in oogonial metaphase preparations should confirm the presence of a Y chromosome in half the regular daughters and specifically in those showing an elevated frequency of nondisjunction (a key argument in Bridges’s proof of the chromosome theory). Bridges confirmed the accuracy of each of these predictions and others, providing sound support for his competitive pairing model. However, there were several other critical predictions that he was not able to test because he lacked the necessary genetic tools at the time. One was that crossing over between the two X’s should be reduced in an XXY female in proportion to the frequency of X-Y pairing at the expense of X-X pairing. Another was that when X-Y pairing occurred, the assumed random segregation of the unpaired X imposed a maximum frequency of nondisjunction of 50% (among viable offspring), because no matter how frequently this pairing occurs, half of the resultant gametes will be regular X and XY eggs.
Both of these key predictions of the competitive pairing model were disproven in another classic paper by Sturtevant and Beadle (1936), which examined crossing over in females heterozygous for inversions (which Sturtevant discovered). Heterozygosity for certain inversions in XXY females led to secondary nondisjunction frequencies far higher than could be explained by Bridges’s competitive pairing model. Sturtevant and Beadle recognized that the only way to explain the results was by preferential (rather than random) segregation of two X chromosomes to one pole, away from the Y chromosome. Moreover, despite the very high frequency of secondary nondisjunction in these females, the frequency of crossing over (even though reduced because of the presence of inversion heterozygosity) was identical in XX and XXY females. The presence of the Y did not affect the ability of X chromosomes to undergo crossing over even though the X chromosomes that did fail to segregate properly were all nonexchange (achiasmate) chromosomes.
On the basis of Sturtevant and Beadle’s observations, as well as his own studies, Cooper (1948) proposed an alternative model of secondary nondisjunction (Figure 2B) that resolved many of the problems. He suggested that in those meioses where X pairings are not “locked in” by crossover events, the two X chromosomes engage in trivalent association with the Y chromosome, such that one X chromosome pairs with each arm of the Y chromosome. Cooper further proposed that segregation from this trivalent at the first meiotic division is such that the Y chromosome directs the two X chromosomes to one pole while the Y moves to the opposite pole. Cooper’s model does not predict an upper limit on the maximum frequency of nondisjunction; the frequency of secondary nondisjunction would simply rise with the frequency of X chromosome bivalents that failed to undergo crossing over. Similarly, there is no competition because the X-Y-X trivalent association is presumed to involve only those X chromosomes that would have been nonexchange anyway, regardless of the presence of the Y. However, homology between the X and Y was still assumed to be crucial for their association in a trivalent. Cooper (1948) offered a strong test of this model by examining secondary nondisjunction in females carrying a single-armed derivative (YL) of the Y chromosome. In these XXYL females, the observed frequency of secondary nondisjunction fell to half of the value observed in XXY females bearing the same X chromosomes but a normal Y, presumably because the single-armed Y could not form a trivalent but was only able to pair with and segregate from one X chromosome, leaving the remaining X to segregate at random.
The unusual meiotic behavior of nonexchange X chromosomes in XXY females was seized upon by Grell, who correctly inferred the existence of a distinct meiotic mechanism in Drosophila females for the segregation of these achiasmate chromosomes. She elaborated a specific model of achiasmate segregation, known as the distributive pairing model, a key feature of which was that chromosomes that failed to undergo exchange could still segregate from one another (Grell 1962a, 1976) even if they were nonhomologs. She proposed that secondary nondisjunction was a specific example of the more general mechanism of distributive segregation (Grell 1962b). In this model, the presumed X-Y-X trivalent association was neither competitive with X-X pairing nor was it dependent on homology between X and Y chromosomes.
Although details of the distributive pairing model were controversial, it was generally accepted that there was a mechanism for segregation of nonexchange chromosomes and that this mechanism was ultimately responsible for secondary nondisjunction. After 90 years of debate, Xiang and Hawley (2006) were finally able to provide definitive cytological evidence for the mechanism of secondary nondisjunction. Cooper’s model appears to have been nearly correct with a slight twist. Xiang and Hawley observed that X-Y-X pairing exists in most, if not all, oocytes from the beginning of meiotic prophase, but persists only if the two X chromosomes fail to crossover (Figure 2C). These data required a reversal of the temporal order of Cooper’s model. According to Xiang and Hawley, trivalent formation, mediated by homologous heterochromatic pairing, is an early event that precedes crossing over but does not interfere with the ability of the euchromatic regions of the X chromosomes to undergo crossing over. If the X chromosomes do crossover, they disengage from the Y chromosome and segregate normally, leaving the unpaired Y to segregate at random. X chromosomes that fail to undergo crossing over remain associated in a trivalent with the Y and preferentially segregate to produce XX and Y eggs, as proposed by Cooper.
Acknowledgments
We gratefully acknowledge the enormous contributions of Angela Miller, who not only drew the figures but also greatly improved the clarity, conciseness, and style of the manuscript with her insightful and skillful editing. We also thank members of the Hawley lab for their helpful comments and suggestions on multiple earlier drafts. Finally, we recall with gratitude and appreciation the incomparable mentorship of our thesis advisor, Larry M. Sandler, who embedded in us a deep love and respect for the art of Drosophila genetics so beautifully exemplified by Bridges.
Footnotes
Communicating editor: M. Johnston
2As an interesting side note, after Sutton made this classic contribution, he did not complete his Ph.D. but went on to become a distinguished surgeon before an early death at age 39 (Crow and Crow 2002).
3Specifically, two doses of X-linked regulatory factors are required to activate expression of the master-switch gene Sex-lethal (Sxl). When Sxl is on, it promotes female development, as in 2X:2A embryos; when it is off, as in 1X:2A embryos, male development ensues. The effect of autosomes on sexual development in flies (or cells) of particular chromosome constitution (e.g., 1X:1A or 2X:3A), thought by Bridges to be due to male-determining genes on the autosomes, now appears instead to depend on subtle advances or delays of the time window in embryogenesis during which regulatory factors affecting Sxl can accumulate, tipping the scale in one direction or the other (Salz and Erickson 2010).
4We cannot help but wonder how many contemporary fly workers (ourselves included) might have dismissed such rare events as due to the use of nonvirgin females as mothers or the presence of a nondiscarded parent scored as progeny. One must admire Bridges’s self-confidence as well as his scientific instincts that enabled him to put aside any concerns and accept the rare exceptions as valid manifestations of an intriguing biological phenomenon.
5A modern view of the mechanism of primary nondisjunction in Drosophila females follows from Bridges’s demonstration that primary exceptions almost always carried two nonexchange X chromosomes. A large-scale study of the origin of spontaneous nondisjunction in Drosophila females by Koehler et al. (1996) confirms Bridges’s observations and demonstrates that the vast majority of primary nondisjunction events arise from bivalents that have not undergone exchange. Segregation of such bivalents is usually mediated by the homologous achiasmate system (Grell 1976; Hawley et al. 1992; Theurkauf and Hawley 1992). Failures of this system result in random segregation of the X chromosomes, such that the two X’s are recovered at the same pole one-half of the time (Carpenter 1973; Theurkauf and Hawley 1992; Hawley and Theurkauf 1993).
6By extension, the term secondary nondisjunction now refers to X chromosome nondisjunction in any XXY female regardless of whether that female arose by primary nondisjunction or from proper X chromosome segregation in an XXY female.
7Bridges did, in fact, observe a small fraction of nondisjunctional events in XXY females that involved recombinant X chromosomes. However, because the genetic map of the X chromosome had not yet been oriented relative to the centromere, Bridges misattributed these so-called “equational exceptions” to nondisjunction at the second meiotic division. We now know that most instances of apparent nondisjunction at the second meiotic division in flies are due to errors in the first meiotic division resulting in precocious separation of sister centromeres, causing random segregation of sister chromatids at the second meiotic division. Recent studies indicate that the vast majority of meiotic errors in aged mouse oocytes are also preceded by the premature dissolution of bivalents into univalents in meiosis I, resulting in missegregation of chromatids in meiosis II (Sakakibara et al. 2015).
Literature Cited
- Baker B. S., 1989. Sex in flies: the splice of life. Nature 340: 521–524. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Carpenter A. T., 1972. Genetic analysis of sex chromosomal meiotic mutants in Drosophilia melanogaster. Genetics 71: 255–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell L. R., Horabin J. I., Schedl P., Cline T. W., 1991. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell 65: 229–239. [DOI] [PubMed] [Google Scholar]
- Benson K. R., 2001. T. H. Morgan’s resistance to the chromosome theory. Nat. Rev. Genet. 2: 469–474. [DOI] [PubMed] [Google Scholar]
- Bridges C. B., 1913. Non-disjunction of the sex chromosomes of Drosophila. J. Exp. Zool. 15: 587–606. [Google Scholar]
- Bridges C. B., 1914. Direct proof through non-disjunction that the sex-linked genes of Drosophila are borne by the X-chromosome. Science 40: 107–109. [DOI] [PubMed] [Google Scholar]
- Bridges, C. B., 1916 Non-disjunction as proof of the chromosome theory of heredity. Genetics 1: 1–52, concluded 1: 107–163. [DOI] [PMC free article] [PubMed]
- Bridges C. B., 1921. Triploid intersexes in Drosophila melanogaster. Science 54: 252–254. [DOI] [PubMed] [Google Scholar]
- Bridges C. B., 1925. Sex in relation to chromosomes and genes. Am. Nat. 59: 127–137. [Google Scholar]
- Carpenter, A. T., 1973 A meiotic mutant defective in distributive disjunction in Drosophila melanogaster. Genetics 73: 393–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T., 1975a Electron microscopy of meiosis in Drosophila melanogaster females. I. Structure, arrangement, and temporal change of the synaptonemal complex in wild-type. Chromosoma 51: 157–182. [DOI] [PubMed] [Google Scholar]
- Carpenter A. T., 1975b Electron microscopy of meiosis in Drosophila melanogaster females. II. The recombination nodule—a recombination-associated structure at pachytene? Proc. Natl. Acad. Sci. USA 72: 3186–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline T. W., 2005. Reflections on a path to sexual commitment. Genetics 169: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock A. G., 1983. William Bateson’s rejection and eventual acceptance of chromosome theory. Ann. Sci. 40: 19–59. [DOI] [PubMed] [Google Scholar]
- Cooper K. W., 1948. A new theory of secondary non-disjunction in female Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 34: 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow E. W., Crow J. F., 2002. 100 years ago: Walter Sutton and the chromosome theory of heredity. Genetics 160: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford C. E., Jones K. W., Polani P. E., De Almeida J. C., Briggs J. H., 1959. A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome). Lancet 1: 711–713. [DOI] [PubMed] [Google Scholar]
- Gilliland W. D., Colwell E. M., Lane F. M., Snouffer A. A., 2015. Behavior of aberrant chromosome configurations in Drosophila melanogaster female meiosis I. G3 (Bethesda) 5: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen M. S., Gowen J. W., 1922. Complete linkage in Drosophila melanogaster. Am. Nat. 56: 286–288. [Google Scholar]
- Grell R. F., 1962a A new hypothesis on the nature and sequence of meiotic events in the female of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 48: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell R. F., 1962b A new model for secondary nondisjunction: the role of distributive pairing. Genetics 47: 1737–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grell R. F., 1976. Distributive pairing, pp. 435–486 in Genetics and Biology of Drosophila, edited by Novitski E. M. A., Academic Press, New York. [Google Scholar]
- Hassold T. J., Jacobs P. A., 1984. Trisomy in man. Annu. Rev. Genet. 18: 69–97. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Theurkauf W. E., 1993. Requiem for distributive segregation: achiasmate segregation in Drosophila females. Trends Genet. 9: 310–317. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Irick H., Zitron A. E., Haddox D. A., Lohe A., et al. , 1992. There are two mechanisms of achiasmate segregation in Drosophila females, one of which requires heterochromatic homology. Dev. Genet. 13: 440–467. [DOI] [PubMed] [Google Scholar]
- Jacobs P. A., Strong J. A., 1959. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 183: 302–303. [DOI] [PubMed] [Google Scholar]
- Janssens, F. A., 1909 The chiasmatype theory. A new interpretation of the maturation divisions. Cellule 25: 389–411. (Translated from the French; reprinted in Genetics 191: 319–346.) [DOI] [PMC free article] [PubMed]
- Koehler K. E., Boulton C. L., Collins H. E., French R. L., Herman K. C., et al. , 1996. Spontaneous X chromosome MI and MII nondisjunction events in Drosophila melanogaster oocytes have different recombinational histories. Nat. Genet. 14: 406–414. [DOI] [PubMed] [Google Scholar]
- Kohler R. E., 1994. Lords of the Fly, University of Chicago Press, Chicago. [Google Scholar]
- Koszul R., Meselson M., Van Doninck K., Vandenhaute J., Zickler D., 2012. The centenary of Janssens’s chiasmatype theory. Genetics 191: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B., 1951. Pseudoallelism and gene evolution. Cold Spring Harb. Symp. Quant. Biol. 16: 159–174. [DOI] [PubMed] [Google Scholar]
- Lewis E. B., 1998. The bithorax complex: the first fifty years. Int. J. Dev. Biol. 42: 403–415. [PubMed] [Google Scholar]
- Morgan T. H., 1910a Chromosomes and heredity. Am. Nat. 477–478. [Google Scholar]
- Morgan, T. H., 1910b Sex-limited inheritance in Drosophila. Science 32: 120–122. [DOI] [PubMed] [Google Scholar]
- Nagaoka S. I., Hassold T. J., Hunt P. A., 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Hashimoto S., Nakaoka Y., Kouznetsova A., Hoog C., et al. , 2015. Bivalent separation into univalents precedes age-related meiosis I errors in oocytes. Nat. Commun. 6: 7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., Erickson J. W., 2010. Sex determination in Drosophila: the view from the top. Fly (Austin) 4: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler L., Lindsley D. L., Nicoletti B., Trippa G., 1968. Mutants affecting meiosis in natural populations of Drosophila melanogaster. Genetics 60: 525–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens N. M., 1905. Studies in Spermatogenesis with Especial Reference to the “Accessory Chromosome.” Carnegie Institution of Washington, Washington, DC. [Google Scholar]
- Sturtevant A. H., 1913. The linear arrangement of six sex-linked factors in Drosophila as shown by their mode of association. J. Exp. Zool. 14: 43–59. [Google Scholar]
- Sturtevant A. H., Beadle G. W., 1936. The relations of inversions in the X chromosome of Drosophila melanogaster to crossing over and disjunction. Genetics 21: 554–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. S., 1903. The chromosomes in heredity. Biol. Bull. 4: 231–251. [Google Scholar]
- Theurkauf W. E., Hawley R. S., 1992. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J. Cell Biol. 116: 1167–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons W. J., Russell L. B., 1959. The Y-chromosome as the bearer of male determining factors in the mouse. Proc. Natl. Acad. Sci. USA 45: 560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. B., 1905. The chromosomes in relation to the determination of sex in insects. Science 22: 500–502. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Hawley R. S., 2006. The mechanism of secondary nondisjunction in Drosophila melanogaster females. Genetics 174: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]