Abstract
Background and Aims Sclerotinia stem rot (SSR, Sclerotinia sclerotiorum) is a damaging disease of oilseed brassicas world-wide. Host resistance is urgently needed to achieve control, yet the factors that contribute to stem resistance are not well understood. This study investigated the mechanisms of resistance to SSR.
Methods Stems of 5-week-old Brassica carinata, B. juncea and B. napus of known resistance were infected via filter paper discs impregnated with S. sclerotiorum mycelium under controlled conditions. Transverse sections of the stem and portions of the stem surface were examined using optical and scanning electron microscopy. The association of anatomical features with the severity of disease (measured by mean lesion length) was determined.
Key Results Several distinct resistance mechanisms were recorded for the first time in these Brassica–pathogen interactions, including hypersensitive reactions and lignification within the stem cortex, endodermis and in tissues surrounding the lesions. Genotypes showing a strong lignification response 72 h post-infection (hpi) tended to have smaller lesions. Extensive vascular invasion by S. sclerotiorum was observed only in susceptible genotypes, especially in the vascular fibres and xylem. Mean lesion length was negatively correlated with the number of cell layers in the cortex, suggesting progress of S. sclerotiorum is impeded by more cell layers. Hyphae in the centre of lesions became highly vacuolate 72 hpi, reflecting an ageing process in S. sclerotiorum hyphal networks that was independent of host resistance. The infection process of S. sclerotiorum was analogous in B. carinata and B. napus. Infection cushions of the highly virulent isolate of S. sclerotiorum MBRS-1 were grouped together in dense parallel bundles, while hyphae in the infection cushions of a less aggressive isolate WW-3 were more diffuse, and this was unaffected by host genotype.
Conclusions A variety of mechanisms contribute to host resistance against S. sclerotiorum across the three Brassica species. These complex interactions between pathogen and host help to explain variable expressions of resistance often observed in the field.
Keywords: Sclerotinia stem rot, Brassica carinata, B. juncea, B. napus, white mould, histopathology, host–pathogen interaction, fungal infection, resistance, lignification, hypersensitive reaction, infection cushion
INTRODUCTION
Sclerotinia stem rot (SSR) of brassicas is a damaging disease of oilseed rape and other oilseed brassicas in many parts of the world (Barbetti et al., 2012). It is caused by the fungus Sclerotinia sclerotiorum, which has over 400 host species and affects a variety of plant parts (Boland and Hall, 1994). Only one study has specifically investigated the infection process of S. sclerotiorum on the stems of oilseed brassicas (Huang et al., 2008), although the infection of other tissues of Brassica napus, such as leaves, petals, pollen and cotyledons (Jamaux et al., 1995; Huang et al., 1998, 2008; Garg et al., 2010c) has been investigated. Additionally, detailed studies have been carried out on several other crop species that are hosts of S. sclerotiorum, such as potatoes and peas (Jones, 1976), bean (Lumsden and Dow, 1973; Abawi et al., 1975; Tariq and Jeffries, 1986) and sunflower (Rodríguez et al., 2004). Together, these studies show that the method by which S. sclerotiorum invades the host and establishes infection is similar across diverse susceptible hosts. The process involves germination of S. sclerotiorum ascospores followed by rapid growth of mycelium over the surface of the plant tissue. Approximately 24 h after growth has commenced, S. sclerotiorum begins to form appressoria and infection cushions. Hyphae from the infection cushions penetrate the plant using mechanical pressure, possibly assisted by the production of oxalic acid and polygalacturonase and other enzymes. The hyphae grow within the susceptible host, with mycelium ramifying from the penetration site through the cortex and sometimes into the vascular tissue of the host. Damage to the host tissue in the expanding lesion is catastrophic (Lumsden, 1979).
Huang et al. (2008) showed that infection processes on B. napus hypocotyls were largely similar to those on other plant parts, although larger infection cushions were found on the hypocotyl than on leaves. There was also evidence of some growth of S. sclerotiorum in the xylem of the hypocotyl 6 d post-infection (dpi).
While the infection process of S. sclerotiorum has been extensively examined, the information available deals almost exclusively with susceptible hosts while the resistance response has been little studied. This appears to be largely due to the lack of highly resistant genotypes available for investigation (Delourme et al., 2011). However, the discovery of high levels of resistance to stem infection in oilseed brassicas has now made investigation of the resistance response possible (Barbetti et al., 2013). Anatomical comparisons between resistant and susceptible Brassicaceae have so far been limited to a single study on B. napus cotyledons, which showed that while there was no difference between the germination of S. sclerotiorum ascospores on a moderately resistant variety ‘Charlton’ or susceptible ‘RQ001-02M2’, its growth was slow on the resistant variety (Garg et al., 2010c). The resistant genotype also suppressed formation of infection cushions, caused extrusion of protoplast from fungal hyphae and produced a hypersensitive reaction (HR). Although these findings in cotyledons are significant, such studies are not directly transposable to stems as there appears to be separate genetic control of stem resistance in seedlings versus adult plants (Zhao et al., 2006; Mei et al., 2012; Uloth et al., 2013a, b; Taylor et al., 2015). There is therefore a need to determine resistance mechanisms directly in oilseed Brassica stems, as stems are the host tissue most affected by SSR, relating directly to the major field losses caused by S. sclerotiorum (Li et al., 2006).
Oilseed Brassica breeders combine genetic material from a variety of Brassica species into breeding lines, which makes the level of resistance to S. sclerotiorum and mechanisms of resistance present in each species and their crosses a matter of great practical importance (Chen et al., 2010; Barbetti et al., 2014). We have therefore examined genotypes of known resistance from three species, namely B. napus, B. juncea and B. carinata, to determine whether their resistance reactions are similar. In addition, isolates of S. sclerotiorum differ in aggressiveness and pathotype but no attempts to reveal variation in infection processes between isolates of differing aggressiveness have been reported (Garg et al., 2010b; Ge et al., 2012). The comparison of highly and mildly aggressive isolates may reveal differences which could potentially lead to the development of novel methods to manipulate the pathogen and reduce disease.
In studies of SSR in field trials, a significant number of distinct stem lesion types have been observed on Brassica hosts with varying levels of resistance (Fig. 1). A consistent observation is that the stem lesions caused by S. sclerotiorum extend further longitudinally along the stem than radially (M. B. Uloth et al., unpubl. res.). In addition, dark ‘shadows’ extending well past the edge of the lesion are observed in many genotypes. These ‘shadows’ appear as sub-surface brown discoloration which runs longitudinally along the stem from the point of infection, under an intact cuticle/stem surface (Fig. 1B). Furthermore, the plant delimits lesions over time (Fig. 1C) in all but the most rampant infections (Fig. 1D). Studies into how B. napus limits the growth of Leptosphaeria maculans on its stem revealed a process of lignification, cambium formation and callose deposition at the edge of the lesion (Hammond and Lewis, 1986, 1987; Li et al., 2007b, 2008). Whether similar processes occur in association with infection by S. sclerotiorum remains unknown and the mechanisms governing the different types of lesions observed in the field need to be elucidated.
Fig. 1.
Reactions by Brassica genotypes of varying susceptibility to infection by Sclerotinia sclerotiorum in a field trial. (A) Extreme resistance reaction at 21 dpi (arrowhead). (B) Stem inoculated with an S. sclerotiorum-infested agar plug, attached to the stem with Parafilm, showing sub-surface brown discoloration (arrowheads) caused by S. sclerotiorum infection running longitudinally along the stem from the point of inoculation at 21 dpi. (C) Moderately resistant reaction, showing zonate lesion at 21 dpi. (D) Highly susceptible reaction in B. carinata SMP3-82 at 21 dpi.
This work was carried out to (1) compare the infection processes of S. sclerotiorum between resistant and susceptible selections of B. carinata, B. napus and B. juncea in order to reveal resistance strategies employed by the host; (2) investigate and compare the infection process for S. sclerotiorum on B. carinata stems with that previously reported for B. napus; (3) investigate reasons for the preferential longitudinal extension of SSR stem lesions; and (4) establish whether differences in infection strategy occur between highly aggressive and mildly aggressive isolates of S. sclerotiorum.
In the course of the investigation it was noted that the cortex of the susceptible B. carinata genotype was consistently shallower than in the resistant genotype, with fewer cortical cell layers. Preliminary examination of the cortex in nine Brassica genotypes across B. carinata, B. juncea and B. napus (M. B. Uloth et al., unpubl. res.) and comparison with historical data on lesion lengths for these genotypes (Uloth et al., 2013a) supported the concept of a relationship between a lesser number of cell layers in the cortex and greater susceptibility to S. sclerotiorum. Therefore, an experiment was also conducted to test the hypothesis that Brassica genotypes with more layers in the cortex of the stem are more resistant to S. sclerotiorum.
MATERIALS AND METHODS
Host genotypes
Two separate experiments were conducted on the stages of the infection processes (Table 1). For Experiment 1, two selections of B. carinata were chosen as resistant and susceptible on the basis of their reaction to S. sclerotiorum in field trials (Uloth et al., 2013a) confirmed by several preliminary experiments in controlled environment growth rooms (Uloth et al., 2015b) (M. B. Uloth et al., unpubl. res.). Brassica carinata SMP3-82 from Pakistan was the most severely affected by S. sclerotiorum out of 108 diverse Brassica genotypes (mean lesion length = 155 mm), while B. carinata 054113 from Ethiopia was highly resistant (mean lesion length = 4·2 mm). In Experiment 2, 26 Brassica genotypes across B. carinata, B. juncea and B. napus (see Table 2) were selected using data from their performance in the Uloth et al. (2013a) field trial to provide a range of resistance levels. In particular, B. napus ZY006 from China was highly resistant in the field trial (mean lesion length = 7·1 mm), while B. napus YM04 from China was susceptible (mean lesion length = 41·6 mm), and these genotypes were chosen for more detailed examination.
Table 1.
Stages of the infection process of Sclerotinia sclerotiorum on Brassica carinata stems following inoculation with filter paper discs impregnated with mycelium (Experiments 1 and 2)
| Infection stage | Infection process | Time observed (hpi) |
|---|---|---|
| 1 | Active growth of hyphae from disc onto stem | 0–24 |
| 2 | Formation of appressoria and infection cushions | 24–48 |
| 3 | Penetration of cuticle | 24–48 |
| 4 | Visible damage to stem | 24–48 |
| 5 | Secondary infections commenced. Growth of subcuticular hyphae in susceptible genotype | 48 |
| 6 | Extension of visible lesion into and along stem | 48–72 |
| 7 | Hyphae in lesion vacuolate. Lignification evident around lesions and in cortex | 72 |
| 8 | No viable hyphae visible on stem surface outside the lesion | 96 |
Table 2.
Stem lesion length 3 dpi, the number of cell layers in the stem cortex and stem diameter, and presence of strong autofluorescence (AF) for 26 Brassica genotypes (stems >4 mm in diameter) inoculated with Sclerotinia sclerotiorum MBRS-1 (Experiment 2); standard errors are indicated in parentheses
| Genotype name | Brassica species | Lesion length (mm) | No. of cortical cell layers | Stem diameter (mm) | Strong AF noted 3 dpi* | No. of plants (stem diameter >4mm) |
|---|---|---|---|---|---|---|
| 054111 | B. carinata | 3·2 (0·8) | 11·8 (0·3) | 6·3 (0·6) | – | 6 |
| Mystic | B. napus | 3·2 (0·6) | 13·4 (0·5) | 6·7 (0·6) | E | 9 |
| PI 197402 | B. carinata | 3·3 (0·6) | 13·5 (1·8) | 7·6 (1·4) | – | 4 |
| Charlton | B. napus | 3·4 (0·7) | 12·2 (0·5) | 6·7 (0·7) | C | 9 |
| RT108 | B. napus | 3·7 (1·0) | 13·0 (0·5) | 7·3 (0·6) | C | 9 |
| ZY006 | B. napus | 3·8 (0·9) | 11·4 (0·5) | 7·2 (0·6) | C | 9 |
| 06-P71–2 | B. napus | 3·9 (0·6) | 11·4 (1·9) | 7·2 (0·5) | E | 8 |
| YM14 | B. napus | 4·3 (0·8) | 14·5 (0·8) | 7·4 (0·6) | E | 10 |
| ZY005 | B. napus | 4·4 (0·5) | 16·1 (1·3) | 9·5 (0·8) | – | 8 |
| YM18 | B. napus | 4·9 (0·6) | 13·0 (0·4) | 6·6 (0·8) | E | 8 |
| JM06018 | B. juncea | 4·9 (0·6) | 9·2 (0·5) | 8·2 (0·6) | – | 9 |
| 054103 | B. carinata | 5·1 (0·8) | 10·7 (0·3) | 7·4 (0·6) | – | 9 |
| PI 193459 | B. carinata | 5·4 (0·8) | 12·0 (0·4) | 7·3 (0·8) | C | 8 |
| YM04 | B. napus | 5·5 (1·1) | 11·4 (0·4) | 7·0 (1·2) | L, E, C | 10 |
| YM12 | B. napus | 5·5 (0·9) | 12·8 (0·7) | 6·0 (0·7) | – | 8 |
| BRA 926/81 | B. carinata | 5·7 (2·0) | 9·3 (0·4) | 7·0 (0·6) | – | 7 |
| 054113 | B. carinata | 6·3 (1·4) | 10·3 (0·4) | 8·0 (0·7) | C | 9 |
| JO006 | B. juncea | 6·5 (0·9) | 8·3 (0·4) | 7·8 (0·5) | – | 8 |
| Montara | B. juncea | 6·7 (1·2) | 8·5 (0·5) | 7·1 (0·7) | L | 10 |
| PI 360884 | B. carinata | 6·8 (1·1) | 11·8 (0·7) | 7·9 (0·3) | – | 10 |
| 054106 | B. carinata | 6·9 (1·3) | 12·1 (1·4) | 7·4 (0·6) | – | 7 |
| SMP3–82 | B. carinata | 7·3(1·6) | 6·6 (0·4) | 7·3 (0·5) | – | 9 |
| JM06006 | B. juncea | 8·3 (2·4) | 7·6 (0·4) | 6·9 (0·6) | – | 9 |
| Xinyou 9 | B. juncea | 8·4 (1·7) | 6·9 (0·5) | 8·9 (0·5) | – | 9 |
| 054104 | B. carinata | 8·6 (2·9) | 12·6 (0·5) | 8·0 (0·5) | – | 9 |
| B. juncea #2 | B. juncea | 9·1 (1·1) | 7·4 (0·5) | 6·7 (0·5) | – | 7 |
| Significance (P) | 0·01 | <0·001 | 0·372 | |||
| Least significant difference (P ≤0·05) | 3·7 | 1·9 | – | |||
*Lignification indicated by the presence of autofluorescence. Letters indicate strong autofluoresence was observed ‘C’ in cortex, ‘L’ around lesion or ‘E’ as a distinct barrier in the endodermis. ‘–’ indicates no strong autofluorescence was observed.
S. sclerotiorum isolates
Two isolates of S. sclerotiorum were used in both experiments, MBRS-1 and WW-3. Both were collected from infected tissue of B. napus at sites where there was significant disease in an oilseed rape crop. MBRS-1 was collected from Mount Barker and WW-3 from the Walkaway region of Western Australia in 2004 (Li et al., 2006; Garg et al., 2010b). Cultures of these isolates were revived from original sclerotia stored in glass ampoules and subsequently refreshed as needed using air-dried mycelium-impregnated filter paper. Previous work has shown that MBRS-1 is highly virulent and represents pathotype 76, the prevailing pathotype in Western Australia, while isolate WW-3 is generally only weakly virulent and represents pathotype 10 in Western Australia (Ge et al., 2012; Uloth et al., 2015b).
Experiment 1: examination of the infection processes for a resistant and a susceptible genotype of B. carinata by S. sclerotiorum
Several microscopy techniques were used to compare the infection processes of S. sclerotiorum in the stems of resistant and susceptible 5-week-old B. carinata plants. Seed was sown into 35 × 29 × 6-cm trays, each having 48 individual cells filled with a pasteurized potting mix. Seedlings were transplanted after 3 weeks of growth in a controlled environment growth room, set to 22 ± 1 °C day and 18 ± 1 °C night, on a 12-h light–dark cycle, with light intensity of 400 µm m−2 s−1 into pots 14 cm in diameter (volume 1·7 L), with four plants per pot, and returned to the same growth room. Plants were grown for a further 3–4 weeks and were inoculated when stems were approx. 30 cm tall with a stem diameter up to 15 mm [growth stage between 2.05 and 3.5 on the Sylvester-Bradley scale (Sylvester-Bradley and Makepeace, 1984)].
Production of inoculum and plant inoculations were carried out as described by Uloth et al. (2015b). Inoculum was produced by placing five agar plugs from the actively growing margin of 3-d-old colonies of S. sclerotiorum grown on potato dextrose agar into 150 mL of liquid growth medium (per litre of water: 24 g potato dextrose broth and 10 g peptone). Cultures were shaken at 150 r.p.m. at 20 °C. After 3 d, actively growing mycelium was harvested by straining the broth through four layers of cheesecloth and washing with deionized water. The mycelium was macerated in the same liquid medium for 3 min using a hand-held blender. The concentration of mycelia in the suspension was measured using a haemocytometer (Superior®, Marienfeld, Berlin, Germany), and adjusted to 1 × 104 fragments per millilitre by diluting with fresh growth medium. Round discs of filter paper 5 mm in diameter (Qualitative no. 2; Advantec MFS, Inc., Dublin, CA, USA) were added to the inoculum and allowed to stand for at least 15 min. The filter paper discs were applied to the stems of the plants above the first internode using Parafilm® as this technique mimics natural field infection from colonized flower petals (Uloth et al., 2015a). Plants were placed in controlled environment growth rooms at either 25/21 or 18/14 °C. Eighty-two pots of B. carinata SMP3-82 and 86 pots of B. carinata 054113 were inoculated. Half the pots were inoculated with each isolate and pots were randomized. The final number of plants examined was 321 for B. carinata SMP3-82 and 342 for B. carinata 054113. After inoculation, the plants were misted until leaves were visibly wet and relative humidity in the rooms was increased from 60 to 95 % night and 90 % day. Lesion length was measured at 72 h post-infection (hpi). Parafilm wrapping was removed and the length of the lesion on each stem (or length of the longest lesion if there were multiple lesions) was measured to the nearest millimetre with a ruler. Results were analysed using a two-factor analysis of variance using Genstat (14th edition, Lawes Agricultural Trust, Rothamsted Research, Harpenden, UK).
At 12, 24 and 72 hpi, sections of inoculated stems approx. 1·5 cm long were cut from the plants and immediately immersed in 2·5 % glutaraldehyde in phosphate-buffered saline. Further stem sections were immersed in acetic acid/ethanol/water (2 : 2 : 1 by volume) to decolourize tissues. Samples were held at 4 °C until required. Transverse sections 200 µm thick were cut from some of the stem sections using a Vibratome 3000 sectioning system fitted with razor blades. A series of sections were cut at known distances from the centre of the lesion. Sections were mounted in distilled water on glass slides and imaged with a Zeiss Axioplan microscope using both UV excitation and brightfield modes. Images were captured using a Zeiss Axiocam digital imaging system. Forty lesions were examined for each host × isolate combination. In other stems, the epidermis and the first few layers of cortical cells were removed from the inoculated area as a single layer using a scalpel. These samples were stained for 90 s in 0·05 % aniline blue, then rinsed in distilled water. Stained samples were placed in a drop of distilled water on a glass slide and observed under an Olympus BX51 microscope. Images were recorded using an Olympus DP71 digital photographic system with brightfield optics.
Small portions of infection sites, approx. 5 mm in length, were cut for observation of the stem surface using scanning electron microscopy (SEM). These were dehydrated in a graded series of ethanol (30, 50, 75, 100, 100 %) using a PELCO BioWave® microwave fitted with PELCO coldspot. For each change in ethanol solution, specimens were microwaved at 250 W for 40 s. Samples were then dried in a Polaron E3000 critical point drier, mounted on aluminium mounts with carbon tabs and coated with both 3 nm platinum and 10 nm carbon. Images were collected at 5 kV using a field emission scanning electron microscope (1555 VP-FESEM; Zeiss, Oberkochen, Germany).
Experiment 2: examination of the effect of the number of cortical cell layers, stem diameter and lignification on infection of Brassica stems by S. sclerotiorum
The average number of cell layers between the epidermis and the outermost vascular element (endodermis) was counted in the stems of 26 Brassica genotypes and related to stem diameter and the severity of infection 3 dpi. Lignification around the infection was also examined in stems at four time points after infection. Plants were grown in a controlled environment growth room set to 22 ± 1 °C day and 18 ± 1 °C night with a 12-h photoperiod. There were three pots each containing four plants for each genotype, and these were randomized in the growth room. Other conditions, growing methods and inoculation methods were as described for Experiment 1. Plants were inoculated when they were 5 weeks old. Growth stages of the different genotypes varied between 2·05 and 3·5 on the Sylvester-Bradley scale (Sylvester-Bradley and Makepeace, 1984) but all had an elongated stem suitable for inoculation. Parafilm wrapping was removed 3 dpi. The longest lesion on each stem was measured with a ruler to the nearest millimetre and 8–10 arbitrarily chosen stem sections were harvested for each genotype. Portions of stem approx. 80 mm long that encompassed the point of inoculation were cut, sealed in a plastic bag and held at 4 °C until observation. In total, 247 stems were included in the final analysis (numbers listed by genotype in Table 2), with a further 29 stems <4 mm in diameter discarded.
Transverse stem sections were cut with a razor blade by hand approx. 30 mm above the point of inoculation, and never within or immediately adjacent to a lesion. Sections were mounted in water on glass slides for observation with an Olympus BX51 microscope. The number of cell layers in the cortex between the endodermis and epidermis was counted (the endodermis and epidermis were not included in the count). The locations for counting were chosen arbitrarily, avoiding ridges and points of leaf attachment, as preliminary work showed little variation in the number of cell layers between repeated counts of a stem using this system (M. B. Uloth et al., unpubl. res.). The number of layers was counted in each stem. The diameter of the stems was measured using Vernier callipers to the nearest millimetre. The number of cell layers was analysed using a single-factor analysis of variance, then the relationship between the mean number of cell layers in the cortex, stem diameter, and mean lesion length for each genotype was determined using linear regression in GenStat (14th edition, Lawes Agricultural Trust).
The same stem portions were used to qualitatively assess anatomical responses at 3 dpi to infection by S. sclerotiorum, including lignification. Transverse sections were cut by hand using a razor blade at or near the centre of the lesion. At least five lesions were examined for each genotype. Sections were mounted in water and examined using an Olympus U-RFL-T burner with a mercury bulb as the fluorescence source, with a dichroic mirror DM400, excitation filter BP330-385 and barrier filter BA420. Other sections were cleared by placing them in a small amount of acetic acid/ethanol/water (2 : 2 : 1 by volume) solution in a lidded Petri dish. The Petri dish was then placed on a rack in a water bath set to 55 °C, not allowing water to enter the dish, and held for 10 min. Cleared sections were stained for 3 min using freshly prepared phloroglucinol-HCl (0·2 g phloroglucinol dissolved in 20 mL 20 % HCl) and examined for the presence of lignin (Gahan, 1984). Ten per cent of images obtained using fluorescence microscopy were cross-checked with stained images to confirm that the autofluorescence observed was due to the presence of lignin. Stems where surface discoloration was observed at the point of inoculation but no lesion had formed were selected and the epidermis and first few layers of cortical cells were excised and examined after staining with 0·05 % aniline blue as described in Experiment 1.
Following the harvest at 3 dpi, relative humidity in the growth room was reduced to 80 % during the day but maintained at 95 % at night and the remaining inoculated plants of B. carinata (SMP3-82, 054113) and B. napus (ZY006, YM04) continued to grow. More stems were harvested at 7, 14 and 19 dpi. At each time point, lesions in hand-cut transverse sections were examined as described above. At 19 dpi, lesion length for B. carinata SMP3-82 and 054113 was measured again with a ruler to the nearest millimetre. Mean lesion lengths were compared using the t-test function in Genstat.
RESULTS
Experiment 1: examination of the infection processes for a resistant and a susceptible genotype of B. carinata by S. sclerotiorum
Stages of infection.
Sclerotinia sclerotiorum MBRS-1 and WW-3 successfully colonized and infected susceptible and resistant B. carinata stems. Mean lesion length for the susceptible B. carinata SMP3-82 was greater than for resistant B. carinata 054113 at 72 hpi [5·2 ± 0·2 mm (n = 321) and 2·6 ± 0·3 mm (n = 342), respectively, P < 0·001]. Mean lesion length for S. sclerotiorum MBRS-1 was greater than for S. sclerotiorum WW-3 over both plant genotypes tested [4·9 ± 0·2 mm (n = 317) and 2·8 ± 0·2 mm (n = 346), respectively, P <0·001].
Several distinct stages were observed in the infection of B. carinata by S. sclerotiorum (Table 1). In the first stages of infection, hyphae grew in all directions from the filter paper disc onto and then along the stem of the host (Fig. 2A). Around 24 hpi, hyphal tips branched to form infection cushions. By 48 hpi, infection cushions were well established and secondary infections were forming (Fig. 2B). From qualitative observations, there were no obvious differences between host genotypes in the number or complexity of infection cushions. Subcuticular hyphae were frequently observed at the edge of lesions caused by MBRS-1 on the stems of B. carinata SMP3-82 (5/10 samples) (Fig. 2C), but were not seen in Brassica carinata 054113. At this stage lesions were visible to the naked eye as small black regions. Sclerotinia sclerotiorum infection then expanded into and along the stem. By 72 hpi the largest lesions (on B. carinata SMP3-82, but not on B. carinata 054113) were sunken and brown with a water-soaked appearance. White fungal growth was sometimes visible in the centre of these lesions. Most lesions on B. carinata 054113 were dry and black but remained visible. In B. carinata SMP3-82, the cytoplasmic contents of hyphae on the surface of the stem began to disappear by 72 hpi. Hyphae in the centre of lesions on both B. carinata genotypes became highly vacuolate or disintegrated completely (Fig. 2D), and by 96 hpi, hyphae were no longer visible on the surface of the stem outside the lesion.
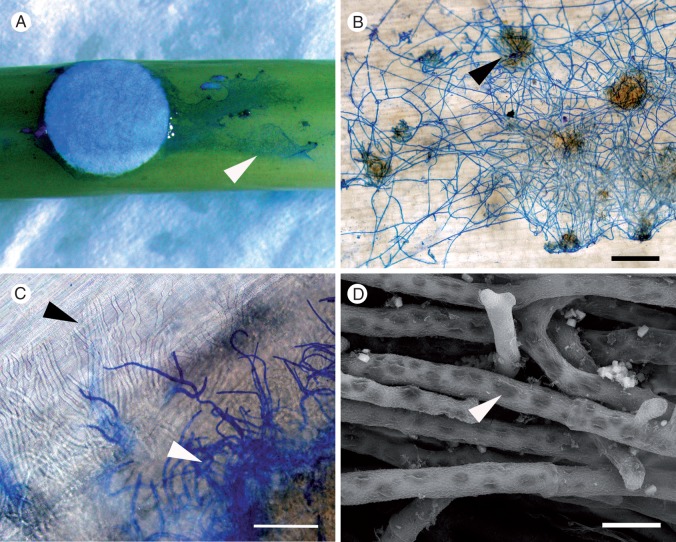
Fig. 2.
Light and scanning electron micrographs showing disease progression caused by Sclerotinia sclerotiorum MBRS-1 (A–C) and WW-3 (D) on the stems of Brassica carinata following inoculation using colonized filter paper discs. (A) Extension of S. sclerotiorum along the surface of the stem of susceptible B. carinata SMP3-82 (arrowhead) at 24 hpi. (B) Established hyphal net on the surface of resistant B. carinata 054113 showing infection cushions (arrowhead) at 48 hpi. (C) Subcuticular hyphae visible in susceptible B. carinata SMP3-82 (black arrowhead) and surface hyphae (stained blue, white arrowhead) at 48 hpi. (D) Hyphae in the lesion and on the stem surface became highly vacuolated (arrowhead) by 72 hpi (susceptible B. carinata SMP3-82). Scale bars (B) = 500 µm, (C) = 66 µm, (D) = 10 µm.
Damage to vascular fibres in advance of lesion development.
Sclerotinia sclerotiorum MBRS-1 preferentially targeted vascular elements in susceptible B. carinata SMP3-82 stems, frequently penetrating bundles of vascular fibres (Fig. 3C, E), or growing directly through the cortex to destroy vascular elements near the site of surface penetration (Fig. 3A). Damage was observed in the vascular fibres longitudinally up to 2·6 mm distal from the end of the lesion on the stem surface. In B. carinata 054113, vascular elements were sometimes damaged, but only where a substantial surface lesion had extended down to the edge of the vascular tissues. Damage to vascular fibres was never observed in advance of surface lesion development in B. carinata 054113, although some damage was evident in the cortex around the surface lesion (Fig. 3D, F).
Fig. 3.
Light micrographs of transverse sections of stems of susceptible Brassica carinata SMP3-82 (A, C, E) and resistant B. carinata 054113 (B, D, F) showing the development of lesions caused by Sclerotinia sclerotiorum MBRS-1 at 72 hpi. At the initial point of infection (A, B), approx. 1 mm away from the initial point of infection (C, D), and approx. 0·6 mm away from the edge of the externally visible lesion (E, F). (A) Susceptible B. carinata SMP3-82 centre of lesion with a fungal mass (arrowhead) showing numerous calcium oxalate crystals. Destruction of cells in the stem cortex and vascular system of the plant is evident. (B) Entry of S. sclerotiorum MBRS-1 into the stem of resistant B. carinata 054113. Stem cuticle around the entry point is depressed by the pressure of fungal penetration and fungal hyphae are evident in the stem, strongly resembling those in infection cushions. (C) Sclerotinia sclerotiorum from a surface lesion advancing to invade vascular fibres (black arrowhead). (D) Restriction of lesion to the stem cortex in a resistant host. (E) Destruction of vascular tissues, including bundles of vascular fibres (black arrowhead), and xylem (white arrowhead) in advance of the surface lesion. (F) Damage to cells in the stem cortex (arrowhead). Scale bars (A–C) = 100 µm; (D–F) = 200 µm.
Difference between highly aggressive and mildly aggressive isolates.
Light micrographs showed that infection cushions of MBRS-1 were consistently more compact than those of WW-3 (Fig. 4). The structure of the infection cushions was not affected by the host genotype. Hyphae in the infection cushion of MBRS-1 were grouped together in dense parallel bundles (Fig. 4A, C), while the hyphae in the infection structures of WW-3 were more diffuse in their arrangement and less organized (Fig. 4B, D). Sometimes, bunched fungal hyphae of MBRS-1 entered the cortex as a group that strongly resembled an infection cushion and the cuticle around the entry point on the stem was depressed (Fig. 3B).
Fig. 4.
Comparison of infection structures formed on susceptible Brassica carinata SMP3-82 by two isolates of Sclerotinia sclerotiorum with different levels of aggressiveness. (A) Isolate MBRS-1 (highly aggressive) infection cushions at 24 hpi (arrowhead), (B) isolate WW-3 (weakly aggressive) infection structure at 24 hpi (arrowhead), (C) isolate MBRS-1 dense infection cushion at 72 hpi (arrowhead), (D) isolate WW-3 diffuse infection cushion at 72 hpi (arrowhead). Scale bars (A–D) = 100 µm.
Experiment 2: examination of the effect of the number of cortical cell layers, stem diameter and lignification on infection of Brassica stems by S. sclerotiorum
Effect of number of cell layers on lesion length.
Lesions were severe in stems <4 mm in diameter regardless of genotype [mean lesion length at 72 hpi was 9·3 ± 0·3 mm for stems <4 mm (n = 67) and 5·6 ± 0·1 mm for stems ≥4 mm (n = 181)]. Therefore, data from plants with thin stems <4 mm were excluded from the analysis.
Mean lesion lengths at 3 dpi, the number of cell layers in the cortex and stem diameter for the 26 Brassica genotypes are shown in Table 2. The number of layers differed significantly between genotypes [P < 0·001, least significant difference (l.s.d.) = 1·9]. The lowest mean number of cells in the cortex was 6.6 for susceptible B. carinata SMP3-82, and the greatest was 16.1 for B. napus ZY005. Mean lesion length also differed significantly between the genotypes (P = 0·01, l.s.d. = 3·7). Brassica juncea #2 had the longest mean lesion length (9·1 mm) and B. carinata 054111 had the shortest (3·2 mm). There was a significant negative correlation between the mean number of cell layers in the cortex and mean lesion length across genotypes (P < 0·001, r2 = 0·434, n = 26; Fig. 5). Mean stem diameter did not vary significantly between genotypes (P = 0·372).
Fig. 5.
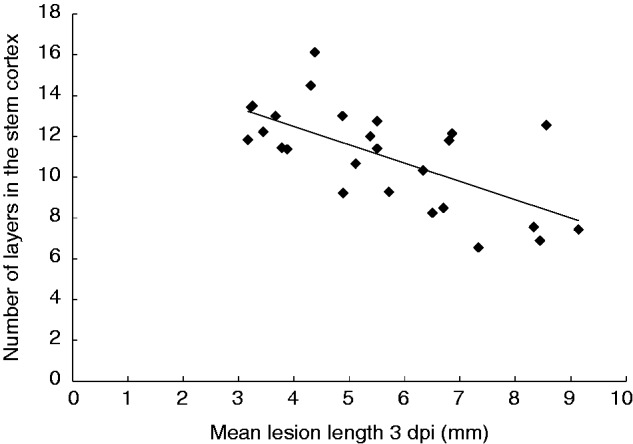
Relationship between number of cell layers in the stem cortex for 26 Brassica genotypes and mean lesion length (mm) at 3 d post-inoculation (dpi) following inoculation with Sclerotinia sclerotiorum MBRS-1. Regression significant at P < 0·001, r = 0·422, n = 26.
Lesion length for B. carinata SMP3-82 and 054113.
Brassica carinata SMP3-82 was among the most susceptible group of genotypes at 72 hpi (7·3 mm). Early infection on resistant B. carinata 054113 was unusually severe for this genotype, with lesions observed on approx. 30 % of stems. This contrasted with several preliminary experiments in which early infection on B. carinata 054113 was very mild and lesions appeared dry by 72 hpi. However, while mean lesion length at 19 dpi was 27·4 ± 8·0 mm for B. carinata SMP3-82, it was only 10·8 ± 1·0 mm for B. carinata 054113. A t-test confirmed the significance of this difference (P = 0·05; t = 2·07 on 36 d.f.), with strong evidence of different sample variances. Brassica carinata SMP3-82 had several very long lesions that encompassed the whole stem. Lesions as long as 230 mm were recorded and 15 % of the stems had lesions >40 mm. By contrast, the longest lesion on B. carinata 054113 was 31 mm. By 19 dpi, almost all lesions on B. carinata 054113 were dry in appearance.
Lignification in stem.
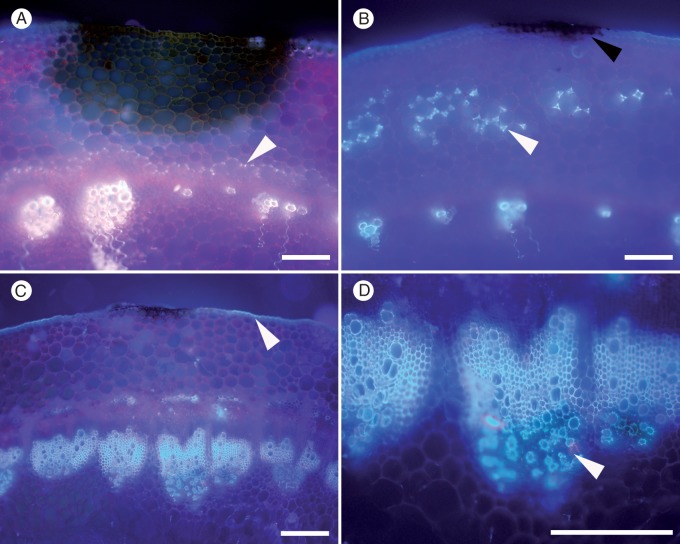
Autofluorescence developed in diverse patterns in the stems of 26 Brassica genotypes tested in response to S. sclerotiorum infection. In general, the pattern of autofluorescence was identical to the pattern of red/pink coloration observed after staining with phloroglucinol, a stain which reveals areas of lignification (Fig. 6B, C). However, there were some exceptions to this, and in 4 % of samples, white autofluorescence was observed in a sample that did not stain with phloroglucinol. Autofluorescence of cell walls developed in different patterns in a range of Brassica genotypes. It was present in the cortex around the edge of the lesion (Fig. 6A, B, C, F), in response to advancing mycelia (Fig. 6D), and in the endodermis, protecting the vascular tissues (Fig. 6A, C). Autofluorescence and phloroglucinol staining also developed in the cuticle in some genotypes (Fig. 6G, I).
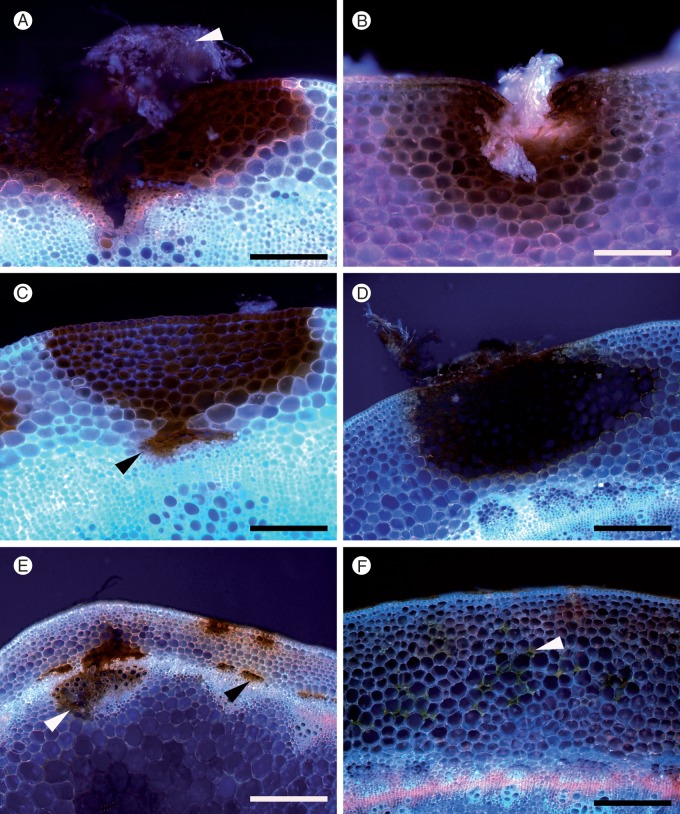
Fig. 6.
Patterns of damage and lignification in transverse sections of Brassica napus stem at 3 or 19 d post-inoculation (dpi) following inoculation with Sclerotinia sclerotiorum MBRS-1. Images captured using fluorescence microscopy or phloroglucinol-HCL staining. (A) Susceptible B. napus YM04 showing strong autofluorescence (AF) surrounding lesion (white arrowhead) and formation of a lignin barrier in the endodermis (black arrowhead) at 3 dpi. (B) Susceptible B. napus YM04 at 19 dpi with very strong AF around the lesion (white arrowhead) and in endodermis (black arrowhead). (C) Susceptible B. napus YM04 at 19 dpi after phloroglucinol-HCl staining, showing wide band of lignification ‘containing’ the lesion (white arrowhead) and in the endodermis (black arrowhead). (D) Resistant B. napus ZY006 AF at 3 dpi showing strong AF in the cortex. (E) Resistant B. napus ZY006 at 3 dpi showing damage in the cortex (not stained). (F) Resistant B. napus ZY006 at 19 dpi showing very strong AF at the edge of a lesion. (G) Susceptible B. carinata SMP3-82 showing damage in vascular fibres (black arrowhead) and AF in the cuticle (white arrowhead) at 3 dpi. (H) Susceptible B. carinata SMP3-82 at 3 dpi (not stained). (I) Susceptible B. carinata SMP3-82 at 7 dpi showing lignification in the cuticle stained pink (arrowhead) following phloroglucinol-HCl staining. Scale bars (A, B, C, F) = 200 µm, (G, H) = 1 mm, (D, E, I) = 100 µm.
Of the genotypes tested, the strongest autofluorescence at 3 dpi was in susceptible B. napus YM04 (Fig. 6A), which showed strong autofluorescence in the cuticle, around the borders of the lesion and also as a distinct barrier in the endodermis. By 19 dpi, the barrier around the surface lesion was extensive and intensive (Fig. 6B, C). Autofluorescence in other genotypes was less comprehensive and mostly comparatively weaker. Resistant B. napus ZY006 showed a strong autofluorescence reaction in the cortex to advancing mycelia (Fig. 6D), but there was no evidence of any defence reaction under brightfield illumination (Fig. 6E). Brassica napus YM18 also displayed strong autofluorescence in the cortex (Fig. 7B). In B. napus ZY006, a strong barrier had formed around the lesion by 19 dpi (Fig. 6F), but not in the endodermis (Fig. 6D, F). Brassica napus ‘Mystic’ developed a distinct barrier in the endodermis, but autofluorescence around the surface lesion was weak (Fig. 7A). The cuticle of B. carinata SMP3-82 fluoresced strongly near the edges of the surface lesion, but weakly around the lesion and in the cortex (Fig. 6G, I).
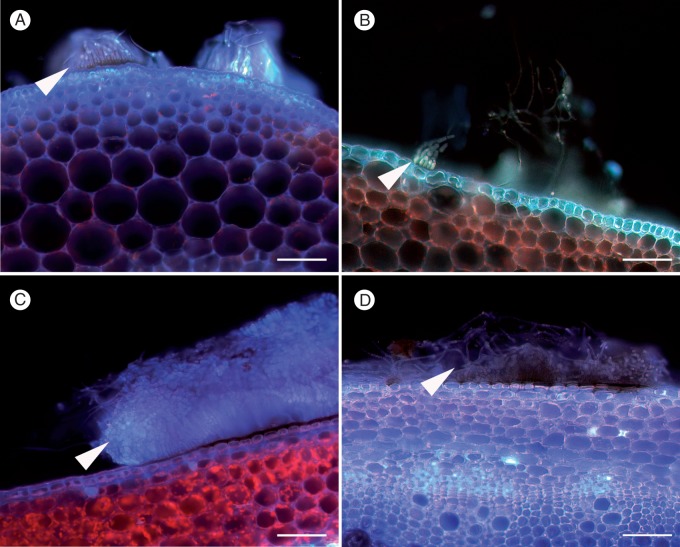
Fig. 7.
Patterns of autofluorescence (AF) in transverse Brassica stem sections at 3 dpi following inoculation with Sclerotinia sclerotiorum MBRS-1. (A) Resistant Brassica napus Mystic showing AF barrier layer (arrowhead) only above the vascular system in the endodermis. (B) Strong AF reaction in stem cortex of susceptible B. napus YM18 at 3 dpi in response to hyphae growing through the cortex (white arrowhead). Hypersensitive reaction on stem surface (black arrowhead). (C) Susceptible B. juncea JM06006 showing weak AF present in cuticle (arrowhead) near the surface lesion and damage to xylem caused by S. sclerotiorum. (D) Susceptible B. juncea JM06006 showing greater detail from C, in particular showing damage to xylem bundles (arrowhead). Scale bars (A–D) = 200 µm.
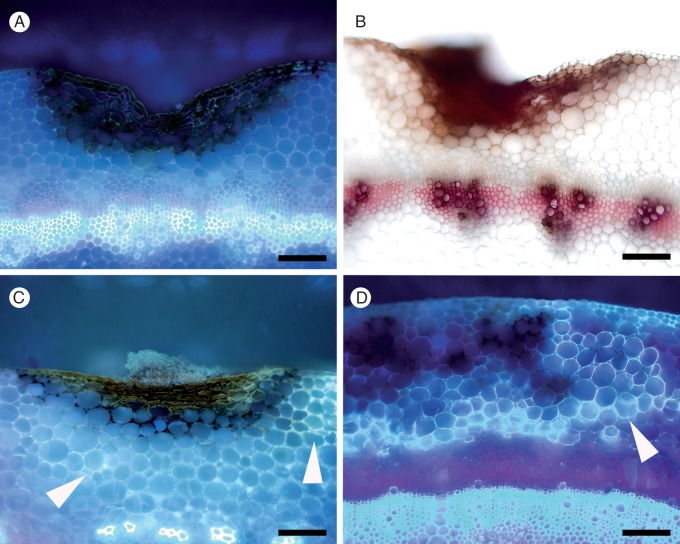
Brassica carinata 054113 generally showed little autofluorescence or lignification by 3 dpi (Fig. 8A, B), although in several samples a strong reaction to advancing mycelia in the cortex was observed. However, by 19 dpi, a distinct lignin layer developed, delimiting the surface lesion (Fig. 8C, D). While there was no clear relationship between the strength of autofluorescence at 3 dpi and lesion length, there was a trend for Brassica genotypes displaying strong autofluorescence in any part of the cortex to have smaller lesions (Table 2). In contrast, there was no evidence that autofluorescence in the cuticle was associated with increased resistance. Strong autofluorescence was not observed in the cuticle, cortex, or endodermis in the absence of S. sclerotiorum in any of the stems.
Fig. 8.
Progression of lignification in resistant Brassica carinata 054113. (A) Minimal autofluorescence (AF) around the lesion at 3 dpi. (B) No lignification around the lesion at 3 dpi following phloroglucinol-HCl staining (lignin in xylem stained pink). (C) Developing AF around the lesion at 7 dpi (arrowheads). (D) Strong AF around the lesion at 19 dpi (arrowhead). Scale bars (A–D) = 200 µm.
In genotypes that failed to stop the encroachment of S. sclerotiorum into the vascular system, fluorescence was reduced in colonized tissues (Fig. 6G), and when the same samples were viewed under brightfield illumination, damage to the stem was evident as dark discoloration (Fig. 6H). Infection sometimes resulted in breakdown within xylem bundles with a marked decrease in autofluorescence (Fig. 7C, D).
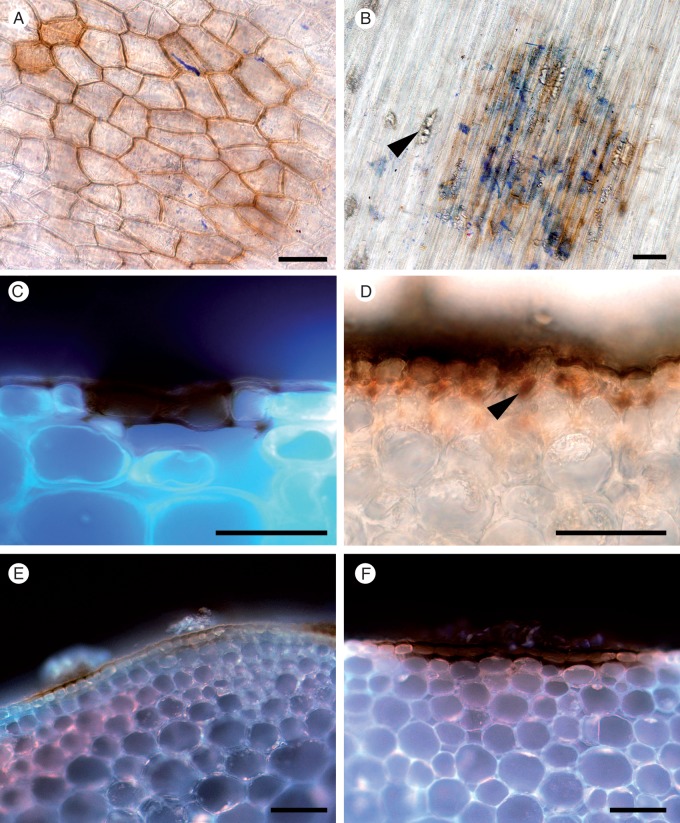
Discoloration of epidermal cell walls and HR.
A typical HR (Ulloa and Hanlin, 2000), comprising dark brown/black dried spots or sometimes a slight discoloration of the stem surface, indicated sites of failed infections on the stems of B. carinata and B. napus (Fig. 9). When decolourized stem surface tissues were observed under the microscope, there was often evidence of past S. sclerotiorum presence in the form of hyphal fragments and/or calcium oxalate crystals (Fig. 9A, B). It was noted that no autofluorescence was visible in the cell layers beneath these sites (Fig. 9C, E, F). In >95 % of samples, no further growth of S. sclerotiorum extended from these sites of discoloration within the epidermal layer (Fig. 9C, E, F). Infection sites that later developed into lesions could be distinguished by the presence of hyphae and/or cell damage in the cortex beneath the discoloured epidermis (Fig. 9D).
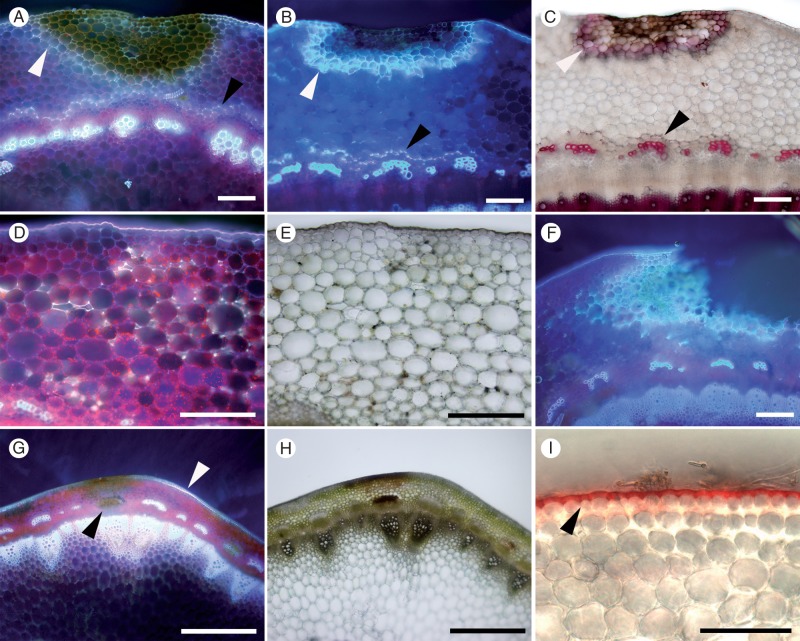
Fig. 9.
Discoloration of epidermal cell walls after resistant and susceptible Brassica carinata and B. napus lines were challenged with S. sclerotiorum isolates MBRS-1 and WW-3 (A, B, C, E, F). Hypersensitive reaction (HR) at sites of failed infection on Brassica stems. (A) Resistant B. carinata 054113 at 3 dpi (WW-3). (B) Disintegrating hyphae, calcium oxalate crystals (arrowhead) and discoloration of cell walls at the site of an attempted infection on the stem of resistant B. napus ZY006 at 3 dpi (MBRS-1). (C) Discoloration of cell walls and cell death at site of a failed infection on resistant B. carinata 054113 at 3 dpi (WW-3). (D) Successful infection, with growth of subcuticular hyphae (arrowhead) in susceptible B. napus YM04 at 3 dpi in (MBRS-1). (E, F) Discoloration of cell walls and cell death at site of a failed infection on (E) susceptible B. carinata SMP3-82 at 3 dpi (MBRS-1) and (F) resistant B. carinata 054113 at 3 dpi (MBRS-1). Scale bars (A–F) = 50 µm.
DISCUSSION
These studies are the first to define host–pathogen interactions involving S. sclerotiorum on stems of B. carinata B. juncea and B. napus. Comparison of resistant and susceptible genotypes of B. carinata, B. napus and B. juncea revealed a range of distinct mechanisms in Brassica stems associated with their relative ability to resist infection by S. sclerotiorum. Specific mechanisms defined in Brassica stems for the first time include HR, lignification within the stem cortex and/or around the edges of lesions, and a greater number of host cortical cell layers. Reasons for the preferential longitudinal extension of SSR stem lesions were explained, and the involvement of extensive vascular invasion in severe stem disease caused by Sclerotinia was demonstrated. Differences in infection strategy between highly aggressive and mildly aggressive isolates of S. sclerotiorum were highlighted. Hyphae of the highly virulent isolate MBRS-1 were shown to group together in dense parallel bundles in the infection cushion compared with more diffuse hyphae and less organized infection cushions of the weakly aggressive isolate WW-3. These findings demonstrate that the variable outcome of field stem infections and stem disease development is a consequence of complex interactions between several host resistance mechanisms and pathogen isolate characteristics. This study also demonstrated that infection processes on stems of S. sclerotiorum are analogous between B. carinata and B. napus.
Invasion of vascular system
Experiment 1 showed the movement of S. sclerotiorum into vascular fibres of susceptible B. carinata SMP3-82 soon after penetration, as well as growth along the vascular fibres or into and along the xylem. Experiment 2 demonstrated that genotypes where S. sclerotiorum extensively invaded the vascular system always had, relatively, very long lesions. Sclerotinia sclerotiorum preferentially targets the plant’s vascular system, which provides not only nutrients but also a convenient conduit for rapid longitudinal growth. There have only been a few reports of Sclerotinia entering the xylem during infection of oilseed brassicas (Buchwaldt et al., 2005; Huang et al., 2008), common bean (Lumsden and Dow, 1973; Tariq and Jeffries, 1986) and clover (Prior and Owen, 1964), and this is the first time that the importance of entry to the vascular system has been demonstrated for any Sclerotinia species. In addition, entry of the fungus to the vascular system was observed earlier (3 dpi) than in the previous report for brassicas (6 dpi) (Huang et al., 2008). Importantly, the current study was able to show the extent of vascular involvement in disease progression for S. sclerotiorum because intact and elongated stems on plants approaching flowering were used, as opposed to the cotyledons (Garg et al., 2010c), hypocotyls (Huang et al., 2008) or detached plant parts (Jamaux et al., 1995) used in previous studies.
Subcuticular growth of S. sclerotiorum
The proliferation of prominent subcuticular hyphae helps S. sclerotiorum to rapidly exploit susceptible host tissues and extend growth up and down the stem. In Experiment 1, subcuticular hyphae were commonly present in the susceptible B. carinata SMP3-82 but not in the resistant B. carinata 054113, confirming the role of effective resistance in impeding this hyphal proliferation. A similar finding was previously made for S. sclerotiorum in Phaseolus coccineus (Lumsden, 1979), where resistant tissue posed a physical barrier to the fungus, while in the susceptible host infection hyphae were capable of rapid intercellular spread and development beneath the surface of the stem.
Utilization of the vascular system by S. sclerotiorum and/or extensive subcuticular growth helps explain the preferential longitudinal, rather than radial, expansion of SSR lesions. Damage associated with largely unimpeded movement of S. sclerotiorum through a stem’s vascular system beneath intact surface tissues can be seen in the field with the naked eye (Fig. 1B) and explains why relative resistance to SSR in the Brassicaceae is best presented in terms of lesion length (Garg et al., 2010a).
Lignification in B. napus and B. carinata
This study provides the first strong evidence for a significant role for S. sclerotiorum-induced lignification in the stems of B. carinata and B. napus. Autofluorescence of cell walls indicated the presence of phenolic compounds that were laid down variously around lesions, within the cortex, in the cuticle and in a protective layer in the endodermis above the vascular elements of the stem when challenged by S. sclerotiorum. No autofluorescence was observed in these tissues in the absence of S. sclerotiorum. Successful staining with phloroglucinol-HCl of the same structures suggests strongly that the changes in cell-wall composition during infection by S. sclerotiorum were due to lignin deposition.
Lignin has often been proposed as an agent used by plants to defend against plant pathogens (Vance et al., 1980; Nicholson and Hammerschimdt, 1992). It is thought to act as a physical barrier and to resist the diffusion of toxins produced by the pathogen (Dushnicky et al., 1998). Within the Brassicaceae, Eynck et al. (2012) presented evidence that a resistant selection of Camelina sativa produced more precursors of lignin biosynthesis and concluded that plant cell-wall strengthening plays a role in resistance to S. sclerotiorum. A gene involved in monolignol synthesis (BnaC.IGMT5.a) has recently been linked to a major resistance quantitative trait locus for S. sclerotiorum resistance in B. napus (Wu et al., 2013), supporting the current finding of lignification as an important mechanism of resistance to SSR. The lignification of cell walls within the stems of infected brassicas appears to impede the advance of S. sclerotiorum by not only stopping the extension of hyphae of S. sclerotiorum within the cortex, but also by building a protective ‘containment’ barrier around the edges of lesions. Previous work in B. napus has shown lignin deposition is an important resistance mechanism in response to infection by pathogens such as L. maculans (Li et al., 2007a, b) and Verticillium longisporum (Eynck et al., 2009). Of particular relevance are studies of L. maculans invasion of stems of B. napus (Li et al., 2007b), which showed post-penetration defence reactions including a HR, lignification, suberization and additional cambium formation in cultivars resistant to L. maculans that together impeded pathogen progress into the vascular tissues. Additional cambium formation was not observed with S. sclerotiorum.
The lignification reaction varied in location, strength and timing in each of the 26 Brassica genotypes tested. There was no direct relationship between the strength of the reaction and mean lesion length for a particular genotype, but there was a clear trend that genotypes showing strong lignification had shorter lesions 3 dpi. This suggests that while increased lignification is not always effective in providing early resistance to S. sclerotiorum, it can successfully curtail early growth of the pathogen where the expression of lignification is strong and rapid. It is likely that lignification becomes even more influential in the later stages of the infection process. This was best illustrated in B. carinata 054113 in Experiment 2, where lesions were completely surrounded by a thick layer of lignin by 14 dpi and were drying out, in spite of a substantial and seemingly uncontrolled infection at 3 dpi. The zonate appearance of many older lesions in the field (e.g. Fig. 1C) also suggests a role for lignification in delimiting S. sclerotiorum. This is in line with the findings of Hammond and Lewis (1986, 1987) for L. maculans. They described a series of attempts on the part of the plant to limit the spread of the fungus through lignification, which are overcome by the pathogen, leading to a similar zonate appearance of lesions. Further elucidation of the role of lignification in host defence against S. sclerotiorum is warranted. Clarification and classification of lignification reactions from a wider range of brassicas may allow the future development of a rating scale for resistance that is measured by the lignification response.
Hypersensitive reaction
Strong evidence was provided in Experiments 1 and 2 that even susceptible Brassica genotypes can sometimes prevent entry of S. sclerotiorum using a HR. The HR is characterized by localized programmed cell death at the site of the attempted invasion by the pathogen and has been identified as the major defence reaction against other Brassica pathogens such as L. maculans (Li et al., 2007b). Garg et al. (2008, 2010c) described a HR for S. sclerotiorum on B. napus and on B. napus and B. juncea containing one or more introgressions from weedy crucifer species. On the former, the expression of a HR was secured by utilizing a weakly virulent isolate in combination with strict control of both inoculum concentration and environmental conditions (Garg et al., 2008). Sutton and Deverall (1984) also reported a HR in soybean affected by S. sclerotiorum at the site of fungal penetration, with the fungus restricted to cells showing the HR. With necrotrophic pathogens such as S. sclerotiorum, which can utilize dead cells as a nutrient source, it could be argued that plant cell death in the initial phases of the HR should promote fungal colonization of susceptible host tissue. However, it appears that there is a balance between promotion and destruction of the invading S. sclerotiorum, as argued by Williams et al. (2011). The lack of autofluorescence around the HR suggests that there was no further stimulation of the plant’s defences after expression of the HR, except in the immediate surrounding cuticle. This autofluorescence reaction in the cuticle may be a response to the growth of S. sclerotiorum on the stem surface.
The observation of a HR in the Brassica genotypes examined is in agreement with information available from proteomic and biochemical studies of the resistance response of brassicas. These studies have revealed up-regulation of genes involved in the oxidative burst that are important in the initial phase of the HR, including evidence of the activation of salicylic acid and jasmonic acid-mediated defence responses and inhibition of H2O2 accumulation (Zhao et al., 2007, 2009; Wang et al., 2009, 2014; Rietz et al., 2012; Garg et al., 2013). Studies have also shown that protein production after infection and up-regulation of genes associated with defence, such as WRKY, zinc finger proteins and plant cell-wall-related proteins (Zhao et al., 2007) and phytoalexin production (Bennett and Wallsgrove, 1994), are all faster in resistant than in susceptible Brassica genotypes. Differences in the speed of the host response may help to explain the variable success of expression of the HR in highly and moderately susceptible Brassica genotypes. Where the HR is slow, it may be successful only in conditions that are not conducive to the pathogen, and fail in conditions that favour the pathogen. Similarly, the HR in a highly resistant genotype may not be successful in preventing infection in every case due to the balance between host and pathogen responses mentioned earlier (Williams et al., 2011). In fact, all Brassica genotypes in the current study were able to sometimes preclude significant colonization by S. sclerotiorum by invoking the HR. This ability may partially explain why inoculum levels are so important in determining the extent of disease development in the field (Clarkson et al., 2014). It follows that if only some of attempted infections are successful due to the HR, then a larger number of propagules will be needed to establish successful infection. In summary, our observations of the HR are consistent with the theory that infection by S. sclerotiorum always invokes a HR in Brassica stems; this sometimes prevents infection, but that at other times the programmed cell death of the HR instead helps the pathogen initiate a lesion. This would explain some of the observed variability in the response of brassicas to infection by S. sclerotiorum (Taylor et al., 2015). A more rigorous investigation of the HR on the stems of resistant and susceptible brassicas would be beneficial.
Variation in the expression of resistance was also seen in the early phases of infection within the stem, with resistant B. napus ZY006 having lesions as long as susceptible B. carinata SMP3-82 at 3 dpi in Experiment 2. In spite of this, by 19 dpi, the resistant variety had contained the lesions. The ability of B. napus ZY006 to reassert its control of the infection, even under conditions where S. sclerotiorum has successfully established an early infection, is likely to contribute to the genotype’s consistently resistant phenotype in field trials and controlled environment experiments (Li et al., 2009; Ge et al., 2012; Uloth et al., 2013a, 2015b; Barbetti et al., 2014).
Number of cell layers in the stem cortex
The observation in initial imaging that a susceptible B. carinata genotype had fewer layers in the cortex than a resistant genotype led to an experiment comparing cell layers, stem diameter and lesion length for 26 Brassica genotypes. It is logical that the larger the number of cell walls the fungus has to breach, the slower its progress will be, and that extra cell layers would impede invasion of the vascular system. This was consistent with the study’s results, and a significant negative correlation was found between the number of cell layers and the severity of infection. The influence of this parameter on Sclerotinia disease warrants study in other relevant host species.
A link between stem diameter and SSR resistance was proposed by Li et al. (2006) after a field trial in which genotypes with either a large (> approx. 14 mm) or a small (< 7 mm) stem diameter were more susceptible to infection. However, subsequent studies found little or no correlation between Brassica stem diameter and resistance (Li et al., 2007; Garg et al., 2010a). In the current study, mean stem diameter did not differ between Brassica genotypes. However, stems < 4 mm in diameter were frequently catastrophically infected, and this is consistent with the finding of Li et al. (2006) that stems with the smallest diameters were more severely infected by S. sclerotiorum. It also implies that the level of resistance to SSR should be checked in Brassica genotypes tending towards particularly small stems before they are utilized in breeding programmes, especially where this is associated with few cell layers in the cortex.
Stage of infection process and ageing in the hyphal network
The techniques used in this study mimic natural infection processes, with the discs of filter paper impregnated with hyphae representing the infested petals from which the majority of stem infections occur in the field (Morrall and Dueck, 1982). The infection process and timing on B. carinata stems was similar to that reported on detached B. napus stems by Huang et al. (2008). A distinct ageing process was observed in the mycelium. Early in the infection process (≤ 24 hpi), hyphae grew rapidly, were easily stained and full of opaque cytoplasmic contents, hyphal walls had a high level of integrity, and diffusion of hyphal protoplast materials onto the stem surface was not evident. However, after some hyphae penetrated the cuticle, integrity of the surface hyphae was lost, and by 72 hpi hyphae visible on the surface of the lesion were highly vacuolated. This pattern was evident across all genotypes and suggests that S. sclerotiorum has an organized hyphal network that follows a distinct ‘ageing’ process. The hyphal network commences with the growth of young hyphae on the stem surface, progresses to penetration phase, and then to the exploitation of plant material and growth within the primary lesion. This leads to the production of a hyphal mass that can support extension along the host’s stem and penetration into the interior of the stem. For hosts with a particularly narrow cortex, such as B. carinata SMP3-82, entry to the plant vascular tissues allows rapid access to these nutrient-rich tissues, and fosters rapid lesion expansion, with a longitudinal path unimpeded by constraints from cells/cell walls. Our observation of highly vacuolated older hyphae confirms the report of De Silva et al. (2009), who found that older hyphae were hard to image even with green fluorescent protein-transformed S. sclerotiorum due to their high level of vacuolation. The presence of heavily vacuolated hyphae in the surface lesions by 72 hpi suggests mobilization of resources to the advancing ‘infection front’ by S. sclerotiorum in the stem. Studies of the movement of radioisotopes through the hyphal network of a variety of fungi have shown that nutrients and organelles can be translocated from source to sink (Lindahl and Olsson, 2004), as the hyphal network ages. Translocation of nutrients from old to young hyphae of Sclerotinia rolfsii has also been demonstrated using radioisotopes (Wilcoxson and Subbarayudu, 1968). This process is analogous to the source/sink relationship between young and mature leaves of higher plants, where young developing leaves import photosynthetic assimilates exported by mature leaves (Fellows and Geiger, 1974). This mode of exploitation of resources by S. sclerotiorum hyphae in one area followed by transportation to the infection front suggests that if this process could be interrupted, then extension of the lesion and perhaps even subsequent production of sclerotia could be impeded. This provides a potential avenue through which control of S. sclerotiorum could be achieved.
Structure of infection cushions
The more aggressive isolate of S. sclerotiorum (MBRS-1) formed infection cushions that were more densely packed than those of the less aggressive isolate (WW-3), and this was independent of host. Infection cushions are known to vary in size and complexity according to the nutrition of the hyphae from which they are formed (Abawi et al., 1975), and the mechanical resistance of the surface under attack (Tariq and Jeffries, 1984). Sclerotinia sclerotiorum relies in part on mechanical pressure to enable penetration of the host (Lumsden, 1979). It is possible that more compact infection cushions of MBRS-1 are able to exert more pressure than those of WW-3 and that this contributes to the greater aggressiveness of MBRS-1. That hyphae of MBRS-1 appear to be able to penetrate through the host surface layers en masse, in association with a depression of the surface layers which appears to have been caused by pressure exerted from the surface, supports this conclusion. Uloth et al. (2015b) recently showed that at high temperatures (28 °C), WW-3 can be equally as aggressive as MBRS-1. It would be interesting to determine whether this is at least partly due to the formation of more robust infection cushions by WW-3 at high temperatures.
Garg et al. (2010c) reported that the formation of infection structures was completely suppressed on the cotyledons of a resistant genotype of B. napus. On the stem, by contrast, there was no obvious difference in the number of infection cushions between resistant and susceptible genotypes. The distorted hyphae and extrusion of cell contents seen on resistant cotyledons (Garg et al., 2010c) were also not observed. Although by no means definitive proof, these contrasting reactions support previous suggestions (Uloth et al., 2013a) that different resistance mechanisms operate in Brassica stems, leaves and cotyledons, and also between seedlings and adult plants.
In conclusion, a range of resistance mechanisms in the stems of B. napus and B. carinata have been revealed, including: deployment of the HR; lignification within the stem cortex, in the endodermis and at the edge of lesions; and the influence of the number of cortical cell layers impeding S. sclerotiorum from reaching the vascular elements of the stem. These studies highlight the variable nature of the resistance reaction in the S. sclerotiorum/Brassica pathosystem. ‘Susceptible’ Brassica genotypes can sometimes impede infection processes in a limited way, and even ‘resistant’ genotypes can succumb where the pathogen isolate is highly virulent and environmental conditions are highly conducive. Further identification and control of factors affecting the expression of resistance to this devastating pathogen are essential to develop oilseed brassicas with high levels of reliable resistance, which would result in yield increases and reduction of pesticide use.
ACKNOWLEDGEMENTS
M.B.U. gratefully acknowledges the financial assistance of an Australian Postgraduate Award. Operational funding support for this research was provided by the School of Plant Biology, University of Western Australia (UWA), and by the Australia Research Council in conjunction with the Department of Agriculture and Food Western Australia (project LP100200113, ‘Factors responsible for host resistance to the pathogen Sclerotinia sclerotiorum for developing effective disease management in vegetable brassicas’). Exceptional technical support from Mr Robert Creasy and Mr William Piasini in the UWA Plant Growth Facilities is also gratefully acknowledged. We acknowledge the facilities and assistance of the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy, Characterisation and Analysis (CMCA), UWA, a facility funded by the University, State, and Commonwealth Governments. Able technical assistance from Mr John Murphy and Mrs Lyn Kirilak at the CMCA was particularly appreciated by M.B.U.
LITERATURE CITED
- Abawi GS, Polach FJ, Molin WT. 1975. Infection of bean by ascospores of Whetzelinia sclerotiorum. Phytopathology 65: 673–678. [Google Scholar]
- Barbetti MJ, Banga SS, Salisbury PA. 2012. Challenges for crop production and management from pathogen biodiversity and diseases under current and future climate scenarios – case study with oilseed Brassicas. Field Crops Research 127: 225–240. [Google Scholar]
- Barbetti MJ, Uloth M, You MP, Liu SY, Banga SS. 2013. Sclerotinia on oilseed, forage and vegetable cruciferous crops—no longer an impossible disease to manage. In: Proceedings of the 15th International Sclerotinia Workshop, 20–24 August 2013, Wuhan, China. Wuhan, China: Huazhong Agricultural University, 62. [Google Scholar]
- Barbetti MJ, Banga SK, Fu TD, et al. 2014. Comparative genotype reactions to Sclerotinia sclerotiorum within breeding populations of Brassica napus and B . juncea from India and China. Euphytica 197: 47–59. [Google Scholar]
- Bennett RN, Wallsgrove RM. 1994. Secondary metabolites in plant defence mechanisms. New Phytologist 127: 617–633. [DOI] [PubMed] [Google Scholar]
- Boland GJ, Hall R. 1994. Index of plant hosts of Sclerotinia sclerotiorum. Canadian Journal of Plant Pathology 16: 93–108. [Google Scholar]
- Buchwaldt L, Li R, Hegedus DD, Rimmer SR. 2005. Pathogenesis of Sclerotinia sclerotiorum in relation to screening for resistance. In Proceedings of the 13th International Sclerotinia Workshop. Monterey, CA, 22. [Google Scholar]
- Chen S, Zou J, Cowling WA, Meng J. 2010. Allelic diversity in a novel gene pool of canola-quality Brassica napus enriched with alleles from B . rapa and B. carinata. Crop and Pasture Science 61: 483–492. [Google Scholar]
- Clarkson JP, Fawcett L, Anthony SG, Young C. 2014. A model for Sclerotinia sclerotiorum infection and disease development in lettuce, based on the effects of temperature, relative humidity and ascospore density. PLoS ONE 9: e94049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva AP, Bolton MD, Nelson BD. 2009. Transformation of Sclerotinia sclerotiorum with the green fluorescent protein gene and fluorescence of hyphae in four inoculated hosts. Plant Pathology 58: 487–496. [Google Scholar]
- Delourme R, Barbetti M, Snowdon R, Zhao J, Manazanares-Dauleux MJ. 2011. Genetics and genomics of disease resistance. In: Edwards D, Batley J, Parkin IAP, Kole C, eds. Genetics, genomics and breeding of oilseed brassicas. Boca Raton, FL: Science Publishers, 276–318. [Google Scholar]
- Dushnicky LG, Ballance GM, Sumner MJ, MacGregor AW. 1998. The role of lignification as a resistance mechanism in wheat to a toxin-producing isolate of Pyrenophora tritici-repentis. Canadian Journal of Plant Pathology 20: 35–47. [Google Scholar]
- Eynck C, Koopmann B, Karlovsky P, von Tiedemann A. 2009. Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum. Phytopathology 99: 802–811. [DOI] [PubMed] [Google Scholar]
- Eynck C, Séguin-Swartz G, Clarke WE, Parkin IAP. 2012. Monolignol biosynthesis is associated with resistance to Sclerotinia sclerotiorum in Camelina sativa. Molecular Plant Pathology 13: 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows RJ, Geiger DR. 1974. Structural and physiological changes in sugar beet leaves during sink to source conversion. Plant Physiology 54: 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahan PB. 1984. Plant histochemistry and cytochemistry - an introduction. London: Academic Press. [Google Scholar]
- Garg H, Sivasithamparam K, Banga SS, Barbetti MJ. 2008. Cotyledon assay as a rapid and reliable method of screening for resistance against Sclerotinia sclerotiorum in Brassica napus genotypes. Australasian Plant Pathology 37: 106–111. [Google Scholar]
- Garg H, Atri C, Sandhu PS, et al. 2010a. High level of resistance to Sclerotinia sclerotiorum in introgression lines derived from hybridization between wild crucifers and the crop Brassica species B . napus and B. juncea. Field Crops Research 117: 51–58. [Google Scholar]
- Garg H, Kohn LM, Andrew M, Li H, Sivasithamparam K, Barbetti MJ. 2010b. Pathogenicity of morphologically different isolates of Sclerotinia sclerotiorum with Brassica napus and B . juncea genotypes. European Journal of Plant Pathology 126: 305–315. [Google Scholar]
- Garg H, Li H, Sivasithamparam K, Kuo J, Barbetti MJ. 2010c. The infection processes of Sclerotinia sclerotiorum in cotyledon tissue of a resistant and a susceptible genotype of Brassica napus. Annals of Botany 106: 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg H, Li H, Sivasithamparam K, Barbetti MJ. 2013. Differentially expressed proteins and associated histological and disease progression changes in cotyledon tissue of a resistant and susceptible genotype of Brassica napus infected with Sclerotinia sclerotiorum. PLoS ONE 8: e65205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XT, Li YP, Wan ZJ, et al. 2012. Delineation of Sclerotinia sclerotiorum pathotypes using differential resistance responses on Brassica napus and B. juncea genotypes enables identification of resistance to prevailing pathotypes. Field Crops Research 127: 248–258. [Google Scholar]
- Hammond KE, Lewis BG. 1986. Ultrastructural studies of the limitation of lesions caused by Leptosphaeria maculans in stems of Brassica napus var . oleifera. Physiological and Molecular Plant Pathology 28: 251–265. [Google Scholar]
- Hammond KE, Lewis BG. 1987. Variation in stem infections caused by aggressive and non-aggressive isolates of Leptosphaeria maculans on Brassica napus var. oleifera. Plant Pathology 36: 53–65. [Google Scholar]
- Huang HC, Kokko EG, Erickson RS, Hynes RK. 1998. Infection of canola pollen by Sclerotinia sclerotiorum. Plant Pathology Bulletin 7: 71–77. [Google Scholar]
- Huang L, Buchenauer H, Han Q, Zhang X, Kang Z. 2008. Ultrastructural and cytochemical studies on the infection process of Sclerotinia sclerotiorum in oilseed rape. Journal of Plant Diseases and Protection 115: 9–16. [Google Scholar]
- Jamaux I, Gelie B, Lamarque C. 1995. Early stages of infection of rapeseed petals and leaves by Sclerotinia sclerotiorum revealed by scanning electron microscopy. Plant Pathology 44: 22–30. [Google Scholar]
- Jones D. 1976. Infection of plant tissue by Sclerotinia sclerotiorum: a scanning electron microscope study. Micron (1969) 7: 275–279. [Google Scholar]
- Li CX, Li H, Sivasithamparam K, et al. 2006. Expression of field resistance under Western Australian conditions to Sclerotinia sclerotiorum in Chinese and Australian Brassica napus and Brassica juncea germplasm and its relation with stem diameter. Australian Journal of Agricultural Research 57: 1131–1135. [Google Scholar]
- Li CX, Li H, Siddique AB, et al. 2007. The importance of the type and time of inoculation and assessment in the determination of resistance in Brassica napus and B . juncea to Sclerotinia sclerotiorum. Australian Journal of Agricultural Research 58: 1198–1203. [Google Scholar]
- Li CX, Liu SY, Sivasithamparam K, Barbetti MJ. 2009. New sources of resistance to Sclerotinia stem rot caused by Sclerotinia sclerotiorum in Chinese and Australian Brassica napus and B . juncea germplasm screened under Western Australian conditions. Australasian Plant Pathology 38: 149–152. [Google Scholar]
- Li H, Kuo J, Barbetti MJ, Sivasithamparam K. 2007a. Differences in the responses of stem tissues of spring-type Brassica napus cultivars with polygenic resistance and single dominant gene-based resistance to inoculation with Leptosphaeria maculans. Canadian Journal of Botany 85: 191–203. [Google Scholar]
- Li H, Stone V, Dean N, Sivasithamparam K, Barbetti M. 2007b. Breaching by a new strain of Leptosphaeria maculans of anatomical barriers in cotyledons of Brassica napus cultivar Surpass 400 with resistance based on a single dominant gene. Journal of General Plant Pathology 73: 297–303. [Google Scholar]
- Li H, Sivasithamparam K, Barbetti M, Wylie S, Kuo J. 2008. Cytological responses in the hypersensitive reaction in cotyledon and stem tissues of Brassica napus after infection by Leptosphaeria maculans. Journal of General Plant Pathology 74: 120–124. [Google Scholar]
- Lindahl BD, Olsson S. 2004. Fungal translocation – creating and responding to environmental heterogeneity. Mycologist 18: 79–88. [Google Scholar]
- Lumsden RD. 1979. Histology and physiology of pathogenesis in plant diseases caused by Sclerotinia species. Phytopathology 69: 890–896. [Google Scholar]
- Lumsden RD, Dow RL. 1973. Histopathology of Sclerotinia sclerotiorum infection of bean. Canadian Journal of Botany 63: 708–715. [Google Scholar]
- Mei J, Ding Y, Lu K, et al. 2012. Identification of genomic regions involved in resistance against Sclerotinia sclerotiorum from wild Brassica oleracea. Theoretical and Applied Genetics 126: 549–556. [DOI] [PubMed] [Google Scholar]
- Morrall RAA, Dueck J. 1982. Epidemiology of sclerotinia stem rot of rapeseed in Saskatchewan. Canadian Journal of Plant Pathology 4: 161–168. [Google Scholar]
- Nicholson RL, Hammerschimdt R. 1992. Phenolic compounds and their role in disease resistance. Annual Review of Phytopathology 30: 369–389. [Google Scholar]
- Prior GD, Owen JH. 1964. Pathological anatomy of Sclerotinia trifoliorum on clover and alfalfa. Phytopathology 54: 784–787. [Google Scholar]
- Rietz S, Bernsdorff F, Cai D. 2012. Members of the germin-like protein family in Brassica napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia sclerotiorum. Journal of Experimental Botany 63: 5507–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez MA, Venedikian N, Bazzalo ME, Godeas A. 2004. Histopathology of Sclerotinia sclerotiorum attack on flower parts of Helianthus annuus heads in tolerant and susceptible varieties. Mycopathologia 157: 291–302. [DOI] [PubMed] [Google Scholar]
- Sutton DC, Deverall BJ. 1984. Phytoalexin accumulation during infection of bean and soybean by ascospores and mycelium of Sclerotinia sclerotiorum. Plant Pathology 33: 377–383. [Google Scholar]
- Sylvester-Bradley R, Makepeace RJ. 1984. A code for stages of development in oilseed rape (Brassica napus L.). Aspects of Applied Biology 6: 399–419. [Google Scholar]
- Tariq VN, Jeffries P. 1984. Appressorium formation by Sclerotinia sclerotiorum: scanning electron microscopy. Transactions of the British Mycological Society 82: 645–651. [Google Scholar]
- Tariq VN, Jeffries P. 1986. Ultrastructure of penetration of Phaseolus spp. by Sclerotinia sclerotiorum. Canadian Journal of Botany 64: 2909–2915. [Google Scholar]
- Taylor A, Coventry E, Jones JE, Clarkson JP. 2015. Resistance to a highly aggressive isolate of Sclerotinia sclerotiorum in a Brassica napus diversity set. Plant Pathology 64: 932–940. [Google Scholar]
- Ulloa M, Hanlin RT. 2000. Illustrated dictionary of mycology. St Paul, MN: APS Press. [Google Scholar]
- Uloth MB, You MP, Finnegan PM, et al. 2013a. New sources of resistance to Sclerotinia sclerotiorum for crucifer crops. Field Crops Research 154: 40–52. [Google Scholar]
- Uloth MB, You MP, Finnegan PM, Banga SS, Yi H, Barbetti MJ. 2013b. Seedling resistance to Sclerotinia sclerotiorum as expressed across diverse cruciferous species. Plant Disease 98: 184–190. [DOI] [PubMed] [Google Scholar]
- Uloth MB, Clode P, You MP, Barbetti MJ. 2015a. Calcium oxalate crystals: an integral component of the Sclerotinia sclerotiorum/Brassica carinata pathosystem. PLoS One 10: e0122362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uloth MB, You MP, Cawthray G, Barbetti MJ. 2015b. Temperature adaptation in isolates of Sclerotinia sclerotiorum affects their ability to infect Brassica carinata. Plant Pathology, doi:10.1111/ppa.12338. [Google Scholar]
- Vance CP, Kirk TK, Sherwood RT. 1980. Lignification as a mechanism of disease resistance. Annual Review of Phytopathology 18: 259–288. [Google Scholar]
- Wang Z, Mao H, Dong C, et al. 2009. Overexpression of Brassica napus MPK4 enhances resistance to Sclerotinia sclerotiorum in oilseed rape. Molecular Plant-Microbe Interactions 22: 235–244. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fang H, Chen Y, et al. 2014. Overexpression of BnWRKY33 in oilseed rape enhances resistance to Sclerotinia sclerotiorum. Molecular Plant Pathology 15: 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxson RD, Subbarayudu S. 1968. Translocation to and accumulation of phosphorus-32 in sclerotia of Sclerotium rolfsii. Canadian Journal of Botany 46: 85–88. [Google Scholar]
- Williams B, Kabbage M, Kim HJ, Britt R, Dickman MB. 2011. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathogens 7: e1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cai G, Tu J, et al. 2013. Identification of QTLs for resistance to Sclerotinia Stem Rot and BnaC.IGMT5.a as a candidate gene of the major resistant QTL SRC6 in Brassica napus. PLoS ONE 8: e67740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Udall J, Quijada P, Grau CR, Meng J, Osborn T. 2006. Quantitative trait loci for resistance to Sclerotinia sclerotiorum and its association with a homeologous non-reciprocal transposition in Brassica napus L. Theoretical and Applied Genetics 112: 509–516. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang J, An L, et al. 2007. Analysis of gene expression profiles in response to Sclerotinia sclerotiorum in Brassica napus. Planta 227: 13–24. [DOI] [PubMed] [Google Scholar]
- Zhao J, Buchwaldt L, Rimmer SR, et al. 2009. Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum. Molecular Plant Pathology 10: 635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]