Abstract
Histones are among the most conserved proteins known, but organismal differences do exist. In this study, we examined the contribution that divergent amino acids within histone H3 make to cell growth and chromatin structure in Saccharomyces cerevisiae. We show that, while amino acids that define histone H3.3 are dispensable for yeast growth, substitution of residues within the histone H3 α3 helix with human counterparts results in a severe growth defect. Mutations within this domain also result in altered nucleosome positioning, both in vivo and in vitro, which is accompanied by increased preference for nucleosome-favoring sequences. These results suggest that divergent amino acids within the histone H3 α3 helix play organismal roles in defining chromatin structure.
Keywords: histone, H3, S. cerevisiae, nucleosome positioning
IN eukaryotes, DNA is packaged into a nucleoprotein structure known as chromatin, which consists of DNA, histones, and nonhistone proteins. The basic unit of chromatin is the nucleosome core particle, which is made up of an octamer of the four core histones, H2A, H2B, H3, and H4, wrapped with ∼147 bp of DNA (Luger et al. 1997; Kornberg and Lorch 1999). Although nucleosomes will form on most sequences in vitro, they are not randomly positioned in vivo. Genome-wide mapping studies have shown that gene promoters and other regulatory regions tend to be nucleosome depleted and two general mechanisms have been proposed to explain this (Hughes and Rando 2014). First, certain DNA sequences, such as AT-rich regions, are refractory to nucleosome formation. Second, trans-acting factors, such as transcriptional activators, RNA polymerases, and ATP-dependent chromatin remodelers can either evict or reposition nucleosomes. Nucleosomes immediately adjacent to nucleosome-depleted regions (NDRs) are generally well positioned, but nucleosome position shows more cell-to-cell variability with increasing distance from NDRs (Yuan et al. 2005; Mavrich et al. 2008). This has led to proposal of the statistical positioning model, which suggests that strongly positioned nucleosomes create barriers against which other nucleosomes are packed into positioned and phased arrays (Kornberg and Stryer 1988; Zhang et al. 2011).
Nucleosomes block the access of proteins to DNA and thus chromatin structure can modulate DNA-dependent processes such as transcription, replication, recombination, and DNA repair. Much of our insight into the role of chromatin in regulating the access of cellular machinery to DNA was driven by work with the budding yeast, Saccharomyces cerevisiae. Genetic analyses in this organism have revealed the roles played by histones and multiprotein complexes in regulating DNA-dependent processes (Rando and Winston 2012). However, although histones are among the most well-conserved proteins known, noted differences do exist between yeast and metazoan histones. First, although histone H4 is 92% conserved between S. cerevisiae and humans, H2A and H2B are less so (77 and 73% identity, respectively), which is suggested to impact nucleosome stability (White et al. 2001). Second, while the majority of eukaryotes express distinct histone H3 variants for replication-coupled (RC) (designated histone H3.1 and H3.2 in humans) and replication-independent (RI) (designated histone H3.3 in humans) histone deposition, S. cerevisiae has retained a histone H3.3-like variant for both pathways.
The amino acid differences between RC- and RI-specific H3 variants are proposed to restrict the histones to their requisite deposition pathways (Szenker et al. 2011), but these variants also directly alter chromatin structure in vitro (Thakar et al. 2009; Chen et al. 2013). This together with the previously established link between H3.3 deposition and active transcription in metazoans (Waterborg 1990; Johnson et al. 2004; McKittrick et al. 2004; Chow et al. 2005; Mito et al. 2005) has led to speculation that yeasts have retained H3.3 due to its ability to promote an open chromatin conformation. In this study, we sought to determine whether the divergent amino acids within histone H3 regulate chromatin structure in yeast. We found that, while H3.3-specific amino acids are dispensable for yeast growth, the α3 helix of histone H3 serves a yeast-specific function. Substitution of this region with the corresponding amino acids from human histone H3 results in a severe growth defect, increased nuclease sensitivity, loss of nucleosome positioning, and relocation of nucleosomes to predicted nucleosome-favoring sequences. Collectively these results suggest that divergent amino acids within the α3 helix of S. cerevisiae histone H3 contribute to a unique chromatin structure in this organism.
Materials and Methods
Yeast strains and plasmids
Strains expressing histone H3 mutations were derived from FY2162, which has deletions of the HHT1-HHF1 and HHT2-HHF2 genes, and carries HHT2-HHF2 on a URA3 plasmid (Duina and Winston 2004). A TRP1 plasmid expressing wild-type HHT2 and HHF2 was constructed by ligation of the SpeI restricted fragment from pDM18 into the SpeI site of pRS414 (Duina and Winston 2004). Plasmids expressing mutant versions of H3 were constructed by replacing the BamHI/XhoI fragment from this plasmid with codon optimized, synthesized DNA fragments (see Supporting Information, File S1). The DNA template containing 25 tandem 197-bp repeats of the 601 nucleosome positioning sequence (Lowary and Widom 1998) was obtained by subcloning a 25x197_601 construct (Huynh et al. 2005) into a modified version of the pWM530 plasmid (Dorigo et al. 2003). Strains and plasmids are available upon request.
MNase-seq analysis
One hundred milliliters of S. cerevisiae cultures were grown to an A600 of 0.8 and cross-linked with 1% formaldehyde for 15 min before quenching the reaction with 125 mM glycine. Cells were washed three times with 1 mL of 10 mM Tris-Cl pH 7.5 and 100 mM NaCl and frozen at −80°. Thawed cell pellets were resuspended in 600 μl of 50 mM HEPES pH 7.5, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, Roche protease inhibitor cocktail (PIC), and 0.2 mM PMSF and lysed by bead beating. The resulting lysate was centrifuged at 15,000 × g for 30 min and the chromatin pellet was washed and resuspended in 900 μl NP-S buffer (0.5 mM sperimidine, 1 mM β-ME, 0.075% NP-40, 50 mM NaCl, 10 mM Tris-Cl pH 7.4, 5 mM MgCl2, 1 mM CaCl2, EDTA-free PIC, and 2 mM PMSF). The resuspended pellet was added to 100 μl NP-S buffer containing 200 units micrococcal nuclease (MNase) and incubated at 37° for 1 hr. The reaction was stopped by addition of EDTA to 10 mM. Cross-links were reversed by the addition of 1% SDS and 40 μg proteinase K followed by incubation at 65° overnight. Following treatment with 20 μg RNase A, “spiked-in” synthetic DNA was added and construction of sequencing libraries was performed as described previously using eight rounds of PCR amplification (Maltby et al. 2012). Indexed samples were pooled and 100 bp paired-end sequencing was performed by using an Illumina HiSequation 2500.
FASTQ files were aligned to saccer3, the most recent build of the yeast genome (R64-1-1, released February 3, 2011; downloaded from http://www.yeastgenome.org), using the Burrows Wheeler aligner (BWA) mem algorithim (Li and Durbin 2010). Samtools was used to filter for reads mapped to the genome and for a mapping quality score of at least 10 (Li et al. 2009). Bedtools “intersect” was used to remove reads mapping to the ribosomal DNA (rDNA) locus on chromosome XII (chrXII) (Quinlan and Hall 2010). Reads longer than 200 bp were filtered out and the remaining data randomly sampled to normalize the numbers of reads. The Java Genomics Toolkit (downloaded from http://palpant.us/java-genomics-toolkit/) and Matrix2png (Pavlidis and Noble 2003) were used for all subsequent analysis.
In vitro analysis of nucleosome positioning
Histones were expressed in Escherichia coli BL21 (DE3) and purified to homogeneity through acid extraction, ion exchange (Macro-Prep Ion Exchange Media, BioRad), and reverse phase HPLC (C18-300, 250 mM × 4.6 mM, 5 μM, ACE). Array DNA was prepared by digesting the pWM530-25x197_601 plasmid with EcoRV, which generates both the desired 25-repeat of the 197-bp 601 positioning sequence as well as plasmid-backbone competitor DNA required for chromatin reconstitution. Individual lyophilized histones were combined in a 1.1:1.1:1:1 H2B:H2A:H3:H4 mole ratio, dissolved in unfolding buffer (7 M GuHCl, 20 mM Tris pH 7.5, 1 mM DTT) and dialyzed against three changes of refolding buffer (2 M NaCl, 10 mM Tris pH 7.5, 1 mM EDTA). Reconstituted histone octamer was purified by size exclusion chromatography using refolding buffer (Superdex 200 10/300 GL, GE Healthcare). Chromatinized arrays were assembled as previously described (Huynh et al. 2005) using a 1.1:1 (hH3) or 1.2:1 (yH3 and H3α3h) octamer:DNA ratio. Each chromatin species was treated with AvaI and HhaI, subjected to a PCR cleanup (EZ-10 spin column PCR purification kit, Biobasic) and resolved on a 0.7% agarose gel in 0.2× TB.
Results
Histone H3.3-defining amino acids are dispensable for growth of S. cerevisiae
Histone H3 in S. cerevisiae shares 90 and 89% identity with human H3.3 and H3.1/2, respectively. We were interested in determining the contribution of divergent amino acids within histone H3 to chromatin structure. To this end, we identified three regions of histone H3 that exhibit sequence differences between yeast and human (Figure 1, A and B) and generated codon-optimized constructs encoding yeast H3 that contained the human sequence for each region. Because human H3.1 and H3.3 differ in regions I and II, we generated two constructs with human versions of regions I and II to reflect differences between these variants. The resulting constructs were introduced into yeast via plasmid shuffle and histone H3 expression was driven from a native H3 promoter. All constructs fully rescued growth on rich media (Figure 1C, bottom panel), with the exception of the one in which region III was substituted with the human counterpart. Histone H3 was expressed at wild-type levels from this construct (Figure 1C, middle panel), suggesting that the slow growth phenotype was due to a defect in H3 function rather than protein production.
Figure 1.
Histone H3.3-defining amino acids are dispensable for growth of S. cerevisiae. (A) Amino acid sequences of histone H3 in S. cerevisiae and humans. Amino acid differences are shown in red text and regions I, II, and III used to generate human versions of H3 are shown as colored boxes with yH3 sequences in red, hH3.1 in yellow, and hH3.3 in blue. Note that regions III for hH3.1 and hH3.3 are highlighted in green to reflect the fact that these sequences are identical in hH3.1 and hH3.3. (B) Schematic representation of the primary sequence of yH3, hH3.1, and hH3.3 with regions I, II, and III shown as boxes colored as in A. (C, middle panel) Protein levels of the indicated H3 chimeras were detected by Western blot. (C, lower panel and D) Fivefold serial dilutions of yeast strains expressing the indicated H3 chimeras as the sole source of histone H3 were plated on YPD (C) or media supplemented with 0.015% MMS, 2% formamide, 4 mM caffeine, 75 mM HU, or 100 μg/mL 6-AU (D) and incubated at 30° for 3 days.
The indistinguishable growth rates of cells expressing histone H3 with the human version of region I or II was intriguing, as these regions include amino acids that differentiate histone H3.3 from H3.1 in metazoans. To further confirm that a histone H3.1-like variant can substitute for H3.3 in yeast, we created constructs expressing histone H3 chimeras with human regions I, II, and yeast region III. These constructs rescued growth of yeast on various growth media (Figure 1D) and thus, despite the conservation of histone H3.3-specific amino acids from yeast to humans, these residues are not critical for cell growth in S. cerevisiae.
The α3 helix of yeast histone H3 mediates nucleosome positioning in vivo
Region III of histone H3 encompasses the third alpha helix within the histone fold, plus four additional carboxyl-terminal amino acids. Yeast and human histone H3 differ at five amino acids within this region (Figure 1A), but mutation of these amino acids individually did not result in a growth defect (Figure 2A and data not shown). The viability of the Q120M and L130I individual substitutions was surprising as others have shown that substitution of these residues with glutamic acid and alanine, respectively, results in lethality (Dai et al. 2008); however, the lack of phenotype in our study was most likely due to the more conservative nature of the substitutions. Three of the divergent residues within region III are located within the third alpha helix, which is in close proximity to the nucleosome dyad (Figure 2B). While yeast-to-human substitutions of these residues rescued growth of yeast on various growth media, mutation of all three residues in combination (designated yH3α3h) largely recapitulated the growth defect of the entire region III swap (Figure 2A). Moreover, cells expressing yH3α3h also appeared to exhibit sensitivity to a range of drugs, including 6-azauracil, hydroxyurea, methyl methanesulfonate, caffeine, and formamide. These phenotypes should be interpreted with caution, however, as they may be a manifestation of the slow growth of the mutant. Histone protein levels were unchanged in the yH3α3h mutant, indicating that the growth defect was not due to reduced histone content (Figure 2, C and D). Collectively, these results suggest a yeast-specific function for the histone H3 α3 helix.
Figure 2.
Mutation of the histone H3 α3 helix confers a growth defect in yeast that is not due to impaired histone deposition. (A) Yeast expressing wild-type histone H3, or histone H3 with yeast-to-human substitutions at the indicated amino acids were plated in fivefold dilutions on YPD or media supplemented with 0.015% MMS, 2% formamide, 4 mM caffeine, 75 mM HU, or 100 μg/mL 6-AU, and incubated at 30° for 3 days. (B) PyMOL (The PyMOL Molecular Graphics System 2010) structure of a yeast nucleosome (White et al. 2001) with both subunits of H3 highlighted in green. Residues Q120 (red), K121 (yellow), and K125 (magenta) of the α3 helix are indicated. (C) Strains expressing wild-type H3 or histone H3 with yeast-to-human substitutions at amino acids 120, 121, and 125 (yH3α3h) were lysed and subjected to Western blot analysis. (D) Quantitative analysis of H3 over H4 signal in C from three biological replicates. (E) Yeast whole cell extracts (input) from the indicated strains were fractionated into soluble (sup) and insoluble (pellet) components and subjected to immunoblot for histone H3.
Newly synthesized histones are deposited on DNA with the aid of histone chaperones and multiple chaperones have been shown to interact with the histone H3 α3 helix, although in the case of the Asf1 histone chaperone, residues critical for interaction are conserved from yeast to human (Antczak et al. 2006; English et al. 2006; Agez et al. 2007). Nevertheless, to rule out the possibility that the growth defect of the yH3α3h mutant was due to a histone deposition defect, we fractionated yeast whole cell extracts into soluble and chromatin-associated fractions. Figure 2E demonstrates equal levels of histone H3 in the chromatin fraction in both the wild-type and mutant strains, suggesting that the growth defect observed in Figure 2A was not due to fewer histones associated with DNA.
Although the yH3α3h mutant did not appear to have a defect in nucleosome assembly, it is possible that the nucleosomes are mislocalized relative to the DNA sequence in this strain. To test this, we mapped nucleosome positions by deep sequencing of micrococcal nuclease (MNase)-resistant fragments. Our first observation was that replacement of the yH3 α3 helix with the human counterpart resulted in increased MNase sensitivity (Figure 3A). Despite the recovery of slightly shorter DNA, the use of spike-in control DNA indicated that we did not recover less MNase-resistant DNA from the mutant, again supporting the fact that there are not fewer nucleosomes in the yH3α3h mutant.
Figure 3.
The α3 helix of yeast histone H3 mediates nucleosome positioning. (A) Histogram of read lengths of sequenced micrococcal nuclease-digested DNA from yeast expressing wild-type histone H3 (yH3) or histone H3 with yeast-to-human substitutions at amino acids 120, 121, and 125 (yH3α3h). Note that the replicates were from independent cultures. (B) The average number of read midpoints (i.e., nucleosome dyads) from sequencing of micrococcal nuclease-resistant DNA plotted relative to 5043 transcriptional start sites (TSSs) (van Bakel et al. 2013). (C) A phasogram (distribution of distance between read midpoints) across the yeast genome for yeast expressing the indicated versions of histone H3.
To determine nucleosome positioning in both the wild-type and yH3α3h cells, the midpoint of each sequence read was calculated and mapped relative to 5043 transcriptional start sites (TSSs) (van Bakel et al. 2013). Figure 3B shows that wild-type cells exhibited a typical nucleosome position pattern with an NDR upstream of the TSS, a well-positioned “plus one” nucleosome and a regular array of nucleosomes across the gene body. The yH3α3h mutant showed a similar NDR, but the peak summits of the nucleosomes were lower and the valleys were shallower, indicative of less regular nucleosome packing. These differences were also observed when mapping full reads and full reads adjusted for read length, demonstrating that the altered nucleosome positioning observed in the mutant was not an effect of reduced MNase protection (data not shown). To further confirm that the nucleosomes were less well positioned in the yH3α3h mutant, we plotted the read midpoints as a phasogram, which displays the average distance between the dyad of each nucleosome and the dyad of adjacent nucleosomes (Figure 3C). Although the phases were similar in wild-type and mutant cells (162 bp), indicative of a similar average nucleosome repeat length, the phasogram confirmed that nucleosomes were not as regularly packed relative to each other in the yH3α3h mutant when compared to wild type.
The human histone H3 α3 helix promotes preference for nucleosome positioning sequences in S. cerevisiae
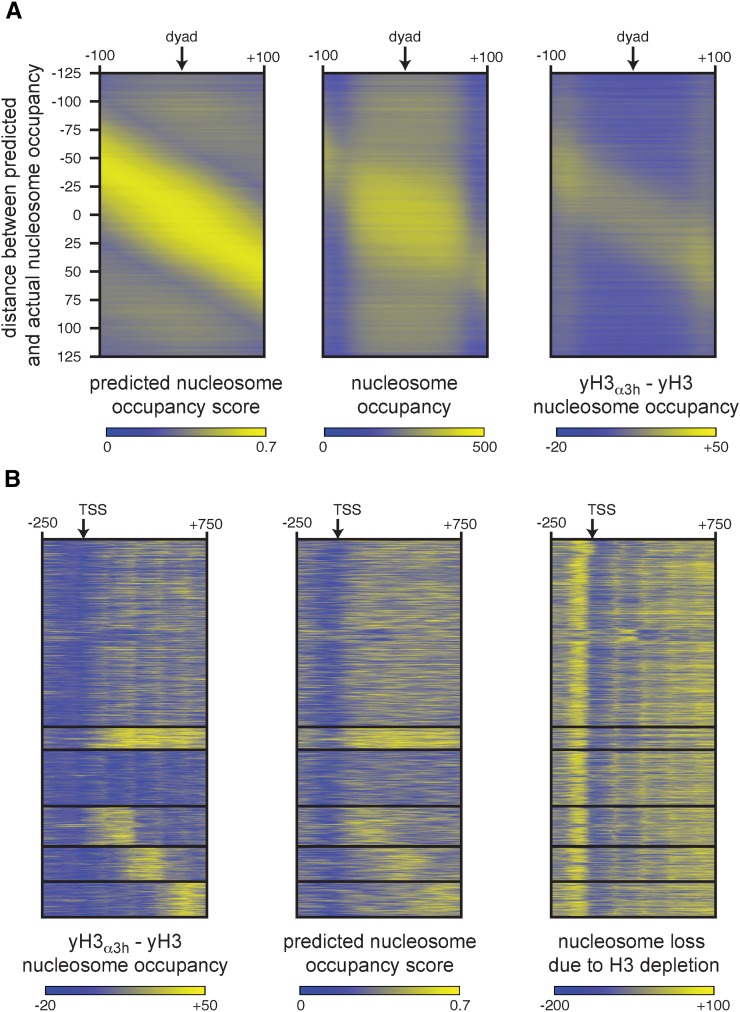
Although nucleosomes will assemble on most sequences, they do show sequence preference. Indeed algorithms have been developed that predict nucleosome occupancy based on sequence alone (Liu et al. 2014). The performance of these algorithms, however, is modest in predicting positions in vivo, presumably due to cellular activities that mobilize nucleosomes away from their sequence-preferred positions. The atypical nucleosome positioning in the yH3α3h mutant led us to hypothesize that humanized H3 shows increased preference for nucleosome favoring sequences. To test this, we called the position of 63,998 nucleosomes in wild-type yeast and determined the optimal predicted nucleosome occupancy within a region 125 bp upstream and 125 bp downstream of the dyad of each nucleosome (Kaplan et al. 2009). Nucleosomes were then sorted based on the distance between the maximum predicted nucleosome occupancy and the actual nucleosome dyad and the predicted and actual occupancies plotted as heat maps. Comparison of the left panel (predicted nucleosome occupancy) with the middle panel (actual nucleosome occupancy) in Figure 4A confirmed that the majority of nucleosomes deviate from the site predicted based on DNA sequence. We then calculated the increase in nucleosome occupancy in the yH3α3h mutant across the same nucleosomes. The right panel of Figure 4A shows that changes in nucleosome occupancy in the mutant closely matched the predicted nucleosome position, suggesting the nucleosomes were shifting to more preferred sequences in the yH3α3h mutant.
Figure 4.
The human histone H3 α3 helix enhances preference for nucleosome positioning sequences in S. cerevisiae. (A) The position of 63,998 nucleosomes in yeast expressing wild-type histone H3 was called using the greedy caller algorithm from the Java Genomics Toolkit, and the nucleosome dyads were determined. The predicted nucleosome occupancy (Kaplan et al. 2009) 125 bp upstream and downstream of the dyad was determined and the nucleosomes were sorted based on the distance between the maximum predicted occupancy and the actual nucleosome dyad. Predicted nucleosome occupancy, actual nucleosome occupancy, and increase in nucleosome occupancy in the yH3α3h mutant were plotted as heat maps. (B) K-means clustering was used to divide yeast genes longer than 750 bp into six bins based on changes in nucleosome occupancy in the yH3α3h mutant. Increase in nucleosome occupancy in the yH3α3h mutant, predicted nucleosome occupancy (Kaplan et al. 2009), and nucleosome loss due to histone H3 depletion (Gossett and Lieb 2012) were plotted as heatmaps.
The relocalization of nucleosomes to preferred sequences was documented in another study examining the effects of histone H3 depletion (Gossett and Lieb 2012). Although our data thus far have ruled out a major loss of nucleosomes in the yH3α3h mutant, subtle changes in the occupancy of neighboring nucleosomes could explain the results observed in Figure 4A. To rule this possibility out, we used K-means clustering to divide genes longer than 750 bp (4455 genes) into six clusters based on the pattern of changes in nucleosome occupancy in the yH3α3h mutant within a region 250 bp upstream to 750 bp downstream of the TSS (Figure 4B, left panel). Again, we observed increases in nucleosome occupancy closely mirrored patterns of predicted nucleosome occupancy (Figure 4B, middle panel). We then compared changes in nucleosome occupancy in the yH3α3h mutant to changes due to depletion of histone H3 (Figure 4B, right panel). Clusters that showed increased nucleosome occupancy in the yH3α3h mutant did not exhibit similar increases upon depletion of histone H3, indicating that the changes in nucleosome position in the yH3α3h mutant were not a manifestation of changes in nucleosome occupancy.
The α3 helix of histone H3 influences nucleosome positioning in vitro
Our data thus far have shown that yeast-to-human changes of amino acids at positions 120, 121, and 125 in the carboxyl terminus of histone H3 (which reduce positive charge) direct nucleosomes toward predicted positioning sequences in vivo. This shift could be the result of altered interactions between histone H3 and the cellular machinery or direct effects on histone–DNA interactions. In support of the latter, it has been shown that neutralization of charge in this area by acetylation of H3 K122 destabilizes the nucleosome in vitro (Manohar et al. 2009). To test between these two possibilities, we monitored the positioning of recombinant nucleosomes containing yeast H3 and yH3α3h on DNA in vitro. Using the standard salt dialysis method, we loaded histone octamers onto a 25-mer repeat of the 197-bp Widom 601 nucleosome positioning sequence (Figure 5A). The positioning of nucleosomes on this array can be readily measured by restriction enzyme digestion with AvaI and HhaI, whose recognition sites are in the linker regions and at the nucleosome dyad axis, respectively (Figure 5, A and B). As expected, we found that recombinant human histone octamers loaded in an ordered fashion onto 601 DNA; almost all AvaI sites in these arrays were digested after 60 min, but all HhaI sites remained protected (Figure 5C, left). By contrast, we found that recombinant octamers containing yH3 exhibited an alternative loading behavior. While all HhaI sites were protected, only ∼55% of AvaI sites were digested, indicating a proportion of nucleosomes in these arrays occlude the linker region (Figure 5C, middle). This positioning difference can be attributed to yeast specific amino acids at positions 120, 121, and 125 in the carboxyl terminus of H3 because yeast nucleosomes bearing yH3α3h behave as human nucleosomes; 94% of these chromatin arrays were reduced to mononucleosome species with AvaI (Figure 5C, right). Collectively these results suggest that amino acid differences within the α3 helix of histone H3 function to directly dictate organismal-specific chromatin structure.
Figure 5.
The α3 helix of histone H3 affects nucleosome positioning in vitro. (A) Schematic representation of chromatin arrays including restriction enzyme site locations. (B) Fifteen-minute AvaI or HhaI digestion of naked array DNA liberates a 197-bp mononucleosome fragment. * indicates competitor DNA fragments generated from the array plasmid that are removed upon preparative chromatin array precipitation. (C) Chromatin arrays assembled from recombinant human (hH3), yeast (yH3), or humanized yH3 (yH3α3h) were digested with AvaI or HhaI for 0, 30, and 60 min. DNAs were purified, resolved, and visualized by ethidium bromide staining. The proportion of array digested to mononucleosome was estimated by the densitometric ratio of mononucleosome species to input (undigested) array.
Discussion
The budding yeast S. cerevisiae has been used extensively as a model organism for the study of chromatin structure with the assumption that, due to the high conservation of histones, data generated is relevant to other eukaryotes. Differences in histone sequences do exist between yeast and humans and in this study we examined the contribution that yeast-specific amino acids within histone H3 have on chromatin structure. Through this analysis we demonstrated that replacement of all but the α3 helix of yeast H3, including the residues that differentiate histone H3.1 from H3.3, with the human counterparts fully rescues growth of yeast in multiple conditions. In contrast, replacing residues within the α3 helix of yeast histone H3 with their human counterpart’s results in a severe growth defect, increased nuclease sensitivity, and altered nucleosome positioning both in vivo and in vitro. Collectively these results confirm other studies that suggest that minor sequence variations within histones function to establish a unique chromatin structure in S. cerevisiae (D’Arcy and Luger 2011).
Despite the differences in sequences of hH3.1 and hH3.3, we found that both equally rescued growth of yeast in numerous conditions, suggesting that S. cerevisiae did not retain an H3.3-like variant to maintain an open chromatin structure. The full rescue of cell growth with an H3.1-like variant is surprising when one considers that others have shown that while ectopically expressed hH3.3 is incorporated into yeast chromatin via the RI pathway, hH3.1 is not (Song et al. 2013). It should be noted however that, in contrast to the aforementioned study, the yeast in our work did not have wild-type histone H3 available and thus a failure to observe integration of hH3.1 in the previous study could have been due to competition effects. Alternately, it is possible that growth of yeast is not strongly dependent on RI histone deposition. Indeed, loss of the HIR complex, responsible for RI deposition of H3.3, does not result in a growth defect (Sherwood et al. 1993). It also should be noted our analysis does not rule out the possibility that hH3.1 may not rescue yeast growth in other media or in competitive growth conditions.
In contrast to the minimal impact of replacing the remainder of histone H3, substitution of the α3 helix of yeast histone H3 had significant consequences on chromatin structure including increased micrococcal nuclease sensitivity, defects in nucleosome phasing, and an increased preference of octamers for nucleosome-favoring sequences. Altered nucleosome positioning was observed both in vitro and in vivo, suggesting intrinsic differences in nucleosome structure in the mutant, as opposed to differing susceptibility to cellular factors such as polymerases or ATP-dependent remodellers. One explanation for our observations is that histone octamers with human histone H3 are more sensitive to sequence positioning effects. Interestingly, the algorithm we used for prediction of nucleosome favoring sequences was trained using DNA reconstituted with Xenopus histones, which share an identical α3 helix with human H3 (Kaplan et al. 2009). If yeast and human histones show different sequence dependencies, one would expect the algorithm to work better in vivo when using the yH3α3h mutant. However, other approaches that disrupt nucleosome packing, such as transcription inhibition or histone depletion, also increase the ability of the Kaplan score to predict nucleosome position (Weiner et al. 2010; Gossett and Lieb 2012), suggesting that factors that govern nucleosome occupancy of Xenopus nucleosomes are relevant to yeast nucleosomes.
A second explanation for our results is that nucleosomes with the human histone H3 α3 helix may be refractory to statistical positioning, and thus relocate to preferred positions as a default. The statistical positioning model suggests that nucleosome position is dictated by the packing of nucleosomes against a strongly positioned nucleosome barrier (Kornberg and Stryer 1988; Mavrich et al. 2008). The +1 nucleosome on protein coding genes is the most well-characterized barrier nucleosome, and our data suggest that the position of the NDR and +1 nucleosome is unchanged in the yH3α3h mutant. We observe the same results when examining other barrier nucleosomes (Hsieh et al. 2015) (data not shown). We therefore propose that it is the packing of nucleosomes that is defective in the yH3α3h mutant. Humanization of the α3 helix involves loss of two lysine residues and the introduction of a proline, which are expected to alter both the charge and secondary structure of this motif. The α3 helix is located at the dyad axis of the nucleosome, where it could conceivably alter the trajectory of linker DNA entering and exiting the nucleosome, thus determining the orientation and packing of adjacent nucleosomes in an array. The α3 helix is unlikely to play as critical a role in statistical positioning in human cells as these cells have longer linker DNA, and thus the trajectory of DNA exiting the nucleosome would not have such an impact. Further, since linker length is dictated by the abundance of linker histones (Schlegel et al. 1980; Fan et al. 2005), S. cerevisiae-specific amino acids within the histone H3 α3 helix may be important due to the substoichiometric amounts of linker histones in this organism (Freidkin and Katcoff 2001; Downs et al. 2003). It will be interesting to determine whether there is a link between α3 helix sequences and linker histone stoichiometry in other organisms.
Acknowledgments
We gratefully acknowledge Julien Bergeron for assistance in generating figures and Fred Winston, Daniela Rhodes, and Timothy Richmond for providing yeast strains and plasmids. This work was supported by Discovery Grants awarded to C.J.N. and L.J.H. from the Natural Sciences and Engineering Research Council (NSERC) and an operating grant from the Canadian Institutes of Health Research (awarded to L.J.H.). B.J.E.M. and J.K.C. are recipients of NSERC Alexander Graham Bell Canada Graduate Scholarships and N.A.T.I. was supported by an NSERC Undergraduate Student Research Award.
Footnotes
Communicating editor: M. Hampsey
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.180810/-/DC1.
The MNase-seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession number: GSE73425).
Literature Cited
- Agez M., Chen J., Guerois R., van Heijenoort C., Thuret J. Y., et al. , 2007. Structure of the histone chaperone ASF1 bound to the histone H3 C-terminal helix and functional insights. Structure 15: 191–199. [DOI] [PubMed] [Google Scholar]
- Antczak A. J., Tsubota T., Kaufman P. D., Berger J. M., 2006. Structure of the yeast histone H3–ASF1 interaction: implications for chaperone mechanism, species-specific interactions, and epigenetics. BMC Struct. Biol. 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Zhao J., Wang Y., Wang M., Long H., et al. , 2013. H3.3 actively marks enhancers and primes gene transcription via opening higher-ordered chromatin. Genes Dev. 27: 2109–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C. M., Georgiou A., Szutorisz H., Maia e Silva A., Pombo A., et al. , 2005. Variant histone H3.3 marks promoters of transcriptionally active genes during mammalian cell division. EMBO Rep. 6: 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcy S., Luger K., 2011. Understanding histone acetyltransferase Rtt109 structure and function: How many chaperones does it take? Curr. Opin. Struct. Biol. 21: 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Hyland E. M., Yuan D. S., Huang H., Bader J. S., et al. , 2008. Probing nucleosome function: a highly versatile library of synthetic histone H3 and H4 mutants. Cell 134: 1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigo B., Schalch T., Bystricky K., Richmond T. J., 2003. Chromatin fiber folding: requirement for the histone H4 N-terminal tail. J. Mol. Biol. 327: 85–96. [DOI] [PubMed] [Google Scholar]
- Downs J. A., Kosmidou E., Morgan A., Jackson S. P., 2003. Suppression of homologous recombination by the Saccharomyces cerevisiae linker histone. Mol. Cell 11: 1685–1692. [DOI] [PubMed] [Google Scholar]
- Duina A. A., Winston F., 2004. Analysis of a mutant histone H3 that perturbs the association of Swi/Snf with chromatin. Mol. Cell. Biol. 24: 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English C. M., Adkins M. W., Carson J. J., Churchill M. E., Tyler J. K., 2006. Structural basis for the histone chaperone activity of Asf1. Cell 127: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Nikitina T., Zhao J., Fleury T. J., Bhattacharyya R., et al. , 2005. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell 123: 1199–1212. [DOI] [PubMed] [Google Scholar]
- Freidkin I., Katcoff D. J., 2001. Specific distribution of the Saccharomyces cerevisiae linker histone homolog HHO1p in the chromatin. Nucleic Acids Res. 29: 4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett A. J., Lieb J. D., 2012. In vivo effects of histone H3 depletion on nucleosome occupancy and position in Saccharomyces cerevisiae. PLoS Genet. 8: e1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T. H., Weiner A., Lajoie B., Dekker J., Friedman N., et al. , 2015. Mapping nucleosome resolution chromosome folding in yeast by micro-C. Cell 162: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. L., Rando O. J., 2014. Mechanisms underlying nucleosome positioning in vivo. Annu. Rev. Biophys. 43: 41–63. [DOI] [PubMed] [Google Scholar]
- Huynh V. A., Robinson P. J., Rhodes D., 2005. A method for the in vitro reconstitution of a defined “30 nm” chromatin fibre containing stoichiometric amounts of the linker histone. J. Mol. Biol. 345: 957–968. [DOI] [PubMed] [Google Scholar]
- Johnson L., Mollah S., Garcia B. A., Muratore T. L., Shabanowitz J., et al. , 2004. Mass spectrometry analysis of Arabidopsis histone H3 reveals distinct combinations of post-translational modifications. Nucleic Acids Res. 32: 6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan N., Moore I. K., Fondufe-Mittendorf Y., Gossett A. J., Tillo D., et al. , 2009. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature 458: 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., Stryer L., 1988. Statistical distributions of nucleosomes: nonrandom locations by a stochastic mechanism. Nucleic Acids Res. 16: 6677–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D., Lorch Y., 1999. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 98: 285–294. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R., 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J. et al, 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang R., Xiong W., Guan J., Zhuang Z., et al. , 2014. A comparative evaluation on prediction methods of nucleosome positioning. Brief. Bioinform. 15: 1014–1027. [DOI] [PubMed] [Google Scholar]
- Lowary P. T., Widom J., 1998. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 276: 19–42. [DOI] [PubMed] [Google Scholar]
- Luger K., Mader A. W., Richmond R. K., Sargent D. F., Richmond T. J., 1997. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389: 251–260. [DOI] [PubMed] [Google Scholar]
- Maltby V. E., Martin B. J. E., Brind’Amour J., Chruscicki A. T., McBurney K. L., et al. , 2012. Histone H3K4 demethylation is negatively regulated by histone H3 acetylation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 109: 18505–18510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar M., Mooney A. M., North J. A., Nakkula R. J., Picking J. W., et al. , 2009. Acetylation of histone H3 at the nucleosome dyad alters DNA-histone binding. J. Biol. Chem. 284: 23312–23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrich T. N., Ioshikhes I. P., Venters B. J., Jiang C., Tomsho L. P., et al. , 2008. A barrier nucleosome model for statistical positioning of nucleosomes throughout the yeast genome. Genome Res. 18: 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKittrick E., Gafken P. R., Ahmad K., Henikoff S., 2004. Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc. Natl. Acad. Sci. USA 101: 1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito Y., Henikoff J. G., Henikoff S., 2005. Genome-scale profiling of histone H3.3 replacement patterns. Nat. Genet. 37: 1090–1097. [DOI] [PubMed] [Google Scholar]
- Pavlidis P., Noble W. S., 2003. Matrix2png: a utility for visualizing matrix data. Bioinformatics 19: 295–296. [DOI] [PubMed] [Google Scholar]
- The PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC. Available at: http://www.pymol.org.
- Quinlan A. R., Hall I. M., 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando O. J., Winston F., 2012. Chromatin and transcription in yeast. Genetics 190: 351–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R. A., Haye K. R., Litwack A. H., Phelps B. M., 1980. Nucleosome repeat lengths in the definitive erythroid series of the adult chicken. Biochim. Biophys. Acta 606: 316–330. [DOI] [PubMed] [Google Scholar]
- Sherwood P. W., Tsang S. V., Osley M. A., 1993. Characterization of HIR1 and HIR2, two genes required for regulation of histone gene transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 13: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Seol J. H., Yang J. H., Kim H. J., Han J. W., et al. , 2013. Dissecting the roles of the histone chaperones reveals the evolutionary conserved mechanism of transcription-coupled deposition of H3.3. Nucleic Acids Res. 41: 5199–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szenker E., Ray-Gallet D., Almouzni G., 2011. The double face of the histone variant H3.3. Cell Res. 21: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakar A., Gupta P., Ishibashi T., Finn R., Silva-Moreno B., et al. , 2009. H2A.Z and H3.3 histone variants affect nucleosome structure: biochemical and biophysical studies. Biochemistry 48: 10852–10857. [DOI] [PubMed] [Google Scholar]
- van Bakel H., Tsui K., Gebbia M., Mnaimneh S., Hughes T. R., et al. , 2013. A compendium of nucleosome and transcript profiles reveals determinants of chromatin architecture and transcription. PLoS Genet. 9: e1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterborg J. H., 1990. Sequence analysis of acetylation and methylation in two histone H3 variants of alfalfa. J. Biol. Chem. 265: 17157–17161. [PubMed] [Google Scholar]
- Weiner A., Hughes A., Yassour M., Rando O. J., Friedman N., 2010. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 20: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. L., Suto R. K., Luger K., 2001. Structure of the yeast nucleosome core particle reveals fundamental changes in internucleosome interactions. EMBO J. 20: 5207–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G. C., Liu Y. J., Dion M. F., Slack M. D., Wu L. F., et al. , 2005. Genome-scale identification of nucleosome positions in S. cerevisiae. Science 309: 626–630. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Wippo C. J., Wal M., Ward E., Korber P., et al. , 2011. A packing mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science 332: 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]