Abstract
Background One of the best-known plant movements, phototropic solar tracking in sunflower (Helianthus annuus), has not yet been fully characterized. Two questions are still a matter of debate. (1) Is the adaptive significance solely an optimization of photosynthesis via the exposure of the leaves to the sun? (2) Is shade avoidance involved in this process? In this study, these concepts are discussed from a historical perspective and novel insights are provided.
Scope and Methods Results from the primary literature on heliotropic growth movements led to the conclusion that these responses cease before anthesis, so that the flowering heads point to the East. Based on observations on 10-week-old plants, the diurnal East–West oscillations of the upper fifth of the growing stem and leaves in relation to the position of the sun (inclusive of nocturnal re-orientation) were documented, and photon fluence rates on the leaf surfaces on clear, cloudy and rainy days were determined. In addition, the light–response curve of net CO2 assimilation was determined on the upper leaves of the same batch of plants, and evidence for the occurrence of shade-avoidance responses in growing sunflower plants is summarized.
Conclusions. Only elongating, vegetative sunflower shoots and the upper leaves perform phototropic solar tracking. Photon fluence response and CO2 assimilation measurements cast doubt on the ‘photosynthesis-optimization hypothesis’ as the sole explanation for the evolution of these plant movements. We suggest that the shade-avoidance response, which maximizes light-driven CO2 assimilation, plays a major role in solar tracking populations of competing sunflower plants, and an integrative scheme of these growth movements is provided.
Keywords: Phototropic solar tracking, photosynthesis, phototropism, plant movement, shade avoidance, solar tracking, sunflower, Helianthus annuus
INTRODUCTION
In the USA and European countries, the common sunflower (Helianthus annuus) is not only a popular garden plant, but also a field crop of considerable economic importance. As the species name indicates, it is an annual herb, characterized by coarsely hairy stems and leaves, as well as large, terminal circular heads composed of numerous, small yellow flowers. In the centre, these flowers can develop into seeds (disc florets), whereas the outer, petal-bearing and non-seed-producing florets (ray flowers) form a yellow wreath that attracts potential pollinators, such as bees and butterflies (Fig. 1) (Heiser, 1976).
Fig. 1.

Upper part of a mature sunflower plant (Helianthus annuus L., ‘Sunspot’), height approx. 2·2 m, with the flowering head facing East (original photograph, Stanford, CA, USA; September 2013).
Sunflower seeds are a major source of vegetable oil worldwide, and commercially available varieties of the species H. annuus (Asteraceae) contain up to 50 % oil in their seeds. Accordingly, the growth of large populations of sunflower plants as an oilseed crop, which began in Russia in around 1860, has developed into an international agricultural business. Currently, the extracted oil accounts for approximately four-fifths of the value of these crop plants (Heiser, 1976; Schneiter, 1997).
In addition to gardeners and agriculturists, botanists have long been interested in the anatomy and physiology of these large, rapidly growing dicotyledonous angiosperms. The founding father of experimental plant physiology, Julius Sachs (1832–1897), used H. annuus individuals to analyse root exudation and sunlight-dependent dry matter accumulation within the leaves (Sachs, 1882, 1884) and, during the 1930s, sunflower seedlings served as important model organisms for the study of blue-light-dependent organ development (see Briggs, 1963; Briggs and Baskin, 1988). More recently, H. annuus has been used to study root development (Josten and Kutschera, 1999), cell wall architecture (Kutschera, 2008), C3 photosynthesis (Kutschera et al., 2010), the biophysical basis of stem elongation (Kutschera and Niklas, 2013), the adaptive significance of the timing of seedling emergence (Mercer et al., 2011), speciation (Levin, 2013) and as a representative genome of the family Asteraceae (Gill et al., 2014).
However, one aspect of growing sunflower plants, the heliotropic movements of the stem and leaves, has sometimes been ignored or misrepresented in the recent literature. For instance, Whippo and Hangarter (2006), Holland et al. (2009), Christie and Murphy (2013), Hohm et al. (2013) and Liscum et al. (2014) reviewed the literature on shoot phototropism in seed plants, but none of these authors mentioned sunflower. Vandenbrink et al. (2014) reviewed some aspects of solar tracking in sunflower plants, but they did not address a number of issues, such as the relative photosynthetic activity of the upper leaves, or consider any possible role for a shade-avoidance response.
In this article, we first provide some basic definitions. Then, we summarize the pertinent literature on solar tracking, discuss misconceptions on the behaviour of H. annuus shoots, and present an integrative hypothesis on the possible adaptive significance of this striking physiological process based on our own unpublished results.
TERMINOLOGY: CIRCUMNUTATION VS. SOLAR TRACKING
The early literature on solar tracking (heliotropism) in land plants (embryophytes) often uses the terms ‘circumnutation’, ‘nutate’ and ‘nutating’ when referring to the physiological phenomena under investigation (see, for instance, Schaffner, 1898, 1900). However, nutation occurs without any change in the direction of the light source and is driven by an internal signal (see the recent review by Vandenbrink et al., 2014). Circumnutation, a term coined by Darwin (1881) in his book The power of movement in plants, also functions strongly in a microgravity environment in the absence of a moving light source (Brown et al., 1990). Solar tracking, on the other hand, is driven by an external signal (changing light direction), although an internal process prompts nocturnal re-positioning (Vandenbrink et al., 2014).
Some years ago, Galston (1979) reported that unilateral blue light did not alter the circumnutation of etiolated seedlings of garden pea (Pisum sativum), although it modified the growth rates of proximal and distal sides of the stem to develop curvature. He also reported that dim red light induces an increase in circumnutation some hours later, while bright white light completely damped circumnutation for several hours, followed by enhancement over dark-control seedlings. These experiments demonstrated that, although light signals could clearly alter circumnutation, the response was not directional. Here we use the terminology ‘solar tracking’ as distinct from circumnutation sensu Darwin (1881). However, as we have retained the nutation terms in quotations and titles of articles, we want to alert the reader to this source of possible confusion.
SOLAR TRACKING: FROM KIRCHER 1643 TO KOLLER 2011
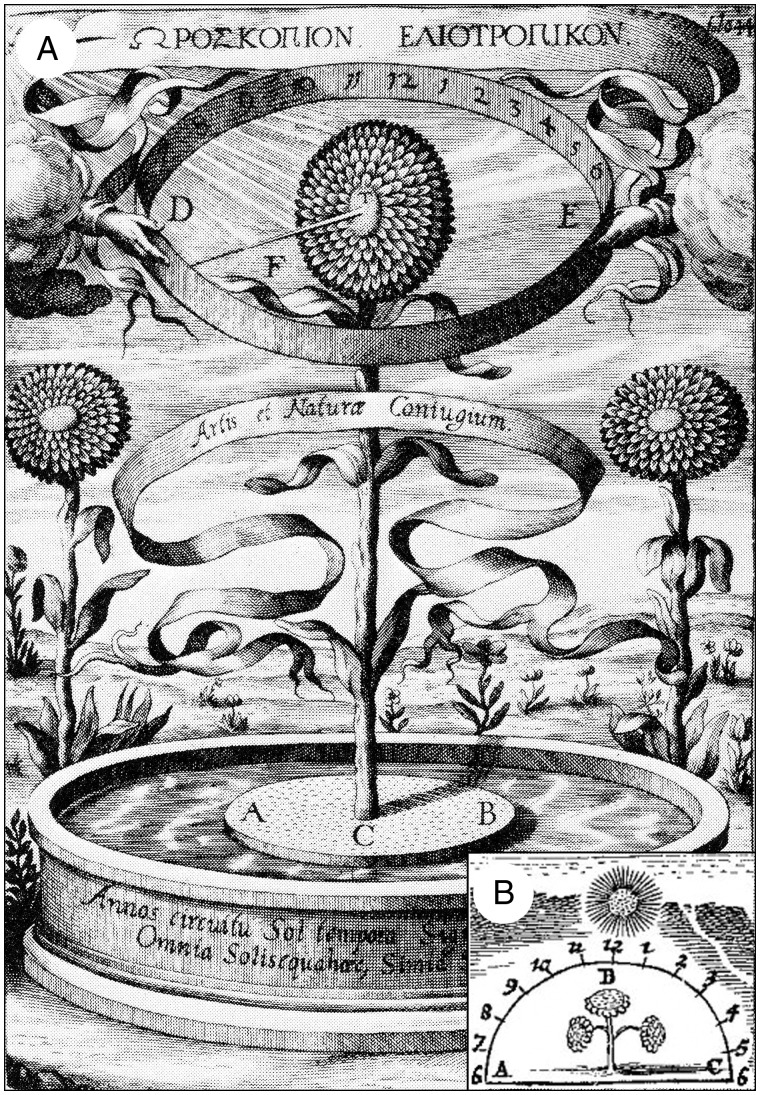
The most popular misconception is that flowering H. annuus heads (Fig. 1) track the moving sun across the sky. This belief can be traced back to the writings of the German Jesuit polymath Athanasius Kircher (1602–1680), who has been described as ‘the last man who knew everything’ (Breidbach and Ghiselin, 2006). In a monograph published in 1643, Kircher depicted a ‘sunflower clock’, which purported to inform humans about the time of day via continuous movements of the mature, flowering head, driven by a mysterious cosmic magnetic force (Fig. 2A). Today, we no longer take this example of early 17th century natural magic seriously, but in Kircher’s time the standards were different. In a subsequent book of 1667 entitled Regnum Naturae Magneticum, Kircher depicted a more realistic version of his ‘sunflower clock’, which is reproduced here (Fig. 2B). This drawing shows a mature sunflower plant the head of which tracks the sun during the day, from 0600 h (6 am), through 1200 h (noon), to 1800 h (6 pm).
Fig. 2.
Two versions of Athanasius Kircher’s ‘sunflower clock’. The famous drawing of 1643 shows a sunflower plant with the stem fixed to a floating disc (ACB), which is growing on water (A). The inset shows a second ‘ABC version’ of the same alleged phenomenon, with a plant growing in soil, published in 1667 (B) (adapted from Athanasius Kircher at Stanford www.stanford.edu/group/kircher/cgi-bin/site/, accessed 24 February 2014).
In a classic monograph on Asteraceae of the genus Helianthus, Heiser (1976) summarized quotations from poets in which Kircher’s ‘sunflower dogma’ had been praised. He referred to the English botanist John Gerard (1545–1611), who was the first to dispute the old misconception of the ‘moving sunflower heads’ (Gerard, 1597), as depicted by Kircher in 1667 (Fig. 2B). Heiser argued that ‘green plants are phototropic and respond by growing toward the source of light. Thus many plants, particularly at early stages, bend toward the east in the morning and toward the west in the evening. The common sunflower shows this tendency more strikingly than most plants, but, once the flower head opens, it no longer bends toward the source of light. Interestingly enough, in my gardens the heads of the giant sunflowers always end up facing the east’ (Heiser, 1976, p. 28).
Despite this and other more recent clarifications (see, for instance, Iino, 1990, 2001; Vandenbrink et al., 2014), Kircher’s alleged ‘sunflower head-movements’ persist in the popular literature. A recent book by Koller (2011) describes the most important plant movements and their biophysical bases (reviewed by Kutschera, 2011). Specifically, Koller (2011) summarizes growth-mediated solar tracking (heliotropism) in angiosperms, a topic on which he had published extensively (e.g. Koller, 1986, 1990; Koller et al., 1985). The author acknowledges that ‘the domestic sunflower … is the most familiar and conspicuous manifestation of … growth-mediated solar tracking’. With reference to a schematic drawing, he described the old ‘sunflower clock-dogma’ (Fig. 2B) in the following words: ‘The apical bud and its cluster of young leaves, and eventually its disc-shaped developing inflorescence, keep moving to remain facing the sun with high fidelity during the course of each day. They do so by growth-mediated positive phototropic curvature of the young, growing part of the subtending stem. Solar tracking is kept up as long as the stem grows, throughout reproductive development, until fruit set’ (Koller, 2011, p. 136).
Koller’s description is not entirely correct. As mentioned by Heiser (1976), the growing vegetative shoot is the solar tracking organ, and tracking ceases at some early time during flower development (Vandenbrink et al., 2014). Mature H. annuus heads are themselves not solar trackers, nor are the flowers of at least one other sunflower relative, Rudbeckia laciniata (W. R. Briggs, pers. obs.). In both cases, tracking ceases as the flowers mature and open, and the mature heads cease tracking the sun and face doggedly east. However, the mature flowers of many species do indeed follow the sun (e.g. Ranunculus adoneus and other alpine flowers, see Sherry and Galen, 1998; Viola pedunculata, W. Briggs, pers. obs.). In the case of the alpine R. adoneus, the interior of the flowers reached temperatures several degrees Celsius above the ambient air temperatures. When flowers were prevented from tracking, their internal temperatures were several degrees lower than their tracking counterparts, and both insect visitation and seed set were significantly reduced (Sherry and Galen 1998).
Koller (2011) summarizes that ‘The developing leaves (of Helianthus annuus) play a role in the diaheliotropic response of the stem, as their excision results in partial loss of the response. However, the site of perception of the solar signal remains unknown’ (Koller, 2011, p. 136). In another chapter of his book, he writes ‘Throughout the day, … (the vegetative shoots) track the sun with high fidelity. At the end of the day, they are oriented toward the direction of sunset. Sometime after sunset, they start reorienting in the opposite direction and end (well before daybreak) by facing in the anticipated direction of sunrise … Nocturnal reorientation is more rapid (ca. 26 °C h–1) than tracking the sun (ca. 15 °C h–1)’ (Koller, 2011, p. 143). In the next section, we describe this process in more detail, with reference to the history of sunflower research.
THE MOVEMENTS OF SUNFLOWERS: FROM KELLERMANN 1890 TO GROWTH-MEDIATED SOLAR TRACKING 2014
Prior to Kellerman (1890), there was little literature on heliotropism, a term that was coined by the French botanist Augustin Pyramus de Candolle (1832). Although Sachs (1882) referred to ‘heliotropism’ on several pages of his classic monograph, and depicted a sunflower plant in the context of root exudation, he only briefly mentioned solar tracking in Helianthus. The reasons for this are unknown. There is evidence to suggest that H. annuus seeds were not readily available in Germany during the 19th century (Heiser, 1976). Hence, the founding father of experimental botany may not have planted sunflowers in his botanical garden where he made so many key discoveries on the growth and behaviour of seed plants (Morton, 1981; Kutschera and Briggs, 2009, 2012). One year before Sachs’s death, Rothert (1896) published a comprehensive review on heliotropism in seed plants (in German). However, the author mentioned the species H. annuus only with reference to hypocotyl development.
Sunflower is a crop species that originated in the Americas, where seeds were available during the 19th century (Blackman et al., 2011). This may be one reason why the scientific study of the ‘solar tracking of Helianthus’ commenced in the USA. To the best of our knowledge, it was Kellerman (1890) who gave the first account of heliotropism in sunflower plants that were cultivated in Kansas (USA), which, incidentally, uses this plant species as its state flower. Kellerman’s account is not very revealing. Despite some valuable observations, the author concluded that ‘It is likely that nutation is more marked in the head previous to anthesis, but this question was not regarded’ (Kellermann, 1890, p. 141). Based on observations on wild/cultivated sunflower plants grown in Clay county (KS) and Columbus (OH), Schaffner (1898, 1900) summarized heliotropism in this angiosperm. According to Schaffner (1900), growing sunflower (cultivated variety) ‘nutate from 60° to 90° west in the evening and from 50° to 70° or more, east in the morning’. At night, the leaves droop and the tips point downward. When anthesis begins nutation ceases and the heads are tilted toward the east or northeast’ (Schaffner, 1900, p. 197). In addition, the author pointed out that only growing shoots display heliotropism, which ceases when the adult plant reaches anthesis. According to Schaffner (1900), the flowering heads generally are oriented toward the east.
Six decades later, the Japanese botanists Shibaoka and Yamaki (1959) documented essentially the same mode of heliotropism in 9-week-old (growing) cultivated sunflower plants (although they failed to cite Schaffner, 1898, 1900). In addition, their description includes the nocturnal re-orientation response. Vandenbrink et al. (2014) published data on the daily cycle of the stem angle, measured at the first node below the apex of 8-week-old sunflower plants. These results are consistent with earlier observations (Schaffner, 1898, 1900; Shibaoka and Yamaki, 1959).
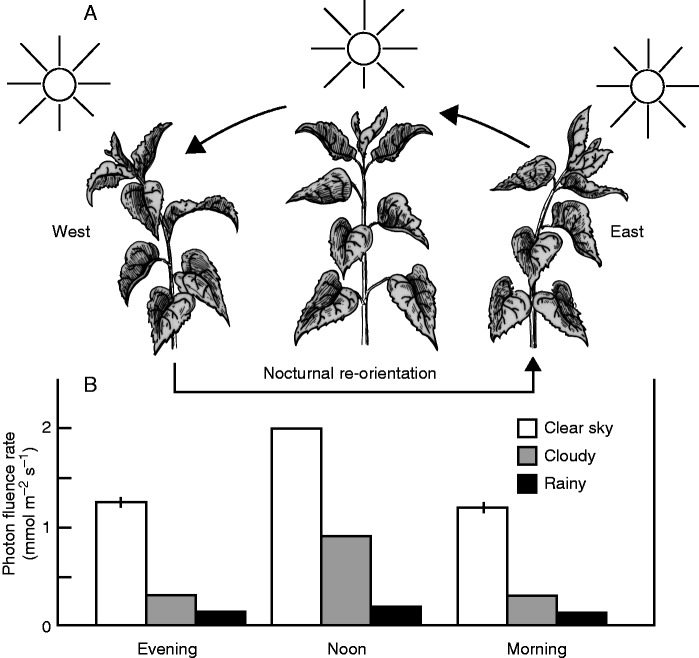
A general scheme of growth-mediated heliotropism in developing Helianthus plants, based on our observations on 10-week-old H. annuus individuals that were raised in an open field in Stanford, CA, is shown in Fig. 3A. The characteristic movements, i.e. diurnal East–West oscillations of the heads (before anthesis) and the upper leaves, are depicted in relation to the position of the sun. It is apparent that the phototropic bending both of the upper part of the stem and of the petiole/midrib of the 3–4 youngest leaves contributes to the movements of the sunflower plant. The East–West oscillations of the upper fifth of the stem, and the nocturnal re-orientations, ceased as the flower head opened and anthesis commenced, so that the heads finally faced to the east, exactly as reported by Schaffner (1898, 1900) over a century earlier. This observation was confirmed by Shibaoka and Yamaki (1959), Heiser (1976) and, more recently, by Vandenbrink et al. (2014) and Harmer et al. (2015).
Fig. 3.
Growth-mediated solar tracking of the stem and upper leaves in a sunflower plant (Helianthus annuus L., ‘Sunspot’). The drawings were plotted from observations on a 10-week-old plant (A). Photon fluence densities of sunlight on the surface of the upper leaves were measured as described by Pieruschka et al. (2011) in the morning (0700 h), at noon, and in the evening (1900 h) under three different weather conditions (7–25 September 2013; means ± s.e.m. of nine measurements each) (B).
In a series of investigations carried out during the 1970s, it was shown that (a) the heads and leaves reach their original position at night, well before sunrise (between 0300 and 0600 h) (Shell et al., 1974; Shell and Lang, 1975); (b) leaf movements lag behind the azimuthal movement of the sun by 12°, corresponding to approx. 48 min (Shell and Lang, 1976); and (c) the easterly direction of the flowering heads has the advantages of reducing the heat load at noon, and provides a larger irradiation during early morning. This may speed up drying of the developing seeds and may decrease the likelihood of fungal attack (Leshem, 1977; Lang and Begg, 1979). These investigators also noted that the East–West oscillations of young plants (Fig. 3A) do not occur on cloudy or rainy days, when the heads remain in an upright position, although they do occur in older plants as long as stem growth persists (Shibaoka and Yamaki, 1959). Moreover, in a growth chamber, under a 16 h daytime overhead illumination (approximately one-third full sunlight), no heliotropism occurs in populations of growing sunflower plants (Shell and Lang, 1976). These facts, consistent with our own observations, suggest that an endogenous rhythm may not be the primary driver of daytime solar tracking in sunflower plants. However, more recent studies indicate that the daytime heliotropic response in sunflower is, at least in part, regulated by the circadian clock (Atamian, 2014; Harmer et al., 2015; S. L. Harmer, pers. com.), as originally suggested by Shibaoka and Yamaki (1959).
Schaffner (1900) proposed that heliotropism in Helianthus is an adaptation for the optimization of leaf photosynthesis. This hypothesis is discussed in the next section.
PHOTON FLUENCE AND LEAF PHOTOSYNTHESIS
It was proposed long ago that the East–West oscillations in sunflower plants (Fig. 3A) result in an improvement of photosynthetic activity of the upper leaves (Schaffner, 1900). Shell and Lang (1976) provided more recent evidence. From field measurements of leaf position, they calculated that, at clear skies, ‘heliotropism of the sunflower head leaves throughout the day could result in an average increase of 9·5 % in photosynthesis compared to the optimum arrangement of fixed leaves, and 23 % increase relative to a (hemi-) spherical distribution. Average daily photosynthesis would be only 14 % less than for leaves which were always aligned perpendicular to the sun’s beam. Therefore, heliotropism could cause an important increase in photosynthesis, particularly in the early morning and late afternoon’ (Shell and Lang, 1976, p. 169). These values were inferred from their calculations, but they are not accompanied by actual measurements of photosynthesis per se.
In a more recent study, heliotropic sunflower plants were compared, under open sky conditions, with modelled ‘non-moving (static)’ controls with respect to light interception throughout the day. The calculations for the amount of photosynthetically active radiation absorbed by the leaves indicated that this parameter was largely identical in both groups of plants. Thus, heliotropic movements apparently had no significant positive effect on light capture (Rey et al., 2008).
In these studies, no direct photosynthetic photon flux density measurements on the surface of the upper leaves on clear, cloudy or rainy days were provided, with respect to the corresponding CO2 assimilation rates. Moreover, the recent finding that, in sunflower leaves, the rate of water loss by transpiration changes in proportion to the radiation load at half to full sunlight (Pieruschka et al., 2011) has not been taken into account.
Using our methods for quantification of photon fluence and photosynthetic CO2 exchange in sunflower leaves, as described recently (Kutschera et al., 2010; Pieruschka et al., 2011), we generated the data set shown in Figs 3B and 4A, B. In Northern California, with clear skies, average photon fluence densities increased from approx. 1200 (morning) to 2050 (noon) μmol photons m–2 s–1 and, in the evening, reached values similar to those in the morning. On cloudy days, the respective values were approx. 300, 900 and 290 μmol photons m–2 s–1, and, on rainy days (dark-grey sky), only 115, 180 and 110 μmol photons m–2 s–1, respectively, were recorded on 9–12 September 2013 (Fig. 3B). If we compare these data with the average rate of photosynthesis (i.e., net CO2 assimilation), measured on the upper leaves (Fig. 4A), the following conclusions for vegetative H. annuus 70 d post-germination can be drawn:
Fig. 4.
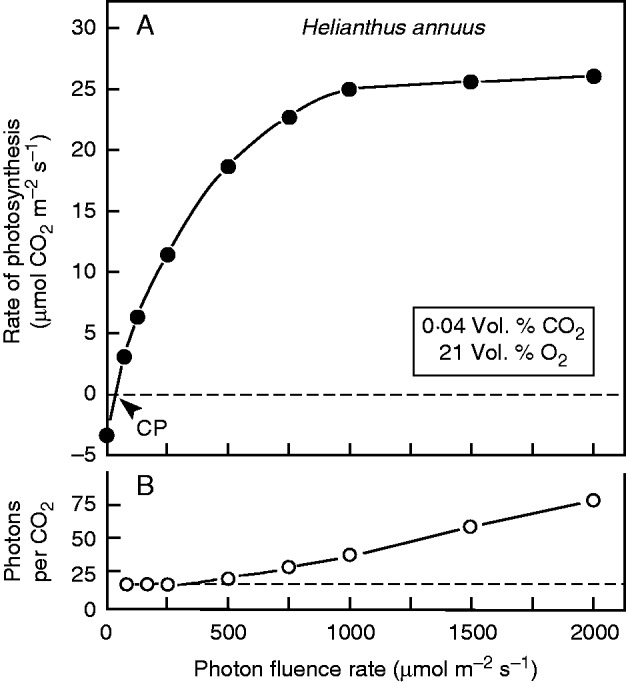
Representative light–response curve of net photosynthesis (CO2 assimilation) rate, measured on an upper, fully expanded leaf of a sunflower plant (see Fig. 1), as described by Kutschera et al. (2010). The light compensation point (CP) is indicated (approx. 40 μmol photons m–2 s–1) (A). The calculated ratio (photons per CO2 assimilated) indicates the growing inefficiency of photosynthesis with increasing irradiance (B).
1. Under clear skies (i.e. half full sunlight providing approx. 1000 μmol photons m–2 s–1): leaf photosynthesis is >98 % saturated, so that the erect position (at noon) does not further enhance photosynthesis, compared with the morning and evening positions. However, it might confer an advantage in the early springtime when days are shorter and fluence rates overall lower, and in geographic areas where sunflower plants are grown under sub-optimal light conditions.
2. Under cloudy skies, in theory, the rate of photosynthesis might be significantly enhanced by phototropic solar tracking, but, in this medium light environment, the movements of these 70-day-old plants are only weak or absent. Shibaoka and Yamaki (1959) also report relatively weak heliotropism on rainy days for 70-day-old plants, but a somewhat stronger response for 100-day-old H. annuus.
3. In sunflower leaves, the light compensation point is approx. 40 μmol photons m–2 s–1 (i.e. 2 % of full sunlight) (Fig. 4A; for details, see Kutschera et al., 2010). In rainy weather, under these limiting-light conditions, net assimilation rates are very low (<5 μmol CO2 m–2 s–1). No heliotropism was observed under these environmental conditions. Based on the fact that, in sunflower seedlings, root respiration reaches values of approximately one-third of that of the entire organism (Kutschera and Niklas, 2012), chances are that the plant as a whole may be heterotrophic during the daytime. In other words, photosynthesis is insufficient to compensate for the respiratory CO2 loss of this C3 species. Heliotropism would make no difference, because, even at noon, net CO2 assimilation of the leaves would probably not compensate for the respiratiory CO2 loss of the root system, plus that of the entire above-ground plant body.
Finally, it should be mentioned that our photosynthesis measurements, which are in accordance with those of other investigators on the leaves of H. annuus (e.g. Demming-Adams et al., 1989), document that above photon fluences of 500 μmol photons m–2 s–1 the efficiency of light-driven CO2 assimilation decreases drastically (Fig. 4B). Our data are in accordance with the much more detailed measurements of Björkman and Demming (1987).
These facts, combined with the enormous latent heat flux from a leaf in full sunlight (and the associated water loss through transpiration), indicate that the erect position of the sunflower plant in full sunlight at noon is of questionable adaptive significance. Numerous studies have shown that green photosynthetic organisms must cope with what can be severe light stress, when absorption of solar radiation exceeds photosynthetic capacity (>1000 μmol photons m–2 s–1 in the case of H. annuus), so that the photosynthetic apparatus must be protected from photodamage (Takahashi and Murata, 2008).
Clearly, solar tracking of leaves can provide a significant improvement in photosynthetic carbon gain in some species. Ehlringer and Forseth (1980) reported that photosynthesis of the desert annual Malvastrum rotundifolium was only saturated at photon fluence rates above the mid-day maximum. They conclude that tracking the sun provides a significant advantage to this species, and it is highly likely that it does so for many other species with solar-tracking leaves. In this sense, the solar-tracking response of H. annuus vegetative shoots, and perhaps other composites, may be an exception.
HELIOTROPISM AND SHADE AVOIDANCE
Based on numerous experimental data, Shirley (1929) concluded that sunflower plants ‘needed more light for survival, more for flowering and fruiting, more for maximum height growth, and more for attaining maximum dry weight’ than other angiosperms raised under the same environmental conditions. Decades later, other investigators corroborated this hypothesis (Wilson, 1966; Sinclair and Muchow, 1999). Moreover, Shirley (1929) documented that, in small populations of 6-week-old H. annuus plants, the stems are longer when the organisms were grown under 48 and 21 % of full sunlight, respectively, compared with unshaded controls. These observations suggest the presence of a photomorphogenic, phytochrome B-regulated shade-avoidance response in populations of sunflower plants (Smith, 1995; Kutschera and Briggs, 2009, 2013). Without reference to Shirley (1929), Shell and Lang (1976, p. 169) concluded that, with respect to ‘more mature and denser plantings, where the tendency for mutual shading of leaves would be greater’, … ‘the main advantage of heliotropism of sunflower plants would be in the early stages of growth, when heliotropism may improve their competitive position.’
The fact that the shade-avoidance responses, i.e. the elongation of the stems and petioles as a result of a lack of red light in dense populations, occurs in H. annuus is obvious. Usually, individual Helianthus plants that grow at the margin of a field are smaller and sturdier than their conspecifics in the centre of the group, which are considerably taller. This observation has been corroborated experimentally. Garrison and Briggs (1972) covered portions of the internodes of growing sunflower plants with foil and found that they were significantly longer than those of untreated controls. The data indicated that light on the internode itself was required to suppress stem elongation. They then showed that direct irradiation of the internode itself with far-red light was sufficient to allow for an increase in growth, although far-red irradiation of the entire plant allowed for a greater increase (Garrison and Briggs, 1975). Subsequently, Morgan et al. (1980) demonstrated that localized irradiation of internodes of growing Sinapis alba seedlings with far-red light elicited a substantial increase in the growth rate of the internode within 10–15 min, whereas similar irradiation of leaves had no effect. These results clearly document the occurrence of a phytochrome-mediated shade-avoidance response in the growing internodes in both sunflower and S. alba, and probably in other angiosperms (Kutschera and Briggs, 2009, 2013). They are also in accordance with the well-characterized far-red enrichment of photosynthetically active radiation under high population density conditions, which results in a promotion of basal leaf senescence (Rousseaux et al., 1996).
In a comprehensive analysis of phototropism in angiosperms, Iino (2001) concluded that, with reference to work on sunflower, shade avoidance is the key process behind heliotropic movements. Our observations and measurements on stem and leaf phototropism in sunflower plants (Figs. 3 and 4) do not add new insights on this topic. Nevertheless, we conclude that a photomorphogenic, phytochrome-regulated shade-avoidance response is a major biological function of the heliotropic growth movements in sunflower plants, and not optimization of photosynthesis per se.
CONCLUSIONS AND OUTLOOK
The annual sunflower, a plant first domesticated about 5000 years ago in a region that is now the South-eastern USA, has been used by indigenous Americans as a symbol of their solar deity (Blackman et al., 2011). More recently, selected strains of sunflower with an enhanced oil content have been employed as a major crop, and this large annual herb is also planted by gardeners as an ornament (Heiser, 1976; Schneiter, 1997). However, despite its economic importance and popularity, the most conspicuous feature of this fast-growing angiosperm, its striking heliotropism, has not yet been fully characterized (Vandenbrink et al., 2014).
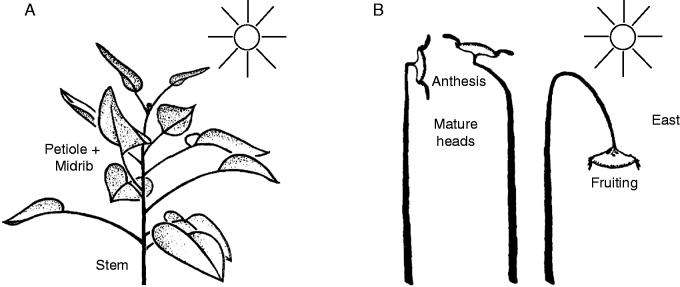
Here we confirm that only growing, vegetative shoot apices and immature flower buds perform growth-mediated heliotropic movements, accompanied by leaf bending responses (Fig. 5A). Mature flowering heads point in a fixed, easterly direction throughout a sunny day, although wind and rain can turn the heads and modify their position. After anthesis, fruiting heads bend downwards (Fig. 5B). In our experience, on cloudy or rainy days, and in a growth chamber with fixed light bulbs, the stems/leaves of growing, juvenile sunflower plants do not change in position. Hence, for these plants, any endogenous rhythm appears to be dominated by the growth-mediated organ movements that occur in the field. However, recent reports provide growing evidence for a role for the ‘internal clock’ in modulating heliotropic movement during the day and driving the reorientation movement at night (Harmer, 2009; Atamian, 2014; Harmer et al., 2015). It was the seminal study of Shibaoka and Yamaki (1959) that first suggested that an endogenous rhythm is involved in the regulation of phototropic solar tracking in H. annuus. The role of the circadian rhythm during the daytime evidently increases as the growing seedlings become older.
Fig. 5.
Schematic summary of phototropic solar tracking in a growing sunflower plant (A), and position of the mature head at anthesis, and during fruiting (B). Note that, in the younger, solar-tracking plant, both the stem and the upper leaves turn towards the sun (adapted from Shibaoka and Yamaki, 1959).
Finally, our data show that the classic ‘photosynthesis-optimization hypothesis’ (Schaffner, 1900; Hart, 1990; Schneiter, 1997; Li et al., 2012) as sole explanation is questionable. We suggest that the shade-avoidance response may play a major role, but acknowledge that, by this means, the rate of light-dependant CO2 assimilation in the upper leaves of solar-tracking plants may be optimized. However, many open questions as to the physiology and adaptive significance of heliotropism in developing sunflower plants (Fig. 5) are unanswered. (1) How is the nocturnal re-orientation regulated? (2) Are the light-dependent shoot and leaf movements independent reactions or co-ordinated processes? (3) Is heliotropism in sunflower a blue light (phototropin)-mediated process, or are other photoreceptors involved (Deng et al., 2014)? (4) Which phytohormones (auxins, brassinosteroids or gibberellins) are causally involved in the re-distribution of growth leading to organ bending (Kurepin et al., 2007, 2012)? (5) What are the sites of photoperception? (6) Are the heliotropic movements of the stem and upper leaves true irreversible growth processes (phototropisms) or, at least in part, turgor-driven nastic bending responses?
These and other questions must be answered before we fully understand one of the most popular physiological processes in the plant kingdom.
ACKNOWLEDGEMENTS
This article is dedicated to our colleague Dov Koller (1925–2007), a pioneer in the field of experimental plant physiology. We thank the Alexander von Humboldt-Stiftung (AvH, Bonn, Germany) for financial support (AvH-Fellowships Stanford 2013/14 to U.K., Institute of Biology, University of Kassel, Germany).
LITERATURE CITED
- Atamian HS. 2014. Sunflower heliotropism: new insights into a classic phenomenon. ASPB-Plant Biology Meeting, 2014. Portland, OR. Abstract-P 12011, [Google Scholar]
- Björkman O, Demmig B. 1987. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170: 489–504. [DOI] [PubMed] [Google Scholar]
- Blackman BK, Scascitelli M, Kane NC, et al. 2011. Sunflower domestication alleles support single domestication center in eastern North America. Proceedings of the National Academy of Sciences of the USA 108: 14360–14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidbach O, Ghiselin MT. 2006. Athanasius Kircher (1602–1680) on Noah’s Ark: Baroque ‘Intelligent Design’ theory. Proceedings of the California Academy of Sciences 57: 991–1002. [Google Scholar]
- Briggs WR. 1963. The phototrophic response of higher plants. Annual Review of Plant Physiology 14: 311–352. [Google Scholar]
- Briggs WR, Baskin T. 1988. Phototropism in higher plants – controversies and caveats. Botanica Acta 101: 133–139. [Google Scholar]
- Brown A, Chapman DK, Lewis RF, Venditti AL. 1990. Circumnutations of sunflower hypocotyls in satellite orbit. Plant Physiology 94: 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Murphy AS. 2013. Shoot phototropism in higher plants: new light through old concepts. American Journal of Botany 100: 35–46. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1881. The power of movements in plants. John Murray: London. [Google Scholar]
- De Candolle AP. 1832. Physiologie végétale. Paris: Béchet. [Google Scholar]
- Demmig-Adams B, Winter K, Krüger A, Czygan F-C. 1989. Light response of CO2 assimilation, dissipation of excess excitation energy, and zeaxanthin content of sun and shade leaves. Plant Physiology 90: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Oses-Prieto JA, Kutschera U, et al. 2014. Blue light-induced proteomic changes in etiolated Arabidopsis seedlings. Journal of Proteome Research 13: 2524–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR, Forseth IN. 1980. Solar tracking by plants. Science 210: 1094–1098. [DOI] [PubMed] [Google Scholar]
- Galston AW. 1979. Circumnutation, rhythms and light-regulated movements in plants. In: Skoog F, eds. Plant growth substances 1979. Berlin: Springer Verlag, 437–442. [Google Scholar]
- Garrison R, Briggs WR. 1972. Internodal growth in localized darkness. Botanical Gazette 133: 270–276. [Google Scholar]
- Garrison R., Briggs WR. 1975. The growth of internodes in Helianthus in response to far-red light. Botanical Gazette 136: 353–357. [Google Scholar]
- Gerard J. 1597. Herball, or general historie of plantes. London: John Norton. [Google Scholar]
- Gill N, Buti M, Kane N, et al. 2014. Sequence-based analysis of structural organization and composition of the cultivated sunflower (Helianthus annuus L.). Biology 3: 295–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL. 2009. The circadian system in higher plants. Annual Review of Plant Biology 60: 357–377. [DOI] [PubMed] [Google Scholar]
- Harmer SL, Atamian H, Creux N. 2015. Circadian rhythms are turning heads. International Symposium on Plant Photobiology, 2015. Austin, Texas, USA. Abstract, p. 69. [Google Scholar]
- Hart JW. 1990. Plant tropisms and other growth movements. London: Unwin Hyman. [Google Scholar]
- Heiser CB. 1976. The sunflower. Norman, OK: University of Oklahoma Press. [Google Scholar]
- Hohm T, Preuten T, Frankhauser C. 2013. Phototropism: translating light into directional growth. American Journal of Botany 100: 47–59. [DOI] [PubMed] [Google Scholar]
- Holland JJ, Roberts D, Liscum E. 2009. Understanding phototropism: from Darwin to today. Journal of Experimental Botany 60: 1969–1978. [DOI] [PubMed] [Google Scholar]
- Iino M. 1990. Phototropism: mechanisms and ecological implications. Plant, Cell and Environment 13: 633–650. [Google Scholar]
- Iino M. 2001. Phototropism in higher plants. In: Häder D-P, Lebert M, eds. Photomovement. Amsterdam: Elsevier Science, 663–811. [Google Scholar]
- Josten P, Kutschera U. 1999. The micronutrient boron causes the development of adventitious roots in sunflower cuttings. Annals of Botany 84: 337–342. [Google Scholar]
- Kellerman WA. 1890. Observations on the nutation of sunflowers. Transactions of the Kansas Academy of Sciences 12: 140–158. [Google Scholar]
- Koller D. 1986. The control of leaf orientation by light. Photochemistry and Photobiology 44: 819–826. [Google Scholar]
- Koller D. 1990. Light-driven leaf movements. Plant, Cell and Environment 13: 615–632. [Google Scholar]
- Koller D. 2011. The restless plant. Cambridge, MA: Harvard University Press. [Google Scholar]
- Koller D, Levitan I, Briggs WR. 1985. The vectorial photo-excitation in solar-tracking leaves of Lavatera cretica (Malvaceae). Photochemistry and Photobiology 42: 717–723. [Google Scholar]
- Kurepin LV, Emery RJN, Pharis RP, Reid DM. 2007. Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: putative roles for plant hormones in leaf and internode growth. Journal of Experimental Botany 58: 2145–2157. [DOI] [PubMed] [Google Scholar]
- Kurepin LV, Joo S-H, Kim S-K, Pharis RP, Back TG. 2012. Interaction of brassinosteroids with light quality and plant hormones in regulating shoot growth of young sunflower and Arabidopsis seedlings. Journal of Plant Growth Regulation 31: 156–164. [Google Scholar]
- Kutschera U. 2008. The outer epidermal wall: design and physiological role of a composite structure. Annals of Botany 101: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U. 2011. Book review: The restless plant. Quarterly Review of Biology 86: 355–356. [Google Scholar]
- Kutschera U, Briggs WR. 2009. From Charles Darwin’s botanical country-house studies to modern plant biology. Plant Biology 11: 785–795. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Briggs WR. 2012. Root phototropism: from dogma to the mechanism of blue light perception. Planta 235: 443–452. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Briggs WR. 2013. Seedling development in buckwheat and the discovery of the photomorphogenic shade-avoidance response. Plant Biology 15: 931–940. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Niklas KJ. 2012. Organ-specific rates of cellular respiration in developing sunflower seedlings and their bearing on metabolic scaling theory. Protoplasma 249: 1049–1057. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Niklas KJ. 2013. Cell division and turgor-driven stem elongation in juvenile plants: a synthesis. Plant Science 207: 45–56. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Pieruschka R, Berry JA. 2010. Leaf development, gas exchange characteristics, and photorespiratory activity in maize seedlings. Photosynthetica 48: 617–622. [Google Scholar]
- Lang ARG, Begg JE. 1979. Movements of Helianthus annuus leaves and heads. Journal of Applied Ecology 16: 299–305. [Google Scholar]
- Leshem YY. 1977. Sunflower: a misnomer? Nature 269: 102. [Google Scholar]
- Levin DA. 2013. The timetable for alloploidy in flowering plants. Annals of Botany 102: 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Liu Y, Huang X, Jiang H. 2012. Direct sun-driven artificial heliotropism for solar energy harvesting based on a photo-thermo-mechanical liquid-crystal elastomer nanocomposite. Advanced Functional Materials 22: 5166–5174. [Google Scholar]
- Liscum E, Askinosie S, Leutman DL, Morrow J, Willenburg KT, Coats DR. 2014. Phototropism: growing towards an understanding of plant movement. The Plant Cell 26: 38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer KL, Alexander HM, Snow AA. 2011. Selection on seedling emergence timing and size in an annual plant, Helianthus annuus (common sunflower). American Journal of Botany 98: 975–985. [DOI] [PubMed] [Google Scholar]
- Morgan DC, O’Brian T, Smith H. 1980. Rapid photomodulation of stem extension in light-grown Sinapis alba L. Studies on kinetics, site of perception and photoreceptor. Planta 150: 95–101. [DOI] [PubMed] [Google Scholar]
- Morton AG. 1981. The history of botanical science. London: Academic Press. [Google Scholar]
- Pieruschka R, Huber G, Berry JA. 2011. Control of transpiration by radiation. Proceedings of the National Academy of Sciences, USA 107: 13372–13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey H, Dauzat J, Chenu K, Barczi J-F, Dosio GAA, Lecoeur J. 2008. Using 3-D virtual sunflower to simulate light capture at organ plant and root levels: contribution of organ interception, impact of heliotropism, and analysis of genotypic differences. Annals of Botany 101: 1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothert W. 1896. Ueber Heliotropismus. Beiträge zur Biologie der Pflanzen 7: 1–212. [Google Scholar]
- Rousseaux MC, Hall AJ, Sánchez RA. 1996. Far-red enrichment and photosynthetically active radiation level influence leaf senescence in field-grown sunflower. Physiologia Plantarum 96: 217–224. [Google Scholar]
- Sachs J. 1882. Vorlesungen über Pflanzen-Physiologie. Leipzig: Verlag Wilhelm Engelmann,. [Google Scholar]
- Sachs J. 1884. Ein Beitrag zur Kenntnis der Ernährungsthätigkeit der Blätter. Arbeiten des Botanischen Instituts Würzburg 3: 1–33. [Google Scholar]
- Schaffner JH. 1898. Oberservations on the nutation of Helianthus annuus. Botanical Gazette 25: 395–403. [Google Scholar]
- Schaffner JH. 1900. The nutation of Helianthus. Botanical Gazette 29: 197–200. [Google Scholar]
- Schneiter AA, ed. 1997. Sunflower technology and production. Madison, WI: American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc. [Google Scholar]
- Shell GSG, Lang ARG. 1975. Description of leaf orientation and heliotropic response of sunflower using directional statistics. Agricultural Meteorology 15: 33–48. [Google Scholar]
- Shell GSG, Lang ARG. 1976. Movements of sunflower leaves over a 24-h period. Agricultural Meteorology 16: 161–170. [Google Scholar]
- Shell GSG, Lang ARG, Sale PJM. 1974. Quantitative measures of leaf orientation and heliotropic response in sunflower, bean, pepper and cucumber. Agricultural Meteorology 13: 25–37. [Google Scholar]
- Sherry RA, Galen C. 1998. The mechanism of floral heliotropism in the snow buttercup, Ranunculus adoneus. Plant, Cell and Environment 21: 983–993. [Google Scholar]
- Shibaoka H, Yamaki T. 1959. Studies on the growth movement of sunflower plant. Scientific Papers of the College of General Education University of Tokyo 9: 105–126. [Google Scholar]
- Shirley HL. 1929. The influence of light intensity and light quality upon the growth of plants. American Journal of Botany 16: 354–390. [Google Scholar]
- Sinclair TR, Muchow RC. 1999. Radiation use efficiency. Advances in Agronomy 65: 215–263. [Google Scholar]
- Smith H. 1995. Physiological and ecological function within the phytochrome family. Annual Review of Plant Physiology and Plant Molecular Biology 46: 289–315. [Google Scholar]
- Takahashi S, Murata N. 2008. How do environmental stresses accelerate photoinhibition? Trends in Plant Science 13: 178–182. [DOI] [PubMed] [Google Scholar]
- Vandenbrink JP, Brown EA, Harmer SL, Blackman BK. 2014. Turning heads: the biology of solar tracking in sunflower. Plant Science 224: 20–26. [DOI] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP. 2006. Phototropism: bending towards enlightenment. The Plant Cell 18: 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JW. 1966. High net assimilation rates of sunflower plants in an arid climate. Annals of Botany 30: 745–751. [Google Scholar]