Abstract
The aim of this study was to determine the role of the endogenous dynorphin/kappa opioid receptor (DYN/KOP) system in ethanol-induced state-dependent conditioned place preference (CPP). To this end, mice lacking the pro-DYN gene and their wild-type littermates/controls were tested for baseline place preference on day 1, received 15-min morning and afternoon conditionings with saline or ethanol (2 g/kg) each day for three consecutive days and were then tested for CPP under a drug-free state on day 5 and following a saline or ethanol (1 or 2 g/kg) challenge on day 8. Given that compensatory developmental changes may occur in knockout mice, the effect of nor-binaltorphimine (nor-BNI), a KOP antagonist, on state-dependent CPP induced by ethanol was also studied in wild-type mice. On day 1, mice were tested for baseline place preference and, 4 h later, treated with saline or nor-BNI (10 mg/kg). On days 2–4, mice received 15-min morning and afternoon conditionings and were tested for CPP under a drug-free state on day 5 and following an ethanol (1 g/kg) challenge on day 8. A comparable CPP was observed in mice lacking the pro-DYN gene and their wild-type littermates/controls as well as in wild-type mice treated with nor-BNI and their saline-treated controls. However, these mice compared to their respective controls exhibited a greater CPP response following an ethanol (1 g/kg) challenge, suggesting that the endogenous DYN/KOP system may negatively regulate ethanol-induced state-dependent CPP.
Keywords: Alcohol reward, Conditioned place preference (CPP), State-dependent, Knockout mouse, Endogenous dynorphin, Kappa opioid receptor
1. Introduction
Alcoholism and alcohol-related disorders represent a major public health and socioeconomic issue. Alcohol is a powerful reinforcing agent and its chronic use leads to compulsive alcohol-taking and alcohol-seeking behaviors. However, only limited pharmacotherapeutic agents are available to treat this chronic relapsing brain disorder. This is, at least in part, due to the fact that the neurobiological mechanisms underlying the rewarding and reinforcing actions of alcohol are not fully understood. Notably, alcohol administration has been shown to affect numerous neurotransmitter/neuropeptide systems. Therefore, understanding factors that regulate the rewarding and addictive action of alcohol has paramount importance for the development and design of pharmacotherapy to curb alcohol addiction.
The opioid peptides (beta-endorphin, enkephalin and dynorphin) and their receptors (namely mu, delta and kappa opioid receptors) have been implicated in the rewarding and addictive actions of ethanol and other drugs of abuse [1–6]. For example, kappa opioid receptor (KOP) agonists are shown to reduce alcohol reward [7] and self-administration [8,9]. In addition, the dynorphin (DYN)/KOP system may be important in stress-mediated reinstatement of ethanol-seeking behaviors [10].
The endogenous DYN/KOP receptor system may also be involved in alcoholism and alcohol-related disorders [3,6,7,10,11]. Thus, mice lacking KOP compared to their wild-type littermates had a higher dopamine outflow in the nucleus accumbens (NAc) following an acute ethanol challenge [7]. Moreover, ethanol withdrawal was reported to be associated with changes in the level of DYN and its precursor in brain areas relevant to reward and reinforcement [8,12]. Surprisingly, however, mice lacking DYN exhibited a conditioned place preference (CPP) response that was comparable to their wild-type controls [13]. Nevertheless, given that changes in the level of DYN and pro-DYN mRNA have been reported shortly after withdrawal from ethanol [8,12], we assessed whether ethanol CPP would be altered if mice were tested at a later time point following ethanol conditioning.
Earlier studies have shown a greater CPP response under a drugged compared to drug-free state, a phenomenon referred to as state-dependent CPP. This phenomenon has been reported with methamphetamine [14] and even with agents that induce aversion [15–17]. However, there appears to be a lack of support for state-dependent CPP following ethanol administration at least in some mouse strains [18]. Given that ethanol possesses both rewarding and aversive effects [19,20], that DYN levels increase following alcohol administration [8,12], and that DYN and related KOP agonists can induce aversion [3], we also determined whether state-dependent CPP induced by ethanol would be regulated by the endogenous DYN/KOP system. Thus, using an unbiased CPP paradigm and mice lacking DYN [21] and their wild-type littermates/controls, we assessed the role of endogenous DYN in ethanol-induced CPP both under a drug-free state as well as in the presence of an ethanol challenge, i.e., state-dependent CPP. Considering that compensatory changes may develop in knockout mice, we also examined whether ethanol-induced state-dependent CPP would be altered in wild-type mice treated with nor-binaltorphimine (nor-BNI), a selective and long-acting KOP antagonist [22], compared to saline-treated controls.
2. Materials and methods
2.1. Subjects
Male and female C57BL/6 mice as well as female mice lacking the pro-DYN gene [21] and/or wild-type littermates/controls (2–4-month-old) fully backcrossed (F12) on the C57BL/6 mouse strain were used for the experiments. Mice were housed 2–4 per cage with free access to laboratory chow and water under a 12-h light/12-h dark cycle. All experiments were conducted according to the National Institute of Health Guideline and approved by the Western University of Health Sciences Animal Care and Use Committee (Pomona, CA, USA).
2.2. Experimental procedures
2.2.1. Experiment 1: to assess the role of gender in ethanol-induced CPP
A three-chambered CPP apparatus (ENV-3013, Med Associates Inc., St. Albans, VT, USA) was used. The apparatus consisted of a smaller (7.2 cm × 12.7 cm × 12.7 cm) central grey chamber with smooth surface, used as the neutral chamber, and two conditioning chambers on either side identical in size (16.8 cm × 12.7 cm × 12.7 cm) but distinguishable from each other by visual (horizontal or vertical 2.54 cm black and white stripes) and tactile (rod surface or mesh floor) cues. The CPP procedure was conducted over a 5-day period. On day 1 (D1), male and female mice were tested for baseline place preference. Mice then received conditioning twice daily (once in the morning and once in the afternoon) on days 2–4, in which they were injected with saline or ethanol (2 g/kg, i.p.) and confined to the saline- or ethanol-paired chamber for 15 min. In the afternoon session, mice received the alternate treatment and were confined to the opposite chamber for 15 min. The assignment of mice to the treatments and conditioning chambers was carried out in a counterbalanced manner in which some animals received alcohol in the chamber with rod floor and some in the chamber with mesh floor. Also, we made sure that some animals received ethanol conditioning in the chamber with horizontal stripes and some in the chamber with vertical stripes. On day 5, mice were tested for postconditioning place preference under a drug-free state. On each test day (days 1 and 5), each mouse was placed in the central neutral chamber and allowed to freely explore all CPP chambers. The amount of time that mice spent in each chamber was recorded and used as a measure of place preference.
2.2.2. Experiment 2: to characterize the role of endogenous DYN in ethanol-induced CPP
Mice lacking DYN and their wild-type littermates were tested for baseline place preference on day 1, received their twice-daily (once in the morning and once in the afternoon) conditionings on days 2–4 and were tested for CPP on day 5, as described above. We used this dose of ethanol to enable us to compare our results to a previous report [13], which also studied the role of endogenous DYN in alcohol reward using mice on a mixed background mouse strain (C57BL/6J × 129/Sv-Tac). Given that changes in the level of DYN and pro-DYN mRNA have been reported shortly after withdrawal from ethanol [8,12], we determined whether ethanol CPP would be altered in mice lacking DYN if mice were tested for CPP at a later time point following the conditionings. Thus, mice were also tested for CPP on day 8.
2.2.3. Experiment 3: to characterize the role of endogenous DYN in ethanol-induced state-dependent CPP
Mice lacking DYN and their wild-type littermates/controls were tested for baseline place preference on day 1, received saline/ethanol or ethanol/saline conditioning on days 2–4 and were tested for CPP on day 5 and state-dependent CPP on day 8. To test for state-dependent CPP, mice were injected with ethanol (1 or 2 g/kg) and immediately thereafter were placed in the central neutral chamber and allowed to explore the CPP chambers. The amount of time that mice spent in each chamber was recorded. Mice used for the state-dependent CPP with the higher dose of ethanol (2 g/kg) were also tested for locomotor activity a week later. In order to reduce the number of mice used, we gave the same animal two doses of ethanol or one dose of ethanol and an injection of saline, so that they received two treatments separated from each other by 48 h. On the test day, mice were habituated to the locomotor activity chambers for 1 h, as described earlier [23], then injected with saline or ethanol (1 or 2 g/kg, s.c.), and locomotor activity was recorded for 1 h. To assure that our results were not skewed by the use of the same mice used for the CPP experiment, we also assessed locomotor activity in naïve DYN null mice and their wild-type littermates following single or repeated (once daily for 4 days) administration of the higher dose of ethanol (2 g/kg, i.p.).
2.2.4. Experiment 4: to examine the effect of nor-BNI, a selective and long-acting KOP antagonist, on ethanol-induced CPP
Wild-type mice were tested for baseline place preference on day 1 and treated with saline or nor-BNI (10 mg/kg) 4 h later. On the following day, mice received morning/afternoon saline/ethanol or ethanol/saline conditionings for three consecutive days and were then tested for postconditioning place preference on day 5, as described above. Mice were also tested for ethanol-induced state-dependent CPP on day 8, in which mice were injected with ethanol (1 g/kg) immediately prior to a CPP test.
3. Data analysis
Data are expressed as mean (±S.E.M.) of the amount of time that mice spent in the CPP chambers on preconditioning (D1), postconditioning (D5) and state-dependent (D8) test days or distance traveled (cm) following ethanol administration. Data were analyzed using two-factor or repeated measures analysis of variance (ANOVA) with the factors being test day and the amount of time that mice spent in the CPP chambers, genotype, or treatment with repeated measures over the test days. The Bonferroni post-hoc test was used to reveal significant differences in the amount of time that mice spent in the ethanol-paired vs. saline-paired chamber. A value of p < 0.05 was considered statistically significant.
4. Results
4.1. Ethanol induced a significant CPP in female but not male mice under the current unbiased CPP paradigm
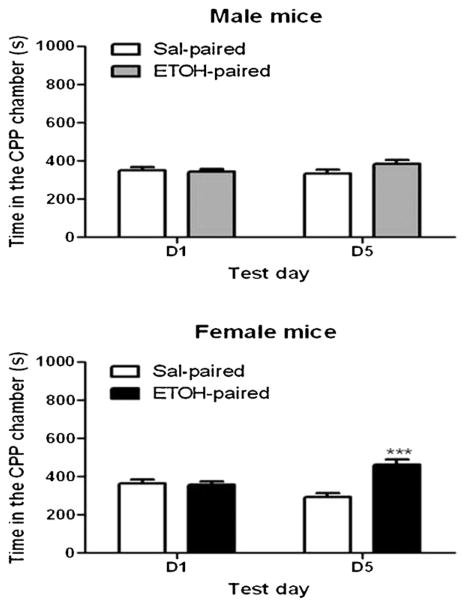
The amount of time that male and female mice spent in the CPP chambers on the preconditioning (D1) and postconditioning (D5) test days is shown in Fig. 1. Repeated measures ANOVA of data in male mice revealed no significant effect of time that mice spent in the CPP chamber (F(1,56) = 1.25; p = NS), no significant effect of test day (F(1,56) = 0.79; p = NS) and no significant interaction between the two factors (F(1,56) = 3.43; p = 0.07), suggesting that male mice did not express CPP (Fig. 1, upper panel). In contrast, female mice exhibited a robust CPP response (Fig. 1, lower panel), as evidenced by a significant interaction between the two factors (F(1,44) = 32.95; p < 0.0001). This result illustrates that female but not male mice exhibited a significant CPP under our current unbiased CPP paradigm. Accordingly, we used female mice for the rest of the experiments.
Fig. 1.
Ethanol-induced CPP in male and female C57BL/6 mice. Mice were tested for baseline place preference on day 1 (D1), received saline/ethanol (2 g/kg) or ethanol/saline conditioning twice daily on days 2–4 and were then tested for postconditioning place preference on day 5 (D5). Data are mean (±S.E.M.) of 23–29 mice per gender.
4.2. Ethanol-induced CPP under a drug-free state was not altered in mice lacking DYN compared to their wild-type littermates
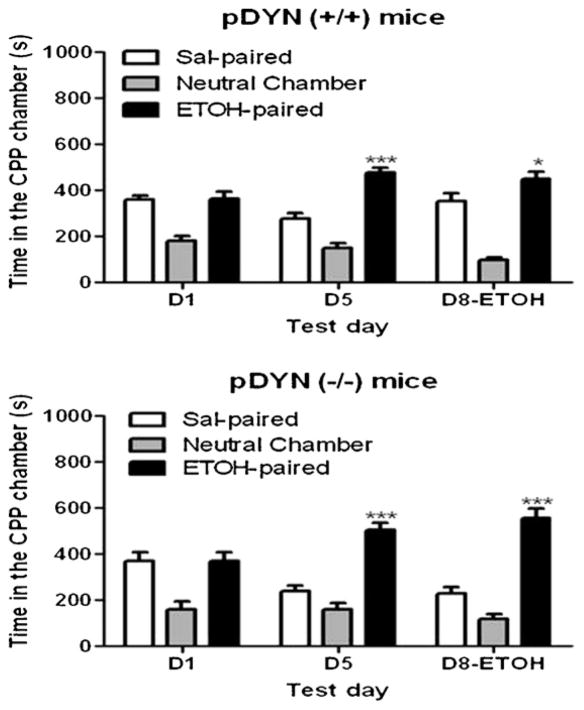
Fig. 2 depicts the amount of time that mice lacking DYN and their wild-type littermates/controls spent in the conditioning chambers on preconditioning (D1) and postconditioning (D5) test days as well as following a saline challenge on day 8 (D8). Repeated measures ANOVA revealed a significant interaction between the amount of time that wild-type mice spent in the CPP chambers and test day (F(4,30) = 10.63; p < 0.0001). The post-hoc analysis showed that mice spent a significantly greater amount of time in the ethanol-paired compared to saline-paired chamber on the postconditioning (D5) and state-dependent CPP (D8) test days (Fig. 2, upper panel). A similar result was observed in mice lacking DYN (Fig. 2, lower panel), as evidenced by a significant interaction between the two factors (F(4,30) = 4.98; p < 0.01). These results suggest that the rewarding action of ethanol was not altered in mice lacking DYN compared to their wild-type littermates/controls under a drug-free state at either time point following conditioning.
Fig. 2.
Ethanol-induced CPP in mice lacking the prodynorphin gene (pDYN−/−) and their wild-type (pDYN+/+) littermates. Mice were tested for baseline place preference on day 1 (D1), received saline/ethanol (2 g/kg) or ethanol/saline conditioning twice daily on days 2–4 and were then tested for postconditioning place preference on day 5 (D5) and again on day 8 (D8). Data are mean (±S.E.M.) of 6 mice per genotype.
4.3. Mice lacking DYN compared to their wild-type littermates exhibited a greater state-dependent CPP response following an ethanol challenge (1 g/kg)
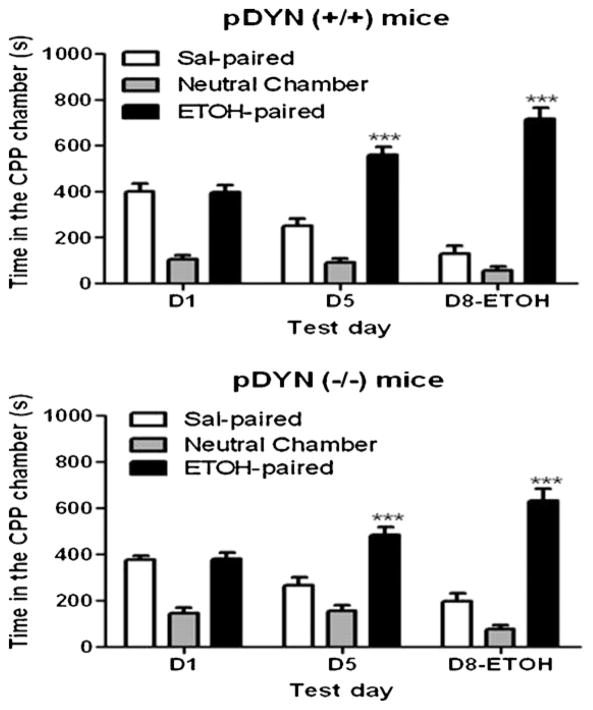
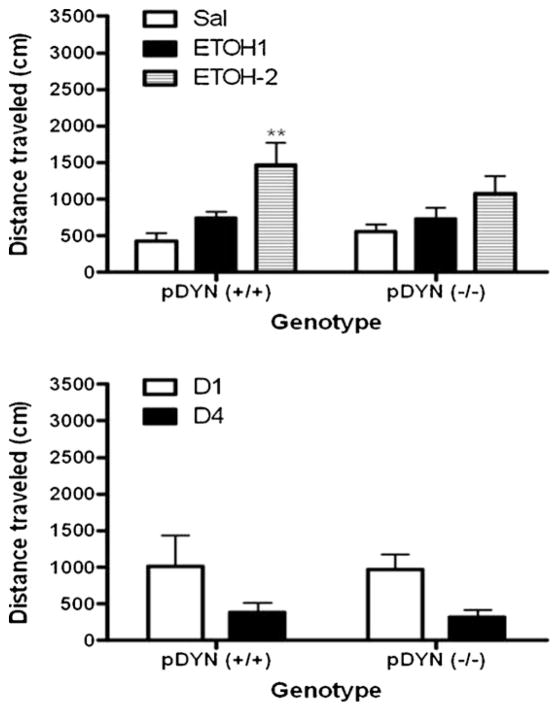
A separate group of DYN null mice and their wild-type littermates/controls was tested for CPP on day 5 and for state-dependent CPP following an ethanol (1 g/kg) challenge on day 8 (Fig. 3). Repeated measures ANOVA revealed a significant interaction between the amount of time that wild-type mice spent in the CPP chambers and test day (F(4,54) = 24.25; p < 0.0001). Post-hoc analysis of the data showed that wild-type mice spent a significantly greater amount of time in the ethanol-paired compared to saline-paired chamber on the postconditioning day (D5) and on the test day for state-dependent CPP following a challenge dose of ethanol (1 g/kg). However, the difference between the amount of time that mice spent in the ethanol-paired compared to saline-paired chamber was reduced on this day compared to day 5 (Fig. 3, upper panel). On the other hand, this difference appeared to be greater in mice lacking DYN on day 8 compared to day 5 (Fig. 3, lower panel). These results illustrate that mice lacking DYN compared to their wild-type littermates exhibited a greater CPP response when tested following an ethanol challenge (1 g/kg). However, this difference was not observed between the two genotypes when a higher dose of ethanol (2 g/kg) was used on day 8 (Fig. 4). On the other hand, mice of both genotypes exhibited a similar locomotor activity following the lower dose of ethanol (1 g/kg), although a somewhat greater response was observed in wild-type mice with the higher dose of ethanol (2 g/kg). However, the level of activity was comparable between the naïve mice of the two genotypes when they were tested for locomotion following single or repeated (once daily for 4 consecutive days) dosing with the higher dose of ethanol (Fig. 5). These results suggest that locomotor activity was comparable between wild-type and DYN null mice.
Fig. 3.
State-dependent CPP in pDYN null mice and their wild-type littermates following a lower dose of ethanol (1 g/kg). Mice were tested for baseline place preference on day 1 (D1), received saline/ethanol (2 g/kg) or ethanol/saline conditioning twice daily for three consecutive days and were then tested for postconditioning place preference on day 5 (D5) and again on day 8 (D8) following an ethanol (1 g/kg) challenge. Data are mean (±S.E.M.) of 10–15 mice per genotype.
Fig. 4.
State-dependent CPP in pDYN null mice and their wild-type littermates following a higher dose of ethanol (2 g/kg). Mice were tested for baseline place preference on day 1 (D1), received saline/ethanol (2 g/kg) or ethanol/saline conditioning twice daily for three consecutive days and were then tested for postconditioning place preference on day 5 (D5) and again on day 8 (D8) following an ethanol (2 g/kg) challenge. Data are mean (±S.E.M.) of 8–13 mice per genotype.
Fig. 5.
Locomotor activity in naïve and ethanol-pretreated mice lacking DYN and their wild-type littermates. Mice lacking DYN and their wild-type littermates used for the experiment described under legend to Fig. 4 were habituated to the locomotor activity chambers for 1 h, then injected with saline or ethanol (1 or 2 g/kg; n = 6–8 mice per dose of each genotype). Locomotor activity was recorded for 1 h (upper panel). Naïve mice of each genotype (n = 4 per genotype) were habituated, injected with ethanol (2 g/kg) and locomotor activity was recorded for 1 h following a single or repeated (once daily for 4 days) ethanol administration (lower panel). Data are mean (±S.E.M.) of distance traveled (cm) during the one-hour test period following saline or ethanol administration.
4.4. Mice treated with nor-BNI compared to their saline-treated controls exhibited a greater CPP following an ethanol challenge (1 g/kg)
Given that compensatory developmental changes may occur in knockout mice, we also studied the effect of nor-BNI on ethanol-induced state-dependent CPP in wild-type mice (Fig. 6). We used the lower dose of ethanol (1 g/kg) since the state-dependent CPP was different between the two genotypes following the lower but not higher challenge dose of ethanol (Figs. 3 and 4). Repeated measures ANOVA revealed a significant interaction between the amount of time that mice spent in the CPP chambers and the test day (F(4,36) = 4.06; p < 0.01). Post-hoc analysis of the data showed that mice spent a significantly greater amount of time in the ethanol-paired compared to saline-paired chamber on the postconditioning day (D5) and following an ethanol challenge on day 8 (Fig. 6, upper panel). On the other hand, wild-type mice treated with nor-BNI showed a robust CPP on day 8 following the ethanol challenge (Fig. 6, lower panel). Repeated measures ANOVA revealed a significant interaction between the amount of time that mice spent in the CPP chambers and the test day (F(4,48) = 26.43; p < 0.0001). Post-hoc analysis showed that mice spent a significantly greater amount of time in the ethanol-paired compared to saline-paired chamber on the postconditioning day (D5) and on the test day for state-dependent CPP (D8). This result suggests that blockade of KOP by nor-BNI during the conditioning sessions enhanced ethanol-induced state-dependent CPP.
Fig. 6.
Ethanol CPP in wild-type mice treated with nor-BNI and their saline-treated controls. Mice were tested for baseline place preference on day 1 (D1) and then 4 h later injected with saline or nor-BNI (10 mg/kg). On days 2–4, mice received saline/ethanol (2 g/kg) or ethanol/saline conditioning twice daily and were then tested for postconditioning place preference on day 5 (D5) and again on day 8 (D8) following an ethanol challenge (1 g/kg, i.p.). Data are mean (±S.E.M.) of 7–9 mice per genotype.
5. Discussion
The main findings of the present study are that mice lacking DYN exhibited a greater CPP in the presence but not absence of ethanol (1 g/kg) compared to their wild-type littermates/controls. Similar results were obtained in wild-type mice treated with nor-BNI, a KOP antagonist, compared to their saline-treated controls. Overall, our results illustrate that the endogenous DYN/KOP system may play a functional role in ethanol-induced state-dependent CPP.
Previous studies have implicated the endogenous DYN/KOP system in ethanol reward and reinforcement. For example, a single administration of nor-BNI, a selective and long-acting KOP antagonist [22], has been shown to increase ethanol consumption [24]. Furthermore, C57BL/6J mice exhibit a higher preference for alcohol than alcohol-avoiding DBA/2J mice [25]. Alcohol administration induces taste aversion in DBA/2J but not C57BL/6J mice [26] although DBA/2J mice exhibit a greater CPP response than C57BL/6 mice [27]. Interestingly, the NAc of alcohol-preferring C57BL/6 mice contains lower levels of DYN compared to alcohol-avoiding DBA/2J mice [8], raising the possibility that the endogenous DYN/KOP system may be involved in alcohol reward and reinforcement. Notably, KOP knockout mice compared to their wild-type littermates showed an enhanced accumbal dopamine overflow [7]. However, mice lacking DYN [13] or KOP [28] expressed reduced rather than increased preference for ethanol in the two-bottle choice paradigm. Furthermore, ethanol reward was not altered in mice lacking DYN compared to their wild-type littermates [13]. Nevertheless, these mice were not fully backcrossed on a C57BL/6 mouse strain. Thus, in the present study, we used fully backcrossed mice to re-address the issue. However, consistent with the former study [13], we found no significant difference in ethanol CPP between DYN-deficient mice and their wild-type littermates when mice were tested for CPP under a drug-free state. The lack of a difference in ethanol CPP between DYN knockout mice and their wild-type controls was not due to compensatory developmental changes in DYN null mice because the CPP response was not altered in wild-type mice treated with nor-BNI compared to their saline-treated controls. Taken together, these results suggest that the endogenous DYN/KOP system may not play a functional role in the motivational valence of contextual cues associated with ethanol administration, at least under the current unbiased CPP paradigm.
Ethanol administration has been associated with changes in the level of DYN and pro-DYN mRNA in brain regions implicated in reward and reinforcement [8,12]. Considering that these alterations were more pronounced following ethanol withdrawal, we proposed that the endogenous DYN/KOP system may become engaged after cessation of ethanol administration and could alter the rewarding action of ethanol following a short-term withdrawal from ethanol. Thus, we tested mice lacking DYN and their wild-type littermates/controls for CPP at a later time point (3 days following the last ethanol conditioning). We selected this time point because higher levels of pro-DYN mRNA have been found in the NAc following this short-term ethanol withdrawal [12]. However, we observed no significant difference in the CPP response between mice lacking the pro-DYN gene and their wild-type littermates at this time point (Fig. 2). Nevertheless, we found that knockout mice exhibited a greater CPP response compared to their wild-type littermates when tested for CPP following a challenge dose of ethanol (1 g/kg). A similar result was observed in wild-type mice treated with nor-BNI (Fig. 6), suggesting that the endogenous DYN/KOP system may be involved in ethanol-induced state-dependent CPP. However, the state-dependent CPP was comparable between wild-type and DYN null mice following a higher dose of ethanol (2 g/kg; Fig. 4). The lack of a difference between the DYN null and wild-type mice in this case could have been due to a ceiling state-dependent CPP in DYN null mice. In fact, a closer look at the CPP response following the two doses of ethanol shows that state-dependent CPP was greater with the higher (2 g/kg) compared to lower (1 g/kg) challenge dose of ethanol in wild-type mice (compare Fig. 3 vs. Fig. 4, upper panels on day 8). However, this difference was not observed in DYN null mice challenged with the higher compared to lower dose of ethanol (compare Fig. 4 to Fig. 3, lower panel, D8). A prior report showed that the level of locomotor activity affects the expression of state-dependent CPP [18]. Our results, however, failed to support this notion since we observed a greater level of locomotor activity with the higher dose of ethanol in wild-type mice which was also associated with a greater state-dependent CPP. Nonetheless, we used female mice and also measured the level of activity under a different experimental paradigm than described in the previous report [18] because our CPP apparatus did not allow simultaneous measurement of CPP and motor activity.
Considering that DYN may suppress learning and memory and KOP agonists reduce long-term potentiation [29], one may argue that the enhanced state-dependent CPP might have been due to enhanced associative learning in knockout mice. However, knockout mice did not show enhanced CPP under a drug-free state, raising the possibility that the enhancement was not due to the inhibitory action of endogenous DYN on memory retrieval. Furthermore, the enhanced state-dependent CPP was not due to a regulatory action of the endogenous DYN/KOP system on ethanol withdrawal because a previous report showed that DYN knockout mice exhibited comparable signs of withdrawal relative to their wild-type littermates/controls [13]. Also, we found no difference in ethanol CPP between mice lacking DYN and their wild-type controls when challenged with saline or a higher dose of ethanol (2 g/kg) on day 8. Thus, our results illustrate that the endogenous DYN/KOP system functions to prevent the development of neuronal adaptive changes that alcohol brings about during the acquisition phase of CPP, leading to a greater state-dependent CPP response.
Previous studies have shown lower levels of dopamine in the NAc in mice lacking DYN compared to their wild-type littermates. Interestingly, DYN null mice exhibit reduced cocaine-induced motor stimulation [30] as well as attenuated morphine-stimulated locomotor activity (Hamid and Lutfy, unpublished data). Also, as stated above, mice lacking DYN or KOP express reduced preference for ethanol [13,28]. It is generally thought that the DYN/KOP system exerts inhibitory effects on the rewarding and reinforcing actions of ethanol and other addictive drugs. However, these data and the present results suggest that the endogenous DYN/KOP system may regulate the function of the mesolimbic dopaminergic neurons as well as the rewarding and reinforcing actions of addictive drugs in a more complex way than previously thought. In fact, a number of recent studies have suggested that KOP antagonists may decrease alcohol intake by suppressing negative emotional states associated with increased activity of the endogenous DYN/KOP system that develops following chronic alcohol administration [10]. Thus, further research is needed to fully characterize the role of endogenous DYN/KOP system in the rewarding action of ethanol and other addictive drugs.
In summary, the current results suggest that the endogenous DYN/KOP system may not play an important role in the motivational valence of contextual cues acquired following ethanol conditioning. However, this system may become engaged and negatively regulate ethanol-induced state-dependent CPP.
Acknowledgments
The current study was supported in part by the departmental fund to KN and in part by the NIDA Grant # R24 DA017298.
References
- 1.Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9(11):999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- 2.Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81(2):339–58. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116(2):306–21. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh AN, Srivastava S, Jainar AK. Pharmacotherapy of chronic alcoholism: a review. Drugs Today (Barc) 1999;35(1):27–33. doi: 10.1358/dot.1999.35.1.522944. [DOI] [PubMed] [Google Scholar]
- 5.Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010;108(3):183–94. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210(2):121–35. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zapata A, Shippenberg TS. Endogenous kappa opioid receptor systems modulate the responsiveness of mesoaccumbal dopamine neurons to ethanol. Alcohol Clin Exp Res. 2006;30(4):592–7. doi: 10.1111/j.1530-0277.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 8.Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22(3):165–71. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- 9.Nestby P, Schoffelmeer AN, Homberg JR, Wardeh G, De Vries TJ, Mulder AH, et al. Bremazocine reduces unrestricted free-choice ethanol self-administration in rats without affecting sucrose preference. Psychopharmacology (Berl) 1999;142(3):309–17. doi: 10.1007/s002130050894. [DOI] [PubMed] [Google Scholar]
- 10.Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33(3):643–52. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruijnzeel AW. Kappa-opioid receptor signaling and brain reward function. Brain Res Rev. 2009;62(1):127–46. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238(1–2):13–6. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- 13.Blednov YA, Walker D, Martinez M, Harris RA. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40(2):73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham CL, Noble D. Methamphetamine-induced conditioned place preference or aversion depending on dose and presence of drug. Ann N Y Acad Sci. 1992;654:431–3. doi: 10.1111/j.1749-6632.1992.tb25989.x. [DOI] [PubMed] [Google Scholar]
- 15.Elliott PJ. Place aversion induced by the substance P analogue, dimethyl-C7, is not state dependent: implication of substance P in aversion. Exp Brain Res. 1988;73(2):354–6. doi: 10.1007/BF00248227. [DOI] [PubMed] [Google Scholar]
- 16.Oberling P, Rocha B, Di Scala G, Sandner G. Evidence for state-dependent retrieval in conditioned place aversion. Behav Neural Biol. 1993;60(1):27–32. doi: 10.1016/0163-1047(93)90677-a. [DOI] [PubMed] [Google Scholar]
- 17.Swerdlow NR, van der Kooy D, Koob GF, Wenger JR. Cholecystokinin produces conditioned place-aversions, not place-preferences, in food-deprived rats: evidence against involvement in satiety. Life Sci. 1983;32(18):2087–93. doi: 10.1016/0024-3205(83)90096-6. [DOI] [PubMed] [Google Scholar]
- 18.Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191(2):195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- 19.Chester JA, Risinger FO, Cunningham CL. Ethanol reward and aversion in mice bred for sensitivity to ethanol withdrawal. Alcohol Clin Exp Res. 1998;22(2):468–73. [PubMed] [Google Scholar]
- 20.Fidler TL, Bakner L, Cunningham CL. Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacol Biochem Behav. 2004;77(4):731–43. doi: 10.1016/j.pbb.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U. Generation of dynorphin knockout mice. Brain Res Mol Brain Res. 2001;86(1–2):70–5. doi: 10.1016/s0169-328x(00)00264-3. [DOI] [PubMed] [Google Scholar]
- 22.Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246(1):255–8. [PubMed] [Google Scholar]
- 23.Bebawy D, Marquez P, Samboul S, Parikh D, Hamid A, Lutfy K. Orphanin FQ/nociceptin not only blocks but also reverses behavioral adaptive changes induced by repeated cocaine in mice. Biol Psychiatry. 2010;68(3):223–30. doi: 10.1016/j.biopsych.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182(3):384–92. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- 25.Belknap JK, Crabbe JC, Riggan J, O’Toole LA. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112(2–3):352–8. doi: 10.1007/BF02244932. [DOI] [PubMed] [Google Scholar]
- 26.Broadbent J, Linder HV, Cunningham CL. Genetic differences in naloxone enhancement of ethanol-induced conditioned taste aversion. Psychopharmacology (Berl) 1996;126(2):147–55. doi: 10.1007/BF02246350. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107(2–3):385–93. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- 28.Kovacs KM, Szakall I, O’Brien D, Wang R, Vinod KY, Saito M, et al. Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res. 2005;29(5):730–8. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- 29.Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363(6428):451–4. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chefer VI, Shippenberg TS. Paradoxical effects of prodynorphin gene deletion on basal and cocaine-evoked dopaminergic neurotransmission in the nucleus accumbens. Eur J Neurosci. 2006;23(1):229–38. doi: 10.1111/j.1460-9568.2005.04525.x. [DOI] [PubMed] [Google Scholar]