Abstract
Background and Aims Additional carbohydrate supply resulting from enhanced photosynthesis under predicted future elevated CO2 is likely to increase symbiotic nitrogen (N) fixation in legumes. This study examined the interactive effects of atmospheric CO2 and nitrate (NO3–) concentration on the growth, nodulation and N fixation of field pea (Pisum sativum) in a semi-arid cropping system.
Methods Field pea was grown for 15 weeks in a Vertosol containing 5, 25, 50 or 90 mg NO3–-N kg–1 under either ambient CO2 (aCO2; 390 ppm) or elevated CO2 (eCO2; 550 ppm) using free-air CO2 enrichment (SoilFACE).
Key Results Under aCO2, field pea biomass was significantly lower at 5 mg NO3–-N kg–1 than at 90 mg NO3–-N kg–1 soil. However, increasing the soil N level significantly reduced nodulation of lateral roots but not the primary root, and nodules were significantly smaller, with 85 % less nodule mass in the 90 NO3–-N kg–1 than in the 5 mg NO3–-N kg–1 treatment, highlighting the inhibitory effects of NO3–. Field pea grown under eCO2 had greater biomass (approx. 30 %) than those grown under aCO2, and was not affected by N level. Overall, the inhibitory effects of NO3– on nodulation and nodule mass appeared to be reduced under eCO2 compared with aCO2, although the effects of CO2 on root growth were not significant.
Conclusions Elevated CO2 alleviated the inhibitory effect of soil NO3– on nodulation and N2 fixation and is likely to lead to greater total N content of field pea growing under future elevated CO2 environments.
Keywords: Nitrogen fixation, nitrate, carbon cycling, nitrogen cycling, free-air CO2 enrichment, FACE, high atmospheric CO2, 15N, Pisum sativum, climate change
INTRODUCTION
Elevated atmospheric CO2 concentrations under future climate scenarios (IPCC, 2001) are predicted to produce major changes in the productivity and sustainability of agricultural cropping systems. It has been widely demonstrated that elevated CO2 stimulates photosynthesis, reduces evapotranspiration and subsequently increases plant productivity when water and nutrients are not limiting (Ainsworth and Long, 2005; de Graaff et al., 2006; Luo et al., 2006; Leakey et al., 2009). The relative responses of different systems are highly dependent on plant species and soil properties.
Legumes generally produce greater responses to elevated CO2 than non-legumes (Rogers et al., 2009). Additional carbohydrate supply under eCO2 resulting from enhanced photosynthesis is likely to increase symbiotic N2 fixation and fulfil any additional nitrogen (N) requirement (Cabrerizo et al., 2001; West et al., 2005). Field pea (Pisum sativum) is an economically important crop in many semi-arid cropping systems and is both mycorrhizal and a legume, and therefore may have a greater ability to avoid photosynthetic acclimation by diverting carbon (C) to its symbioses (Aranjuelo et al., 2013). The increase in N2 fixation under eCO2 could be derived from an increase in the number, size and/or activity of root nodules (Rogers et al., 2009). Lam et al. (2012) reported no change in number or mass of field pea root nodules under elevated CO2 despite 44–51 % increases in N2 fixation. In contrast, field pea grown under elevated CO2 had greater nodule biomass but not a greater number of root nodules (Cabrerizo et al., 2001). However, few studies have examined the effects of elevated CO2 on field pea.
The response of legumes to elevated CO2 in Australian agricultural systems could be less predictable than other systems since they are frequently water limited and soil N levels are highly variable. Preceding cereal crops generally have poor N fertilizer use efficiency (Chen et al., 2008), and consequently high soil N levels during legume establishment could limit responses to elevated CO2. Small amounts of soil N at the start of the growing season can enhance legume production, possibly due to bridging the interval between seed N utilization and N2 fixation and the lower energy requirement of nitrate (NO3–) uptake and assimilation than of N2 fixation (Jensen, 1986; Streeter and Wong, 1988; Peoples et al., 2012). Alternatively, plants supplied with small amounts of N may establish greater leaf area and photosynthesis during early growth stages that provides a greater capacity for subsequent nodulation and N2 fixation, although this is not always the case (Peoples et al., 1995).
High levels of soil N, predominantly NO3– from fertilizers, are known to inhibit the initiation, development and function of legume symbioses. Nodule number is depressed in the presence of NO3–, possibly due to a reduction in root hair growth, signalling molecules (flavonoids) and subsequent expression of nodulation genes in Rhizobium that disrupt the infection process (Waterer and Vessey, 1993; Bollman and Vessey, 2006). Nodule formation and hence nodule number is often reported to be less sensitive to NO3– than nodule growth and activity (Streeter and Wong, 1988), although this is not always the case (Waterer and Vessey, 1993). For established nodules, NO3– is thought to retard nodule growth and activity by causing preferential diversion of photosynthate to NO3– assimilation, by reducing oxygen transport within nodules or via toxic effects once reduced to nitrite (NO2–), although these are highly debated (Streeter and Wong, 1988; Arrese-Igor et al., 1997). Moreover, the relative effect of NO3– on crop legumes differs widely between species and between cultivars (Cowie et al., 1990; Peoples et al., 2012).
The combined effects of elevated CO2 and soil N level on the development and function of field pea root nodules is not clear. Previous studies on the mechanisms of NO3– inhibition of legumes under artificial and controlled conditions are not likely to reflect the response of plants grown in soil under field conditions. Naudin at al. (2010) showed that N2 fixation of field pea intercropped with wheat could recover from NO3– inhibition at all growth stages. This could be due to temporal reduction of bulk soil N or to spatial depletion of rhizosphere NO3– that allows nodulation and N2 fixation to proceed. Furthermore, greater C flow to the roots under elevated CO2 could alter the response of field pea to the inhibitory effects of soil NO3–. This experiment aimed to quantify the interactive effects of soil NO3– concentration and CO2 concentration on nodulation and N2 fixation of field pea under field [free-air CO2 enrichment (FACE)] conditions. We hypothesized that nodule establishment (nodule number), development (nodule mass) and function (nitrogenase activity, N derived from the atmosphere) would be progressively inhibited with increasing NO3– concentration, but these effects would be reduced under elevated CO2 via enhanced N demand due to greater photosynthetic activity and plant biomass accumulation.
MATERIALS AND METHODS
Field site and sampling details
This study was carried out at the SoilFACE facility at the Department of Economic Development, Jobs, Transport and Resources (DEDJTR) Plant Breeding Centre, Horsham (36 °44'57''S, 142 °06'50''E), Victoria, Australia. The SoilFACE system is part of the Australian Grains FACE (AgFACE) facility in the Victorian Wimmera region (Mollah et al., 2009). A detailed description of SoilFACE is given in Butterly et al. (2015). Soil was collected in June 2011 by excavating small areas of a nearby roadside (0–15 cm) following the removal of plant material and debris from the soil surface. Soil was sieved (<4 mm), thoroughly mixed and air-dried. This virgin soil was classified as a Vertosol (Isbell, 1996) or Vertisol (FAO/ISRIC/ISSS, 1998), respectively, and had the following physiochemical properties: total C, 14 mg kg–1; total N, 0·9 mg kg–1; organic C, 9·4 mg kg–1; Colwell P, 6 mg kg–1; NH4-N, 3 mg kg–1; NO3-N, 5 mg kg–1; pH (1:5 in 0·01 m CaCl2), 7·8; and clay, 50 % (Butterly et al., 2015).
Column experiment design and establishment
A column experiment was conducted using longitudinally sectioned PVC columns (10 cm ID × 60 cm high rejoined with silicon and PVC tape and fitted with PVC end caps at the base). Vertosol was mixed 1:1 (w/w) with triple-washed white sand (mean diameter 430 μm) to facilitate root sampling. Each column was filled with 5·5 kg of soil–sand mix containing basal nutrients (mg kg–1) (KH2PO4, 70·4; K2SO4, 103; CaCl2, 186; MgSO4, 122; MnSO4, 6; ZnSO4, 8; CuSO4, 6; CoCl2, 0·4; FeCl3, 0·6; Na2B4O7, 1·6; and Na2MoO4, 0·4) at a bulk density of 1·2 g cm–3. Columns also contained four NO3-N levels; 5, 25, 50 and 90 mg N kg–1, with 20, 20, 4 and 4 %, respectively, of the NO3– added as Ca(15NO3)2 (20 % atom excess, Shanghai Research Institute of Chemical Industry, China). Each column was adjusted to 80 % field capacity (θg = 0·182 g g–1) using reverse osmosis (RO) water and allowed to equilibrate.
Columns were hand sown with field pea (Pisum sativum ‘PBA Twilight’) (six per column) on 27 June 2011 at a depth of 2 cm using uniform germinated seeds. Field pea was inoculated using a commercial Group E peat inoculum (Rhizobium leguminosarum). Columns were placed within one of the eight SoilFACE bunkers treated with either ambient CO2 (aCO2; 390 ppm) or elevated CO2 (eCO2; 550 ppm) concentration, replicated four times on 1 July 2011. After 3 weeks, field pea seedlings were thinned to two plants per column. Overall, the experiment consisted of a randomized split-plot design with 2 CO2 concentrations (main plots) × 4 NO3-N levels (sub-plots) × 4 replicates (32 columns in total). Wheat (Triticum aestivum L. ‘Yitpi’) (eight seeds were sown and seedlings thinned to three per column) was included as a non-legume reference plant in the same design but with only two NO3-N levels (5 and 90 mg N kg–1) (eight columns in total).
Column harvest and processing
Columns were removed from SoilFACE on 13 October, after 15 weeks of growth (late pod development; 50–60 % of pods at maximum size) and destructively sampled. Prior to removal, the youngest fully emerged leaf was cut from each plant, weighed and placed in liquid N (two leaves per column). Plant shoots were cut off at the soil surface, washed three times with RO water and dried at 70 °C for 3 d. One half of each PVC column was removed and the soil was sectioned into depths of 0–10 and 10–55 cm. Roots within each sample were carefully removed, washed free of soil and debris with RO water, and stored at 5 °C. The soil was thoroughly mixed, stored at 5 °C overnight, and soil N was extracted the following day on field moist soil as outlined below. The remaining soil samples were air-dried at 25 °C for subsequent analyses. After morphological measurements (within 4 d), root samples were dried at 70 °C for 3 d for subsequent analyses.
Nodulation and leghaemoglobin content
For surface (0–10 cm) roots, nodules on the main root and lateral roots were counted; the nodules were then removed using a scalpel and weighed, before being stored at –20 °C. Nodule mass and number of nodules in the subsoil (10–55 cm) were independently assessed by three individuals using a visual ranking system (1–5; lowest/smallest–greatest/largest).
The leghaemoglobin concentration in nodules collected from 0–10 cm was determined using the method of Riley and Dilworth (1985). Briefly, approx. 300 mg of nodule material was ground in 5 mL of 0·1 m sodium phosphate buffer (pH 6·8; 5 °C) using a pre-cooled mortar and pestle. The macerate was passed through muslin and centrifuged at 500 g for 10 min at 5 °C to remove coarse material. The supernatant was then centrifuged at 4400 g for 25 min at 5 °C, and the leghaemoglobin concentration was determined spectrophotometrically at 555 nm (Cary 50, UV-Visible Spectrophotometer, Agilent, USA) following conversion to pyridine haemochromogen (1:1 supernatant/reagent; containing 0·2 m KOH in 50 % pyridine) according to Paul et al. (1953).
Nitrate reductase activity
Nitrate reductase activity (NRA) in field pea leaves was determined using the approach of Kaiser et al. (2000) but with some modifications. Leaves (approx. 500 mg) were ground with 2 mL of extraction buffer [50 mm potassium phosphate buffer, pH 7·6; 1 mm dithiothreitol (DTT); 10 μm FAD; 10 mm MgCl2] using a mortar and pestle pre-cooled with liquid N. Extracts were transferred to tubes and centrifuged at 16 000 g for 10 min at 4 °C. Aliquots (100 μL) were added to 900 μL of reaction buffer (50 mm potassium phosphate buffer, pH 7·6; 1 mm DTT; 10 μm FAD; 10 mm MgCl2; 5 mm KNO3; 9·2 mm NADH) and incubated for 3 min at 24 °C. The reaction was stopped using 125 μL of 0·5 m zinc acetate, and solutions were centrifuged as previously described. Initial and incubated extracts were mixed 1:1 with colour reagent [1·5 g L–1 NED (naphthylethylenediamine dihydrochloride); 2·5 g L–1 sulphanilamide; 1·13 n HCl), allowed to stand for 30 min, and nitrite concentrations were determined colorimetrically at 540 nm (Cary 50, UV-Visible Spectrophotometer, Agilent). The desalting of extracts prior to the assay performed by Kaiser et al. (2000) did not show any benefit and hence was omitted. Furthermore, potassium phosphate buffer was used instead of HEPES-KOH due to greater enzyme activity in preliminary tests (data not shown).
Soil and plant analyses
Soil texture was characterized by determining the particle size distribution using a Laser Particle Size Analyser (Malvern Mastersizer 2000, Worcestershire, UK) following dispersion of soil (approx. 10 g) with 10 mL of 0·164 m Na6P5O18 (VWR, Australia) in 800 mL of RO water. Soil N extractions were performed on field moist soil using 25 g of soil (dry weight basis) with 2 m KCl (1:1) and shaking end-over-end for 1 h, centrifuging at 2000 g for 5 min and filtering through Whatman #1 filter papers (Whatman International, UK). Filtered extracts were frozen and later analysed for nitrate (NO3–) and ammonium (NH4+) using a QuickChem 8500 Flow Injection Analyser (Lachat Instruments, USA).
Dried plant samples were ground (<2 mm) using a Retsch ZM200 centrifugal mill (Retsch GmbH, Haan, Germany) to reduce sample volume. Sub-samples of both ground plant material and whole soil were then finely ground using a Retsch MM400 mixer mill (Retsch GmbH).
Isotope ratio mass spectrometry (IRMS) (Hydra 20-20; SerCon, Crewe, UK) was used to determine the total C and N concentration and 15N abundance (atom% 15N) of plant and soil samples. Total N content (mg N per column) and the amount of N derived from either the atmosphere or the fertilizer were calculated using combined N and 15N in the whole plant and the entire soil column. The percentage of N derived from the atmosphere (% Ndfa) and fertilizer (% Ndff) was calculated according to McAuliffe et al. (1958) using wheat as the reference (Supplementary Data Table S1). The isotope dilution approach can overestimate % Ndfa as it does not account for N derived from soil. In the current study, the soil–sand mix contained 4 mg of NO3– + NH4+ kg–1 which constitutes an average error of approx. 6·3 %.
Statistical analyses
A two-way analysis of variance (ANOVA) using a split-plot design was used to test the effects of CO2 concentration (main plots) and NO3-N level (sub-plots) on plant and soil parameters. Differences between means were tested using the least significance difference (LSD) test at P = 0·05.
RESULTS
Plant growth
Shoot growth of field pea was significantly (P = 0·016) greater under eCO2 than under aCO2. Shoot biomass ranged from 11 to 15 g per column under aCO2 and on average increased by 32 % under eCO2 (Fig. 1A). Overall, shoot biomass was ten times greater than root biomass. Since the root biomass in the topsoil (0–10 cm) was small (15 % of the total root mass) and was not affected by the treatments, the pooled root biomass for each column was used for analyses. For root biomass, there was an apparent increase under eCO2; however, this was not significant (P = 0·086). Higher rates of NO3–-N significantly (P = 0·038) increased root mass. In particular, 25 mg NO3–-N kg–1 soil had a lower root mass than 90 mg NO3–-N kg–1 (Fig. 1B). No effect of CO2 or N level on the root:shoot ratio was observed (Fig. 1C). For shoot and root biomass, no significant interactions between CO2 and NO3–-N concentration were observed.
Fig. 1.
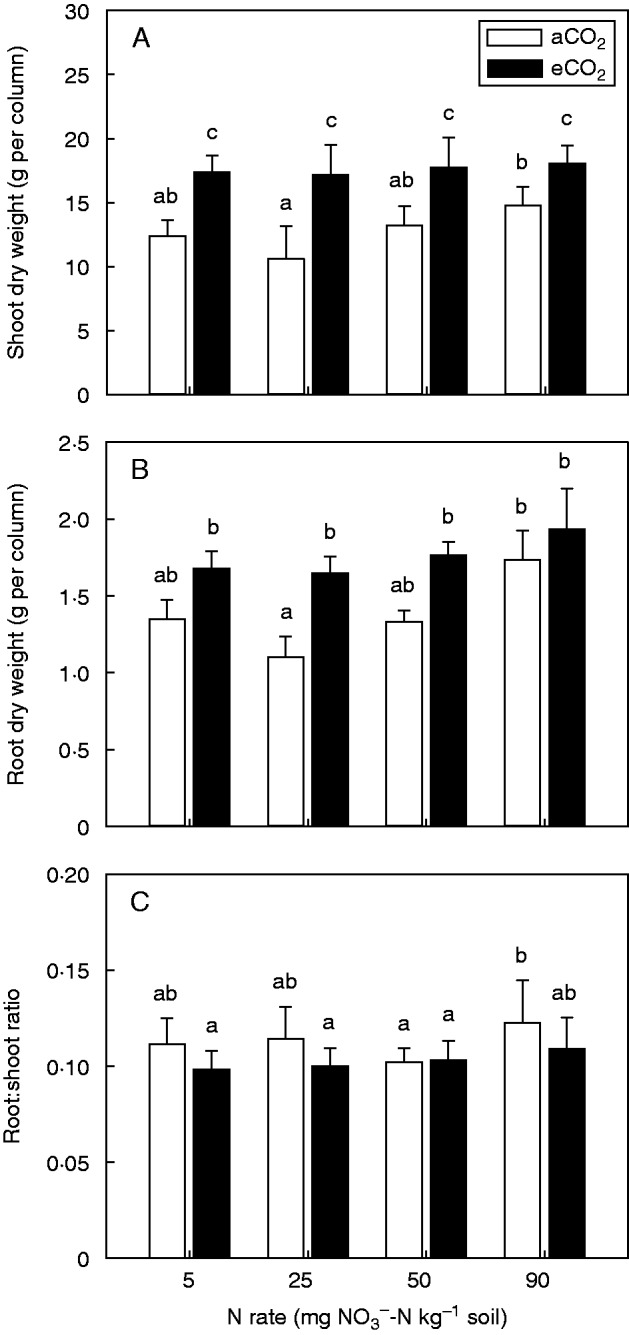
Shoot (A) and root (B) biomass and root:shoot ratio (C) of field pea grown for 15 weeks under either an aCO2 or eCO2 concentration and with 5, 25, 50 or 90 mg NO3–-N kg–1 soil. Root mass includes nodules. Error bars indicate the s.e.m. (n = 4). Means with the same lower case letter are not significantly (P < 0·05) different.
Nodule number, mass and leghaemoglobin content
Nodulation of field pea was affected by NO3–-N more than CO2 concentration, and no interactions were observed (Table 1). For surface roots (0–10 cm), nodule numbers on primary and lateral roots were not different between aCO2 and eCO2. Under aCO2, both the number and mass of nodules on lateral roots significantly (P < 0·05) decreased with increasing N level; however, at eCO2, this was only significant for 50 mg NO3–-N kg–1. The mass of field pea nodules on roots in the 0–10 cm layer was reduced by 85 % between 5 and 90 mg NO3–-N under aCO2. While the mass of nodules in the 0–10 cm layer was also reduced under eCO2, the reduction was much lower in magnitude (72 %) and only significantly lower in the 90 mg NO3–-N treatment. Hence, the decrease in nodule mass under eCO2 was not as sharp when NO3–-N was increased compared with aCO2. The decrease in nodule mass with increasing NO3–-N was largely due to a reduction in the size of nodules on the primary root. However, NO3–-N and CO2 concentration did not alter the leghaemoglobin concentration expressed per mass of nodule tissue (mmol g–1) or normalized to account for nodule size (mmol per 1000 nodules) (Table 1). Due to the volume of roots in the deeper soil layer (10–55 cm), visual ranking was used to assess the number and size of root nodules. Consistent with surface soil, the number and size of nodules in the deeper layer were significantly reduced when the NO3–-N concentration was increased (Table 1). In general, nodules in the subsoil were more numerous but much smaller in size than in the topsoil.
Table 1.
Number, mass and leghaemoglobin content of root nodules in the 0–10 cm layer (Root1) and visual ranking (1–5; lowest to highest and smallest to largest) of root nodules in the 10–55 cm layer (Root2) of field pea grown for 15 weeks under either an aCO2 or eCO2 concentration and with 5, 25, 50 or 90 mg NO3–-N kg–1 soil
| Factor | N rate (mg kg–1) | Primary root1 nodules (per plant) | Lateral root1 nodules (per plant) | Root1 nodule mass (mg per column) | Root1 nodule size (mg per nodule) | Root2 nodule ranking (1–5) |
Root1 leghaemoglobin concentration (µmol) |
||
|---|---|---|---|---|---|---|---|---|---|
| n | Size | g–1 | Per nodule | ||||||
| N rate (mg kg–1) | 5 | 28·0 | 127·9 | 512 | 1·26 | 3·4 | 4·0 | 628 | 1·66 |
| 25 | 27·2 | 103·8 | 327 | 1·29 | 3·1 | 3·5 | 575 | 2·42 | |
| 50 | 27·7 | 70·4 | 267 | 1·01 | 2·5 | 2·8 | 557 | 2·27 | |
| 90 | 25·8 | 93·4 | 108 | 0·42 | 1·2 | 1·5 | 616 | 2·77 | |
| CO2 level | aCO2 | 27·1 | 97·7 | 272 | 0·97 | 2·4 | 2·9 | 567 | 2·5 |
| eCO2 | 27·2 | 100·0 | 335 | 1·02 | 2·8 | 3·0 | 621 | 2·1 | |
| N rate | n.s. | * | * | *** | ** | *** | n.s. | n.s. | |
| LSD (P = 0·05) | – | 31 | 236 | 0·32 | 0·38 | 0·73 | – | – | |
| CO2 level | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| LSD (P = 0·05) | – | – | – | – | – | – | – | – | |
No CO2 × N interactions were observed (P > 0·05).
n.s., P > 0·05; *P < 0·05; **P < 0·01; ***P < 0·001.
–, not applicable; LSD, least significant difference.
N concentration and 15N abundance
The concentration of N in shoot and roots of field pea was not affected by CO2 or N level (Table 2). Consequently, the C:N ratio of plant material was also not affected by the treatments. However, the N concentration of deeper roots (10–55 cm) was higher than that of surface roots (0–10 cm), resulting in lower C:N ratios. In contrast to overall N concentration, 15N abundance (atom%) of plant material was significantly affected by CO2 concentration (P < 0·05) and N level (P < 0·001). For shoot, 15N abundance was greater under aCO2 than eCO2, except for 90 mg NO3–-N kg–1. In general, greater 15N abundance under aCO2 was observed for roots, except surface roots (0–10 cm) with 5 mg NO3–-N kg–1 and deep roots (10–55 cm) with 5 and 90 mg NO3–-N kg–1.
Table 2.
N concentration, C:N ratio and 15N abundance in shoot and root of field pea grown for 15 weeks under either an aCO2 or eCO2 concentration and with 5, 25, 50 or 90 mg NO3–-N kg–1 soil
| Factor | N rate (mg kg–1) | N concentration (g kg–1) |
C:N |
15N (atom%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root1 | Root2 | Shoot | Root1 | Root2 | Shoot | Root1 | Root2 | ||
| N rate (mg kg–1) | 5 | 20·7 | 24·3 | 29·3 | 20·4 | 16·3 | 14·0 | 1·6 | 1·2 | 2·5 |
| 25 | 20·3 | 24·3 | 29·5 | 20·8 | 16·1 | 14·1 | 6·0 | 4·1 | 7·4 | |
| 50 | 21·9 | 22·3 | 29·3 | 20·7 | 16·9 | 14·1 | 2·6 | 2·0 | 2·9 | |
| 90 | 20·5 | 24·2 | 29·2 | 20·7 | 16·4 | 13·6 | 3·1 | 2·4 | 3·3 | |
| CO2 level | aCO2 | 20·8 | 24·1 | 30·1 | 20·1 | 16·0 | 13·4 | 3·8 | 2·7 | 4·3 |
| eCO2 | 20·9 | 23·4 | 28·5 | 21·3 | 16·8 | 14·6 | 2·9 | 2·2 | 3·8 | |
| N rate | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | *** | *** | *** | |
| LSD (P = 0·05) | – | – | – | – | – | – | 1·08 | 0·32 | 0·63 | |
| CO2 level | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | * | * | |
| LSD (P = 0·05) | – | – | – | – | – | – | 0·72 | 0·30 | 0·30 | |
Roots from 0–10 cm (root1) and 10–55 cm (root2) were analysed separately.
No CO2 × N interactions were observed (P > 0·05).
n.s., P > 0·05; *P < 0·05; ***P < 0·001.
–, not applicable; LSD, least significant difference
Total N content and N derived from fertilizer
Total N content was significantly (P = 0·011) greater under eCO2; however, NO3–-N concentration was less (Fig. 2A), and was only different between 25 and 90 mg NO3–-N kg–1. On average, total N content was 294 mg N per column under aCO2, and was increased by 23–54 % under eCO2. Fertilizer recovery was generally high (55 and 74 %) since inorganic N (NOx– + NH4+) (45·7 mg N per column) was relatively low compared with the amount of fertilizer added (495 mg N per column in the 90 mg kg–1 treatment) (data not shown). The percentage of N derived from fertilizer (% Ndff) showed a significant (P = 0·002) CO2 × N interaction (Fig. 2B). Specifically, field pea plants grown under aCO2 derived a higher proportion of N from fertilizer and the maximal increase was 61 % for the 50 mg NO3–-N kg–1 treatment.
Fig. 2.
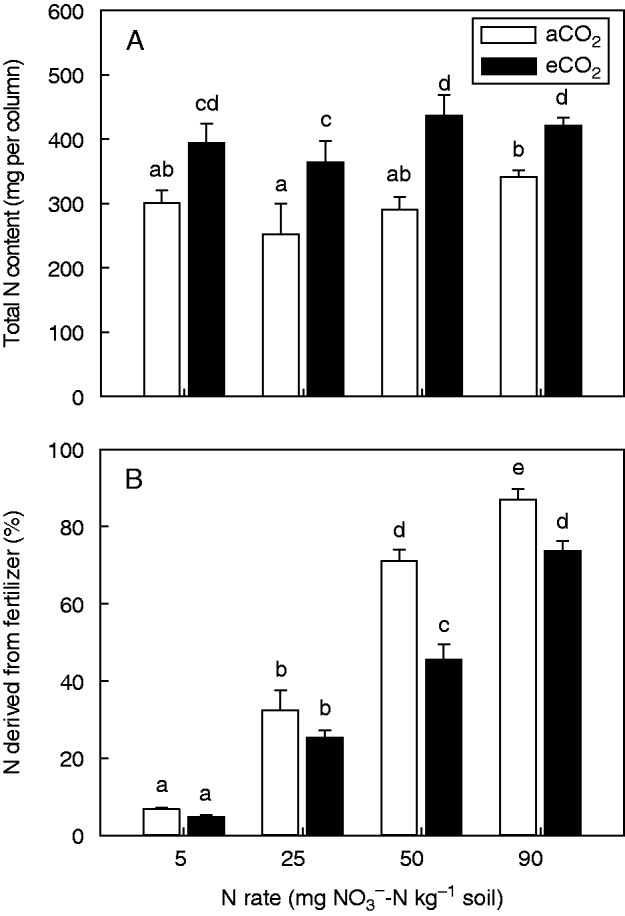
Total N content (A) and percentage of N derived from the fertilizer (B) for field pea grown for 15 weeks under either an aCO2 or eCO2 concentration and 5, 25, 50 or 90 mg NO3–-N kg–1 soil. Bars indicate the s.e.m. (n = 4). Means with the same lower case letter are not significantly (P < 0·05) different.
N2 fixation
Consistent with reduced fertilizer utilization, the percentage of N derived from the atmosphere (% Ndfa) was significantly (P = 0·011) greater for field pea grown under eCO2 than under aCO2 (Fig. 3). An increase in Ndfa of 10 and 139 % under eCO2 was observed for 5 and 90 mg NO3–-N kg–1, respectively. In contrast, a greater NO3– concentration strongly (P < 0·001) reduced Ndfa, indicating that N fixation was severely inhibited. At 90 mg NO3–-N kg–1, the mean Ndfa was five times lower than that measured at 5 mg. Thus, the relative effect of eCO2 was greater at the higher N rate than the lower one, despite a much lower Ndfa overall.
Fig. 3.
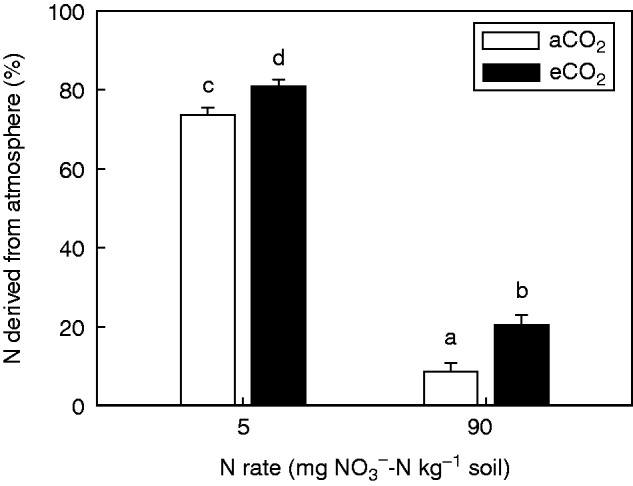
Percentage of N derived from the atmosphere for field pea grown for 15 weeks under either an aCO2 or eCO2 concentration and 5 or 90 mg NO3–-N kg–1 soil. Bars indicate the s.e.m. (n = 4). Means with the same lower case letter are not significantly (P < 0·05) different.
Nitrate reductase activity
Nitrate reductase activity was determined in the youngest fully expanded leaves of field pea. There was an apparent increase in NRA between 5 and 50 mg NO3–-N kg–1 and then a sharp drop in NRA at 90 mg NO3–-N kg–1. However, the data obtained for NRA was highly variable, and consequently no effects of NO3– and CO2 concentration were observed (P > 0·05) (data not presented).
Soil inorganic N
Inorganic N (NOx– + NH4+) in the soil was pooled since NH4+ concentration was low (<4 % of NO3–) most probably due to the neutral pH. The inorganic N concentration in the soil was significantly (P < 0·001) affected by the N rate (Fig. 4). The inorganic N concentration increased with increasing rate of NO3–; however, except for the 90 mg NO3–-N kg–1 treatment, all other N rates were not significantly different (P > 0·05). In particular, the 90 mg NO3–-N kg–1 treatment had approx. 310 mg inorganic N per column, which was between six and 43 times more than the other treatments. While the inorganic N concentration of soil appeared to be lower under eCO2, this was not significant (P > 0·05).
Fig. 4.
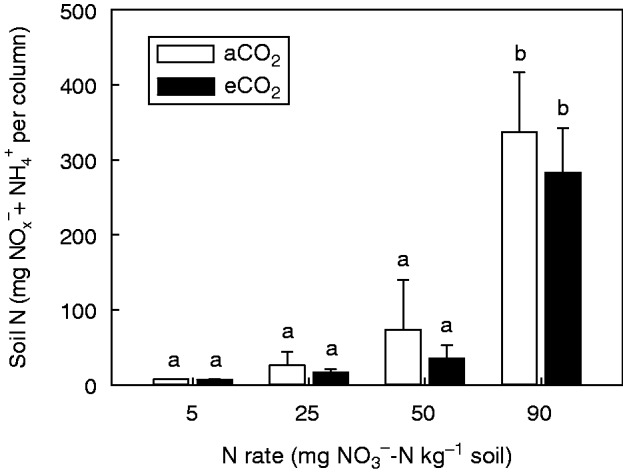
Inorganic N (NOx– + NH4 +) in soil following harvest of field pea grown for 15 weeks under either an aCO2 or eCO2 concentration and 5, 25, 50 and 90 mg NO3–-N kg–1 soil. Bars indicate the s.e.m. (n = 4). Means with the same lower case letter are not significantly (P < 0·05) different.
DISCUSSION
Effects of CO2 and N level on plant growth and nodulation
Shoot and root biomass of field pea grown under aCO2 was lower at 25 mg N kg–1 than at 90 mg N kg–1 (Fig. 1). The relative difference between these treatments was probably a function of contrasting N source and C allocation by plants. Moderate (approx. 25 mg N kg–1) levels of soil NO3– are known to inhibit legume symbiosis and nodule function (Streeter and Wong, 1988; Cowie et al., 1990; Naudin et al., 2011), resulting in insufficient N acquisition to meet the plant’s requirement. At high (approx. 90 mg N kg–1) levels of soil NO3–, field pea derived less N from N2 fixation (Fig. 3) but still maintained greater biomass due to greater availability of soil N. Photosynthates are known to be preferentially diverted to nodule morphogenesis rather than growth, particularly during the early growth stages (Voisin et al., 2002a). Therefore, lower biomass of the 25 mg N kg–1 than the 90 mg N kg–1 treatment could be attributed to either lower levels of photosynthate C or greater allocation of C to the production and maintenance of root nodules. This phenomenon has been used to explain greater biomass production, particularly of roots, in NO3–-fed field pea plants (Aranjuelo et al., 2013). In the current study, the N level did not alter the root:shoot ratio, which is similar to the findings other studies (Voisin et al., 2003a; Aranjuelo et al., 2013), and the low root biomass (root:shoot 0·6–0·7) is consistent for this species (Nuruzzaman et al., 2006) (Fig. 1C).
The inhibitory effects of NO3– on nodule formation and development observed in the current study are consistent with previous reports (Tang et al., 1999; Bollman and Vessey, 2006; Aranjuelo et al., 2013). Although the numbers of nodules on the primary roots were the same for all treatments, nodulation of lateral roots and total nodule mass in the topsoil (0–10 cm) were significantly reduced as the NO3– level was increased (Table 1). The reduction in nodule number on the lateral roots but not the primary root may be due to spatial and temporal depletion of soil NO3–. It is likely that primary root nodules of high N treatments were formed but their development was delayed until later stages of growth once the NO3– concentration in the rhizosphere soil was sufficiently depleted. The reduction in nodule mass with increased NO3– level, predominantly due to lower primary root nodule mass, supports the idea of an inhibition of nodule growth and development. In contrast, lateral roots that are much finer and constantly grow into new areas of soil may not have been able to deplete the soil NO3– concentration sufficiently to allow nodulation and/or nodule function. Reductions in the number and size of root nodules with increasing NO3– level in the 10–55 cm soil layer were consistent with field pea nodulation in the topsoil (0–10 cm) (Table 1).
Field pea plants grown under eCO2 had greater biomass (approx. 32 %) than those grown under aCO2 and were not affected by the soil NO3– level (Fig. 1). Therefore, field pea grown under eCO2 achieved the same biomass irrespective of N source. While eCO2 appeared to alleviate the inhibitory effects of NO3– on nodule number and mass (as indicated by a greater % Ndfa, discussed later), these effects were not significant (Table 1). Changes in nodulation and nodule morphology were expected under eCO2, particularly for the 25 mg N kg–1 treatment that showed the largest gain in biomass production. Other studies have shown increased mass and activity of field pea nodules under eCO2 (Cabrerizo et al., 2001; Aranjuelo et al., 2014). Enhanced nodule function is likely to have contributed to productivity gains under eCO2, although the concentration of leghaemoglobin in nodules, used here as an indicator of nodule activity, did not support this (Table 1). However, nodule activity is known to decrease during reproductive growth due to greater allocation of assimilates to grain (Voisin et al., 2002b). Hence, the leghaemoglobin concentrations observed may be a poor indicator of actual nodule activity during the growing season. Furthermore, primary root nodules dominated the nodule biomass, and the greater activity of more numerous but smaller lateral root nodules under eCO2 may have been masked. Importantly, the parameters used to assess treatment effects on field pea nodules were not related to overall nodule function (Ndfa), as discussed later.
Enhanced productivity of field pea under eCO2 was probably facilitated by greater availability of photosynthates, particularly to root nodules. Using a 13CO2 pulse-labelling approach, Voisin et al. (2003b) showed that C allocated to field pea roots increased with atmospheric CO2 concentration due to enhanced net photosynthesis. Similarly, field pea nodules are a major C sink and are generally substrate limited under aCO2 conditions (Aranjuelo et al., 2013). Furthermore, the additional N demand of field pea grown under eCO2 could have resulted in a more rapid depletion of soil N and subsequently allowed the establishment of N2 fixation earlier than plants grown under aCO2. Since field pea has a relatively small root system and excretes less carbohydrates and enzymes compared with other crop legumes (Nuruzzaman et al., 2006), the contribution of microbial N immobilization to reductions in rhizosphere NO3– concentration are expected to be small compared with plant N demand. Nodulation rapidly commences once the NO3– concentration falls below a critical level (Voisin et al., 2002b), but this recovery is dependent on C availability to nodules (Naudin et al., 2011). Evidence suggests that C supply to field pea roots is independent of the N source and extent of nodulation; however, nodulated roots have lower C use efficiency due to greater respiration losses (Voisin et al., 2003a, b). In the current study, the relative increase in biomass of field pea grown under eCO2 was greater for 25 mg N kg–1 than for 90 mg N kg–1, and this could be due to the lower C use efficiency of well-nodulated plants in the lower N aCO2 treatment. Alternatively, photosynthetic acclimation or downregulation of photosynthesis could have occurred in the high NO3– treatment due to lower sink strength for photosynthate C (Gavito et al., 2000; Aranjuelo et al., 2013).
Effects of CO2 and N level on legume performance
The current study highlighted that the total N content of field pea grown under eCO2 was enhanced (Fig. 2A). As expected, the N concentration and C:N ratio of shoots and roots were not affected by either treatment (Table 2) and, subsequently, the total N content was a function of plant biomass. Generally, the N concentration of legumes is maintained under eCO2 unless environmental or nutritional limitations to N2 fixation, such as phosphorus, exist (Jin et al., 2012; Lam et al., 2012). Greater N content was primarily achieved by N2 fixation since N fertilizer recovery (data not shown) and the soil N concentration at harvest (Fig. 4) did not differ between the CO2 treatments. The % Ndfa values for field pea grown at the low N level (74 % aCO2, 81 % eCO2) were consistent with other studies (average ≥80 %) (Voisin et al., 2002b; Hauggaard-Nielsen et al., 2010) but higher than commonly observed in southern Australia (average 68 %) (Unkovich et al., 1997). Accordingly, the amount of N2 fixed under aCO2 and eCO2 ranged from 263 to 376 mg N per column at the low N level and from 49 to 111 mg N per column for the high N level, respectively. However, it is unclear whether specific increases in N2 fixation efficiency occurred since this process was not directly quantified. The N concentration did not affect the rate of N2 fixation of field pea but simply delayed the onset of N2 fixation (Voisin et al., 2002b). Similarly, greater Ndfa of white clover under eCO2 was due to greater nodule mass rather than specific nodule activity (Zanetti et al., 1998). However, NO3– added after legume establishment can reduce both nodule biomass and specific activity (Naudin et al., 2011).
Importantly, this study showed that Ndfa was greater under eCO2 irrespective of N level. In the current study, Ndfa decreased and was inversely proportional to Ndff as the N level increased (Fig. 3). This substitution of N2 fixation with soil N has been well established (Streeter and Wong, 1988; Naudin et al., 2011). In fact, Voisin et al. (2002b) suggest that Ndfa can be predicted across the growing season from initial mineral N at sowing. Results obtained in the current study conform with the relationship between N level and Ndfa reported by Jensen (1986) (data not shown). In contrast to other studies, N2 fixation was less inhibited at the highest N level (equivalent to 630 kg N ha–1) and may be due to the fact that fertilizer was mixed to a greater depth (uniformly through the 55 cm soil columns). Greater inhibition of N2 fixation by inorganic N in other studies could also to be due to high existing soil N pools (Lam et al., 2012) or the use of NH4NO3– as the N fertilizer source (Voisin et al., 2002b). NH4+ has been shown to reverse the inhibitory effects of NO3– in artificial growth media (Bollman and Vessey, 2006). However, in soil and when added at the start of the growing season in the absence of living roots, NH4+ is likely to be converted to NO3–, thereby underestimating the actual NO3- concentration.
Conclusions
Using FACE combined with a 15N isotope dilution approach, this study showed for the first time that eCO2 reduced the inhibitory effects of soil NO3– on N2 fixation of field pea. Under eCO2, the N content of field pea was enhanced via greater biomass production and higher % Ndfa, although the physiological (number, size and activity of nodules) differences were not evident at the time of sampling. It is possible that eCO2 increased the critical concentration at which N2 fixation of field pea was inhibited and/or field pea was more efficient at switching between fertilizer N and N2 fixation under eCO2 due to more rapid depletion of the rhizosphere NO3– concentration. The current results indicate that field pea may perform well in semi-arid agricultural systems under future CO2 concentrations irrespective of soil N status, and subsequent gains in N input via enhanced N2 fixation will be important for maintaining the N fertility of cropping systems.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Table S1: biomass, N concentration, C:N ratio and 15N abundance in shoot and root of wheat grown under either aCO2 or eCO2 concentrations and with 5 or 90 mg NO3–-N kg–1 soil.
ACKNOWLEDGEMENTS
This research was supported by an Australian Research Council Linkage Project (LP100200757), and was conducted at the SoilFACE facility of the Department of Economic Development, Jobs, Transport and Resources (DEDJTR), Victoria at Horsham. We are grateful to Dr Xiaojuan Wang for help in establishing experiments. Joseph Conheady worked on this study as part of his Honours project.
LITERATURE CITED
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165: 351–371. [DOI] [PubMed] [Google Scholar]
- Aranjuelo I, Cabrerizo PM, Arrese-Igor C, Aparicio-Tejo PM. 2013. Pea plant responsiveness under elevated CO2 is conditioned by the N source (N2 fixation versus NO3– fertilization). Environmental and Experimental Botany 95: 34–40. [Google Scholar]
- Aranjuelo I, Cabrerizo PM, Aparicio-Tejo PM, Arrese-Igor C. 2014. Unravelling the mechanisms that improve photosynthetic performance of N2-fixing pea plants exposed to elevated CO2. Environmental and Experimental Botany 99: 167–174. [Google Scholar]
- Arrese-Igor C, Minchin FR, Gordon AJ, Nath AK. 1997. Possible causes of the physiological decline in soybean nitrogen fixation in the presence of nitrate. Journal of Experimental Botany 48: 905–913. [Google Scholar]
- Bollman MI, Vessey JK. 2006. Differential effects of nitrate and ammonium supply on nodule initiation, development, and distribution on roots of pea (Pisum sativum). Canadian Journal of Botany 84: 893–903. [Google Scholar]
- Butterly CR, Armstrong R, Chen D, Tang C. 2015. Carbon and nitrogen partitioning of wheat and field pea grown with two nitrogen levels under elevated CO2. Plant and Soil 391: 367–382. [Google Scholar]
- Cabrerizo PM, Gonzalez EM, Aparicio-Tejo PM, Arrese-Igor C. 2001. Continuous CO2 enrichment leads to increased nodule biomass, carbon availability to nodules and activity of carbon-metabolising enzymes but does not enhance specific nitrogen fixation in pea. Physiologia Plantarum 113: 33–40. [Google Scholar]
- Chen D, Suter H, Islam A, Edis R, Freney JR, Walker CN. 2008. Prospects of improving efficiency of fertiliser nitrogen in Australian agriculture: a review of enhanced efficiency fertilisers. Soil Research 46: 289–301. [Google Scholar]
- Cowie A, Jessop R, MacLeod D. 1990. Effect of soil nitrate on the growth and nodulation of winter crop legumes. Australian Journal of Experimental Agriculture 30: 651–654. [Google Scholar]
- FAO/ISRIC/ISSS. 1998. World reference base for soil resources. Rome: FAO. [Google Scholar]
- Gavito ME, Curtis PS, Mikkelsen TN, Jakobsen I. 2000. Atmospheric CO2 and mycorrhiza effects on biomass allocation and nutrient uptake of nodulated pea (Pisum sativum L.) plants. Journal of Experimental Botany 51: 1931–1938. [DOI] [PubMed] [Google Scholar]
- de Graaff M-A, van Groenigen K-J, Six J, Hungate B, van Kessel C. 2006. Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Global Change Biology 12: 2077–2091. [Google Scholar]
- Hauggaard-Nielsen H, Holdensen L, Wulfsohn D, Jensen ES. 2010. Spatial variation of N2-fixation in field pea (Pisum sativum L.) at the field scale determined by the 15N natural abundance method. Plant and Soil 327: 167–184. [Google Scholar]
- IPCC. 2001. Climate change 2001: impacts, adaptation and vulnerability. New York: Cambridge University Press. [Google Scholar]
- Isbell RF. 1996. The Australian soil classification. Melbourne: CSIRO Publishing. [Google Scholar]
- Jensen ES. 1986. The influence of rate and time of nitrate supply on nitrogen fixation and yield in pea (Pisum sativum L.). Fertilizer Research 10: 193–202. [Google Scholar]
- Jin J, Tang CX, Armstrong R, Sale P. 2012. Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient vertisol. Plant and Soil 358: 86–99. [Google Scholar]
- Kaiser WM, Kandlbinder A, Stoimenova M, Glaab J. 2000. Discrepancy between nitrate reduction rates in intact leaves and nitrate reductase activity in leaf extracts: what limits nitrate reduction in situ? Planta 210: 801–807. [DOI] [PubMed] [Google Scholar]
- Lam SK, Chen D, Norton R, Armstrong R. 2012. Does phosphorus stimulate the effect of elevated [CO2] on growth and symbiotic nitrogen fixation of grain and pasture legumes? Crop and Pasture Science 63: 53–62. [Google Scholar]
- Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR. 2009. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. Journal of Experimental Botany 60: 2859–2876. [DOI] [PubMed] [Google Scholar]
- Luo YQ, Hui DF, Zhang DQ. 2006. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87: 53–63. [DOI] [PubMed] [Google Scholar]
- McAuliffe C, Chamblee DS, Uribe-Arango H, Woodhouse WW. 1958. Influence of inorganic nitrogen on nitrogen fixation by legumes as revealed by 15N. Agronomy Journal 50: 334–337. [Google Scholar]
- Mollah M, Norton R, Huzzey J. 2009. Australian grains free-air carbon dioxide enrichment (AGFACE) facility: design and performance. Crop and Pasture Science 60: 697–707. [Google Scholar]
- Naudin C, Corre-Hellou G, Pineau S, Crozat Y, Jeuffroy M-H. 2010. The effect of various dynamics of N availability on winter pea–wheat intercrops: crop growth, N partitioning and symbiotic N2 fixation. Field Crops Research 119: 2–11. [Google Scholar]
- Naudin C, Corre-Hellou G, Voisin AS, et al. 2011. Inhibition and recovery of symbiotic N2 fixation by peas (Pisum sativum L.) in response to short-term nitrate exposure. Plant and Soil 346: 275–287. [Google Scholar]
- Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ. 2006. Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant and Soil 281: 109–120. [Google Scholar]
- Paul KG, Theorell H, Akeson A. 1953. The molar light absorption of pyridine ferroprotoporphyrin (Pyridine haemochromogen). Acta Chemica Scandinavica 7: 1284–1287. [Google Scholar]
- Peoples MB, Gault RR, Lean B, Sykes JD, Brockwell J. 1995. Nitrogen fixation by soybean in commercial irrigated crops of central and southern New South Wales. Soil Biology and Biochemistry 27: 553–561. [Google Scholar]
- Peoples MB, Brockwell J, Hunt JR, et al. 2012. Factors affecting the potential contributions of N2 fixation by legumes in Australian pasture systems. Crop and Pasture Science 63: 759–786. [Google Scholar]
- Riley IT, Dilworth MJ. 1985. Cobalt requirement for nodule development and function in Lupinus angustifolius L. New Phytologist 100: 347–359. [Google Scholar]
- Rogers A, Ainsworth EA, Leakey ADB. 2009. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiology 151: 1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J, Wong PP. 1988. Inhibition of legume nodule formation and N2 fixation by nitrate. Critical Reviews in Plant Sciences 7: 1–23. [Google Scholar]
- Tang C, Unkovich MJ, Bowden JW. 1999. Factors affecting soil acidification under legumes. III. Acid production by N2-fixing legumes as influenced by nitrate supply. New Phytologist 143: 513–521. [DOI] [PubMed] [Google Scholar]
- Unkovich MJ, Pate JS, Sanford P. 1997. Nitrogen fixation by annual legumes in Australian Mediterranean agriculture. Australian Journal of Agricultural Research 48: 267–293. [Google Scholar]
- Voisin AS, Salon C, Munier-Jolain NG, Ney B. 2002a. Effect of mineral nitrogen on nitrogen nutrition and biomass partitioning between the shoot and roots of pea (Pisum sativum L.). Plant and Soil 242: 251–262. [Google Scholar]
- Voisin AS, Salon C, Munier-Jolain NG, Ney B. 2002b. Quantitative effects of soil nitrate, growth potential and phenology on symbiotic nitrogen fixation of pea (Pisum sativum L.). Plant and Soil 243: 31–42. [Google Scholar]
- Voisin AS, Salon C, Jeudy C, Warembourg FR. 2003a. Root and nodule growth in Pisum sativum L. in relation to photosynthesis: analysis using 13C-labelling. Annals of Botany 92: 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin AS, Salon C, Jeudy C, Warembourg FR. 2003b. Seasonal patterns of 13C partitioning between shoots and nodulated roots of N2- or nitrate-fed Pisum sativum L. Annals of Botany, 91: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterer JG, Vessey JK. 1993. Effect of low static nitrate concentrations on mineral nitrogen uptake, nodulation, and nitrogen fixation in field pea. Journal of Plant Nutrition 16: 1775–1789. [Google Scholar]
- West JB, HilleRisLambers J, Lee TD, Hobbie SE, Reich PB. 2005. Legume species identity and soil nitrogen supply determine symbiotic nitrogen-fixation responses to elevated atmospheric [CO2]. New Phytologist 167: 523–530. [DOI] [PubMed] [Google Scholar]
- Zanetti S, Hartwig UA, Nosberger J. 1998. Elevated atmospheric CO2 does not affect per se the preference for symbiotic nitrogen as opposed to mineral nitrogen of Trifolium repens L. Plant, Cell and Environment 21: 623–630. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


