Innate immunity provides a first line of defense against invading pathogens. The host cell is equipped with a set of germ-line-encoded pattern-recognition receptors (PRRs) that recognizes the basic structural units that make up microorganisms. These receptors are located on the cell surface, endosomal membrane, and in the cytoplasm. The membrane-bound toll-like receptors (TLRs) serve to detect ligands from the extracellular and endosomal milieu, whereas nucleotide-binding oligomerization domain and leucine-rich repeat-containing receptors (NLRs), the AIM2-like receptors (ALRs) and RIG-I-like receptors provide immune surveillance in the cytoplasm. Of these, NLRs and ALRs have the ability to form an inflammasome, a multi-protein entity with the capacity to activate the cysteine protease caspase-1, and ultimately induce proteolytic cleavage of the proinflammatory cytokines pro-interleukin-1β (pro-IL-1β) and pro-IL-18. Inflammasome activation also engages a violent and proinflammatory form of cell death known as pyroptosis.
In this issue of Immunological Reviews, we take an in-depth look at the development of the inflammasome field as a whole from its inception. We also explore the latest and most exciting advances and highlight future directions. In 2002, a landmark paper by Martinon et al. (1) described for the first time in an overexpression or cell free system the existence of a molecular platform comprised of caspase-1, caspase-5, ASC (apoptosis-associated speck-like protein containing a CARD), and NLRP1 (NLR family, pyrin domain-containing 1), a complex now known as the NLRP1 inflammasome. In 2003, Mariathasan et al. (2) provided genetic evidence to demonstrate the importance of inflammasomes in endogenous settings. They generated mice deficient in ASC or NLRC4 (NLR family, CARD domain-containing 4) and found that macrophages lacking these components have an impaired ability to activate caspase-1 and induce robust pro-IL-1β processing in response to infection by Salmonella enterica serovar Typhimurium. In addition, macrophages deficient in ASC failed to activate caspase-1 following lipopolysaccharide (LPS) and adenosine triphosphate (ATP) stimulation. The sensor that mediates LPS and ATP-induced inflammasome activation was later identified to be NLRP3 by same group in 2006 (3). At the same time, two other groups independently identified that bacterial RNA molecules or uric acid crystals trigger NLRP3 inflammasome activation (4, 5).
Since these seminal discoveries, the field has expanded at an unprecedented rate, illustrated by the publication of over 3000 papers describing the biology of this macromolecular complex. It is well established that at least five inflammasome receptors exist: NLRP1, NLRP3, NLRC4, AIM2, and Pyrin (Fig. 1). Emerging evidence suggests that NLRP7 and IFI16 are among a handful of other PRRs that could activate caspase-1 by forming an inflammasome complex. These inflammasome receptors sense not only microbial-associated molecular patterns but also a diverse range of damage-associated molecular patterns released by the host during tissue stress.
Fig. 1.
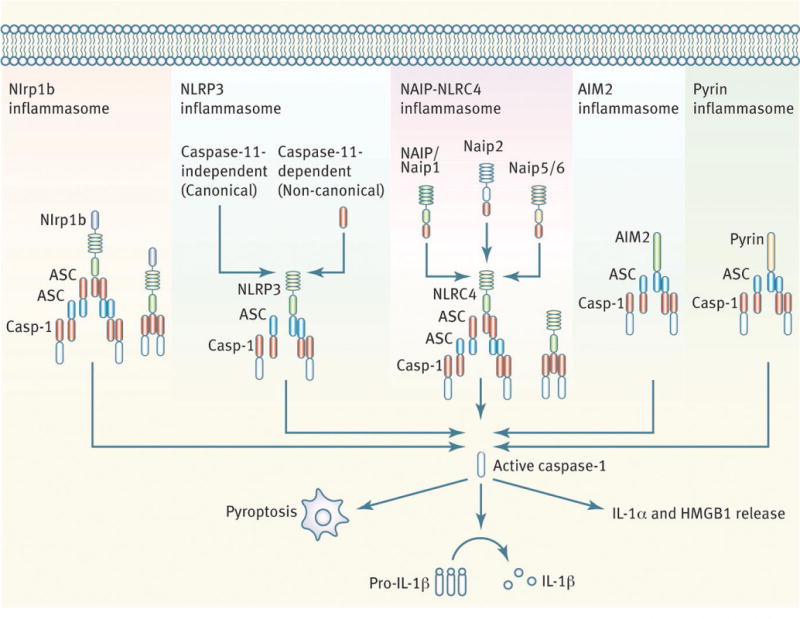
Established inflammasome complexes.
As a result of the remarkable ability to recognize microbial patterns and physiological aberration, the inflammasome is crucial for providing host protection against infectious diseases and the development of cancer. In contrast, inappropriate recognition of endogenous activators by inflammasome receptors triggers inflammation, a detrimental cue often leading to autoinflammatory, metabolic, and other chronic disorders. While mouse genetic models have unraveled the mechanistic details underpinning inflammasome activities, genetic studies within the human population uncover mutations in inflammasome components that are associated with devastating consequences, including the development of often-fatal autoinflammatory conditions and cancer. My own review (6) provides a general overview on the latest developments in the inflammasome field and highlights the functional roles of established and newly described inflammasome receptors in health and disease.
The founding member of the inflammasome family, NLRP1, responds to only a limited set of activators, including the Bacillus anthracis lethal toxin and Toxoplasma gondii. The review by Chavarría-Smith and Vance (7) describes comprehensively the immunobiology of the NLRP1 inflammasome. They provide detailed and opinionated discussions on the different models by which NLRP1 is activated upon sensing lethal toxin and muramyl dipeptide (MDP), whether cleavage of NLRP1 itself is sufficient for its activation, and how the host and pathogen regulate NLRP1 responses (7).
Unlike the NLRP1 inflammasome, the NLRP3 inflammasome responds to a variety of activators, including pathogens and endogenous signals such as ATP, ROS, saturated fatty acid, and amyloid polypeptides. The observation that seemingly unrelated activators are all able to engage the NLRP3 inflammasome is one of the most puzzling phenomena that is yet to be fully understood. Furthermore, the relevance of NLRP3 to almost all aspects of health and disease has attracted immense research interest. While the search for a unified model for activation of the NLRP3 inflammasome is ongoing, several mechanisms have been proposed: a common ion efflux signal driven by known activators, mitochondria-associated dysfunctions driven by cellular damage, and changes in cell volume. The review by Elliott and Sutterwala (8) expertly summarizes and discusses these proposed models in detail. The authors further dissect the proposed molecular models describing the assembly of the NLRP3 inflammasome, which they have elegantly categorized as the ‘spoked wheel’, ‘branching tree’, ‘layered speck’, and ‘sandwich’ models.
The large selection of activators that can ignite the NLRP3 inflammasome increases the likelihood that unchecked activation could lead to chronic diseases. Indeed, inflammation driven by the NLRP3 inflammasome contributes to the development of obesity, type I diabetes, aging, and atherosclerosis. The review by Haneklaus and O’Neill (9) describes the importance of NLRP3 in these clinical manifestations and examines the discrepant metabolic phenotypes between different animal studies as well as disease-specific roles for IL-1 and IL-18. Importantly, the authors (9) highlight an ongoing clinical trial based on IL-1 therapy for the treatment of atherosclerosis. Goldberg and Dixit (10) echo the message for the importance of the NLRP3 inflammasome in chronic diseases; their insightful review examines the consequence of NLRP3 activities and persistent inflammation from the perspective of age-related disease. An important observation from research studies is that dietary modulation can suppress aging caused by inflammasome-mediated inflammation. In agreement with the authors, it is evident that the field needs to identify inhibitors to reduce functional decline without compromising the anti-microbial activities of inflammasomes. This is of paramount importance as we search for more options to treat chronic diseases for the aging population.
An alternative mode of NLRP3 inflammasome activation, also known as ‘non-canonical’ NLRP3 inflammasome activation, is a pathway defined by its requirement for caspase-11. This pathway was identified as a result of an important discovery made by Kayagaki et al. (11) showing that previously generated Casp1-deficient mouse strains lack caspase-11. The Casp1 gene was originally knocked out from embryonic stem cells of the 129 mouse strain, which harbors a mutation in the Casp11 locus and is devoid of caspase-11 protein expression. The Casp1 and Casp11 genes are too close in the genome (separated by approximately 1500 base pairs) to have been segregated, despite extensive backcrossing onto the C57BL/6 strain. As a result, conventional Casp1−/−mice lack both caspase-1 and caspase-11. Stowe et al. (12) provide a timely historical overview on the discovery of caspase-11, followed by an in-depth discussion on the mechanisms involved in the activation and regulation of caspase-11 and its emerging role as a direct LPS sensor. Although there is some progress in elucidating the distinct contribution of caspase-1 and caspase-11 in health and disease, much work is needed to dissect the functional roles of these inflammatory caspases.
The ligands for two other established inflammasome receptors, NLRC4 and AIM2, have been identified. NLRC4, together with the members of the NAIP family, binds bacterial flagellin and components of the Type III secretion systems. The mouse genome encodes seven NAIP proteins, whereas the human encodes only one. Zhao and Shao (13) review the literature on the biochemical and molecular events governing the assembly of the NAIP-NLRC4 inflammasome. They also summarize recent advances on the role of this inflammasome in infectious diseases (13). The ligand specificity of and the molecular mechanism regulating the human NAIP receptor remain poorly defined and are exciting areas for future research.
In 2009, four groups independently identified AIM2 as an inflammasome receptor for cytosolic double-stranded DNA. Xiao (14) elegantly summarizes the molecular, biochemical, and structural features of AIM2 and other nucleic acid sensing inflammasomes. Salmonella enterica serovar Typhimurium and Francisella tularensis are two important human pathogens recognized by the NLRC4 and AIM2 inflammasome, respectively. Complementing the two reviews on the molecular and structural regulation of NLRC4 and AIM2 inflammasomes is the contribution by Storek and Monack (15). The authors discuss the importance of cell-type specific roles for these complexes in the host defense against Salmonella and Francisella infection (15). So far, most studies on inflammasomes and infectious diseases have focused on myeloid cells, such as macrophages and dendritic cells. However, investigations into the role of inflammasomes in adaptive immune cells and epithelial cells are now surfacing.
A major consequence of inflammasome activation in myeloid cells is pyroptosis. It has been proposed that pyroptosis of infected macrophages removes the intracellular niche for intracellular bacteria, which are then taken up and killed by neutrophils. Jorgensen and Miao (16) examine the dynamic relationship between pyroptosis and killing of intracellular bacteria and how bacteria have evolved various ways to evade inflammasome and pyroptotic responses to ensure survival and dissemination. How pyroptosis contributes to chronic inflammatory diseases in humans independently of IL-1 and IL-18 is unknown and would be an intriguing research area to pursue.
In addition to bacteria, inflammasomes are critical for the host defense against viruses, parasites, and fungi. Each of these important groups of pathogens is featured in one of the reviews in this issue. Negash and Gale (17) provide an overview on the immunobiology and clinical importance of the inflammasome in liver diseases, with a particular emphasis on hepatitis B virus (HBV), hepatitis C virus (HCV), and non-alcoholic fatty liver disease (NAFLD). The authors discuss the beneficial and detrimental properties of IL-1 and IL-18 production in HBV and HCV infections and speculate the possibility of using anti-IL-1 therapies as a potential treatment option.
Parasitic infections are a major concern in the developing world. A number of these infections have been classified as neglected tropical diseases (NTDs). NTDs affect more than a billion people, particularly those who live in the poorest communities in the world. The World Health Organization and Centers for Disease Control and Prevention both list leishmaniasis and Chagas disease, caused by Leishmania and Trypanosoma cruzi, respectively, as NTDs. Lima-Junior and Zamboni (18) explore the importance of innate immunity in the host protection against Leishmania, Trypanosoma cruzi, Plasmodium, and Toxoplasma gondii. It is clear that additional research efforts are required to elucidate how innate immunity contributes to the host defense against this group of organisms.
Another group of eukaryotic pathogens is represented by fungi, including Candida albicans and Aspergillus fumigatus. van de Veerdonk et al. (19) discuss all aspects of inflammasome biology in the context of fungal infections. Interestingly, inflammasome responses are tailored to the fungal morphology encountered by the cell. The authors also highlight the latest development on the regulation of the inflammasome, in particular, the role of Syk and caspase-8 in shaping the immunological profile during fungal infection. Caspase-8, an apoptotic caspase, has emerged as a key player in the regulation of inflammation and inflammasome functions. The new roles ascribed to caspase-8 challenge the traditional classification of caspases being either apoptotic or inflammatory. Monie and Bryant (20) provide a timely contribution on the novel roles of caspase-8 in pro-IL-1β processing and the induction of cell death and assess the debate in the published literature over whether caspase-8 is an activator or a suppressor of inflammasome activity.
Excessive inflammation is detrimental to the host, because it may potentiate the development of autoinflammatory diseases. Therefore, inflammation must be strictly controlled or resolved at the conclusion of an infection or physiological aberration. Specialized strategies exist to control overt inflammation in the cell. Post-translational modifications and autophagy are two examples of immunological checkpoints used by the cell to prevent or suppress inflammasome activities. Abdelaziz et al. (21) closely examine the functional relationships between inflammasome activities and autophagy. It is apparent that the synergy between the two is required to control infection while preventing the development of chronic inflammation. In addition to autophagy, the human genome encodes six or more pyrin-only proteins (POPs) and CARD-only proteins (COPs). Interestingly, members of the Poxviridae family encode viral POPs (vPOPs) to manipulate the host immune system. The review by Dorfleutner et al. (22) features information on the biological activities of POPs, COPs, and vPOPs in the inhibition or engagement of inflammasome functions and NF-κB signaling. The authors provide an in-depth look at the biochemistry and structural elements of POPs and COPs that gives insight into the complexity of the mammalian immune system. Complementary to this review is a contribution by Matusiak et al. (23). They focus on the functional aspects of POPs, COPs, and vPOPs in innate immunity. Importantly, the authors spotlight the possibility of using myxoma virus for cancer virotherapy. Myxoma virus is a rabbit pathogen that is non-pathogenic in humans and has the capacity to replicate in human cancer cells. Furthermore, vPOPs may hold therapeutic potential as a means to modulate inflammation in chronic disorders. Finally, Pedraza-Alva et al. (24) highlight the anti-inflammatory and potentially therapeutic properties of organic and inorganic compounds derived from traditional medicinal plants, including green tea and ginseng.
This selection of reviews provides a snapshot of all areas of inflammasome research in its entirety. It truly showcases the growth and development of the research field since the discovery of the inflammasome a little more than a decade ago. We celebrate the remarkable progress that the field has made and the contribution of this knowledge to medical science. I personally thank all the contributors who have generously contributed to this issue of Immunological Reviews. I acknowledge all my colleagues for their tireless research contributions to the field. I hope that the readers find this issue exciting and informative.
Acknowledgments
Work from the lab is supported by grants from the National Institutes of Health (grants AR056296, CA163507, and AI101935) and the American Lebanese Syrian Associated Charities (to T.-D. K).
Footnotes
The author has no conflicts of interest to declare.
References
- 1.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 3.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 4.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 5.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 6.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:???–???. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavarría-Smith J, Vance RE. The NLRP1 inflammasomes. Immunol Rev. 2015;265:???–???. doi: 10.1111/imr.12283. [DOI] [PubMed] [Google Scholar]
- 8.Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:???–???. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haneklaus M, O’Neill LAJ. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265:???–???. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg EL, Dixit VD. Drivers of age-related inflammation and strategies for healthspan extension. Immunol Rev. 2015;265:???–???. doi: 10.1111/imr.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 12.Stowe I, Lee B, Kayagaki N. Caspase-11: arming the guards against bacterial infection. Immunol Rev. 2015;265 doi: 10.1111/imr.12292. ???–??? [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Shao F. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion. Immunol Rev. 2015;265 doi: 10.1111/imr.12293. ???–??? [DOI] [PubMed] [Google Scholar]
- 14.Xiao TS. The nucleic acid-sensing inflammasomes. Immunol Rev. 2015;265 doi: 10.1111/imr.12281. ???–??? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storek KM, Monack DM. Bacterial recognition pathways that lead to inflammasome activation. Immunol Rev. 2015;265 doi: 10.1111/imr.12289. ???–??? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265 doi: 10.1111/imr.12287. ???–??? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negash AA, Gale M. Hepatitis regulation by the inflammasome signaling pathway. Immunol Rev. 2015;265 doi: 10.1111/imr.12279. ???–??? [DOI] [PubMed] [Google Scholar]
- 18.Zamboni DS, Lima-Junior DS. Inflammasomes in host response to protozoan parasites. Immunol Rev. 2015;265 doi: 10.1111/imr.12291. ???–??? [DOI] [PubMed] [Google Scholar]
- 19.van de Veerdonk FL, Joosten LAB, Netea MG. The interplay between inflammasome activation and antifungal host defense. Immunol Rev. 2015;265 doi: 10.1111/imr.12280. ???–??? [DOI] [PubMed] [Google Scholar]
- 20.Monie TP, Bryant CE. Caspase-8 functions as a key mediator of inflammation and pro-IL-1β processing via both canonical and non-canonical pathways. Immunol Rev. 2015;265 doi: 10.1111/imr.12284. ???–??? [DOI] [PubMed] [Google Scholar]
- 21.Abdelaziz DHA, Khalil H, Cormet-Boyaka E, Amer AO. The cooperation between the autophagy machinery and the inflammasome to implement an appropriate innate immune response: do they regulate each other? Immunol Rev. 2015;265 doi: 10.1111/imr.12288. ???–??? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorfleutner A, Chu L, Stehlik C. Inhibiting the inflammasome: one domain at a time. Immunol Rev. 2015;265 doi: 10.1111/imr.12290. ???–??? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matusiak M, Van Opdenbosch N, Lamkanfi M. CARD- and pyrin-only proteins regulating inflammasome activation and immunity. Immunol Rev. 2015;265 doi: 10.1111/imr.12282. ???–??? [DOI] [PubMed] [Google Scholar]
- 24.Pedraza-Alva G, Pérez-Martínez L, Valdez-Hernández A, Meza-Sosa KF, Ando-Kuri M. Negative regulation of the inflammasome: keeping inflammation under control. Immunol Rev. 2015;265 doi: 10.1111/imr.12294. ???–??? [DOI] [PubMed] [Google Scholar]


