Abstract
Objective
To determine whether, among women with gestational diabetes (GDM), gestational weight gain above Institute of Medicine (IOM) guidelines increases the risk of large for gestational age (LGA) neonates.
Study Design
We conducted a retrospective cohort study of singleton term pregnancies with GDM delivered at University of North Carolina Women’s Hospital, Chapel Hill, NC from January 2002 to May 2010. We used Poisson regression modeling to estimate LGA risk (birth weight > 90th percentile for gestational age), by body mass index class and adherence to 2009 IOM weight gain guidelines. Women meeting IOM guidelines were the referent group. Final adjusted models included race/ethnicity, medical management of GDM, and gestational age at delivery.
Results
Among the 466 women studied, mean ± standard deviation birth weight was 3,526 ± 544 g; 18% (82/466) delivered LGA neonates. Birth weight was greatest among women exceeding, compared with meeting or gaining less than, IOM guidelines (3,703 ± 545 vs. 3,490 ± 505 vs. 3,328 ± 503, p = 0.001). Exceeding IOM guideline was associated with LGA among obese women (adjusted risk ratio 2.62, 95% confidence interval 1.25, 5.50) but not among overweight or normal weight women.
Conclusion
Targeting gestational weight gain, a modifiable risk factor, independent of GDM treatment, may decrease LGA risk. Women with GDM may benefit from tailored weight gain recommendations.
Keywords: gestational diabetes, gestational weight gain, large for gestational age, overweight, obesity, obesity
Women with gestational diabetes (GDM) are at an increased risk of large for gestational age (LGA) neonates due to hyperglycemia-mediated in utero fetal overgrowth.1 LGA, defined as birth weight > 90th percentile for gestational age, is associated with short- and long-term adverse outcomes, and these risks may be further compounded by maternal prepregnancy weight and weight gain, each of which is an independent risk factor for fetal overgrowth.2–4 Such fetal overgrowth increases the risk of shoulder dystocia and birth injury among women witha vaginal birth and increases the risk of both primary cesarean birth and unsuccessful trial of labor after a cesarean delivery.5,6 For the neonate, in utero overgrowth and LGA at birth are associated with increased risks of neonatal hypoglycemia as well as overweight, obesity, and glucose intolerance in later life.7
In 2009, the Institute of Medicine (IOM) replaced the former 1990 gestational weight gain guidelines.8,9 Under the new guidelines, recommended gestational weight gain remains specific to prepregnancy body mass index (BMI).8,9 More women are classified as above-normal weight, and recommended weight gain for obese women was lowered from “at least 15 lb” to “11 to 20 lb.”9 These guidelines aim to optimize maternal and infant outcomes in a low-risk population. It is not known whether adherence to these guidelines is equally applicable for women with the increasingly prevalent complication of GDM. A positive association between early excessive weight gain and later GDM3,10,11 as well as infant birth weight12–14 has been reported. However, GDM is not routinely diagnosed until the third trimester, and guidelines for a non-GDM population may no longer be appropriate for those women.
Management strategies to achieve optimal glycemic control and thereby decrease the risk of adverse outcomes exist for women with GDM. However, whether adherence to gestational weight gain guidelines is also associated with improved outcome is not known. In the current analysis, we sought to determine whether gestational weight gain above IOM weight gain guidelines increases the risk of LGA neonates among women with GDM.
Material and Methods
We performed a retrospective cohort study of women with singleton pregnancies who were diagnosed with GDM and had a term delivery at University of North Carolina Women’s Hospital (UNC), Chapel Hill, NC from January 1, 2002 to May 31, 2010. Multiple gestations, preterm birth < 37 0/7 weeks, women with pre-gestational diabetes mellitus and those without documented GDM screening and diagnostic test results were excluded. University of North Carolina Institutional Review Board approval was obtained for this study.
During the study period, GDM was diagnosed using a two-step process. Universal screening with a 50 g, 1-hour oral glucose load was performed between 24 and 28 weeks’ gestation, with plasma glucose 2: 140 mg/dL considered screen positive. Diagnostic testing included a 100 g, 3-hour oral glucose tolerance test (OGTT). Women meeting National Diabetes Group (NDDG) criteria15 were diagnosed with GDM and received nutritional counseling and instruction for glucose self-monitoring. Only women whose GDM screening followed this standard protocol were captured for this analysis. During the study period, the clinical protocol at our institution defined adequate glycemic control as 50% or more of blood glucose levels at goal levels (fasting < 105 mg/dL and 1-hour postprandial < 140 mg/dL or 2-hour postprandial < 130 mg/dL). Medical therapy was initiated (subcutaneous insulin or oral glyburide) or escalated if adequate glycemic control was not achieved, as determined by the primary obstetrical provider.
Data for this analysis were obtained from the UNC perinatal database (PD) and direct chart abstraction. The UNC PD is maintained by trained abstractors who enter information for each birth at UNC, abstracting data from the prenatal and intrapartum clinical record. Use of the UNC PD for research purposes has been described elsewhere.16 We obtained maternal demographic and pregnancy data from the PD, including self-reported race/ethnicity (choices in prenatal record were Caucasian, African American, Hispanic, Asian, or specify other). Gestational age was established by last menstrual period, if reliable, or by earliest ultrasound. Data abstractors entered the “final estimated due date” as recorded by the primary obstetric provider. Measured or self-reported height, prepregnancy self-reported weight, initial prenatal visit measured weight, and final prenatal visit measured weight was abstracted directly from the prenatal record.
Our primary study outcome was birth of a LGA neonate defined as gestational-age–specific birth weight above the 90th percentile, according to Oken et al.17 BMI (kg/m2) and gestational weight gain were calculated and classified as follows. BMI was calculated using height and self-reported prepregnancy weight, available for 75% of women. If pre-pregnancy weight was not available, measured first prenatal visit weight < 20 weeks was used. For the about 25% of women for whom we abstracted first prenatal visit weight, the median gestational age was less than 12 weeks, and BMI was similar between women with self-reported weight and first visit weight. Gestational weight gain was calculated by subtracting weight used for BMI calculation from weight at last prenatal visit 2: 35 weeks and within 2 weeks of delivery. All women with a term delivery had a prenatal care visit with weight recorded within 2 weeks of delivery.
We characterized our cohort by adherence to BMI-specific 2009 IOM gestational weight gain guidelines.9 Women who lost weight were classified as gaining less than IOM guidelines and included 1 normal weight woman, 1 overweight woman, and 19 obese women. We compared characteristics and laboratory data among women who gained less than, within, or more than the 2009 IOM guidelines. We compared proportion of women who adhered to 2009 IOM guidelines by BMI class (normal, overweight, and obese). These bivariate analyses were performed with one way ANOVA test for normally distributed continuous and Pearson chi-square for categorical variables. Pairwise comparisons were performed with Student t-test.
For all other analyses, we a priori classified women by BMI class as normal weight, overweight, and obese. Analyses were performed separately for each BMI class. We excluded the 1% (6/472) of women who were underweight from all analyses. Women who gained within IOM guidelines were the reference group. We estimated unadjusted and adjusted risk ratios (RR, aRR) with 95% confidence intervals (95% CI) for LGA using Poisson regression modeling, allowing for nonconvergence of statistical models. Covariates considered for inclusion in models included maternal demographics and laboratory data that differed by adherence to IOM guidelines. Covariates were considered significant if excluding them changed the effect estimate by > 10% or if they were independently associated with the outcome. Fasting blood glucose of the OGTT, while significant in bivariate analysis, did not impact magnitude of aRRs, significance, or precision of confidence intervals and was not forced into adjusted models. Final aRRs included race/ethnicity and need for medical management of GDM (dichotomized as diet-control vs. any pharmacologic treatment that included insulin and/or glyburide), which remained significant in the model, and gestational age at delivery, which the authors chose to force into the model.
Results
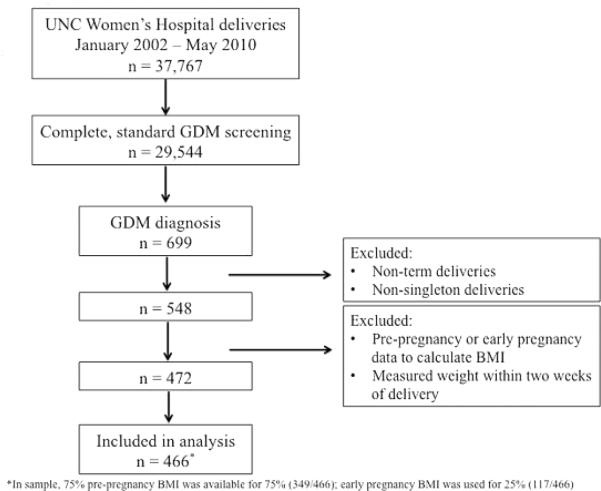
Over the 8-year study period, 37,767 women delivered at UNC Women’s Hospital, 29,544 had complete GDM screening results that followed the institution’s standard algorithm and 2.4% (699/29,544) were diagnosed with GDM. Of these, 78.4% (548/699) had term, singleton deliveries. Complete prepregnancy BMI and weight gain data were available for 86% (472/548), and the final cohort for analysis included 466 women after exclusion of the 6 underweight women. Women missing complete BMI and weight gain data, compared with the 466 included in analysis, were of similar maternal age at delivery, ethnicity, and gestational age at term delivery. Among these 466 women, prepregnancy BMI data were used for 75% (349/466), and early pregnancy BMI data were used for 25% (117/466). Study fiow chart is shown in Fig. 1. Demographic data are shown in Table 1 with bivariate analysis by adherence to IOM weight gain guidelines.
Fig. 1.
Flow chart of the study.
Table 1.
Maternal demographic and laboratory data by adherence to 2009 IOM guidelines
| < IOM recs (n = 149) |
= IOM recs (n = 124) |
> IOM recs (n = 193) |
|
|---|---|---|---|
| Mean ± SD or n (%) | |||
| Maternal age at delivery (y) | 32.0 ± 5.3 | 31.1 ± 5.5 | 30.5 ± 6.0 |
| Ethnicitya | |||
| Caucasian | 38 (26) | 28 (23) | 51 (26) |
| African American | 12 (8) | 6 (5) | 35 (18) |
| Hispanic | 99 (66) | 90 (73) | 107 (55) |
| Gestational age at term delivery (wks) | 39.1 ± 0.99 | 39.0 ± 0.97 | 39.2 ± 1.0 |
| 50 g 1-h oral glucose load | 175 ± 27.4 | 174 ± 23.8 | 174 ± 23.3 |
| 100 g 3-h oral glucose tolerance test | |||
| Fastinga | 82 ± 17.2 | 97 ± 20.0 | 99 ± 15.6 |
| 1 h | 207 ± 28.7 | 213 ± 30.2 | 211 ± 31.0 |
| 2 h | 191 ± 35.1 | 191 ± 39.0 | 190 ± 34.3 |
| 3 h | 146 ± 38.6 | 142 ± 43.0 | 138 ± 44.1 |
| Pharmacologic management (insulin or oral medication) | 69 (46) | 67 (54) | 115 (60) |
Abbreviations: IOM, Institute of Medicine; SD, standard deviation.
p < 0.05.
By baseline BMI, 24% (110/466), 36% (167/466), and 41% (189/466) of women were normal weight, overweight, and obese, respectively. Among three prepregnancy BMI classes absolute maternal weight gain differed (p = 0.01). In pairwise comparisons, mean ± standard deviation (SD) weight gain among normal weight women was 27 ± 15.6 lb, similar to mean weight gain among overweight women (25 ± 20.1 lb; p = 0.4) but significantly more than obese women (20 ± 18.5 lb; p = 0.002). Gestational weight gain was also significantly different between overweight and obese women in pairwise comparisons (p = 0.02).
Adherence to IOM weight gain guidelines also differed by prepregnancy BMI class as shown in Table 2 (overall p = 0.001). Normal weight women were most likely to gain less than IOM guidelines, compared with either overweight (45 vs. 28%, p = 0.002) or obese women (45 vs. 28%, p = 0.003). Obese women were more likely to gain more than IOM guidelines, compared with normal weight women (49 vs. 29%, p < 0.001). Other pairwise comparisons were not significant.
Table 2.
Adherence to 2009 IOM guidelines by BMI class
| Adherence to IOM guidelines | |||
|---|---|---|---|
| < IOMa
(n = 149) |
= IOM (n = 124) |
> IOM (n = 193) |
|
| BMI class | n (%) | ||
| Normal weight (n = 110) | 50 (45) | 28 (25) | 32 (29) |
| Overweight (n = 167) | 46 (28) | 55 (32) | 68 (41) |
| Obese (n = 189) | 53 (28) | 42 (23) | 93 (49) |
Abbreviations: BMI, body mass index; IOM, Institute of Medicine.
21 women (1 normal weight, 1 overweight, and 19 obese) lost weight and were classified as gaining < IOM guidelines; overall Pearson chi-square, p = 0.001.
Mean ± SD birth weight was 3,526 ± 544 g and was greatest among obese women, compared with overweight or normal weight women (3,703 ± 545 vs. 3,490 ± 505 vs. 3,328 ± 503 g, p < 0.001). A total of 18% (82/466) of women delivered a LGA neonate. Obese women delivered significantly more LGA neonates (49/189, 26%) than either overweight (24/167, 14%) or normal weight (9/110, 8%) women (p < 0.001). Using the women in each prepregnancy BMI class who met IOM weight gain guidelines as the reference group, we examined LGA risk by weight gain that was less than or exceeded IOM guidelines. These data are presented in Table 3. Exceeding IOM guidelines was associated with LGA in obese women in unadjusted (RR 2.44, 95% CI 1.18, 5.04) and adjusted analyses (aRR 2.62, 95% CI 1.25, 5.50) that included race/ethnicity, medical management of GDM, and gestational age at delivery. Among overweight and normal weight women, exceeding IOM guidelines was not associated with risk of LGA in unadjusted or adjusted analyses.
Table 3.
Risk of LGA by IOM guidelines in each BMI class
| Birth Weight (g)a | LGA (n)/ sample (n) |
< IOM guidelines (149/466) |
> IOM guidelines (193/466) |
|||
|---|---|---|---|---|---|---|
| RR (95% CI) | aRR (95% CI) | RR (95% CI) | aRR (95% CI) | |||
| Normal | 3,328 ± 503 | 9/110 | 0.28 (0.05, 1.44) | 0.53 (0.10, 2.74) | 0.66 (0.16, 2.70) | 0.42 (0.13, 1.40) |
| Overweight | 3,490 ± 505 | 24/167 | 0.96 (0.31, 2.95) | 1.05 (0.68, 4.19) | 1.69 (0.69, 4.16) | 1.68 (0.68, 4.19) |
| Obese | 3,703 ± 545 | 49/189 | 0.58 (0.20, 1.70) | 0.62 (0.21, 1.84) | 2.44 (1.18, 5.04) | 2.62 (1.25, 5.50) |
Abbreviations: aRR, adjusted risk ratio; BMI, body mass index; IOM, Institute of Medicine; LGA, large for gestational age; RR, unadjusted risk ratio.
Notes: Reference group, IOM weight gain guidelines met (124/466).
Normal (BMI 18.5–24.9), overweight (BMI 25.0–29.9), obese (BMI > 30.0); final models adjusted for race/ethnicity, medical management of GDM, and gestational age at delivery.
p < 0.001 for birth weight different by BMI class.
Discussion
Among a cohort of women diagnosed with GDM, obese women gained the least amount of weight during pregnancy but were more likely than normal weight women to exceed BMI-specific recommended weight gain guidelines. Nearly one in five neonates was LGA, with the greatest proportion among obese women. Exceeding recommended weight gain was associated with LGA among obese women with GDM, regardless of need for medical treatment. An increased LGA risk was also observed among overweight women but this was not statistically significant, perhaps due to a smaller number of women in this BMI class. These findings suggest weight gain in excess of IOM guidelines modifies LGA risk among obese women with GDM.
Our analysis, specific to women already diagnosed with GDM, is unique among others examining prepregnancy BMI, gestational weight gain, and adverse outcome risk. Several studies have demonstrated an association between early excessive weight gain and subsequent GDM or glucose intolerance.10,11,18,19 Maternal prepregnancy BMI has also been positively associated with GDM, independent of early weight gain,20 and excessive weight gain is independently associated with infant birth weight.12–14 These findings have prompted suggestions for preconception and early pregnancy counseling regarding maternal weight gain.
Focusing on women with GDM, who are typically already in the third trimester of pregnancy, has important clinical applications. Ouzounian et al reported excessive weight gain was associated with macrosomia, defined as birth weight > 4,000 g for their analysis, risk among women with diet-controlled GDM.21 While we report LGA instead of a precise birth weight, study comparisons are valid, as LGA and neonates > 4,000 g were almost identical in our cohort; only seven LGA neonates were not also > 4,000 g. Similar to our findings, while weight gain was lowest among obese women, they were most likely to exceed recommendations.21 However, while Ouzounian et al found an increased risk among all BMI classes and the greatest risk among overweight women, our findings were only significant among obese women. These differences may refiect cohort differences, as Ouzounian et al evaluated only diet-controlled gestational diabetics, a large proportion of Asian women, and an about 12% prevalence of macrosomia.21 Horosz et al evaluated a GDM cohort by adherence to IOM guidelines and reported an association between excessive GWG through time of third trimester diagnosis and LGA neonates among overweight and obese women, but their association was no longer significant when total GWG was considered.22 Treatment may have attenuated this relationship, and may explain differences in our findings.
Our cohort may represent women at higher baseline risk and with poorer glycemic control where gestational weight gain was not as substantial a factor in LGA risk. Nonetheless, our data suggest, among women with GDM, weight gain in excess of current guidelines may increase risk of neonatal LGA. Whether women with GDM may still benefit from third trimester interventions to decrease gestational weight gain is unknown. Women with GDM may be ideal candidates for interventions to slow their weight gain trajectory. Small trials in European cohorts have already suggested weight gain may be modified in obese women.23,24
Strengths of our study include a large sample size of women with GDM at a single institution. Clinical care guidelines were consistent during the 8 years included in our analysis. In addition, a single author abstracted weight data directly from the patient medical record. Limitations of this study include its retrospective design. Height and prepregnancy weight was self-reported, and weight may be under-reported. However, as routine clinical practice typically collects prepregnancy weight data by patient self-report, our research results may still be generalizable.
By including only term births, we may have excluded women whose preterm births were associated with either too little or too much weight gain. Excluding 15% of women otherwise meeting study inclusion criteria but without data to calculate BMI and weight gain likely did not impact results, as these women did not differ on other abstracted variables from those included. We did not differentiate weight gain before versus after GDM diagnosis. Thus, we do not know from our data whether the driving factor for LGA is weight gain before diagnosis, after initiation of GDM treatment, or a combination of both. Importantly, while exceeding recommended weight gain was not significantly associated with LGA among overweight women, this may be primarily due to our smaller sample of overweight women. Among normal weight women, the number of LGA infants was too small to provide meaningful information. Despite limitations, our data offer important insight for third trimester counseling and care of women with GDM, especially those who enter pregnancy obese and who are on a trajectory to exceed current guidelines for weight gain in pregnancy.
Up to 30% of women enter pregnancy obese, and even more are overweight.25 GDM prevalence has also risen in parallel with the increasing obesity epidemic.26 These factors, along with gestational weight gain, each contribute to infant birth weight. Current 2009 IOM guidelines for weight gain in pregnancy have classified more women as above normal BMI and lowered weight gain recommendations for obese women. Concern for small for gestational age (SGA) infants with too little weight gain among obese women has appeared to be of little concern,9 until a recent publication by Catalano et al readdressed this outcome and showed obese, as well as overweight, women gaining under 5 kg had increased SGA risk.27 These findings, as well as other neonatal anthropometrics, held true when diabetic status was considered.27 Whether current guidelines optimize neonatal outcomes without increasing risks and whether tailored guidelines may benefit women following a GDM diagnosis remains uncertain. Nonetheless, it remains an essential question to tackle for such a prevalent outcome as LGA, as close to one in five had an LGA infant in our cohort.
For women who enter pregnancy overweight or obese and are diagnosed with GDM, addressing gestational weight gain, in conjunction with current standard-of-care glycemic control efforts, may improve outcomes. Further, earlier screening and diagnosis of GDM may allow more time to implement tailored weight gain recommendations. Further studies should identify optimal weight gain for women with GDM. Interventions should be patient-centered, evaluate innovative ways of engaging patients to follow our evidence-based recommendations, and assess whether achieving recommended weight gain can modify risk of an LGA neonate and reduce the long-term consequences of maternal overweight and glucose intolerance for child health.
Footnotes
Note
The preliminary findings were presented at the 33rd Annual Meeting of The Society for Maternal-Fetal Medicine; February 11–16, 2013; San Francisco, CA.
Confiict of Interest
None of the authors report a confiict of interest.
References
- 1.Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. 2005;192(4):989–997. doi: 10.1016/j.ajog.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 3.Hedderson MM, Weiss NS, Sacks DA, et al. Pregnancy weight gain and risk of neonatal complications: macrosomia, hypoglycemia, and hyperbilirubinemia. Obstet Gynecol. 2006;108(5):1153–1161. doi: 10.1097/01.AOG.0000242568.75785.68. [DOI] [PubMed] [Google Scholar]
- 4.Heude B, Thiébaugeorges O, Goua V, et al. EDEN Mother-Child Cohort Study Group. Pre-pregnancy body mass index and weight gain during pregnancy: relations with gestational diabetes and hypertension, and birth outcomes. Matern Child Health J. 2012;16(2):355–363. doi: 10.1007/s10995-011-0741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan SP, Grobman WA, Gherman RA, et al. Suspicion and treatment of the macrosomic fetus: a review. Am J Obstet Gynecol. 2005;193(2):332–346. doi: 10.1016/j.ajog.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine . Nutrition during Pregnancy: Part I, Weight Gain; Part II, Nutrient Supplements. National Academy Press; Washington, DC: 1990. (Subcommittees on Nutritional Status and Weight Gain during Pregnancy and Dietary Intake and Nutrient Supplements During Pregnancy, Committee on Nutritional Status During Pregnancy and Lactation, Food and Nutrition Board) [Google Scholar]
- 9.Institute of Medicine . Weight Gain during Pregnancy: Reexamining the Guidelines. National Academy Press; Washington, DC: 2009. (Committee to Reexamine IOM Pregnancy Weight Guidelines, Food and Nutrition Board and Board on Children, Youth, and Families.) [Google Scholar]
- 10.Carreno CA, Clifton RG, Hauth JC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Excessive early gestational weight gain and risk of gestational diabetes mellitus in nulliparous women. Obstet Gynecol. 2012;119(6):1227–1233. doi: 10.1097/AOG.0b013e318256cf1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson KS, Waters TP, Catalano PM. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet Gynecol. 2012;119(3):560–565. doi: 10.1097/AOG.0b013e31824758e0. [DOI] [PubMed] [Google Scholar]
- 12.Ferraro ZM, Barrowman N, Prud’homme D, et al. Excessive gestational weight gain predicts large for gestational age neonates independent of maternal body mass index. J Matern Fetal Neonatal Med. 2012;25(5):538–542. doi: 10.3109/14767058.2011.638953. [DOI] [PubMed] [Google Scholar]
- 13.Simas TA, Waring ME, Liao X, et al. Prepregnancy weight, gestational weight gain, and risk of growth affected neonates. J Womens Health (Larchmt) 2012;21(4):410–417. doi: 10.1089/jwh.2011.2810. [DOI] [PubMed] [Google Scholar]
- 14.Crane JM, White J, Murphy P, Burrage L, Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J Obstet Gynaecol Can. 2009;31(1):28–35. doi: 10.1016/s1701-2163(16)34050-6. [DOI] [PubMed] [Google Scholar]
- 15.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 16.Berggren EK, Boggess KA, Stuebe AM, Jonsson Funk M. National Diabetes Data Group vs Carpenter-Coustan criteria to diagnose gestational diabetes. Am J Obstet Gynecol. 2011;205(3):e1–e7. doi: 10.1016/j.ajog.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115(3):597–604. doi: 10.1097/AOG.0b013e3181cfce4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herring SJ, Oken E, Rifas-Shiman SL, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol. 2009;201(1):e1–e7. doi: 10.1016/j.ajog.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 21.Ouzounian JG, Hernandez GD, Korst LM, et al. Pre-pregnancy weight and excess weight gain are risk factors for macrosomia in women with gestational diabetes. J Perinatol. 2011;31(11):717–721. doi: 10.1038/jp.2011.15. [DOI] [PubMed] [Google Scholar]
- 22.Horosz E, Bomba-Opon DA, Szymanska M, Wielgos M. Maternal weight gain in women with gestational diabetes mellitus. J Perinat Med. 2013;41(5):523–528. doi: 10.1515/jpm-2012-0254. [DOI] [PubMed] [Google Scholar]
- 23.Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34(12):2502–2507. doi: 10.2337/dc11-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bogaerts AF, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van den Bergh BR. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes (Lond) 2013;37(6):814–821. doi: 10.1038/ijo.2012.162. [DOI] [PubMed] [Google Scholar]
- 25.Guelinckx I, Devlieger R, Beckers K, Vansant G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes Rev. 2008;9(2):140–150. doi: 10.1111/j.1467-789X.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 26.Langer O, Yogev Y, Xenakis EM, Brustman L. Overweight and obese in gestational diabetes: the impact on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1768–1776. doi: 10.1016/j.ajog.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 27.Catalano PM, Mele L, Landon MB, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]