Abstract
Background/Objectives
The rise in the number of elderly kidney transplant recipients over the past decade makes it increasingly important to understand factors affecting post-transplant outcomes in this population. Our objective was to investigate the racial/ethnic differences in graft and patient survival among elderly kidney transplant recipients.
Design
Retrospective Cohort.
Setting & Participants
All first-time, kidney-only transplant recipients ≥60 years of age at transplantation in the United Network for Organ Sharing (UNOS) database, transplanted between July 1996 and October 2010, N=44,013.
Measurements
Time to graft failure and death obtained from the UNOS database and linkage to the Social Security Death Index. Neighborhood poverty from 2000 U.S. Census geographic data.
Results
Of the 44,013 recipients in the sample, 20% were African American, 63% non-Hispanic white, 11% Hispanic, 5% Asian and the rest “other racial groups”. In adjusted Cox models, we found that compared to whites, African Americans were more likely to experience graft failure (HR: 1.23, 95%CI: 1.15, 1.32), while Hispanics, (HR: 0.77, 95%CI: 0.70, 0.85) and Asians (HR: 0.70, 95%CI: 0.61, 0.81) were less likely to experience graft failure. Secondly, compared to whites, African Americans (HR: 0.84, 95%CI: 0.80, 0.88), Hispanics (HR: 0.68, 95%CI: 0.64, 0.72), and Asians (HR: 0.62, 95%CI: 0.57, 0.68) all were less likely to die after renal transplantation.
Conclusion
Elderly African Americans are at increased risk of graft failure compared to white transplant recipients, but survive longer after transplantation. Asians have the highest patient and graft survival followed by the Hispanics. Further studies are needed to assess additional factors affecting graft and patient survival including outcomes such as quality of life.
Keywords: elderly, racial disparities, patient survival, graft survival, kidney transplantation
INTRODUCTION
Over the past decade, the number of elderly patients living with End Stage Renal Disease (ESRD) has increased, with about 48% of the ESRD population now over the age of 60 (1, 2). Similarly, the number of kidney transplant recipients who are over age 65 has increased from 2.4% to 16% over the past two decades (3–6). Kidney transplantation is the preferred treatment for most patients with ESRD because it offers increased patient survival and improved quality of life as compared to dialysis for both younger and older recipients (2, 7, 8).
The rise in the number of older kidney transplant recipients makes it increasingly important to understand the factors affecting graft and patient survival in the elderly population. Race has been shown to be an important factor affecting graft and patient survival in kidney transplant recipients (9–11). In the general population, African American kidney transplant recipients have been shown to have worse graft and patient survival compared to white recipients for living and deceased donor transplants (10–14). However, it is not known if the racial differences in graft and patient survival in the general kidney transplant population is also seen among the elderly transplant recipient population. A consensus workshop held on organ transplantation in the elderly emphasized the critical need to identify factors underlying disparities in transplant outcomes in the elderly (15). Understanding the role of race and other factors in graft and patient survival in the elderly is crucial in reducing disparities and improving outcomes in this unique patient population
The purpose of this study was to evaluate the assosciation of race/ethnicity with graft and patient survival following transplantation among elderly (age >60) kidney transplant recipients. We also aimed to determine potentially modifiable factors that may be associated with graft and patient survival in the elderly kidney transplant population. We hypothesized that, consistent with results in the general kidney transplant recipient population, elderly African Americans would have worse graft and patient survival compared to white recipients and Asians and Hispanics would have better graft and patient survival compared to white recipients.
METHODS
Data Sources
Data was obtained from three databases: the United Network for Organ Sharing (UNOS), Social Security Death Index (SSDI) and US Census geographic data for year 2000. UNOS is a private organization contracted by the government to manage the transplant waiting list, match donors to recipients, and maintain information about every transplant recipient in the U.S. including follow-up data. The SSDI database is a publicly available national database of death records extracted from the U.S. Social Security Administration’s Death Master File. The Census 2000 data on neighborhood poverty were linked to the recipient’s residential zip codes in the UNOS data using zip code tabulation areas.
Data from UNOS was merged with death date information from the SSDI using unique encrypted recipient codes to calculate post-transplant survival time.
Study Population
Our study population was drawn from the Organ Procurement and Transplantation Network (OPTN) data received from UNOS. We restricted our population to kidney transplant recipients aged 60 and older who were transplanted between July 1996 and October 2010. Patients were followed for outcomes through December 2011. A total of 44,013 patients were included in our analysis.
Measures
The main study outcomes were time from kidney transplantation until death (patient survival time) and time from kidney transplantation until graft failure (graft survival time). People who did not experience any of the two study outcomes were censored at the end of the follow-up period (December 31, 2011). In the analysis of graft survival, recipients who died before graft failure occurred were censored at the time of death (i.e. death with a functioning graft was censored rather than treated as graft failure). This was done in an attempt to capture only those with a recorded graft failure event, since death could be a result of multiple causes and not just a failed allograft.
The primary variable of interest was recipient race/ethnicity (self-reported in most cases or as assessed by the transplant center coordinator). Race/ethnicity was classified into five groups: Black/African American, White, Hispanic, Asian and Other. Recipient demographic factors examined included age at transplant and sex. Primary health insurance at transplantation and neighborhood poverty were considered proxies for socio-economic status (SES). Insurance was categorized as private, public (Medicaid, Medicare Fee for Service, Medicare & Choice, Department of VA, Other government insurance and Medicare (further detail not collected)) or Other (self, donation, free care, pending). The proportion of individuals residing below the federal poverty level in each 5-digit zip code was used to estimate the neighborhood poverty level using the 2000 U.S Census Bureau data. High neighborhood poverty were defined as areas in which more than 20% of the households were assigned below the federal poverty level (16).
Additional covariates of interest included recipient clinical characteristics including: the primary assigned cause of ESRD; categorized into five major groups (diabetes, hypertension, cystic kidney disease, glomerulonephritis and other) and years on dialysis defined as the number of years on dialysis prior to transplantation. Donor characteristics assessed included age and type of donor kidney (living or deceased) and for deceased donor whether the implanted allograft was a standard criteria or extended criteria donor (ECD).
Transplant characteristics were also assessed as covariates as described below. In order to account for changes in kidney transplantation practices over time such as immunosuppression medications and allocation systems, a covariate for the year of kidney transplantation was created and then categorized into three time periods; 1996–2000, 2001–2005 and 2006–2010. Other transplant factors evaluated were HLA mismatch, cold ischemia time and reported incidence of any acute rejection. An individual was classified as having experienced an acute rejection episode if they were reported in UNOS to have experienced acute, or hyper-acute rejection prior to graft failure or any episode of acute rejection recorded (whether or not they were treated for it) or if they had a kidney biopsy that confirmed acute rejection. Recipient age, donor age and cold ischemic time were analyzed as continuous variables.
Analysis
We performed a retrospective cohort analysis of the UNOS database. Kaplan-Meier product-limit curves were generated and stratified by race/ethnicity and we calculated log-rank statistics for differences between groups. All predictor variables were evaluated for adherence to the proportional hazards assumption using log-log survival curves, an extended Cox approach using time dependent variables, and a correlation analysis between Schoenfeld residuals and ranked follow-up time.
To evaluate the effect of race/ethnicity on patient and graft survival, separate multivariable Cox proportional hazard regression models were constructed for graft and patient survival time as a function of race/ethnicity. Crude and adjusted hazard ratios, along with 95% confidence intervals (CI), were computed for race/ethnicity and for all other covariates. Effect estimates for continuous variables, such as recipient age, donor age, years on dialysis and cold ischemia time, were calculated for a 10-unit change. Variables were considered to be confounders if they were associated with the exposure, race/ethnicity and the outcomes; graft failure and death. Employment was excluded because more than 30% of the variables were missing. We used the fully conditional specification method of multiple imputation for other missing covariate information (n=15,903 individuals) (17). For sensitivity analysis, we also conducted a complete case analysis. Our final model was adjusted for recipient age, gender, insurance, ESRD etiology, years on dialysis, neighborhood poverty level, donor type, donor age, period of transplantation, cold ischemia time, HLA mismatch and any acute rejection. All analyses were conducted using SAS 9.4 and data were evaluated at the 0.05 significance level.
Approval for this study was obtained from the Emory University IRB (#IRB00065148).
RESULTS
Table 1 shows the baseline demographic and clinical characteristics of patients in our sample stratified by racial/ethnic groups. Of the 44,013 transplant recipients analyzed, the median age was 65 (inter-quartile range = 7.0). The population was predominantly male (62.5%) and white (62.4%). Diabetes was the most common cause of ESRD (33.7%) among all races/ethnicities except African Americans, where it was hypertension (40.0%). African Americans had the highest percentage of acute rejection episodes (11.8%); pre-transplant dialysis (93.9%) and non-ECD kidneys (55.2%). Asians received the largest percentage of ECD kidneys (31.5%). Hispanics had the largest (41.1%) and whites had the lowest (7.8%) percentage of people living in the high neighborhood poverty areas.
Table 1.
Baseline Characteristics of Elderly (≥60 years) Renal Transplant Recipients from July 1996 - October 2010 By Race/Ethnicity
| Characteristic | Overall- n (%) | White- n (%) | Black- n (%) | Hispanic- n (%) | Asian- n (%) | Other- n (%) | |
|---|---|---|---|---|---|---|---|
| 44,013 (100) | 27,481 (62.4) | 8,903 (20.2) | 4,669 (10.6) | 2,095 (4.8) | 865 (2.0) | ||
| Recipient Demographics | |||||||
| Recipient Age | Median (IQR) | 65 (7.0) | 65 (7.0) | 64 (6.0) | 64 (6.0) | 65 (6.0) | 64 (5.0) |
| Gender | Male | 27,512 (62.5) | 17,669 (64.3) | 5,128 (57.6) | 2,957 (63.3) | 1,238 (59.1) | 520 (60.1) |
| Total | 44,013 | 27,481 | 8,903 | 4,669 | 2,095 | 865 | |
| Insurance Type# | Private | 13,585(31.1) | 9,407(34.3) | 2,183(24.5) | 1,097(23.5) | 1,664(31.7) | 234(27.1) |
| Public or other | 30,394(68.9) | 18,050(65.77) | 6,716(75.5) | 3,568(76.5) | 1,429(68.3) | 631(72.9) | |
| Total | 43,979 | 27,457(100) | 8,899(100) | 4,665(100) | 2,093(100) | 865(100) | |
| Neighborhood Poverty# | >20% (high) | 7,653 (17.39) | 2,008 (7.56) | 3,267 (37.83) | 1,835 (41.08) | 245 (12.09) | 298 (35.65) |
| ≤20% (low) | 34,858 (79.20) | 24,536 (92.44) | 5,370 (62.17) | 2,632 (58.92) | 1,782 (87.91) | 538 (64.35) | |
| Total | 42,511 | 26,437 | 8,623 | 4,456 | 2,022 | 833 | |
| Employment# | No | 19,393 (81.1) | 11,341 (77.9) | 4,212 (86.2) | 2,417 (88.1) | 1,053 (82.0) | 370 (84.7) |
| Yes | 4,513 (18.9) | 3,211 (22.1) | 676 (13.8) | 328 (11.9) | 231 (18.0) | 67 (15.3) | |
| Total | 23,906 | 14,552 | 4,888 | 2,745 | 1,284 | 437 | |
| Recipient Clinical Characteristics | |||||||
| ESRD Etiology# | Diabetes | 13,488 (33.7) | 6,759 (27.8) | 3,233 (38.1) | 2,225 (50.8) | 782 (38.9) | 489 (60.4) |
| Hypertension | 10,039 (25.1) | 5,082 (20.9) | 3,389 (40.0) | 935 (21.4) | 503 (25.0) | 130 (16.0) | |
| Cystic kidney Disease | 3,598 (9.0) | 2,964 (12.2) | 290 (3.4) | 228 (5.2) | 91 (4.5) | 25 (3.1) | |
| Glomerulonephritis | 6,128 (15.3) | 4,536 (18.6) | 703 (8.3) | 469 (10.7) | 321 (16.0) | 99 (12.2) | |
| Other | 6,774 (16.9) | 5,011 (20.6) | 862 (10.2) | 519 (11.9) | 315 (15.7) | 67 (8.3) | |
| Total | 40,027 | 24,352 | 8,477 | 4,376 | 2,012 | 810 | |
| Pre-transplant Dialysis# | No | 6,122 (13.9) | 5,045 (18.4) | 545 (6.1) | 294 (6.3) | 178 (8.5) | 60 (7.0) |
| Yes | 37,848 (86.1) | 22,408 (81.6) | 8,355 (93.9) | 4,366 (93.7) | 1,916 (91.5) | 803 (93) | |
| Total | 43,970 | 27,453 | 8,900 | 4,660 | 2,094 | 863 | |
| Years on Dialysis# | Median (IQR) | 2.47 (2.81) | 2.02(2.29) | 3.44(3.27) | 3.15(3.30) | 3.09(3.11) | 3.35(3.09) |
| Donor Characteristics | |||||||
| Type of Donor# | Living | 13,212 (30.0) | 9,761 (35.5) | 1,588 (17.8) | 1,234 (26.4) | 422 (20.1) | 207 (23.9) |
| Deceased (non-ECD) | 21,387 (48.6) | 12,646 (46.0) | 4,914 (55.2) | 2,375 (50.9) | 1,014 (48.4) | 438 (50.6) | |
| ECD- Cadaveric | 9,412 (21.4) | 5,073 (18.5) | 2,400 (27) | 1,060 (22.7) | 659 (31.5) | 220 (25.4) | |
| Total | 44,011 | 27,480 | 8,902 | 4,669 | 2,095 | 865 | |
| Donor Age | Mean (SD) | 43.2 (16.0) | 43.3 (15.7) | 43.1 (16.4) | 42.2 (16.0) | 44.7 (17.6) | 43.4 (16.0) |
| Transplant Characteristics | |||||||
| Period of Transplant | 1996–2000 | 8,389 (19.1) | 5,546 (20.2) | 1,614 (18.1) | 775 (16.6) | 282 (13.5) | 172 (19.9) |
| 2001–2005 | 15,210 (34.6) | 9,606 (35) | 2,960 (33.2) | 1,626 (34.8) | 688 (32.8) | 330 (38.2) | |
| 2006–2010 | 20,414 (46.4) | 12,329 (44.9) | 4,329 (48.6) | 2,268 (48.6) | 1,125 (53.7) | 363 (42) | |
| Total | 44,013 | 27,481 | 8,903 | 4,669 | 2,095 | 865 | |
| HLA Mismatch# | Zero | 3,897 (8.9) | 3,046 (11.1) | 294 (3.3) | 419 (9.0) | 80 (3.8) | 58 (6.7) |
| One | 1,901 (4.3) | 1,446 (5.3) | 222 (2.5) | 157 (3.4) | 46 (2.2) | 30 (3.5) | |
| Two | 4,605 (10.5) | 3,300 (12.1) | 609 (6.9) | 470 (10.1) | 142 (6.8) | 84 (9.7) | |
| Three | 8,535 (19.5) | 5,834 (21.3) | 1,411 (15.9) | 880 (18.9) | 254 (12.2) | 156 (18.1) | |
| Four | 8,948 (20.4) | 5,342 (19.5) | 2,003 (22.6) | 950 (20.4) | 479 (23.0) | 174 (20.1) | |
| Five | 10,402 (23.7) | 5,557 (20.3) | 2,745 (30.9) | 1,184 (25.5) | 691 (33.2) | 225 (26.0) | |
| Six | 5,536 (12.6) | 2,827 (10.3) | 1,590 (17.9) | 591 (12.7) | 391 (18.8) | 137 (15.9) | |
| Total | 43,824 | 27,352 | 8,874 | 4,651 | 2,083 | 864 | |
| Cold Ischemia Time | Median (IQR) | 15 (18.0) | 14 (19.0) | 16.3 (13.0) | 15.5 (15.0) | 15 (14.3) | 14 (13.5) |
| Any Acute Rejection | No | 39,577 (89.9) | 24,698 (89.9) | 7,852 (88.2) | 4,289 (91.9) | 1,956 (93.4) | 782 (90.4) |
| Yes | 4,436 (10.1) | 2,783 (10.1) | 1,051 (11.8) | 380 (8.1) | 139 (6.6) | 83 (9.6) | |
| Total | 44,013 | 27,481 | 8,903 | 4,669 | 2,095 | 865 | |
Missing data insurance type=0.08%, neighborhood poverty=3.41%, employment =46%, ESRD etiology =9.06%, pre-transplant dialysis=0.01%, years on dialysis= 20.24%, donor type=0.004%, HLA mismatch=0.43%, cold ischemia time = 7.88%.
Significant p value <0.05, ECD = Extended criteria donor, IQR= Interquartile range, ESRD = End Stage Renal Disease, HLA= Human Leukocyte Antigen, hrs. =hours.
Graft survival
Race/Ethnicity
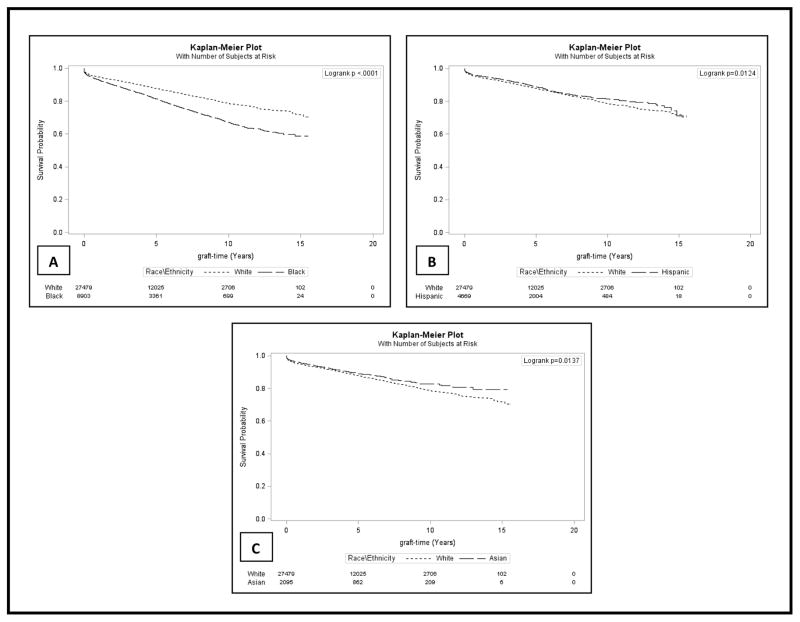
A total of 14.1% of patients experienced a graft failure event over the median of 4.3 years of follow up (IQR 2.2 – 7.0 years), and a greater proportion of African Americans had graft failure compared to whites, Hispanics, and other races (19.2% vs. 13%, 11.6%, and 16.6%) respectively. Table 2 depicts the crude and adjusted hazard ratios for graft failure for all the covariates examined. Compared to whites, African Americans were more likely to experience graft failure (HR: 1.23, 95%CI: 1.15, 1.32) while Hispanics (HR: 0.77, 95%CI: 0.70, 0.85) and Asians (HR: 0.70, 95%CI: 0.61, 0.81) were less likely to experience graft failure after adjusting for covariates (Table 2).
Table 2.
Unadjusted and Adjusted Hazard Ratios for Death and Graft Failure among Elderly (≥60 years) Renal Transplant Recipients from July 1996 - October 2010 by Race/Ethnicity
| Characteristic | Death | Graft Failure | |||
|---|---|---|---|---|---|
| Crude HR (95% CI) | Adjusted HR (95% CI) | Crude HR (95% CI) | Adjusted HR (95% CI | ||
| Recipient Demographics | |||||
| Race/Ethnicity | White | Ref | Ref | Ref | Ref |
| African American | 1.02 (0.98, 1.07) | 0.84 (0.80, 0.88) | 1.60 (1.51, 1.69) | 1.23 (1.15, 1.32) | |
| Hispanic | 0.81 (0.76, 0.86) | 0.68 (0.64, 0.72) | 0.89 (0.81, 0.98) | 0.77 (0.70, 0.85) | |
| Asian | 0.71 (0.65, 0.78) | 0.62 (0.57, 0.68) | 0.85 (0.74, 0.97) | 0.70 (0.61, 0.81) | |
| Other | 1.02 (0.92, 1.14) | 0.82 (0.74, 0.92) | 1.27 (1.08, 1.50) | 1.05 (0.88, 1.25) | |
| Recipient Age a | 1.55 (1.50, 1.60) | 1.47 (1.42, 1.52) | 1.02 (0.96, 1.08) | 0.94 (0.89, 1.00) | |
| Gender | Female | Ref | Ref | Ref | Ref |
| Male | 1.23 (1.19, 1.27) | 1.15 (1.11, 1.19) | 1.07 (1.02, 1.13) | 1.04 (0.98, 1.09) | |
| Insurance Type | Private | Ref | Ref | Ref | Ref |
| Public and other | 1.39 (1.34, 1.44) | 1.15 (1.11, 1.19) | 1.26 (1.19, 1.33) | 1.09 (1.03, 1.16) | |
| Neighborhood Poverty | ≤20% (low poverty) | Ref | Ref | Ref | Ref |
| >20% (high poverty) | 1.03 (0.99, 1.07) | 1.01 (0.97, 1.06) | 1.25 (1.18, 1.33) | 1.06 (0.99, 1.14) | |
| Recipient Clinical Characteristics | |||||
| ESRD Etiology | Diabetes | Ref | Ref | Ref | Ref |
| Hypertension | 0.79 (0.76, 0.82) | 0.72 (0.69, 0.75) | 1.05 (0.98, 1.12) | 0.95 (0.89, 1.02) | |
| Cystic kidney Disease | 0.46 (0.43, 0.50) | 0.43 (0.40, 0.46) | 0.66 (0.59, 0.73) | 0.65 (0.59, 0.73) | |
| Glomerulonephritis | 0.59 (0.56, 0.62) | 0.54 (0.51, 0.57) | 0.82 (0.75, 0.89) | 0.84 (0.77, 0.91) | |
| Other | 0.76 (0.72, 0.80) | 0.67 (0.64, 0.70) | 0.95 (0.88, 1.03) | 0.93 (0.87, 1.01) | |
| Years on Dialysisb | 1.39 (1.33, 1.46) | 1.39 (1.33, 1.45) | 1.49(1.39, 1.59) | 1.27 (1.17, 1.38) | |
| Donor Characteristics | |||||
| Type of Donor | Living | Ref | Ref | Ref | Ref |
| Deceased non-ECD | 1.43 (1.38, 1.49) | 1.33 (1.25, 1.41) | 1.54 (1.44, 1.65) | 1.38 (1.26, 1.51) | |
| Deceased - ECD- | 1.88 (1.80, 1.97) | 1.42 (1.33, 1.52) | 3.06 (2.85, 3.28) | 1.78 (1.61, 1.97) | |
| Donor Agea | 1.09 (1.08, 1.10) | 1.06 (1.05, 1.08) | 1.25 (1.23, 1.27) | 1.16 (1.13, 1.19) | |
| Transplant Characteristics | |||||
| Period of Transplant | 1996–2000 | Ref | Ref | Ref | Ref |
| 2001–2005 | 0.88 (0.85, 0.92) | 0.86 (0.83, 0.90) | 0.89 (0.84, 0.95) | 0.94 (0.88, 1.01) | |
| 2006–2010 | 0.73 (0.69, 0.76) | 0.66 (0.63, 0.70) | 0.74 (0.69, 0.80) | 0.74 (0.69, 0.80) | |
| HLA Mismatch | Zero | Ref | Ref | Ref | Ref |
| One | 1.05 (0.97, 1.15) | 1.10 (1.01, 1.20) | 0.99 (0.84, 1.17) | 1.04 (0.87, 1.23) | |
| Two | 0.93 (0.86, 1.00) | 1.07 (0.99, 1.15) | 1.06 (0.93, 1.20) | 1.16 (1.01, 1.33) | |
| Three | 1.00 (0.94, 1.07) | 1.10 (1.03, 1.17) | 1.22 (1.09, 1.37) | 1.25 (1.11, 1.40) | |
| Four | 1.16 (1.09, 1.23) | 1.12 (1.05, 1.20) | 1.68 (1.50, 1.88) | 1.35(1.20, 1.51) | |
| Five | 1.19 (1.12, 1.27) | 1.15 (1.08, 1.22) | 1.76 (1.58, 1.96) | 1.39 (1.24, 1.56) | |
| Six | 1.22 (1.14, 1.31) | 1.18 (1.10, 1.26) | 1.99 (1.77, 2.23) | 1.52 (1.34, 1.71) | |
| Cold Ischemia Timec | 1.14 (1.12, 1.15) | 1.03 (1.01, 1.05) | 1.21 (1.19, 1.24) | 1.08 (1.05, 1.11) | |
| Any Acute Rejection | No | Ref | Ref | Ref | Ref |
| Yes | 1.35 (1.29, 1.41) | 1.26 (1.20, 1.32) | 2.53 (2.38, 2.69) | 2.25 (2.11, 2.40) | |
Effect estimate calculated for a 10-year change in age.
Effect estimate calculated for a 10-year change in time on dialysis
Effect estimate calculated for a 10-hr change in time ischemia time. HR- Hazard ratio, Bold numbers represent statistically significant estimates Adjusted model adjusted for race/ethnicity, any acute rejection, ESRD etiology, Gender, HLA mismatch, pre-transplant dialysis, type of donor, recipient age, donor age and cold ischemia time, insurance and period of transplantation HR- Hazard ratio, Bold numbers represent statistically significant estimates.
Recipient Demographics
Compared to those with private insurance, recipients with public/other insurance were more likely to experience graft failure after adjusting for covariates (HR:1.09, 95%CI: 1.03, 1.16). Compared to those with low neighborhood poverty, those with high neighborhood poverty were more likely to experience graft failure, (HR: 1.25, 95%CI 1.18, 1.33) in the crude model but this association was not significant in the adjusted model (1.06, 0.99, 1.14).
Recipient Clinical Characteristics
After adjusting for covariates, compared to diabetics, patients with cystic kidney disease (HR: 0.65, 95%CI: 0.59, 0.73) and glomerulonephritis (HR: 0.84, 95%CI: 0.77, 0.91) were less likely to experience graft failure. Hypertension was not associated with graft failure (HR: 0.95, 95%CI 0.89, 1.02). A 10-year increase in years on dialysis was associated with a 27% higher risk of graft failure (HR: 1.27, 95%CI: 1.17, 1.38) (Table 2).
Donor Characteristics
Compared to persons who received living donor kidneys, those who received a deceased non-ECD kidneys (HR: 1.38, 95%CI: 1.26, 1.51) and those who received ECD kidneys (HR: 1.78, 95%CI: 1.61, 1.97) were more likely to experience graft failure (Table 2). A 10-year increase in donor age, was associated with a 16% higher risk of graft failure (HR: 1.16, 95%CI: 1.13, 1.19) in the adjusted models.
Transplant Characteristics
Compared to individuals transplanted between 1996 and 2000, recipients transplanted between 2001 and 2005 were less likely to experience graft failure in the crude model (HR: 0.89, 95%CI: 0.84, 0.95) but in the adjusted model, the HR was not statistically significant (HR: 0.94, 95%CI: 0.88, 1.01). Those transplanted between 2006 and 2010 were also less likely to experience graft failure when compared to those transplanted between 1996 and 2000, both in crude (HR: 0.74, 95%CI: 0.69, 0.80) and adjusted models (HR: 0.74, 95%CI: 0.69, 0.80).
Having 2 or more HLA mismatches was significantly associated with a higher likelihood of graft failure compared to zero HLA mismatches.
Recipients who experienced any acute rejection episode were significantly more likely to have graft failure compared to those without an acute rejection episode in crude (HR: 2.53; 95%CI: 2.38, 2.69) and adjusted models (HR: 2.25, 95%CI: 2.11, 2.40).
Figure 1 shows Kaplan-Meier estimates for unadjusted graft survival comparing African Americans, Hispanics and Asians to whites among elderly renal transplant recipients from July 1996 to October 2010 with a follow up until December 2011 (median follow up of 4.3 years).
Figure 1. Graft Survival among Elderly (>60 years) U.S. Renal Transplant Recipients from July 1996 to October 2010 with Follow-up through December 2011.
Kaplan-Meier estimates showing Unadjusted Graft Survival among Elderly Renal Transplant Recipients from July 1996 - October 2010 with recipient follow-up until December 2011
Patient Survival
Race/Ethnicity
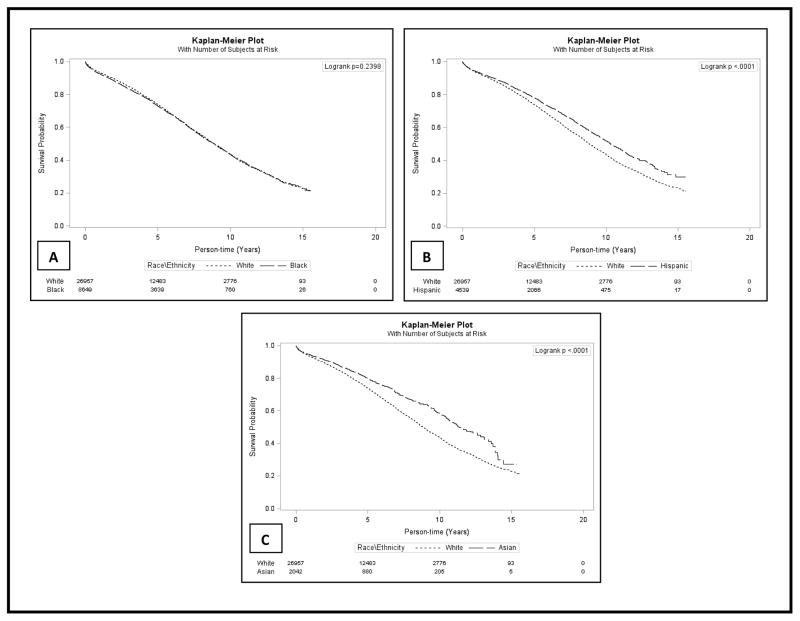
A total of 37.4% of patients died over the median of 4.6 years of follow up (IQR 2.5–7.3years), and a greater proportion of whites died compared to African Americans, Hispanics, and other races (38.8% vs. 37.7%, 32.3%, and 27.6%), respectively. The median survival time for all patients in our study population was 9.1 years, in whites it was 8.8 years, African Americans at 8.9 years, Asians at 11.3 years, Hispanics at 10.3 years and persons of other race/ethnicities at 8.7 years. Table 2 depicts the crude and adjusted hazard ratios for patient death by race and other covariates examined. Compared to whites, African Americans (HR: 0.84, 95%CI: 0.80, 0.88), Hispanics (HR: 0.68, 95%CI: 0.64, 0.72), and Asians (HR: 0.62, 95%CI: 0.57, 0.68) all had a lower likelihood of death.
Recipient Demographics
Compared to females, male recipients had a 15% higher likelihood of death (HR: 1.15, 95%CI: 1.11, 1.19). Compared to those with private insurance, recipients with public insurance had a 15% higher likelihood of death (HR: 1.15, 95%CI: 1.11, 1.19) in the adjusted model. A 10-year increment in recipient age was associated with a 55% (HR: 1.55, 95%CI: 1.50, 1.60) and 47% (HR: 1.47, 95%CI: 1.42, 1.52) higher rate of death in the crude and adjusted models, respectively. Neighborhood poverty was not associated patient survival (HR1.01, 95%CI: 0.97, 1.06).
Recipient Clinical Characteristics
After adjusting for covariates, recipients with hypertension as the primary etiology of ESRD (HR: 0.72, 95%CI: 0.69, 0.75), cystic kidney disease (HR: 0.43, 95%CI: 0.40, 0.46) and glomerulonephritis (HR: 0.54, 95%C.I: 0.51, 0.57) had a lower likelihood of death compared to persons with diabetes. A 10-year increase in years on dialysis, was associated with a 39% higher likelihood of death (HR: 1.39, 95%CI: 1.33, 1.45). Having 3 or more HLA mismatches was significantly associated with a higher likelihood of death compared to those with zero HLA mismatches.
Donor Characteristics
Persons who received deceased non-ECD kidneys (HR: 1.33, 95%CI: 1.25, 1.41) and more so, those who received deceased ECD kidneys (HR: 1.42, 95%CI: 1.33, 1.52) were at a higher risk of death compared to those who received living donor kidneys (Table 2). A 10-year increase in donor age was associated with a 6% higher likelihood of death (HR: 1.06, 95%CI: 1.05, 1.08).
Transplant Characteristics
Compared to individuals transplanted from 1996–2000, those transplanted from 2001 – 2005 (HR: 0.86, 95%CI: 0.83, 0.90) and 2006–2010 (HR: 0.66, 95%CI: 0.63, 0.70) all had a lower likelihood of death. Persons who experienced any acute rejection episode had a higher likelihood of death in the crude (HR: 1.35, 95%CI: 1.29, 1.41) and adjusted (HR: 1.26, 95%CI: 1.20, 1.32) model. Figure 2 a–c shows Kaplan-Meir estimates for unadjusted patient survival in African Americans, Hispanics and Asians compared to whites respectively over a median follow up of 4.6 years.
Figure 2. Patient Survival among Elderly (>60 years) U.S. Renal Transplant Recipients from July 1996 to October 2010 with Follow-up through December 2011.
Kaplan-Meier estimates showing Unadjusted Patient Survival among Elderly Renal Transplant Recipients from July 1996 - October 2010 with recipient follow-up until December 2011
Using complete case analysis, multivariable results for effect of race/ethnicity on patient or graft survival did not differ significantly from the main analysis, similarly, stratifying by age did not yield significant differences in trends for patient and graft survival in ages 60–70 vs >70 (supplemental tables 1 & 2).
DISCUSSION
This study sought to evaluate the effect of race/ethnicity on graft and patient survival among elderly (age >60) kidney transplant recipients and also to determine other factors that may be associated with graft and patient survival. A consensus paper on organ transplantation in the elderly observed that there was lack of data addressing age in minority and non-minority transplant recipients and stressed the need for studies identifying biologic, behavioral, and social mechanisms contributing to long- term post-transplant outcomes in the elderly (15).
The major findings in this study were, compared to whites, African Americans were more likely to experience graft failure (HR: 1.23, 95%CI: 1.15, 1.32), while Hispanics, (HR: 0.77, 95%CI: 0.70, 0.85) and Asians (HR: 0.70, 95%CI: 0.61, 0.81) were less likely to experience graft failure. Secondly, compared to whites, African Americans (HR: 0.84, 95%CI: 0.80, 0.88), Hispanics (HR: 0.68, 95%CI: 0.64, 0.72), and Asians (HR: 0.62, 95%CI: 0.57, 0.68) all were less likely to die after renal transplantation. These results indicate that race/ethnicity may be an independent risk factor for graft failure in the elderly, but does not necessarily impact patient survival in the same manner. Our findings support previous studies in the general kidney transplant population showing superior patient and graft survival in Hispanics (18–20), and Asians (21) and worse graft survival in African Americans compared to whites (9, 11, 22). Although patient survival has been shown to be equivalent (11, 23) or worse (10, 24, 25) in blacks versus non-blacks, we demonstrate higher patient survival in African Americans aged 60 and above compared to whites.
Previous studies have shown that the half-life of renal allografts after transplantation appears to be 30%–40% shorter in African Americans compared to whites (10, 26). In pediatric transplant recipients, Patzer et al showed that African Americans had the lowest graft survival rates prior to the three-year mark when Medicare eligibility ends (27). One may speculate that biological factors more than socio-economic factors affect graft loss in the elderly African Americans since these patients remain eligible for Medicare even after 3-years post-transplant and are better able to afford immunosuppression medication.
Our results on graft survival support the “Hispanic paradox” which has shown that although Hispanics have similarly low SES as African Americans, they have comparable or lower mortality rates than non-Hispanic whites in the US (18, 28). This paradox is described in the transplant population and hypothesized to be related to age, occurring predominantly in middle-aged and older Hispanics (18, 29, 30). Previous studies showed that characteristics of individual neighborhoods may have a vital but underappreciated impact on health outcomes even above individual-level SES (31–34). This study showed that insurance but not neighborhood poverty was associated with graft and patient survival. However, SES is complex and multifactorial and may not be fully represented by a single variable (34, 35). Therefore, an observed racial/ethnic disparity may not be completely independent of SES, (36) neither can these differences be completely accounted for by socioeconomic issues (37–39). In addition to psycho-social factors and access to care, other biological factors such as genetic, immune and pharmacokinetic factors can contribute to poorer graft outcomes in African Americans.
Our findings differ from previous studies which showed no difference in five-year patient survival rates between African Americans and white recipients (11). Foster et al. also found no significant difference in one and five year patient survival rates in African American and non-African Americans for both living and deceased donor kidney transplants (23). Our study showed that patients receiving ECD kidneys have the highest likelihood of death in the elderly (40, 41). This may be explained by increased immunosuppression use and a higher rate of acute rejection which also contributes to heightened immunosuppression and thus, more deaths (40).
African Americans, Hispanics and Asians were less likely to die after renal transplantation compared to whites, however; African Americans had worse graft survival than whites. We do not know the reason for this paradox, however, one possible explanation is that African Americans and Hispanics who are selected for transplant may have a lower cardiovascular disease burden and less severe co-morbidities compared to whites thus creating a survival bias as the healthiest candidates are being presented for transplantation. It has also been suggested that older, sicker Hispanics return to their country of origin resulting in less reported deaths (42, 43). On the other hand, heightened immunologic response to the allograft may cause a higher rate of graft loss in African Americans (44–47) but not necessarily lead to an increased risk of death. It is interesting to note that a survival paradox also exists in Hispanics and older non-Hispanics blacks on dialysis. Compared to non-Hispanic whites, Yan et al found that Hispanic dialysis patients had the lowest mortality risk, followed by non-Hispanic blacks above 30 years (48). Rhee et al found that Hispanics on dialysis had lower mortality compared to whites in all age groups but in blacks the lower mortality was only evident over the age of 40 years (42).
Hypertension was the leading cause of ESRD among African American recipients supporting previous studies showing that blacks are more likely to be labelled as having hypertensive renal disease even when other causes for ESRD exist (49, 50).
Our study is limited by its retrospective nature and the inability to adjust for unknown or unmeasured confounders. The acute rejection variable may not have captured everyone with an acute rejection. Also, according to UNOS, as of November, 2011, the percentage of deaths accessible in the SSDMF data decreased significantly due to data release issues with the Social Security Administration. As such, persons who died during the last month of follow up in our study, i.e. between November 2011 and December 2011, might not have been accurately captured. In addition, since we were interested in actual records of graft failure, recipients who died with a functioning graft were censored and not counted as failed grafts, even though it is possible that incipient graft failure was the reason for death. A unique strength of our study is the fact that we examined all persons in the UNOS database who received a transplant during the specified period and thus our results are representative of the elderly US transplant population.
Conclusion
In elderly transplant recipients, compared to whites, African Americans, Hispanics and Asians had higher patient survival, but only African Americans had worse graft survival relative to whites. Further studies may be needed to identify specific immunologic and non-immunological factors such as currently unmeasured socio-economic effects that may be implicated in graft loss. Identifying potentially novel factors that may explain these differences is essential in order to improve graft survival in the elderly and ensure equity in outcomes across all racial/ethnic groups.
Supplementary Material
Acknowledgments
Funding Sources: The author responsibilities were as follows: Titilayo O. Ilori, William McClellan, and Rachel E. Patzer designed the research study, Titilayo O. Ilori was responsible for writing the manuscript and had full access to all of the data in the study and takes primary responsibility for the integrity of the data, accuracy of the data analysis and final content of the manuscript. Demilade A. Adedinsewo, Titilayo O. Ilori, and Nosayaba Enofe performed the data analysis; Demilade A. Adedinsewo, Oluwaseun Odewole, Akinlolu Ojo, William McClellan, Nosayaba Enofe, and Rachel E. Patzer participated in writing the manuscript and all authors read and approved the final manuscript for submission. Titilayo O. Ilori receives educational support from the ACTSI. This study was supported in part from divisional funds of the Department of Nephrology, Emory University Atlanta GA. This publication was also supported in part by the National Heart, Lung, Blood and Sleep Institute, National Institutes of Health, through Grant Number R25 HL105401. REP is supported in part by the National Institute of Minority Health and Health Disparities (R24MD008077). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Special thanks to Dr. Laura Plantinga for proof-reading the manuscript.
Footnotes
Conflict of Interest: The authors declare no competing interests.
Author Contributions
The author responsibilities were as follows: Titilayo O. Ilori, William McClellan and Rachel Patzer designed the research study. Titilayo O. Ilori was responsible for writing the manuscript and had full access to all of the data in the study and takes primary responsibility for the integrity of the data, accuracy of the data analysis and final content of the manuscript. Demilade Adedinsewo, Nosayaba Enofe and Titilayo O. Ilori performed the data analysis; Demilade Adedinsewo, Oluwaseun Odewole, Nosayaba Enofe, Akinlolu Ojo, William McClellan and Rachel Patzer participated in writing the manuscript. All authors read and approved the final manuscript for submission.
Sponsor’s Role “none”.
References
- 1.USRDS; National Institutes of Health NIoDaDaKD, editor. US Renal Data System 2012. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: 2012. [Google Scholar]
- 2.Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQI (TM)) Conference. Clin J Am Soc Nephrol. 2008;3:471–480. doi: 10.2215/CJN.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartmann EL, Wu C. The evolving challenge of evaluating older renal transplant candidates. Adv Chronic Kidney Dis. 2010;17:358–367. doi: 10.1053/j.ackd.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Jager KJ, van Dijk PC, Dekker FW, et al. The epidemic of aging in renal replacement therapy: An update on elderly patients and their outcomes. Clin Nephrol. 2003;60:352–360. doi: 10.5414/cnp60352. [DOI] [PubMed] [Google Scholar]
- 5.Danovitch GM, Cohen DJ, Weir MR, et al. Current status of kidney and pancreas transplantation in the United States, 1994–2003. Am J Transplant. 2005;5(4 Pt 2):904–915. doi: 10.1111/j.1600-6135.2005.00835.x. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe RA, Merion RM, Roys EC, et al. Trends in organ donation and transplantation in the United States, 1998–2007. Am J Transplant. 2009;9(4 Pt 2):869–878. doi: 10.1111/j.1600-6143.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 7.Oniscu GC, Brown H, Forsythe JL. How great is the survival advantage of transplantation over dialysis in elderly patients? Nephrol Dial Transplant. 2004;19:945–951. doi: 10.1093/ndt/gfh022. [DOI] [PubMed] [Google Scholar]
- 8.Mendonca HM, Dos Reis MA, de Castro de Cintra Sesso R, et al. Renal transplantation outcomes: a comparative analysis between elderly and younger recipients. Clin Transplant. 2007;21:755–760. doi: 10.1111/j.1399-0012.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs RB, Nock SL, Spencer CE, et al. Racial disparities in renal transplant outcomes. Am J Kidney Dis. 1999;34:706–712. doi: 10.1016/S0272-6386(99)70397-5. [DOI] [PubMed] [Google Scholar]
- 10.Eckhoff DE, Young CJ, Gaston RS, et al. Racial disparities in renal allograft survival: a public health issue? J Am Coll Surg. 2007;204:894–902. doi: 10.1016/j.jamcollsurg.2007.01.024. discussion -3. [DOI] [PubMed] [Google Scholar]
- 11.Moore DE, Feurer ID, Rodgers S, Jr, et al. Is there racial disparity in outcomes after solid organ transplantation? Am J Surg. 2004;188:571–574. doi: 10.1016/j.amjsurg.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Patzer RE, Pearson TC. Racial disparities in kidney graft survival: Does donor quality explain the difference? Am J Transplant. 2012;12:1670–1671. doi: 10.1111/j.1600-6143.2012.04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakkera HA, O’Hare AM, Johansen KL, et al. Influence of race on kidney transplant outcomes within and outside the department of veterans affairs. J Am Soc Nephrol. 2005;16:269–277. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]
- 14.Fan PY, Ashby VB, Fuller DS, et al. Access and outcomes among minority transplant patients, 1999–2008, with a focus on determinants of kidney graft survival. Am J Transplant. 2010;10:1090–1107. doi: 10.1111/j.1600-6143.2009.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abecassis M, Bridges ND, Clancy CJ, et al. Solid-organ transplantation in older adults: current status and future research. Am J Transplant. 2012;12:2608–2622. doi: 10.1111/j.1600-6143.2012.04245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patzer RE, Amaral S, Wasse H, et al. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20:1333–1340. doi: 10.1681/ASN.2008030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 18.Gordon EJ, Caicedo JC. Ethnic advantages in kidney transplant outcomes: The Hispanic Paradox at work? Nephrol Dial Transplant. 2009;24:1103–1109. doi: 10.1093/ndt/gfn691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjertson DW. Determinants of long-term survival of adult kidney transplants: a 1999 UNOS update. Clin Transpl. 1999:341–352. [PubMed] [Google Scholar]
- 20.Gjertson DW. A multi-factor analysis of kidney graft outcomes at one and five years posttransplantation: 1996 UNOS Update. Clin Transpl. 1996:343–360. [PubMed] [Google Scholar]
- 21.Katznelson S, Gjertson DW, Cecka JM. The effect of race and ethnicity on kidney allograft outcome. Clin Transpl. 1995:379–394. [PubMed] [Google Scholar]
- 22.Butkus DE, Meydrech EF, Raju SS. Racial differences in the survival of cadaveric renal allografts. Overriding effects of HLA matching and socioeconomic factors. N Engl J Med. 1992;327:840–845. doi: 10.1056/NEJM199209173271203. [DOI] [PubMed] [Google Scholar]
- 23.Foster CE, Philosophe B, Schweitzer EJ, et al. A decade of experience with renal transplantation in African-Americans. Ann Surg. 2002;236:794–804. doi: 10.1097/00000658-200212000-00012. discussion -5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. New Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 25.Ojo AO, Hanson JA, Wolfe RA, et al. Long-term survival in renal transplant recipients with graft function. Kidney Int. 2000;57:307–313. doi: 10.1046/j.1523-1755.2000.00816.x. [DOI] [PubMed] [Google Scholar]
- 26.Young CJ, Gaston RS. Renal transplantation in Black Americans. New Engl J Med. 2000;343:1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 27.Patzer RE, Mohan S, Kutner N, et al. Racial and ethnic disparities in pediatric renal allograft survival in the United States. Kidney Int. 2015;87:584–592. doi: 10.1038/ki.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101:253–265. [PMC free article] [PubMed] [Google Scholar]
- 29.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis. 2001;11:496–518. [PubMed] [Google Scholar]
- 30.Markides KS, Eschbach K. Aging, migration, and mortality: Current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2005;60 (Spec2):68–75. doi: 10.1093/geronb/60.special_issue_2.s68. [DOI] [PubMed] [Google Scholar]
- 31.Massey DS. American apartheid - segregation and the making of the underclass. Am J Sociol. 1990;96:329–357. [Google Scholar]
- 32.Sampson RJ, Sharkey P. Neighborhood selection and the social reproduction of concentrated racial inequality. Demography. 2008;45:1–29. doi: 10.1353/dem.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenstein R. The Truly Disadvantaged, the Inner-City, the Underclass, and Public-Policy. Wilson, WJ: New York Times Book Review; 1987. Oct 25, p. 1. [Google Scholar]
- 34.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research - One size does not fit all. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 35.Winker MA. Measuring race and ethnicity: Why and how? JAMA. 2004;292:1612–1614. doi: 10.1001/jama.292.13.1612. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: The problem of residual confounding and the resiliency of race. Epidemiology. 1997;8:621–628. [PubMed] [Google Scholar]
- 37.Williams DR. Race/ethnicity and socioeconomic status: Measurement and methodological issues. Int J Health Serv. 1996;26:483–505. doi: 10.2190/U9QT-7B7Y-HQ15-JT14. [DOI] [PubMed] [Google Scholar]
- 38.Williams DR, Collins C. Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Rep. 2001;116:404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braveman P, Cubbin C, Marchi K, et al. Measuring socioeconomic status/position in studies of racial/ethnic disparities: Maternal and infant health. Public Health Rep. 2001;116:449–463. doi: 10.1093/phr/116.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier-Kriesche HU, Ojo A, Magee JC, et al. African-American renal transplant recipients experience decreased risk of death due to infection: Possible implications for immunosuppressive strategies. Transplantation. 2000;70:375–379. doi: 10.1097/00007890-200007270-00024. [DOI] [PubMed] [Google Scholar]
- 41.Miles CD, Schaubel DE, Jia X, et al. Mortality experience in recipients undergoing repeat transplantation with expanded criteria donor and non-ECD deceased-donor kidneys. Am J Transplant. 2007;7:1140–1147. doi: 10.1111/j.1600-6143.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- 42.Rhee CM, Lertdumrongluk P, Streja E, et al. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol. 2014;39:183–194. doi: 10.1159/000358497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peralta CA, Risch N, Lin F, et al. The Association of African Ancestry and elevated creatinine in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Nephrol. 2010;31:202–208. doi: 10.1159/000268955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerman RH, Kimball PM, Vanburen CT, et al. Possible contribution of pretransplant immune responder status to renal-allograft survival differences of black versus white recipients. Transplantation. 1991;51:338–342. doi: 10.1097/00007890-199102000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Gaston RS, Hudson SL, Deierhoi MH, et al. Improved survival of primary cadaveric renal-allografts in blacks with quadruple immunosuppression. Transplantation. 1992;53:103–109. doi: 10.1097/00007890-199201000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Neylan JF. Immunosuppressive therapy in high-risk transplant patients - Dose-dependent efficacy of mycophenolate mofetil in African-American renal allograft recipients. Transplantation. 1997;64:1277–1282. doi: 10.1097/00007890-199711150-00008. [DOI] [PubMed] [Google Scholar]
- 47.Ojo AO, Port FK, Held PJ, et al. inferior outcome of 2-haplotype matched renal-transplants in blacks - role of early rejection. Kidney Int. 1995;48:1592–1599. doi: 10.1038/ki.1995.452. [DOI] [PubMed] [Google Scholar]
- 48.Yan G, Norris KC, Yu AJ, et al. The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol. 2013;8:953–961. doi: 10.2215/CJN.09180912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittle JC, Whelton PK, Seidler AJ, et al. Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Arch Intern Med. 1991;151:1359–1364. [PubMed] [Google Scholar]
- 50.McClellan W, Tuttle E, Issa A. Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis. 1988;12:285–290. doi: 10.1016/s0272-6386(88)80221-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.