Abstract
Objective
To determine dose-dependent effects of testosterone on sexual function, body composition, muscle performance, and physical function in hysterectomized women with and without oophorectomy.
Methods
71 menopausal women who previously underwent hysterectomy with or without oophorectomy with total testosterone<31ng/dl or free testosterone<3.5 pg/ml received a standardized transdermal estradiol regimen during the 12-week run-in period, and were then randomized to receive weekly IM injections of placebo, or 3, 6.25, 12.5 or 25 mg testosterone enanthate for 24 weeks. Total and free testosterone levels were measured by LC-MS/MS and equilibrium dialysis, respectively. The primary outcome was change in sexual function measured using Brief Index of Sexual Function (BISF-W); Secondary outcomes included changes in sexual activity, sexual distress, DeRogatis Inventory of Sexual Function, lean (LBM) and fat mass, muscle strength and power, and physical function.
Results
71 women were randomized; five groups were similar at baseline. 62 women with analyzable data for the primary outcome were included in the final analysis. Mean on-treatment total testosterone concentrations were 19, 78, 102, 128 and 210ng/dl in the placebo, 3, 6.25, 12.5 and 25-mg groups, respectively. Changes in composite BISF-W scores, thoughts-desire, arousal, frequency of sexual activity, LBM, chest-press power and loaded stair-climb power were significantly related to increases in free testosterone concentrations; changes were significantly greater in women assigned to the 25-mg group when compared to placebo but not at the lower dose groups. Sexual activity increased by 2.7 encounters per week in 25-mg group. Frequency of androgenic adverse events was low.
Conclusion
Testosterone administration in hysterectomized women with and without oophorectomy for 24-weeks was associated with dose and concentration-dependent gains in several domains of sexual function, LBM, chest-press power and loaded stair-climb power. Long-term trials are needed to weigh improvements in these outcomes against potential long-term adverse effects.
Keywords: Testosterone, menopause, oophorectomy, hysterectomy, sexual function, body composition, muscle performance, physical function
INTRODUCTION
The clinical applications of testosterone for the treatment of sexual dysfunction, physical dysfunction, and osteoporosis in menopausal women are predicated upon the assumption that testosterone dose-response relationships are different in women than in men, and that clinically significant effects on health-related outcomes can be achieved safely at testosterone doses and concentrations that are substantially lower than those required to produce similar effects in men; these assumptions have not been tested rigorously.1 Thus, an investigation of the testosterone dose-response relationships in women is needed to determine whether improvements in sexual function, muscle mass and performance, and physical function can be achieved at testosterone doses and concentrations that do not produce dose-limiting androgenic adverse effects.
Despite the loss of estrogen production during natural menopause, the climacteric ovary continues to secrete a substantial amount of androgens 2 so that circulating testosterone levels change very little during the perimenopausal period. However, both hysterectomy and bilateral oophorectomy results in a substantial decline in testosterone levels.3 Women who have undergone surgical oophorectomy experience greater deterioration in sexual function than naturally menopausal women despite estrogen therapy 4–8 leading to speculation that surgically menopausal women with sexual dysfunction might benefit from testosterone replacement.
Several well-designed placebo-controlled trials using a transdermal testosterone patch have reported significant improvements in some domains of sexual function in both surgically and naturally menopausal women.9–15 However, two phase III trials using a transdermal testosterone gel failed to meet the co-primary endpoints of a greater increase in the total number of days with a satisfying sexual event and in sexual desire when compared to placebo. Very few studies have investigated the effects of testosterone administration on body composition, muscle performance and physical function in women. Natural menopause is associated with an increase in fat mass and a decrease in lean body mass.16, 17 The Study of Women’s Health Across the Nation (SWAN) found higher rates of physical limitation in surgically menopausal women compared to naturally menopausal women,18 Furthermore, the age-related decline in testosterone levels in older women has been associated with frailty.19 Thus, surgically menopausal women with low testosterone levels may be at excess risk for physical disability. Clinical trials using various formulations and doses of testosterone or other androgens have reported some improvements in lean body mass and muscle strength;20–23 however, these results have not been consistent across studies and none has demonstrated improvements in physical function. The dose-dependent effects of testosterone on skeletal muscle mass, measures of muscle performance and physical function have not been studied.
Here, we evaluated the dose-response relationships of testosterone with several domains of sexual function and measures of body composition, muscle performance and physical function in hysterectomized women with or without oophorectomy with low serum testosterone concentrations. The primary objective was to determine the testosterone dose-dependent effects for a wide range of androgen-dependent outcomes in androgen-deficient women; thus unlike other studies, we did not specifically recruit women with hypoactive sexual desire disorder or other forms of sexual dysfunction in this trial. We carefully monitored the androgenic side effects using structured instruments – hair growth using the Ferriman-Gallwey scale, sebum production using the Sebu-Tape, acne using the Palatsi scale, and clitoral size using a caliper scale. We used intramuscular injections of graded doses of testosterone esters to achieve high level of bioavailability and compliance, and a wide range of circulating testosterone concentrations, extending from the physiologic to the supraphysiologic range. Testosterone levels were measured using liquid chromatography tandem mass spectrometry, widely considered the method with the highest sensitivity and specificity.
METHODS
Study Design
The Testosterone Dose Response in Surgically Menopausal Women (TDSM) trial was a multi-center, parallel group, placebo-controlled, double-blind randomized trial consisting of a 12-week run-in period of transdermal estradiol administration, a 24-week treatment period, and a 16-week recovery period. The study was conducted at Boston University Medical Center (BUMC) and Charles Drew University of Medicine and Science (Los Angeles, CA) and was approved by the institutional review board at each study site. All participants provided written informed consent. The subject enrollment started in July 2005 and the last participant completed the trial on January 4, 2011. The trial was registered on June 28, 2007. An independent Data and Safety Monitoring Board reviewed the study progress and safety data every six months.
Participants
The participants were healthy women, 21–60 years of age who had undergone hysterectomy with or without partial or total oophorectomy. The participants had serum total testosterone concentrations less than 31ng/dl or free testosterone concentrations less than 3.5pg/ml (less than the median for healthy young women 24). Women who had had hysterectomy alone or partial oophorectomy were included if their FSH levels were 30 U/L or higher or if they were already receiving estrogen therapy. Inclusion required a documented normal Pap smear and mammogram within the last 12 months.
Women with major psychiatric illness, including new onset depression in the previous 3 months or untreated depression, recent hospitalization, active cancers, poorly controlled diabetes mellitus (HbA1c >8.5%), uncontrolled hypertension, severe obesity (BMI >40 kg/m2), illicit drug use, alcohol dependence and abnormal liver function were excluded. Women who had been on a stable regimen of anti-depressant therapy for 3 months or more were allowed to continue their antidepressant medications through the study. Women with a history of breast, ovarian, endometrial or cervical cancer, hyperandrogenic disorders, physical disabilities, cardiac disease or thromboembolic disorders, and those taking glucocorticoids, androgens, spironolactone and GnRH agonists were also excluded.
Interventions and Randomization
All eligible women were administered a regimen of transdermal estradiol (E2) patch applied twice a week and designed to achieve nominal delivery of 50-ug estradiol daily (Alora, Watson Pharmaceuticals) for a 12-week run-in phase. Women taking a different form of estrogen therapy prior to study entry were switched to the transdermal patch. This standardized estrogen patch regimen was employed to minimize the confounding symptoms of low estrogen that may overlap with those of testosterone deficiency. After run-in, the participants were randomized to one of 5 groups to receive weekly IM injections of placebo, 3, 6.25, 12.5 or 25 mg testosterone enanthate (ENDO Pharmaceuticals, Malvern, PA) for 24 weeks. To achieve blinding, all injections were preloaded into a syringe by the Investigational Drug Pharmacy and administered in the same volume by the research staff. A concealed computer-generated randomization table was used to allocate individuals to one of the 5 groups, with a block size of 6.
The trial was designed to obtain data on 128 women, assuming 15% prior loss to follow-up; 128 evaluable subjects would have yielded 90% power to detect differences across groups if the cumulative variation in BISF-W accounted for by randomized assignment (R2 in a model including only randomized assignment) was 40%, but was under-enrolled. With an a priori assumption that the standard deviation of BISF-W is 15, the enrollment actually achieved provides 80% power to detect an increase in BISF-W of 5 points for each successive dose group.
Blinding
The investigators, the study staff, and the participants were blinded. The intervention assignment was known only to the Investigational Drug Pharmacy. All doses were prepared by the Investigational Drug Pharmacy in a similar volume of vehicle to maintain blinding.
Outcomes
The primary outcome was the change from baseline in the composite and individual domain scores for sexual function, measured by the Brief Index of Sexual Functioning for Women (BISF-W). The BISF-W was selected as our primary outcome because this instrument has been validated for the evaluation of sexual function in surgically menopausal women and shown to be androgen-responsive in pivotal trials.9, 25 Sexual activity was assessed using weekly Sexual Activity Log (SAL) and personal distress/bother associated with sexual dysfunction using the Female Sexual Distress Scale (FSDS). To obtain a more comprehensive assessment of some domains that are not well covered by BISF, we also used the Derogatis Interview for Sexual Functioning-Self Report (DISF-SR). Mood and well-being were assessed using the Psychological General Well-Being Index (PGWBI).26–28
Additional secondary outcomes included changes in lean mass, fat mass, maximal voluntary muscle strength, muscle power and several performance-based measures of physical function.
Body Composition was measured using dual-energy x-ray absorptiometry (Hologic QDR 4500A), calibrated using a soft tissue phantom 29. Seated leg-press and chest-press strength were measured by the 1 repetition-maximum (1-RM) method 30 using pneumatic resistance machines (Keiser Sport, Fresno, CA). Following a 5-minute warm-up, loads were progressively increased until the subject was no longer able to complete a full range of motion repetition in good form. The 1-RM was defined as the greatest amount of resistance that could be successfully moved one time only. Grip strength was measured in both hands using an adjustable Jamar hydraulic dynamometer (Sammons Preston, Inc., Bolingbrook, IL), described elsewhere.31 Muscle power, the rate of exerting force, was determined at resistances equivalent to 50–70% of the 1-RM for the leg press and chest press using the equipment and software described for capturing strength.32 Both tests were repeated within 7 days of the initial test; if the measurements were within 5%, the better of the 2 measures was recorded. Physical function was assessed using a 12-step stair-climb, 40-m walk, and a lift-and lower task, described previously.33–35 The stair-climb and walk tests were performed with and without a load equal to 20% of the participant’s body weight.
Blood counts, liver function tests, fasting glucose, lipids, presence of acne, hirsutism, sebum production, clitoral size, voice change and adverse events were monitored. Sebum production was measured using sebu tapes applied to the forehead, nose and back for 12-hours.36 Hair growth was measured by the Ferriman-Gallwey scale,37 and acne using the Palatsi scale.38
Hormone Assays
Serum total testosterone levels were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) with sensitivity of 2 ng/dl, as described elsewhere.39 The cross-reactivity of DHEA, DHEAS, DHT, androstenedione and estradiol in the testosterone assay was negligible at ten times the circulating concentrations of these hormones. The interassay coefficient of variation was 15.8%, at 12.0 ng/dL, 10.6%, at 23.5 ng/dL, 7.9%, at 48.6 ng/dL, 7.7% at 241 ng/dL, 4.4% at 532 ng/dL, and 3.3% at 1016 ng/dL respectively. As part of the Centers for Disease Control’s (CDC) Testosterone Assay Harmonization Initiative, quality control samples provided by the CDC were run every three months; the bias in quality control samples in the 3.47-to-34.7nmol/L (100-to-1000ng/dL) range was <6.2%. Free testosterone was measured using equilibrium dialysis with an interassay coefficient of variation of 12.3%.24, 40 Sex hormone binding globulin levels were measured using an immunofluorometric assay with a sensitivity of 0.5 nmol/L.41 The inter-assay CVs were 8.3%, 7.9%, and 10.9%, and intra-assay CVs 7.3%, 7.1% and 8.7%, respectively, in the low, medium, and high pools.
Statistical Analysis
All variables were examined using summary statistics. Compliance with treatment was assessed in terms of the number of testosterone injections administered to the participant, expressed as percent of the total number of doses intended by design. Percent compliance was averaged within each treatment group to obtain group means.
The primary analysis included all participants with at least a baseline and 1 post-randomization measurement of the primary outcome. Mean change in outcomes was compared across treatment doses by linear regression incorporating adjustment for baseline outcome measurements. Response at each dose was estimated using a treatment contrast and 95% confidence interval.
Missing data were addressed using multiple imputations by chained equations.42 Treatment assignment, age, lean and fat mass, and total testosterone levels were chosen as covariates for the imputation model providing estimates for missing values. Outcome questionnaires were imputed at the level of domains (subscores), so that partially complete records were retained and utilized. Ordinal variables were imputed using polytomous logistic regression and continuous measures using a linear regression model. Fixed quantitative associations–for instance, relationships between domain scores and overall scores in the BISF-W - were maintained for the full course of the estimation of missing records through the use of passive imputation.43 Twenty imputed values were generated for each record; this number was chosen to exceed 100 times the proportion of subject missing data in at least one domain of the primary outcome (12/62 = 19.4%), typically an overestimate of the proportion of missing information for an outcome measure44, and which greatly exceeded the total proportion of combined baseline and follow-up BISF-W questions missing in aggregate (9.9%). Each imputation was allowed to converge over 25 iterations. Estimates of regression effects were computed incorporating each of the imputed values, and the cumulative statistical significance of dose effects determined using likelihood ratio statistics.45 Comparisons of individual active doses to placebo utilized Wald statistics. Analyses of adverse event data were on observed data only.
Generalized additive models (GAM) were used to evaluate the association between change in each outcome measure with changes in total and free testosterone levels.46 These models allow for curvelinearity in associations if it is present, with preference for a simpler model if it is consistent with the data. GAM models were restricted to observed data. Analyses were conducted using R version 2.14.2 (R Foundation for Statistical Computing, Vienna, Austria)); multiple imputations were generated using the mice library 43 and GAM fits derived using the mgcv package.46
RESULTS
Flow of Participants Through the Study
Of the 850 women who underwent telephone screening, 218 met eligibility criteria, 85 entered the estrogen run-in-period, 71 were randomized, and 62 who had the baseline and at least one post-randomization measurement constituted the analytic sample for the primary analysis (placebo (n=13), 3 mg (n=12), 6.25 mg (n=13), 12.5 mg (n=13) or 25 mg (n=11) (CONSORT diagram, Figure 1)
Figure 1.
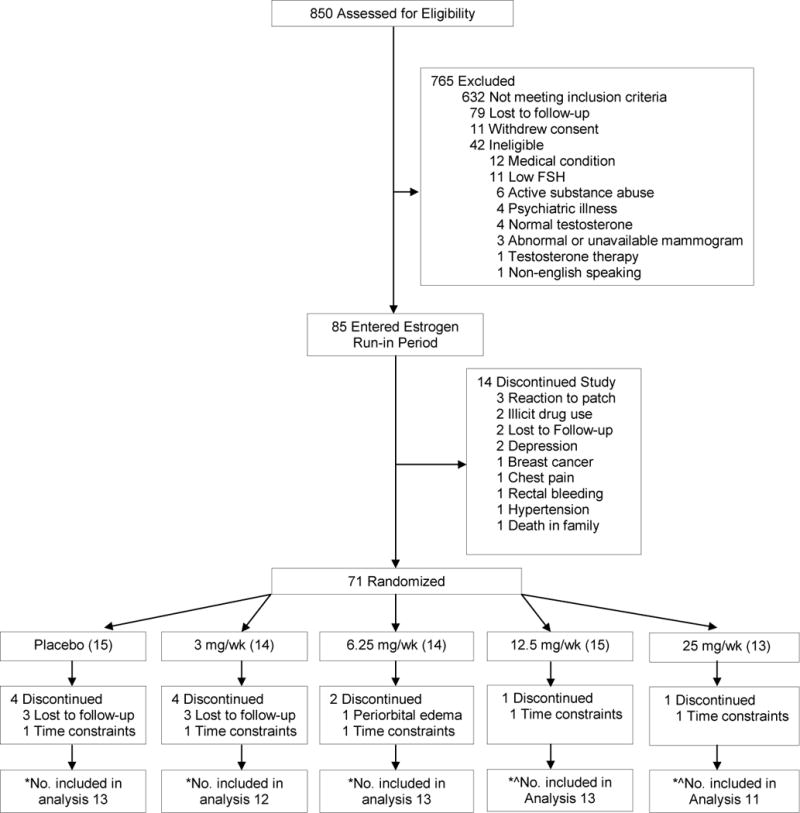
Flow of Participants Through the Trial
* Represents number of participants who had a baseline and at least one post-randomization visit assessment of the Brief Index of Sexual Functioning Questionnaire, the primary outcome of the trial. ^In each of these groups, one subject did not have an evaluable post-randomization assessment.
Baseline Characteristics
The treatment groups were similar in their baseline characteristics (Table 1). Mean age was 53 years with an average BMI of 29.8 kg/m2. 80% of the women had undergone bilateral oophorectomy. The overall baseline sexual function scores of the study population were significantly lower than those observed previously in healthy women (Supplementary Table 1).25
Table 1.
Baseline Characteristics of the Participants by Randomized Assignment (N = 62)
| Dose of Testosterone Enanthate, mg/wk | Placebo (n = 13) |
3 (n= 12) |
6.5 (n = 13) |
12.5 (n= 13) |
25 (n= 11) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, yr | 53 ± 5 | 54 ± 5 | 52 ± 5 | 52 ± 5 | 53 ± 5 |
| Height, inch | 63 ± 2 | 64 ± 4 | 64 ± 3 | 63 ± 2 | 65 ± 1 |
| Weight, lb | 182 ± 27 | 181 ± 50 | 169 ± 34 | 161 ± 32 | 164 ± 41 |
| BMI, kg/m2 | 33 ± 5 | 31 ± 6 | 29 ± 5 | 29 ± 5 | 28 ± 7 |
| Hysterectomy alone, N (%) | 3 (23) | 3 (25) | 1 (8) | 1 (8) | 4 (36) |
| Partial Oophorectomy, N (%) | 1 (8) | 1 (8) | 0 (0) | 0 (0) | 0 (0) |
| Bilateral Oophorectomy, N (%) | 9 (69) | 8 (67) | 12 (92) | 12 (92) | 7 (64) |
| Use of Antidepressants, N (%) | 3 (23) | 3 (25) | 3 (23) | 5 (38) | 4 (36) |
|
| |||||
| Baseline Blood Levels | |||||
| Total Testosterone, ng/dl | |||||
| Screening | 11 ± 8 | 17 ± 14 | 17 ± 15 | 15 ± 16 | 16 ± 11 |
| Post-Estrogen run-in | 14 ± 8 | 12 ± 5 | 14 ± 12 | 10 ± 6 | 15 ± 10 |
| Free Testosterone, pg/ml | |||||
| Screening | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 2 ± 3 |
| Post-Estrogen run-in | 2.5 ± 1.6 | 1.9 ± 0.8 | 2.3 ± 2.4 | 1.9 ± 0.9 | 2.0 ± 1.3 |
| SHBG, nmol/L | 64 ± 23 | 73 ± 35 | 59 ± 24 | 64 ± 36 | 92 ± 39 |
| Hematocrit, % | 39 ± 3 | 39 ± 2 | 38 ± 3 | 39 ± 3 | 40 ± 2 |
| Total Cholesterol, mg/dl | 195 ± 29 | 215 ± 46 | 218 ± 40 | 198 ± 33 | 199 ± 48 |
| Low-density lipoprotein, mg/dl | 113 ± 23 | 119 ± 47 | 108 ± 29 | 118 ± 28 | 104 ± 32 |
| High-density lipoprotein, mg/dl | 57 ± 16 | 64 ± 23 | 70 ± 15 | 56 ± 16 | 74 ± 18 |
| Triglycerides, mg/dl | 133 ± 62 | 99 ± 36 | 125 ± 49 | 174 ± 130 | 80 ± 37 |
| Fasting glucose, mg/dl | 99 ± 35 | 93 ± 10 | 92 ± 5 | 97 ± 23 | 89 ± 13 |
|
| |||||
| Body Composition and Muscle Performance | |||||
| Lean mass, kg | 44 ± 5 | 45 ± 10 | 45 ± 8 | 42 ± 7 | 43 ± 6 |
| Fat mass, kg | 35 ± 8 | 33 ± 13 | 28 ± 9 | 29 ± 9 | 29 ± 12 |
| Leg press strength, Newton | 836 ± 192 | 819 ± 208 | 809 ± 186 | 686 ± 174 | 744 ± 172 |
| Chest press strength, Newton | 152 ± 29 | 157 ± 32 | 161 ± 27 | 139 ± 31 | 151 ± 31 |
| Leg Press Power, Watts | 395 ± 85 | 440 ± 168 | 472 ± 138 | 365 ± 129 | 374 ± 132 |
| Chest Press Power, Watts | 100 ± 22 | 114 ± 49 | 108 ± 43 | 96 ± 28 | 100 ± 26 |
| Lift-Lower Score | 21 ± 12 | 21 ± 15 | 35 ± 7 | 22 ± 11 | 31 ± 10 |
| Dominant Hand Grip Strength, kg | 23 ± 4 | 21 ± 7 | 24 ± 9 | 19 ± 7 | 23 ± 3 |
| Unloaded Stair Climb Power, W | 372 ± 78 | 346 ± 80 | 378 ± 78 | 330 ± 89 | 361 ± 66 |
| Loaded Stair Climb Power, W | 418 ± 99 | 423 ± 121 | 473 ± 82 | 339 ± 151 | 406 ± 77 |
| Unloaded 40-m walking speed, m/s2 | 23 ± 3 | 21 ± 4 | 21 ± 6 | 26 ± 5 | 20 ± 3 |
| Loaded 40-m walking speed, m/s2 | 25 ± 3 | 23 ± 4 | 22 ± 4 | 27 ± 6 | 22 ± 4 |
|
| |||||
| Sexual Function | |||||
| Brief Index of Sexual Functioning | |||||
| Composite | 18 ± 11 | 32 ± 15 | 22 ± 11 | 10 ± 12 | 22 ± 15 |
| Thoughts/Desire | 5 ± 3 | 4 ± 3 | 5 ± 2 | 2 ± 2 | 4 ± 3 |
| Arousal | 3 ± 2 | 5 ± 2 | 4 ± 3 | 2 ± 2 | 5 ± 3 |
| Frequency of Sexual Activity | 2 ± 1 | 2 ± 2 | 3 ± 2 | 1 ± 1 | 2 ± 1 |
| Receptivity/Initiation | 5 ± 4 | 7 ± 6 | 6 ± 4 | 5 ± 4 | 6 ± 5 |
| Pleasure/Orgasm | 2 ± 2 | 4 ± 3 | 3 ± 3 | 1 ± 2 | 4 ± 3 |
| Relationship Satisfaction | 5 ± 4 | 7 ± 4 | 7 ± 3 | 6 ± 4 | 6 ± 4 |
| Problems Affecting Sexual Function | 5 ± 3 | 3 ± 3 | 5 ± 2 | 5 ± 3 | 5 ± 3 |
| Derogatis Interview for Sexual Functioning | |||||
| Composite | 49 ± 20 | 43 ± 30 | 59 ± 26 | 30 ± 23 | 54 ± 29 |
| Female Sexual Distress Scale | |||||
| Composite | 17 ± 11 | 8 ± 8 | 18 ± 17 | 19 ± 15 | 17 ± 14 |
| Sexual Activity Log | |||||
| # Sexual Encounter/wk | 1.6 ± 2.1 | 1.3 ± 2.1 | 2.2 ± 2.9 | 0.8 ± 1.1 | 1.3 ± 1.6 |
| Psychological General Well Being | |||||
| Composite | 81 ± 13 | 83 ± 22 | 83 ± 12 | 64 ± 29 | 71 ± 19 |
Data represent mean ± SD or N (%). BMI, body mass index; SHBG, sex hormone-binding globulin.
Compliance
Overall compliance with testosterone injections was 99% in the placebo group, 100% in the 3-mg TE dose group, 98% in the 6.25-mg, 98% in the 12.5-mg group and 98% in the 25-mg group.
Hormone Levels
Baseline mean total and free testosterone concentrations of all randomized subjects were 13.0 ng/dl and 2.2 pg/ml, respectively, well below the range for healthy, menstruating women. Serum nadir total and free testosterone levels, measured during week 24, one week after the previous injection increased from baseline in a dose-dependent fashion (Figure 2). Mean on-treatment nadir total testosterone concentrations were 19, 78, 102, 128 and 210 ng/dl at the 0, 3, 6.25, 12.5 and 25-mg doses, respectively. On-treatment testosterone concentrations were significantly greater compared to levels reported in healthy cycling women.47
Figure 2.
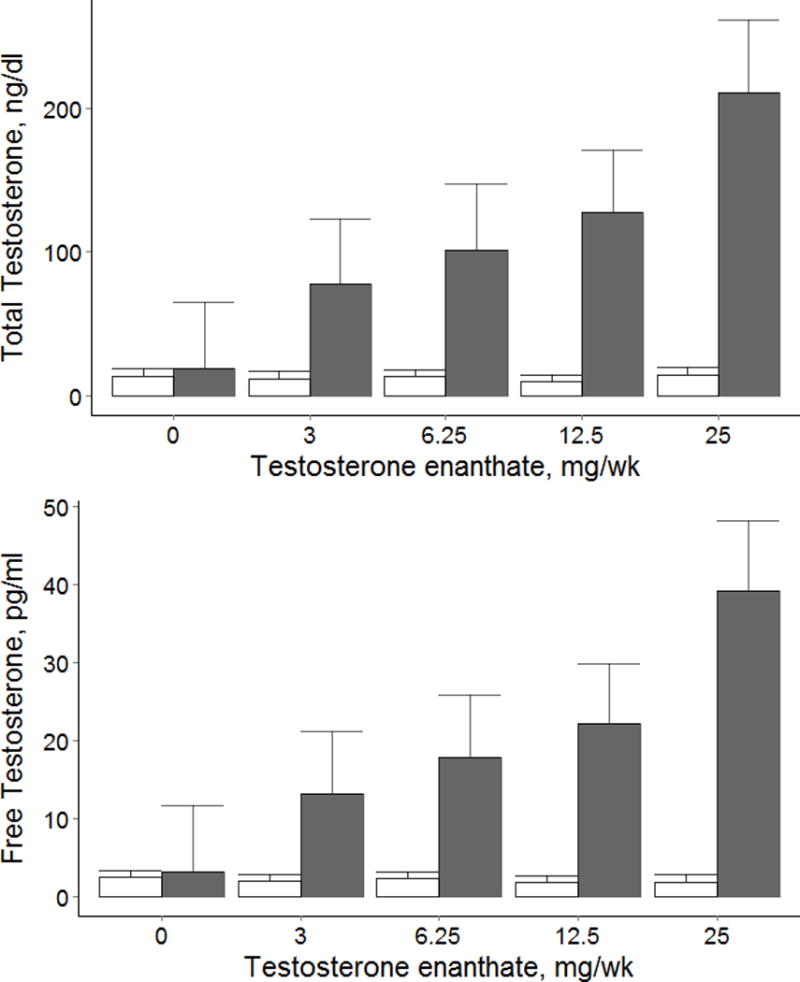
On−Treatment Total and Free Testosterone Concentrations.
Data represents means and standard errors at baseline and on−treatment for each testosterone dose group.
The reference ranges for total and free testosterone in healthy, cycling women from the Framingham Heart Study presented as the median (2.5th, 97.5th precentile range) are as follows: Total testosterone: Follicular Phase 23.7 (8.3, 48.3)ng/dl, Ovulatory Phase (10.1, 48.3) ng/dl, Luteal Phase 28.5 (11.3, 62.5) ng/dl. Free Testosterone: Follicular Phase 1.8 (0.7, 4.4) pg/ml, Ovulatory Phase 2.8 (1.1, 4.6) pg/ml, Luteal Phase 2.4 (0.9, 7.2) pg/ml.47
Sexual Function
The changes in the composite scores for the BISF-W – the primary outcome of the trial - were significantly associated with testosterone dose and with the change from baseline in free testosterone concentrations (Dose Effect p<0.01) (Figure 3A). The changes in sexual thoughts and desire and frequency of sexual activity were significantly greater only in women assigned to the 25-mg group than in those assigned to placebo group (p<0.05 vs placebo). The changes in other domains of sexual function – receptivity/initiation, relationship satisfaction, pleasure/orgasm, and problems affecting sexual function – were not related to on-treatment testosterone concentrations and did not differ from those in the placebo group. (Supplementary Figure 1A) The changes in the domain scores for thoughts-desire, arousal, and the frequency of sexual activity also were significantly related to increases in free testosterone concentrations (p<0.05). The sexual activity scores measured by weekly logs showed an average increase of 2.7 sexual encounters per week in the 25-mg testosterone dose group; the changes in sexual activity scores were significantly related to increases in free testosterone concentrations (p<0.01). (Figure 3B)
Figure 3A.
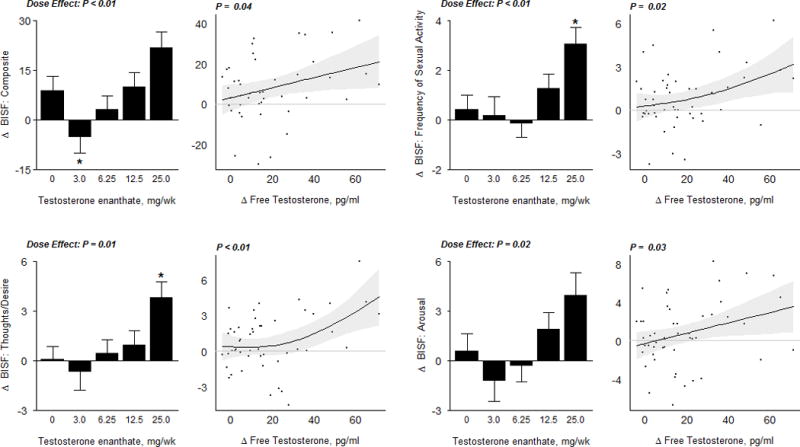
Sexual Function Outcome Measures
In the bar graphs on the left, data represent absolute mean changes (± SE) from baseline for each treatment group. The * represents a significant difference between mean on treatment change in dose group vs. placebo at a 0.05 level; the significance level for the overall dose effect (by likelihood ratio test) is also shown. Scatterplots on the right display estimates and 95% confidence regions for the Generalized Additive Model (GAM) of change in sexual function outcomes as a function of free testosterone levels. The p−values displayed here are from a significance test of no association. BISF; Brief Index of Sexual Functioning
Figure 3B.
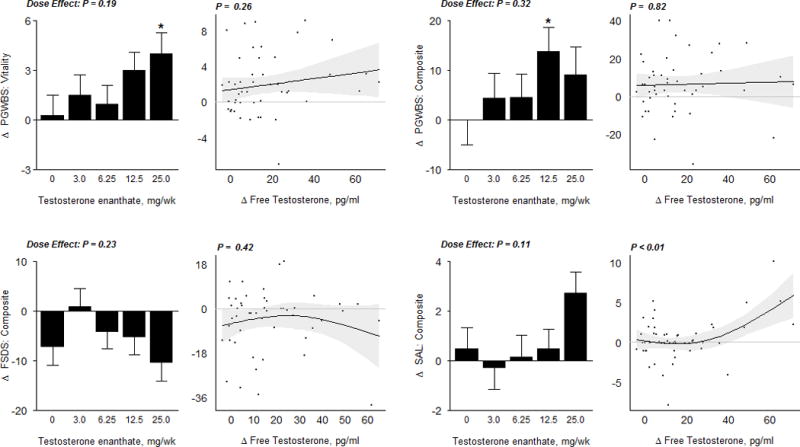
Sexual Function Outcome Measures
In the bar graphs on the left, data represent absolute mean changes (± SE) from baseline for each treatment group. The * represents a significant difference between mean on treatment change in dose group vs. placebo at a 0.05 level; the significance level for the overall dose effect (by likelihood ratio test) is also shown. Scatterplots on the right display estimates and 95% confidence regions for the Generalized Additive Model (GAM) of change in sexual function outcomes as a function of free testosterone levels. The p−values displayed here are from a significance test of no association. SAL, Sexual Activity Log; FSDS, Female Sexual Distress Scale; PGWBI, Psychological General Well-Being Index
The scores on the FSDS (Figure 3B) and the DISF-SR (Supplementary Figure 1A) did not significantly change when compared to placebo, and were not related to on-treatment testosterone concentrations. The Psychological General Well-Being Index (PGWBI) composite and vitality domain score significantly increased at the 12.5mg and 25mg dose-group, respectively when compared to placebo (p<0.05 vs placebo). (Figure 3B)
Body Composition
Changes in LBM were significantly related to change in free testosterone concentrations. The gains in lean body mass in women assigned to 25-mg weekly dose averaged 1.8 kg and were significantly greater than in the placebo group (p <0.01) (Figure 4). The estimated between-person difference in LBM was 0.6 kg for each 100 ng/dl change in total testosterone concentrations (95% CI: 0.2, 1.1; p = 0.003). There were no significant changes in total fat mass during intervention.
Figure 4.
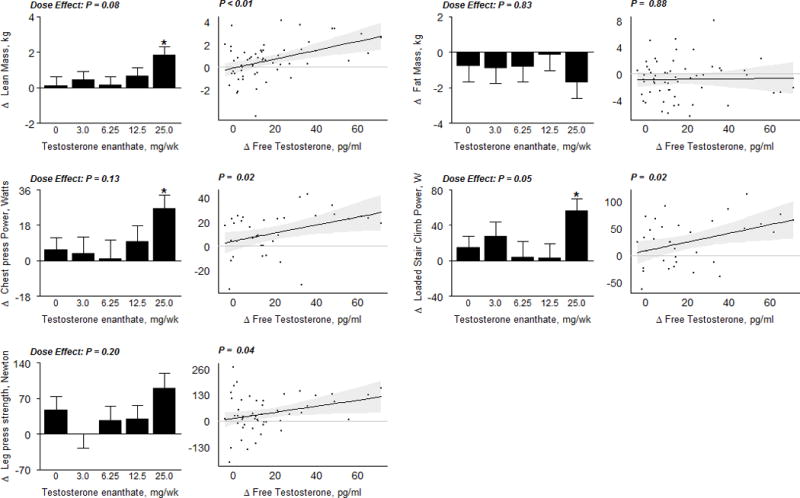
Body Composition and Muscle Performance Measures
In the bar graphs on the left, data represent absolute mean changes (± SE) from baseline for each treatment group. The * represents a significant difference between mean on treatment change in dose group vs. placebo at a 0.05 level; the significance level for the overall dose effect (by likelihood ratio test) is also shown. Scatterplots on the right display estimates and 95% confidence regions for the Generalized Additive Model (GAM) of change in body composition compartments as a function of free testosterone levels. The p−values displayed here are from a significance test of no association. Kg, kilogram; N, Newton; W, Watt.
Muscle Performance and Physical Function
Chest press power (CPP) and loaded stair climb power (LSCP) significantly increased in the 25-mg group compared to placebo (average increase in CPP = 27 watts, p = 0.03; average increase in LSCP =57 watts, p = 0.03; Figure 4). GAM indicated a significant relationship between changes in leg press strength, chest press power, and loaded stair climbing power with changes in free testosterone concentrations after adjusting for baseline muscle function. Other performance-based measures of physical function - gait speed, lift-and-reach, unloaded stair climb speed or power did not change significantly in any group and were not related to testosterone dose or on-treatment concentrations in these otherwise healthy women without any functional limitations (Supplementary Figure 2).
Results obtained in sensitivity analyses that controlled for baseline BMI did not meaningfully differ from those described above.
Hematocrit and Metabolic Parameters
Hemoglobin and hematocrit levels increased in a dose-dependent fashion (Figure 5); the increases in hemoglobin and hematocrit were significantly greater in women assigned to the 25-mg dose group than in those assigned to placebo (p<0.05 vs placebo). Fasting glucose, lipid profile and liver function tests did not significantly change from baseline and did not differ among groups.
Figure 5.
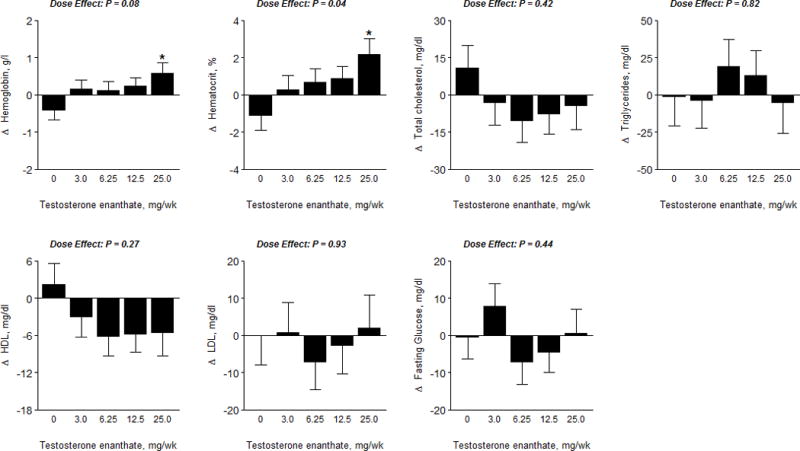
Hematocrit, Hemoglobin and Metabolic Parameters
The data represent absolute mean changes (± SE) of data for each treatment group. The * represents a significant difference between mean on treatment change in dose group vs. placebo at a 0.05 level. The p−value displayed is an overall F-test for significance of dose level, obtained via ANCOVA. LDL, Low Density Lipoprotein; HDL, High Density Lipoprotein.
Adverse Events
The overall frequency of adverse events by physiologic system was similar among the treatment groups (Supplementary Table 2). There were no serious or life-threatening testosterone-related adverse events. Clitoral size and Palatsi acne scores did not differ among groups. There were small but significant increases in Ferriman-Gallwey score in the 12.5 and 25-mg dose groups when compared to placebo. Sebum production in the forehead, nose and back did not change and did not differ significantly among groups. (Figure 6)
Figure 6.
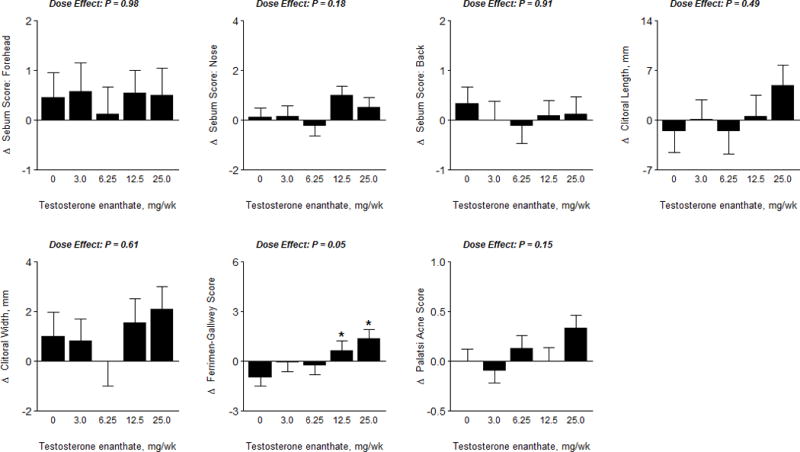
Androgenic Adverse Events
The data represent absolute mean changes (± SE) of data for each treatment group. The * represents a significant difference between mean on treatment change in dose group vs. placebo at a 0.05 level. The p−value displayed is an overall F-test for significance of dose level, obtained via ANCOVA.
Nine women assigned to testosterone arms reported acne, four of these nine women were in the 6.25 mg dose group. Three women – all in the 12.5 mg dose group - reported increased hair growth. Two women in the 25 mg dose group reported voice changes that were attributed to gastroesophageal reflux disease and resolved with proton-pump inhibitor therapy. There was no report of clitoromegaly. One woman reported increased libido as a negative complaint in the 12.5 mg dose group.
DISCUSSION
The concept of the “female androgen deficiency syndrome” is predicated upon the notion that a certain physiologic range of testosterone levels is necessary for the maintenance of androgen-dependent measures in women (e.g., some aspects of sexual function, and muscle mass and performance), that testosterone levels below this range are associated with an increased risk of impairment of these androgen-dependent measures (e.g., sexual dysfunction), and that raising testosterone levels in women with low testosterone levels into the physiologic range would improve these androgen-dependent measures.48, 49 Indeed, several well-designed randomized, controlled trials in surgically menopausal women with hypoactive sexual desire have reported that administration of transdermal testosterone improves modestly some domains of sexual function such as sexual desire, satisfaction and frequency.9–13 However, two large phase III trials using a transdermal testosterone gel (Libi-Gel, Bio-Sante, Inc) failed to show significant improvements in sexual function in women with hypoactive sexual desire disorder. In our dose-response study, in which we created a range of testosterone concentrations in oophorectomized and hysterectomized women, testosterone administration was associated with concentration-related improvements in some domains of sexual function – sexual thoughts and desires, sexual activity scores, and arousal, but not in other domains. Significant improvements were observed only at the highest dose of testosterone enanthate which was associated with supraphysiologic on-treatment testosterone concentrations. In our trial of women not presenting with hypoactive sexual desire disorder or other forms of sexual dysfunction, physiologic testosterone doses resulted in no significant improvements.
Testosterone administration at supraphysiologic doses was associated with significant gains in lean body mass, chest press power, and loaded stair climbing power. Miller et al have reported modest increases in lean body mass in women with hypopituitarism in response to testosterone administration.50 In the relatively healthy women without any physical dysfunction, who participated in this trial, significant changes in other performance-based measures of physical function were not observed. Furthermore, significant improvements in LBM, chest press power and stair climbing power were observed only at the highest dose of 25-mg*week-1 that was associated with nadir testosterone concentrations of 211 ng/dl (nearly 5-to-6-times the physiologic level); however, there were more consistent linear and nonlinear patterns of increases in lean body mass and muscle performance measures (Figure 4) when changes in these outcomes were modeled as a function of change in circulating free testosterone concentrations. These relationships were maintained even after adjusting for baseline BMI. Overall, the observed dose-response relationships of testosterone concentrations and changes in lean body mass, chest press power, and stair climbing power render it unlikely that significant gains in these outcomes could be achieved by raising testosterone levels from low into the physiologic range for healthy women.
We did not find significant changes with 24-weeks of exogenous testosterone administration on fat mass at any dose. These results obtained in our population of women are consistent with the findings of other testosterone trials in HIV-infected women and in women with hypopituitarism,40, 50, 51 but are in contrast to those obtained from testosterone trials in men, in whom testosterone administration is associated with significant loss of whole body, subcutaneous and visceral fat. Further research is needed to determine the mechanistic basis of this sex difference in response of adipose tissue to testosterone administration. It is possible that larger testosterone doses than those used in this trial or longer treatment duration may be necessary to achieve reductions in fat mass in women.
We observed few androgenic effects even at supraphysiologic doses and there were no serious adverse events related to intervention during the 6-month period of the study. Hemoglobin and hematocrit levels increased dose-dependently, but the changes were small, and both measures stayed within the normal range for women. Total cholesterol and LDL cholesterol, triglycerides and fasting glucose did not change significantly. HDL cholesterol levels decreased with all testosterone dose groups in comparison to placebo, but the changes in HDL cholesterol were non-significant. It is possible that concurrent estrogen therapy may have mitigated some of the androgenic side effects in our trial. The effects of testosterone therapy on cardiovascular and metabolic risk in women need further investigation in long-term, adequately powered trials 52; this dose response study was not designed or powered to determine long-term cardiovascular safety of testosterone in women. Long-term testosterone administration at doses that raise testosterone levels to 5 -6 times physiologic range may have adverse consequences on cardiovascular safety, either related to direct effects of testosterone or due to concurrent estrogen therapy.
Our study had notable strengths and some limitations. The trial had many features of a good trial design: concealed randomization, placebo-control, blinding and oversight by an independent DSMB. The administration of testosterone by injections by the study staff ensured high rates of compliance and high level of bioavailability. Furthermore, testosterone injections were effective in raising testosterone concentrations in a dose-dependent fashion over a wide range. We measured total testosterone levels using LC-MS/MS, widely considered the reference method with the highest sensitivity and specificity. Free testosterone was measured using equilibrium dialysis method, also considered the reference method. Sexual function was measured using the BISF-W, which has been validated in surgically menopausal women, and the findings of BISF-W were corroborated using sexual activity logs. The sexual function domains that improved with testosterone administration – sexual desire/thoughts, sexual activity, arousal, and overall sexual activity scores – are consistent with previous reports. The sample size of this mechanistic dose response study was guided by the primary aim to detect significant relationships between testosterone concentrations and significant changes in measures of sexual function, and muscle mass and performance. Our trial was limited by small sample size due to low recruitment as well as large placebo effects that may have reduced the power to detect significant improvements, particularly at the lower dose groups. We did encounter a moderate proportion of missingness of data within the BISF-W questionnaire that we addressed through imputation modeling. Although the BISF-W has been well-validated, this measure has tendency toward a high noise-to-signal ratio which may prevent detection of subtle effects. To account for between-group variation at baseline, our assessments of sexual function (and other outcomes) controlled for baseline. The women in our study were healthy, medically stable women who were not recruited for hypoactive sexual desire disorder or other forms of sexual dysfunction; it is possible that lower testosterone doses or concentrations would have been effective in improving sexual function in women with hypoactive sexual desire disorder_ENREF_25. It is also possible that women with undiagnosed depression in our sample may have influenced our findings as we did not actively screen for depression with a validated instrument. In addition, a minority of women were on antidepressant therapy (29%) in our sample which may have influenced their sexual function.
CONCLUSION
By creating a wide range of testosterone concentrations from physiologic to highly supraphysiologic range, our study provides novel information about the range of testosterone doses associated with potential beneficial effects on a variety of androgen-dependent outcomes in women. Our findings demonstrate that improvements in some measures of sexual function, lean body mass, and some domains of muscle performance and physical function in hysteretomized women with and without oophorectomy are related to testosterone dose and increases in testosterone concentrations. Although significant gains were observed only at the highest dose group associated with supraphysiologic testosterone concentrations, few androgenic adverse effects were observed. Long-term randomized trials are needed to determine whether patient-important improvements in sexual and physical function can be achieved safely with testosterone doses that do not induce virilization or worsen cardio-metabolic risk.
Supplementary Material
Acknowledgments
Data Safety Monitoring Board: Dr. Jan Shifren, Massachusetts General Hospital, Boston, Massachusetts (Chair); Dr. Raja Sayegh, Boston Medical Center; and Dr. Anita Nelson, Harbor-UCLA Medical Center.
Additional Contributions: We thank the staff of the General Clinical Research Unit of Boston University’s Clinical and Translational Science Institute and the Clinical Research Center of Charles Drew University of Medicine and Science for their help with these studies, and the study participants for their commitment and generosity
Funding/Support: This study was supported by grants 5U54HD041748-04 (to Charles Drew University of Medicine and Science) and 2008 TF D2274G (sub award to Boston University) from the National Institute of Child Health and Human Development and the Boston Claude D. Pepper Older Americans Independence Center grant #5P30AG031679 from the National Institute of Aging. Watson Pharmaceuticals provided the transdermal estradiol patch for this trial.
Footnotes
Conflict of Interest Disclosure: Dr. Bhasin has received research grant support from Abbott Pharmaceuticals and Eli Lilly and Co. for investigator-initiated research which is unrelated to this study. Dr. Bhasin has served as a consultant for Regeneron, Merck and Eli Lilly and Co. No other potential conflict of interest relevant to this article was reported.
Clinical Trials Registration Number: NCT00494208
References
- 1.Padero MC, Bhasin S, Friedman TC. Androgen supplementation in older women: too much hype, not enough data. Journal of the American Geriatrics Society. 2002;50(6):1131–40. doi: 10.1046/j.1532-5415.2002.50273.x. [DOI] [PubMed] [Google Scholar]
- 2.Basaria S, Dobs AS. Clinical review: Controversies regarding transdermal androgen therapy in postmenopausal women. The Journal of Clinical Endocrinology and Metabolism. 2006;91(12):4743–52. doi: 10.1210/jc.2006-0740. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. The Journal of Clinical Endocrinology and Metabolism. 2000;85(2):645–51. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 4.Maserejian NN, Shifren J, Parish SJ, Segraves RT, Huang L, Rosen RC. Sexual arousal and lubrication problems in women with clinically diagnosed hypoactive sexual desire disorder: preliminary findings from the hypoactive sexual desire disorder registry for women. Journal of Sex & Marital Therapy. 2012;38(1):41–62. doi: 10.1080/0092623X.2011.569642. [DOI] [PubMed] [Google Scholar]
- 5.Zussman L, Zussman S, Sunley R, Bjornson E. Sexual response after hysterectomy-oophorectomy: recent studies and reconsideration of psychogenesis. American Journal of Obstetrics and Gynecology. 1981;140(7):725–9. doi: 10.1016/0002-9378(81)90730-4. [DOI] [PubMed] [Google Scholar]
- 6.Nathorst-Boos J, von Schoultz B. Psychological reactions and sexual life after hysterectomy with and without oophorectomy. Gynecologic and Obstetric Investigation. 1992;34(2):97–101. doi: 10.1159/000292735. [DOI] [PubMed] [Google Scholar]
- 7.Celik H, Gurates B, Yavuz A, Nurkalem C, Hanay F, Kavak B. The effect of hysterectomy and bilaterally salpingo-oophorectomy on sexual function in post-menopausal women. Maturitas. 2008;61(4):358–63. doi: 10.1016/j.maturitas.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Nathorst-Boos J, von Schoultz B, Carlstrom K. Elective ovarian removal and estrogen replacement therapy–effects on sexual life, psychological well-being and androgen status. Journal of Psychosomatic Obstetrics and Gynaecology. 1993;14(4):283–93. doi: 10.3109/01674829309084451. [DOI] [PubMed] [Google Scholar]
- 9.Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. The New England Journal of Medicine. 2000;343(10):682–8. doi: 10.1056/NEJM200009073431002. [DOI] [PubMed] [Google Scholar]
- 10.Davis SR, Moreau M, Kroll R, et al. Testosterone for low libido in postmenopausal women not taking estrogen. The New England Journal of Medicine. 2008;359(19):2005–17. doi: 10.1056/NEJMoa0707302. [DOI] [PubMed] [Google Scholar]
- 11.Buster JE, Kingsberg SA, Aguirre O, et al. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstetrics and Gynecology. 2005;105(5 Pt 1):944–52. doi: 10.1097/01.AOG.0000158103.27672.0d. [DOI] [PubMed] [Google Scholar]
- 12.Braunstein GD, Sundwall DA, Katz M, et al. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Archives of internal medicine. 2005;165(14):1582–9. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- 13.Simon J, Braunstein G, Nachtigall L, et al. Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. The Journal of clinical endocrinology and metabolism. 2005;90(9):5226–33. doi: 10.1210/jc.2004-1747. [DOI] [PubMed] [Google Scholar]
- 14.Panay N, Al-Azzawi F, Bouchard C, et al. Testosterone treatment of HSDD in naturally menopausal women: the ADORE study. Climacteric : the journal of the International Menopause Society. 2010;13(2):121–31. doi: 10.3109/13697131003675922. [DOI] [PubMed] [Google Scholar]
- 15.Shifren JL, Davis SR, Moreau M, et al. Testosterone patch for the treatment of hypoactive sexual desire disorder in naturally menopausal women: results from the INTIMATE NM1 Study. Menopause. 2006;13(5):770–9. doi: 10.1097/01.gme.0000243567.32828.99. [DOI] [PubMed] [Google Scholar]
- 16.Douchi T, Yamamoto S, Nakamura S, et al. The effect of menopause on regional and total body lean mass. Maturitas. 1998;29(3):247–52. doi: 10.1016/s0378-5122(98)00035-8. [DOI] [PubMed] [Google Scholar]
- 17.Aloia JF, McGowan DM, Vaswani AN, Ross P, Cohn SH. Relationship of menopause to skeletal and muscle mass. The American Journal of Clinical Nutrition. 1991;53(6):1378–83. doi: 10.1093/ajcn/53.6.1378. [DOI] [PubMed] [Google Scholar]
- 18.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. The Journal of Clinical Endocrinology and Metabolism. 2007;92(3):895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women’s Health and Aging studies. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2009;64(2):243–8. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobs AS, Nguyen T, Pace C, Roberts CP. Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. The Journal of Clinical Endocrinology and Metabolism. 2002;87(4):1509–16. doi: 10.1210/jcem.87.4.8362. [DOI] [PubMed] [Google Scholar]
- 21.Miller KK, Grieco KA, Klibanski A. Testosterone administration in women with anorexia nervosa. The Journal of Clinical Endocrinology and Metabolism. 2005;90(3):1428–33. doi: 10.1210/jc.2004-1181. [DOI] [PubMed] [Google Scholar]
- 22.Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21(3):227–36. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- 23.Gruber DM, Sator MO, Kirchengast S, Joura EA, Huber JC. Effect of percutaneous androgen replacement therapy on body composition and body weight in postmenopausal women. Maturitas. 1998;29(3):253–9. doi: 10.1016/s0378-5122(98)00031-0. [DOI] [PubMed] [Google Scholar]
- 24.Sinha-Hikim I, Arver S, Beall G, et al. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. The Journal of Clinical Endocrinology and Metabolism. 1998;83(4):1312–8. doi: 10.1210/jcem.83.4.4718. [DOI] [PubMed] [Google Scholar]
- 25.Mazer NA, Leiblum SR, Rosen RC. The brief index of sexual functioning for women (BISF-W): a new scoring algorithm and comparison of normative and surgically menopausal populations. Menopause. 2000;7(5):350–63. doi: 10.1097/00042192-200007050-00009. [DOI] [PubMed] [Google Scholar]
- 26.Grossi E, Groth N, Mosconi P, et al. Development and validation of the short version of the Psychological General Well-Being Index (PGWB-S) Health and Quality of Life Outcomes. 2006;4:88. doi: 10.1186/1477-7525-4-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derogatis LR. The Derogatis Interview for Sexual Functioning (DISF/DISF-SR): an introductory report. Journal of Sex & Marital Therapy. 1997;23(4):291–304. doi: 10.1080/00926239708403933. [DOI] [PubMed] [Google Scholar]
- 28.Derogatis L, Clayton A, Lewis-D’Agostino D, Wunderlich G, Fu Y. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. The Journal of Sexual Medicine. 2008;5(2):357–64. doi: 10.1111/j.1743-6109.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. The American Journal of Clinical Nutrition. 2002;76(2):378–83. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- 30.Storer TW, Magliano L, Woodhouse L, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. The Journal of Clinical Endocrinology and Metabolism. 2003;88(4):1478–85. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 31.Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. The Journal of Clinical Endocrinology and Metabolism. 2010;95(6):2790–9. doi: 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeBrasseur NK, Lajevardi N, Miciek R, Mazer N, Storer TW, Bhasin S. Effects of testosterone therapy on muscle performance and physical function in older men with mobility limitations (The TOM Trial): design and methods. Contemporary Clinical Trials. 2009;30(2):133–40. doi: 10.1016/j.cct.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2011;66(10):1090–9. doi: 10.1093/gerona/glr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. The New England Journal of Medicine. 2010;363(2):109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeBrasseur NK, Bhasin S, Miciek R, Storer TW. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. Journal of the American Geriatrics Society. 2008;56(11):2118–23. doi: 10.1111/j.1532-5415.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierard G, Pierard-Franchimont C. The Sebutape technique for monitoring androgen dependent disorders. The European Journal of Medicine. 1992;1(2):109–12. [PubMed] [Google Scholar]
- 37.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. The Journal of Clinical Endocrinology and Metabolism. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 38.Palatsi R, Hirvensalo E, Liukko P, et al. Serum total and unbound testosterone and sex hormone binding globulin (SHBG) in female acne patients treated with two different oral contraceptives. Acta Dermato-Venereologica. 1984;64(6):517–23. [PubMed] [Google Scholar]
- 39.Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. The Journal of Clinical Endocrinology and Metabolism. 2011;96(8):2430–9. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi HH, Gray PB, Storer TW, et al. Effects of testosterone replacement in human immunodeficiency virus-infected women with weight loss. The Journal of Clinical Endocrinology and Metabolism. 2005;90(3):1531–41. doi: 10.1210/jc.2004-1677. [DOI] [PubMed] [Google Scholar]
- 41.Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. American Journal of Physiology Endocrinology and Metabolism. 2001;281(6):E1172–81. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 42.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–42. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 43.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 44.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in medicine. 2011;30(4):377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 45.Li KH, Meng XL, Raghunathan TE, Rubin DB. Significance Levels from Repeated P-Values with Multiply-Imputed Data. Stat Sinica. 1991;1(1):65–92. [Google Scholar]
- 46.Wood SN. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman & Hall/CRC; 2006. [Google Scholar]
- 47.Coviello AD, Nelson KP, Bhasin S. Reference Ranges for Circulating Testosterone in the Follicular and Luteal Phases of the Menstrual Cycle in a Healthy, Community-Based Sample of Women in the Framingham Heart Study [abstract] Endocrine Reviews. 2012;33 [Google Scholar]
- 48.Bhasin S. Female androgen deficiency syndrome–an unproven hypothesis. The Journal of Clinical Endocrinology and Metabolism. 2005;90(8):4970–2. doi: 10.1210/jc.2005-1328. [DOI] [PubMed] [Google Scholar]
- 49.Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertility and Sterility. 2002;77(4):660–5. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- 50.Miller KK, Biller BM, Beauregard C, et al. Effects of testosterone replacement in androgen-deficient women with hypopituitarism: a randomized, double-blind, placebo-controlled study. The Journal of Clinical Endocrinology and Metabolism. 2006;91(5):1683–90. doi: 10.1210/jc.2005-2596. [DOI] [PubMed] [Google Scholar]
- 51.Herbst KL, Calof OM, Hsia SH, et al. Effects of transdermal testosterone administration on insulin sensitivity, fat mass and distribution, and markers of inflammation and thrombolysis in human immunodeficiency virus-infected women with mild to moderate weight loss. Fertility and Sterility. 2006;85(6):1794–802. doi: 10.1016/j.fertnstert.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Brand JS, van der Schouw YT. Testosterone, SHBG and cardiovascular health in postmenopausal women. International Journal of Impotence Research. 2010;22(2):91–104. doi: 10.1038/ijir.2009.64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


