Abstract
Background and Aims Tiller production and survival determine final spike number, and play key roles in grain yield formation in wheat (Triticum aestivum). This study aimed to understand the genetic and physiological basis of the tillering process, and its trade-offs with other yield components, by introducing genetic variation in tillering patterns via a mapping population of wheat × spelt (Triticum spelta).
Methods The dynamics of tillering and red/far-red ratio (R:FR) at the base of a canopy arising from neighbouring plants in a bread wheat (Triticum aestivum ‘Forno’) × spelt (Triticum spelta ‘Oberkulmer’) mapping population were measured in the field in two growing seasons. Additional thinning and shading experiments were conducted in the field and glasshouse, respectively. Yield components were analysed for all experiments, followed by identification of quantitative trait loci (QTL) associated with each trait.
Key Results Large genetic variation in tillering was observed, and more fertile shoots per plant were associated with more total shoots initiated, faster tillering rate, delayed tillering onset and cessation, and higher shoot survival. A total of 34 QTL for tillering traits were identified, and analysis of allelic effects confirmed the above associations. Low R:FR was associated with early tillering cessation, few total shoots, high infertile shoot number and shoot abortion, and these results concurred with the thinning and shading experiments. These effects probably resulted from an assimilate shortage for tiller buds or developing tillers, due to early stem elongation and enhanced stem growth induced by low R:FR. More fertile tillers normally contributed to plant yield and grain number without reducing yield and grain set of individual shoots. However, there was a decrease in grain weight, partly because of smaller carpels and fewer stem water-soluble carbohydrates at anthesis caused by pleiotropy or tight gene linkages.
Conclusions Tillering is under the control of both genetic factors and R:FR. Genetic variation in tillering and tolerance to low R:FR can be used to optimize tillering patterns for yield improvement in wheat.
Keywords: Carpel, grain number, grain weight, quantitative trait locus, QTL, red/far-red ratio, spelt, stem water-soluble carbohydrates, tillering, Triticum aestivum, Triticum spelta, wheat, yield
INTRODUCTION
Tillering in wheat (Triticum aestivum) determines plant canopy size, photosynthetic area and, more importantly, the number of spikes bearing grains at maturity (fertile shoots), which is a key component of yield. Wheat plants undergo several events to form final fertile shoots: axillary bud initiation, first bud outgrowth, tillering cessation, tiller abortion and the development of surviving tillers. Tiller buds are initiated from the axillary meristems in the axils of developing leaves on the main shoots, and bud number is associated with total number of leaves (Baker and Gallagher, 1983; Longnecker et al., 1993). Early tillers can also be parent shoots producing secondary buds and tillers (Evers and Vos, 2013).
Outgrowth of the first tiller buds represents the onset of apparent tillering. In the field, this can occur from autumn to spring, depending on sowing date and temperature thereafter (Sylvester-Bradley et al., 2008). Tillering normally ceases just before stem elongation (Baker and Gallagher, 1983; Gomez-Macpherson et al., 1998; Sylvester-Bradley et al., 2008), and the remaining axillary buds become dormant. However, dormancy is not definitive, and can be released in cases such as early lodging and damage to the apices of parent shoots (Rameau et al., 2015). The timing of tillering cessation and number of total tillers initiated are regulated by many genetic, physiological and environmental factors. A tiller inhibition gene (tin1), which has been mapped on chromosome 1AS (Richards, 1988; Spielmeyer and Richards, 2004), has been found to reduce tillering through the early cessation of axillary bud outgrowth (Duggan et al., 2002; Kebrom et al., 2012). This inhibition may result from the sugar deficit for lateral tiller buds due to precocious internode elongation (Kebrom et al., 2012), concurring with the report of Langer et al. (1973). Environmental factors such as plant density and shade also affect tillering cessation. A larger plant population has been shown to be associated with earlier tillering cessation and fewer maximum tillers per plant (Evers et al., 2006; Sparkes et al., 2006). In dense communities, the red and blue wavelengths of light are absorbed by surrounding plants, and most far red is reflected and transmitted, resulting in a reduction in light intensity and quality (red/far-red ratio, R:FR), or shade. There is evidence that tiller bud outgrowth responds to light quality and, to a lesser extent, light intensity (Sparkes et al., 2006). Cessation of axillary bud outgrowth coincides with a relatively conservative R:FR of 0·20–0·40 (Evers et al., 2006; Sparkes et al., 2006; Dreccer et al., 2013). High R:FR delays tillering cessation, and increases total tiller number (Toyota et al., 2014). Treatment with far-red light has the opposite effects, which can be reversed by adding red light, suggesting phytochrome perception (Kasperbauer and Karlen, 1986; Casal, 1988; Ugarte et al., 2010). Tillering (branching) response to low R:FR or shade has also been observed in ryegrass (Lolium multiflorum) (Casal et al., 1990), barley (Hordeum vulgare) (Davis and Simmons, 1994), sorghum (Sorghum bicolor) (Kebrom et al., 2006), soybean (Glycine max) (Kasperbauer, 1987) and arabidopsis (Arabidopsis thaliana) (Reddy et al., 2013). Shade acts as a warning signal of impending competition from neighbouring plants, and the consequent reduction of shoot branching is able to enhance apical growth for more incident light, known as a part of the shade avoidance syndrome (Gommers et al., 2013; Rameau et al., 2015).
Tiller abortion ensues immediately after the arrest of tiller bud outgrowth. Of the total tillers initiated, 10–80 % are destined to die, as affected by genotype, season, growing location, seeding rate and nutrient supply (Ishag and Taha, 1974; Hucl and Baker, 1989; Sharma, 1995; Berry et al., 2003). Tiller abortion usually takes place between the onset of stem elongation and anthesis, and those appearing last die first (Sylvester-Bradley et al., 2008). As there is a net loss of dry matter from non-surviving tillers, they have been thought to be detrimental for yield potential, especially when a further increase in harvest index is required (Sharma, 1995; Berry et al., 2003; Foulkes et al., 2011). Therefore, tiller survival needs to be improved in future breeding, and a first step would be to clarify its genetic and physiological basis, which remains unknown. In contrast, fertile shoot or spike number at maturity has been widely investigated. Three genes, tin1 on chromosome 1AS (Richards, 1988; Duggan et al., 2005), tin2 on 2A (Peng et al., 1998) and tin3 on 3AmL (Kuraparthy et al., 2007), have been identified to reduce final tiller number. This trait is often expressed quantitatively, and many quantitative trait loci (QTL) have been detected on at least 12 chromosomes (Kato et al., 2000; Deng et al., 2011; Naruoka et al., 2011; Jia et al., 2013; Zhang et al., 2013).
Despite the importance of tillering dynamics in terms of yield formation in wheat, knowledge of the genetic and environmental factors regulating this process remains scarce. The questions that need to be addressed include: (1) what are the genes or QTL controlling the timing and rate of tillering, tillering capacity, and the degree of tiller abortion and survival; (2) whether and how the shade from neighbouring plants affects tillering dynamics, particularly tiller abortion, and if so, what is the genetic basis of the shade kinetics arising from a genotype grown in the field; and (3) whether more fertile tillers contribute to plant productivity, given the possible negative effects on other yield components. In this study, we aimed to address these questions in a recombinant inbred line mapping population of bread wheat (Triticum aestivum) × spelt (T. spelta). Dynamics of the tillering and R:FR were measured consecutively in the field in two seasons, and this was also done in the thinning study. In the third season, a shading experiment was carried out in the glasshouse to determine its effect on fertile tiller number. Yield components of each genotype in all seasons were then analysed. Subsequently, the QTL underlying these traits were identified.
MATERIALS AND METHODS
Plant materials
A mapping population of Swiss winter bread wheat (Triticum aestivum) ‘Forno’ and Swiss winter spelt (T. spelta) ‘Oberkulmer’ was used to introduce genetic variation in tillering patterns. This population consists of 226 F5 recombinant inbred lines (RILs) (Messmer et al., 1999), and showed large variation in tiller number at different developmental stages in the preliminary field trials. Based on these observations, a subset including 72 RILs was selected in the 2011–2012 season (hereafter 2012), with considerable difference in tillering but similar flowering time (± 4 d in 2009–2010 and ± 1 d in 2010–2011) to minimize the confounding effect of different phasic development. This subset was enlarged to 110 RILs in the 2012–2013 and 2013–2014 seasons (hereafter 2013 and 2014, respectively).
Growth conditions for field experiments
Field experiments were carried out at University of Nottingham Farm, Leicestershire, UK (52°50′N, 1°15′W, 50 m above sea level) in 2012 and 2013. The soil was a sandy loam (soil indices: N = 0, P = 4, K = 4, Mg = 4, pH = 7·6 in 2012; N = 0, P = 5, K = 4, Mg = 4, pH = 7·3 in 2013). An additional 140 and 160 kg N ha−1 were applied in three splits between March and May in 2012 and 2013, respectively. The whole population, including ‘Forno’ and ‘Oberkulmer’, was arranged in a randomized complete block design with three replicates. The seeds of each RIL were sown in 6 × 1·6 -m plots on 19 October 2011 and in 12 × 1·6 -m plots on 31 October 2012, with 250 seeds m−2. Herbicides, fungicides and insecticides were applied when necessary to maintain undisturbed plant growth.
Tillering, R:FR and yield components in the field experiments
Ten central plants per plot were selected and labelled after emergence in 2012 and 2013. To create relatively uniform populations among plots, plant density was adjusted by removing extra surrounding plants. When the tiller buds grew out at the leaf–stem junctions and became new tillers, the shoot number of each plant was counted approximately every 100 degree days (°Cd, base temperature 0 °C) until tillering cessation. Dying tillers, whose newest leaves started yellowing, were tagged using wires so that all shoots produced during tillering were taken into account. At the late stage of grain filling, the fertile shoots bearing spikes were counted. Immediately after each shoot count, R:FR at the base of each plant was measured using a two-channel radiometer (SKR 116, Skye Instruments, Llandrindod Wells, UK), following the method of Sparkes et al. (2006). Measurements were made under sunny days, with the sensor facing north against the stem bases, which allowed the light reflected and transmitted from the neighbouring plants to reach the sensor.
Data of shoots per plant and R:FR from each plot were then fitted over the accumulated thermal time from sowing using a logistic function (Fig. 1) (Sparkes et al., 2006).
where S is the numbers of shoots per plant, R is R:FR, A is the lower asymptote, (A + C) is the upper asymptote, B is the doubled relative rate of tillering or R:FR reduction at time M, M is the accumulated thermal time when tillering rate or R:FR decline rate is at maximum and when shoot number or R:FR reaches (A + 0·5C), and t is the accumulated thermal time after sowing. The parameters used to describe the kinetics of tillering and R:FR are: total shoots per plant (A + C), fertile shoots per plant (counted at late grain filling), shoot survival (fertile shoots divided by total shoots, %), infertile shoots per plant (the difference between total and fertile shoots), shoot abortion (infertile shoots divided by total shoots, %), tillering onset (tto, when A + 0·1C is reached, tto = M – 2·1972/B), tillering cessation (ttc, when A + 0·9C is reached, ttc = M + 2·1972/B), tillering duration (ttd, ttd = ttc – tto), tillering rate (0·8C/ttd), the onset of R:FR reduction (tR:FRor, when A + 0·9C is reached tR:FRor = M + 2·1972/B), the end of R:FR reduction (tR:FRer, when A + 0·1C is reached tR:FRer = M – 2·1972/B) and stabilized R:FR (the lower asymptote A). In addition, R:FR at tillering onset and cessation were calculated.
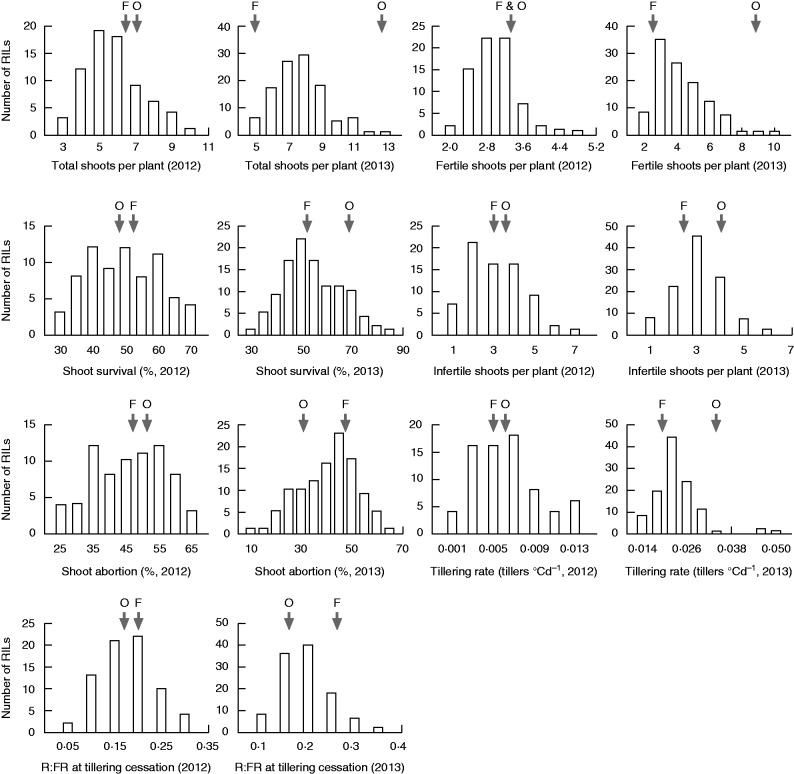
Fig. 1.
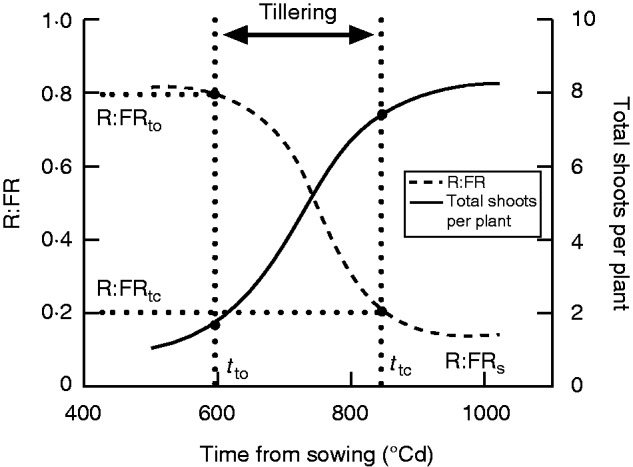
Dynamics of tillering and red/far-red ratio (R:FR) at the base of the canopy in the mapping population of ‘Forno’ and ‘Oberkulmer’. Data of shoot number per plant and R:FR from each plot were fitted over the accumulated thermal time from sowing using a logistic function. Definitions of the parameters: tto, the time at tillering onset; ttc, the time at tillering cessation; R:FRto, R:FR at tillering onset; R:FRtc, R:FR at tillering cessation; R:FRs, stabilized R:FR.
Another key event during tillering is the onset of stem elongation (Growth Stage 31, GS31) (Zadoks et al., 1974). Five plants in central rows from each plot were split to observe the first internodes every 4 d. A line was judged to enter this stage when three or more main shoots had the first internodes longer than 1 cm. R:FR at GS31 was then calculated. In 2013, 15 RILs were selected randomly; five plants from each plot were measured for R:FR, counted for shoot number, and split for initial stem length (removing leaf sheaths and spikes) on 9 May (around GS31).
Thinning was carried out in five RILs selected randomly in 2013. Plant density in these lines was reduced to 50 % by removing every other plant after emergence. Ten central plants in the thinned area in each plot were selected, and another ten plants without thinning taken as control. The dynamics of shoot number and R:FR of these plants were recorded for curve fitting, as described above.
Plant height, carpel size and stem water-soluble carbohydrate (WSC) content at anthesis were analysed in both seasons. For each plot of the subsets, five (in 2012) and ten (in 2013) shoots with the first anthers on spikes just visible were collected. Plant height was measured from the shoot bases to spike tips, excluding awns. Five spikes of each sample were used for carpel analysis. Two middle spikelets of each spike in 2012 and three spikelets (the third spikelets counted from the bases and tips, and the middle one between them on one side of a spike) in 2013 were dissected carefully. The carpels in the first three florets of a spikelet counting from the rachis were removed, oven-dried at 85 °C for 48 h, and weighed using an electronic balance (0·0001 g) (125A, Precisa, Dietikon, Switzerland). Average dry weight of individual carpels was then calculated. After removing leaves, all the stems (plus leaf sheaths) from the same shoots were collected, oven-dried immediately, weighed and finely ground. Stem carbohydrates were extracted (80 % ethanol and water), and WSCs were measured using the anthrone method, following the protocols of van Herwaarden et al. (1998), and Yemm and Willis (1954). Average dry weight of stem WSC per shoot was then calculated.
At maturity, five and 20 spikes from each plot in 2012 and 2013, respectively, were collected and threshed by a thresher and then by hand. The grains were oven-dried at 85 °C for 48 h and weighed, and yield per shoot was calculated. Then, approx. 200 grains were counted to calculate thousand grain weight (TGW) and grains per shoot. Yield and grains per plant were obtained by multiplying yield and grains per shoot by fertile shoot number, respectively.
Shading experiment in the glasshouse
A glasshouse experiment was conducted to test the effects of shade on tiller number and yield components in 2014. Green shade was achieved by using a green plastic filter (122 Fern Green; LEE Filters, Andover, UK) (Kegge et al., 2013). This green filter reduced photosynthetically active radiation (PAR, measured with a ceptometer: AccuPAR, Decagon Devices, Pullman, WA, USA) to 220 µmol m−2 s−1 and R:FR (SKR 116, Skye Instruments, Llandrindod Wells, UK) to 0·2, compared with the control using clear filters (PAR = 680 µmol m−2 s−1 and R:FR = 1·0). The filters were fixed on four sides and top of a wooden frame, but a 15-cm gap was left at the top of each side for ventilation. Daily temperature inside the frames during treatment was recorded using a data logger (Tinytag Ultra 2, Gemini Data Loggers, Chichester, UK), and the average temperature between shading and control was the same (15·3 °C). The seeds of the subset (110 RILs) were sown on 17 December 2013. The seedlings were vernalized at 6 °C for 9 weeks, and then transplanted into 1-litre pots (one plant per pot) filled with a loam-based compost (No. 3, John Innes, Norwich, UK). The RILs were arranged in a randomized complete block design with three replicates for both the shading and the control. Frames were put on the plants from 27 March (onset of tillering) to 2 May 2014. The plants were watered frequently, and individually fed with 40 kg N ha−1 at the beginning of stem elongation. At maturity, fertile shoots of each plant were counted, and all spikes were threshed. Total grains were oven-dried at 85 °C for 48 h, weighed and counted. Yield per plant, yield per shoot, grains per plant, grains per shoot and TGW were then calculated.
Statistical analysis of phenotypic data
Analysis of variance (ANOVA) was used to test the differences between RILs and between treated (thinned and shaded) and control plants. Pearson correlations and regression analysis were carried out to determine the phenotypic relationships between different traits. Data were transformed to improve their normality, if necessary. Statistical analyses, including curve fitting, were performed with Genstat v17 and GraphPad Prism v6.05.
QTL analysis
A genetic map of ‘Forno’ × ‘Oberkulmer’ is available in the GrainGenes database (http://wheat.pw.usda.gov/GG2/index.shtml). Linkage analysis was repeated with 182 polymorphic markers [restriction fragment length polymorphism (RFLP) and simple sequence repeat (SSR)] using the JoinMap v4 (Van Ooijen, 2006), resulting in the same genetic map, with slightly different total coverage. This map included 230 segregating loci and 23 linkage groups, covering 2469 cM (approx. two-thirds of the whole genome of bread wheat and spelt) with an average marker density of 13·6 cM (Messmer et al., 1999). QTL analysis was performed with the MapQTL v6 (Van Ooijen, 2009), using the mean values of quantitative traits over replicates in each year. Interval mapping was used to estimate the QTL locations, logarithm of the odds (LOD) scores, additive effects and phenotypic variation explained by individual QTL (R2). A genome-wide significance threshold (P < 0·05) was computed by the permutation test with 1000 iterations. Co-factors, which were the markers nearest to QTL peaks, were selected, tested for significance (P < 0·02), and used for the multiple-QTL model (MQM) mapping. QTL symbols were designed according to the Catalogue of Gene Symbols for Wheat (http://wheat.pw.usda.gov/GG2/Triticum/wgc/2008/). Locations of the significant QTL were presented using the 1-LOD support intervals (fall-off from the QTL maximum LOD peaks), and drawn using MapChart v2.2 (Voorrips, 2002). For each QTL, the allele increasing the quantitative trait value was defined as increasing allele, and the other one as decreasing allele. Those parents conferring increasing or decreasing alleles were analysed.
RESULTS
Phenotypic variation in tillering dynamics in the ‘Forno’ × ‘Oberkulmer’ mapping population
Tillering traits, including total shoots per plant, fertile and infertile shoots per plant, shoot survival and abortion, and tillering rate, were similar between the bread wheat ‘Forno’ and spelt ‘Oberkulmer’ in the field in 2012 (Fig. 2). However, ‘Oberkulmer’ had many more total, fertile and infertile shoots per plant, higher shoot survival and tillering rate but lower shoot abortion than ‘Forno’ in 2013. In the glasshouse experiment, fertile shoots per plant of ‘Oberkulmer’ (5.3 shoots) was similar to that of ‘Forno’ (5.0 shoots) under control conditions, but ‘Oberkulmer’ had 4·7 fertile shoots per plant under shading treatment, compared with 3·3 for ‘Forno’. These indicate that the spelt can produce equal numbers of or more shoots than the bread wheat, depending on growth environments. Large genetic variation in all tillering traits was found in the RILs in each year (Fig. 2). In addition to genotype, year also affected tillering patterns: total shoots per plant (+38 %), fertile shoots per plant (+60 %), shoot survival (+9 %), infertile shoots per plant (+16 %) and tillering rate (+316 %) were higher in 2013 than in 2012 (P < 0·01). The differences in tillering traits for the parents and RILs between years could result from the colder weather from sowing to March in 2013 (–2·6 °C for mean daily temperature) than in 2012, leading to delayed tillering and other consequent effects on the remaining traits, as demonstrated below. ‘Oberkulmer’ was more responsive to the growing years than ‘Forno’ in terms of tillering. Averaged across years, shoot survival was only 55 % over all the genotypes in the field.
Fig. 2.
Distributions of the recombinant inbred line (RIL) values for tillering and red/far-red ratio (R:FR) at tillering cessation. Parental values are indicated by arrows: F, ‘Forno’; O, ‘Oberkulmer’. A significant difference in each trait among the RILs was found (P < 0·01).
Phenotypic correlations between tillering traits
Total shoots per plant was largely dependent on tillering rate rather than its duration (Table 1). There was no (in 2012) or weak (in 2013) negative relationship between fertile and infertile shoot number, indicating large independence. Both traits were positively associated with tillering rate. In addition, delayed onset and cessation of tillering appeared to be associated with more fertile shoots and higher shoot survival, and with fewer infertile shoots and lower shoot abortion. Tillering onset showed a positive relationship with tillering rate, but a negative one with tillering duration, suggesting that the later tillering onset, the faster tillering rate and the shorter tillering duration.
Table 1.
Correlations between tillering traits in the mapping population of ‘Forno’ and ‘Oberkulmer’
| Tillering trait† | Total shoots per plant | Fertile shoots per plant | Shoot survival | Infertile shoots per plant | Shoot abortion | Tillering rate | Tillering onset | Tillering cessation | Tillering duration |
|---|---|---|---|---|---|---|---|---|---|
| Total shoots per plant | 1 | 0·46** | −0·76** | 0·94** | 0·76** | 0·90** | 0·23* | −0·02 | −0·21 |
| Fertile shoots per plant | 0·80** | 1 | 0·19 | 0·14 | −0·19 | 0·33** | 0·36** | 0·24* | −0·12 |
| Shoot survival | 0·31** | 0·80** | 1 | −0·92** | −1·00** | −0·76** | 0·00 | 0·21 | 0·16 |
| Infertile shoots per plant | 0·35** | −0·28** | −0·77** | 1 | 0·92** | 0·88** | 0·12 | −0·12 | −0·19 |
| Shoot abortion | −0·31** | −0·80** | −1·00** | 0·77** | 1 | 0·76** | 0·00 | −0·21 | −0·16 |
| Tillering rate | 0·64** | 0·50** | 0·15 | 0·25** | −0·15 | 1 | 0·29* | −0·23* | −0·43** |
| Tillering onset | 0·22* | 0·40** | 0·43** | −0·27** | −0·43** | 0·43** | 1 | 0·26* | −0·64** |
| Tillering cessation | 0·48** | 0·66** | 0·61** | −0·26** | −0·61** | 0·01 | 0·25** | 1 | 0·57** |
| Tillering duration | 0·26** | 0·28** | 0·21* | −0·02 | −0·21* | −0·31** | −0·54** | 0·69** | 1 |
* Significant at P < 0·05, ** significant at P < 0·01.
† Top right matrix: 2012 season; bottom left matrix: 2013 season.
Identification of the QTL associated with tillering traits
A total of 34 QTL were identified for the tillering traits in the ‘Forno’ × ‘Oberkulmer’ mapping population, including one QTL for total shoots per plant, six for fertile shoots per plant, two for infertile shoots per plant, five for each of shoot survival and abortion, one for tillering rate, ten for tillering onset (containing nine for initial shoots per plant, which were recorded from the second tiller count at the beginning of tillering and used to measure tillering progress) and four for tillering cessation (Fig. 3 and Table 2). These QTL were scattered on ten chromosomes (1A, 2D, 3A, 3B, 4A, 4B, 5A, 5B, 7AL and 7B), and most of them (76 %) were located in the A genome. Phenotypic variation explained by individual QTL varied, ranging from 6·3 to 22·6 %.
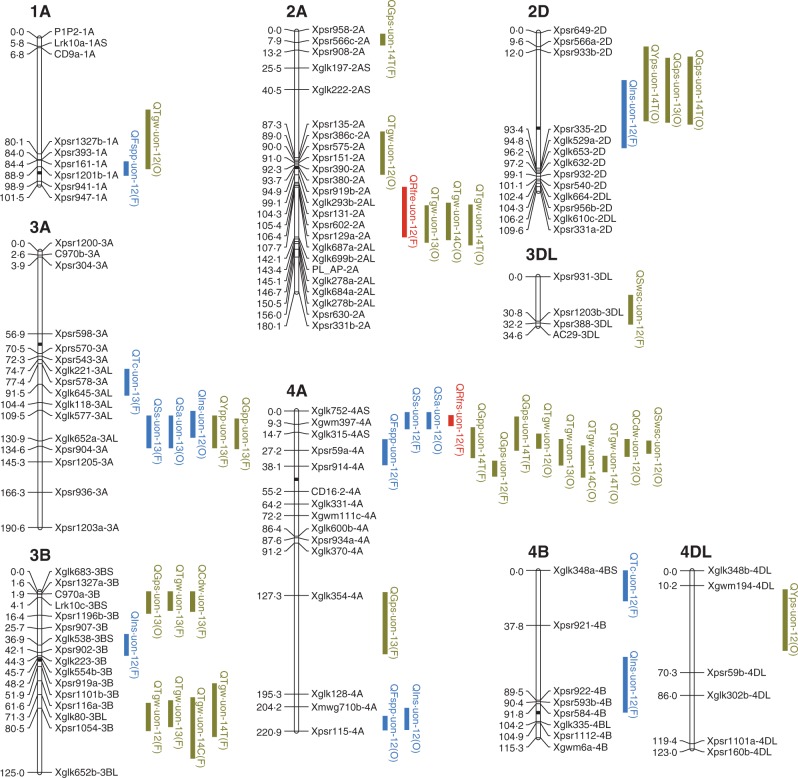
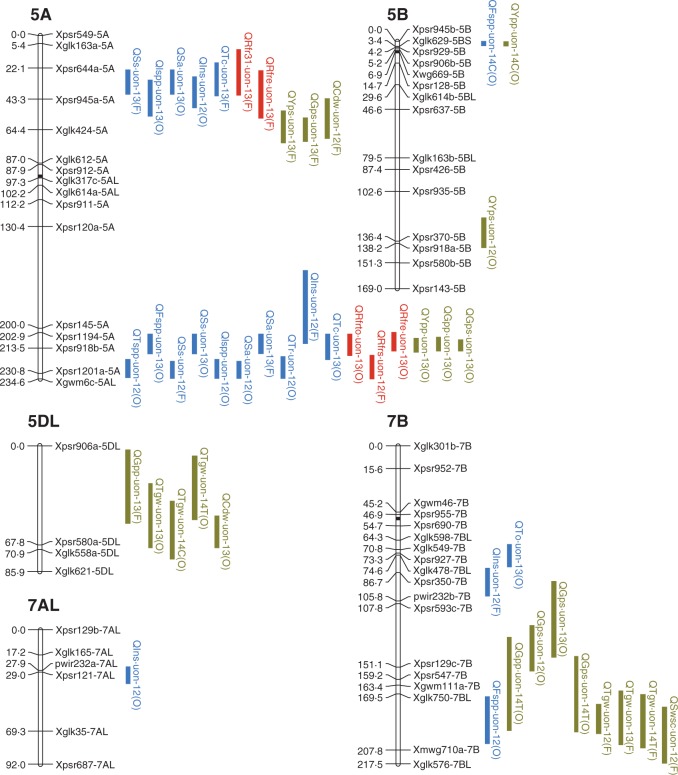
Fig. 3.
Quantitative trait loci (QTL) for tillering, red/far-red ratio (R:FR) and yield components in the mapping population of ‘Forno’ and ‘Oberkulmer’. The 1-LOD support intervals of significant QTL are indicated by blue (tillering), red (R:FR) and green (yield components) vertical bars. For QTL symbols, the ‘Q’ is followed by the abbreviated names of quantitative traits and laboratory (uon). Abbreviations for traits: Tspp, total shoots per plant; Fspp, fertile shoots per plant; Ss, shoot survival (%); Ispp, infertile shoots per plant; Sa, shoot abortion (%); Tr, tillering rate; Ins, initial shoots per plant; To, the time at tillering onset; Tc, the time at tillering cessation; Rfrto, R:FR at tillering onset; Rfr31, R:FR at GS31 (onset of stem elongation); Rfrs, stabilized R:FR; Rfre, the time at the end of R:FR reduction; Ypp, yield per plant; Yps, yield per shoot; Gpp, grains per plant; Gps, grains per shoot; Tgw, thousand grain weight; Cdw, carpel dry weight at anthesis; Swsc, stem water-soluble carbohydrate dry weight at anthesis. The QTL found in 2012 (field), 2013 (field), 2014 (glasshouse, control) and 2014 (glasshouse, shading treatment) are indicated by the suffixes 12, 13, 14C and 14T, respectively. The parental lines providing the alleles increasing trait values are given in parentheses: F, ‘Forno’; O, ‘Oberkulmer’.
Table 2.
Quantitative trait loci (QTL) for tillering, red/far-red ratio (R:FR) and yield components in the ‘Forno’ × ‘Oberkulmer’ mapping population
| Trait/chromosome | Year* | Position (cM) | LOD | R2† | Additive effect‡ | Closest marker |
|---|---|---|---|---|---|---|
| Tillering | ||||||
| Total shoots per plant | ||||||
| 5A | 2012 | 229·5 | 3·68 | 21·5 | −0·7 | Xpsr1201a-5A |
| Fertile shoots per plant | ||||||
| 1A | 2012 | 88·9 | 3·28 | 19·4 | 0·2 | Xpsr1201b-1A |
| 4A | 2012 | 30·2 | 3·13 | 18·6 | 0·2 | Xpsr59a-4A |
| 2012 | 219·2 | 3·56 | 20·9 | −0·2 | Xpsr115-4A | |
| 5A | 2013 | 209·9 | 5·85 | 21·7 | −0·8 | Xpsr918b-5A |
| 5B | 2014C | 0·1 | 3·42 | 13·3 | −0·4 | Xpsr945b-5B |
| 7B | 2012 | 188·5 | 3·45 | 20·3 | −0·2 | Xmwg710a-7B |
| Shoot survival (%) | ||||||
| 3A | 2013 | 124·5 | 3·28 | 12·8 | 5·1 | Xglk652a-3AL |
| 4A | 2012 | 8·0 | 3·33 | 19·7 | 5·2 | Xgwm397-4A |
| 5A | 2012 | 230·8 | 2·99 | 17·9 | 4·6 | Xpsr1201a-5A |
| 2013 | 32·1 | 5·29 | 19·9 | 6·3 | Xpsr644a-5A | |
| 2013 | 209·9 | 6·12 | 22·6 | −6·4 | Xpsr918b-5A | |
| Infertile shoots per plant | ||||||
| 5A | 2012 | 231·8 | 3·62 | 21·2 | −0·6 | Xpsr1201a-5A |
| 2013 | 37·1 | 5·49 | 20·5 | −0·5 | Xpsr945a-5A | |
| Shoot abortion (%) | ||||||
| 3A | 2013 | 124·5 | 3·28 | 12·8 | −5·1 | Xglk652a-3AL |
| 4A | 2012 | 8·0 | 3·33 | 19·7 | −5·2 | Xgwm397-4A |
| 5A | 2012 | 230·8 | 2·99 | 17·9 | −4·6 | Xpsr1201a-5A |
| 2013 | 32·1 | 5·29 | 19·9 | −6·3 | Xpsr644a-5A | |
| 2013 | 209·9 | 6·12 | 22·6 | 6·4 | Xpsr918b-5A | |
| Tillering rate (tillers °Cd−1) | ||||||
| 5A | 2012 | 228·5 | 3·28 | 19·4 | −0·0016 | Xpsr1201a-5A |
| Initial shoots per plant | ||||||
| 2D | 2012 | 55·9 | 3·53 | 7·8 | 0·3 | Xpsr335-2D |
| 3A | 2012 | 119·5 | 4·78 | 10·4 | −0·2 | Xglk577-3AL |
| 3B | 2012 | 38·9 | 3·51 | 7·8 | 0·1 | Xglk538-3BS |
| 4A | 2012 | 213·2 | 4·24 | 9·3 | −0·2 | Xpsr115-4A |
| 4B | 2012 | 91·8 | 3·54 | 7·8 | 0·1 | Xpsr584-4B |
| 5A | 2012 | 35·1 | 5·88 | 12·7 | −0·2 | Xpsr945a-5A |
| 2012 | 205·9 | 4·59 | 10·0 | 0·2 | Xpsr1194-5A | |
| 7AL | 2012 | 27·9 | 3·18 | 7·1 | −0·1 | pwir232a-7AL |
| 7B | 2012 | 91·7 | 2·84 | 6·3 | 0·1 | Xpsr350-7B |
| Time at tillering onset (°Cd) | ||||||
| 7B | 2013 | 75·6 | 3·24 | 12·7 | −12 | Xglk478-7BL |
| Time at tillering cessation (°Cd) | ||||||
| 3A | 2013 | 89·4 | 3·29 | 12·9 | 14 | Xglk645-3AL |
| 4B | 2012 | 8·0 | 3·10 | 18·4 | 72 | Xglk348a-4BS |
| 5A | 2013 | 30·1 | 3·35 | 13·1 | 15 | Xpsr644a-5A |
| 2013 | 210·9 | 3·67 | 14·2 | −15 | Xpsr918b-5A | |
| R:FR | ||||||
| R:FR at tillering onset | ||||||
| 5A | 2013 | 211·9 | 3·37 | 13·2 | −0·02 | Xpsr918b-5A |
| R:FR at GS31 (onset of stem elongation) | ||||||
| 5A | 2013 | 31·1 | 3·59 | 13·9 | 0·02 | Xpsr644a-5A |
| Stabilized R:FR | ||||||
| 4A | 2012 | 9·3 | 3·13 | 18·6 | 0·01 | Xgwm397-4A |
| 5A | 2012 | 225·5 | 4·46 | 25·5 | 0·02 | Xpsr1201a-5A |
| Time at the end of R:FR reduction (°Cd) | ||||||
| 2A | 2012 | 125·7 | 2·59 | 15·7 | 91 | Xglk699b-2AL |
| 5A | 2013 | 35·1 | 3·45 | 13·5 | 15 | Xpsr945a-5A |
| 2013 | 208·9 | 4·73 | 17·9 | −16 | Xpsr918b-5A | |
| Yield components | ||||||
| Yield per plant (g) | ||||||
| 3A | 2013 | 124·5 | 3·26 | 12·8 | 1·37 | Xglk652a-3AL |
| 5A | 2013 | 211·9 | 6·94 | 25·2 | −1·76 | Xpsr918b-5A |
| 5B | 2014C | 1·0 | 3·15 | 12·3 | −0·80 | Xpsr945b-5B |
| Yield per shoot (g) | ||||||
| 2D | 2014T | 40·9 | 3·60 | 14·0 | −0·22 | Xpsr933b-2D |
| 4DL | 2012 | 37·2 | 3·15 | 18·7 | −0·15 | Xgwm194-4DL |
| 5A | 2013 | 63·3 | 3·38 | 13·2 | 0·10 | Xglk424-5A |
| 5B | 2012 | 136·4 | 3·30 | 19·5 | −0·07 | Xpsr370-5B |
| Grains per plant | ||||||
| 3A | 2013 | 125·5 | 3·08 | 12·1 | 32 | Xglk652a-3AL |
| 4A | 2014T | 21·7 | 4·21 | 16·1 | 13 | Xpsr59a-4A |
| 5A | 2013 | 209·9 | 7·74 | 27·7 | −46 | Xpsr918b-5A |
| 5DL | 2013 | 31·0 | 3·68 | 14·3 | 60 | Xpsr906a-5DL |
| 7B | 2014T | 156·1 | 4·24 | 16·3 | −12 | Xpsr547-7B |
| Grains per shoot | ||||||
| 2A | 2014T | 7·0 | 3·08 | 12·1 | 2 | Xpsr566c-2A |
| 2D | 2013 | 45·9 | 4·69 | 17·8 | −6 | Xpsr933b-2D |
| 2014T | 44·9 | 4·23 | 16·2 | −5 | Xpsr933b-2D | |
| 3B | 2013 | 8·1 | 3·72 | 14·4 | −3 | Lrk10c-3BS |
| 4A | 2012 | 38·1 | 3·49 | 20·5 | 2 | Xpsr914-4A |
| 2013 | 152·3 | 3·61 | 14·0 | 6 | Xglk354-4A | |
| 2014T | 10·3 | 2·93 | 11·5 | 2 | Xgwm397-4A | |
| 5A | 2013 | 64·4 | 4·53 | 17·3 | 3 | Xglk424–5A |
| 2013 | 213·5 | 5·10 | 19·2 | −3 | Xpsr918b-5A | |
| 7B | 2012 | 138·8 | 3·15 | 18·7 | −3 | Xpsr129c-7B |
| 2013 | 128·8 | 3·09 | 12·1 | −3 | Xpsr593c-7B | |
| 2014T | 165·4 | 3·85 | 14·9 | −2 | Xgwm111a-7B | |
| Thousand grain weight (g) | ||||||
| 1A | 2012 | 80·1 | 3·57 | 20·9 | −2·55 | Xpsr1327b-1A |
| 2A | 2012 | 94·9 | 3·47 | 20·4 | −2·50 | Xpsr919b-2A |
| 2013 | 133·7 | 3·54 | 13·8 | −2·00 | Xglk699b-2AL | |
| 2014C | 133·7 | 2·98 | 11·7 | −2·01 | Xglk699b-2AL | |
| 2014T | 143·4 | 3·52 | 13·7 | −1·60 | PL_AP-2A | |
| 3B | 2012 | 80·5 | 4·73 | 26·8 | 2·97 | Xpsr1054-3B |
| 2013 | 2·9 | 3·44 | 13·4 | 1·60 | C970a-3B | |
| 2013 | 80·5 | 5·11 | 19·3 | 1·91 | Xpsr1054-3B | |
| 2014C | 100·5 | 3·39 | 13·2 | 2·90 | Xpsr1054-3B | |
| 2014T | 78·3 | 3·02 | 11·9 | 1·62 | Xpsr1054-3B | |
| 4A | 2012 | 20·7 | 7·17 | 37·6 | −3·95 | Xglk315-4AS |
| 2013 | 31·2 | 4·76 | 18·1 | −2·09 | Xpsr59a-4A | |
| 2014C | 32·2 | 4·10 | 15·8 | −2·13 | Xpsr59a-4A | |
| 2014T | 34·2 | 7·93 | 28·2 | −2·61 | Xpsr914-4A | |
| 5DL | 2013 | 45·0 | 3·88 | 15·0 | −3·17 | Xpsr580a-5DL |
| 2014C | 58·0 | 5·54 | 20·7 | −3·05 | Xpsr580a-5DL | |
| 2014T | 32·0 | 4·17 | 16·0 | −3·62 | Xpsr906a-5DL | |
| 7B | 2012 | 187·5 | 5·97 | 32·5 | 4·64 | Xglk750-7BL |
| 2013 | 187·5 | 3·24 | 12·7 | 2·24 | Xglk750-7BL | |
| 2014T | 189·5 | 3·15 | 12·4 | 2·27 | Xmwg710a-7B | |
| Carpel dry weight at anthesis (mg) | ||||||
| 3B | 2013 | 0·1 | 4·37 | 16·7 | 0·03 | Xglk683-3BS |
| 4A | 2012 | 27·2 | 3·46 | 20·4 | −0·06 | Xpsr59a-4A |
| 5A | 2012 | 56·3 | 3·78 | 22·0 | 0·07 | Xglk424-5A |
| 5DL | 2013 | 67·8 | 4·29 | 16·4 | −0·03 | Xpsr580a-5DL |
| Stem water soluble carbohydrate dry weight at anthesis (g) | ||||||
| 3DL | 2012 | 23·0 | 3·49 | 20·5 | 0·069 | Xpsr1203b-3DL |
| 4A | 2012 | 27·2 | 4·88 | 27·5 | −0·069 | Xpsr59a-4A |
| 7B | 2012 | 192·5 | 3·51 | 20·6 | 0·080 | Xmwg710a-7B |
* 2012 and 2013: field experiments; 2014: glasshouse experiment (C, control; T, shading treatment).
† The proportion of phenotypic variation explained by individual QTL.
‡ Positive additive effects indicate that the alleles from ‘Forno’ increase the values of the traits, whereas negative additive effects indicate that the alleles from ‘Oberkulmer’ increase the values of the traits.
The QTL coincidences between tillering traits were mainly found on chromosomes 3A, 4A and 5A (Fig. 3). For the QTL cluster on 3A, the alleles from the bread wheat ‘Forno’ delayed tillering onset and cessation, and increased shoot survival. There were two regions of QTL coincidences on 4A: one was located on 4AS, where ‘Forno’ conferred the alleles increasing fertile shoot number and shoot survival; the other was located on the distal region of 4AL, where ‘Oberkulmer’ conferred the alleles increasing initial and fertile shoot number. Likewise, there were also two regions of QTL coincidences on 5A: one was located on 5AS, where the alleles from ‘Forno’ delayed tillering onset and cessation, increased shoot survival, and decreased infertile shoots; the other was located on 5AL, where the alleles from ‘Oberkulmer’ delayed tillering onset and cessation, accelerated tillering rate, and increased total, fertile and infertile shoot number. However, one increasing and one decreasing allele for shoot survival were also identified from ‘Oberkulmer’ in this region; in other words, there were two closely linked alleles with the opposite effects on shoot survival, and their expressions depended on year.
Tillering dynamics as related to low R:FR
R:FR at the base of the canopy in the field showed relationships with tillering dynamics (Table 3). R:FR at tillering onset was positively associated with total and fertile shoot number, and shoot survival in 2013. Higher R:FR at that time was also associated with delayed tillering cessation across years. R:FR at tillering cessation and GS31, and stabilized R:FR, showed negative relationships with infertile shoots per plant and shoot abortion, indicating that low R:FR established after tiller initiation promotes tiller death. R:FR at tillering cessation differed between the RILs, indicating the different responses of genotypes to low R:FR (Fig. 2). R:FR at tillering cessation was slightly higher in 2013 (0·21) than in 2012 (0·19) (P < 0·05).
Table 3.
Correlations between tillering traits and red/far-red ratio (R:FR) in the mapping population of ‘Forno’ and ‘Oberkulmer’
| Tillering trait | R:FRto |
R:FRtc |
R:FR31 |
R:FRs |
R:FRor |
R:FRer |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | |
| Total shoots per plant | 0·12 | 0·33** | −0·55** | −0·34** | −0·59** | 0·11 | −0·59** | 0·08 | −0·22 | 0·03 | 0·06 | 0·31** |
| Fertile shoots per plant | −0·04 | 0·43** | −0·30** | −0·22* | −0·11 | 0·45** | −0·24* | 0·30** | −0·12 | −0·01 | 0·15 | 0·60** |
| Shoot survival | 0·08 | 0·38** | 0·40** | −0·05 | 0·59** | 0·62** | 0·50** | 0·41** | 0·16 | −0·04 | 0·04 | 0·66** |
| Infertile shoots per plant | −0·10 | −0·14 | −0·50** | −0·21* | −0·62** | −0·52** | −0·56** | −0·33** | −0·19 | 0·06 | 0·01 | −0·45** |
| Shoot abortion | −0·08 | −0·38** | −0·40** | 0·05 | −0·59** | −0·62** | −0·50** | −0·41** | −0·16 | 0·04 | −0·04 | −0·66** |
| Tillering rate | −0·13 | −0·18 | −0·47** | 0·12 | −0·57** | 0·03 | −0·51** | 0·06 | −0·17 | −0·03 | 0·03 | 0·18 |
| Tillering onset | 0·33** | 0·10 | −0·33** | 0·02 | 0·18 | 0·25** | 0·01 | 0·29** | 0·09 | 0·04 | 0·21 | 0·37** |
| Tillering cessation | 0·34** | 0·44** | −0·30** | −0·61** | 0·32** | 0·49** | −0·04 | 0·30** | 0·20 | 0·10 | 0·18 | 0·58** |
| Tillering duration | 0·15 | 0·31** | −0·49** | −0·55** | 0·10 | 0·24* | −0·04 | 0·04 | 0·08 | 0·06 | −0·03 | 0·23* |
R:FRto, R:FR at tillering onset; R:FRtc, R:FR at tillering cessation; R:FR31, R:FR at GS31 (onset of stem elongation); R:FRs, stabilized R:FR; R:FRor, the time at the onset of R:FR reduction; R:FRer, the time at the end of R:FR reduction.
* Significant at P < 0·05, ** significant at P < 0·01.
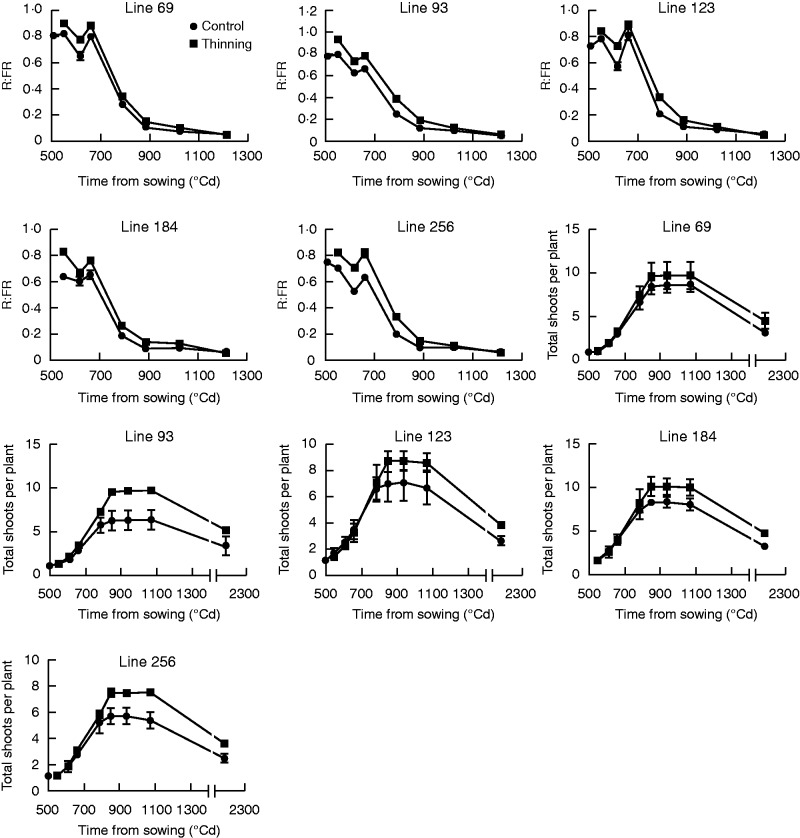
As expected, thinning across the five RILs selected randomly raised R:FR at tillering onset (+17 %), leading to more total (+31 %) and fertile (+47 %) shoots per plant, higher shoot survival (+12 %) and lower shoot abortion (–8 %) (Fig. 4 and Table 4). A detailed analysis showed that thinning did not change the onset and rate of tillering, but delayed tillering cessation. These results are consistent with the above observations. There was no difference between thinned and control lines in R:FR at either tillering cessation or GS31, as well as stabilized R:FR.
Fig. 4.
Dynamics of tillering and red/far-red ratio (R:FR) in the control (circles) and thinned (squares) lines. Values of shoot number per plant and R:FR at each time point are shown as mean ± standard error of the mean (bars). The last count, representing the fertile shoot number per plant, was taken at late grain filling.
Table 4.
Thinning effects on tillering and red/far-red ratio (R:FR)
| Trait | Mean across five lines (n = 3) |
P-value (n.s., not significant; *P < 0·05; **P < 0·01) |
Thinning effect (%) | |||
|---|---|---|---|---|---|---|
| Control | Thinning | Treatment | Line | Treatment × line | ||
| Total shoots per plant | 7·2 | 9·4 | ** | n.s. | n.s. | + 31 |
| Fertile shoots per plant | 3·0 | 4·4 | ** | n.s. | n.s. | + 47 |
| Shoot survival (%) | 42·2 | 47·1 | * | * | n.s. | + 12 |
| Infertile shoots per plant | 4·2 | 5·0 | n.s. | * | n.s. | n.s. |
| Shoot abortion (%) | 57·8 | 52·9 | * | * | n.s. | –8 |
| Tillering rate (tillers °Cd−1) | 0·024 | 0·027 | n.s. | n.s. | n.s. | n.s. |
| Tillering onset (°Cd) | 580 | 590 | n.s. | n.s. | n.s. | n.s. |
| Tillering cessation (°Cd) | 789 | 838 | ** | n.s. | n.s. | +6 |
| Tillering duration (°Cd) | 210 | 248 | n.s. | n.s. | n.s. | n.s. |
| R:FR at tillering onset | 0·71 | 0·83 | ** | * | n.s. | +17 |
| R:FR at tillering cessation | 0·25 | 0·20 | n.s. | n.s. | n.s. | n.s. |
| Onset of stem elongation (°Cd, GS31) | 882 | 930 | ** | ** | n.s. | +5 |
| R:FR at GS31 | 0·10 | 0·12 | n.s. | n.s. | n.s. | n.s. |
| End of R:FR reduction | 832 | 854 | ** | ** | * | +3 |
| Stabilized R:FR | 0·08 | 0·09 | n.s. | n.s. | n.s. | n.s. |
R:FR around GS31 was measured in the 15 RILs on a given day in 2013, and showed a positive relationship with fertile shoots per plant (Fig. 5). In addition, shading in the glasshouse also reduced fertile shoots per plant by 12 % (Table 5).
Fig. 5.
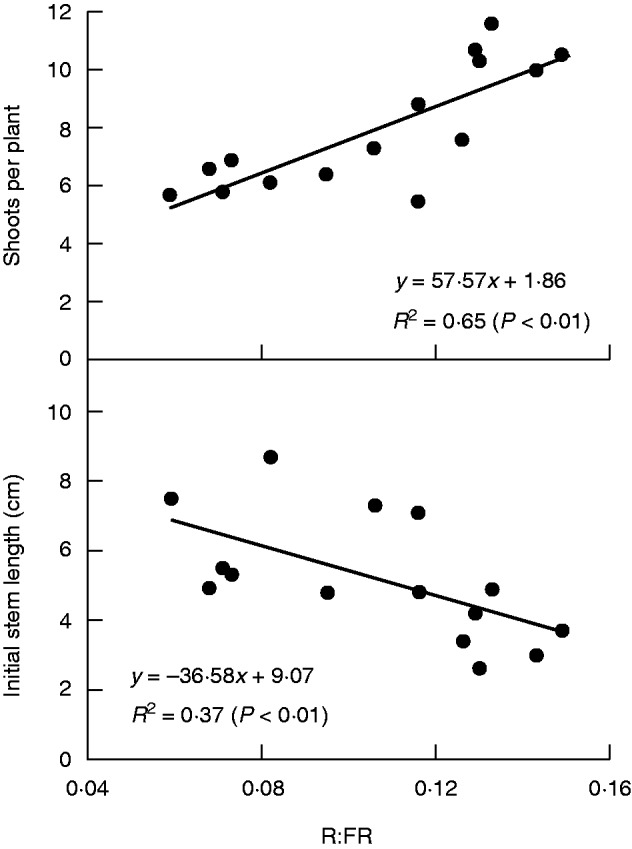
Relationships between red/far-red ratio (R:FR), shoots per plant and initial stem length around the onset of stem elongation.
Table 5.
Shading effects on fertile shoot number and other yield components
| Trait | Mean across 112 lines (n = 3) |
P-value (n.s., not significant; *P < 0·05; **P < 0·01) |
Shading effect (%) | |||
|---|---|---|---|---|---|---|
| Control | Shading | Treatment | Line | Treatment × line | ||
| Fertile shoots per plant | 5·1 | 4·5 | ** | ** | n.s. | –12 |
| Yield per plant (g) | 7·60 | 5·23 | ** | ** | n.s. | –31 |
| Yield per shoot (g) | 1·47 | 1·21 | ** | ** | n.s. | –18 |
| Grains per plant | 162 | 111 | ** | ** | n.s. | –31 |
| Grains per shoot | 31 | 25 | ** | ** | n.s. | –19 |
| Thousand grain weight (g) | 47·4 | 47·8 | n.s. | ** | n.s. | n.s. |
Genetic analysis revealed a total of seven QTL for R:FR, including one QTL for each of R:FR at tillering onset and GS31 on chromosome 5A, two for stabilized R:FR on 4A and 5A, and three for the timing of R:FR reduction on 2A and 5A (Fig. 3 and Table 2). A QTL for stabilized R:FR was coincident with those for tillering traits on 4A; the increasing alleles from ‘Forno’ raised stabilized R:FR, fertile shoot number and shoot survival. In addition, the QTL coincidences between R:FR and tillering occurred on chromosome 5A. ‘Forno’ provided the alleles on 5AS increasing R:FR at GS31, delaying tillering onset and cessation, increasing shoot survival, and decreasing infertile shoot number and shoot abortion. In contrast, ‘Oberkulmer’ provided the alleles on 5AL increasing R:FR at tillering onset, delaying tillering onset and cessation, and increasing total and fertile shoots per plant, as well as shoot survival. A QTL for stabilized R:FR was also coincident with the other QTL for shoot survival in this region, with the increasing alleles from ‘Forno’. These results support the above phenotypic relationships between R:FR and tillering.
Responses of the onset of stem elongation and plant height to low R:FR
There were positive relationships between the R:FR just before GS31 and the accumulated thermal time for GS31 (r = 0·40, P < 0·01 in 2012; r = 0·33, P < 0·01 in 2013), indicating that the lower R:FR, the earlier onset of stem elongation. Consistent with this, R:FR around GS31 was negatively associated with the initial stem length at the same time (Fig. 5). In addition, R:FR was increased by thinning, resulting in a delay of the onset of stem elongation (Table 4).
Plant height at anthesis was negatively associated with R:FR at tillering cessation (r = –0·28, P < 0·05 in 2012; r = –0·20, P < 0·05 in 2013), and with stabilized R:FR in 2012 (r = –0·31, P < 0·01).
Synchrony among tillering cessation, R:FR stabilization and the onset of stem elongation
Tillering ceased at 1196 and 844 °Cd after sowing over all RILs in 2012 and 2013, respectively, coincident with R:FR stabilization (1273 °Cd in 2012 and 862 °Cd in 2013) and GS31 (1214 °Cd in 2012 and 905 °Cd in 2013). This was also found in the thinning experiment, including both control and treatment (Table 4). The onset of stem elongation was slightly later than tillering cessation and R:FR stabilization. However, taking account of the measurement of GS31 (first internodes > 1 cm), the exact beginning of stem elongation might coincide with the other two events.
Relationships between tillering and yield components
Total shoots per plant contributed to yield and grain number per plant, but did not affect yield or grain number per shoot, or TGW (Table 6). Similarly, fertile shoots per plant and shoot survival in the field in 2012 and 2013, and fertile shoots per plant in the glasshouse in 2014 (both control and shading), were closely and positively associated with yield and grains per plant. More fertile shoots and higher shoot survival did not reduce yield per shoot, and even showed associations with slightly increased grains per shoot, despite an accompanying slight decline in TGW. One exception was the fertile shoots per plant in the shading treatment, where more fertile shoots were associated with lower yield per shoot, which resulted mainly from reduced grains per shoot (Tables 5 and 6).
Table 6.
Correlations between tillering traits and yield components in the mapping population of ‘Forno’ and ‘Oberkulmer’
| (A) Field | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tillering trait | Yield per plant | Yield per shoot | Grains per plant | Grains per shoot | Thousand grain weight | |||||
| 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | |
| Total shoots per plant | 0·31** | 0·69** | −0·07 | −0·12 | 0·26* | 0·67** | −0·11 | −0·01 | 0·01 | −0·16 |
| Fertile shoots per plant | 0·70** | 0·94** | −0·07 | 0·04 | 0·80** | 0·93** | 0·20 | 0·20* | −0·28* | −0·26** |
| Shoot survival | 0·15 | 0·81** | 0·04 | 0·17 | 0·27* | 0·82** | 0·26* | 0·33** | −0·20 | −0·27** |
| Infertile shoots per plant | 0·09 | −0·36** | −0·05 | −0·25** | −0·01 | −0·39** | −0·20 | −0·33** | 0·12 | 0·15 |
| Shoot abortion | −0·15 | −0·81** | −0·04 | −0·17 | −0·27* | −0·82** | −0·26* | −0·33** | 0·20 | 0·27** |
| Tillering rate | 0·25* | 0·44** | −0·03 | −0·06 | 0·20 | 0·41** | −0·07 | −0·02 | 0·01 | −0·05 |
| Tillering onset | 0·47** | 0·37** | 0·24* | −0·04 | 0·41** | 0·40** | 0·23* | 0·12 | 0·05 | −0·24** |
| Tillering cessation | 0·31** | 0·64** | 0·19 | 0·08 | 0·21 | 0·64** | 0·07 | 0·19* | 0·16 | −0·20* |
| Tillering duration | −0·15 | 0·28** | −0·05 | 0·10 | −0·19 | 0·25** | −0·14 | 0·08 | 0·08 | 0·00 |
|
(B) Glasshouse (2014) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Shading | Control | Shading | Control | Shading | Control | Shading | Control | Shading | |
| Fertile shoots per plant | 0·71** | 0·54** | 0·11 | −0·46** | 0·76** | 0·53** | 0·22* | −0·40** | −0·16 | −0·12 |
* Significant at P < 0·05, ** significant at P < 0·01.
To understand how more fertile shoots reduced TGW, carpel size and stem WSC content at anthesis were analysed (Table 7). Both carpel size and stem WSC content were positively associated with TGW, confirming their roles in determining grain weight. Furthermore, they showed negative relationships with fertile shoots per plant, so more fertile shoots tended to produce smaller carpels and less stem WSC per shoot, and in turn smaller grains.
Table 7.
Correlations between carpel size and stem water soluble carbohydrates (WSC) at anthesis, thousand grain weight and fertile shoots per plant at maturity in the mapping population of ‘Forno’ and ‘Oberkulmer’
| Trait |
Thousand grain weight |
Fertile shoots per plant |
||
|---|---|---|---|---|
| 2012 | 2013 | 2012 | 2013 | |
| Carpel dry weight | 0·46** | 0·34** | −0·31** | −0·19* |
| Stem WSC | 0·55** | 0·20* | −0·52** | −0·16 |
* Significant at P < 0·05, ** significant at P < 0·01.
A total of 44 QTL for yield components were identified in the field and glasshouse experiments, including three QTL for yield per plant, four for yield per shoot, five for grains per plant, 12 for grains per shoot and 20 for TGW (Fig. 3 and Table 2). These QTL were scattered on 11 chromosomes (1A, 2A, 2D, 3A, 3B, 4A, 4DL, 5A, 5B, 5DL and 7B), individually explaining 11·5–37·6 % of the phenotypic variation. The QTL for grains per shoot on 2D, 4A and 7B were stable across 2–3 environments, while those for TGW on 2A, 3B, 4A, 5DL and 7B were stable across 3–4 environments. In the glasshouse experiment, one QTL for yield per shoot, two for grains per plant, four for grains per shoot and one for TGW were identified only under the shading treatment, indicating that they may be involved in shade responses. In terms of carpel size and stem WSC content at anthesis, four and three QTL were detected, respectively, individually explaining 16·4–27·5 % of the phenotypic variation (Fig. 3 and Table 2).
The QTL coincidences between tillering traits and yield components were found on seven chromosomes (1A, 2D, 3A, 4A, 5A, 5B and 7B) (Fig. 3). One QTL for total shoots per plant was coincident with one for each of yield and grains per plant as well as grains per shoot on 5A, with their increasing alleles conferred by ‘Oberkulmer’. Likewise, eight QTL for fertile shoots per plant and shoot survival were coincident with those for yield and grains per plant, and grains per shoot on 3A, 4A, 5A, 5B and 7B; their increasing alleles were provided by the same parents. In contrast, four QTL for fertile shoots per plant and shoot survival were also coincident with eight QTL for TGW on 1A, 4A and 7B, but their increasing alleles were provided by the opposite parents, confirming the negative relationships between them. A further analysis showed that three QTL for carpel size and two for stem WSC content at anthesis were coincident with 11 QTL for TGW on 3B, 4A, 5DL and 7B, with the increasing alleles provided by the same parents; additionally, one QTL for carpel size and two for stem WSC content were coincident with two QTL for fertile shoots per plant on 4A and 7B, with the increasing alleles conferred by the opposite parents. There was no QTL coincidence between total and fertile shoot number, nor yield per shoot; only one QTL for shoot survival was coincident with one for yield per shoot on 5AS, with the increasing alleles conferred by ‘Forno’. These results agree with the above physiological relationships between tillering and yield components: more total and fertile shoots, and higher shoot survival, were associated with higher yield and grain number per plant without reducing those of individual shoots; however, more fertile shoots and higher shoot survival were associated with reduced TGW because of smaller carpels and less stem WSC per shoot.
DISCUSSION
Large variation in tillering dynamics and its genetic control
Significant variation in tillering traits between genotypes has been observed in the present and previous studies (Ishag and Taha, 1974; Hucl and Baker, 1989; Sharma, 1995; Berry et al., 2003; Dreccer et al., 2013). Thus, it is possible to optimize wheat tillering patterns by genetic selection. A major target of tillering optimization is to increase fertile shoot number per plant, an important component of grain number to enlarge sink size. Fertile shoots per plant was positively associated with total shoots per plant, tillering rate, and the time for tillering onset and cessation, indicating that genetic selection for delayed but fast tillering, and high tillering capacity, can result in more fertile shoots. An additional strategy to increase fertile shoot number is to improve tiller survival. The present study showed that only 55 % of the total shoots initiated produced spikes, and there was large variation in shoot survival among the RILs (31–87 %). This variation has been demonstrated in several studies, for example 37–68 % by Berry et al. (2003) and 70–93 % by Sharma (1995), suggesting an opportunity to select genotypes with high shoot survival for more spikes. Likewise, only about half of the florets initiated within spikelets set grains, and the remaining ones (mainly those at distal positions) are aborted just before anthesis (Kirby, 1988; González-Navarro et al., 2015). Floret fertility has been known to largely determine grains per shoot at maturity, the other key component of grain number per unit land area (González et al., 2011). It has been found that shoot and floret fertility respond to the availability of environmental resources such as nutrients and radiation (Ishag and Taha, 1974; Fischer and Stockman, 1980; Thorne and Wood, 1987; Alzueta et al., 2012), indicating plasticity. This attribute of wheat plants may play a crucial role in accommodating various environments and forming yield (Sadras and Rebetzke, 2013).
The QTL for tillering dynamics were reported here for the first time, except the trait of fertile shoots per plant, which has been widely studied (Kato et al., 2000; Deng et al., 2011; Naruoka et al., 2011; Jia et al., 2013; Zhang et al., 2013). Most QTL for tillering dynamics were located on chromosomes 3A, 4A and 5A. The most important QTL cluster was detected on 5AL, where the alleles from the spelt ‘Oberkulmer’ were associated with increased total, fertile and infertile shoot number, accelerated tillering rate, and delayed tillering onset and cessation. In a single-chromosome (spelt 5A) recombinant line mapping population, Kato et al. (2000) also mapped a QTL for fertile tiller number per plant at this location. Another QTL coincidence for fertile shoots per plant and shoot survival was found in the distal region of 4AS, where the QTL for tillers per plant was identified in a previous study (Jia et al., 2013). In addition, the present study revealed a QTL for initial shoots per plant on 2D, corresponding to the Ppd-D1 gene, indicating that the photoperiod response gene probably regulates the progress of tillering (Borras-Gelonch et al., 2012). Two QTL for fertile shoots per plant were coincident with those for total shoots per plant, shoot survival, tillering rate, and tillering onset and cessation, and their increasing alleles were conferred by the same parents. This is in line with the above conclusion that more fertile shoots per plant can be achieved by increasing tillering capacity and survival, accelerating tillering rate, and delaying tillering onset and cessation. Although many QTL for tillering were identified here, none of them was stable over years, indicating the genetic complexity of the tillering process and the important roles of environmental factors such as shade, as discussed below. Future work is needed to dissect genetic elements for tillering per se and those responding to environmental cues. The QTL presented in this study provide an initial step for this purpose.
Low R:FR inhibits tiller production, and increases tiller abortion
It seems that wheat plants can sense R:FR at an early stage of tillering. Low R:FR at the beginning of tillering was associated with fewer total shoots per plant, as confirmed in the thinning experiment, indicating an inhibition of tiller production. Detailed analysis showed that low R:FR did not reduce tillering rate, but led to early tiller cessation. The same results have been observed with the treatments of low R:FR, far-red light, shade or high plant density (Evers et al., 2006; Sparkes et al., 2006; Ugarte et al., 2010; Toyota et al., 2014). The threshold of R:FR for tillering cessation in the field was on average 0·20, similar to that of previous reports (0·20–0·40) (Evers et al., 2006; Sparkes et al., 2006; Dreccer et al., 2013). However, significant variation in this trait among the RILs (0·07–0·37) was also determined, suggesting genetic difference in the tolerance of tiller bud outgrowth to low R:FR. This difference has previously been reported between the tiller inhibition (tin1) lines and free-tillering lines. The tin1 lines become more sensitive to light quality (0·18–0·22), compared with the free-tillering lines (0·09–0·11) (Moeller et al., 2014). The tin1 gene appears to be involved in the perception of R:FR. This gene inhibits tiller bud outgrowth by limiting sugar supply due to precocious internode development (Kebrom et al., 2012). Early stem elongation can be induced by low R:FR, as shown in the present study. Therefore, it can be hypothesized that a low R:FR promotes the onset of stem elongation, leading to assimilate deprivation from growing tiller buds and, in turn, bud dormancy. The tin1 mutants respond to low R:FR earlier, and start stem elongation earlier, resulting in earlier cessation of axillary tiller bud outgrowth, fewer buds growing out and hence fewer total tillers. Thus, R:FR may function as a direct signal inhibiting tillering by inducing stem elongation in the tin1 lines. This model can also be used to explain the coincidence between tillering cessation and the onset of stem elongation in the present and previous studies (Baker and Gallagher, 1983; Gomez-Macpherson et al., 1998; Sylvester-Bradley et al., 2008).
The results showed that low R:FR not only inhibits tiller bud outgrowth, but also promotes the abortion of young tillers initiated, which extends our understanding of the effect of low R:FR. The underlying mechanism is not yet clear. Tiller death normally starts from the onset of stem elongation, and ends around flowering (Sylvester-Bradley et al., 2008). During this period, stems and spikes are growing rapidly, suggesting source limitation (Gomez-Macpherson et al., 1998; González et al., 2011). More carbohydrates have to be diverted to these expanding sinks, leading to a shortage for developing young tillers and, in turn, tiller death (Gomez-Macpherson et al., 1998). On the other hand, a release in intraplant competition by increasing resource availability such as radiation improves tiller survival (Thorne and Wood, 1987). In this study, it was found that low R:FR was associated with early stem elongation and taller plants at anthesis. These responses have been well known as part of the shade avoidance syndrome in many other species, involved in phytochrome perception (mainly PHYB) and hormonal regulation (Gommers et al., 2013; Rameau et al., 2015). Therefore, low R:FR may increase stem sink, and intensify intraplant competition indirectly; as a result, tiller abortion is enhanced.
To improve tiller survival, the genotypes with either high tolerance to shade or well-established light environment can be selected. Genetic variation in shade tolerance has been determined in this study. For the latter, light quality under a canopy is a complex trait, depending on plant architecture, such as leaf characteristics (number, size, thickness, insertion angle, shape, stiffness and colour) and plant height. Redesigning these traits using a 3-D imaging and modelling method may improve the light environment at the bottom of the canopy.
Increasing fertile shoot number while maintaining other yield components
Fertile shoot number per plant largely contributed to plant productivity, confirming its role as a key yield determinant (Sharma, 1995; Kato et al., 2000). This resulted from an increase in grain number per plant, rather than individual grain weight. A close look revealed that more fertile shoots did not significantly reduce yield and grains per shoot, as seen in previous studies (Kato et al., 2000; Jia et al., 2013); there was even a positive relationship between fertile shoots per plant and grains per shoot. In full sunlight, fertile shoots per plant were only negatively associated with individual grain weight, as supported by analyses of the QTL coincidences and allelic effects. Grains develop from the carpels growing mainly between booting and anthesis, and carpel size at anthesis has been considered as an upper limit to grain weight (Calderini et al., 1999). Another pre-anthesis trait affecting grain weight is stem WSC remobilized into grains during grain filling (van Herwaarden et al., 1998). Each of these two traits was positively associated with grain weight in this study, consistent with the results of QTL analysis, confirming their roles as grain weight determinants. Carpel growth and stem WSC accumulation concur with tiller death and final tiller formation before anthesis. More fertile shoots produced was associated with smaller carpels and less stem WSC. Genetic analysis showed the QTL coincidences between fertile shoots per plant, carpel size and stem WSC content on chromosomes 4A and 7B, indicating that the negative relationships between them at least partly result from the pleiotropic effects or tight gene linkages. To break the negative relationships, these genes may be excluded, and/or more independent ones have to be added; at the same time, leaf photosynthesis and soil nutrient supply during the pre-anthesis period should be improved to increase source availability.
CONCLUSIONS
This study describes the tillering dynamics of wheat in detail, and its genetic and environmental control. Large genetic variation in tillering traits was determined, and it is proposed that the genotypes with higher tillering capacity, faster tillering rate, delayed tillering onset and cessation, and higher tiller survival can be selected to increase fertile shoot number. Based on this variation, the QTL for tillering traits were identified, and QTL coincidence analysis agrees with the above proposition for fertile shoot improvement. R:FR has significant effects on tillering: low R:FR generated from neighbouring plants inhibits tiller production by accelerated tillering cessation, and promotes infertile tillers and tiller abortion, probably resulting from an assimilate shortage due to early stem elongation and enhanced stem growth induced by low R:FR. A few QTL for R:FR kinetics in the field were also detected. After these processes, final shoot number is defined. More fertile shoots at maturity contribute to plant yield and grain number, without reducing single-shoot productivity and grain set. However, this is accompanied by a slight decrease in individual grain weight, partly as an outcome of reduced carpel size and stem WSC content at anthesis. Therefore, this study improves our knowledge of the genetic and environmental determination of the tillering process and, in turn, grain yield formation in wheat.
ACKNOWLEDGEMENTS
We thank Beat Keller (University of Zurich, Switzerland) for providing the ‘Forno’ × ‘Oberkulmer’ mapping population, and Monika Messmer (Research Institute of Organic Agriculture, Switzerland) for providing the molecular marker data. We also thank John Alcock, Matthew Tovey and Mark Meacham for their help with field and glasshouse experiments. This work was supported by a joint grant from the China Scholarship Council and University of Nottingham.
LITERATURE CITED
- Alzueta I, Abeledo LG, Mignone CM, Miralles DJ. 2012. Differences between wheat and barley in leaf and tillering coordination under contrasting nitrogen and sulfur conditions. European Journal of Agronomy 41: 92–102. [Google Scholar]
- Baker CK, Gallagher JN. 1983. The development of winter wheat in the field. 1. Relation between apical development and plant morphology within and between seasons. Journal of Agricultural Science 101: 327–335. [Google Scholar]
- Berry PM, Spink JH, Foulkes MJ, Wade A. 2003. Quantifying the contributions and losses of dry matter from non-surviving shoots in four cultivars of winter wheat. Field Crops Research 80: 111–121. [Google Scholar]
- Borras-Gelonch G, Rebetzke GJ, Richards RA, Romagosa I. 2012. Genetic control of duration of pre-anthesis phases in wheat (Triticum aestivum L.) and relationships to leaf appearance, tillering, and dry matter accumulation. Journal of Experimental Botany 63: 69–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderini DF, Abeledo LG, Savin R, Slafer GA. 1999. Effect of temperature and carpel size during pre-anthesis on potential grain weight in wheat. Journal of Agricultural Science 132: 453–459. [Google Scholar]
- Casal JJ. 1988. Light quality effects on the appearance of tillers of different order in wheat (Triticum aestivum). Annals of Applied Biology 112: 167–173. [Google Scholar]
- Casal JJ, Sanchez RA, Gibson D. 1990. The significance of changes in the red/far-red ratio, associated with either neighbour plants or twilight, for tillering in Lolium multiflorum Lam. New Phytologist 116: 565–572. [Google Scholar]
- Davis MH, Simmons SR. 1994. Tillering response of barley to shifts in light quality caused by neighboring plants. Crop Science 34: 1604–1610. [Google Scholar]
- Deng S, Wu X, Wu Y, et al. 2011. Characterization and precise mapping of a QTL increasing spike number with pleiotropic effects in wheat. Theoretical and Applied Genetics 122: 281–289. [DOI] [PubMed] [Google Scholar]
- Dreccer MF, Chapman SC, Rattey AR, et al. 2013. Developmental and growth controls of tillering and water-soluble carbohydrate accumulation in contrasting wheat (Triticum aestivum L.) genotypes: can we dissect them? Journal of Experimental Botany 64: 143–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan BL, Richards RA, Tsuyuzaki H. 2002. Environmental effects on stunting and the expression of a tiller inhibition (tin) gene in wheat. Functional Plant Biology 29: 45–53. [DOI] [PubMed] [Google Scholar]
- Duggan BL, Richards RA, Van Herwaarden AF, Fettell NA. 2005. Agronomic evaluation of a tiller inhibition gene (tin) in wheat. I. Effect on yield, yield components, and grain protein. Australian Journal of Agricultural Research 56: 169–178. [Google Scholar]
- Evers JB, Vos J. 2013. Modeling branching in cereals. Frontiers in Plant Science 4: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers JB, Vos J, Andrieu B, Struik PC. 2006. Cessation of tillering in spring wheat in relation to light interception and red:far-red ratio. Annals of Botany 97: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RA, Stockman YM. 1980. Kernel number per spike in wheat (Triticum aestivum L.): responses to preanthesis shading. Functional Plant Biology 7: 169–180. [Google Scholar]
- Foulkes MJ, Slafer GA, Davies WJ, et al. 2011. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. Journal of Experimental Botany 62: 469–486. [DOI] [PubMed] [Google Scholar]
- Gomez-Macpherson H, Richards RA, Masle J. 1998. Growth of near-isogenic wheat lines differing in development—plants in a simulated canopy. Annals of Botany 82: 323–330. [Google Scholar]
- Gommers CMM, Visser EJW, Onge KRS, Voesenek LACJ, Pierik R. 2013. Shade tolerance: when growing tall is not an option. Trends in Plant Science 18: 65–71. [DOI] [PubMed] [Google Scholar]
- González FG, Miralles DJ, Slafer GA. 2011. Wheat floret survival as related to pre-anthesis spike growth. Journal of Experimental Botany 62: 4889–4901. [DOI] [PubMed] [Google Scholar]
- González-Navarro OE, Griffiths S, Molero G, Reynolds MP, Slafer GA. 2015. Dynamics of floret development determining differences in spike fertility in an elite population of wheat. Field Crops Research 172: 21–31. [Google Scholar]
- Hucl P, Baker RJ. 1989. Tillering patterns of spring wheat genotypes grown in a semiarid environment. Canadian Journal of Plant Science 69: 71–79. [Google Scholar]
- Ishag HM, Taha MB. 1974. Production and survival of tillers of wheat and their contribution to yield. Journal of Agricultural Science 83: 117–124. [Google Scholar]
- Jia H, Wan H, Yang S, et al. 2013. Genetic dissection of yield-related traits in a recombinant inbred line population created using a key breeding parent in China's wheat breeding. Theoretical and Applied Genetics 126: 2123–2139. [DOI] [PubMed] [Google Scholar]
- Kasperbauer MJ. 1987. Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiology 85: 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperbauer MJ, Karlen DL. 1986. Light-mediated bioregulation of tillering and photosynthate partitioning in wheat. Physiologia Plantarum 66: 159–163. [Google Scholar]
- Kato K, Miura H, Sawada S. 2000. Mapping QTLs controlling grain yield and its components on chromosome 5A of wheat. Theoretical and Applied Genetics 101: 1114–1121. [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA. 2006. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiology 140: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Chandler PM, Swain SM, King RW, Richards RA, Spielmeyer W. 2012. Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiology 160: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegge W, Weldegergis BT, Soler R, et al. 2013. Canopy light cues affect emission of constitutive and methyl jasmonate-induced volatile organic compounds in Arabidopsis thaliana. New Phytologist 200: 861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby EJM. 1988. Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis. Field Crops Research 18: 127–140. [Google Scholar]
- Kuraparthy V, Sood S, Dhaliwal H, Chhuneja P, Gill B. 2007. Identification and mapping of a tiller inhibition gene (tin3) in wheat. Theoretical and Applied Genetics 114: 285–294. [DOI] [PubMed] [Google Scholar]
- Langer RHM, Prasad PC, Laude HM. 1973. Effects of kinetin on tiller bud elongation in wheat (Triticum aestivum L.). Annals of Botany 37: 565–571. [Google Scholar]
- Longnecker N, Kirby EJM, Robson A. 1993. Leaf emergence, tiller growth, and apical development of nitrogen-deficient spring wheat. Crop Science 33: 154–160. [Google Scholar]
- Messmer MM, Keller M, Zanetti S, Keller B. 1999. Genetic linkage map of a wheat × spelt cross. Theoretical and Applied Genetics 98: 1163–1170. [Google Scholar]
- Moeller C, Evers JB, Rebetzke G. 2014. Canopy architectural and physiological characterization of near-isogenic wheat lines differing in the tiller inhibition gene tin. Frontiers in Plant Science 5: 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruoka Y, Talbert LE, Lanning SP, Blake NK, Martin JM, Sherman JD. 2011. Identification of quantitative trait loci for productive tiller number and its relationship to agronomic traits in spring wheat. Theoretical and Applied Genetics 123: 1043–1053. [DOI] [PubMed] [Google Scholar]
- Peng Z, Yen C, Yang J. 1998. Genetic control of oligo-culms in common wheat. Wheat Information Service 86: 19–24. [Google Scholar]
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, Sakr S. 2015. Multiple pathways regulate shoot branching. Frontiers in Plant Science 5: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SK, Holalu SV, Casal JJ, Finlayson SA. 2013. Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiology 163: 1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RA. 1988. A tiller inhibitor gene in wheat and its effect on plant growth. Australian Journal of Agricultural Research 39: 749–757. [Google Scholar]
- Sadras VO, Rebetzke GJ. 2013. Plasticity of wheat grain yield is associated with plasticity of ear number. Crop and Pasture Science 64: 234–243. [Google Scholar]
- Sharma RC. 1995. Tiller mortality and its relationship to grain yield in spring wheat. Field Crops Research 41: 55–60. [Google Scholar]
- Sparkes DL, Holme SJ, Gaju O. 2006. Does light quality initiate tiller death in wheat? European Journal of Agronomy 24: 212–217. [Google Scholar]
- Spielmeyer W, Richards RA. 2004. Comparative mapping of wheat chromosome 1AS which contains the tiller inhibition gene (tin) with rice chromosome 5S. Theoretical and Applied Genetics 109: 1303–1310. [DOI] [PubMed] [Google Scholar]
- Sylvester-Bradley R, Berry P, Blake J, et al. 2008. The wheat growth guide. London: HGCA. [Google Scholar]
- Thorne GN, Wood DW. 1987. Effects of radiation and temperature on tiller survival, grain number and grain yield in winter wheat. Annals of Botany 59: 413–426. [Google Scholar]
- Toyota M, Tatewaki N, Morokuma M, Kusutani A. 2014. Tillering responses to high red/far-red ratio of four Japanese wheat cultivars. Plant Production Science 17: 124–130. [Google Scholar]
- Ugarte CC, Trupkin SA, Ghiglione H, Slafer G, Casal JJ. 2010. Low red/far-red ratios delay spike and stem growth in wheat. Journal of Experimental Botany 61: 3151–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Herwaarden AF, Angus JF, Richards RA, Farquhar GD. 1998. ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser. II. Carbohydrate and protein dynamics. Australian Journal of Agricultural Research 49: 1083–1093. [Google Scholar]
- Van Ooijen JW. 2006. JoinMap® 4, software for the calculation of genetic linkage maps in experimental populations. Wageningen: Kyazma BV. [Google Scholar]
- Van Ooijen JW. 2009. MapQTL® 6, software for the mapping of quantitative traits loci in experimental populations of diploid species. Wageningen: Kyazma BV. [Google Scholar]
- Voorrips RE. 2002. MapChart: software for the graphical presentation of linkage maps and QTLs. Journal of Heredity 93: 77–78. [DOI] [PubMed] [Google Scholar]
- Yemm EW, Willis AJ. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal 57: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research 14: 415–421. [Google Scholar]
- Zhang J, Wu J, Liu W, et al. 2013. Genetic mapping of a fertile tiller inhibition gene, ftin, in wheat. Molecular Breeding 31: 441–449. [Google Scholar]