Abstract
Background and Aims Unrelated organisms that share similar niches often exhibit patterns of convergent evolution in functional traits. Based on bimodal distributions of hawkmoth tongue lengths and tubular white flowers in Africa, this study hypothesized that long-tongued hawkmoths comprise a pollination niche (ecological opportunity) that is distinct from that of shorter-tongued hawkmoths.
Methods Field observations, light trapping, camera surveillance and pollen load analysis were used to identify pollinators of plant species with very long-tubed (>8 cm) flowers. The nectar properties and spectral reflectance of these flowers were also measured. The frequency distributions of proboscis length for all captured hawkmoths and floral tube length for a representative sample of night-blooming plant species were determined. The geographical distributions of both native and introduced plant species with very long floral tubes were mapped.
Key Results The convolvulus hawkmoth Agrius convolvuli is identified as the most important pollinator of African plants with very long-tubed flowers. Plants pollinated by this hawkmoth species tend to have a very long (approx. 10 cm) and narrow flower tube or spur, white flowers and large volumes of dilute nectar. It is estimated that >70 grassland and savanna plant species in Africa belong to the Agrius pollination guild. In South Africa, at least 23 native species have very long floral tubes, and pollination by A. convolvuli or, rarely, by the closely related hawkmoth Coelonia fulvinotata, has been confirmed for 11 of these species. The guild is strikingly absent from the species-rich Cape floral region and now includes at least four non-native invasive species with long-tubed flowers that are pre-adapted for pollination by A. convolvuli.
Conclusions This study highlights the value of a niche perspective on pollination, which provides a framework for making predictions about the ecological importance of keystone pollinators, and for understanding patterns of convergent evolution and the role of floral traits in plant colonization.
Keywords: Agrius convolvuli, biological invasions, ecological opportunity, flower colour, functional traits, long-tongued hawkmoth, moth pollination, mutualism, nectar, plant–pollinator interactions, pollination ecology, proboscis length, regeneration niche, specialization
INTRODUCTION
The concept of the niche as an ecological opportunity has had a major influence on evolutionary biology and ecology (Schluter, 2000; Chase and Leibold, 2003; Devictor et al., 2010; Losos, 2010). Pollinators are niches in that they represent ecological opportunities for plants, but the niche concept has historically been focused on competition and thus has been slow to infiltrate the study of mutualisms. This is surprising because plant floral traits can readily be linked to pollinator niches (Johnson, 2010), while the relationships of other plant functional traits such as height, leaf dimensions and seed size to particular environmental niches often are much harder to establish. As a component of the regeneration niche (Grubb, 1977), pollinators can be likened to canopy gaps and other ecological opportunities that plants exploit under selection for enhanced reproduction.
Shifts between pollinator niches have been a key driver of adaptive radiation in angiosperms (Whittall and Hodges, 2007; van der Niet and Johnson, 2012). These ecological niche shifts provide examples of evolutionary diversification that are arguably as compelling as those in Galapagos finches and Caribbean lizards (Schluter, 2000). Pollinator niches can be defined according to axes of pollinator morphology such as proboscis length, as well as phenology and perception of colour and scent. As examples, evolutionary shifts between bird and bee pollinators will typically involve floral modifications along several of these niche axes (Bradshaw and Schemske, 2003), shifts between different hummingbird species may involve floral adjustment to both the length and curvature of bills (McDade, 1992; Maglianesi et al., 2014), while shifts between short- and long-tongued moths may involve adjustments only of the floral tube length and nectar volume (Boberg et al., 2014).
For an outbreeding plant with a highly specialized pollination system, a particular pollinator may be considered part of its fundamental niche requirement. However, in most cases interactions among species will shape a more complex realized pollination niche. For example, the colour preference of pollinators may be altered by the dominant nectar plants in a community, such that the floral colour traits that a plant requires to attract pollinators may vary among communities (Newman et al., 2012). For generalist plants, there is no single pollinator niche, and floral trait combinations will be selected according to the composition of local assemblages of pollinators (Gomez et al., 2015). In both specialized and generalized plants, floral adaptation to pollinators can be mitigated or constrained by opposing selective pressures provided by floral enemies (Theis et al., 2007; Galen et al., 2011).
The identification of proboscis length as a key niche dimension and floral tube length as a key functional floral trait stems back to Darwin and his famous prediction of a very long-tongued species of hawkmoth in Madagascar, based on the floral dimensions of an orchid flower (Darwin, 1877). Subsequent work has confirmed the importance of pollinator proboscis length for floral tube length evolution (Nilsson, 1988; Alexandersson and Johnson, 2002), although the role of coevolution in the evolution of the pollinator niche itself (Nilsson et al., 1987; Anderson and Johnson, 2008; Pauw et al., 2009) vs. shifts between pre-existing pollinator niches (Wasserthal, 1997; Whittall and Hodges, 2007) is still much debated.
In this study we examine the niche axis of hawkmoth proboscis length in Africa. Previous studies in Madagascar (Nilsson et al., 1987) and Costa Rica (Haber and Frankie, 1989; Agosta and Janzen, 2005) suggest that the distribution of hawkmoth tongue lengths in these regions is multimodal, reflecting the rich diversity of hawkmoths in these regions, and also right-skewed, reflecting dominance of shorter-tongued hawkmoths, while recent studies indicated that there may be a simple bimodal distribution of both tongue lengths of hawkmoths and tube lengths of flowers on the African mainland (Anderson et al., 2010; Martins and Johnson, 2013). On the basis of this bimodal distribution, we hypothesized that there is a distinct niche of long-tongued hawkmoths to which African plants have become adapted. Our interest here is the classical macroevolutionary patterns of divergence due to niche shifts and convergent evolution among species that occupy a fundamental niche (Losos and Mahler, 2010), rather than questions about finer scale patterns of character displacement and species sorting that may contribute to species coexistence within communities (Silvertown, 2004; Pauw, 2013). We do, however, note a possible case of species sorting involving long-tubed invasive plant species in Africa that are pollinated by local long-tongued moths.
As emphasized by Stebbins (1970), the geographical distribution of pollinators is an important factor underlying floral divergence in plants. For example, in his review of hawkmoth pollination in North America, Grant (1983) noted a concentration of hawkmoth-pollinated species in south-western North America. The biogeography of hawkmoth pollination has not been formally investigated in South Africa, but a near absence of hawkmoth-pollinated Amaryllidaceae in the south-west of the sub-continent has been noted (Manning and Snijman, 2002).
The specific aims of this study were as follows: (1) to determine if there is a distinct long-tongued hawkmoth pollinator niche in Africa; (2) to investigate whether African plant species with very long-tubed (>8 cm) flowers are pollinated by specific hawkmoth species; (3) to document patterns of convergent evolution in the floral traits of plants pollinated by long-tongued hawkmoths in southern Africa; and (4) to determine the biogeographical distribution of long-tongued hawkmoth pollination systems in South Africa.
MATERIALS AND METHODS
Trait measurements
Interactions between hawkmoths and plants with long-tubed flowers (Fig. 1) were studied by us between 2002 and 2015 at 16 sites distributed across the eastern half of South Africa (Supplementary Data Table S1). Hawkmoths were recorded by light trapping, direct observations at flowers and through use of motion-activated cameras. Data from a further eight sites described by Anderson et al. (2010) were used for analyses of the morphometrics of light-trapped hawkmoths. To estimate the frequency distribution of proboscis lengths in the general hawkmoth fauna, measurements to the nearest 1 mm using a steel ruler or digital calipers were taken of the proboscides of 1199 hawkmoths captured by light trapping.
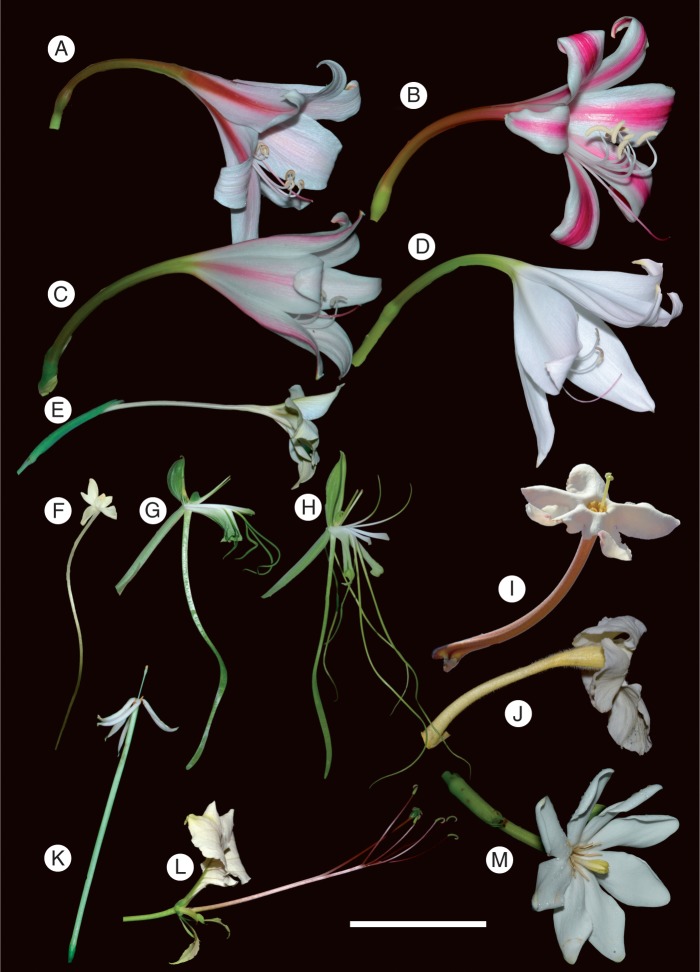
Fig. 1.
Long-tubed flowers of South African plant species. (a) Crinum bulbispermum. (B) Crinum delagoaense. (C) Crinum macowanii. (D) Crinum moorei. (E) Gladiolus longicollis. (F) Rangaeris muscicola. (G) Bonatea steudneri. (H) Bonatea lamprophylla. (I) Sesamothamnus lugardii. (J) Harveya speciosa. (K) Oxyanthus pyriformis. (L) Cladostemon kirkii. (M) Gardenia thunbergia. Scale bar = 5 cm.
To estimate the frequency distribution of floral tube length in moth-pollinated plants in eastern South Africa, we used botanical descriptions in guides to wildflowers and trees of the region (Pooley, 1998; Boon, 2010). Plants were considered likely to be moth pollinated if they have flowers that are long-tubed, white, scented and night blooming. If the tube length was given as a range, we used midpoint values. To identify the entire set of plants in southern Africa that have long-tubed flowers, we contacted taxonomic authorities and consulted descriptions in general taxonomic accounts of the flora. Data for Kenya were obtained from Martins and Johnson (2013).
For each of 18 plant species, we measured, to the nearest 1 mm using a steel ruler, the tube length and distance from the stigma to the base of the nectar column of 3–289 flowers (median = 9 flowers per species), each taken from a different plant wherever possible. Nectar volume was sampled using micropipettes, and nectar concentration was measured using a Bellingham and Stanley 0–50 % refractometer. This was done immediately prior to dusk to estimate the nectar reward available at the start of hawkmoth foraging.
Spectral reflectance of flowers of 11 of the study species was measured using an Ocean Optics spectrometer, as described by Johnson and Andersson (2002). We recorded floral spectra from one to 12 (median = 4) plants per species.
Voucher specimens of the plant species are deposited in the Bews Herbarium (NU), Pietermaritzburg, and hawkmoth specimens are deposited in the insect pollinator collection in the School of Life Sciences on the Pietermaritzburg campus of the University of KwaZulu-Natal.
Pollinator observations
We used dimmed flashlights to make observations of hawkmoth visitors (range from one to >150 individuals per plant species). A sub-set of the captured hawkmoths (range from one to 33 individuals per plant species) was identified and examined for pollen placement while measurements were taken of the proboscis, as described above. Swabs of pollen taken using blocks of fuschin gel were examined under a compound microscope and compared with pollen standards taken from the study species to confirm pollen identity. In a few cases (Supplementary Data Table S1) we used field cameras (Bushnell Natureview) equipped with passive infra-red sensors and close up lenses to record hawkmoth activity at sites where wild animals made it unsafe to make observations in the dark.
Floral tube length as a mechanical filter
To establish whether the diversity of hawkmoth visitor species decreases with increasing floral tube length, we used the data obtained in this study as well as data from previous studies of hawkmoth pollination in southern Africa and Kenya (Johnson, 1995, 1997; Johnson et al., 2002, 2004; Peter et al., 2009; Anderson et al., 2010; Martins and Johnson, 2013; van der Niet et al., 2015). We also combined data from those studies with our own to establish the relationship between floral tube length and the volume of nectar available at the start of hawkmoth foraging. We used Poisson regression implemented in SPSS 22 (IBM Corp.) to analyse relationships between hawkmoth number and floral tube length, and linear regression to analyse the relationships between nectar volume and floral tube length.
Biogeography
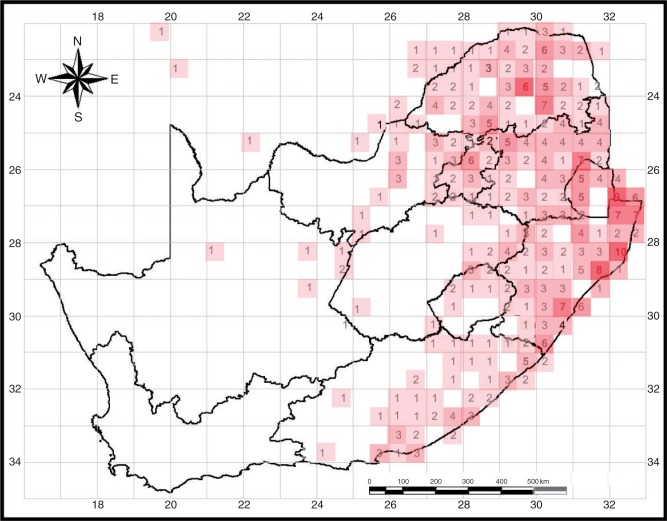
The geographical distribution of long-tubed (>8 cm) plant species in South Africa was determined by downloading available herbarium distribution records from SIBIS – the South African Biodiversity Institute Integrated Biodiversity Information System (http://sibis.sanbi.org). We then mapped the number of these long-tubed plant species that occur in each quarter degree square. This was done separately for native and invasive species. The geographical distribution of Agrius convolvuli in South Africa was mapped at a degree grid level using 817 records from the Ditsong National Musuem of Natural History (formerly Transvaal Museum) and Iziko South African museum. To control for collecting effort, we mapped the percentage of Agrius specimens out of the total number of hawkmoth specimens collected in each degree square.
RESULTS
Trait measurements
The proboscis lengths of hawkmoths captured in South Africa had a distinctly bimodal distribution (Fig. 2A). The longer mode was made up almost entirely of individuals of Agrius convolvuli which had proboscides that varied from 64.8 to 15.1 cm in length (mean ± s.e. 10.1 ± 0.7 cm, n = 537). A small number of individuals of Coelonia fulvinotata contributed to this mode and had proboscides that varied from 8.3 to 8.9 cm in length (mean ± s.e. 8.60 ± 0.5 cm, n = 12). The shorter mode was made up of a diverse assemblage of hawkmoth species with proboscides that ranged from 30.2 to 45.6 cm (mean ± s.e. 38.2 ± 0.1, n = 650)
Fig. 2.
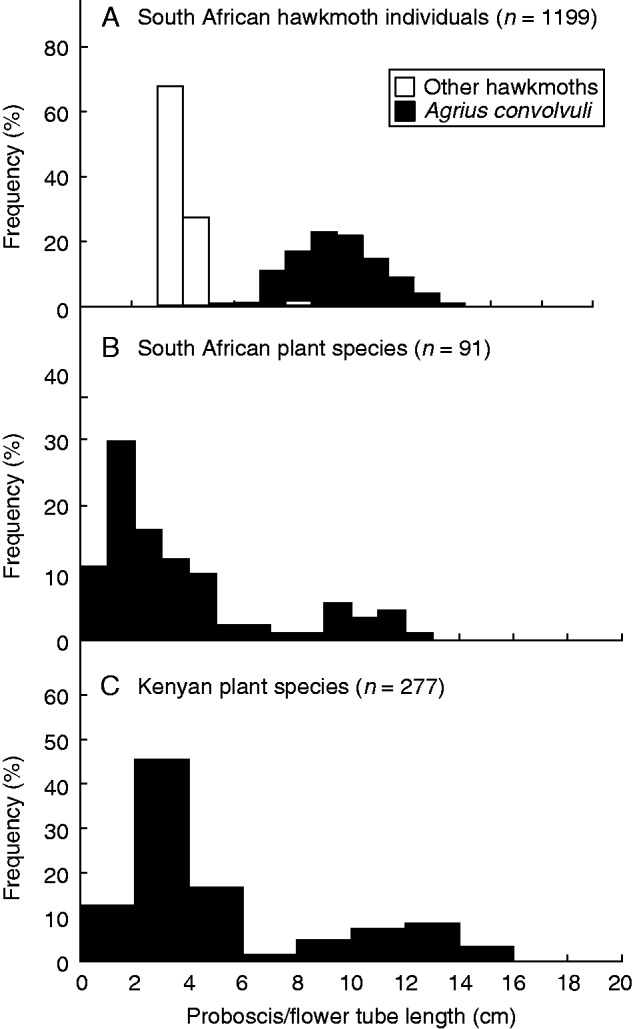
Frequency distributions of hawkmoth tongue and flower tube lengths. (A) Hawkmoths captured by light Trapping in KwaZulu-Natal (KZN), South Africa. (B) Plant species in South Africa. (C) Plant species in Kenya (after Martins and Johnson, 2013).
The bimodal distribution of hawkmoth proboscis length was matched closely by a similar bimodal distribution in the floral tube length of night-blooming plant species in South Africa (Fig. 2B). A bimodal distribution of floral tube length also occurs in the flora of Kenya (Fig. 2C).
We identified 23 native plant and four invasive plant species in South Africa that have floral tubes >8 cm in length (Table 1). The native species represent 12 genera in seven families (Amaryllidaceae, Orchidaceae, Iridaceae, Orobanchaceae, Pedaliaceae, Rubiaceae and Capparaceae), while the invasive species represent four genera in four families (Liliaceae, Convolvulaceae, Solanaceae and Zingiberaceae). Flowers sampled from these species had relatively large volumes of nectar per flower at dusk (grand means ± s.e.: native species, 26.50 ± 5.2 μL; invasive species, 17.95 ± 2.3 μL, F = 2.2, P = 0.15; Supplementary Data Table S2). The sugar concentration (g g–1) grand means were 18.56 ± 1.1 % for nectar of the native species and 34.76 ± 2.5 % for nectar of the invasive species (F = 35.3, P < 0.001).
Table 1.
Plant species in South Africa with floral tubes >80 mm in length
| Family/species | Tube length (cm) | Confirmation of pollination by Agrius convolvuli or Coelonia fulvinotata |
|---|---|---|
| Native taxa | ||
| Amaryllidaceae | ||
| Crinum buphanoides Welw. ex Bak. | 7–10 | – |
| C. paludosum Verdoorn. | 9–12 | – |
| C. moorei Hook. F. | 8–10 | – |
| C. acaule Bak. | 10–12 | – |
| C. minimum Milne-Redh. | 8–12 | – |
| C. foetidum Verdoorn | Approx. 10 | – |
| C. graminicola Verdoorn | 7–11 | – |
| C. delagoanse Verdoorn | 7–12 | This study |
| C. lugardiae N. E. Br. | 8–13 | – |
| C. macowanii Bak. | 7–12 | This study, Martins and Johnson (2013) |
| C. bulbispermum (Burm. f.) Milne-Redh. & Schweick. | 7–12 | This study |
| Orchidaceae | ||
| Aerangis somalensis (Schltr.) Schltr. | 10–12 | – |
| Aerangis verdickii (De Wil.) Schltr. | 12–16 | – |
| Angraecum stella-africae P.J. Cribb | 12–15 | – |
| Bonatea lamprophylla J. Stewart | 9–13 | – |
| B. steudneri (Rchb.f) T. Durand & Schinz | 8–23 | Martins and Johnson (2013) |
| Centrostigma occultans (Reichb.f.) Schltr. | Approx.12 | – |
| Rangaeris muscicola (Reichb.f) Summerh | 9–12 | This study |
| Iridaceae | ||
| Gladiolus longicollis subsp platypetalus (Baker) Goldblatt & J.C.Manning | 7–12 | Goldblatt and Manning (1999), Alexandersson and Johnson (2002, unpubl. res.), this study |
| Orobanchaceae | ||
| Harveya speciosa Bernh. ex Krauss | 7–9 | This study |
| Cycnium adonense E. mey. ex Benth. | 4–9 | Martins and Johnson (2013) |
| Pedaliaceae | ||
| Sesamothamnus lugardii N. E. Br. ex Stapf. | Approx. 10 | This study, A. Bijl (unpubl. res.) |
| Rubiaceae | ||
| Oxyanthus pyriformis subsp. pyriformis (Hochst.) Skeels | 8–10 | Johnson et al. (2003) |
| Gardenia thunbergii Thunb. | 9–10 | This study |
| Gardenia volkensii K. Schum. | 10–13 | |
| Capparaceae | ||
| Cladostemon kirkii (Oliv.) Pax & Gilg. | 8–10 | This study |
| Introduced taxa | ||
| Liliaceae | ||
| Lilium formosanum A. Wallace | Approx. 10 | Rodger and Johnson (2011) |
| Convolvulaceae | ||
| Ipomoea alba L. | Approx. 10 | This study |
| Solanaceae | ||
| Datura stramonium L. | Approx. 10 | Martins and Johnson (2013) |
| Zingiberaceae | ||
| Hedychium gardnerianum Ker Gawl. | Approx. 12 | This study |
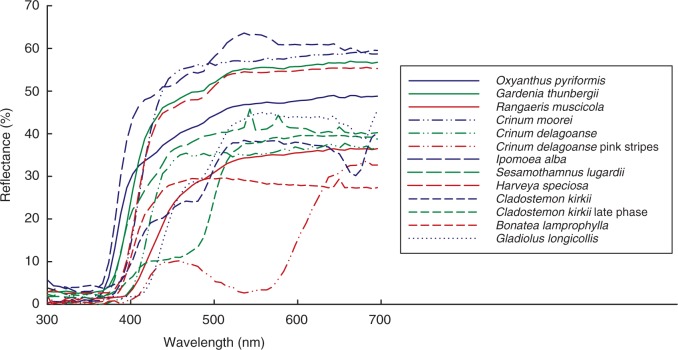
Flowers of the study species are almost all white or cream in human visual perception, sometimes with pink stripes (Crinum delagoanse); they reflect light of wavelengths ranging from 400 to 700 nm and absorb UV wavelengths (300–399 nm; Fig. 3). Older flowers of Cladostemon kirkii, Rangaeris muscicola, Gardenia thunbergii, Oxyanthus speciosa and Sesamothamnus lugardii undergo a colour change in human perception from white to yellow, as illustrated by the contrasting spectra for C. kirkii (Fig. 3).
Fig. 3.
Spectral reflectance of long-tubed flowers in the flora of South Africa.
Pollinator observations
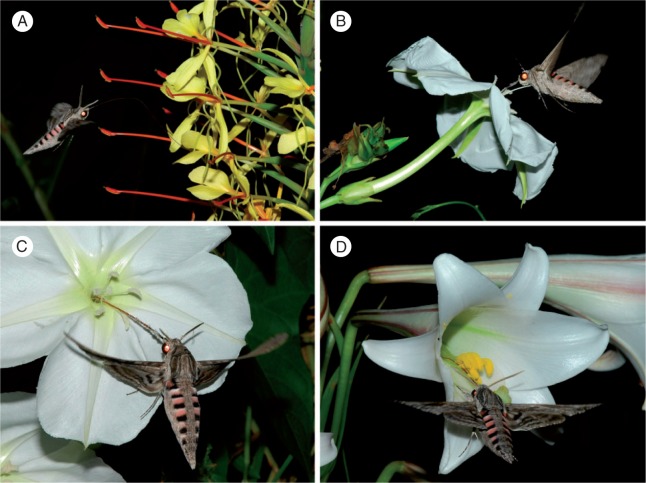
We observed hawkmoths to visit the flowers of 13 plant species with long-tubed flowers (Figs. 4 and 5; Supplementary Data Table S3). Almost all of the visits to these species were by the convolvulus hawkmoth A. convolvuli. This hawkmoth species was the only observed visitor for ten of the 13 plant species (Supplementary Data Table S3). The only other hawkmoth species recorded were the fulvous hawkmoth Coelonia fulvinotata observed on flowers of two of the species, and a single individual of the accented hawkmoth Nephele accentifera accentifera observed on flowers of one species. Hawkmoths generally carried large pollen loads of the flowers they visited, and these were visible even in photographs (Figs. 4 and 5).
Fig. 4.
Long-tongued hawkmoth visitors to native South African plant species with long-tubed flowers. (A) The convolvulus hawkmoth Agrius convolvuli feeding on Crinum macowanii. (B) A. convolvuli on Crinum bulbispermum. (C, D) A. convolvuli on Gardenia thunbergia. (E). A. convolvuli on Harveya speciosa. (F) The fulvous hawkmoth Coelonia fulvinotata on Cladostemon kirkii.
Fig. 5.
Long-tongued hawkmoth visitors to long-tubed flowers of exotic plant species that are invasive in South Africa. (A) The convolvulus hawkmoth Agrius convolvuli feeding on Hedychium gardnerianum. (B, C). A. convolvuli on Ipomoea alba. (D) A. convolvuli on Lilium formosanum.
Video footage of G. thunbergii showed that convolvulus hawkmoths feed from flowers through most of the night. We obtained video footage of ten foraging bouts between 19.00 and 21.00 h, 16 bouts between 21.00 and 23.00 h, zero bouts between 23.00 and 01.00 h, two bouts between 01.00 and 03.00 h, and two bouts between 03.00 and 05.00 h.
Floral tube length as a mechanical filter
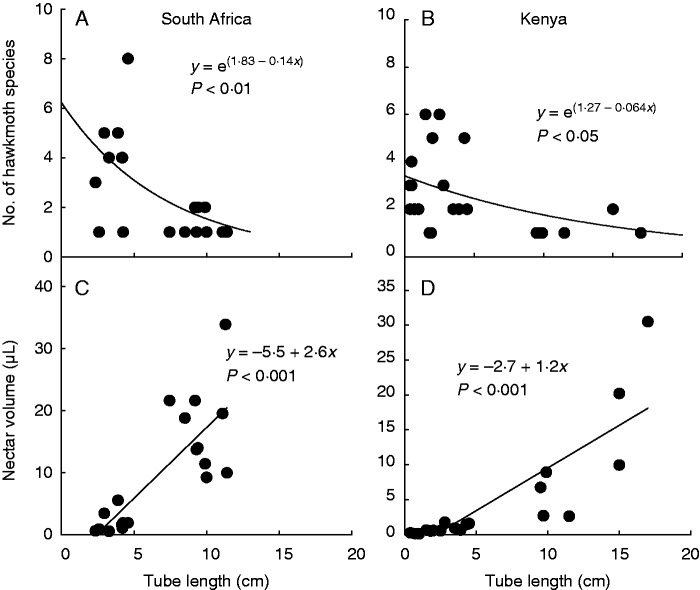
Combining the data from this study with those of other studies of hawkmoth pollination in South Africa showed that the number of hawkmoth visitor species declines with increasing floral tube length (Fig. 6A). This closely matches a similar trend in Kenya (Fig. 6B). In both South Africa and Kenya, flowers visited by hawkmoths show a strong linear relationship between floral tube length and the volume of nectar available just before dusk (Fig. 6C, D).
Fig. 6.
Relationships between floral tube length and the number of hawkmoth visitor species for plants in South Africa (A) and Kenya (B), and between floral tube length and the volume of nectar available at the start of moth foraging for plants in South Africa (C) and Kenya (D). Data for Kenya from Martins and Johnson (2013).
Biogeography
Plant species with long floral tubes (>8 cm) are found only in the eastern half of South Africa and are conspicuously absent from the Cape floral region (Fig. 7). The density of species with long-tubed flowers is greatest along the sub-tropical coastal belt and far-eastern regions of South Africa (Fig. 7). The distribution of invasive species with long-tubed flowers closely matches that of the native species with long-tubed flowers (Supplementary Data Fig. S1). Although hawkmoth collection records are not available for all degree squares in South Africa, available data indicate that A. convolvuli makes up a high percentage of hawkmoth museum accessions (frequently in excess of 50 %) from degree grid squares in the east, but has only very rarely been recorded in the Greater Cape Floral region in the west (Supplementary Data Fig. S2).
Fig. 7.
The geographical distribution of native plant species with long-tubed flowers in South Africa. The number of species is indicated in each quarter degree grid square and is also indicated by the depth of the pink shading.
DISCUSSION
This study conducted in South Africa, together with the findings of Martins and Johnson (2013) in East Africa, demonstrates the existence of a guild of African plants that appears to rely almost exclusively on the long-tongued convolvulus hawkmoth Agrius convolvuli for pollination. This guild of plants is characterized by the shared possession of a very long (approx. 10 cm) floral tube or spur (or androphore in the case of Cladostemon kirkii; Fig. 1). Previous studies (cf. Alexandersson and Johnson, 2002) have shown that the nectar column in these guild members typically occupies about 10 % of the tube length, meaning that a tongue of at least 9 cm would be required for moths to obtain any nectar. The long floral tubes thus appear to act as a mechanical filter that excludes shorter-tongued moths from flowers (Fig. 6A, B), as was shown to be the case for long-tubed flowers visited by hawkmoths in Costa Rica (Agosta and Janzen, 2005). Apart from A. convolvuli, the only other local hawkmoth species that appeared to be able to access nectar in deep tubular flowers at our study sites was the fulvous hawkmoth Coelonia fulvinotata, but this species was seldom recorded, either among light-trapped specimens (Fig. 2A) or in observations made at flowers (Supplementary Data Table S2), and appears to be restricted to wooded habitats, while the convolvulus hawkmoth is common in open grassland and savanna habitat. In the geographical range of the study plant species, there are no other insect groups that compete with the convolvulus and fulvous hawkmoths in terms of proboscis length. There is one long-proboscid fly species in Africa that has a proboscis with a length of up to 85 mm in some populations, but this species is localized to the south-western Cape and pollinates a guild of plants with day-opening unscented flowers with floral tubes that are typically about 40–80 mm in length (Johnson and Steiner, 1997; Pauw et al., 2009). The maximum length of the proboscis among long-proboscid fly species in the eastern part of South Africa is about 50 mm (Anderson and Johnson, 2009). The convolvulus and fulvous hawkmoths have proboscides that are more than twice as long as those of all other hawkmoths at the study sites (Fig. 2A) and thus seem to represent a distinct pollinator niche to which a wide range of unrelated plants have become adapted.
The importance of floral tube length as an adaptation for pollination by A. convolvuli is evident not only from convergent evolution among guild members, but also from selection studies that show that plants of the iris Gladious longicollis that have floral tubes that match or exceed the proboscis length of A. convolvuli have higher fitness than those that have shorter floral tubes (Alexandersson and Johnson, 2002). Further supporting our contention that there are at least two different hawkmoth pollination systems in South Africa, recent studies have identified two largely geographically separated ecotypes of G. longicollis, one with short floral tubes adapted to short-tongued hawkmoths (e.g. Hippotion celerio) and another with very long floral tubes adapted to A. convolvuli (Anderson et al., 2010).
The results of this study are consistent with our contention that mouthpart (bill/proboscis/tongue) length is a key aspect of the pollinator niche. For groups of animals that have similar sensory systems and therefore select for similar floral advertising traits, mouthpart length is the key niche axis that determines plant floral adaptations and specialization. Animals belonging to the same higher taxon, e.g. hummingbirds, but that vary in the length of their mouthparts are, in most cases, likely to act as different functional groups. This has become evident from studies of specialized pollination systems involving bumble-bees (Ranta and Lundberg, 1980; Harder, 1985), euglossine bees (Borrell, 2005), long-proboscid flies (Johnson and Steiner, 1997), hummingbirds (Maglianesi et al., 2014), bats (Muchhala and Thomson, 2009) and hawkmoths (Anderson et al., 2010; Martins and Johnson, 2013). This simple niche axis of mouthpart length obviously applies mainly to relatively specialized pollination systems. Pollinator niche dimensions for plants with generalized pollination systems are far more complex (and also much less stable in time) and mainly involve the relative abundance of different pollinator functional groups in local assemblages (Gomez et al., 2015)
Besides having very long floral tubes, plants pollinated by A. convolvuli have strong similarities in spectral reflectance (Fig. 3). However, similar spectra are known from plants pollinated by other insects (Kevan et al., 1996) and so we cannot be certain that our study species possess these spectral reflectance traits as a result of specific adaptations to long-tongued moths. It does, however, seem likely that white floral coloration is important for attraction of A. convolvuli. A simple experiment decoupling the olfactory and visual display of invasive Datura inoxia (Solanaceae) flowers in the former Yugoslavia revealed a strong attraction and probing response of wild A. convolvuli moths to white objects (Kugler, 1971), a preference that is shared by the closely related American species Agrius cingulatus (Ippolito et al., 2004), as well as other long-tongued hawkmoths in the sub-family Sphinginae (Raguso and Willis, 2003).
Volumes of nectar in the long-tubed flowers of guild members are much larger than those in shorter-tubed flowers pollinated by other hawkmoths (Fig. 6C, D). Observations by Martins and Johnson (2013) in East Africa showed that A. convolvuli is an extremely polyphagous insect that feeds from both short- and long-tubed flowers. The higher volume of nectar in long-tubed flowers can thus be interpreted as selection for traits that entice A. convolvuli to visit long-tubed flowers in communities where these hawkmoths also have access to short-tubed flowers. This enticement is particularly critical given that these long-tubed flowers filter out shorter-tongued hawkmoths and thus rely solely on long-tongued hawkmoths for pollination. Although we did not attempt to control for phylogeny in our analyses of the South African flora, analysis of a similar data set for Kenyan plants showed that the trend for flowers with longer tubes to have more nectar was still evident after phylogenetic correction (Martins and Johnson, 2013).
Agrius convolvuli appears to be an exceptionally common hawkmoth throughout sub-Saharan Africa. Of the 1199 individual hawkmoths that were captured in light traps in South Africa, 535 (44.6 %) were individuals of A. convolvulus. Even museum accessions for particular degree grid squares are often dominated by this species (Supplementary Data Fig. S2). A similar high proportion of individuals of A. convolvuli was found among light-trapped catches of hawkmoths in Tanzania (Robertson, 1977) and in direct observations of flowers by Martins and Johnson (2013). Agrius convolvuli has also been recorded as an important pollinator of orchids and baobabs that occur in open woodland habitats in Madagascar (Nilsson and Rabakonandrianina, 1988; Baum, 1995; Ryckewaert et al., 2011). In contrast, A. convolvuli made up a very small percentage of the hawkmoths light-trapped in a forest in Madagascar by Nilsson et al (1985). This may reflect the fact that A. convolvuli prefers open habitats (Pinhey, 1962). In studies performed by D. F. Owen at forested sites in Sierra Leone, A. convolvuli made up 3.3 % of the hawkmoth individuals trapped at mercury vapour lamps and showed strong seasonal (and potentially migratory) patterns of abundance (Owen, 1969, 1972). Hawkmoth pollination has not been studied in the closed canopy forests of central Africa and it is possible that other hawkmoth species play a role in the pollination of long-tubed flowers in this habitat. The nominal subspecies of Xanthopan morgani, the famous long-tongued hawkmoth that fitted Darwin’s prediction of a pollinator for the orchid Angraecum sesquipedale in Madagascar, occurs on the African mainland (Pinhey, 1962; Owen, 1969) and, together with the fulvous hawkmoth C. fulvinotata, is likely to play a role in the pollination of long-tubed forest plant species in tropical Africa.
The total number of species reliant on A. convolvuli for pollination is hard to estimate on account of the fragmentary nature of the botanical literature for many African countries. We identified 23 native species in South Africa with floral tubes longer than 8 cm, while Martins and Johnson (2013) identified 51 species with floral tubes longer than 8 cm that are native to Kenya (Supplementary Data Table S4). Four taxa (Crinum macowanii, Cladostemon kirkii, Bonatea steudneri and Gardenia volkensii) are common to both countries, so there are at least 70 distinct native taxa that are either known or likely to be pollinated by A. convolvuli in these two countries alone. These taxa represent 15 plant families and 30 genera, suggesting that the guild had multiple evolutionary origins. Given that South Africa and Kenya make up just a small fraction of the landmass and flora of Africa, we consider it likely that several hundred African plant species are pollinated by A. convolvuli.
In South Africa, the Agrius plant guild is distributed in the eastern sub-tropical regions (Fig. 7). The guild is absent from the Cape floral region and the arid south-west. This appears to be a general pattern for hawkmoth pollination systems in the region, as previous studies have shown that hawkmoth-pollinated Amaryllidaceae are concentrated in the east of South Africa (Manning and Snijman, 2002). It is notable that the Cape fynbos vegetation lacks families such as Rubiaceae, Balsaminaceae, Vitaceae, Convolvulaceae and Loganiaceae that comprise the larval food plants for many hawkmoths (Attie et al., 2010). The scarcity of A. convolvuli in the semi-arid region is somewhat harder to explain, as this hawkmoth species is known to have considerable ability to disperse beyond its natal habitat (Ballesteros-Mejia et al., 2011) and has been observed to pollinate white, long-tubed flowers in the semi-arid, Mediterranean habitats of Israel (Eisikowitch and Galil, 1971).
The niche concept for pollinators, applied using the idea of unique morphospace (Fig. 2), may be a powerful way of predicting whether or not plants are likely to co-opt functionally equivalent substitute pollinators when introduced to new regions. Data from this study and that by Rodger et al. (2010) show that at least four introduced plants with floral tubes approx. 10 cm in length are pollinated by A. convolvuli in Africa. Two of these species (Ipomoea alba and Datura stramonium) originate from the Americas, which are outside of the range of A. convolvuli, while two others (Hedychium gardnerianum and Lilium formosanum) originate from Asia within the range of A. convolvuli. Some of these invasive species are facultative selfers (Rodger et al., 2013), so we cannot exclude the possibility that invasion by these species would have occurred even without hawkmoth pollination. Nevertheless, outcrossing by hawkmoths is likely to lead to genetic recombinations and accelerate the pace of invasion. The distribution of the four long-tubed invasive species is tightly correlated with that of the native species in the guild (Supplementary Data Fig. S1), although this may be due to adaptations of the invasive species to sub-tropical climates rather than to interactions with long-tongued hawkmoths and guild members. The impacts of invasive long-tubed plant species on the native guild members pollinated by A. convolvuli are unknown and worth examining in future studies.
The presence of a guild of long-tubed night-blooming flowers provides a potential niche for invasion by other hawkmoths with a very long proboscis. The long-tubed flowers of guild members have relatively large amounts of nectar that can only be accessed by long-tongued animals. The long-tongued American hawkmoth species A. cingulata has recently become naturalized in West Africa, and bioclimatic models suggest that it could spread across much of Africa (Ballesteros-Mejia et al., 2011). Given the availability of larval food plants and a guild of nectar-rich long-tubed flowers for this hawkmoth, such an invasion scenario seems highly likely.
In conclusion, this study shows that the hawkmoth A. convolvuli and, to a much lesser extent, the related species C. fulvinotata comprise a distinct long-tongued pollinator niche in Africa that is occupied by a large guild of plant species. The key functional trait that enables both native and introduced plants to exploit this ecological opportunity is the possession of a very long (approx. 10 cm) floral tube. Shorter-tubed plants are also visited by long-tongued hawkmoths, but this seldom results in efficient pollination (Alexandersson and Johnson, 2002). From the patterns of convergent evolution among guild members it seems likely that white floral coloration and copious amounts of nectar are also important for establishing mutualisms with long-tongued hawkmoths. The functional roles of these floral traits, as well as of floral scent, remain to be investigated. This study highlights the value of a niche perspective for understanding the geographical context and functional significance of floral traits. This perspective may also have value for predicting patterns of species sorting when plants become naturalized in new regions.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: localities of study sites, plant species studied and methods used. Table S2: corolla dimensions and nectar properties of plant species in South Africa with long-tubed flowers. Table S3: hawkmoths observed on long-tubed flowers of plant species in South Africa, indicating their proboscis length and site of pollen placement. Table S4: plant species in Kenya with floral tubes greater than 80 mm in length (adapted from Martins and Johnson, 2013). Figure S1: the geographical distribution of invasive plant species with long-tubed flowers in South Africa. Figure S2: the geographical distribution of the convolvulus hawkmoth Agrius convolvuli in South Africa.
ACKNOWLEDGEMENTS
We thank Ronny Alexandersson, Bruce Anderson and James Rodger for their contributions to this study, The National Research Foundation (South Africa) for funding, and National Geographic Foundation grant 7534-03 and Fulbright scholar award 10371 for supporting R.A.R.’s visits to S.D.J.’s lab.
LITERATURE CITED
- Agosta SJ, Janzen DH. (2005). Body size distributions of large Costa Rican dry forest moths and the underlying relationship between plant and pollinator morphology. OIKOS 108: 183–193. [Google Scholar]
- Alexandersson R, Johnson SD. (2002). Pollinator mediated selection on flower-tube length in a hawkmoth-pollinated Gladiolus (Iridaceae). Proceedings of the Royal Society B: Biological Sciences 269: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Alexandersson R, Johnson SD. (2010). Evolution and coexistence of pollination ecotypes in an African Gladiolus (Iridaceae). Evolution 64: 960–972. [DOI] [PubMed] [Google Scholar]
- Anderson B, Johnson SD. (2008). The geographical mosaic of coevolution in a plant–pollinator mutualism. Evolution 62: 220–225. [DOI] [PubMed] [Google Scholar]
- Anderson B, Johnson SD. (2009). Geographical covariation and local convergence of flower depth in a guild of fly-pollinated plants New Phytologist 182: 533–540. [DOI] [PubMed] [Google Scholar]
- Attie M, Kitching IJ, Veslot J. (2010). Patterns of larval hostplant usage among hawkmoths (Lepidoptera, Sphingidae) from La Réunion, with a comparison of the Mascarenes with other regions of the world. Revue d’écologie 65: 3–44. [Google Scholar]
- Ballesteros-Mejia L, Kitching IJ, Beck J. (2011). Projecting the potential invasion of the Pink Spotted Hawkmoth (Agrius cingulata) across Africa. International Journal of Pest Management 57: 153–159. [Google Scholar]
- Baum DA. (1995). The comparative pollination and floral biology of baobabs (Adansonia Bombacaceae). Annals of the Missouri Botanical Garden 82: 322–348. [Google Scholar]
- Boberg E, Alexandersson R, Jonsson M, Maad J, Agren J, Nilsson LA. (2014). Pollinator shifts and the evolution of spur length in the moth-pollinated orchid Platanthera bifolia. Annals of Botany 113: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon R. (2010). Pooley’s trees of eastern South Africa – a complete guide. Durban: Flora & Fauna Publications Trust. [Google Scholar]
- Borrell BJ. (2005). Long tongues and loose niches: evolution of euglossine bees and their nectar flowers. Biotropica 37: 664–669. [Google Scholar]
- Bradshaw HD, Schemske DW. (2003). Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426: 176–178. [DOI] [PubMed] [Google Scholar]
- Chase JM, Leibold MA. (2003). Ecological niches: linking classical and contemporary approaches. Chicago: The University of Chicago Press. [Google Scholar]
- Darwin CR. 1877. The various contrivances by which orchids are fertilised by insects , 2nd edn, revised edn. London: John Murray. [Google Scholar]
- Devictor V, Clavel J, Julliard R, et al. (2010). Defining and measuring ecological specialization. Journal of Applied Ecology 47: 15–25. [Google Scholar]
- Eisikowitch D, Galil J. (1971). Effect of wind on the pollination of Pancratium maritimum L. (Amaryllidaceae) by hawkmoths (Lepidoptera: Sphingidae). Journal of Animal Ecology 40: 673–678. [Google Scholar]
- Galen C, Kaczorowski R, Todd SL, Geib J, Raguso RA. (2011). Dosage-dependent impacts of a floral volatile compound on pollinators, larcenists, and the potential for floral evolution in the alpine skypilot Polemonium viscosum. American Naturalist 177: 258–272. [DOI] [PubMed] [Google Scholar]
- Gomez MJ, Perfectti F, Abdelaziz M, Lorite J, Munoz-Pajares AJ, Valverde J. (2015). Evolution of pollination niches in a generalist plant clade. New Phytologist 205: 440–453. [DOI] [PubMed] [Google Scholar]
- Grant V. (1983). The systematic and geographical distribution of hawkmoth flowers in the temperate North American flora. Botanical Gazette 144: 439–449. [Google Scholar]
- Grubb PJ. (1977). The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52: 107–145. [Google Scholar]
- Haber WA, Frankie GW. (1989). A tropical hawkmoth community: Costa Rican dry forest Sphingidae. Biotropica 21: 155–172. [Google Scholar]
- Harder LD. (1985). Morphology as a predictor of flower choice by bumble bees. Ecology 66: 198–210. [Google Scholar]
- Ippolito A, Fernandes GW, Holtsford TP. (2004). Pollinator preferences for Nicotiana alata, N. forgetiana, and their F-1 hybrids. Evolution 58: 2634–2644. [DOI] [PubMed] [Google Scholar]
- Johnson SD. (1995). Observations of hawkmoth pollination in the South African orchid Disa cooperi. Nordic Journal of Botany 15: 121–125. [Google Scholar]
- Johnson SD. (1997). Pollination ecotypes of Satyrium hallackii (Orchidaceae) in South Africa. Botanical Journal of the Linnean Society 123: 225–235. [Google Scholar]
- Johnson SD. (2010). The pollination niche and its role in the diversification and maintenance of the southern African flora. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Andersson S. (2002). A simple field method for manipulating ultraviolet reflectance of flowers. Canadian Journal of Botany-Revue Canadienne De Botanique 80: 1325–1328. [Google Scholar]
- Johnson SD, Steiner KE. (1997). Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae). Evolution 51: 45–53. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Edwards TJ, Carbutt C, Potgieter C. (2002). Specialization for hawkmoth and long-proboscid fly pollination in Zaluzianskya section Nycterinia (Scrophulariaceae). Botanical Journal of the Linnaean Society 138: 17–27. [Google Scholar]
- Johnson SD, Neal PR, Peter CI, Edwards TJ. (2004). Fruiting failure and limited recruitment in remnant populations of the hawkmoth-pollinated tree Oxyanthus pyriformis subsp pyriformis (Rubiaceae). Biological Conservation 120: 31–39. [Google Scholar]
- Kevan P, Giurfa M, Chittka L. (1996). Why are there so many and so few white flowers? Trends in Plant Science 1: 280–284. [Google Scholar]
- Kugler H. (1971). Zur bestäubung grossblumiger Datura arten. Flora 160: 511–517. [Google Scholar]
- Losos JB. (2010). Adaptive radiation, ecological opportunity, and evolutionary determinism. American Naturalist 175: 623–639. [DOI] [PubMed] [Google Scholar]
- Losos JB, Mahler DL. (2010). Adaptive radiation: the interaction of ecological opportunity, adaptation, and speciation. In: Bell MA, Futuyma DJ, Eanes WF, Levinton JS, eds. Evolution since Darwin: the first 150 years. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Maglianesi AM, Bluethgen N, Boehning-Gaese K, Schleuning M. (2014). Morphological traits determine specialization and resource use in plant–hummingbird networks in the neotropics. Ecology 95: 3325–3334. [Google Scholar]
- Manning J, Snijman D. (2002). Hawkmoth pollination in Crinum variabile (Amaryllidaceae) and the biogeography of sphingophily in southern African Amaryllidaceae. South African Journal of Botany 68: 212–216. [Google Scholar]
- Martins DJ, Johnson SD. (2013). Interactions between hawkmoths and flowering plants in East Africa: polyphagy and evolutionary specialization in an ecological context. Biological Journal of the Linnean Society 110: 199–213. [Google Scholar]
- McDade LA. (1992). Pollinator relationships, biogeography, and phylogeny. Bioscience 42: 21–26. [Google Scholar]
- Muchhala N, Thomson JD. (2009). Going to great lengths: selection for long corolla tubes in an extremely specialized bat–flower mutualism. Proceedings of the Royal Society B: Biological Sciences 276: 2147–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Anderson B, Johnson SD. (2012). Flower colour adaptation in a mimetic orchid. Proceedings of the Royal Society B: Biological Sciences 279: 2309–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Niet T, Johnson SD. (2012). Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends in Ecology and Evolution 27: 353–361. [DOI] [PubMed] [Google Scholar]
- van der Niet T, Jurgens A, Johnson SD. (2015). Is the timing of scent emission correlated with insect visitor activity and pollination in long-spurred Satyrium species? Plant Biology 17: 226–237. [DOI] [PubMed] [Google Scholar]
- Nilsson LA. (1988). The evolution of flowers with deep corolla tubes. Nature 334: 147–149. [Google Scholar]
- Nilsson LA, Jonsson L, Rason L, Randrianjohany E. (1985). Monophily and pollination in Angaecum arachnites Schlrt. (Orchidaceae) in a guild of long-tongued hawk-moths (Sphingidae) in Madagascar. Biological Journal of the Linnean Society 26: 1–19. [Google Scholar]
- Nilsson LA, Jonsson L, Rolison L, Randrianjohany E. (1987). Angraecoid orchids and hawkmoths in central Madagascar: specialized pollination systems and generalist foragers. Biotropica 19: 310–318. [Google Scholar]
- Nilsson LA, Rabakonandrianina E. (1988). Hawk-moth scale analysis and pollination specialization in the epilithic Malagasy endemic Aerangis ellisii (Reichenb. fil.) Schltr. (Orchidaceae). Botanical Journal of the Linnean Society 97: 49–61. [Google Scholar]
- Owen DF. (1969). Species diversity and seasonal abundance in tropical Sphingidae (Lepidoptera). Proceedings of the Royal Entomological Society of London. Series A, General Entomology 44: 162–168. [Google Scholar]
- Owen DF. (1972). Species diversity in tropical Sphingidae and a systematic list of species collected in Sierra Leone. Journal of Natural History 6: 177–194. [Google Scholar]
- Pauw A. (2013). Can pollination niches facilitate plant coexistence? Trends in Ecology and Evolution 28: 30–37. [DOI] [PubMed] [Google Scholar]
- Pauw A, Stofberg J, Waterman RJ. (2009). Flies and flowers in Darwin’s race. Evolution 63: 268–279. [DOI] [PubMed] [Google Scholar]
- Peter CI, Coombs G, Huchzermeyer CF, et al. (2009). Confirmation of hawkmoth pollination in Habenaria epipactidea: Leg placement of pollinaria and crepuscular scent emission. South African Journal of Botany 75: 744–750. [Google Scholar]
- Pinhey E. (1962). Hawkmoths of central and southern Africa. Cape Town: Longmans. [Google Scholar]
- Pooley E. (1998). A field guide to wildflowers of KwaZulu-Natal and the eastern region. Durban: Natal Flora Publications Trust. [Google Scholar]
- Raguso R, Willis MA. (2003). Hawkmoth pollination in Arizona’s Sonoran Desert: behavioral responses to floral traits. In: Boggs CL, Watt WB, Ehrlich PR, eds. Evolution and ecology taking flight: butterflies as model systems Chicago, IL: Chicago University Press. [Google Scholar]
- Ranta E, Lundberg H. (1980). Resource partitioning in bumblebees: the significance of differences in proboscis length. OIKOS 35: 298–302. [Google Scholar]
- Robertson I. (1977). Records of insects taken at light traps in Tanzania. Ministry of Overseas Development, 1–14. [Google Scholar]
- Rodger JG, van Kleunen M, Johnson SD. (2010). Does specialized pollination impede plant invasions. International Journal of Plant Sciences 171: 382–391. [Google Scholar]
- Rodger JG, van Kleunen M, Johnson SD. (2013). Pollinators, mates and Allee effects: the importance of self-pollination for fecundity in an invasive lily. Functional Ecology 27: 1023–1033. [Google Scholar]
- Ryckewaert P, Razanamaro O, Rasoamanana E, Rakotoarimihaja T, Ramavovololona P, Danthu P. (2011). Sphingidae as likely pollinators of Madagascar’s baobabs. Bois et Forets des Tropiques: 55–68. [Google Scholar]
- Schluter D. (2000). The ecology of adaptive radiation. Oxford: Oxford University Press. [Google Scholar]
- Silvertown J. (2004). Plant coexistence and the niche. Trends in Ecology and Evolution 19: 605–611. [Google Scholar]
- Stebbins GL. (1970). Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326. [Google Scholar]
- Theis N, Lerdau M, Raguso RA. (2007). The challenge of attracting pollinators while evading floral herbivores: patterns of fragrance emission in Cirsium arvense and Cirsium repandum (Asteraceae). International Journal of Plant Sciences 168: 587–601. [Google Scholar]
- Wasserthal LT. (1997). The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Botanica Acta 110: 343–359. [Google Scholar]
- Whittall JB, Hodges SA. (2007). Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447: 706–709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.