Abstract
Background and Aims Trait–environment relationships are commonly interpreted as evidence for local adaptation in plants. However, even when selection analyses support this interpretation, the mechanisms underlying differential benefits are often unknown. This study addresses this gap in knowledge using the broadly distributed South African shrub Protea repens. Specifically, the study examines whether broad-scale patterns of trait variation are consistent with spatial differences in selection and ecophysiology in the wild.
Methods In a common garden study of plants sourced from 19 populations, associations were measured between five morphological traits and three axes describing source climates. Trait–trait and trait–environment associations were analysed in a multi-response model. Within two focal populations in the wild, selection and path analyses were used to test associations between traits, fecundity and physiological performance.
Key Results Across 19 populations in a common garden, stomatal density increased with the source population’s mean annual temperature and decreased with its average amount of rainfall in midsummer. Concordantly, selection analysis in two natural populations revealed positive selection on stomatal density at the hotter, drier site, while failing to detect selection at the cooler, moister site. Dry-site plants with high stomatal density also had higher stomatal conductances, cooler leaf temperatures and higher light-saturated photosynthetic rates than those with low stomatal density, but no such relationships were present among wet-site plants. Leaf area, stomatal pore index and specific leaf area in the garden also co-varied with climate, but within-population differences were not associated with fitness in either wild population.
Conclusions The parallel patterns of broad-scale variation, differences in selection and differences in trait–ecophysiology relationships suggest a mechanism for adaptive differentiation in stomatal density. Densely packed stomata may improve performance by increasing transpiration and cooling, but predominately in drier, hotter climates. This study uniquely shows context-dependent benefits of stomatal density – a trait rarely linked to local adaptation in plants.
Keywords: Protea repens, Proteaceae, sugarbush, South Africa, Cape Floristic Region, stomatal density, functional traits, trait–environment associations, local adaptation, leaf morphology, ecophysiology, photosynthesis, selection analysis
INTRODUCTION
Environmentally linked differences in leaf size and shape are often thought to reflect a legacy of adaptive evolution in plants. Broad-scale comparisons across plant communities, for example, show that plants in arid or infertile sites often have thick, tough leaves (Wright et al., 2004; Ordoñez et al., 2009) and that plants in high-elevation sites often have shorter statures and smaller leaves that are also thick and tough (Korner, 2003). Although these trends are well established, their evolutionary origins are less clear; community-wide correlations between traits and environments could arise through a combination of adaptive evolution in situ, differences in colonization success, and phylogenetic constraints. Studies that control for phylogeny or focus on single lineages or species are better able to disentangle these effects (e.g. Lamont et al., 2002; Yates et al., 2010) and they often, but not always, find that morphological differences predominantly reflect adaptive rather than non-adaptive divergence (adaptive: Verboom et al., 2004; Friar et al., 2006; Ellis et al., 2006; Nakazato et al., 2008; Ramirez-Valiente et al., 2009; Carlson et al., 2011; Frei et al., 2012; Brouillette et al., 2014; non-adaptive: Comes et al., 2008; Britton et al., 2014). Yet even when trait–environment associations are linked to adaptation, this does not explain why a given morphology works better in some sites than others. Despite growing evidence for adaptive differentiation in plant morphology, surprisingly few studies address its biological or physiological impetus, i.e. the context-dependent effects of morphological traits on plant performance (Dudley, 1996a; Brouillette et al., 2014).
Morphological leaf traits that mediate environmental effects on plant fitness are often regarded as ‘functional traits’ (Geber and Griffen, 2003; Reich et al., 2003). These often easy-to-measure traits, such as leaf shape, size or thickness, influence plant physiological responses to their immediate environment (e.g. through photosynthetic rates), which in turn affect performance, growth and survival. The ways in which functional traits indirectly affect fitness will vary with the environmental context, however, and this could be a starting point for adaptive differentiation. For example, plants in relatively arid sites may benefit from having densely packed, small stomata because of their intrinsically higher rates of gas exchange, increased capacity for transpirational cooling and more rapid stomatal closure in response to desiccation (Hetherington and Woodward, 2003; Franks and Beerling, 2009; Franks et al., 2009). In sites where conditions are consistently moist, however, plants with fewer, larger stomata may invest less energy in stomatal production and maintenance, yet achieve equivalent rates of CO2 assimilation. Although these ideas are not new, there is relatively little evidence for context-dependent functions of stomatal or leaf traits, and even less evidence that such functional differences lead to natural selection in the wild.
In this study we explore the links between trait–environment variation, ecophysiology and context-dependent selection using a widespread shrub, Protea repens, in the Cape Floristic Region (CFR) of South Africa. This 90 000-ha region is characterized by steep mountain ranges (0–2000 m a.s.l.) and a marked rainfall gradient, from winter-concentrated in the west to aseasonal in the east. Such contrasts are attractive for studies of trait environment associations, but they also present unique challenges. Due to the covariance between rainfall seasonality and distance eastward in particular, a legacy of drift or stepping-stone colonization could produce non-adaptive trait clines that resemble adaptive ones. Our study addresses this challenge by providing evidence of – and possible mechanisms for – adaptation along environmental gradients, by measuring fecundity selection and ecophysiology in two environmentally distinct natural populations. We first identify several trait–environment associations across 19 populations, using morphological traits measured in common garden plants. We then assess whether these traits are differentially associated with fecundity or with ecophysiology in two wild populations that differ in water availability. Differences in trait selection gradients between the two populations indicate that morphology–fitness relationships are context-dependent, and site-specific relationships with eco-physiological traits could help explain why. The research questions of our study are as follows. (1) Do leaf traits and stomatal morphology in a common garden co-vary with the climate from which plants were sourced? (2) Do selection gradients on morphological traits differ between two natural populations, as would be expected if trait–environment associations evolved through local adaptation? (3) Are there site-specific phenotypic associations between morphology and ecophysiology, and, if so, are differences consistent with any context-dependent selection gradients that we identify?
MATERIALS AND METHODS
Study species
Protea repens is an evergreen, broad-leaved, woody perennial of semi-arid shrub lands, i.e. fynbos, in southern South Africa (Fig. 1). It is broadly distributed in comparison with most other co-occurring Protea, spanning 800+ km east to west and from sea level to 1500 m (Rebelo, 2001). Like many Protea, P. repens has erect sympodial growth (reaching up to 4·5 m), with annual flowering from May to October in western sites and September to March in eastern sites (Rebelo, 2001). Inflorescences contain an average of 90 protandrous florets (range 39–172; J. E. Carlson, unpubl. res.) and are subtended by showy involucral bracts that may be pink, white, or white with pink tips. Each floret produces an achene (i.e. a single-seeded fruit), although in the wild only about 27 % of achenes contain a viable seed (Coetzee and Giliomee, 1987). Seed development occurs over ∼7 months (Jordaan, 1972; from Van Staden, 1978), during which time the inflorescence closes and hardens into a serotinous cone, henceforth ‘seed head’. Achenes are retained in seed heads until fire kills adult plants or stems are otherwise damaged. Fires occur at ∼20-year intervals in the fynbos, with some variation among regions (Forsyth and van Wilgen, 2007).
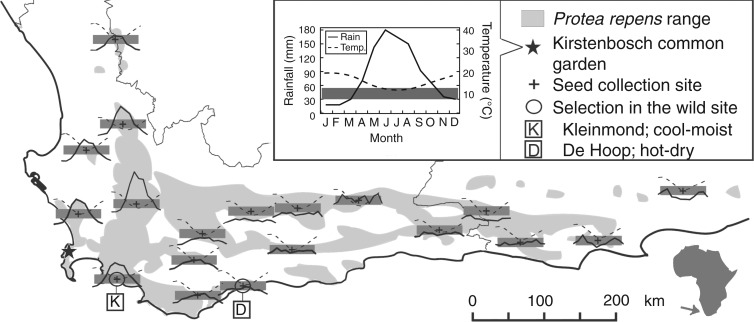
Fig. 1.
Seed source sites of Protea repens used in the common garden at Kirstenbosch, South Africa, and their respective annual rainfall and temperature patterns from 30-year climate averages. For each of the 19 sites, the mean monthly rainfall and temperature are plotted on a consistent scale, following the inset for the common garden (vertical height of shaded grey blocks is 30–55 mm rainfall or 8–14 °C). The two ‘Selection in the wild’ sites were used for detailed study of traits, ecophysiology and fecundity. For Kleinmond, seed collection and ecophysiology were from the same local population, but for De Hoop the two sites were 13 km apart.
Common gardens
Between February and April 2011, we collected seed heads from 19 wild populations from across the distribution of P. repens (Fig. 1). From 40–50 plants per population (mean n = 45 plants; total N = 871 plants), we collected one or two mature seed heads from the previous year’s growth and allowed them to air-dry until achenes were released. We retained all plump, non-shrivelled achenes (∼70 % of the total), which included both viable, endosperm-containing seeds as well as indistinguishable woody seeds that lacked endosperm. In May 2011 we sowed five to ten achenes per plant into shallow trays (6 cm deep) filled with low-nutrient sandy soil mix (1 part loam, 8 parts bark, 3 parts sand) in a completely randomized design within a greenhouse at Kirstenbosch Botanical Garden, Cape Town, South Africa. Watering occurred twice weekly until plants were transplanted into outdoor tilled beds at Kirstenbosch in July 2011. Germination rates were low (21 % on average), probably because woody inviable seeds could not be distinguished from viable seeds. At planting, each population was represented by an average of 43 plants (range 12–81; total N = 819) and 20 different maternal lines (range 7–34). Some plants were dead or unmeasurable by the time of our first sampling 2 years later, but per population sample sizes remained high (June 2013: mean per population n = 36, range 12–75, total N = 691; June 2014: n = 23, range 9–39, total N = 444).
In the austral winter of the second and third years post-planting (June 2013 and 2014), we measured functional leaf morphology in the common garden. We collected from each plant one fully expanded leaf from the most recent growth interval, which we scanned fresh and measured digitally for leaf area, maximum length and maximum width. We also dried leaves at 60 °C for 48 h and weighed them. These values were used to calculate leaf length : width ratio (LWR; leaf length · leaf width−1) and specific leaf area, a measure of sclerophylly or leaf thickness and toughness (SLA; fresh area · dry mass−1). From at least ten plants per source population per year, we also collected a peel of clear acrylic nail polish from the abaxial surface of the leaf (average n per population = 13; total N = 511). We viewed peels at × 40 using a light microscope and NIS Elements 3.1 Imaging Software (Nikon, NY), and we counted the number of stomata per square millimetre and measured stomatal pore length on each of three views per leaf (see also Carlson and Holsinger, 2012). The averages from three views produced stomatal density, pore length, and stomatal pore index (SPI; stomatal pore length2 × stomatal density). As shown by Reynoso-Castillo et al. (2001), P. repens has equivalent stomatal densities on both leaf surfaces, and the sunken stomata are within an epistomatal cavity covered by a projecting flange with a small apical opening. The flange often obscured the stomatal aperture in peels (Supplementary Data Fig. S1), such that stomatal pore length had to be approximated by measuring the outer edge of one epistomatal cavity wall to the other, along its longest axis.
Our study focused on five morphological measurements that were relatively uncorrelated with each other: LWR, stomatal density, SPI, SLA and leaf area. None of the trait pairs had Pearson’s correlations coefficients (R) > 0·43 in the common garden or wild population datasets. Stomatal pore length and leaf width were excluded from most analyses, because each was highly correlated with one or more of the preceding five traits in all datasets. The strongest correlation coefficients for these unused variables were as follows, in order of 2013 garden, 2014 garden, Kleinmond, De Hoop: pore length with SPI (0·64, 0·42, 0·83, 0·70), pore length with stomatal density (−0·44, −0·53, −0·47, −0·33), leaf width with leaf area (0·79, 0·85, 0·68, 0·57) and leaf width with LWR (−0·76, −0·80, −0·83, −0·68).
Although common garden plants were sampled in earlier developmental stages than their wild adult parents, they strongly resembled adult plants by the time of garden measurement. Garden leaf sizes and shapes in both sampling years overlapped in range with those of wild adults in their source populations, although garden leaves were smaller on average (Supplementary Data Appendix S1, Table S1 and Fig. S2). By mid-2014, many plants had also reached reproductive maturity: only eight plants had developed inflorescences by the 2013 sampling, in contrast to 324 plants by the 2014 sampling.
Traits and fecundity in the wild
Between June and July 2012 we visited two environmentally contrasting sites to measure the traits and physiology of wild adult P. repens. The cooler, moister site was in Kleinmond Coast and Mountain Reserve and the hotter, drier site was in De Hoop Nature Reserve (Fig. 1). Both sites were within 5 km of the coast and close to sea level (27 and 34 m a.s.l., respectively), but De Hoop had hotter, drier austral summers with less rainfall annually, based on 30-year means (mean annual temperature 17·3 versus 15·6 °C; rain/year 424 versus 746 mm; Schulze, 2007) (Fig. 1). Soil chemistry also differed between sites, with the De Hoop population growing in more shallow, alkaline, limestone-derived soil (pH 6·3, phosphorus 16 mg kg−1, potassium 56 mg kg−1) and the Kleinmond population growing in deeper, more acidic, sandstone-derived soil (pH 4·2, phosphorus 4·2 mg kg−1, potassium 41 mg kg−1).
Within each population, we selected 20 adult P. repens for study. Our focal plants represented intensive sampling within a 0·3 to 0·4 km2 sampling area, which had a homogeneous most-recent fire history and spanned observed variation in soils and elevation. In De Hoop this area encompassed almost all plants in an isolated stand, but in Kleinmond our sampling area covered less than a quarter of the total population area because of differences in fire history. On each plant, we counted the total number of reproductive heads, including both fresh inflorescences and seed heads, and we collected one or two seed heads. We then selected two fully expanded leaves, each in full sun and from a different apical shoot produced during the most recent growth interval. Using the same techniques as the common garden study, we measured LWR, stomatal density, SPI, SLA and leaf area on each leaf. The seed heads were allowed to air dry, and then we counted the total number of achenes per seed head (including fertile and infertile fruits, most of which were difficult to distinguish non-destructively). We multiplied this count by the number of heads per plant as our measure of current lifetime achene production per plant, a relevant fitness component for Protea.
Ecophysiology in the wild
On half of the morphology plants in each site (n = 10), we collected physiological data on two to four leaves per plant. On two leaves sampled consecutively, we recorded the following physiological measurements: light-saturated photosynthetic rate per unit area (µmol CO2 m−2 s−1), transpiration (mol H2O m−2 s−1), stomatal conductance to water vapour (mol H2O m−2 s−1), leaf surface temperature (°C) and photosystem II quantum yield, which is an inverse measure of light-adapted fluorescence (relative units). Instantaneous water use efficiency (WUE) was calculated later as light-saturated photosynthetic rate divided by stomatal conductance. Field-based measurements were taken in order of increasing disturbance to the leaf, beginning with temperature of the leaf’s adaxial surface using an infrared thermometer (LS, Micro-Epsilon, Germany), followed by light-adapted fluorescence using the Junior PAM portable chlorophyll fluorometer (Walz GmbH, Germany) and ending with transpiration, conductance and photosynthetic rate using a Li-Cor 6400 XT with CO2 mixing system and red/blue LED light source (Li-Cor, Lincoln, NE). The Li-Cor 6400 XT was set at a CO2 concentration of 400 p.p.m., photosynthetically active radiation =1500 μmol m−2 s−1 and ambient air temperature and relative humidity, which were similar between sites during the measurement period (20·3 ± 2·3 °C and 56·6 ± 7·2 % for Kleinmond and 19·5 ± 2·5 °C and 52·8 ± 8·4 % for De Hoop; mean ±s.d.). After measuring photosynthesis, each leaf was collected for the morphology measurements described above.
All physiological measurements were taken between 1000 and 1400 hours on clear days during 2 weeks in mid-June (weeks 1 and 2) and 2 weeks in mid-July (weeks 3 and 4). Up to four plants were measured per day; plants in De Hoop were measured in weeks 1 and 4, whereas those in Kleinmond were measured in weeks 2 and 3. Most plants were measured on only one day (two leaves per plant), but four plants were measured a second time in the following sampling week (total of four leaves per plant). There was slightly more rain during the 5 d preceding each measurement in Kleinmond than in De Hoop (18 versus 6 mm on average), but rainfall totals for June and July 2012 were similar between sites (165·4 and 173·8 mm respectively; data from South African Weather Service and C. Beattie, Cape Nature, South Africa, pers. comm.). Physiological measurements were made in the austral winter to get the best estimates possible of a plant’s true maximum photosynthetic rate. During the austral summer, heat and drought cause Protea plants to reduce or halt stomatal conductance early in the day, which results in lower light-saturated photosynthetic rates (Carlson and Holsinger, 2012).
Statistical analyses
Climate axes. We obtained seven environmental descriptors for all 19 populations of common garden plants by intersecting GPS coordinates with climate or elevation layers. Climate layers came from the South African atlas of Agrohydrology and Climatology (Schulze, 1997, 2007), and elevation came from an NASA digital elevation model (LPDAAC, 2011). The focal climate layers were mean annual rainfall, mean annual temperature, average daily maximum temperature for January, average daily minimum temperature for July, an index of rainfall seasonality (the percentage of annual precipitation to fall within a single month) and an inverse measure of peak summertime drought (mean monthly rainfall summed for December to February). We reduced these seven environmental descriptors to three variables using PC analysis (PRINCOM procedure in SAS 9.1.3).
Common garden
We tested for significant associations between leaf traits in the common garden and seed source climates using a multi-response multiple regression (Mitchell et al., 2015) implemented in JAGS 3.4.0 (Plummer, 2003). The five morphological traits – LWR, stomatal density, SPI, SLA and leaf area – were each regarded as a single, vector-valued response, and plant was the sampling unit (one leaf per plant per year). The multi-response model allowed us to account for co-variation among traits, while simultaneously regressing each trait against climate variables. Our model included the three PC axes as environmental predictors and the effects of source population and sampling year (2013 or 2014). We did not account for differences associated with maternal lines or individual plants, because stomatal traits were measured on an average of one plant per line per year (range for included lines: one to four), and different plants were measured each year. Source code and data used for this analysis are available at https://github.com/kholsinger/Protea-repens-physiology/releases/tag/v1.0.
Traits and fecundity in the wild
We performed a separate selection gradient analysis for each site, using multiple regression of fecundity (current lifetime achene production) on five morphological traits. We also calculated selection differentials using single linear regressions between each trait and fecundity. Prior to analysis, we averaged the two to four leaves per plant, and we standardized each trait and fecundity to a mean of 0 and a standard deviation of 1, so that selection gradients (β) could be compared among traits (Lande and Arnold, 1983). If a coefficient was significant in our selection gradient analysis, we tested whether patterns of selection on that trait differed between sites. We did so with an additional regression for that trait using data on both sites combined, with variables standardized within sites. A significant interaction between site and trait fixed effects was interpreted as evidence of differences in selection.
In selection gradient analyses, a significant coefficient (β) indicates directional phenotypic selection on the trait; however, similar associations can arise from the influence of favourable microsites on traits and fecundity (Rausher, 1992). To partially control for this, we performed each selection gradient analysis with and without a spatial random effect (Proc MIXED Method = ML; type = sp(sph); SAS 9.3.1; Littell et al., 2006), which was based on field-drawn maps and GPS locations (relative location accuracy ±3 m). Because the results were indistinguishable and the non-spatially explicit model had lower Akaike information criterion scores, we only present the output of the non-spatial model.
Mean trait differences in the wild
We compared site means between Kleinmond and De Hoop for seven leaf traits and five physiology variables. Leaf traits included the five focal morphological traits (LWR, stomatal density, SPI, SLA and leaf area) plus stomatal pore length and leaf width. Physiology variables were photosystem II yield, stomatal conductance, transpiration, light-saturated photosynthetic rate and instantaneous water use efficiency. To test for ecotypic differences between sites, we ran a separate ANOVA of each trait variable, with site as the only fixed effect (Proc MIXED SAS 9.1.3). A Bonferroni-adjusted P-value of 0·0038 (for 13 tests) was used to determine significance.
Trait-ecophysiology associations in the wild
We developed a path-analysis model to explore associations between morphological traits and ecophysiology in each site (n = 10 plants per site, total of 48 leaves). Although path analyses are often used to test different causal structures (e.g. Shipley, 2009), our application of these models is more basic: to account for associations we know to be likely a priori and to better estimate the direct associations between each pair of variables. This follows the tradition of Wright (1934) and many others. In this context, we consider all path coefficients significant ‘associations’ if they are statistically distinguishable from 0, and we do not differentiate between causal correlations and non-causal associations.
The two sites were analysed in separate models, allowing for different slopes, intercepts and covariance matrices in each. Within each model, five morphological traits were used to predict four physiological variables: transpiration, conductance, leaf surface temperature and photosystem II yield. The latter three physiology variables were then used to predict light-saturated photosynthetic rates (excluding transpiration due to autocorrelation with conductance). Relative humidity and ambient temperature were additional covariates predicting the four physiology variables, and all associations between pairs of traits and pairs of physiological variables were also estimated. Individual leaves were the sampling unit and repeated sampling on plants was accounted for with a random plant effect in the models. See the repository cited above for source code and data used for this analysis.
We excluded WUE from the path models because it is a deterministic function of photosynthesis and conductance. We thought it important, however, to examine significant associations in our models in the context of WUE. We therefore selected morphological traits that were significantly associated with a physiological variable in either path model, and we used these morphological traits in pairwise linear comparisons with WUE (Proc MIXED in SAS 9.3; source plant as a random effect).
RESULTS
Climate axes
The climate of the 19 seed source sites was described in three PC axes, which together accounted for 87 % of the variability. Axis 1 was a measure of the three temperature variables and elevation (loading was 0·56 for mean annual temperature, 0·44 for average daily maximum temperature for January, 0·50 for average daily minimum temperature for July and −0·49 for elevation); low values reflect cooler average annual temperatures, cooler summers and winters, and higher elevations. Axis 2 was associated with rainfall seasonality and summertime rainfall (loadings, −0·67 and 0·69); low values reflect intense summertime drought and a high percentage of total rain falling in the winter. Axis 3 was positively associated with just one variable, mean annual rainfall (loading, 0·84).
Common garden
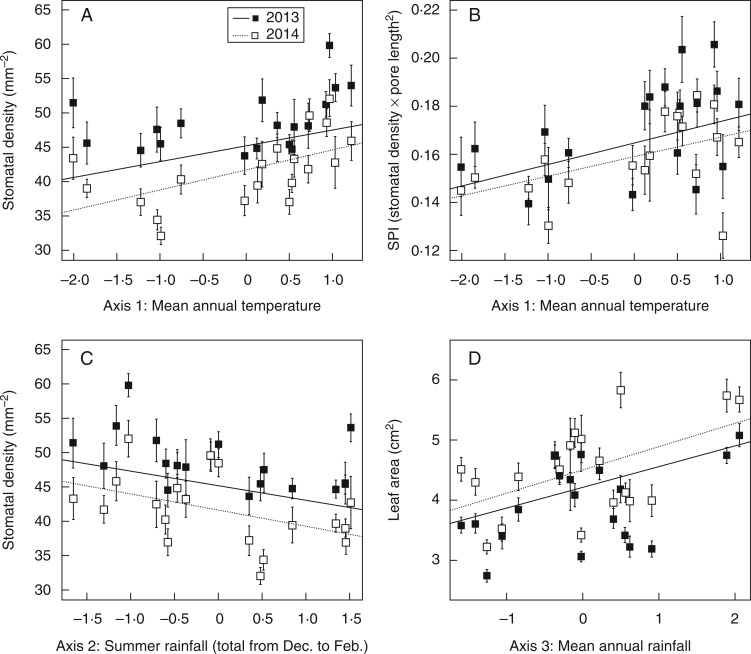
There were four significant trait–environment associations in the multi-response model, and three of these involved stomatal morphology (Figs 2 and 3; statistical results are shown in Supplementary Data Table S2). Stomatal density and SPI were higher in plants sourced from warmer climates (positive with Axis 1; Fig. 3A, B), and stomatal density was also higher in plants sourced from climates with more intense summertime drought (negative with Axis 2; Fig. 3C). Finally, leaf area was higher in plants sourced from sites that received more rain annually (positive with Axis 3; Fig. 3D). There was an additional, marginally significant relationship between SLA and Axis 2 (Supplementary Data Table S1). Plants sourced from sites with more arid summers appeared to have thicker, tougher leaves, although the evidence for this association was weak. No significant environmental associations were detected for LWR.
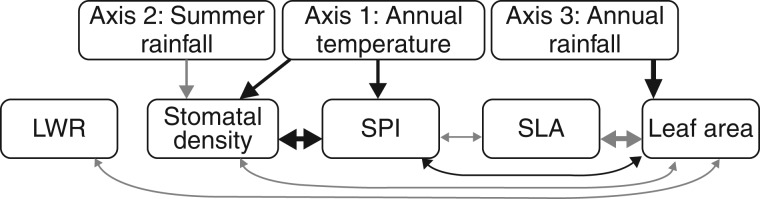
Fig. 2.
Output of multi-response multiple regression testing relationships between population means of leaf traits of Protea repens, measured on plants grown in the Kirstenbosch common garden, and the home climate for the 19 seed collection sites. The upper row of three climate axes are from a principal components analysis of seven environmental variables measuring temperature and rainfall for the seed source climate. See Materials and methods for descriptions of climate variables and leaf trait abbreviations. Morphological leaf traits are from 2 and 3 years post-planting, and year and source population are random effects in the model. The thickness of the line is proportional to the regression coefficient estimate. Only significant relationships are shown, with black and grey lines representing positive and negative relationships, respectively. There was also a marginally significant positive relationship between SLA and Axis 2, which is not shown.
Fig. 3.
Population-averaged leaf trait values for Protea repens plants grown in a common garden at Kirstenbosch, South Africa, in relation to three principle component axes describing seed source climates for (A) stomatal density versus Axis 1, which is positively correlated with mean annual temperature, (B) stomatal pore index versus Axis 1, (C) stomatal density versus Axis 2, which is positively correlated with the total amount of rainfall during December to February, and (D) leaf area versus Axis 3, which is strongly correlated with mean annual rainfall. Plants were measured at 2 and 3 years post-planting (2013 and 2014), and both sets of measurements were compared simultanously in a single multi-response model of five traits and the three climate axes (see Materials and methods for details). The trait–environment relationships in (A)–(D) were significant in the model (Fig. 2), although plotted here are least-squares regressions lines and raw means ±1 s.e.
The multi-response model also showed that leaf traits were strongly inter-correlated in the common garden (Fig. 2), and each trait showed cross-year and cross-population differences (Supplementary Data Table S2, Supplementary Data Fig. S2). The population effect was significant for all five traits, providing evidence for a genetic component to trait variation among sites (see Supplementary Data Appendix S2 for detailed comparison of cross-population differences). Between the 2013 and 2014 measurements, there was a decrease in SLA, SPI and stomatal density and an increase in leaf area and LWR. For inter-trait correlations, leaf area was significantly correlated with many other traits: larger leaves tended to be broader (lower LWR), to be more sclerophyllous (lower SLA), to have more stomata per square millimetre and to dedicate more surface area to stomata (higher SPI). Stomatal density and SPI had a strong positive relationship when compared directly (Fig. 2), such that plants with more stomata per square millimetre also dedicated more of that area to stomatal structures, as opposed to pavement cells. Thicker, tougher leaves also tended to have higher SPI.
If the trait-climate associations detected in the common garden are adaptive, then traits associated with hot, arid sites should be favoured in De Hoop and those associated with cooler, moister sites should be favoured in Kleinmond. Thus, we expect selection in De Hoop to favour increased stomatal density, increased SPI and decreased leaf area, while selection in Kleinmond should act in the opposite direction.
Traits and fecundity in the wild
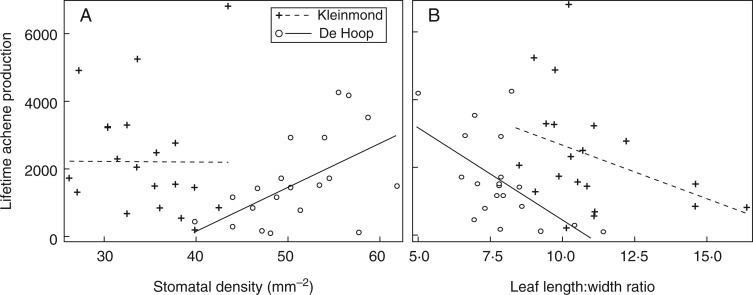
In our selection gradient analysis of five traits simultaneously regressed against fecundity, we detected significant selection gradients on two morphological traits in the De Hoop site, but no traits had significant selection gradients in Kleinmond (Fig. 4A, B; Table 1). Current lifetime achene production in De Hoop was positively correlated with stomatal density and negatively correlated with LWR, i.e. plants with more stomata per square millimetre and narrower leaves were more fecund. Narrow-leaved plants also appeared more fecund in Kleinmond, but the relationship was not significant (Fig. 4B). Analyses of selection differentials, as opposed to selection gradients, revealed no additional significant associations (Table 1). When data for LWR and stomatal density were combined between sites and their selection differentials were re-analysed, we detected a significant site × trait interaction for stomatal density, but not for LWR (Table 1).
Fig. 4.
Stomatal density (A) and leaf length : width ratio (B) of adult Protea repens plants in De Hoop and Kleinmond reserves, in relation to the total number of achenes produced over the plant’s lifetime thus far (n = 20 per site). The single linear regression lines drawn here on raw data illustrate both significant (solid lines) and non-signficant (dotted lines) slopes from selection gradient analyses, i.e. mulitple regressions of all five standardized morphological traits, which also included SLA, SPI and leaf area. In these selection gradient analyses, stomatal density and leaf length : width ratio (LWR) were significantly correlated with achene production at De Hoop (P < 0·05), but no traits were significant at Kleinmond (not significant, P > 0·05; results in Table 1). When the two sites were compared in a single model for each trait, regression coefficients were significantly different between sites for stomatal density but not for LWR.
Table 1.
Selection gradients and differentials from two wild Protea repens populations in the Western Cape of South Africa (see Fig. 1 for locations). Gradients were estimated for each population using five morphological traits regressed simultaneously against current lifetime total achene production as the fitness measure (achenes per seed head × seed heads per plant; n = 20). Selection differentials were estimated from single linear regressions of each trait against the fitness measure. Values in bold type are significant slopes, and asterisks mark traits that had significantly different slopes between sites, based on individual trait regressions on fecundity in a dataset of both sites
| Effect | Kleinmond (cooler, moister) |
De Hoop (hotter, drier) |
||||||
|---|---|---|---|---|---|---|---|---|
| Selection gradients |
Selection differentials |
Selection gradients |
Selection differentials |
|||||
| β | P-value | β | P-value | β | P-value | β | P-value | |
| Length:width ratio (LWR) | −0·186 | 0·13 | −0·186 | 0·08 | −0·311 | 0·02 | −0·417 | 0·005 |
| Stomatal density (mm−2)* | 0·086 | 0·71 | −0·082 | 0·72 | 0·704 | 0·01 | 0·529 | 0·01 |
| Stomatal pore index | 0·107 | 0·62 | 0·214 | 0·34 | −0·429 | 0·12 | 0·149 | 0·51 |
| Specific leaf area (cm2 g−1) | 0·100 | 0·63 | 0·095 | 0·66 | 0·0547 | 0·74 | 0·160 | 0·47 |
| Leaf area (cm2) | 0·030 | 0·89 | 0·130 | 0·57 | −0·120 | 0·51 | 0·055 | 0·81 |
Mean trait differences in the wild
Morphological traits differed significantly between Kleinmond and De Hoop, but eco-physiological measures did not (Table 2). In De Hoop, leaves were smaller and broader for their length, on average. Their stomata were also smaller and denser, and SPI was lower, relative to Kleinmond. Leaf width and SLA were statistically indistinguishable between sites, and the same was true for all physiological measures except WUE (Table 2). Leaves from De Hoop plants had greater WUE than leaves from Kleinmond plants at P < 0·05, but, based on the Bonferroni-adjusted P-value, this difference was non-significant.
Table 2.
Trait and ecophysiology values from two wild Protea repens populations in the Western Cape of South Africa. Morphological traits were measured on 20 plants per site, half of which were measured for ecophysiology (two to four leaves per plant) in June–July 2012. Data are raw means and standard deviations. Double asterisks indicate a significant difference between sites at the Bonferroni-adjusted level of P < 0·0038 and a single asterisk is P < 0·05
| Effect | Kleinmond (cooler, moister) |
De Hoop (hotter, drier) |
P-value | ||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | ||
| LWR (leaf length·leaf width−1) | 10·99 | 2·05 | 7·92 | 1·38 | <0·0001** |
| Stomatal density (mm−2) | 34·59 | 4·97 | 50·90 | 5·60 | <0·0001** |
| SPI (stomatal density · stomatal pore length2) | 0·14 | 0·03 | 0·11 | 0·02 | <0·0001** |
| SLA (cm2 g−1) | 33·62 | 2·50 | 34·66 | 2·25 | 0·18 |
| Leaf area (cm2) | 6·94 | 1·20 | 4·92 | 0·84 | <0·0001** |
| Stomatal pore length (mm) | 0·064 | 0·008 | 0·046 | 0·002 | <0·0001** |
| Leaf width (mm) | 9·90 | 1·28 | 10·39 | 1·87 | 0·34 |
| Light-saturated photosynthetic rate (µmol CO2 m−2 s−1) | 16·84 | 2·88 | 17·43 | 3·00 | 0·50 |
| Leaf surface temperature (°C) | 16·77 | 2·98 | 18·40 | 2·79 | 0·06 |
| Photosystem II quantum yield | 0·34 | 0·04 | 0·36 | 0·07 | 0·26 |
| Stomatal conductance per unit area (mol H2O m−2 s−1) | 0·224 | 0·051 | 0·206 | 0·063 | 0·30 |
| Transpiration per unit area (mol H2O m−2 s−1) | 3·30 | 0·71 | 3·06 | 0·42 | 0·15 |
| Instantaneous water use efficiency (light-saturated photosynthetic rate · stomatal conductance−1) | 77·30 | 14·45 | 88·54 | 15·52 | 0·014* |
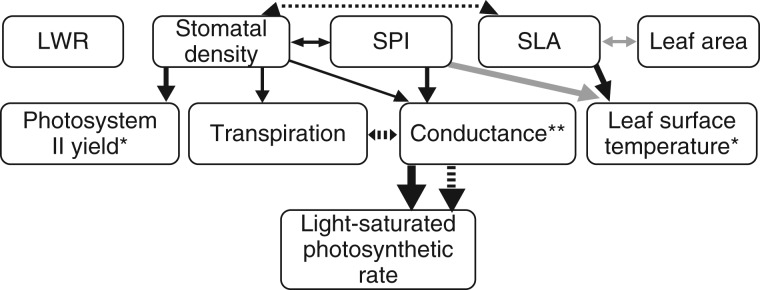
Trait–ecophysiology associations in the wild
In our two path models, the number of associations (i.e. significant coefficients) between morphological and physiological traits differed notably between the two study sites, with most associations being detected only at De Hoop (Fig. 5; Table 3A, B). Stomatal density, SPI and SLA of De Hoop plants were all associated with at least one physiological measure. Plants with more stomata per square millimetre had higher stomatal conductances, higher transpiration rates and higher photosystem II quantum yield. Similarly, plants with higher SPI had increased stomatal conductance and maintained lower leaf surface temperatures (Fig. 5; Table 3A). Finally, plants with more sclerophyllous leaves (lower SLA) also had lower leaf surface temperatures. As expected, photosynthetic rates increased with stomatal conductances at both De Hoop and Kleinmond (Table 3B), but at Kleinmond there were no significant associations between morphological traits and any physiological measure.
Fig. 5.
Output of path models estimating associations between leaf morphological traits and ecophysiology of Protea repens in two sites with distinct climates. The relatively arid De Hoop Reserve has solid lines and the relatively moist Kleinmond Reserve has dotted lines. The thickness of each line is proportional to the estimated regression coefficient. Only significant relationships are shown, with black and grey lines representing positive and negative relationships, respectively. Relative humidity and air temperature were included as covariates on the four middle ecophysiological variables, and they showed significant associations in De Hoop only. Asterisks mark variables that had negative correlations with relative humidity (*) or air temperature (**). Coefficients and 95 % CI from this diagram are in Table 3A and B.
Table 3.
Coefficients and 95 % credible intervals (in parentheses) from path models comparing relationships among functional traits and ecophysiology of adult Protea repens plants in Kleinmond (K) and De Hoop (D) Nature Reserves, South Africa. (A) Trait–physiology associations and trait-trait associations; (B) associations with photosynthesis and between pairs of physiology variables. Within each site, coefficients in (A) and (B) were estimated with the same path model. Variables in the first three rows of (A) and the first row of (B) were response variables; otherwise, coefficients were from non-directional associations. Significant associations are indicated by asterisks; see Materials and methods for details.
| (A) Trait–physiology associations and trait–trait associations | ||||||||
|---|---|---|---|---|---|---|---|---|
| Air temperature | Relative humidity | LWR | Stomatal density | SPI | SLA | Leaf area | ||
| Photosystem II yield | K | −0·22 (−0·90, 0·48) | −0·05 (−0·93, 0·74) | 0·07 (−0·35, 0·48) | 0·39 (−0·32, 1·11) | 0·16 (−0·22, 0·53) | 0·04 (−0·54, 0·64) | −0·14 (−0·5, 0·28) |
| D | −0·36 (−0·96, 0·21) | −0·98* (−1·75, −0·19) | 0·54 (−0·19, 1·28) | 0·51* (0·03, 0·97) | −0·21 (−0·73, 0·32) | −0·15 (−0·65, 0·35) | −0·23 (−0·90, 0·46) | |
| Leaf surface temperature | K | 0·27 (−0·91, 1·12) | 0·02 (−1·10, 1·02) | −0·22 (−0·75, 0·32) | 0·39 (−0·52, 1·34) | −0·09 (−0·58, 0·39) | −0·29 (−1·03, 0·45) | −0·15 (−0·69, 0·39) |
| D | 0·23 (−0·37, 0·78) | −0·82* (−1·59, −0·08) | 0·40 (−0·18, 0·96) | 0·12 (−0·20, 0·45) | −0·59* (−1·00, −0·19) | 0·45* (0·05, 0·85) | −0·05 (−0·53, 0·42) | |
| Stomatal conductance | K | −0·67 (−1·38, 0·03) | −0·52 (−1·38, 0·28) | −0·16 (−0·60, 0·27) | 0·12 (−0·60, 0·83) | −0·02 (−0·44, 0·39) | 0·39 (−0·22, 1·02) | 0·32 (−0·14, 0·76) |
| D | −0·91* (−1·35, −0·44) | 0·36 (−0·25, 0·97) | −0·42 (−0·92, 0·11) | 0·32* (0·01, 0·60) | 0·52* (0·16, 0·88) | −0·23 (−0·59, 0·13) | 0·28 (−0·17, 0·73) | |
| Transpiration | K | −0·38 (−1·33, 0·56) | −0·79 (−1·88, 0·36) | −0·12 (−0·69, 0·45) | −0·28 (−1·23, 0·64) | −0·057 (−0·58, 0·45) | 0·52 (−0·28, 1·37) | 0·33 (−0·25, 0·89) |
| D | −0·14 (−0·56, 0·28) | 0·01(−0·57, 0·56) | −0·01 (−0·53, 0·50) | 0·41* (0·11, 0·71) | 0·36 (−0·01, 0·71) | 0·05 (−0·30, 0·41) | 0·24 (−0·23, 0·69) | |
| LWR | K | – | – | – | 0·16 (−0·24, 0·53) | 0·13 (−0·29, 0·50) | −0·13 (−0·50, 0·29) | 0·02 (−0·37, 0·414) |
| D | – | – | – | 0·21 (−0·14, 0·54) | −0·06 (−0·41, 0·31) | −0·09 (−0·45, 0·27) | 0·05 (−0·31, 0·41) | |
| Stomatal density | K | – | – | – | – | 0·35 (−0·06, 0·67) | 0·46* (0·10, 0·73) | 0·09 (−0·32, 0·47) |
| D | – | – | – | – | 0·40* (0·06, 0·67) | 0·25 (−0·12, 0·57) | −0·17 (−0·50, 0·20) | |
| SPI | K | – | – | – | – | – | 0·25 (−0·16, 0·59) | −0·13 (−0·49, 0·29) |
| D | – | – | – | – | – | 0·24 (−0·14, 0·56) | −0·12 (−0·45, 0·26) | |
| SLA | K | – | – | – | – | – | – | 0·14 (−0·27, 0·52) |
| D | – | – | – | – | – | – | −0·41* (−0·68, −0·07) | |
|
(B) Associations with photosynthesis and between pairs of physiology variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Stomatal conductance | Photosystem II yield | Leaf surface temp. | ||||||
| Light-saturated photosynthetic rate | K | 0·76* (0·28, 1·26) | −0·20 (−0·82, 0·43) | −0·01 (−0·43, 0·42) | ||||
| D | 0·89* (0·53, 1·26) | 0·13 (−0·09, 0·36) | 0·11 (−0·33, 0·56) | |||||
| Transpiration | K | 0·71* (0·34, 0·92) | 0·30 (−0·26, 0·73) | 0·11 (−0·46, 0·63) | ||||
| D | 0·39 (−0·11, 0·74) | 0·07 (−0·42, 0·54) | −0·20 (−0·67, 0·36) | |||||
| Stomatal conductance | K | – | 0·25 (−0·34, 0·72) | 0·18 (−0·41, 0·68) | ||||
| D | – | 0·36 (−0·14, 0·73) | 0·02 (−0·50, 0·52) | |||||
| Photosystem II yield | K | – | – | −0·06 (−0·58, 0·47) | ||||
| D | – | – | 0·24 (−0·28, 0·67) | |||||
Associations among morphological traits also differed between Kleinmond and De Hoop plants, and we detected more associations at De Hoop than at Kleinmond (Fig. 5; Table 3A). At De Hoop, stomatal density and SPI were positively associated, and SLA and leaf area were negatively associated, as was also observed in the common garden dataset. At Kleinmond, stomatal density and SLA were positively associated, i.e. more sclerophyllous leaves had fewer stomata.
None of the morphological traits that were significantly associated with physiology in a path model were significantly related to instantaneous WUE in pairwise comparisons. Specifically, relationships with WUE in De Hoop were non-significant for SLA (F1,15 = 0·04, P = 0·84), SPI (F1,15 = 2·23, P = 0·16) and stomatal density (F1,15 = 0·25, P = 0·62).
DISCUSSION
This study provides strong and multifaceted evidence that cross-populational trait differences in P. repens evolved adaptively. First, we identified several trait–environment associations in common garden plants, including an increase in stomatal density with mean annual temperature and summertime drought intensity. Second, we detected a positive relationship between stomatal density and fecundity in the hotter, drier De Hoop but not in the cooler, moister Kleinmond, which was the direction expected based on trait–environment relationships. Third, we showed that denser stomata in De Hoop were associated directly or indirectly with increased stomatal conductances, increased photosystem II output, cooler leaf temperatures and higher photosynthetic rates during the austral winter, providing a mechanistic explanation for the significant selection gradient on stomatal density in that site. In combination, these three findings suggest that among-population differences in stomatal density in P. repens may evolve through positive selection in hotter, drier sites, where dense (and relatively small) stomata are favoured for their improved transpirational leaf cooling and gas exchange capacity. For traits other than stomata, evidence is equivocal for any mechanisms promoting differences. Even so, the environmental associations we document for leaf size and sclerophylly are parallel to patterns found within other Protea species (Yates et al., 2010; Carlson et al., 2011; Mitchell et al., 2015) and within Proteaceae in South Africa and Australia (Lamont et al., 2002; Thuiller et al., 2004).
The generality of trait–environment relationships across and within species
Across species
The trait–environment associations we detected for leaf area and SLA in P. repens are consistent with most broad-scale, cross-species analyses of these traits. Although the decline in SLA with aridity was only weakly supported in our study, this pattern is well-documented in many cross-species studies (e.g. Cunningham et al., 1999; Wright et al., 2004), including a recent, phylogenetically explicit study of Protea and Pelargonium by Mitchell et al. (2015). Similarly, leaf area and leaf width both tend to decline with decreasing rainfall in cross-species studies (leaf area: McDonald et al., 2003; Thuiller et al., 2004; leaf width: Cunningham et al., 1999; Fonseca et al., 2000; Yates et al., 2010; but see Mitchell et al., 2015). In P. repens, we detected an aridity-related decline for leaf area but not for LWR. These parallels reveal a surprisingly uniform response to aridity both within and across species. In other words, plants appear to exhibit similar trait values under arid conditions across several phylogenetic scales. If the trait–environment associations found in P. repens are representative, it also suggests that ecotypic variation may influence community mean trait values more than is sometimes assumed (e.g. Merow et al., 2011). If among-species differences are much larger than within-species differences, this effect may be small, but variability within species and its contribution to community means is often overlooked (see also Cornwell and Ackerly, 2009; Albert et al., 2010; Jung et al., 2010; Messier et al., 2010).
Within species
Beginning with the foundational work by Hiesey et al. (1942), ecotypic differences among populations have been linked to environmental differences within many plant species. Cross-population studies of other Protea species parallel our findings for P. repens for leaf area and SLA, and to a lesser extent for stomatal density. In a study of 35 populations from seven species in Protea section Exsertae, Carlson et al. (2011) found that leaves of common garden seedlings sourced from drier climates were thicker (lower SLA) and smaller (lower area) than those from moister climates, which is the same pattern seen in P. repens. Carlson et al. (2011) also found that stomatal density was positively associated with rainfall seasonality in common garden plants and with dry-season drought across wild populations. Our results for P. repens were similar, in that plants sourced from hotter sites with stronger summer drought had higher stomatal density, and those from hotter sites also dedicated more leaf surface area to stomata.
The patterns that Carlson et al. (2011) detected in Protea section Exsertae were driven by both cross-species and within-species differences. Even so, the same trait–environment relationships have been found in several single-species studies. In a greenhouse study of Protea section Exsertae, Prunier et al. (2012) showed that leaf area was positively associated with winter temperature and rainfall across five P. mundii populations and LWR was negatively associated with rainfall across five P. aurea populations, i.e. leaves were narrower in drier environments. Within non-Protea species, low SLA and low leaf area are commonly linked to drier climates (e.g. Etterson, 2004; Ramirez-Valiente et al., 2009), but high stomatal density is less often linked with drier sites. Some studies detect no associations between stomatal density and measures of increasing aridity (Franks et al., 2009; Skelton et al., 2012), and for those that detect associations the relationship is sometimes positive (Clay and Quinn, 1978; Pearce et al., 2006; Maes et al., 2009; Carins Murphy et al., 2014) and sometimes negative (Ashton and Berlyn, 1994; Pääkkönen et al., 1998; Stenström et al., 2002; Yu et al., 2008). In a study by Xu and Zhou (2008), both stomatal density and stomatal pore index were highest at intermediate levels of drought, but then declined as drought intensity strengthened.
Context-dependent selection can reveal the evolutionary underpinnings of trait–environment relationships
The selection regimes on stomata differed significantly between De Hoop and Kleinmond, suggesting that a history of divergent selection may contribute to the distribution-wide patterns of stomatal variation. Specifically, selection on stomatal density was positive in the drier climate of De Hoop and indistinguishable from zero in the moister climate of Kleinmond, consistent with the observation that common garden plants from hotter, more drought-prone sites had higher stomatal density. Dudley (1996a, b) also detected positive or non-linear selection in a drier site but no selection in a moister site. In two experimental populations of Cakile edulenta, selection favoured high WUE and small – but not the smallest – leaves in the dry site, which was interpreted as evidence of adaptive differentiation. Condition-dependent relationships between traits and fitness measures have been linked to local adaptation in other studies as well (Ackerly et al., 2000; Etterson, 2004; Agrawal et al., 2008; Donovan et al., 2009; Carlson et al., 2011).
Relationships between traits and physiological responses are also context-dependent
Populations that have long grown under contrasting conditions may differ in how traits relate to performance, and these differences may reflect plastic responses, genetic divergence or, most probably, some of both (Ackerly et al., 2000; Picotte et al., 2007). In P. repens, plasticity is presumed to influence site-specific relationships in Kleinmond and De Hoop to some degree, likely following local differences in soil chemistry, weather and microclimate. Even so, common garden results show that morphological differences in the traits we measured include a genetic component. We thereby infer that mean trait values in Kleinmond and De Hoop are subject to ongoing selection and have been shaped by past selection, as has been suggested in other studies (Dudley, 1996a, b; Brouillette et al., 2014). Future studies are needed, however, to clarify the relative contribution of plasticity in the observed site-specific eco-physiological responses. Unravelling these complex interactions may require direct manipulation of soil moisture, soil chemistry or ambient temperature in a laboratory setting (e.g. Shane et al., 2008) or outdoor measurement of trait–physiology relationships at different times of year (e.g. Carlson and Holsinger, 2012; West et al., 2012).
Photosynthetic performance of dry-site P. repens plants increased with stomatal density, but we did not detect such a relationship in Kleinmond. Stomatal density and the rate at which molecules enter and exit the leaf are often thought to co-vary, although additional variables, like pore size, pore depth and pubescence, can weaken this relationship (Reich, 1984). Indeed, a tight relationship is not consistently found (stomatal density not related to CO2 uptake or H2O loss: Carlson et al., 2011; Carlson and Holsinger, 2012; stomatal density related to H2O loss: Pearce et al., 2006; Galmez et al., 2007; Franks et al., 2009). Yates et al. (2010) attributed variation in these relationships to the influence of leaf size on transpiration, and in particular to narrow leaves having thinner air boundary layers at the leaf surface, which allows greater stomatal control over transpiration (see also Gutschick, 1999). They posit that water loss from larger leaves is limited by thicker boundary layers and is therefore less responsive to differences in stomatal density or size.
The interplay described by Yates et al. (2010) between leaf morphology and boundary layer thickness could contribute to physiological differences between Kleinmond and De Hoop. De Hoop leaves are smaller than Kleinmond leaves (though leaf widths are similar), which could promote thicker boundary layers in Kleinmond and thinner boundary layers in De Hoop. If so, this would explain why stomatal density and conductance (or conductance and air temperature) are correlated in De Hoop but decoupled in Kleinmond. An ability to lose water rapidly may at first appear maladaptive for dry site plants, yet these dense, small stomata should also respond more rapidly to changing conditions, thus reducing desiccation risk (Hetherington and Woodward, 2003; Drake et al., 2013; Giday et al., 2013; Raven, 2014). This may be particularly important in De Hoop, where stem water potentials were more negative (mean ± s.d. measured at mid-day on 13 plants per site in De Hoop versus Kleinmond: −0·60 ± 0·15 versus –0·54 ± 0·18 MPa; J. E. Carlson, unpubl. data), possibly because the shorter depth to bedrock limits soil water retention (e.g. Richards et al., 1995).
In De Hoop there is a second potential advantage to having many small, dense stomata (i.e. higher SPI): these plants maintained cooler leaf temperatures, likely because of their higher stomatal conductances. When leaves experience temperatures above their optimum for photosynthesis, CO2 is captured less efficiently, membranes loosen, electron transport slows and thermal damage may occur (Gutschick, 1999; Lambers et al., 2008; Heschel et al., 2014). Assuming that both P. repens populations reach maximum photosynthesis at similar temperatures, high rates of transpirational cooling may be more beneficial in hotter, drier De Hoop and less beneficial in cooler, moister Kleinmond (e.g. Yates et al., 2010; Skelton et al., 2012). Franks et al. (2009) also suggest that maintaining many stomata increases metabolic costs, meaning that fewer, larger stomata will be favoured when transpirational cooling is less important.
Summary
Trait–environment relationships are often presumed to reflect environmental adaptation, yet studies showing direct evidence of context-dependent fitness consequences are relatively rare. Even rarer are studies that begin to dissect the mechanisms behind fitness differences or identify the traits that have different physiological or functional consequences in different environments (but see Dudley, 1996a). Our study addresses this gap by demonstrating parallel patterns of trait–physiology–environment matching at broad and narrow geographical scales. We show that, across populations of P. repens, higher stomatal densities are associated with more arid climates. We also show that, within a more arid population, but not a more mesic one, higher stomatal densities are associated, directly or indirectly, with higher rates of conductance and photosynthesis, cooler leaf temperatures and higher fecundity. Our use of interlinking datasets makes a unique and strong case that natural selection rather than drift is driving trait divergence in P. repens, thus highlighting the importance of adaptive evolution in one of the richest, most unique floras in the world.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: stomatal structures of the adaxial leaf surface of Protea repens as seen under light microscope and SEM. Figure S2: traits of Protea repens plants from 19 wild populations and of their offspring in a common garden at Kirstenbosch Botanical Gardens, South Africa. Table S1: model output to assess whether Protea repens leaves in the common garden differ significantly from those of wild plants. Table S2: results of a multi-response multiple regression comparing seed source climate with functional traits of Protea repens seedlings from 19 populations grown in a common garden. Appendix S1: comparing leaf traits between wild adults and common garden plants at 1, 2 and 3 years post-planting. Appendix S2: comparing mean trait values across populations in the common garden.
ACKNOWLEDGEMENTS
We thank N. Mitchell for her major contribution to data collection for this study, as well as for editorial comments. Additional thanks for field or laboratory assistance go to H. Martinez-Cabrera, M.A. Carlson, C.E.T. Paine, C. Schlichting, K. Mocko, T. Williams, E. Bergeron, K. Kellermann and J. Slingsby. C. Jones produced the SEM image in Fig. S1. L. Nurrish and T. Rebelo of SANBI generously provided logistical support and garden access at Kirstenbosch. Several trait and physiological measures were made possible through equipment loans from G. Midgley, E. February and E. Melancon. We thank the reserve managers and property owners for access to field sites. This work was supported by the National Science Foundation (DEB-1046328). Data were collected under Cape Nature permits AAA005-00214-0028 and AAA005-00224-0028 and Eastern Cape Province permit CRO 4/11 CR.
LITERATURE CITED
- Ackerly DD, Dudley SA, Sultan SE, et al. 2000. The evolution of plant ecophysiological traits: recent advances and future directions. Bioscience 50: 979–995. [Google Scholar]
- Agrawal AA, Erwin AC, Cook SC. 2008. Natural selection on and predicted responses of ecophysiological traits of swamp milkweed (Asclepias incarnata). Journal of Ecology 96: 536–542. [Google Scholar]
- Albert CH, Thuiller W, Yoccoz NG, et al. 2010. Intraspecific functional variability: extent, structure and sources of variation. Journal of Ecology 98: 604–613. [Google Scholar]
- Ashton PMS, Berlyn GP. 1994. A comparison of leaf physiology and anatomy of Quercus (section Erythrobalanus – Fagaceae) species in different light environments. American Journal of Botany 81: 589–597. [Google Scholar]
- Britton MN, Hedderson TA, Verboom GA. 2014. Topography as a driver of cryptic speciation in the high-elevation cape sedge Tetraria triangularis (Boeck.) C. B. Clarke (Cyperaceae: Schoeneae). Molecular phylogenetics and Evolution 77: 96–109. [DOI] [PubMed] [Google Scholar]
- Brouillette LC, Mason CM, Shirk RY, Donovan LA. 2014. Adaptive differentiation of traits related to resource use in a desert annual along a resource gradient. New Phytologist 201: 1316–1327. [DOI] [PubMed] [Google Scholar]
- Carins Murphy MR, Jordan GJ, Brodribb TJ. 2014. Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant, Cell & Environment 37: 124–131. [DOI] [PubMed] [Google Scholar]
- Carlson JE, Holsinger KE. 2012. Developmental plasticity in Protea as an evolutionary response to environmental clines in the Cape Floristic Region. PLoS ONE 7: e52035 doi:10.1371/journal.pone.0052035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JE, Holsinger KE, Prunier R. 2011. Plant responses to climate in the Cape Floristic Region of South Africa: evidence for adaptive differentiation in the Proteaceae. Evolution 65: 108–124. [DOI] [PubMed] [Google Scholar]
- Clay K, Quinn JA. 1978. Density of stomata and their responses to a moisture gradient in Danthonia sericea populations from dry and wet habitats. Bulletin of the Torrey Botanical Club 105: 45–49. [Google Scholar]
- Coetzee JH, Giliomee JH. 1987. Seed predation and survival in the infructescences of Protea repens (Proteaceae). South African Journal of Botany 53: 61–64. [Google Scholar]
- Comes HP, Tribsch A, Bittkau C. 2008. Plant speciation in continental island floras as exemplified by Nigella in the Aegean archipelago. Proceedings of the Royal Society Series B: Biological Sciences 363: 3083–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell WK, Ackerly DD. 2009. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecological Monographs 79: 109–126. [Google Scholar]
- Cunningham SA, Summerhayes B, Westoby M. 1999. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecology 69: 569–588. [Google Scholar]
- Donovan LA, Ludwig F, Rosenthal DM, Rieseberg LH, Dudley SA. 2009. Phenotypic selection on leaf ecophysiological traits in Helianthus. New Phytologist 183: 868–879. [DOI] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ. 2013. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. Journal of Experimental Botany 64: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley SA. 1996a. Differing selection on plant physiological traits in response to environmental water availability: a test of adaptive hypotheses. Evolution 50: 92–102. [DOI] [PubMed] [Google Scholar]
- Dudley SA. 1996b. The response to differing selection on plant physiological traits: evidence for local adaptation. Evolution 50: 103–110. [DOI] [PubMed] [Google Scholar]
- Ellis AG, Weis AE, Gaut BS. 2006. Evolutionary radiation of ‘stone plants’ in the genus Argyroderma (Aizoaceae): unraveling the effects of landscape, habitat, and flowering time. Evolution 60: 39–55. [PubMed] [Google Scholar]
- Etterson JR. 2004. Evolutionary potential of Chamaecrista fasciculata in relation to climate change. 1. Clinal patterns of selection along an environmental gradient in the great plains. Evolution 58: 1446–1458. [DOI] [PubMed] [Google Scholar]
- Fonseca CR, Overton JM, Collins B, Westoby M. 2000. Shifts in trait-combinations along rainfall and phosphorus gradients. Journal of Ecology 88: 964–977. [Google Scholar]
- Forsyth GG, van Wilgen BW. 2007. An analysis of fire history records from protected areas in the western cape. CSIR Report Number CSIR/NRE/ECO/ER/2007/0118/C. Stellenbosch, South Africa: CSIR Natural Resources and the Environment. [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences of the USA 106: 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Drake PL, Beerling DJ. 2009. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: an analysis using Eucalyptus globulus. Plant, Cell & Environment 32: 1737–1748. [DOI] [PubMed] [Google Scholar]
- Frei ES, Scheepens JF, Armbruster GFJ, Stöcklin J. 2012. Phenotypic differentiation in a common garden reflects the phylogeography of a widespread Alpine plant. Journal of Ecology 100: 297–308. [Google Scholar]
- Friar EA, Prince LM, Roalson EH, et al. 2006. Ecological speciation in the East Maui-endemic Dubautia (Asteraceae) species. Evolution 60: 1777–1792. [PubMed] [Google Scholar]
- Galmes J, Flexas J, Sav R, Medrando H. 2007. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant and Soil 290: 139–155. [Google Scholar]
- Geber MA, Griffen LR. 2003. Inheritance and selection on plant functional traits. International Journal of Plant Sciences 164 (Supplement 3): S21–S42. [Google Scholar]
- Giday H, Kjaer KH, Fanourakis D, Ottosen CO. 2013. Smaller stomata require less severe leaf drying to close: a case study in Rosa hydrida. Journal of Plant Physiology 170: 1309–1316. [DOI] [PubMed] [Google Scholar]
- Gutschick VP. 1999. Biotic and abiotic consequences of differences in leaf structure. New Phytologist 143: 3–18. [Google Scholar]
- Heschel MS, Evankow A, Wolfson KB, Carlson JE, Holsinger KE. 2014. Drought response diversification in African Protea species. International Journal of Plant Sciences 175: 442–449. [Google Scholar]
- Hetherington AM, Woodward FI. 2003. The role of stomata in sensing and driving environmental change. Nature 424: 901. [DOI] [PubMed] [Google Scholar]
- Hiesey WM, Clausen J, Keck DD. 1942. Ecological aspects of evolution – relations between climate and intraspecific variation in plants. American Naturalist 76: 5–22. [Google Scholar]
- Jordaan PG. 1972. Die generatiewe voorplanting van die Proteaceae. Jaarblad van die Botaniese Vereniging van Suid-Afrika 58: 48–56. [Google Scholar]
- Jung V, Violle C, Mondy C, Hoffmann L, Muller S. 2010. Intraspecific variability and trait-based community assembly. Journal of Ecology 98: 1134–1140. [Google Scholar]
- Korner C. 2003. Alpine plant life: functional ecology of high mountain ecosystems. New York: Springer. [Google Scholar]
- Lambers H, Chapin FSI, Pons TL. 2008. Plant physiological ecology, 2nd edn New York: Springer. [Google Scholar]
- Lamont BB, Groom PK, Cowling RM. 2002. High leaf mass per area of related species assemblages may reflect low rainfall and carbon isotope discrimination rather than low phosphorus and nitrogen concentrations. Functional Ecology 16: 403–412. [Google Scholar]
- Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37: 1210–1226. [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O. 2006. SAS for mixed models. Cary, NC: SAS Publishing. [Google Scholar]
- LP DAAC. 2011. ASTER GDEM, METI and NASA release version 2. Sioux Falls, SD: USGS/Earth Resources Observation and Science (EROS) Center. [Google Scholar]
- Maes WH, Achten WMJ, Reubens B, Raes D, Samson R, Muys B. 2009. Plant-water relationships and growth strategies of Jatropha curcas L. seedlings under different levels of drought stress. Journal of Arid Environments 73: 877–884. [Google Scholar]
- McDonald PG, Fonseca CR, Overton JM, Westoby M. 2003. Leaf-size divergence along rainfall and soil-nutrient gradients: is the method of size reduction common among clades? Functional Ecology 17: 50–57. [Google Scholar]
- Merow C, Latimer AM, Silander JA. 2011. Can entropy maximization use functional traits to explain species abundances? A comprehensive evaluation. Ecology 92: 1523–1537. [DOI] [PubMed] [Google Scholar]
- Messier J, McGill BJ, Lechowicz MJ. 2010. How do traits vary across ecological scales? A case for trait-based ecology. Ecology Letters 13: 838–848. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Moore T, Mollman HK, et al. 2015. Functional traits in parallel evolutionary radiations and trait–environment associations in the Cape Floristic Region of South Africa. American Naturalist 185: 525–537. [DOI] [PubMed] [Google Scholar]
- Nakazato T, Bogonovich M, Moyle LC. 2008. Environmental factors predict adaptive phenotypic differentiation within and between two wild Andean tomatoes. Evolution 62: 774–792. [DOI] [PubMed] [Google Scholar]
- Ordoñez JC, van Bodegom PM, Witte JPM, Wright IJ, Reich PB, Aerts R. 2009. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecology and Biogeography 18: 137–149. [Google Scholar]
- Pääkkönen E, Günthardt-Goerg MS, Holopainen T. 1998. Responses of leaf processes in a sensitive birch (Betula pendula Roth) clone to ozone combined with drought. Annals of Botany 82: 49–59. [Google Scholar]
- Pearce DW, Millard S, Bray DF, Rood SB. 2006. Stomatal characteristics of riparian poplar species in a semi-arid environment. Tree Physiology 26: 211–218. [DOI] [PubMed] [Google Scholar]
- Picotte JJ, Rosenthal DM, Rhode JM, Cruzan MB. 2007. Plastic responses to temporal variation in moisture availability: consequences for water use efficiency and plant performance. Oecologia 153: 821–832. [DOI] [PubMed] [Google Scholar]
- Plummer M. 2003. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. http://citeseer.ist.psu.edu/plummer03jags.html. [Google Scholar]
- Prunier R, Holsinger KE, Carlson JE. 2012. The effect of historical legacy on adaptation: do closely related species respond to the environment in the same way? Journal of Evolutionary Biology 25: 1636–1649. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valiente JA, Lorenzo Z, Soto A, Valladares F, Gil L, Aranda I. 2009. Elucidating the role of genetic drift and natural selection in cork oak differentiation regarding drought tolerance. Molecular Ecology 18: 3803–3815. [DOI] [PubMed] [Google Scholar]
- Rausher MD. 1992. The measurement of selection on quantitative traits – biases due to environmental covariances between traits and fitness. Evolution 46: 616–626. [DOI] [PubMed] [Google Scholar]
- Raven JA. 2014. Speedy small stomata? Journal of Experimental Botany 65: 1415–1424. [DOI] [PubMed] [Google Scholar]
- Rebelo AG. 2001. Proteas: a field guide to the proteas of southern Africa. Vlaeberg, South Africa: Fernwood Press. [Google Scholar]
- Reich PB. 1984. Leaf stomatal density and diffusive conductance in three amphistomatous hybrid poplar cultivars. New Phytologist 98: 231–239. [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, et al. 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences 164: S143–S164. [Google Scholar]
- Reynoso-Castillo GA, Morokuma M, Kuyama M, Hasegawa A, Goi M. 2001. Morphological features and stomatal density in Proteaceae species. ISHS Acta Horticulturae 545: 245–249. [Google Scholar]
- Richards MB, Stock WD, Cowling RM. 1995. Water relations of seedlings and adults of two fynbos Protea species in relation to their distribution patterns. Functional Ecology 9: 575–583. [Google Scholar]
- Schulze RE. 1997. South African atlas of agrohydrology and climatology. Technical Report. Report TT82/96. Pretoria, South Africa: Water Resource Commission. [Google Scholar]
- Schulze RE. 2007. South African atlas of climatology and agrohydrology: WRC Report 1489/1/06. Pretoria, South Africa: Water Research Commission. [Google Scholar]
- Shane MW, Cramer MD, Lambers H. 2008. Root of edaphically controlled Proteaceae turnover on the Agulhas Plain, South Africa: phosphate uptake regulation and growth. Plant, Cell & Environment 31: 1825–1833. [DOI] [PubMed] [Google Scholar]
- Shipley B. 2009. Confirmatory path analysis in a generalized multilevel context. Ecology 90: 363–368. [DOI] [PubMed] [Google Scholar]
- Skelton RP, Midgley JJ, Nyaga JM, Johnson SD, Cramer MD. 2012. Is leaf pubescence of Cape Proteaceae a xeromorphic or radiation-protective trait? Australian Journal of Botany 60: 104–113. [Google Scholar]
- Van Staden J. 1978. Seed viability in Protea neriifolia. I. The effects of time of harvesting on seed viability. Agroplantae 10: 65–67. [Google Scholar]
- Stenström A, Jónsdóttir IJ, Augner M. 2002. Genetic and environmental effects on morphology in clonal sedges in the Eurasian Arctic. American Journal of Botany 89: 1410–1421. [DOI] [PubMed] [Google Scholar]
- Thuiller W, Lavorel S, Midgley G, Lavergne S, Rebelo T. 2004. Relating plant traits and species distributions along bioclimatic gradients for 88 Leucadendron taxa. Ecology 85: 1688–1699. [Google Scholar]
- Verboom GA, Linder HP, Stock WD. 2004. Testing the adaptive nature of radiation: growth form and life history divergence in the African grass genus Ehrharta (Poaceae: Ehrhartoideae). American Journal of Botany 91: 1364–1370. [DOI] [PubMed] [Google Scholar]
- West AG, Dawson TE, February EC, Midgley GF, Bond WJ, Aston TL. 2012. Diverse functional responses to drought in a Mediterranean-type shrubland in South Africa. New Phytologist 195: 396–407. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wright S. 1934. The method of path coefficients. Annals of Mathematical Statistics 5: 161–215. [Google Scholar]
- Xu Z, Zhou G. 2008. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany 59: 3317–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MJ, Verboom GA, Rebelo AG, Cramer MD. 2010. Ecophysiological significance of leaf size variation in Proteaceae from the Cape Floristic Region. Functional Ecology 24: 485–492. [Google Scholar]
- Yu H, Chen X, Hong Y, et al. 2008. Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. Plant Cell 20: 1134–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.