Abstract
Background
Given the success of immunotherapeutic approaches in hematologic malignancies, the COG designed a phase I/II study to determine whether the addition of epratuzumab (anti-CD22) to an established chemotherapy platform improves rates of second remission (CR2) in pediatric patients with B-lymphoblastic leukemia (B-ALL) and early bone marrow relapse.
Procedure
Therapy consisted of 3 established blocks of re-induction chemotherapy. Epratuzumab (360 mg/m2/dose) was combined with chemotherapy on weekly × 4 (B1) and twice weekly × 4 [8 doses] (B2) schedules during the first re-induction block. Remission rates and minimal residual disease (MRD) status were compared to historical rates observed with the identical chemotherapy platform alone.
Results
CR2 was achieved in 65% and 66%, of the evaluable B1 (n=54) and B2 patients (n=60), respectively; unchanged from that observed historically without epratuzumab. Rates of MRD negativity (< 0.01%) were 31% in B1 (P=0.4128)and 39% in B2 patients (P=0.1731), compared to 25% in historical controls. The addition of epratuzumab was well tolerated, with a similar toxicity profile to that observed with the re-induction chemotherapy platform regimen alone.
Conclusions
Epratuzumab was well tolerated in combination with re-induction chemotherapy. While CR2 rates were not improved compared to historical controls treated with chemotherapy alone, there was a non-significant trend towards improvement in MRD response with the addition of epratuzumab (twice weekly for 8 doses) to re-induction chemotherapy.
Keywords: Epratuzumab, monoclonal antibody, relapsed ALL
INTRODUCTION
Survival for children with relapsed acute lymphoblastic leukemia (ALL) remains poor, especially when relapses occur in close proximity to initial diagnosis. [1–3] These poor outcomes are associated with intrinsic drug resistance, as manifested by much lower rates of successful re-induction of second complete remission (CR2) and very high rates of detectable minimal residual disease (MRD) among those who do attain CR2. [4] Because most re-induction chemotherapy platform regimens already have significant toxicity, one strategy that can be adopted to improve CR2 and MRD rates in patients with first marrow relapse is to add agents with favorable side effect profiles and unique mechanisms of action. Several monoclonal antibodies have been pursued based on this rationale. [5]
CD22 is an attractive target for antibody therapy because it is expressed almost universally in children with B-ALL. Epratuzumab is a humanized anti-CD22 monoclonal antibody with unique mechanisms of action. It is internalized after binding to cell surface CD22 and modulates B-cell activation and signaling in contrast to eliciting direct cytotoxicity. [6–9] Epratuzumab initially entered clinical trials in adults with indolent and aggressive non-Hodgkin lymphoma (NHL) approximately a decade ago. [10,11] Favorable outcomes were observed and treatment was generally well tolerated. Since this time, epratuzumab has continued to be studied extensively in adults with autoimmune diseases and hematologic malignancies and its safety and therapeutic activity have been demonstrated when administered as a single agent and also in combination with other monoclonal antibodies and chemotherapy. [12–22]
Based on promising early results in adults with NHL, the COG initiated the ADVL04P2 phase I/II trial (www.clinicaltrials.gov identifier NCT00098839) combining epratuzumab with re-induction chemotherapy in children, adolescents and young adults with first marrow relapses of B lymphoblastic leukemia (B-ALL). The chemotherapy backbone used in this trial was tested in the same patient population in the prior COG AALL01P2 study (2003–2005). [4] We previously demonstrated the safety of combining epratuzumab with chemotherapy in the Phase I pilot portion of this study, where 15 patients received 360 mg/m2 epratuzumab initially twice-weekly as a single agent for 2 weeks (‘reduction phase’) followed by 4 once-weekly doses administered during the first of 3 blocks of a standard re-induction chemotherapy platform. [23] Here, we report the results of the Phase II portion of the COG ADVL04P2 study, the primary aim of which was to determine if addition of epratuzumab to an established re-induction chemotherapy platform improves CR2 rates in patients with B-ALL and early bone marrow relapse, defined as occurring less than 36 months from initial diagnosis.
METHODS
Patient Selection
Children, adolescents and young adults, ages 2–30 years with first early (< 36 months from initial diagnosis) marrow relapse (≥ 25% marrow blasts) of B-ALL, with or without extramedullary disease, were eligible for this study provided ≥ 25% of the blast population expressed CD22 and adequate performance levels and organ function requirements were met. Patients who underwent allogeneic hematopoietic stem cell transplantation (HSCT) in first remission were eligible provided there was no evidence of graft vs. host disease and at least 4 months had elapsed. Patients with Down syndrome, mature B-ALL, refractory ALL who had received prior re-induction attempts, and those who had received treatment for prior isolated extramedullary disease were excluded. Additionally, patients who could not receive asparaginase on this study were ineligible. Institutional review boards at participating COG centers approved the study and informed consent was obtained from patients or from parents/legal guardians.
Study Design
In Phase II, patients received the 3-block re-induction chemotherapy platform previously used in COG AALL01P2, plus epratuzumab (360 mg/m2/dose) during Block 1 (Table I). Epratuzumab was administered as a slow intravenous infusion starting at a rate of 0.5 mg/kg/h, with gradual incremental increases in the rate to a maximum rate of 400 mg/h, as tolerated. Premedication with corticosteroids, an antihistamine and acetaminophen were advised prior to the first dose of epratuzumab to minimize infusion reactions. Initially, epratuzumab was administered weekly for 4 doses starting on day 1 based on pharmacokinetic (PK) data from adults with lymphoma. However, after the Phase I PK data showed that the half-life of epratuzumab was shorter in children with ALL, the dosing schedule was amended to administer epratuzumab twice weekly for a total of 8 doses over 4 weeks, starting on day 1. This alternate schedule was chosen because it provided the same number of doses, but in a somewhat altered schedule, as given in Phase I and because the safety of twice weekly dosing was established in the Phase I portion of the study. Additionally, no improvements in response rates were observed with higher doses of epratuzumab in early phase trials in adults with NHL. [10,11] Here we report Phase II results of both the weekly (B1) and twice weekly (B2) epratuzumab dosing schedules. ADVL04P2 included 3 blocks of re-induction therapy only with any further treatment at the discretion of treating physicians. All toxicities were graded according to the National Cancer Institute’s Common Toxicity Criteria version 4.0
Table I.
Study Treatment
| Study Phase | Drug Dosing |
|---|---|
| Block 1 | |
| Epratuzumab 360 mg/m2 IV (B1 Cohort) | Days 1, 8, 15, and 22 |
| Epratuzumab 360 mg/m2 IV (B2 Cohort) | Days 1, 4, 8, 11, 15, 18, 22 and 25 |
| Vincristine 1.5 mg/m2 IV | Days 1, 8, 15, and 22 |
| Prednisone 40 mg/m2/day PO | Days 1–29 |
| PEG-asparaginase 2500 IU/m2 IM | Days 2, 9, 16 and 23 |
| Doxorubicin 60 mg/m2 IV | Day 1 |
| IT therapy | Days 1 and 29 (Days 8 and 15 also if CNS +) |
| Block 2 | |
| Cyclophosphamide 440 mg/m2 IV | Days 1–5 |
| Etoposide 100 mg/m2 IV | Days 1–5 |
| Methotrexate 5000 mg/m2 IV | Day 22 |
| IT therapy | Days 1 and 22 |
| Block 3 | |
| Cytarabine 3000 mg/m2 IV q 12 hours | Days 1, 2, 8 and 9 |
| L-asparaginase 6000 IU/m2 IM | Days 2 and 9 (at hour 42 after cytarabine) |
Abbreviations: IT, intrathecal; PEG, pegylated. Intrathecal therapy consisted of cytarabine on Day 1 of block 1 and methotrexate alone for all subsequent doses in CNS-negative patients. Patients with CNS disease received IT methotrexate, hydrocortisone and cytarabine. All intrathecal dosing was based on age. G-CSF was administered during blocks 2 and 3. Blood count criteria to begin blocks 2 and 3 were an absolute neutrophil count ≥ 750/µL and platelet count ≥ 75,000/µL.
Response Assessment
Response to therapy was assessed at the completion of each of the 3 treatment blocks by routine morphological assessments of the bone marrow and cerebrospinal fluid (CSF). MRD was also measured by flow cytometry at the COG Reference Laboratory (Johns Hopkins), as described previously, using a 0.01% cutoff to define positivity. [24] CR2 was defined as <5% bone marrow blasts (M1 marrow) with an absolute neutrophil count >1000/µL and platelet count >100,000/µL, and no extramedullary disease. Per protocol, patients should not have started Block 2 until blood count parameters were met to assess response at the end of Block 1. Patients who attained an M1 marrow, but started Block 2 chemotherapy without waiting at least 2 weeks to potentially meet blood count parameters used to define CR, were deemed inevaluable for response at the end of Block 1.
Serum Epratuzumab Concentration and Human Anti-Human Antibodies
Participation in studies to assess phamacokinetics of epratuzumab was optional for patients receiving drug on the B2 twice weekly × 4 weeks (8 doses) schedule. Blood samples (2 mL) were obtained before and 60 minutes after epratuzumab infusions. Blood samples were drawn prior to doses of epratuzumab on days 1, 4, 8, 11, 15, 18, 22, and 25. Post-infusion samples were drawn after doses on days 1 and 25. Blood samples were additionally collected 1, 4, 8, and 12 weeks after the last dose of epratuzumab. Epratuzumab concentrations were determined by enzyme-linked immunosorbent assays (ELISA) at Immunomedics, Inc. Human anti-human antibody (HAHA) titers were measured prior to the start of treatment, and 4 weeks after the last dose of epratuzumab was given in Block 1, and after that point, every 3 months for one year. Human anti-human antibodies (HAHA) were measured by an ELISA based immunoassay at Immunomedics, Inc.
Statistical Considerations
Phase II was designed to determine if epratuzumab had sufficient activity to pursue further in a randomized prospective Phase III setting in newly diagnosed B-ALL patients. The primary outcome of interest was CR2 rate at the end of the first block of re-induction chemotherapy plus epratuzumab in pediatric patients with first early marrow recurrences < 36 months from diagnosis. Due to substantial differences in CR2 rate between patients with very early (<18 months from diagnosis) vs. early (18 to <36 months from diagnosis) relapse, the study considered a stratified 2-stage design to determine if the overall CR2 rates among all evaluable patients enrolled on the B2 cohort were higher than the 68% CR2 rate (40% in very early relapse and 78% in early relapse) for historical controls treated on COG AALL01P2, which included a similar pediatric population treated with the identical backbone chemotherapy 3-block regimen, but without epratuzumab. [25] The actual decision boundaries depended on the observed numbers of very early and early relapse patients, and were computed after the numbers of patients in the two strata were observed.
The trial outcomes for the B1 cohort were evaluated separately in secondary analyses and further compared with the results from the prior AALL01P2 study. Other secondary analyses included a comparison of MRD negativity rates, EFS and overall survival (OS) rates between cohorts overall, as well as within each stratum. CR2 rates, MRD negativity rates and toxicity rates were compared using one-sided Fisher’s exact test. EFS and OS rates were estimated using the Kaplan-Meier method, with standard errors calculated by the method of Peto et al. [26] EFS time was calculated as the time from study entry to the time of first event (disease progression, which was defined an M2/M3 marrow on Day 15 of Block 2, relapse, second malignancy, or death) or the date of last contact. OS time was calculated as the time from study entry to death or date of last contact. EFS and OS curves were compared using the two-sided Log-rank test. The dataset was locked as of December 31, 2012. All analyses were performed using SAS version 9.2.
RESULTS
Patient Characteristics and Accrual
Between 1/07 and 1/11, 114 patients were treated; 54 on B1 and 60 on B2 (Table II). Median age at relapse was 10.2 years and 8.4 years for the B1 and B2 cohorts, respectively. Concomitant extramedullary disease was present in 3 and 9 of the B1 and B2 patients, respectively. Seven patients underwent prior HSCT in first remission.
Table II.
Patient Characteristics
| B1 Cohort (weekly × 4) |
B2 Cohort (twice weekly × 8) |
|
|---|---|---|
| Patients Enrolled | 54 | 60 |
| Median Age at Relapse (years) | 10.2 (2.1–21.8) | 8.4 (2.4–23.6) |
| Sex, Male | 31 (57%) | 31 (52%) |
| Prior HSCT | 2 | 5 |
| Extramedullary Disease | 3 (6%) | 9 (15%) |
| Response-evaluable Patients | 48 | 50 |
| Relapse < 18months | 20 (42%) | 17 (34%) |
| Relapse 18–36 months | 28 (58%) | 33 (66%) |
Toxicity
The addition of epratuzumab to re-induction chemotherapy was well tolerated and the rates of grade 3/4 non-hematologic toxicities were similar to those observed with the AALL01P2 re-induction chemotherapy platform regimen alone (Table III). While the rates of documented infections and hyperglycemia were higher with epratuzumab, other toxicities, including febrile neutropenia, were less common. Toxic deaths occurred in 3 patients (2.6%) during Block 1 (2 in B1, 1 in B2), compared to 2.4% with Block 1 chemotherapy alone in AALL01P2 (P=1.0). [4] These deaths were attributed to a disseminated aspergillus infection; an intestinal perforation with sepsis and multi-organ failure within the context of refractory leukemia. Some of the most frequently observed grade 3/4 non-hematologic toxicities among the 114 patients treated with epratuzumab in combination with chemotherapy were: infections (50%); fever and neutropenia (26.3%); hyperglycemia (24.6%); ALT elevation (9.6%) and pancreatitis (6.1%).
Table III.
Common Grade 3/4 Non-Hematologic Toxicities
| Toxicity | B1 Cohort (weekly × 4) (n=54) |
B2 Cohort (twice weekly × 8) (n=60) |
B1 and B2 Cohorts (n=114) |
AALL01P2* (n=63) |
P-value** |
|---|---|---|---|---|---|
| Infection (documented clinically or microbiologically) | 35 (64.8%) | 22 (36.7%) | 57 (50.0%) | 21 (33.3%) | 0.0233 |
| Febrile neutropenia | 9 (16.7%) | 21 (35%) | 30 (26.3%) | 28 (44.4%) | 0.0114 |
| Hyperglycemia | 17 (31.5) | 11(18.3%) | 28 (24.6%) | 7 (11.1%) | 0.0227 |
| ALT elevation | 4 (7.4%) | 7 (11.7%) | 11 (9.6%) | 12 (19.0%) | 0.0629 |
| Pancreatitis | 6 (11.1%) | 1 (1.7%) | 7 (6.1%) | 4 (6.3%) | 0.5953 |
Analysis limited to the 63 patients on AALL01P2 with early marrow B-lineage relapses;
One-sided P-values are provided for comparisons of toxicites in the B1 and B2 cohorts combined vs. AALL01P2.
Response to Re-induction Therapy
Six B1 and 10 B2 patients were inevaluable for response. Reasons for inevaluability included failure to complete Block 1 therapy (n=9; 5 from the B1 cohort and 4 from the B2 cohort), either due to toxicity (5); refusal of protocol therapy (1); or because their treating physicians determined it was not in their best interest (3). Additional reasons that patients were inevaluable for response were failure to assess disease at the completion of Block 1 (1 from the B1 cohort) and the initiation of Block 2 therapy prior to achieving full count recovery (6 from the B2 cohort). At the end of Block 1, 48 B1 patients and 50 B2 patients were evaluable for response with CR2 achieved in 65% (31/48) of B1 and 66% (33/50) of B2 patients, compared to 74% for B-lineage patients (68% for B and T-lineage ALL combined) treated with chemotherapy alone on AALL01P2 (Table IV). Among the evaluable B1 and B2 patients who achieved CR2 and had MRD data available at the end of Block 1, 8/26 (31%) B1 and 12/31 (39%) B2 patients were MRD negative (< 0.01%), compared to 9/36 (25%) with chemotherapy alone on AALL01P2 (P=0.2148 for B1+B2 vs. AALL01P2; P=0.4128 for B1 vs. AALL01P2; P=0.1731 for B2 vs. AALL01P2). For very early relapse patients who achieved CR2 at the end of Block 1, no significant differences in MRD rates were observed in either the B1 or B2 cohort compared to AALL01P2. However, for early relapse patients (18–36 months from diagnosis), an improvement in MRD response rate was observed for the B2 cohort compared to AALL01P2, although this did not reach statistical significance due to the small sample size (44% vs. 25%; P=0.1215).
Table IV.
Treatment Response Compared to Historical Controls
| Stratum | AALL01P2 (chemotherapy only) |
B1 Cohort (weekly × 4) |
B2 Cohort (twice weekly × 8) |
|
|---|---|---|---|---|
| Response-evaluable Patients | 58 | 48 | 50 | |
| CR2 after Block 1 | Overall | 74% (43/58) | 65% (31/48) (P=0.1964) | 66% (33/50) (P=0.2380) |
| Very early relapse (< 18 months) | 56% (9/16) | 50% (10/20) (P=0.4854) | 35% (6/17) (P=0.1956) | |
| Early relapse (18 – 36 months) | 81% (34/42) | 75% (21/28) (P=0.3795) | 82% (27/33) (P=0.5602) | |
| MRD <0.01% | Overall | 25% (9/36) | 31% (8/26) (P=0.4128) | 39% (12/31) (P=0.1731) |
| Very early relapse (< 18 months) | 25% (2/8) | 30% (3/10) (P=0.6176) | 17% (1/6) (P=0.8462) | |
| Early relapse (18 – 36 months) | 25% (7/28) | 31% (5/16) (P=0.4560) | 44% (11/25) (P=0.1215) |
P-values are provided for comparisons of CR2 rates and MRD response in the B1 and B2 cohorts to AALL01P2. P-values for MRD responses were based on the one-sided Fisher’s exact test.
Outcomes of B1 and B2 Cohorts
Two-year EFS for the evaluable patients in the B1 and B2 cohorts was 25.9% ± 6.4% and 39.9 ± 8.0% (P=0.1660), respectively and OS was 34.2 ± 6.9% and 49.3 ± 8.1% (P=0.1433), respectively. Notably this study was not powered to detect differences in survival compared to AALL01P2, but there appeared to be no differences in 2-year EFS or OS between the B1 cohort and AALL01P2. However, in the B2 cohort, while no differences in outcome were observed among patients with very early relapse, patients with relapses 18–36 months from diagnosis had significant improvements in outcome when compared to a similar population on AALL01P2 with 2-year EFS 54.6% ± 9.5% vs. 25.7% ± 7.0%, (P=0.0268) and 2-year OS 69.0% ± 8.8% vs. 40.6% ± 7.8%, (P=0.0146).
Correlative Studies
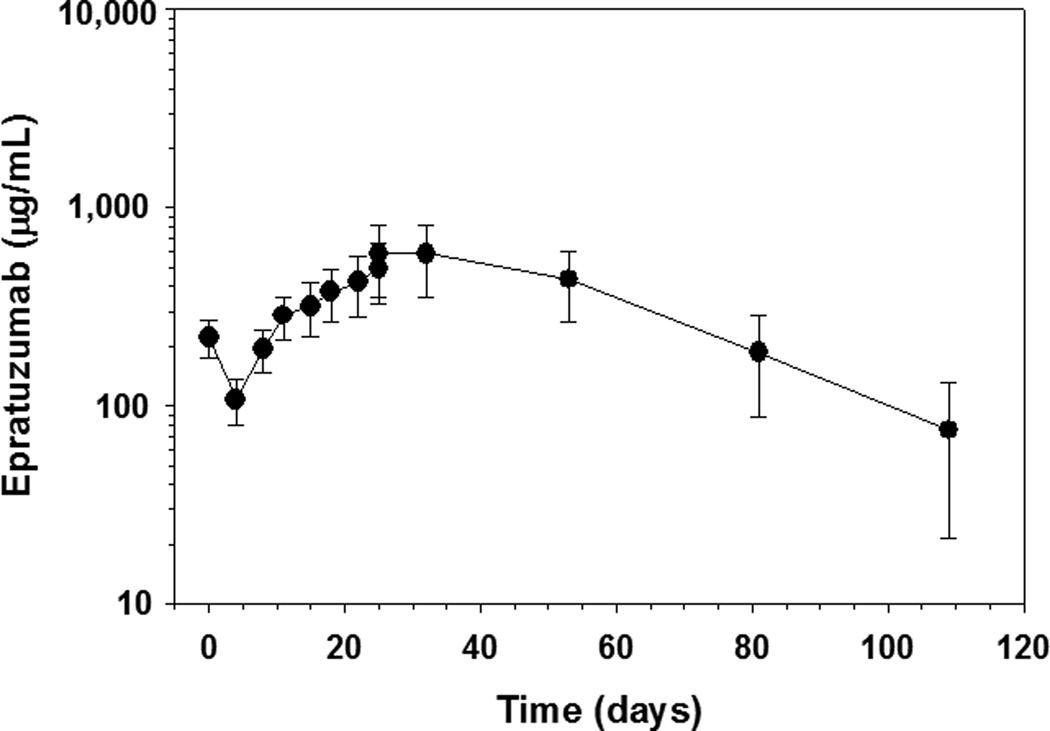
Pharmacokinetic analyses in a subset of B2 patients showed that the epratuzumab trough serum concentration steadily increased to 501 ± 149 µg/mL before the final dose on day 25 (n=26) (Figure 1). The mean serum half-life of epratuzumab was 17.0 ± 4.9 days (n=17) and was shorter than the value of 23 days observed in adults treated with epratuzumab for indolent non-Hodgkin lymphoma. [10] No patient developed human anti-human antibodies among 57 patients with one or more serum samples analyzed 4 weeks to 12 months post treatment.
Figure 1. Graph of Epratuzumab Serum Concentration versus Time.
Semi log plot of serum epratuzumab concentrations are shown following twice-weekly administration for a total of 8 doses demonstrating an increase in the mean plasma concentration of epratuzumab over time before the final dose on day 25.
DISCUSSION
In an effort to study new agents more efficiently in the relapsed childhood ALL population, the COG adopted a strategy of incorporating drugs in combination with an established re-induction platform. The aim was to determine if there was significant improvement in the CR2 rate at the end of Block 1 as a screen for activity and to potentially prioritize new agents of promise in future studies. The ADVL04P2 trial was the first groupwide COG study to combine a novel agent, epratuzumab, with a multiagent re-induction chemotherapy platform, piloted in the predecessor AALL01P2 study. Epratuzumab was selected for study in this trial because of its unique mechanism of action, favorable safety profile, therapeutic efficacy in adults with hematologic malignancies and autoimmune diseases, and broad applicability, since >95% of patients with B-lineage ALL express CD22. Theoretically, the activity of epratuzumab in modulating B-cells may augment the effects of cytotoxic chemotherapy. Preclinical studies have demonstrated that anti-CD19 and anti-CD22 antibodies can potentiate the response to chemotherapy and it has been hypothesized that the combination of agents that inhibit distinct cellular pathways could result in more effective tumor destruction. [27]. Our experience with epratuzumab confirmed its favorable safety profile where the spectrum and severity of toxicities observed when the drug was administered either weekly × 4, or twice weekly × 4 for 8 doses, generally did not exceed those observed with the chemotherapy platform alone. Although the addition of epratuzumab to chemotherapy did not improve CR2 rates and meet the study benchmarks established to define activity, there are several interesting observations from this study.
The study highlights the challenges in using a historical control population as the comparator to assess efficacy, as it is difficult to ascertain how the populations have changed over time, and there was no randomization, which would presumably balance out unforeseen factors between arms. Another potential limitation of this study was the selection of CR2 as the primary endpoint. Although no improvement in CR2 rates was observed compared to chemotherapy alone with either epratuzumab dosing schedule, data from a randomized trial comparing idarubicin to mitoxantrone in first relapse of childhood ALL suggest that CR2 rates do not necessarily correlate with EFS. [2]
ADVL04P2 did show an intriguing non-significant trend towards an advantage in MRD response with the twice weekly × 4 epratuzumab dosing schedule. It is difficult to draw conclusions about the significant improvement in outcome, which was observed among B2 cohort patients with relapses 18–36 months from diagnosis because patient numbers were small and importantly, therapy after re-induction varied and was not a part of this study. Our findings confirm that patients with very early marrow relapses of ALL are highly refractory and raise the question of whether a different impact of epratuzumab might be observed, if it were studied at a time point in therapy when there is a reduced disease burden. Perhaps this agent might be used more effectively in patients already in remission.
In conclusion, epratuzumab, as given on the B1 and B2 schedules, was tolerable in combination with re-induction chemotherapy in pediatric and young adult patients with early relapsed CD22-positive B-ALL, but did not improve CR2 rates when compared to historical controls treated with re-induction chemotherapy alone. However, there were improvements (non-significant) in MRD response when epratuzumab was administered more frequently (twice weekly × 4 for 8 doses). Two Phase II trials in adults (www.clinicaltrials.gov identifiers NCT01219816 and NCT01279707) and a randomized Phase III trial in children with relapsed ALL (www.clinicaltrials.gov identifier NCT01802814) are underway in Europe and will provide additional insight into the activity of this agent.
ACKNOWLEDGMENTS
This work was supported in part by Children’s Oncology Group grants U10 CA98543 and U10 CA98413. The authors also acknowledge Immunomedics, Inc., for providing epratuzumab for this study and BD Biosciences for providing reagents for flow cytometry. SPH is the Ergen Family Chair in Pediatric Cancer and JAW is the Women’s Auxiliary Millennium Chair in Haematology/Oncology.
Footnotes
CONFLICT OF INTEREST STATEMENT
Dr. Goldenberg is the Chairman and Chief Scientific Officer of Immunomedics, Inc.; has finanical holdings in the company and has various patents on epratuzumab. Dr. Wegener is an employee of Immunomedics, Inc.
REFERENCES
- 1.Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, Winick NJ, Hunger SP, Gaynon PS, Loh ML. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, Ancliff P, Morgan M, Masurekar A, Goulden N, Green N, Revesz T, Darbyshire P, Love S, Saha V. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376:2009–2017. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, Ebell W, Escherich G, Schrappe M, Klingebiel T, Fengler R, Henze G, von Stackelberg A. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28:2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 4.Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, Camitta BM, Gaynon PS, Carroll WL. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected] J Clin Oncol. 2008;26:3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth M, Raetz E, Cairo MS. The future role of monoclonal antibody therapy in childhood acute leukaemias. Br J Haematol. 2012;159:3–17. doi: 10.1111/bjh.12002. [DOI] [PubMed] [Google Scholar]
- 6.Carnahan J, Wang P, Kendall R, Chen C, Hu S, Boone T, Juan T, Talvenheimo J, Montestruque S, Sun J, Elliott G, Thomas J, Ferbas J, Kern B, Briddell R, Leonard JP, Cesano A. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res. 2003;9:3982S–3990S. [PubMed] [Google Scholar]
- 7.Coleman M, Goldenberg DM, Siegel AB, Ketas JC, Ashe M, Fiore JM, Leonard JP. Epratuzumab: targeting B-cell malignancies through CD22. Clin Cancer Res. 2003;9:3991S–3994S. [PubMed] [Google Scholar]
- 8.Daridon C, Blassfeld D, Reiter K, Mei HE, Giesecke C, Goldenberg DM, Hansen A, Hostmann A, Frolich D, Dorner T. Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus. Arthritis Res Ther. 2010;12:R204. doi: 10.1186/ar3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi EA, Goldenberg DM, Michel R, Rossi DL, Wallace DJ, Chang CH. Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab. Blood. 2013;122:3020–3029. doi: 10.1182/blood-2012-12-473744. [DOI] [PubMed] [Google Scholar]
- 10.Leonard JP, Coleman M, Ketas JC, Chadburn A, Ely S, Furman RR, Wegener WA, Hansen HJ, Ziccardi H, Eschenberg M, Gayko U, Cesano A, Goldenberg DM. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin's lymphoma. J Clin Oncol. 2003;21:3051–3059. doi: 10.1200/JCO.2003.01.082. [DOI] [PubMed] [Google Scholar]
- 11.Leonard JP, Coleman M, Ketas JC, Chadburn A, Furman R, Schuster MW, Feldman EJ, Ashe M, Schuster SJ, Wegener WA, Hansen HJ, Ziccardi H, Eschenberg M, Gayko U, Fields SZ, Cesano A, Goldenberg DM. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: phase I/II clinical trial results. Clin Cancer Res. 2004;10:5327–5334. doi: 10.1158/1078-0432.CCR-04-0294. [DOI] [PubMed] [Google Scholar]
- 12.Advani A, McDonough S, Coutre S, Wood BL, Radich JP, Mims M, O'Donnell M, Elkins S, Becker MW, Othus M, Appelbaum FR. Southwest Oncology Group Study S0910: A Phase 2 Trial of Clofarabine/Cytarabine/Epratuzumab for Relapsed/Refractory Acute Lymphocytic Leukemia. ASH Annual Meeting Abstracts. 2012;120:2603. [Google Scholar]
- 13.Dorner T, Kaufmann J, Wegener WA, Teoh N, Goldenberg DM, Burmester GR. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther. 2006;8:R74. doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant BW, Jung SH, Johnson JL, Kostakoglu L, Hsi E, Byrd JC, Jones J, Leonard JP, Martin SE, Cheson BD. A phase 2 trial of extended induction epratuzumab and rituximab for previously untreated follicular lymphoma: CALGB 50701. Cancer. 2013;119:3797–3804. doi: 10.1002/cncr.28299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard JP, Coleman M, Ketas J, Ashe M, Fiore JM, Furman RR, Niesvizky R, Shore T, Chadburn A, Horne H, Kovacs J, Ding CL, Wegener WA, Horak ID, Goldenberg DM. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:5044–5051. doi: 10.1200/JCO.2005.13.821. [DOI] [PubMed] [Google Scholar]
- 16.Leonard JP, Schuster SJ, Emmanouilides C, Couture F, Teoh N, Wegener WA, Coleman M, Goldenberg DM. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 17.Micallef IN, Kahl BS, Maurer MJ, Dogan A, Ansell SM, Colgan JP, Geyer S, Inwards DJ, White WL, Habermann TM. A pilot study of epratuzumab and rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated, diffuse large B-cell lymphoma. Cancer. 2006;107:2826–2832. doi: 10.1002/cncr.22342. [DOI] [PubMed] [Google Scholar]
- 18.Micallef IN, Maurer MJ, Wiseman GA, Nikcevich DA, Kurtin PJ, Cannon MW, Perez DG, Soori GS, Link BK, Habermann TM, Witzig TE. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118:4053–4061. doi: 10.1182/blood-2011-02-336990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinfeld SD, Tant L, Burmester GR, Teoh NK, Wegener WA, Goldenberg DM, Pradier O. Epratuzumab (humanised anti-CD22 antibody) in primary Sjogren's syndrome: an open-label phase I/II study. Arthritis Res Ther. 2006;8:R129. doi: 10.1186/ar2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace DJ, Goldenberg DM. Epratuzumab for systemic lupus erythematosus. Lupus. 2013;22:400–405. doi: 10.1177/0961203312469692. [DOI] [PubMed] [Google Scholar]
- 21.Wallace DJ, Gordon C, Strand V, Hobbs K, Petri M, Kalunian K, Houssiau F, Tak PP, Isenberg DA, Kelley L, Kilgallen B, Barry AN, Wegener WA, Goldenberg DM. Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up. Rheumatology (Oxford) 2013;52:1313–1322. doi: 10.1093/rheumatology/ket129. [DOI] [PubMed] [Google Scholar]
- 22.Wallace DJ, Kalunian K, Petri MA, Strand V, Houssiau FA, Pike M, Kilgallen B, Bongardt S, Barry A, Kelley L, Gordon C. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Annals of the rheumatic diseases. 2014;73:183–190. doi: 10.1136/annrheumdis-2012-202760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA, Reid JM, Goldenberg DM, Wegener WA, Carroll WL, Adamson PC. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study. J Clin Oncol. 2008;26:3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borowitz MJ, Pullen DJ, Shuster JJ, Viswanatha D, Montgomery K, Willman CL, Camitta B. Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: relation to other risk factors. A Children's Oncology Group study. Leukemia. 2003;17:1566–1572. doi: 10.1038/sj.leu.2403001. [DOI] [PubMed] [Google Scholar]
- 25.London WB, Chang MN. One- and two-stage designs for stratified phase II clinical trials. Stat Med. 2005;24:2597–2611. doi: 10.1002/sim.2139. [DOI] [PubMed] [Google Scholar]
- 26.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanciu-Herrera C, Morgan C, Herrera L. Anti-CD19 and anti-CD22 monoclonal antibodies increase the effectiveness of chemotherapy in Pre-B acute lymphoblastic leukemia cell lines. Leukemia Research. 2008;32:625–632. doi: 10.1016/j.leukres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]