Abstract
Objective:
To evaluate the effect of dimethyl fumarate (DMF; Tecfidera, Biogen, Weston, MA) on CD4+ and CD8+ T cell subsets in patients with multiple sclerosis (MS).
Methods:
Peripheral lymphocyte subsets, including CD4+ and CD8+ memory cells and T helper (TH) cells TH1, TH2, TH17, and peripheral regulatory T cell (pTreg) subpopulations were analyzed before and 6 months after onset of DMF treatment.
Results:
CD4+ and CD8+ memory T cells were preferentially decreased compared to naive CD4+ and CD8+ T cell populations. Within the CD4+ memory T cell population, frequencies of TH1 cells were decreased, whereas those of TH2 cells were increased and those of TH17 cells remained unaltered. Accordingly, we observed decreased production of interferon γ, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor α, and interleukin (IL)-22 by CD4+ T cells under DMF treatment, whereas the frequency of IL-4- and IL-17A-producing CD4+ T cells remained unchanged. With regard to regulatory T cells, proportions of pTreg increased following DMF treatment.
Conclusion:
Our data demonstrate that DMF treatment of patients with MS affects predominantly memory T cells accompanied by a shift in TH cell populations, resulting in a shift toward anti-inflammatory responses. These findings indicate that monitoring of memory subsets might enhance vigilance of impaired antiviral immunity and that patients with TH1-driven disease might preferentially benefit from DMF treatment.
Classification of Evidence:
This study provides Class IV evidence that DMF might preferentially reduce CD4+ and CD8+ memory T cells in MS.
Delayed-release dimethyl fumarate (DMF; Tecfidera, Biogen, Weston, MA) is a newly approved immune-modulatory drug for treatment of relapsing-remitting multiple sclerosis (RRMS) whose mechanism of action has not been fully resolved.1,2 Anti-inflammatory and neuroprotective effects of DMF have been documented, including a reduction in lymphocyte cytokine production, a reduction in lymphocyte counts presumably by an apoptosis-related mechanism, a downregulation of the migratory activity of immune cells at the blood-brain-barrier, and activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) transcriptional pathway mediating antioxidative and potentially neuroprotective effects.3–7
Immunologic data from patients with RRMS treated with DMF are still sparse. In the clinical study program, a mean reduction in lymphocyte counts of about 50% after 1 year of treatment has been described,1,2 which could be replicated by others.8 The reason a more pronounced reduction in lymphocytes occurs in about 6% of individuals9 remains unclear but should be highlighted because lymphopenia in the context of fumaric ester treatment has been associated with rare cases of progressive multifocal leukoencephalopathy (PML) in both patients with psoriasis and RRMS.9–13 Recently, it has been observed that CD8+ T cells are more affected by DMF treatment–induced lymphopenia than CD4+ T cells.8 However, a detailed analysis of lymphocyte subset changes under DMF treatment in patients with RRMS has not been provided. We therefore aimed to provide a detailed characterization of changes in lymphocyte subset composition as a consequence of DMF treatment in order to increase our knowledge of DMF-mediated immune alterations in the context of MS.
METHODS
Patients.
All patients were recruited at the Department of Neurology at the University Hospital Münster, Germany. Fifteen stable patients with RRMS (ages 24–54 years, mean age 40.7 years; 7 female, 8 male) were included and treated with a standard treatment regimen of DMF for 6 months. Forty-six percent of the patients were treatment naive, whereas 27% each had been previously treated with glatiramer acetate or interferon (IFN) α. All patients switching from glatiramer acetate or IFN-α underwent a washout period of at least 4 weeks.
Standard protocol approvals, registrations, and patient consents.
This study was performed according to the Declaration of Helsinki and was approved by the local ethics committee (# 2010-236-f-S). All patients gave written informed consent.
Cells.
Ethylenediaminetetraacetic acid (EDTA) blood was taken from each patient immediately before the first dose of DMF as well as after 6 months of therapy. Peripheral blood mononuclear cells (PBMCs) were isolated and stored in liquid nitrogen according to our standard operating procedure (SOP).14 Samples from baseline and after 6 months of therapy were thawed following our SOP.14
Cell culture.
For cytokine stimulation assays, freshly thawed PBMCs were centrifuged at 300g for 5 minutes, resuspended in X-Vivo 15 ± 10 μL/mL Leukocyte Activation Cocktail (phorbol 12-myristate 13-acetate, ionomycin, and Brefeldin A; BD Biosciences, Franklin Lakes, NJ) at a concentration of 5 Χ 106 PBMC/mL, and incubated at 37°C/5% CO2 for 6 hours. Finally, PBMCs were washed and stained for flow cytometry.
Flow cytometry.
Freshly thawed or stimulated PBMCs were centrifuged at 300g for 5 minutes, resuspended in phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO) supplemented with 2% heat-inactivated fetal bovine serum (GE Healthcare/PAA, Little Chalfont, UK) and 2 mM EDTA (Sigma-Aldrich) with fluorochrome-conjugated antibodies at the indicated working concentrations (see table e-1 at Neurology.org/nn) or isotype-matched controls, and incubated at 4°C for 30 minutes. Staining of chemokine receptors was performed at 37°C for 30 minutes. Subsequently, cells were washed twice and either analyzed by flow cytometry (Navios; Beckman Coulter, Brea, CA) or stained for intracellular proteins with fixation/permeabilization solution (eBioscience, San Diego, CA) following the manufacturer's instructions. Resulting data were analyzed using Kaluza Flow Cytometry Analysis software version 1.2 (Beckman Coulter) and Prism software version 5.04 (GraphPad, La Jolla, CA).
Gating strategy.
CD14− lymphocytes were selected in a CD14 vs side scatter plot. Lymphocytes were then displayed in a CD3 vs CD56 plot and CD3+CD56− T cells were selected. T cells were further divided into CD4+CD8− and CD8+CD4− subsets using a CD4 vs CD8 plot. Naive T cells were defined as CD45RO−CD27+, whereas memory T cells were defined as CD45RO+ CD4 or CD8 T cells and distinguished by expression of CCR4/CD194, CCR6/CD196, and CXCR3/CD183 into CCR4−CCR6−CXCR3+ T helper (TH)1 cells, CCR4+CCR6−CXCR3− TH2 cells, and CCR4+CCR6+CXCR3− TH17 cells according to the literature.15 Peripheral regulatory T cells (pTreg) were defined as CD127lowCD25highFoxP3+Helios− CD4 T cells.16,17
RESULTS
Six months of DMF treatment did not alter the proportion of CD3+CD56− T cells within the lymphocyte population (figure 1A, left), whereas it resulted in a significant decrease in CD8+ T cells within the T cell subpopulation (figure 1B, left, 19.39%, p = 0.024), in accordance with an earlier study.8 Further analysis revealed that memory T cells (CD45RO+) were predominantly affected within the CD4+ and the CD8+ T cell population, resulting in a highly significant reduction in CD8+ as well as CD4+ memory T cells (figure 1B; 29.90%, p = 0.0079 and 31.13%, p = 0.0002, respectively). Of note, DMF resulted in a decrease of both CD45RO+CD27+ central memory as well as CD45RO+CD27− effector memory cells (data not shown). In contrast, percentages of naive (CD45RO−CD27+) CD4+ and CD8+ T cell subsets increased under DMF treatment (19.26%, p = 0.001 and 18.26%, p = 0.0035, respectively).
Figure 1. Effect of DMF therapy on T cell subsets.

Peripheral blood mononuclear cells from 12 patients with relapsing-remitting multiple sclerosis (RRMS) at baseline (0M) and after 6 months of therapy (6M) with dimethyl fumarate (DMF; Tecfidera, Biogen, Weston, MA) were thawed and analyzed by flow cytometry for changes in the T cell compartment. (A) Left: CD3+CD56− T cells as percentage of total lymphocytes; right: ratio of CD4+ to CD8+ T cells. (B) DMF-induced changes in CD8+ T cells (top left: CD8+CD4− T cells as percentage of total T cells; top center: CD8+CD45RO− naive T cells as percentage of total CD8+ T cells; top right: CD8+CD45RO+ memory T cells as percentage of total CD8+ T cells) and in CD4+ T cells (bottom left: CD4+CD8− T cells as percentage of total T cells; bottom center: CD4+CD45RO− naive T cells as percentage of total CD4+ T cells; bottom right: CD4+CD45RO+ memory T cells as percentage of total CD4+ T cells). Statistical analysis was done by paired Student t test, *p < 0.05, **p < 0.01, ***p < 0.001.
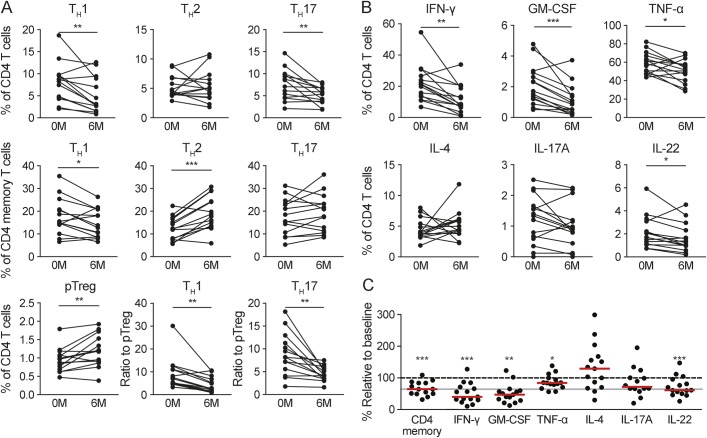
We further addressed the impact of DMF treatment on distinct CD4+ T cell subsets (figure 2A). Whereas proportions of TH1 and TH17 cells were significantly reduced at 6 months after onset of DMF treatment (36.77%, p = 0.0045 and 32.10%, p = 0.0059, respectively), the proportion of TH2 cells was not altered (figure 2A, top). Because DMF treatment decreased CD4 memory T cells in general (figure 1B, bottom), we also analyzed DMF-induced alterations within the cytokine-producing CD4 memory subset (figure 2A, middle). We observed a significant decrease of TH1 cells (17.02%, p = 0.035) accompanied by an increase of TH2 cells (53.67%, p = 0.0003), whereas TH17 cells remained unchanged. With regard to the regulatory subsets, the proportion of pTreg cells was significantly increased (24.6%, p = 0.0087). As a consequence, DMF treatment resulted in a significant reduction of the TH1/pTreg (52.14%, p = 0.0029) and TH17/pTreg (48.21%, p = 0.002) ratios (figure 2A, bottom).
Figure 2. Effect of DMF therapy on the T helper cell repertoire and cytokine production.
Peripheral blood mononuclear cells (PBMCs) from patients at baseline (0M) and after 6 months of therapy (6M) with dimethyl fumarate (DMF) were analyzed by flow cytometry for changes in the T cell compartment with focus on helper T (TH) cells (A, n = 14) and their cytokine production (B, C, n = 15). (A) TH1 (left), TH2 (center), and TH17 cells (right) as percentage of CD4+ T cells (top) and memory CD4+ T cells (middle) at baseline and after 6 months of treatment; bottom: peripheral regulatory T cells (pTreg) as percentage of total CD4+ T cells (left) and TH1 as well as TH17 (in percent of CD4+ T cells) cells as ratio to pTreg (center and right). (B) PBMCs were stimulated with Leukocyte Activation Cocktail for 6 hours and analyzed by flow cytometry for the intracellular amount of interferon (IFN)-γ (top left), granulocyte-macrophage colony-stimulating factor (GM-CSF) (top center), tumor necrosis factor α (TNF-α) (top right), interleukin (IL)-4 (bottom left), IL-17A (bottom center), and IL-22 (bottom right). The given results are the percentage of total CD4+ T cells. (C) CD4+ memory cells and data from (B) depicted as percentage relative to baseline after 6 months of treatment. Red bars indicate the median, and the gray line marks the median reduction of CD4+ memory T cells. Statistical analysis was done by paired Student t test for matched samples and Wilcoxon signed rank test for the comparison of the median cytokine production to baseline, *p < 0.05, **p < 0.01, ***p < 0.001.
Based on these findings, we investigated the functional capacity of CD4+ T cells to produce different cytokines upon ex vivo stimulation (figure 2B). We observed that in patients treated with DMF, the percentage of CD4+ T cells producing tumor necrosis factor α (TNFα) (reduction of 14.21%, p = 0.0231), IFN-γ (47.71%, p = 0.0014), interleukin (IL)-22 (24.41%, p = 0.0244), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (48.05%, p = 0.0008) was significantly reduced when compared to baseline (figure 2, B and C). In contrast, production of IL-17A and IL-4 was not affected.
DISCUSSION
Inhibitory effects of fumaric esters, in particular DMF, on lymphocyte counts are well known and have already been described as frequent adverse events in phase 3 clinical trials in patients with RRMS.1,3 DMF-related lymphopenia has been a recent area of focus, as it is suspected to be associated with rare cases of PML in patients receiving fumaric esters for different indications.10–13 However, the underlying cause of such lymphopenia and the impact on different lymphocyte subpopulations have not been addressed in great detail. Recently, it was described that this effect preferentially affects CD8+ T cells rather than CD4+ T cells.8 We now extend these investigations by demonstrating that DMF treatment in patients with RRMS causes distinct and reciprocal alterations of different CD4+ T cell subsets, characterized by reduced TH1 and TH17 cells and increased pTreg cell populations. The composition of CD4 memory T cells was skewed from TH1 toward TH2 cells by DMF treatment. These changes in subset composition were paralleled by a change in the CD4+ T cell cytokine secretion pattern, with decreased IFN-γ, GM-CSF, and TNFα production.
This study addresses the impact of in vivo DMF treatment on different effector vs regulatory T cell subsets in RRMS. We demonstrated that the impact of DMF treatment on antigen-experienced memory CD4+ and CD8+ T cells is more pronounced compared to total CD4+ and CD8+ T cells. Although the reason for this preferential effect still needs to be elucidated, our study revealed that additional analysis of these populations is important not only with regard to the mechanisms of DMF-mediated immune regulation but also with regard to immune vigilance aspects such as lymphopenia-associated PML,13,18,19 because memory T cell subsets are particularly relevant for maintenance of antiviral immunity.20
In order to elucidate the potential impact of in vivo DMF treatment on different CD4+ T cell subpopulations in more detail, we used established strategies to determine TH1, TH2, TH17,15 and pTreg16 subpopulations via multicolor flow cytometry without further manipulation of cells by short-term culturing and stimulation of cells. Our analysis revealed a distinct and reciprocal regulation of different subsets. This interesting observation has 2 important implications. First, it indicates a rather selective effect of DMF treatment on distinct subsets rather than a broad suppressive activity on all T cells, as suggested by several authors.5,21 Second, it might reflect a normalization of disturbed effector vs regulatory T cell subpopulations in MS. Such a disturbance characterized by augmented proinflammatory TH1 or TH17 responses, impaired regulatory T cell functions, and an association of disease remission with pronounced TH2 responses has already been proposed by several studies in the context of MS.22–28
With regard to the impact of DMF treatment on T cell functions in vivo, only 1 pilot study comprising 10 patients with RRMS, of whom only 6 completed the study, showed enhanced production of IL-10 by restimulated CD4+ T cells at different time points after onset of DMF treatment, whereas IFN-γ production was found to be unchanged.29 The small sample size might explain the discrepancy compared to our data with regard to IFN-γ production. Other studies investigated the impact of in vitro DMF or mycophenolate mofetil exposure on cytokine production by human T cells. Some of these studies observed reduced proinflammatory cytokine production (i.e., IFN-γ or TNFα) with DMF exposure,21,30 whereas others did not observe such reductions.31 It is interesting that at least a few studies also suggested an enhancement of anti-inflammatory cytokine production with DMF,29–32 which is in line with our finding of enhanced TH2 and pTreg frequencies in patients treated with DMF. Together, these data support the notion that DMF exerts differential effects on T cell cytokine production, although the different experimental settings in these in vitro studies did not reveal a conclusive picture of DMF-induced cytokine changes and did not take into account the impact of MS-specific immunologic characteristics. Hence, our study extends these findings by providing data on DMF-induced changes in T cell cytokine production in a defined and larger cohort of patients with MS. It has been shown that DMF acts on different intracellular pathways, such as interference with nuclear factor κB–mediated transcription of proinflammatory genes,33 induction of Nrf2 antioxidant pathways,34 and induction of alternatively activated anti-inflammatory microglial cells through activation of the hydroxycarboxylic acid receptor 2.35 Future studies will reveal whether these important transcriptional pathways might be differentially affected in different TH cell subsets during DMF treatment in patients with RRMS.
We are aware that interpretation of this confined immunologic pilot study is limited by several aspects. First, we cannot distinguish between direct DMF-mediated effects on T cells (e.g., during differentiation) and indirect DMF effects via influence on antigen-presenting cells, which has already been suggested in 2 studies, albeit not involving immune cells from patients treated with DMF.36,37 Second, we were able to address effects on peripheral blood–derived immune cells but not on immune cells within the CNS or CSF from patients treated with DMF. Third, because our observational period was limited to 6 months after treatment onset, a correlation with the treatment response was not possible.
Our study demonstrates distinct effects of DMF treatment on different T cell subsets in patients with MS, with a pronounced reduction of memory subsets and a differential effect on TH cell subsets with a shift toward anti-inflammatory subsets. Our findings suggest that closer monitoring of memory T cell populations might enhance vigilance toward immune-mediated side effects such as PML and that patients with more TH1-driven disease might preferentially benefit from DMF treatment compared to those with TH17-driven disease.
Supplementary Material
ACKNOWLEDGMENT
The excellent technical assistance of Verena Schütte, Schumina Säuberlich, Kerstin Gottschalk, and Anke Schwabe is gratefully acknowledged. We sincerely thank all participants who donated blood in order to complete this study.
GLOSSARY
- DMF
dimethyl fumarate
- EDTA
ethylenediaminetetraacetic acid
- IFN
interferon
- IL
interleukin
- MS
multiple sclerosis
- Nrf2
nuclear factor erythroid 2-related factor 2
- PBMC
peripheral blood mononuclear cell
- PML
progressive multifocal leukoencephalopathy
- pTreg
peripheral regulatory T cell
- RRMS
relapsing-remitting multiple sclerosis
- SOP
standard operating procedure
- TH
T helper cell
- TNFα
tumor necrosis factor α
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
C.C.G., A.S.-M., and S.K. performed research and collected and analyzed data. C.C.G. and L.K. conceptualized the study and analyzed and interpreted the data. A.P.-F. and S.K. organized patient recruitment and logistics and provided clinical information. L.K. designed the project, was responsible for the concept, designed research, generated funding, organized patient recruitment, provided clinical information, and wrote the manuscript. H.W. helped to conceptualize the project, generated funding, and critically edited the manuscript. All authors wrote the manuscript.
STUDY FUNDING
This study has been supported by the Krankheitsbezogene Kompetenznetz Multiple Sklerose (Disease Related Competence Network for Multiple Sclerosis) funded by the Federal Ministry of Education and Research (FKZ 01GI0907, Project B1 to H.W.), the Collaborative Research Centre CRC TR128, Initiating/Effector vs Regulatory Mechanisms in Multiple Sclerosis—Progress towards Tackling the Disease (Project A8 to L.K., Projects A9 and Z2 to H.W.), and a research grant from Biogen to L.K. The work of C.C.G. has been funded by the individual research grant “The role of natural killer cells in the immunoregulation of multiple sclerosis” by the German Research Foundation (DFG, GR3946/2-1).
DISCLOSURE
C. C. Gross received speaker honoraria and travel funding from Genzyme, Novartis, and Bayer; is a review editor for Frontiers in Immunology, and received research support from German Research Foundation, University of Münster. A. Schulte-Mecklenbeck and S. Klinsing report no disclosures. A. Posevitz-Fejfár received speaker honoraria and travel funding from Genzyme. H. Wiendl serves on the scientific advisory board for Bayer, Biogen, Sanofi-Genzyme, Merck Serono, Novartis, Roche, and Teva; is on the editorial board for Journal of Clinical Practice, Journal of Neuroinflammation, and PLOS ONE; has consulted for Biogen, Merck Serono, Novartis, Omnamed, Roche, Sanofi-Genzyme; and received research support from Bayer Healthcare, Bayer Vital, Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme, Sanofi US, Teva, German Ministry for Education and Research, Deutsche Forschungsgesellschaft, European Union, Else Kroner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies Muenster, RE Children's Foundation, Sanofi Aventis, and NovoNordisk. L. Klotz is on the scientific advisory board for Genzyme; received travel funding and speaker honoraria from Novartis, Merck Serono, and CSL Behring; and received research support from Novartis, Biogen, and CRC. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Fox RJ, Philips JT, Hutchinson M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–1097. [DOI] [PubMed] [Google Scholar]
- 2.Kappos L, Radue EW, O'Connor P, et al. ; FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- 3.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–1107. [DOI] [PubMed] [Google Scholar]
- 4.Schilling S, Goetz S, Linker R, Luehder F, Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol 2006;145:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treumer F, Zhu K, Gläser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol 2003;121:1383–1388. [DOI] [PubMed] [Google Scholar]
- 6.Vandermeeren M, Borgers M, Geysen J. Dimethylfumarate is an inhibitor of cytokine-induced E-selectin, VCAM-1, and ICAM-1 expression in human endothelial cells. Biochem Biophys Res Commun 1997;234:19–23. [DOI] [PubMed] [Google Scholar]
- 7.Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 2012;341:274–284. [DOI] [PubMed] [Google Scholar]
- 8.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BAC, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015;2:e76. 10.1212/NXI.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler 2014;21:796–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med 2013;368:1657–1658. [DOI] [PubMed] [Google Scholar]
- 11.van Oosten BW, Killestein J, Barkhof F. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med 2013;358:1658–1659. [DOI] [PubMed] [Google Scholar]
- 12.Sweetser MT, Dawson KT, Bozic C. Case reports of PML in patients treated for psoriasis. N Engl J Med 2013;369:1082. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med 2015;372:1476–1478. [DOI] [PubMed] [Google Scholar]
- 14.Posevitz-Fejfár A, Posevitz V, Gross CC, et al. Effects of blood transportation on human peripheral mononuclear cell yield, phenotype and function: implications for immune cell biobanking. PLoS One 2014;9:e115920. 10.1371/journal.pone.0115920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 2012;484:514–518. [DOI] [PubMed] [Google Scholar]
- 16.Breuer J, Schwab N, Schneider-Hohendorf T, et al. Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann Neurol 2014;75:739–758. [DOI] [PubMed] [Google Scholar]
- 17.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010;184:3433–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasnault J, de Göer de Herve MG, Michot JM, et al. Efficacy of recombinant human interleukin 7 in a patient with severe lymphopenia-related progressive multifocal leukoencephalopathy. Open Forum Infect Dis 2014;1:ofu074. 10.1093/ofid/ofu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgado-Alvado M, Sedano MJ, Gonzáles-Quintanilla V, et al. Progressive multifocal leukoencephalopathy and idiopathic CD4 lymphocytopenia. J Neurol Sci 2013;327:75–79. [DOI] [PubMed] [Google Scholar]
- 20.Antoniol C, Stankoff B. Immunological markers for PML prediction in MS patients treated with natalizumab. Front Immunol 2014;5:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann JCU, Listopad JJ, Rentzsch CU, et al. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol 2007;127:835–845. [DOI] [PubMed] [Google Scholar]
- 22.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology 1987;37:1097–1102. [DOI] [PubMed] [Google Scholar]
- 23.Neukirch F, Lyon-Caen O, Clanet M, et al. Asthma, nasal allergies, and multiple sclerosis. J Allergy Clin Immunol 1997;99:270–271. [DOI] [PubMed] [Google Scholar]
- 24.Simpson CR, Anderson WJ, Helms PJ, et al. Coincidence of immune-mediated diseases driven by Th1 and Th 2 subsets suggests a common aetiology. A population-based study using computerized general practice data. Clin Exp Allergy 2002;32:37–42. [DOI] [PubMed] [Google Scholar]
- 25.Adorini L, Guéry JC, Trembleau S. Manipulation of the Th1/Th2 cell balance: an approach to treat human autoimmune diseases? Autoimmunity 1996;23:53–68. [DOI] [PubMed] [Google Scholar]
- 26.Matusevicius D, Kivisäkk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999;5:101–104. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz A, Schumacher M, Pfaff D, et al. Fine-tuning of regulatory T cell function: the role of calcium signals and naive regulatory T cells for regulatory T cell deficiency in multiple sclerosis. J Immunol 2013;190:4965–4970. [DOI] [PubMed] [Google Scholar]
- 28.Trinschek B, Luessi F, Haas J, et al. Kinetics of IL-6 production defines T effector cell responsiveness to regulatory T cells in multiple sclerosis. PLoS One 2013;8:e77634. 10.1371/journal.pone.0077634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schirmrigk S, Brune N, Hellwig K, et al. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur J Neurol 2006;13:604–610. [DOI] [PubMed] [Google Scholar]
- 30.Moed H, Stoof TJ, Boorsma DM, et al. Identification of anti-inflammatory drugs according to their capacity to suppress type-1 and type-2 T cell profiles. Clin Exp Allergy 2004;34:1868–1875. [DOI] [PubMed] [Google Scholar]
- 31.Zoghi S, Amirghofran Z, Nikseresht A, et al. Cytokine secretion pattern in treatment of lymphocytes of multiple sclerosis patients with fumaric acid esters. Immunol Invest 2011;40:581–596. [DOI] [PubMed] [Google Scholar]
- 32.Ockenfels HM, Schultewolter T, Ockenfels G, Funk R, Goos M. The antipsoriatic agent dimethylfumarate immunomodulates T-cell cytokine secretion and inhibits cytokines of the psoriatic cytokine network. Br J Dermatol 2008;139:390–395. [DOI] [PubMed] [Google Scholar]
- 33.Gold R, Linker RA, Stangel M. Fumaric acid and its esters: an emerging treatment for multiple sclerosis with antioxidative mechanism of action. Clin Immunol 2012;142:44–48. [DOI] [PubMed] [Google Scholar]
- 34.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134:678–692. [DOI] [PubMed] [Google Scholar]
- 35.Parodi B, Rossi S, Morando S, et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol 2015;130:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu K, Mrowietz U. Inhibition of dendritic cell differentiation by fumaric acid esters. J Invest Dermatol 2001;116:203–208. [DOI] [PubMed] [Google Scholar]
- 37.Peng H, Guerau-de-Arellano M, Mehta VB, et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J Biol Chem 2012;287:28017–28026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.